Abstract
Atypical enteropathogenic Escherichia coli (aEPEC) are heterogeneous strains in terms of serotypes, adherence patterns and the presence of novel virulence factors. This heterogeneity is intriguing, promoting studies trying to characterize these novel proteins and to better comprehend this pathotype group. In a previous study analyzing low‐molecular mass proteomes of four representative aEPEC strains of three different adhesion phenotypes, we classified proteins according to their annotated function, with most of them being involved in metabolism and transport; while some of them were classified as hypothetical proteins. The majority of the hypothetical proteins were homologue products of genes identified in the genome of enterohemorrhagic E. coli. One of the hypothetical proteins was annotated as Z2335, with orthologue in EPEC, and by bioinformatics analysis, this protein was revealed to be the universal stress protein F (UspF). Thus, herein we successfully obtained a recombinant UspF protein from aEPEC, which is a α/β, ATP‐binding protein involved in stress response, with comparable protein production among the four studied strains, but showing noteworthy differences when cultivated in different stress conditions, also present in other enterobacterial species, such as Shigella sonnei and Citrobacter freundii. Furthermore, our results confirm that the Usp protein superfamily encompasses a conserved group of proteins involved in stress resistance in aEPEC and other Enterobacteriaceae.
Keywords: atypical EPEC, hypothetical proteins, UspF protein, cloning, expression, α/β protein, stress
Abbreviations
- aEPEC
Atypical enteropathogenic Escherichia coli
- CAPS
N‐cyclohexyl‐3‐aminopropanesulfonic acid
- CD
circular dichroism
- CFU
colony‐forming unit
- EAEC
enteroaggregative E. coli
- EAF
EPEC adherence factor plasmid
- ETEC
enterotoxigenic E. coli
- LEE
Locus of Enterocyte Effacement
- STEC
Shiga toxin‐producing E. coli
- UspF
universal stress protein F
Introduction
Enteropathogenic Escherichia coli (EPEC) remain one the most important enteric pathogens infecting children and they are considered one of the main causes of persistent diarrhea worldwide.1 The hallmark of EPEC pathogenesis is the ability to cause attaching and effacing (A/E) lesions, which results from intimate bacterial adhesion to the intestinal epithelium, the effacement of local microvilli, with subsequent accumulation of polymerized actin and other host cytoskeleton proteins at the site of bacterial attachment, forming pedestal‐like structures.2 A/E lesion‐related genes are located in a pathogenicity island named the Locus of Enterocyte Effacement (LEE).3, 4
EPEC strains have been categorized into two subgroups, termed typical and atypical EPEC (aEPEC) based on the presence and the absence of EPEC adherence factor plasmid (EAF).5 The aEPEC subgroup has been considered an emerging bacterial pathogen, associated with both sporadic cases and outbreaks of diarrhea.6 Indeed, aEPEC isolates have been associated with diarrhea in several worldwide countries, including Brazil.7, 8, 9, 10, 11, 12, 13 The emergence of this pathogen has been intriguing and has triggered new studies in epidemiology and pathogenicity, which have been performed to characterize their virulence profiles and better comprehend this pathogroup.6, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28
One of the most interesting features of aEPEC isolates is their variability in the adherence patterns upon contact with epithelial cells in vitro. A previous study from our group analyzed and compared the low‐molecular mass proteomes of four representative aEPEC strains by 2D gel electrophoresis and LC–MS/MS, study that comprised three different adhesion phenotypes (localized‐like, aggregative and diffuse) and one non‐adherent isolate. We identified a total of 59 proteins, according to their annotated function, some of them were conserved in the four studied strains (Ec292/84, 9100/83, BA320, BA4013), with most of them being involved in metabolism, stress protection and transport; and some of them were still classified as hypothetical proteins. We also found that the majority of the hypothetical and filamentous proteins identified in these isolates were previously identified in the genome of enterohemorrhagic E. coli.29
One of the hypothetical proteins (Z2335, orthologue in EPEC) was revealed by bioinformatics analysis to be the multispecies universal stress protein F (UspF), also identified as the YnaF protein found in soluble E. coli K12 extracts, which has conserved an ATP‐binding site.30 The orthologue protein UspF in Salmonella spp is designated YnaF.31 Universal stress proteins (Usp) are widely spread proteins in nature. Usp proteins belong to the PF00582 superfamily (COG0589).32 The universal stress protein (Usp) superfamily represents a group of small cytoplasmic proteins whose expression is affected by a wide variety of internal or external stresses. As example, UspA is involved in protection of DNA from UV damage. In S. enterica serovar Typhimurium UspA is important for resistance to metabolic and oxidative stress and other types of stresses, like starvation, and the protein UspA contribute to pathogenicity of S. Typhimurium.33
Also, in Mycobacterium smegmatis the Usp Rv1636 was isolated and characterized, this protein binds to cAMP specifically with high affinity and to ATP with lower affinity.34 In M. tuberculosis, proteomic analysis revealed that an increase in protein levels of mycobacterial Usp causes an increase in KatG protein levels, in turn increasing phenotypic susceptibility to isoniazid which is a first line drug for the treatment of active and latent tuberculosis.35 The UspA protein plays a significant role in protecting Acinetobacter baumannii from H2O2, low pH, and the respiratory toxin 2,4‐dinitrophenol. In a mouse model of pneumonia, UspA is essential for A. baumannii pneumonia pathogenesis.36
Because in aEPEC no further information about Usp family proteins is available, the aim of the present work was to investigate the function and the prevalence in enterobacterial isolates of the hypothetical protein (Z2335). Thus, uspF gene was cloned and the UspF protein was expressed and purified. Herein, we successfully obtained a recombinant UspF protein from aEPEC, which is a α/β, ATP‐binding protein, involved in stress responses, with no production differences among the four studied aEPEC strains, but showing significant differences when cultivated in diverse stress conditions. Furthermore, the high prevalence of this protein among the enterobacterial species strength its universal function.
Results
Protein prediction by computational analysis
The uspF gene (435 bp) encodes a predicted protein of 168 amino acids, but the signal peptide was not detected by analysis with the SignalP 4.1 server (http://www.cbs.dtu.dk/services/SignalP/). The computational analysis, based on the presence of conserved structural and functional domains,37 revealed that UspF belongs to the Universal Stress Protein Family. This analiysis showed high similarity values with universal stress proteins of E. coli, Shigella and Salmonella (Table 1).
Table 1.
Identity Analysis of Multispecies UspF With Other Proteins Found in the BLASTp Analysis
| Protein | Gene identification | Multispecies UspF (Shigella) |
|---|---|---|
| Multispecies UspF (Shigella) | gi|15801753 | 100% |
| Putative Filament Protein (S. flexneri 2a str. 2457T) | gi|30062868 | 100% |
| Universal Stress Family Protein (E. coli MS 69‐1) | gi|301020540 | 99,4% |
| Filament Protein (E. coli O157:H7 str. Sakai) | gi|38703971 | 85,7% |
| Stress‐induced ATP‐binding protein (E. coli str. K‐12) | gi|89108222 | 85,1% |
| Putative Universal Stress Protein (Salmonella enterica serovar Tennessee str CDC07‐0191) | gi|238911963 | 78,6% |
| Hypothetical Protein (Salmonella enterica serovar Paratyphi B str SPB7) | gi|161613879 | 78% |
Recombinant multispecies universal stress protein F
The recombinant UspF protein was expressed in E. coli BL21 (DE3) pLyS as a cytosolic protein (HT‐UspF) fused at the N‐terminal with a His‐tag and an additional sequence (20 aa) defining thrombin cleavage site, resulting in protein yields of approximately 100–150 mg/L [Fig. 1(A)]. The protein was purified in a single step after elution of bound protein from loaded resin with imidazole containing buffer at concentrations ranging from 30 to 200 mM [Fig. 1(B)]. The purified protein remained completely soluble at high concentrations (20–30 mg/mL) even after prolonged storage at 4°C.
Figure 1.
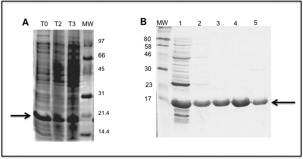
Expression of UspF protein by an IPTG‐inducible E. coli BL21 (DE3) pLyS strain and purification of soluble fractions containing UspF protein. A – T0 total protein extract before induction; T2, total protein extract after induction (3 h); T3, total protein extract after induction (16–18 h). The arrow indicates position of the UspF protein (18.4 kDa). B – Purification of soluble fractions or UspF protein by affinity chromatography using a nickel‐containing resin. 1–Flow through; 2–30 mM, 3–50 mM, 4–100 mM; 5–200 mM of imidazole.
Prediction of secondary structure in different conditions
Further evidence that the recombinant HT‐UspF preserved the structure of the native multispecies UspF was obtained by determination of circular dichroism analyses (CD) of the recombinant protein at different pH values. The CD spectrum of HT‐UspF (Fig. 2) is characteristic of a α/β protein with minimum at 208 and 222 nm. The CD spectra show that the protein UspF is more stable in neutral and basic pH, but in acid pH a structural loss occurs.
Figure 2.
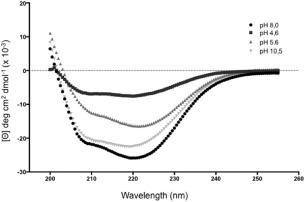
Circular dichroism analyses of the UspF recombinant protein in different pHs. 20 mM phosphate buffer at pH 8.0, 5 mM sodium acetate pH 4.6, 5 mM sodium citrate pH 5.6 and 5 mM of CAPS pH 10.5.
UspF protein three‐dimensional model
The UspF three‐dimensional model was generated with the structural coordinates from putative filament protein/universal stress protein of K. pneumoniae (PDB code 3fdx), the orthologues that had the highest sequence identity (76% over the complete sequence of the mature protein) [Fig. 3(A,B)]. The quality of the model is expressed by Z score and showed per residue in Figure 3(C). In the model, it was verified that the protein could bind ATP [Fig. 3(B)], similar to UspG protein that belong to the same sub‐family.
Figure 3.
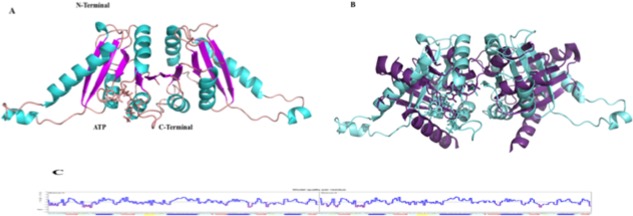
Model of multispecies UspF. A. Schematic representation of the multispecies UspF, ATP is represented in stick. B. Multispecies UspF superimposed with the PDB putative filament protein/universal stress protein F of K. pneumoniae (3fdx), ATP is represented in stick. C. Overall quality of the predicted UspF model.
UspF is involved in stress responses
Therefore, we investigate if the UspF protein was expressed at different serotypes of atypical EPEC (Ec292/84, 9100/83, BA320, BA4013) in different stress conditions. Immunoblotting assay of the heat‐extracted proteins of the strains showed that UspF was detected similarly in all strains and in different stress conditions, such as oxidative stress, low pH, high salt concentration and heat (Fig. 4). In addition to this qualitative analysis; we also performed a survival assay, in which colony‐forming units (CFU) were counted after stressors exposure. The results showed that differences in grow patterns of the strains in different stress conditions. In presence of 3 M NaCl and H2O2, all strains growth was decreased, except in BA4013, where H2O2 stimulated it. The growth for BA4013 and 9100/83 strains was also impaired in low pH. On the other hand, change in temperature only decreased the growth of BA4013 (Fig. 5). The presence of UspF indicates the potential role of this protein in resistance and survival of strains in response to adverse conditions.
Figure 4.
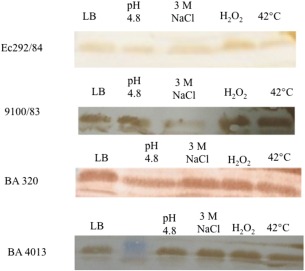
Bacterial lysates from strains Ec292/84, 9100/83, BA320 and BA4013 submitted or not to stress conditions. LB; LB pH 4.8; 3 M NaCl; 0.045% of H2O2; LB at 42°C (10 µg) were separated by SDS‐PAGE (12,5%) and transferred to a nitrocellulose membrane. Nitrocellulose membranes were incubated with anti‐UspF rabbit polyclonal serum followed by goat anti‐rabbit IgG peroxidase‐conjugate. Immunodetection signals were visualized by addition of DAB/H2O2.
Figure 5.
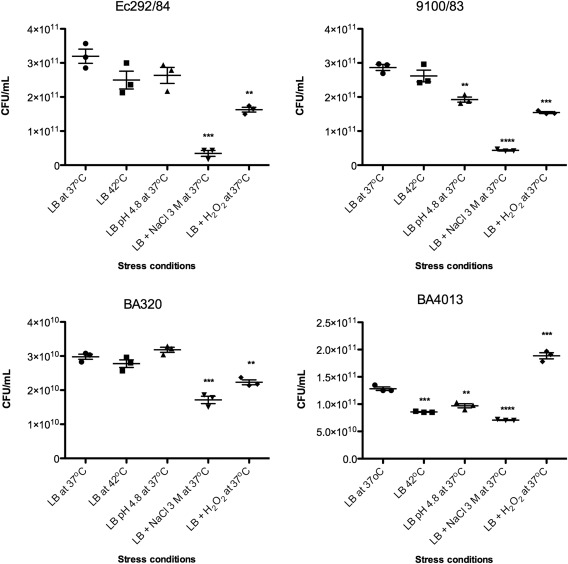
Stress survival assay. After stressors exposure the aEPEC strains: Ec292/84, 9100/83, BA320 and BA4013 were plated on LB agar and then the CFU were counted. The CFU values from triplicates of three independent experiments were analyzed by Graph Prism® 5.01, using unpaired Student's t‐test. Differences were statistically significant compared the strains incubation in LB at 37oC. **** (P < 0.0001); *** (P = 0.0002 to 0.0008); ** (P = 0.002 to 0.0034) or non‐significant.
Presence of the gene as a multispecies universal stress protein F
The uspF gene presence was detected by PCR in the majority of analyzed pathotypes, with some exceptions. The gene was 100% present either in pathogenic bacteria such tEPEC and enteroaggregative E. coli (EAEC), or in non‐pathogenic bacteria, e.g., in E. coli isolates that do not carry virulence factors found in diarrheagenic E. coli. In Shiga toxin‐producing E. coli (STEC) and enterotoxigenic E. coli (ETEC) isolates, the gene was present at 95.6 and 91%, respectively. Half of the tested aEPEC isolates presented the gene and in other enterobacterial species, the presence of uspF gene was less frequent (33.3%), herein detected only in S. sonnei and C. freundii (Table 2).
Table 2.
Presence of UspF (%) Gene in Bacterial Isolates Detected by PCR
| Pathotypes | No. of bacterial isolates | uspF gene presence | Gene presence (%) | Total |
|---|---|---|---|---|
| aEPEC | 72 | 43 | 59.7 | 43/72 |
| tEPEC | 37 | 37 | 100 | 37/37 |
| STEC | 46 | 44 | 95.6 | 44/46 |
| ETEC | 11 | 10 | 90.9 | 10/11 |
| EAEC | 10 | 10 | 100 | 10/10 |
| NVF E. coli | 6 | 6 | 100 | 6/6 |
| Enterobacterial species | 6 | 2 | 33.3 | 2/6 |
aEPEC = atypical enteropathogenic E. coli; tEPEC = typical enteropathogenic E. coli; STEC = Shiga‐toxin producing E. coli; ETEC = enterotoxigenic E. coli; EAEC = enteroaggregative E. coli; NVF E. coli = non‐DEC virulence factors E. coli.
Discussion
Different proteomic studies on E. coli have been used to compare and to identify proteins differentially expressed in tEPEC E2348/69 versus EHEC EDL933 strains38; pathogenic versus commensal E. coli strains39; tEPEC versus aEPEC25 and different strains of aEPEC.29 The proteins identified in our previous data29 consisted of the outer membrane protein OmpX, caseinolytic protease, chain A of the phosphocarrier protein and structure–function of iron superoxide dismutase, described before in E. coli without strain specification. Further, a flavoprotein, Trp repressor‐binding protein, glucose‐specific enzyme IIA component of PTS and the iron‐containing superoxide dismutase were designated in E. coli K12 strain.
Concerning proteins previously described in pathogenic E. coli, we identified in aEPEC different 50S ribosomal proteins, the alkyl hydroperoxide reductase subunit C, autonomous glycyl radical cofactor, caseinolytic protease, DNA starvation/stationary phase protection protein, DNA‐binding transcriptional dual regulator H‐NS, ferritin, galactose‐binding transport protein, the hypothetical proteins Z0175, Z2335 and Z3776, KHG/KDPG aldolase, universal stress protein A and D, stringent starvation protein A, putative transport protein, a protein of the fucose operon, peptidase E; also a putative filament protein defined in enterohemorrhagic E. coli O157:H7 EDL933 and in Sakai strains. Further, hypothetical proteins c1034, c2185 and c4636, dihydropteridine reductase, ribose‐5‐phosphate isomerase A previously described in uropathogenic E. coli CFT073 strain were identified. The inorganic pyrophosphatase described in tEPEC O127:H6 E2348/69 strain, the molybdenum cofactor biosynthesis protein described in atypical EAEC 101‐1 strain and the hypothetical protein O2ColV76 described in a bird pathogenic E. coli strain A2363.
In this previous work from our group, we described the first low‐molecular mass comparative proteomic study of extracted proteins from four representative aEPEC isolates. After fimbrial extraction, we sought that analyzing low‐molecular proteins one can found some fimbrial adhesin involved in the in vitro interaction between bacteria and cell lines, but we observed proteins usually involved in cell structure, protection, metabolism, transport, as well as in gene regulation.29 One of the identified proteins was annotated with the hypothetical name of Z2335 orthologue in EPEC, and by bioinformatics analyses we observed that this protein belongs to the UspF. The annotation has changed recently and now the protein (NP_287771.1) in PubMed is denominated the multispecies universal stress protein F (Shigella). The alignment of the multispecies UspF (Shigella) with UspF of E. coli K12 showed a high identity (99.3%). No signal peptide was observed by using signal 4.1 P server, although a 24 residues in the N‐terminal region of the Z2335 sequence does not seems to be part of the codifying sequence as observed by the ORF finder program.
Herein, for the first time a hypothetical protein from aEPEC was successfully cloned, expressed, purified and characterized. The newly characterized UspF preserved the structure of the native multispecies UspF as a α/β and it is an ATP binding protein. The production of this protein was detected using a specific rabbit serum in the four studied strains in diverse stress conditions, which indicates its potential role in resistance and survival of the strains in response to adverse conditions.
Our result emphasizes previous data showing that the Usp protein superfamily encompasses a conserved group of proteins involved in stress resistance, these proteins promote cell survival during prolonged exposure to stress and may activate a general mechanism for stress endurance. UspA in Salmonella was showed causing resistance to oxidative stress.33 Cells of E. coli BL21 harboring a SbUSP gene from Salicornia brachiata, an extreme halophyte, showed about 1.5‐ to 1.8‐fold increased stress tolerance (1.8 and 1.4‐fold for NaCl and KCl, respectively, and 1.5‐fold for osmotic stress) compared to control E. coli BL21‐DE cells and cells expressing GST only.40 Salmonella needs to enter in host organism, and therefore, the bacteria is exposed to a hostile environment with low pH, lack of oxygen and need to survive to the immune response of the host, so the tolerance to diversity of stress in Salmonella is probably mediated by Usps proteins (UspA, UspE, UspF).41
Indeed, the stress assays showed that when the strains were exposed to different conditions, such as oxidative, temperature, osmolarity and low pH, significant changes in growth were observed, mainly increase with osmolarity and oxidative. It's worth to mention that when the aEPEC strains were cultivated 24 h in those stressors conditions, the growth was completely abolished (data not shown). These data suggest that this UspF is important for maintenance of aEPEC in adverse conditions. Indeed, Usps are among the most highly induced genes when bacteria are subjected to several stress conditions, such as heat shock, nutrient starvation or the presence of oxidants or other stress agents.42 Therefore, expression of this protein is plausible and important for bacterial stress resistance.32, 43, 44, 45, 46, 47
Here, we showed a high prevalence of UspF either in commensal or in the different E. coli pathotypes, which reinforces that the UspF presence might be important for E. coli. By circular dichroism it was verified that the protein is more stable in basic pH, which is in agreement with the intestine pH, where aEPEC strains colonize humans. In conclusion, a hypothetical protein from aEPEC was characterized showing that it is UspF which preserved the structure of the native multispecies UspF as a α/β and ATP binding protein which is involved in bacterial stress.
Material and Methods
Bacterial strains and plasmids
The following E. coli K12 strains were used: DH10b (Stratagene, USA) and BL21 (DE3) pLyS (Novagen, USA). The plasmid vector pET28a (Novagen, USA) and the pGEM‐T Easy Vector System kit (Promega, USA) were used in order to construct the pGEM_uspF and pET28a_uspF plasmids, respectively. Bacterial isolates used in this study consisted of four atypical EPEC strains presenting different adhesion patterns, i.e., Ec292/84, 9100/83, BA320 and BA4013.29 Also, a collection of different bacterial pathogroups were analyzed for the presence of the uspF gene, i.e., typical EPEC (tEPEC), atypical EPEC (aEPEC), ETEC and STEC,17, 48, 49, 50, 51 as well as other Enterobacteriaceae isolates, including Morganella morganii, Klebsiella pneumoniae, Shigella boydii, Proteus mirabilis, Salmonella spp., and Citrobacter freundii. Further, groups of E. coli isolates that do not carry virulence factors found in diarrheagenic E. coli and belonging to our bacterial collection were also analyzed.
Computational analysis
The nucleotide sequence and corresponding amino acid sequence of UspF protein [E. coli O157:H7 strain EDL933] and gene (Gene ID: 961019) were retrieved from gene bank (Accession No. NP_287771.1). Search of orthologs sequences were carried out using BLASTP, available at the National Center of Biotechnology Information server (http://www.ncbi.nlm.nih.gov/BLAST) and ClustalW (www.ebi.ac.uk/clustalw/). Prediction of signal peptide and transmembrane sequences were determined with SignalP and DAS programs, respectively (http://www.cbs.dtu.dk/services/SignalP/) and (http://www.sbc.su.se/~miklos/DAS/). Protein parameters of UspF were calculated applying programs available at the Expasy Bioinformatics Portal (http://www.expasy.org/).
Cloning of the gene that encodes the multispecies uspF gene
The nucleotide sequence (gi|16445223:2115267‐2115773) (without the first 72 base pairs, as indicated by ORF finder program) was amplified by PCR (forward primer 5′ GGA TCC ATG AAC AGA ACG ATT CTT GTC C 3′ and reverse primer 5′ AAG CTT TCA GCG CAC AAC CAG CAC 3′) using Platinum Taq High Fidelity (Invitrogen) and standard amplification conditions: an initial step at 95°C for 5 min, 95°C for 1 min; followed by 30 cycles at 55°C for 1 min, at 68°C for 2 min; and followed by a final extension at 68°C for 10 min. The forward primer included a BamHI site, and the reverse primer a HindIII site (underlined). The resulting amplified fragment, with a total length of 435 nucleotides, was first cloned into the vector pGEM T‐Easy Vector (Promega).
After transformation into E. coli DH10b cells and screening of recombinant colonies, a recombinant plasmid, named pGEM_uspF, was selected, amplified, and cleaved with BamHI and HindIII enzymes (Invitrogen) to release the 435 bp fragment, which was purified in agarose gels and subsequently cloned into the expression vector pET28a (Novagen), previously treated with BamHI/HindIII. Transformation efficiencies of approximately 107 CFU/log DNA were routinely achieved with chemically competent E. coli DH10b cells. One recombinant colony, selected out of 10 chosen colonies, was subjected to restriction analysis and nucleotide sequencing. The recombinant plasmid, named pET_UspF, was further purified and transformed into the E. coli BL21 (DE3) pLyS strain (Novagen). One recombinant clone was chosen at random among the recombinant colonies and selected for further analysis for protein expression and purification. The recombinant UspF protein was expressed as a His6‐tagged cytoplasmic protein genetically fused at the N‐terminal end (HT‐UspF).
Expression and purification of HT‐UspF recombinant protein
Cultures of the recombinant E. coli BL21 (DE3) pLyS strain‐carrying pET_UspF were grown aerobically in Erlenmeyer flasks containing LB medium with 50 μg/mL kanamycin until mid‐log phase (OD600 0.4–0.6) before adding the inducer (0.1 mM IPTG). The cultures were induced aerobically (200 rpm) either, for 4 h at 37°C. Cells were collected by centrifugation and stored at −20°C for approximately 16 h before cell extracts preparation.
Cell pellets from 1 L of bacterial culture were resuspended in 10 mL in 20 mM Tris–HCl, pH 8.0, containing 500 mM of NaCl, 0.5 mM of PMSF and 20 mM of imidazole and incubated with lysozyme (final concentration of 100 μg/mL) for 30 min on an ice bath. Cells were maintained on ice and disrupted by sonication after 4 pulses of 20 s in a cell disruptor (Bandelin) with 30% amplitude, followed by centrifugation at 12,000 g for 30 min, in order to obtain the soluble and non‐soluble cellular fractions. The HT‐UspF protein was purified from soluble protein extracts after addition of a nickel‐charged Sepharose (ProBond, Invitrogen) slurry (1 mL of resin for 15 mg of total protein) previously washed with two volumes of water and one volume of 20 mM Tris–HCl or NaCl, pH 8.0, containing 500 mM of NaCl, 0.5 mM of PMSF and 20 mM of imidazole. The charged resin was transferred to a plastic column and washed with 10 volumes with 20 mM Tris–HCl, pH 8.0, containing 500 mM of NaCl, 0.5 mM of PMSF and 20 mM of imidazole followed by washing with three volumes of 20 mM Tris–HCl or NaCl, pH 8.0, containing 500 mM of NaCl, 0.5 mM of PMSF and 30 mM of imidazole.
The bound HT‐UspF was eluted with buffers containing increasing imidazole concentrations (50 mM, 100 mM, 200 mM, 500 mM). Eluted HT‐UspF fractions were dialyzed with 20 mM Tris–HCl pH 8.0 and 50 mM NaCl. Samples were concentrated with Ultrafree MWCO 10,000 centrifugal filters (Amicon Millipore) to a final concentration of 15 mg/mL. The eluted protein fractions were analyzed by 12% SDS–PAGE gels.52
Prediction of secondary structure in different conditions
All experiments were carried out using a JASCO J‐810 spectropolarimeter equipped with a Peltier‐type temperature controller and a thermostatic cell holder, interfaced with a thermostatic bath. CD spectra were recorded using 0.1 cm path length quartz cells at a protein concentration of 0.25 mg/mL (10 μM). The protein stability was determined at four different pHs (25 mM sodium acetate at pH 4.6; 5 mM sodium citrate at pH 5.6; 20 mM sodium phosphate at pH 8.0, and 5 mM N‐cyclohexyl‐3‐aminopropanesulfonic acid (CAPS) at pH 10.5.
Polyclonal antibody
Polyclonal serum was obtained from a New Zealand white female rabbit (60 days old) which was immunized intramuscularly three times at 2‐week intervals with a dose of 200 μg of UspF recombinant protein adsorbed to 2.5 mg alum (Al3+) as adjuvant. Serum was obtained 45 days after immunization. Immune serum reactivity was tested by indirect ELISA. Serum samples were obtained in order to be used as negative control in specific antibody evaluation, just before immunization by auricular‐venom method. The experiments were conducted in agreement with the Ethical Principles in Animal Research, adopted by the Brazilian College of Animal Experimentation, and they were approved by the Ethical Committee for Animal Research of Butantan Institute (571/09).
Stress assays and heat extracted proteins analyses
The aEPEC strains: Ec292/84, 9100/83, BA320 and BA4013, were grown 16–18 h on LB media. Thus, the bacterial cultures concentrations were adjusted spectrophotometrically (600 nm) to 4 x 109 CFU. The cultures were pelleted by centrifugation at 3,000 g for 10 min, and then the pellets were resuspended in media (LB, LB pH 4.8, LB with 0.045% H2O2, LB with 3 M NaCl),33, 44 and submitted to 30 min of stress conditions at 37°C. Also, one tube containing only LB was placed at 42°C for the same time. After that bacterial cultures were diluted and plated onto Luria Bertani (LB) agar plates. The number of bacteria was determined by counting the CFU.53 The CFU values from triplicates of three independent experiments were analyzed by Graph Prism® 5.01, using unpaired Student's t‐test. The differences were considered statistically significant when P ≤ 0.05.
For protein heat extraction, after the bacteria was submitted to the above mentioned stress conditions, the samples were incubated at 60°C for 30 min, and then pelleted by centrifugation at 3,000g for 10 min. The supernatant was transferred to a new tube, SDS‐sample buffer was added, and samples were boiled at 100°C for 10 min. The samples were separated in 12.5% SDS‐PAGE gels and immunoblotting was performed using the anti‐UspF polyclonal antibody.
Presence of the uspF gene in different bacterial pathotypes
The presence of uspF gene was investigated by PCR reaction in a collection of different bacterial pathotypes. The PCR reaction was performed using the primers sequence (forward 5′ GGA TCC ATG AAC AGA ACG ATT CTT GTC C 3′ and reverse 5′ AAG CTT TCA GCG CAC AAC CAG CAC 3′) using Taq recombinant enzyme (Invitrogen) and amplified using standard conditions: an initial step at 95°C for 5 min, 95°C for 1 min; followed by 30 cycles at 55°C for 1 min, 72°C for 2 min; and a final extension at 72°C for 10 min. The gene amplification in different bacterial isolates was analyzed in agarose gels staining with Gel Red (Biotium).
Modeling
The structural model of the UspF protein was constructed using the Yasara software. The pdb used to construct the model was the 3fdx (Putative filament protein/universal stress protein F of Klebsiella pneumoniae). For model validation, the Yasara used the WHAT CHECK Program.54
Acknowledgments
The authors thank Dr Waldir P. Elias and Dr Roberto Nepomuceno for helpful suggestions and discussions.
References
- 1. Ochoa TJ, Barletta F, Contreras C, Mercado E (2008) New insights into the epidemiology of enteropathogenic Escherichia coli infection. Trans R Soc Trop Med Hyg 102:852–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moon HW, Whipp SC, Argenzio RA, Levine MM, Giannella RA (1983) Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun 41:1340–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McDaniel TK, Jarvis KG, Donnenberg MS, Kaper JB (1995) A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci USA 92:7996–8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaper JB, Nataro JP, Mobley HL (2004) Pathogenic Escherichia coli . Nat Rev Microbiol 2:123–140. [DOI] [PubMed] [Google Scholar]
- 5. Kaper JB (1996) Defining EPEC. Rev Microbiol 27:130–133. [Google Scholar]
- 6. Hernandes RT, Elias WP, Vieira MA, Gomes TA (2009) An overview of atypical enteropathogenic Escherichia coli . FEMS Microbiol Lett 297:137–144. [DOI] [PubMed] [Google Scholar]
- 7. Afset JE, Bevanger L, Romundstad P, Bergh K (2004) Association of atypical enteropathogenic Escherichia coli (EPEC) with prolonged diarrhea. J Med Microbiol 53:1137–1144. [DOI] [PubMed] [Google Scholar]
- 8. Robins‐Browne RM, Bordun AM, Tauschek M, Bennett‐Wood VR, Russell J, Oppedisano F, Lister NA, Bettelheim KA, Fairley CK, Sinclair MI, Hellard ME (2004) Escherichia coli and community‐acquired gastroenteritis, Melbourne, Australia. Emerg Infect Dis 10:1797–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Franzolin MR, Alves RC, Keller R, Gomes TA, Beutin L, Barreto ML, Milroy C, Strina A, Ribeiro H, Trabulsi LR (2005) Prevalence of diarrheagenic Escherichia coli in children with diarrhea in Salvador, Bahia, Brazil. Mem Inst Oswaldo Cruz 100:359–363. [DOI] [PubMed] [Google Scholar]
- 10. Nguyen RN, Taylor LS, Tauschek M, Robins‐Browne RM (2006) Atypical enteropathogenic Escherichia coli infection and prolonged diarrhea in children. Emerg Infect Dis 12:597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bueris V, Sircili MP, Taddei CR, dos Santos MF, Franzolin MR, Martinez MB, Ferrer SR, Barreto ML, Trabulsi LR (2007) Detection of diarrheagenic Escherichia coli from children with and without diarrhea in Salvador, Bahia, Brazil. Mem Inst Oswaldo Cruz 102:839–844. [DOI] [PubMed] [Google Scholar]
- 12. Estrada‐Garcia T, Lopez‐Saucedo C, Thompson‐Bonilla R, Abonce M, Lopez‐Hernandez D, Santos JI, Rosado JL, DuPont HL, Long KZ (2009) Association of diarrheagenic Escherichia coli Pathotypes with infection and diarrhea among Mexican children and association of atypical Enteropathogenic E. coli with acute diarrhea. J Clin Microbiol 47:93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moreno AC, Filho AF, Gomes TA, Ramos ST, Montemor LP, Tavares VC, Filho LS, Irino K, Martinez MB (2010) Etiology of childhood diarrhea in the northeast of Brazil: significant emergent diarrheal pathogens. Diagn Microbiol Infect Dis 66:50–57. [DOI] [PubMed] [Google Scholar]
- 14. Gomes TA, Irino K, Girão DM, Girão VB, Guth BE, Vaz TM, Moreira FC, Chinarelli SH, Vieira MA (2004) Emerging enteropathogenic Escherichia coli strains? Emerg Infect Dis 10:1851–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moreira FC, Vieira MA, Ferreira AJ, Girão DM, Vaz TM, Rosa AC, Knobl T, Irino K, Freymüller E, Gomes TA (2008) Escherichia coli strains of serotype O51:H40 comprise typical and atypical enteropathogenic E. coli strains and are potentially diarrheagenic. J Clin Microbiol 46:1462–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hernandes RT, Silva RM, Carneiro SM, Salvador FA, Fernandes MC, Padovan AC, Yamamoto D, Mortara RA, Elias WP, da Silva Briones MR, Gomes TA (2008) The localized adherence pattern of an atypical enteropathogenic Escherichia coli is mediated by intimin omicron and unexpectedly promotes HeLa cell invasion. Cell Microbiol 10:415–425. [DOI] [PubMed] [Google Scholar]
- 17. Abe CM, Trabulsi LR, Blanco J, Blanco M, Dahbi G, Blanco JE, Mora A, Franzolin MR, Taddei CR, Martinez MB, Piazza RM, Elias WP (2009) Virulence features of atypical enteropathogenic Escherichia coli identified by the eae(+) EAF‐negative stx(‐) genetic profile. Diagn Microbiol Infect Dis 64:357–365. [DOI] [PubMed] [Google Scholar]
- 18. Bando SY, Andrade FB, Guth BE, Elias WP, Moreira‐Filho CA, Pestana de Castro AF (2009) Atypical enteropathogenic Escherichia coli genomic background allows the acquisition of non‐EPEC virulence factors. FEMS Microbiol Lett 299:22–23. [DOI] [PubMed] [Google Scholar]
- 19. Mora A, Blanco M, Yamamoto D, Dahbi G, Blanco JE, López C, Alonso MP, Vieira MA, Hernandes RT, Abe CM, Piazza RM, Lacher DW, Elias WP, Gomes TA, Blanco J (2009) HeLa‐cell adherence patterns and actin aggregation of enteropathogenic Escherichia coli (EPEC) and Shiga‐toxin‐producing E. coli (STEC) strains carrying different eae and tir alleles. Int Microbiol 12:243–251. [PubMed] [Google Scholar]
- 20. Vieira MA, Gomes TA, Ferreira AJ, Knöbl T, Servin AL, Liévin‐Le Moal V (2010) Two atypical enteropathogenic Escherichia coli strains induce the production of secreted and membrane‐bound mucins to benefit their own growth at the apical surface of human mucin‐secreting intestinal HT29‐MTX. Infect Immun 78:927–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yamamoto D, Hernandes RT, Blanco M, Greune L, Schmidt MA, Carneiro SM, Dahbi G, Blanco JE, Mora A, Blanco J, Gomes TA (2009) Invasiveness as a putative additional virulence mechanism of some atypical enteropathogenic Escherichia coli strains with different uncommon intimin types. BMC Microbiol 9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scaletsky IC, Aranda KR, Souza TB, Silva NP, Morais MB (2009) Evidence of pathogenic subgroups among atypical enteropathogenic Escherichia coli strains. J Clin Microbiol 47:3756–3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scaletsky IC, Aranda KR, Souza TB, Silva NP (2010) Adherence factors in atypical enteropathogenic Escherichia coli strains expressing the localized adherence‐like pattern in HEp‐2 cells. J Clin Microbiol 48:302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Magalhães CA, Rossato SS, Barbosa AS, Santos TO, Elias WP, Sircili MP, Piazza RM (2011) The ability of haemolysins expressed by atypical enteropathogenic Escherichia coli to bind to extracellular matrix components. Mem Inst Oswaldo Cruz 106:146–152. [DOI] [PubMed] [Google Scholar]
- 25. Taddei CR, Oliveira FF, Piazza RMF, Paes Leme AF, Klitzke CF, Serrano SM, Martinez MB, Elias WP, Sant Anna OA (2011) A comparative study of the proteomic profile from an atypical and a typical enteropathogenic Escherichia coli . Open Microbiol J 5:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Piazza RM, Delannoy S, Fach P, Saridakis HO, Pedroso MZ, Rocha LB, Gomes TA, Vieira MA, Beutin L, Guth BE (2013) Molecular and phenotypic characterization of Escherichia coli O26:H8 among diarrheagenic E. coli O26 strains isolated in Brazil. Appl Environ Microbiol 79:6847–6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bueris V, Huerta‐Cantillo J, Navarro‐Garcia F, Ruiz RM, Cianciarullo AM, Elias WP (2015) Late establishment of the attaching and effacing lesion caused by atypical enteropathogenic Escherichia coli depends on protein expression regulated by Per. Infect Immun 83:379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moraes CT, Polatto JM, Rossato SS, Izquierdo M, Munhoz DD, Martins FH, Pimenta DC, Farfan MJ, Elias WP, Barbosa AS, Piazza RM (2015) Flagellin and GroEL mediates in vitro binding of an atypical enteropathogenic Escherichia coli to cellular fibronectin. BMC Microbiol 15:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nara JM, Pimenta DC, Abe CM, Abreu PA, Moraes CT, Freitas NC, Elias WP, Piazza RM (2012) Low‐molecular mass comparative proteome of four atypical enteropathogenic Escherichia coli isolates showing different adherence patterns. Comp Immunol Microbiol Infect Dis 35:539–549. [DOI] [PubMed] [Google Scholar]
- 30. Saveanu C, Miron S, Borza T, Craescu CT, Labesse G, Gagyi C, Popescu A, Schaeffer F, Namane A, Laurent‐Winter C, Bârzu O, Gilles AM (2002) Structural and nucleotide‐binding properties of YajQ and YnaF, two Escherichia coli proteins of unknown function. Protein Sci 11:2551–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sagurthi SR, Panigrahi RR, Gowda G, Savithri HS, Murthy MR (2007) Cloning, expression, purification crystallization and preliminar X‐ray diffaction analysis of universal stress protein F (YnaF) from Salmonella typhimurium . Acta Cryst F 63:957–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nachin L, Nannmark U, Nyström T (2005) Differential roles of the universal stress proteins of Escherichia coli in oxidative stress resistance, adhesion, and motility. J Bacteriol 187:6265–6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu WT, Karavolos MH, Bulmer DM, Allaoui A, Homaeche RD, Lee JJ, Khan CM (2007) Role of the universal stress protein A of Salmonella in growth arrest, stress and virulence. Microb Pathog 42:2–10. [DOI] [PubMed] [Google Scholar]
- 34. Banerjee A, Adolph RS, Gopalakrishnapai J, Kleinboelting S, Emmerich C, Steegborn C, Visweswariah SS (2015) A universal stress protein (Usp) in mycobacteria bind cAMP. J Biol Chem 290:12731–12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hu X, Li X, Huang L, Chan J, Chen Y, Deng H, Mi K (2015) Quantitative proteomic reveals novel insights into isoniazid susceptibility in Mycobacteria mediated by a Universal Stress Protein. J Proteome 14:1445–1454. [DOI] [PubMed] [Google Scholar]
- 36. Elhosseiny NM, Amim MA, Yassim AS, Attia AS (2015) Acinetobacter baumannii universal stress protein A plays a pivotal role in stress response and is essential for pneumonia e sepsis pathogenesis. Int J Med Microbiol 305:114–123. [DOI] [PubMed] [Google Scholar]
- 37. Marchler‐Bauer A, Bryant SH (2004) CD‐Search: protein domain annotations on the fly. Nucleic Acids Res 32:W327–W331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li M, Rosenshine I, Tung SL, Wang XH, Friedberg D, Hew CL, Leung KY (2004) Comparative proteomic analysis of extracellular proteins of enterohemorrhagic and enteropathogenic Escherichia coli strains and their ihf and ler mutants. Appl Environ Microbiol 70:5274–5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sommer U, Petersen J, Pfeiffer M, Schrotz‐King P, Morsczeck C (2010) Comparison of surface proteomes of enterotoxigenic (ETEC) and commensal Escherichia coli strains. J Microbiol Methods 83:13–19. [DOI] [PubMed] [Google Scholar]
- 40. Udawat P, Mishra A, Jha B (2014) Heterologous expression of an uncharacterized universal stress protein gene (SbUSP) from the extreme halophyte, Salicornia brachiata, which confers salt and osmotic tolerance to E. coli . Gene 536:163–170. [DOI] [PubMed] [Google Scholar]
- 41. Bangera M, Panigrahi R, Sagurthi SR, Savithri HS, Murphy MRN (2015) Structural and functional analysis of two universal stress proteins YdaA and YnaF from Salmonella typhimurium: possible roles in microbial stress tolerance. J Struct Biol 189:238–250. [DOI] [PubMed] [Google Scholar]
- 42. Kvint K, Nachin L, Diez A, Nyström T (2003) The bacterial universal stress protein: function and regulation. Curr Opin Microbiol 6:140–145. [DOI] [PubMed] [Google Scholar]
- 43. Nyström T, Neidhart FC (1994) Expression and role of the universal stress protein, UspA, of Escherichia coli during growth arrest. Mol Microbiol 11:537–544. [DOI] [PubMed] [Google Scholar]
- 44. Gustavsson N, Diez A, Nyström T (2002) The universal stress protein paralogues of Escherichia coli are coordinately regulated and co‐operate in the defense against DNA damage. Mol Microbiol 43:107–117. [DOI] [PubMed] [Google Scholar]
- 45. Persson O, Valadi A, Nyström T, Farewell A (2007) Metabolic control of the Escherichia coli universal stress protein response through fructose‐6‐phosphate. Mol Microbiol 65:968–978. [DOI] [PubMed] [Google Scholar]
- 46. Raspoet R, Gantois I, Devloo R, Martel A, Haesebrouck F, Pasmans F, Ducatelle R, Van Immerseel F (2011) Salmonella Enteritidis universal stress protein (usp) gene expression is stimulated by egg white and supports oviduct colonization and egg contamination in laying hens. Vet Microbiol 153:186–190. [DOI] [PubMed] [Google Scholar]
- 47. Xu Y, Quan CS, Jin X, Jin X, Zhao J, Li X, Zheng W, Jin L, Liu D, Fan S, Ha NC (2014) Crystallization and preliminary X‐ray diffraction analysis of UspE from Escherichia coli . Acta Cryst F70:1640–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Menezes MA, Rocha LB, Koga PC, Fernandes I, Nara JM, Magalhães CA, Abe CM, Ayala CO, Burgos YK, Elias WP, Castro AF, Piazza RM (2010) Identification of enteropathogenic and enterohaemorrhagic Escherichia coli strains by immunoserological detection of intimin. J Appl Microbiol 108:878–887. [DOI] [PubMed] [Google Scholar]
- 49. Nara JM, Cianciarullo AM, Culler HF, Bueris V, Horton DS, Menezes MA, Franzolin MR, Elias WP, Piazza RM (2010) Differentiation of typical and atypical enteropathogenic Escherichia coli using colony immunoblot for detection of bundle‐forming pilus expression. J Appl Microbiol 109:35–43. [DOI] [PubMed] [Google Scholar]
- 50. Rocha LB, Luz DE, Moraes CT, Caravelli A, Fernandes I, Guth BE, Horton DS, Piazza RM (2012) Interaction between Shiga toxin and monoclonal antibodies: binding characteristics and in vitro neutralizing abilities. Toxins 4:729–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rocha LB, Ozaki CY, Horton DS, Menezes CA, Silva A, Fernandes I, Magnoli FC, Vaz TM, Guth BE, Piazza RM (2013) Different assay conditions for detecting the production and release of heat‐labile and heat‐stable toxins in enterotoxigenic Escherichia coli isolates. Toxins 5:2384–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. [DOI] [PubMed] [Google Scholar]
- 53. Farfan MJ, Inman KG, Nataro JP (2008) The major pilin subunit of the AAF/II fimbriae from enteroaggregative Escherichia coli mediates binding to extracellular matrix proteins. Infect Immun 76:4378–4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hooft RWW, Vriend G, Sander C, Abola EE (1996) Errors in proteins structures. Nature 381:272. [DOI] [PubMed] [Google Scholar]


