Abstract
Background
The basic defect in long QT syndrome type-III (LQT3) is an excessive inflow of sodium current during phase-3 of the action potential caused by mutations in the SCN5A gene. Most sodium channel blockers reduce the early (peak) and late components of the sodium current (INa and INaL) but ranolazine preferentially reduces INaL. We therefore evaluated the effects of ranolazine in LQT3 caused by the D1790G mutation in SCN5A.
Methods and Results
We performed an experimental study of ranolazine in TSA201 cells expressing the D1790G mutation. We then performed a long-term clinical evaluation of ranolazine in LQT3 patients carrying the D1790G mutation. In the experimental study, INaL was significantly higher in D1790G than in wild type channels expressed in the TSA201 cells. Ranolazine exerted a concentration-dependent block of INaL of the SCN5A-D1790G channel without reducing peak INa significantly. In the clinical study, among 8 patients with LQT3 and confirmed D1790G mutation, ranolazine had no effects on the sinus rate or QRS width but shortened the QTc from 509±41 msec to 451±26 msec, a mean decrease of 56±52 msec (10.6%, p=0.012). The QT shortening effect of ranolazine remained effective throughout the entire study period of 22.8±12.8 months. Ranolazine reduced the QTc at all heart rates but less so during extreme nocturnal bradycardia. A type I Brugada ECG was never noticed.
Conclusions
Ranolazine blocks INaL in experimental models of LQT3 harboring the SCN5A D1790G mutation and shortened the QT interval of LQT3 patients.
Keywords: long QT syndrome, torsade de pointes, drug therapy, ranolazine, SCN5A
The long QT syndrome (LQTS) type III (LQT3) is the third most common variant of the congenital LQTS.1 LQT3 differs from the more common (LQT1 and LQT2) variants of LQTS by important clinical and electrophysiological characteristics: 1) Patients with LQT3 develop arrhythmias less often but these are more likely to be lethal when they ultimately occur.2 2) In contrast to LQT1 and LQT2 patients, who develop arrhythmias primarily during stress or sudden startling, patients with LQT3 typically develop arrhythmias at rest, often while asleep.3 3) Experimental models of LQTS suggest that a) heart rate slowing is particularly arrhythmogenic in LQT3 and b) ß-adrenergic stimulation (rather than ß-blockade) is antiarrhythmic in LQT3.4, 5 Consequently, clinicians are often reluctant to initiate ß-blocker therapy in patients with LQT3 who are not protected from bradycardia by cardiac pacing.6, 7
The basic electrophysiologic defect in LQT3 is an excessive inflow of sodium current during phase 3 of the action potential caused by mutations in the SCN5A gene.1 Thus, sodium channel blockers, including lidocaine,8 mexiletine9-11 and flecainide12, 13 have been tested in LQT3. Specifically, in a large family with LQT3 caused by the D1790G mutation described in Israel,14 Benhorin demonstrated impressive QT-shortening effects with flecainide therapy.12 The enthusiasm to treat LQT3 patients with flecainide lessened, however, following reports of flecainide-induced Brugada pattern in patients with LQT3.15 Concern about this potentially proarrhythmic effect of flecainide in LQT3 increased when the same D1790G mutation described by Benhorin in patients with LQT3 was recognized in patients with a spontaneous type-I Brugada electrocardiogram (ECG) in the absence of drug therapy.16
Ranolazine is a relatively new medication (approved for the treatment of angina pectoris) that has unique electrophysiological properties.17-20 As opposed to other sodium channel blockers, which reduce both the early (peak) and late components of the sodium current (INa and INaL, respectively) during phase 0, 2 and phase 3 of the action potential, ranolazine preferentially reduces INaL.17, 21-24 Indeed, in a short-term clinical study involving 5 patients with LQT3, intravenous ranolazine shortened the QT interval without widening the QRS complex.25 Moreover, ranolazine reduces calcium overload of myocardial cells, which contributes to the triggering of LQT-mediated arrhythmias.26 Based on the above, ranolazine is a particularly attractive candidate therapy for LQT3. We therefore performed the present experimental and clinical study that included long-term evaluation of ranolazine in high-risk patients with LQT3.
Methods
We performed an experimental study of ranolazine in a TSA201 cells expressing the D1790G variant responsible for the LQT3 phenotype (Masonic Medical Research Laboratory, Utica, NY) followed by long-term clinical evaluation of ranolazine in LQT3 patients carrying the D1790G mutation (Tel Aviv Medical Center, Israel).
Experimental work
Functional characteristics of SCN5A-D1790G channels
Wild type (WT) and mutant constructs were cloned into a pcDNA3.1+ expression vector. The SCN5A-D1790G plasmid was a kind gift from Dr. R.S. Kass (NY, USA). Sodium channels were expressed in modified human embryonic kidney cell line (TSA201) as previously described.27 Briefly, TSA201 cells were co-transfected with SCN5A (WT or D1790G) and SCN1B using the Fugene6 method. In addition, CD8 cDNA was co-transfected as a reporter gene to visually identify transfected cells using Dynabeads (M-450 CD8 Dynal, Invitrogen, Carlsbad, CA, USA). Cells were cultured in Dulbecco's Modified Eagle's Medium with 10% FBS and 2 mM glutamine at 5% CO2. The cells were grown on polylysine coated 35 mm culture dishes and placed in a temperature-controlled chamber at 37°C for 24 to 72 hours. The biophysical properties of WT and mutant channels were then evaluated at room temperature. Membrane currents were measured using whole-cell patch-clamp techniques for cardiac INa.28 All recordings were obtained using an Axopatch 200B amplifier equipped with a CV-201A head stage (Axon Instruments, San Francisco, CA, USA). Macroscopic whole cell INa was recorded with bath solution containing (in mmol/L) 140 NaCl, 5 KCl, 1.8 CaCl2, 1 MgCl2, 2.8 Na Acetate, 10 HEPES, and 10 Glucose (pH 7.3 with NaOH). Tetraethylammonium Chloride (5 mmol/ L) was added to block TEA-sensitive native currents. 5pipettes were fabricated from borosilicate glass capillaries (1.5 mm O.D., Fisher Scientific, Pittsburgh, PA, USA). They were pulled using a gravity puller (Model PP-89, Narishige Corp, Tokyo, Japan) to obtain a resistances between 1-2 MΩ when filled with a solution containing (in mmol/L) 5 NaCl, 5 KCl, 130 CsF, 1.0 MgCl2, 5 EGTA and 10 HEPES (pH 7.2 with CsOH). Measurements were started 10 minutes after obtaining the whole-cell configuration to allow the current to stabilize. Currents were filtered with a four pole Bessel filter at 5 kHz and digitized at 50 kHz. Series resistance was electronically compensated at 75-85%. All patch-clamp data acquisition and analysis were performed using pCLAMP V10.0 (Axon Instruments, Foster City, CA, USA), EXCEL 2010 (Microsoft, Redmond, WA, USA) and ORIGIN 7.5 (Microcal Software, Northampton, MA, USA). Late sodium channel current (INaL) was measured at the end of a 300 ms depolarization from a holding potential of −120 mV to – 20 mV in the presence INa was measured as the TTX-sensitive current. The effect of ranolazine on late INa was evaluated at concentrations of 3.0, 10.0, and 100.0 μM. Ranolazine was dissolved in distilled water and freshly prepared as a stock of 10 mM before each experiment.
Clinical evaluation of ranolazine
Patient population
We approached carriers of the D1790G mutation described in Israel14 who had a history of LQTS-related symptoms or a QTc ≥500 msec at baseline ECG or during nocturnal bradycardia (in Holter recordings). Eight patients agreed to participate in this prospective study. Ranolazine (ranexa, Gilead Science, Inc.) was started at 500 mg twice daily and increased after 3 days to 1000 mg twice daily, a dose that was continued throughout the study.
Electrocardiographic measurements
The following measurements were made before and after the initiation of ranolazine therapy (every day during the first week and then every 3 months). Sinus rate, PR interval, QRS interval, QT and QTc were evaluated at rest (after 10 minutes supine), and during maximal “QT stretching”29, 30 and “QT stunning”31 in response to quick standing. Symptom limited exercise tests were conducted with the precordial leads placed on the second, third and fourth intercostal space to increase the ability to detect a type I Brugada pattern.32 We measured the QT interval when the sinus rate reached 100 beats/min, at maximal exercise and every minute during the recovery period for 5 minutes.33 Finally, we performed 12-lead Holter recordings at baseline and at least twice a year to monitor for the presence of nocturnal type I Brugada ECG and to study the effects of ranolazine on the QT interval during nocturnal bradycardia; specifically, for each patient we manually measured the QT interval and its preceding RR interval at 100 different points in time during the Holter recordings, with particular emphasis on steady slow heart rates recorded at night. We then estimated the QTc for each heart rate category using the Bazett formula34 and also the Fridericia35 and Framingham36 formulas that tend to over-correct the QTc to a lesser degree during bradycardia. For each 10-beats/min heart-rate range, we calculated the respective ΔQT (and ΔQTc), which is the difference between the QT (and QTc) measured before drug therapy and the QT (QTc) measured during ranolazine therapy for the specific heart rate range.
Statistical methods
Statistical significance of differences between unrelated variables was calculated using Mann–Whitney U test and between matched variables using a Wilcoxon rank sum test. Correlations between HR category and QT or QTc lowering were assessed using the Spearman's rank correlation coefficient. All clinical data were analyzed using SPSS for Windows version 15.0 by IBM.
The Internal Review Board of the Tel Aviv Medical Center and the Israel Ministry of Health approved the study protocol, including the off-labeled use of ranolazine for LQT3. All participants provided informed consent. The drug manufacturer (Gilead Sciences, Inc.) provided supplies of the study medication at no cost but provided no funding for the study and had no access to the study results. The study is registered in https://clinicaltrials.gov as NCT01728025.
Results
Experimental studies
Functional characteristics of ranolazine on SCN5A-D1790G channels
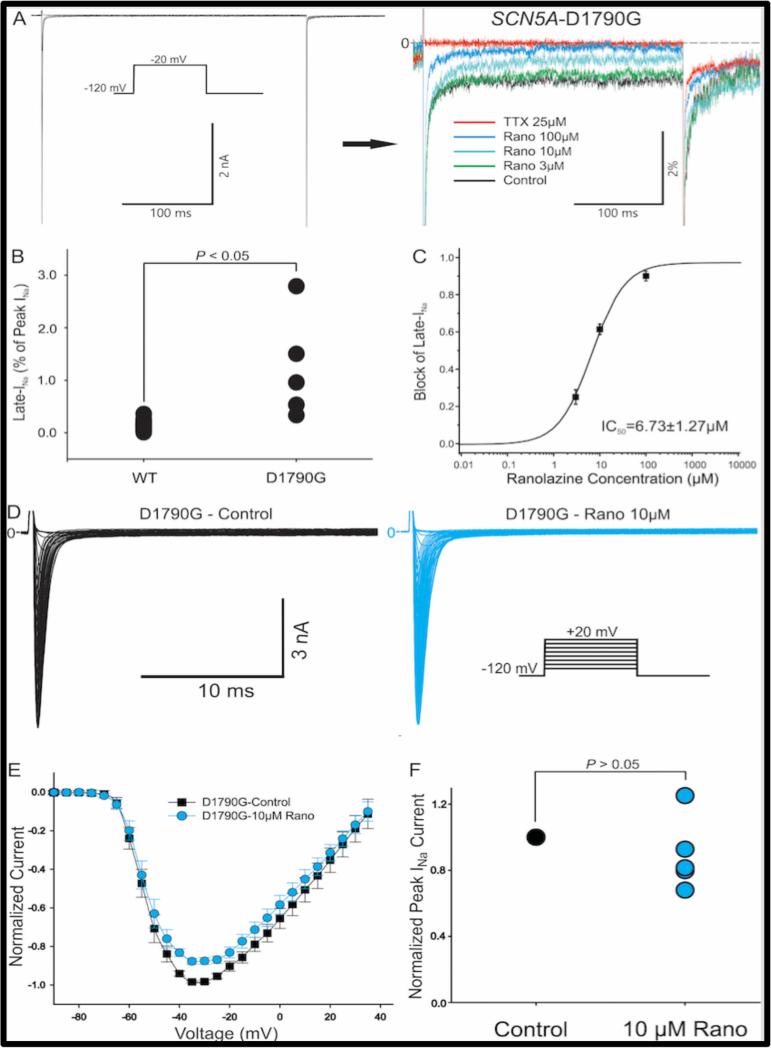
The magnitude of INaL (expressed as percentage of peak INa) was significantly higher in D1790G than in WT channels expressed in the TSA201 cells (1.22% ± 0.44% for D1790G, n=5; 0.14% ± 0.02% for WT, n=22, Figure 1B). Ranolazine exerted a concentration-dependent block of INaL of the SCN5A-D1790G channel (Figure 1A). The half maximal inhibitory concentration (IC50) was 6.73 ±1.27 μM (Figure 1C). Figure 1D shows the original traces of peak INa recorded before and after applying 10 μM Ranolazine. The normalized I-V relationship (Figure 1E) demonstrates that, compared to the control state, peak INa was not significantly reduced with the drug. Peak INa amplitude at −35 mV was decreased by 10.6% ± 9.8% after 10 μM ranolazine (n=5, P=0.31, Figure 1F).
Figure 1.
Effect of ranolazine on late sodium current (INa) of SCN5A-D1790G channels. A: Representative original traces depicting late INa recorded before and after increasing concentrations of ranolazine. B: Dot plot showing late INa as a % of peak INa in WT and SCN5AD1790G channels (n = 22 and 5, p < 0.05). C. Concentration–response relationship of the effect of ranolazine to inhibit late INa in SCN5A-D1790G channels. D. Representative traces depicting peak INa recorded before and after ranolazine. E: Normalized I-V relationship of the effect of 10 μM ranolazine on peak INa in SCN5A-D1790G channels. F: Dot plot of blocking effect of 10 μM ranolazine on peak INa of SCN5A-D1790G mutant (n = 5, p > 0.05).
Clinical studies
Baseline characteristics
Eight patients (4 males and 4 females, aged 41 ± 20 years, range 16-66 years) were enrolled in the study. Two patients already had an implanted ICD: a 24-year-old female with recurrent syncope and documented torsade de pointes and a 66-year-old male with symptomatic sinus node dysfunction. Six patients (including the 2 patients with implanted ICD) had previously received flecainide and have been reported.12 Additional medications included aspirin and ramipril for hypertension (2 patients) and thyroid supplement hormones (1 patient). For the entire patient cohort, the baseline RR interval was 1,105 ± 130 msec, the mean QT was 535 ± 48 msec and the mean QTc 509 ± 41 msec. The QTc at resting ECG was ≥500 msec in all but one patient, who had a QTc >500 msec during nocturnal bradycardia.
Compliance with study medications
Two female patients discontinued the study medication: patient 7 stopped after 1 month because of constipation and patient 1 stopped after 6 months because of pregnancy. A third (male) patient admitted temporary discontinuation of the study drug after missing one follow-up appointment and consequently running out of drug supplies. Compliance with drug therapy was otherwise excellent based on pill counting and stable QT intervals.
Effects of ranolazine on resting-ECG parameters
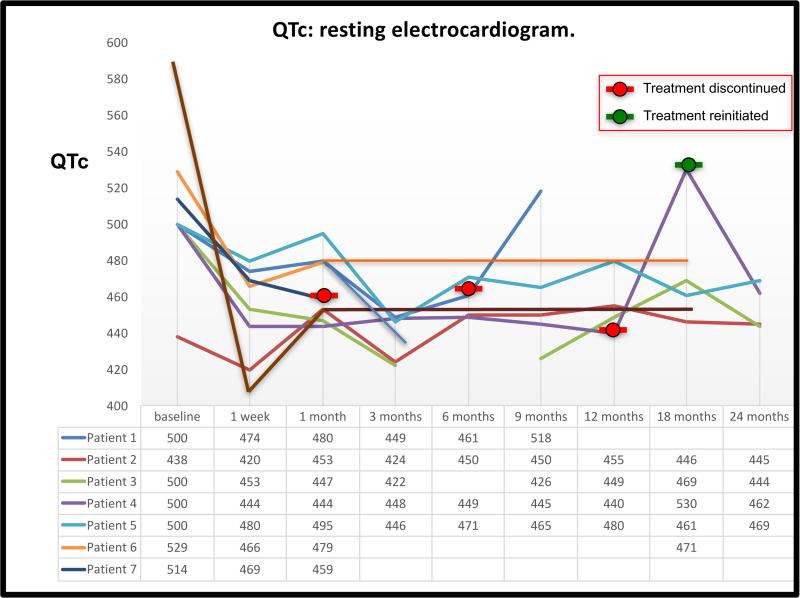
Ranolazine had no significant effects on the sinus rate or on the QRS width during repeated resting ECGs. After one week of therapy with ranolazine 1,000 mg BID the resting QTc shortened from 509 ± 41 msec to 451 ± 26 msec, a mean decrease of 56 ± 52 msec [11%] (p=o.012, Figure 2). After 6 months of therapy, the mean QTc during resting ECG was 455 ± 15 msec, representing a mean decrease of 52 ± 40 msec [9.9%] (p=0.017) from baseline. The QT shortening effect of ranolazine remained effective throughout the entire study period of 22.8 ± 12.8 months except for 3 patients in whom the QTc prolonged back to baseline values during drug discontinuation (Figure 2). At the time of last follow-up on therapy the mean QTc was shorter than baseline by 50 ± 38 msec (p=0.017). The three patients who had a return of QTc to baseline values include 2 female patients who dropped out of the study (because of constipation or because of pregnancy) and one male patient who ran out of study medications for 10 days (Figure 2). Importantly, 87% (7 out of 8) had a QTc ≥500 msec at resting ECG before therapy and this percentage dropped to zero during all follow-up visits while on therapy.
Figure 2.
QTc values (during resting ECG) before and during ranolazine therapy in 8 patients with LQT3. Each line represents QTc values of a single patient at baseline and during follow-up. Patient 6 (orange line) discontinued ranolazine after the 1-month follow-up and declined to come for follow-up ECG. Patient 1 (blue line) discontinued ranolazine after the 6 months follow-up and her QTc returned to her baseline values. Patient 4 (purple line) temporarily stopped ranolazine after the 12 months follow-up visit so his QTc increased (even above his baseline QTc), only to significantly decrease again after he restarted ranolazine.
Effects of ranolazine on the QTc during provocative tests
The QT shortening effect of ranolazine on the maximal QT stretching and QT stunning in response to quick standing was consistent across all patients studied and throughout the study period. As shown in Table 1, ranolazine significantly shortened the QTc during maximal QT stretching, whereas the QT-shortening effects did not reach statistical significance effects during QT-stunning, during exercise or during recovery from exercise. No patient exhibited a type I Brugada ECG in any ECG, either at rest, during exercise, during recovery from exercise or during nocturnal bradycardia (assessed by 12-lead Holter recordings).
Table 1.
Effects of ranolazine on the QT interval of LQT3 patients during provocative tests
| Before therapy (n=8) | Ranolazine 2000 mg/day (one week) (n=8) | Ranolazine 2000 mg/day (last follow up)* (n=8) | |
|---|---|---|---|
| Resting QTc | 509 ± 41 | 452 ± 26† | 458 ± 10† |
| Resting QRS | 96 ± 18 | 104 ± 20 | 106 ± 22 |
| QTc at maximal QT-stretching | 536 ± 32 | 483 ± 24‡ | 483 ± 36† |
| QTc at QT-stunning | 524 ± 56 | 454 ± 28 | 469 ± 23 |
| Exercise QTc during sinus rate 100/min | 465 ± 12 | 457 ± 16 | 448 ± 21 |
| Exercise QTc at maximal exercise | 446 ± 50 | 435 ± 48 | 435 ± 37 |
| Exercise QTc at 1 min recovery | 449 ± 103 | 404 ± 35 | 436 ± 18 |
| Exercise QTc at 5 min recovery | 465 ± 36 | 451 ± 31 | 458 ± 19 |
All values are presented in milliseconds.
Last follow-up denotes the last follow up on therapy, that is, without the values recorded after discontinuation of ranolazine in 3 patients (see text).
Denotes p <0.05 and
denotes p<0.005 in comparison to values before therapy.
Effects of ranolazine during nocturnal bradycardia
As expected, in the absence of therapy, our LQT3 patients had a disproportionate QT prolongation during nocturnal bradycardia, leading to bizarre T-wave morphologies (Figure 3A, 3C and 4A). Ranolazine partially prevented this nocturnal QT-prolongation (Figures 3D and 4D). Ranolazine significantly decreased the QT at every heart-rate range including during extreme bradycardia (Figure 4A). However, the QT and QTc shortening effects of ranolazine lessened during sinus bradycardia (Figure 4A). The QT/RR points measured during nocturnal bradycardia for heart rate <40 beats min (mean heart rate 35 ± 3 betas/min) are important. Within this heart rate category, the QT shortening effects of ranolazine reached statistical significance for the uncorrected QT (p=0.01, Figure 4A, 4C) and for the corrected QT using the Fridericia and Framingham formulas (p=0.005 and p=0.010, respectively). However, the QT shortening effects of ranolazine, when assessed by the Bazzet formula, reached statistical significance except for the <40 beats/min and the 60-70 beats/min subcategories (p=0.093 and p=0.063 respectively) (Figure 4B).
Figure 3.
Effects of ranolazine on the QTc during resting ECG and during nocturnal bradycardia. Resting ECG (A) and 12-lead Holter recording during maximal sinus bradycardia at night (C) in the same patient in the absence of drugs. Note that the baseline electrocardiogram, although showing a long QT interval (QTc 549 msec during sinus rate of 60/min) is not representative of the severe QT prolongation recorded during nocturnal bradycardia, when the QTc increases to 566 msec with distinctly abnormal T-waves. Panels B and D show ECG recordings during ranolazine therapy in the same patient. Note that ranolazine shortens the QT interval not only during resting ECG (B) but also during nocturnal bradycardia, partially normalizing the nocturnal T-wave morphology (E).
Figure 4.
QT-shortening effects of ranolazine at different heart rates. Box plots of the QT (panel A) and QTc (panel B) during different heart-rates in Holter recordings. The colored boxes (blue at baseline and red during ranolazine therapy) represent the interquartile range (25th to 75th percentiles); the thick black line in the box is the 50th percentile, and the bars represent the range of results excluding outliers. Open circles indicate outliers and * signs indicate extreme outliers. The ΔQT (QT at baseline minus QTc during ranolazine) and the ΔQTc for the different heart rate categories, including the calculated high and low 95% confidence intervals, are shown in panels C and D, respectively. Note that the ΔQTc is greater during sinus tachycardia than during nocturnal sinus bradycardia (4D).
The ΔQT showed a U-shaped curve (Spearman's rank correlation coefficient ρ=0.333, p=0.420, Figure 4C) whereas the ΔQTc (with the Bazett formula) demonstrated maximal QTc shortening effects by ranolazine during spontaneous sinus tachycardia but less QTc shortening effects during nocturnal bradycardia (Spearman's rank correlation coefficient ρ=0.714 , p= 0.047, Figure 4D). Importantly, although a QTc >500 msec was never observed in any resting ECG recorded while on ranolazine therapy, this highly abnormal value was seen in all patients at least once during nocturnal bradycardia while on ranolazine.
Inter-observer variability
The QTc values calculated by the two investigators were different (512.11 ± 13 Vs. 519.69 ± 42 for a total of 2089 QT/RR measurements in ECG and Holter recordings). Although this difference was of statistical significance (p=0.002), the absolute inter-observer difference of 7 msec had limited impact on the averaged 50 msec QTc shortening attributed to ranolazine therapy. Moreover, each of the two investigators independently found that ranolazine significantly shortens the QT interval in our patient population (data not shown).
Follow-up
Our patients have been followed for 19.3 patient years (28.8 ± 7.7 months per patient). Follow-up on therapy (after censoring for drug discontinuation) was 15.2 patient-years (22.8 ± 12.8 months per patient). There were no documented or suspected arrhythmias during follow-up. Adverse effects included constipation in two patients (leading to drug discontinuation in one).
Discussion
LQT3 patients are unique among patients with congenital LQTS because their arrhythmic events tend to occur predominantly at rest (often during sleep),3 a clinical characteristic reflecting the bradycardia-dependent proarrhythmic effects of vagal stimulation in LQT3.5 Consequently, whereas ß-blocker therapy is the mainstay of therapy in the more common types of LQTS, sodium channel blocker therapy with either mexiletine9-11 or flecainide12, 13 is increasingly being used to treat patients with LQT3. We report the first long-term study of ranolazine (a more specific blocker of INaL17, 21-24) for LQT3.
Main findings
We first show in experimental expression studies that the SCN5A mutation D1790G is a gain of function mutation with ≈3-fold increased INaL and demonstrate that ranolazine blocks this increased INaL current in a concentration dependent manner (Figure 1). At the concentration close to the IC50 on INaL, ranolazine displayed little effect on peak INa, inferring increased safety of ranolazine for treating LQT3 cases, who are at risk for developing Brugada syndrome during sodium channel blocker therapy.15, 16 We then demonstrate that ranolazine shortens the QT interval of D1790G carriers with LQT3 and show that the QT-shortening effect of ranolazine persisted during the entire 20 patient-year follow-up period. Importantly, the QT shortening effect of ranolazine persisted during nocturnal bradycardia, albeit to a lesser degree than during faster heart rates (Figure 4).
Previous studies
Experimental studies
Previous studies have reported that flecainide, but not lidocaine, corrects the disease phenotype in D1790G carriers by abbreviating QTc.12 Further investigation revealed that the D1790G mutation confers a unique pharmacological response in expressed channels.37 Lidocaine and flecainide differ in the way they block the cardiac sodium channel. Lidocaine interacts preferentially with the inactivated state of the channel so that block does not require channel openings,38, 39 whereas flecainide blocks the open state of the channel and does not depend on channels entering the inactivated state.39, 40 An open state block mechanism underlies the use–dependent blocking effect of flecainide on D1790G channels.41 Like flecainide, ranolazine is an open state blocker that unbinds rapidly from the closed state of the sodium channel but is trapped when the channel is in the inactivated state.42 Flecainide and ranolazine both reduce peak INa as well as INaL, but in the ventricular myocardium ranolazine more selectively blocks INaL and this selective INaL underlies the QTc shortening in D1790G carriers. The greater selectivity for INaL, rather than peak INa, makes ranolazine a better choice in this situation.
Previous clinical studies
The long-term effects of sodium channel blockers in LQT3 have been reported for flecainide12 and mexiletine.11 Benhorin et al recently updated their long-term experience with LQT3 patients (all carriers of the D1790G mutation)12 now including 30 patients treated with flecainide for 83 ± 73 months (Benhorin, personal communication). At the same time, Priori's group expanded their original experience with mexiletine10 to 34 LQT3 patients (with various mutations) treated for a median of 3 years.11 The baseline QTc of the patients treated with flecainide or mexiletine where similar to the baseline QTc of our patients (blue columns in Figure 5); it is therefore possible to compare the effects of these 3 medications on the resting QTc. The QT shortening effects of all 3 sodium channel blockers are comparable: 10% shortening for flecainide, 12% shortening for mexiletine11 and 11% shortening for ranolazine in the present study (red columns in Figure 5). Any drug studies of LQT3 patients should focus on the drug effects during nocturnal sinus bradycardia because cardiac arrest events in LQT3 tend to occur at night and it is reasonable to assume that these tragic events occur during when the heart rate is slowest and the QT prolongation is maximal. Of note, during extreme nocturnal bradycardia our patients had a mean uncorrected QT >700 msec (Figure 4A) with highly abnormal T-wave morphology suggesting “impending TdP” (Figure 3C). Detailed evaluation of the effects of sodium channel blockers on the maximal QT interval recorded during extreme nocturnal bradycardia is only available for ranolazine (Figure 4, present study) because previous studies only reported the effects of sodium-channel blockers on the QTc of the resting electrocardiogram (flecainide12 and mexiletine)11 or on the mean nocturnal QTc (mexiletine).11 Ranolazine shortened the QT and QTc at practically all heart rates but the degree of QT and QTc shortening (i.e., the ΔQT and ΔQTc) lessened as the sinus rate slowed down to <50 beats/min (Figures 4C, 4D). At slow heart rates, the IKr blocking effects of ranolazine increase,20 while its INaL blocker diminish. A new multicenter study is now evaluating the clinical effects of the highly selective INaL blocker eleclazine (GS-6615, Gilead Sciences, Inc., U.S.A) in a multicenter study of patients with LQT3.
Figure 5.
QT-shortening effects of different sodium channel blockers in LQT3. The data on flecainide and mexiletine are as reported by the groups of Benhorin12 and Priori,11 whereas data on ranolazine are from the present study. Although direct comparison between the QT shortening effects of flecainide, mexiletine and ranolazine has not been performed, the similar baseline QT values (blue columns in all three studies) suggest that all three drugs shorten the QT interval in LQT3 patients to a similar degree (red columns). Values shown for mexiletine represent the median values rather than the mean.
Limitations
1) The present study was limited to a single variant in SCN5A (the D1790G mutation). The efficacy of ranolazine to block late INa may be mutation-specific and may be diminished in cases in which the mutation is at or near the binding site of the drug. Additional studies are needed to assess the degree to which efficacy of the drug is compromised by mutations in close proximity to its binding site within the channel. 2) In contrast to data on mexiletine11 and flecainide (Benhorin, personal communication) demonstrating that mexiletine and flecainide significantly reduce the risk of symptomatic arrhythmic events in LQT3 patients, we could not present data on the antiarrhythmic effects of ranolazine because we included only one patient with documented TdP prior to the onset of therapy. This limitation is important because, despite the QT shortening effects of ranolazine during nocturnal bradycardia, QTc values >500 msec (a value that, when recorded during resting ECG correlates with persistent risk of spontaneous arrhythmic events during mexiletine therapy),11 were often observed in our patients during nocturnal bradycardia. 3) Automatic analysis of T-wave morphology changes observed during ranolazine therapy was not performed; this is a potential avenue for future research.
Conclusions
Ranolazine blocks INaL in experimental models of LQT3 harboring the D1790G mutation in SCN5A and significantly shortened the QT interval of LQT3 patients in this long-term prospective study. The QT shortening effects of ranolazine are comparable to the results published for flecainide and mexiletine. In contrast to flecainide, we never observed a type I Brugada ECG during long-term ranolazine therapy.
WHAT IS KNOWN
The long QT syndrome type-III (LQT3), the third most common type of long QT syndrome, is due to mutations in the SCN5A gene resulting in excessive inflow of the late sodium current during phase-3 of the action potential.
Most sodium channel blockers reduce the early (peak) and late components of the sodium current (INa and INaL) but ranolazine preferentially reduces INaL.
WHAT THE STUDY ADDS
In an experimental study of ranolazine in TSA201 cells expressing the D1790G mutation in SCN5A, ranolazine exerted a concentration-dependent block of INaL of the SCN5A-D1790G channel without reducing peak INa significantly.
In a clinical study of 8 patients with LQT3 and confirmed D1790G mutation, ranolazine had no effects on the sinus rate or QRS width but shortened the QTc by 56±52 msec (10.6%). The QT shortening effect of ranolazine remained effective throughout the entire 2-year study period. Ranolazine reduced the QTc at all heart rates but less so during extreme nocturnal bradycardia.
Acknowledgments
Sources of Funding: The experimental part of the study was conducted with funding from NIH grant HL47678 and a research grant from Gilead Sciences, Inc. The drug manufacturer (Gilead Sciences, Inc.) provided supplies of the study medication no cost but provided no funding for the clinical study and had no access to the study results.
Footnotes
Clinical Trial Registration - https://clinicaltrials.gov; Unique Identifier: NCT01728025.
Disclosures: Dr. Belardinelli was Senior Vice President, Cardiovascular Therapeutics at Gilead Sciences, manufacturer of the study drug (ranolazine) that was used in the study. Dr. Antzelevitch was also a consultant to Gilead Sciences, Inc. at the time of the study.
References
- 1.Wang Q, Shen J, Splawski I, Atkinson D, Li Z, Robinson JL, Moss AJ, Towbin JA, Keating JA. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995;80:805–811. doi: 10.1016/0092-8674(95)90359-3. [DOI] [PubMed] [Google Scholar]
- 2.Goldenberg I, Moss AJ. Long QT syndrome. J Am Coll Cardiol. 2008;51:2291–2300. doi: 10.1016/j.jacc.2008.02.068. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz PJ, Priori SG, Spazzolini C, Moss AJ, Vincent GM, Napolitano C, Denjoy I, Guicheney P, Breithardt G, Keating MT, Towbin JA, Beggs AH, Brink P, Wilde AA, Toivonen L, Zareba W, Robinson JL, Timothy KW, Corfield V, Wattanasirichaigoon D, Corbett C, Haverkamp W, Schulze-Bahr E, Lehmann MH, Schwartz K, Coumel P, Bloise R. Genotype phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103:89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 4.Shimizu W, Antzelevitch C. Cellular basis for the ECG features of the LQT1 form of the long QT syndrome. Effects of beta-adrenergic agonists and antagonists and sodium channels blockers on transmural dispersion of repolarization and torsade de pointes. Circulation. 1998;98:2314–2322. doi: 10.1161/01.cir.98.21.2314. [DOI] [PubMed] [Google Scholar]
- 5.Fabritz L, Damke D, Emmerich M, Kaufmann SG, Theis K, Blana A, Fortmuller L, Laakmann S, Hermann S, Aleynichenko E, Steinfurt J, Volkery D, Riemann B, Kirchhefer U, Franz MR, Breithardt G, Carmeliet E, Schafers M, Maier SK, Carmeliet P, Kirchhof P. Autonomic modulation and antiarrhythmic therapy in a model of long QT syndrome type 3. Cardiovasc Res. 2010;87:60–72. doi: 10.1093/cvr/cvq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz PJ, Spazzolini C, Crotti L. All LQT3 patients need an ICD: true or false? Heart Rhythm. 2009;6:113–120. doi: 10.1016/j.hrthm.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz PJ, Spazzolini C, Priori SG, Crotti L, Vicentini A, Landolina M, Gasparini M, Wilde AA, Knops RE, Denjoy I, Toivonen L, Monnig G, Al-Fayyadh M, Jordaens L, Borggrefe M, Holmgren C, Brugada P, De Roy L, Hohnloser SH, Brink PA. Who are the long-QT syndrome patients who receive an implantable cardioverter-defibrillator and what happens to them?: data from the European Long-QT Syndrome Implantable Cardioverter-Defibrillator (LQTS ICD) Registry. Circulation. 2010;122:1272–1282. doi: 10.1161/CIRCULATIONAHA.110.950147. [DOI] [PubMed] [Google Scholar]
- 8.An RH, Bangalore R, Rosero SZ, Kass RS. Lidocaine block of LQT-3 mutant human Na+ channels. Circ Res. 1996;79:103–108. doi: 10.1161/01.res.79.1.103. [DOI] [PubMed] [Google Scholar]
- 9.Priori SG, Napolitano C, Cantu F, Schwartz PJ. Differential response to Na+ channel blockade, beta-adrenergic stimulation and rapid pacing in a cellular model mimicking the SCN5A and HERG defects present in the long QT syndrome. Experimental basis for gene-specific therapy. Circ Res. 1996;78:1009–1015. doi: 10.1161/01.res.78.6.1009. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz PJ, Priori SG, Locati EH, Napolitano C, Cantu F, Towbin JA, Keating MT, Hammoude H, Brown AM, Chen LS, Colatsky TJ. Long QT syndrome patients with mutations of the SCN5A and HERG genes have differential responses to Na+ channel blockade and to increases in heart rate. Implications for gene-specific therapy. Circulation. 1995;92:3381–3386. doi: 10.1161/01.cir.92.12.3381. [DOI] [PubMed] [Google Scholar]
- 11.Mazzanti A, Maragna R, Faragli A, Monteforte N, Bloise R, Memmi M, Novelli V, Baiardi P, Bagnardi V, Etheridge SP, Napolitano C, Priori SG. Gene-Specific Therapy With Mexiletine Reduces Arrhythmic Events in Patients With Long QT Syndrome Type 3. J Am Coll Cardiol. 2016;67:1053–1058. doi: 10.1016/j.jacc.2015.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benhorin J, Taub R, Goldmit M, Kerem B, Kass RS, Windman I, Medina A. Effects of flecainide in patients with new SCN5A mutation: mutation-specific therapy for long-QT syndrome? Circulation. 2000;101:1698–1706. doi: 10.1161/01.cir.101.14.1698. [DOI] [PubMed] [Google Scholar]
- 13.Moss AJ, Windle JR, Hall WJ, Zareba W, Robinson JL, McNitt S, Severski P, Rosero S, Daubert JP, Qi M, Cieciorka M, Manalan AS. Safety and efficacy of flecainide in subjects with Long QT-3 syndrome (DeltaKPQ mutation): a randomized, double-blind, placebo-controlled clinical trial. Ann Noninvasive Electrocardiol. 2005;10:59–66. doi: 10.1111/j.1542-474X.2005.00077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benhorin J, Goldmit M, MacCluer JW, Blangero J, Goffen R, Leibovitch A, Rahat A, Wang Q, Medina A, Towbin J, Kerem B. Identification of a new SCN5A mutation, D1840G, associated with the long QT syndrome. Hum Mutat. 1998;12:72. doi: 10.1002/(SICI)1098-1004(1998)12:1<72::AID-HUMU19>3.0.CO;2-T. Mutations in brief no. 153. Online. [DOI] [PubMed] [Google Scholar]
- 15.Beinart R, Michailidis A, Gurevitz OT, Glikson M. Is flecainide dangerous in long QT-3 patients? Pacing Clin Electrophysiol. 2009;32:143–145. doi: 10.1111/j.1540-8159.2009.02190.x. [DOI] [PubMed] [Google Scholar]
- 16.Blich M, Efrati E, Marai I, Suleiman M, Gepstein L, Boulous M. Novel Clinical Manifestation of the Known SCN5A D1790G Mutation. Cardiology. 2015;132:228–232. doi: 10.1159/000437089. [DOI] [PubMed] [Google Scholar]
- 17.Antzelevitch C, Belardinelli L, Zygmunt AC, Burashnikov A, Di Diego JM, Fish JM, Cordeiro JM, Thomas G. Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation. 2004;110:904–910. doi: 10.1161/01.CIR.0000139333.83620.5D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antzelevitch C, Burashnikov A, Sicouri S, Belardinelli L. Electrophysiologic basis for the antiarrhythmic actions of ranolazine. Heart Rhythm. 2011;8:1281–1290. doi: 10.1016/j.hrthm.2011.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viskin S, Adler A. Ranolazine: Deja vu of the amiodarone story. Heart Rhythm. 2011;8:1291–1292. doi: 10.1016/j.hrthm.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Antzelevitch C, Belardinelli L, Wu L, Fraser H, Zygmunt AC, Burashnikov A, Di Diego JM, Fish JM, Cordeiro JM, Goodrow RJ, Jr., Scornik F, Perez G. Electrophysiologic properties and antiarrhythmic actions of a novel antianginal agent. J Cardiovasc Pharmacol Ther. 2004;9(Suppl 1):S65–83. doi: 10.1177/107424840400900106. [DOI] [PubMed] [Google Scholar]
- 21.Wu L, Shryock JC, Song Y, Li Y, Antzelevitch C, Belardinelli L. Antiarrhythmic Effects of Ranolazine in a Guinea Pig in Vitro Model of Long-QT Syndrome. J Pharmacol Exp Ther. 2004;310:599–605. doi: 10.1124/jpet.104.066100. [DOI] [PubMed] [Google Scholar]
- 22.Wu J, Cheng L, Lammers WJ, Wu L, Wang X, Shryock JC, Belardinelli L, Lei M. Sinus node dysfunction in ATX-II-induced in-vitro murine model of long QT3 syndrome and rescue effect of ranolazine. Prog Biophys Mol Biol. 2008;98:198–207. doi: 10.1016/j.pbiomolbio.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Burashnikov A, Di Diego JM, Zygmunt AC, Belardinelli L, Antzelevitch C. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation: differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation. 2007;116:1449–1457. doi: 10.1161/CIRCULATIONAHA.107.704890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antzelevitch C. Ranolazine: a new antiarrhythmic agent for patients with non-ST-segment elevation acute coronary syndromes? Nat Clin Pract Cardiovasc Med. 2008;5:248–249. doi: 10.1038/ncpcardio1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moss AJ, Zareba W, Schwarz KQ, Rosero S, McNitt S, Robinson JL. Ranolazine shortens repolarization in patients with sustained inward sodium current due to type-3 long-QT syndrome. J Cardiovasc Electrophysiol. 2008;19:1289–1293. doi: 10.1111/j.1540-8167.2008.01246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindegger N, Hagen BM, Marks AR, Lederer WJ, Kass RS. Diastolic transient inward current in long QT syndrome type 3 is caused by Ca2+ overload and inhibited by ranolazine. J Mol Cell Cardiol. 2009;47:326–334. doi: 10.1016/j.yjmcc.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu D, Barajas-Martinez H, Burashnikov E, Springer M, Wu Y, Varro A, Pfeiffer R, Koopmann TT, Cordeiro JM, Guerchicoff A, Pollevick GD, Antzelevitch C. A mutation in the beta 3 subunit of the cardiac sodium channel associated with Brugada ECG phenotype. Circ Cardiovasc Genet. 2009;2:270–278. doi: 10.1161/CIRCGENETICS.108.829192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu D, Barajas-Martinez H, Terzic A, Park S, Pfeiffer R, Burashnikov E, Wu Y, Borggrefe M, Veltmann C, Schimpf R, Cai JJ, Nam GB, Deshmukh P, Scheinman M, Preminger M, Steinberg J, Lopez-Izquierdo A, Ponce-Balbuena D, Wolpert C, Haissaguerre M, Sanchez-Chapula JA, Antzelevitch C. ABCC9 is a novel Brugada and early repolarization syndrome susceptibility gene. Int J Cardiol. 2014;171:431–442. doi: 10.1016/j.ijcard.2013.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viskin S, Postema PG, Bhuiyan ZA, Rosso R, Kalman JM, Vohra JK, Guevara-Valdivia ME, Marquez MF, Kogan E, Belhassen B, Glikson M, Strasberg B, Antzelevitch C, Wilde AA. The response of the QT interval to the brief tachycardia provoked by standing: a bedside test for diagnosing long QT syndrome. J Am Coll Cardiol. 2010;55:1955–1961. doi: 10.1016/j.jacc.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chorin E, Havakuk O, Adler A, Steinvil A, Rozovski U, van der Werf C, Postema PG, Topaz G, Wilde AA, Viskin S, Rosso R. Diagnostic value of T-wave morphology changes during “QT stretching” in patients with long QT syndrome. Heart Rhythm. 2015;12:2263–2271. doi: 10.1016/j.hrthm.2015.06.040. [DOI] [PubMed] [Google Scholar]
- 31.Adler A, van der Werf C, Postema PG, Rosso R, Bhuiyan ZA, Kalman JM, Vohra JK, Guevara-Valdivia ME, Marquez MF, Halkin A, Benhorin J, Antzelevitch C, Wilde AA, Viskin S. The phenomenon of “QT stunning”: the abnormal QT prolongation provoked by standing persists even as the heart rate returns to normal in patients with long QT syndrome. Heart Rhythm. 2012;9:901–908. doi: 10.1016/j.hrthm.2012.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veltmann C, Papavassiliu T, Konrad T, Doesch C, Kuschyk J, Streitner F, Haghi D, Michaely HJ, Schoenberg SO, Borggrefe M, Wolpert C, Schimpf R. Insights into the location of type I ECG in patients with Brugada syndrome: correlation of ECG and cardiovascular magnetic resonance imaging. Heart Rhythm. 2012;9:414–421. doi: 10.1016/j.hrthm.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz PJ, Crotti L. QTc behavior during exercise and genetic testing for the long-QT syndrome. Circulation. 2011;124:2181–2184. doi: 10.1161/CIRCULATIONAHA.111.062182. [DOI] [PubMed] [Google Scholar]
- 34.Bazett HC. An analysis of the time-relations of electrocardiograms. Heart. 1920;7:353–370. [Google Scholar]
- 35.Fridericia LS. Dir Systolendaeur in Elektrokardiogram bei normalen Menchen und bei Herzkranken. Acta Med Scand. 1920;53:469–486. [Google Scholar]
- 36.Sagie A, Larson MG, Goldberg RJ, Bengtson JR, Levy D. An improved method for adjusting the QT interval for heart rate (the Framingham Heart Study). Am J Cardiol. 1992;70:797–801. doi: 10.1016/0002-9149(92)90562-d. [DOI] [PubMed] [Google Scholar]
- 37.Abriel H, Wehrens XH, Benhorin J, Kerem B, Kass RS. Molecular pharmacology of the sodium channel mutation D1790G linked to the long-QT syndrome. Circulation. 2000;102:921–925. doi: 10.1161/01.cir.102.8.921. [DOI] [PubMed] [Google Scholar]
- 38.Bean BP, Cohen CJ, Tsien RW. Lidocaine block of cardiac sodium channels. J Gen Physiol. 1983;81:613–642. doi: 10.1085/jgp.81.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc Natl Acad Sci U S A. 1996;93:9270–9275. doi: 10.1073/pnas.93.17.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anno T, Hondeghem LM. Interactions of flecainide with guinea pig cardiac sodium channels. Importance of activation unblocking to the voltage dependence of recovery. Circ Res. 1990;66:789–803. doi: 10.1161/01.res.66.3.789. [DOI] [PubMed] [Google Scholar]
- 41.Zhu Y, Kyle JW, Lee PJ. Flecainide sensitivity of a Na channel long QT mutation shows an open-channel blocking mechanism for use-dependent block. Am J Physiol Heart Circ Physiol. 2006;291:H29–37. doi: 10.1152/ajpheart.01317.2005. [DOI] [PubMed] [Google Scholar]
- 42.Zygmunt AC, Nesterenko VV, Rajamani S, Hu D, Barajas-Martinez H, Belardinelli L, Antzelevitch C. Mechanisms of atrial-selective block of Na(+) channels by ranolazine: I. Experimental analysis of the use-dependent block. Am J Physiol Heart Circ Physiol. 2011;301:H1606–1614. doi: 10.1152/ajpheart.00242.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]