Abstract
The flavoprotein iodotyrosine deiodinase (IYD) was first discovered in mammals through its ability to salvage iodide from mono‐ and diiodotyrosine, the by‐products of thyroid hormone synthesis. Genomic information indicates that invertebrates contain homologous enzymes although their iodide requirements are unknown. The catalytic domain of IYD from Drosophila melanogaster has now been cloned, expressed and characterized to determine the scope of its potential catalytic function as a model for organisms that are not associated with thyroid hormone production. Little discrimination between iodo‐, bromo‐, and chlorotyrosine was detected. Their affinity for IYD ranges from 0.46 to 0.62 μM (K d) and their efficiency of dehalogenation ranges from 2.4 – 9 x 103 M−1 s−1 (k cat/K m). These values fall within the variations described for IYDs from other organisms for which a physiological function has been confirmed. The relative contribution of three active site residues that coordinate to the amino acid substrates was subsequently determined by mutagenesis of IYD from Drosophila to refine future annotations of genomic and meta‐genomic data for dehalogenation of halotyrosines. Substitution of the active site glutamate to glutamine was most detrimental to catalysis. Alternative substitution of an active site lysine to glutamine affected substrate affinity to the greatest extent but only moderately affected catalytic turnover. Substitution of phenylalanine for an active site tyrosine was least perturbing for binding and catalysis.
Keywords: iodotyrosine, halotyrosine, deiodinase, dehalogenase, flavoprotein, Drosophila
Abbreviations
- Br‐Tyr
3‐bromo‐L‐tyrosine
- Cl‐Tyr
3‐chloro‐L‐tyrosine
- dmIYD
Drosophila melanogaster IYD isoform B lacking its membrane anchor sequence
- F‐Tyr
3‐fluoro‐L‐tyrosine
- HPLC
high performance liquid chromatography
- I2‐Tyr
3,5‐diiodo‐L‐tyrosine
- I‐Tyr
3‐iodo‐L‐tyrosine
- IYD
iodotyrosine deiodinase
- SUMO
small ubiquitin‐like modifier
- TH
thyroid hormone.
Introduction
Deiodination of iodotyrosines was first demonstrated in slices of thyroid tissue over 60 years ago.1 The enzyme iodotyrosine deiodinase (IYD) responsible for this activity was ultimately purified to homogeneity from thyroids and identified as a flavoprotein that promotes reductive deiodination.2, 3 This remains the only example of a flavoprotein known to catalyze such a reductive process in mammals.2, 4 NADPH serves as the electron donor for catalysis in vivo but enzyme reduction is indirect and requires a reductase that has yet to be identified.2 Consequently, all studies in vitro remain focused on the dehalogenation half reaction driven by the artificial donor dithionite.5
IYD was initially recognized for its ability to salvage iodide from mono‐and diiodotyrosine (I‐Tyr and I2‐Tyr) that form as byproducts of thyroid hormone (TH) synthesis.5 Mutation of this enzyme can result in congenital thyroid disease.6 All chordates including vertebrates as well as invertebrates such as amphioxus contain a thyroid gland or its evolutionary precursor entitled an endostyle for synthesize of TH.7, 8, 9 The presence of IYD was originally expected to correlate with the presence of TH but functional IYDs have been discovered in numerous additional phyla including arthropods (insects and crustaceans) and cnidarians (radially symmetrical lower invertebrates such as anemones and jelly fish).10 These lower organisms lack thyroids and endostyles and little evidence for endogenous synthesis of TH has been reported.11, 12
The role for IYD may extend beyond iodide salvage and contribute more generally to the metabolism of halotyrosines since the mouse and human homologs have now been shown in vitro to promote reductive dehalogenation of bromo‐ and chlorotyrosine (Br‐Tyr and Cl‐Tyr) (Fig. 1).13, 14 These alternative derivatives are generated during the oxidative bursts from stimulation of our innate immune response15, 16 and recent data suggest that at least Br‐Tyr may also be reductively dehalogenated in vivo.17 In conjunction with a putative IYD reductase, IYD may act more broadly than its name implies to dehalogenate a range of halotyrosines found throughout nature. For example, Cl‐Tyr has been detected in the cuticles of insects and the operculae of molluscs.18 Similarly, Br‐Tyr and I‐Tyr have been found in scleroproteins of many different organisms.18 Both halogenation and cross‐linking of tyrosyl residues are associated with hardening of the jaws of certain marine worms19 and the fertilization envelope of sea urchins.20 The prevalence of IYD in a surprisingly diverse set of organisms may therefore correlate best with the nearly ubiquitous presence of various endogenous and exogenous halotyrosines in nature and not limited solely to metabolism of I‐Tyr and I2‐Tyr.
Figure 1.
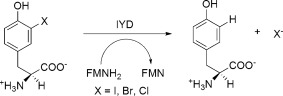
Dehalogenation of halotyrosines by IYD.
When the lineage of IYD was first traced back through the tree of life, Apis mellifera (honeybee) was selected as a representative for insects based on the small size and low cysteine content of its presumptive IYD.10 Once its dehalogenase activity was confirmed, further interest turned to an organism that is more amenable to biological studies, namely Drosophila melanogaster. This was not initially selected since its gene for IYD now designated Condet (cdt) and originally annotated as CG6279 in the FlyBase contained a large N‐terminal domain of unknown function in addition to the deiodinase domain in a splice variant isoform A. However, all of the markers for IYD activity were evident including a specific Glu, Tyr, Lys and Ala for substrate binding and a Thr for hydrogen bonding to the active site FMN.10 Moreover, an alternative splicing pattern was predicted to generate isoform B. This encoded a protein that lacks the entire N‐terminal domain and conforms to the standard IYD architecture.21 Hence, isoform B offered an ideal representative of an IYD from a non‐chordate invertebrate. A nutritional requirement for iodide has not yet been determined in Drosophila but feeding experiments with radiolabeled iodide have detected I‐Tyr and I2‐Tyr in vivo.22 The radiolabel was also observed to localize in specific areas of the larval cuticle especially in posterior ventral regions and near air passages and mouth regions suggesting incorporation by a controlled process. Precedence from other organisms would suggest that additional halotyrosines such as Cl‐Tyr and Br‐Tyr also likely form as well.18
In this article, we examine monohalotyrosines as possible substrates for Drosophila IYD through their equilibrium binding and steady‐state kinetics of turnover. The relative importance of the evolutionarily conserved active site residues Glu, Tyr and Lys that coordinate to the zwitterion of substrate are also evaluated by site‐directed mutagenesis to refine the predictive nature of these residues for IYD function. Halotyrosines represent only a fraction of the halophenols generated from natural and industrial sources23 and the potential for identifying catalytic diversity in IYD homologs increases as more genomic and meta‐genomic data become available for its nitro‐FMN reductase structural superfamily.
Results
Expression and purification of dmIYD
In analogy to other IYD homologs,10 dmIYD was initially expressed as a fusion with SUMO but this required subsequent proteolysis and removal of SUMO by size exclusion chromatography. These procedures generated dmIYD in relatively low yields (4‐7 mg/L) and subsequently, dmIYD was expressed in a soluble form without the SUMO fusion. This simplified its purification and generated higher yields of homogeneous protein (8–11 mg/L). All dmIYD mutants were also expressed without a SUMO fusion and their yields ranged from 5–12 mg/L (Supporting Information Fig. S2). FMN occupancy in dmIYD and its mutants was relatively constant between 82 to 90%.
Substrate recognition and turnover specificity of dmIYD
The biological function of IYD in non‐chordates has not been established and therefore the physiological substrate for dmIYD may be a halotyrosine other than I‐Tyr. To define the potential scope of catalysis promoted by dmIYD, its binding and steady‐state turnover was characterized for each halotyrosine. The intrinsic fluorescence of the active site FMN provided a convenient sensor for association of these potential ligands.13, 24 Independent titration of I‐Tyr, Br‐Tyr and Cl‐Tyr all quenched the fluorescence efficiently and exhibited nearly identical K d values of less than 1 µM (Fig. 2, Table 1). Equivalent studies with F‐Tyr revealed a significantly (> 30‐fold) weaker affinity for dmIYD (Supporting Information Fig. S3).
Figure 2.
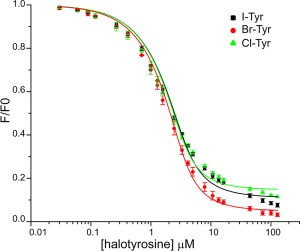
Quenching of FMN fluorescence is induced by ligand binding to the active site of dmIYD. Solutions containing 3 µM dmIYD (as measured by the bound FMN cofactor) in the presence of 200 mM KCl and 100 mM potassium phosphate pH 7.4 were titrated with the halotyrosines. Fluorescence emission was monitored at 520 nm with an excitation at 450 nm. Each data point represents an average of three individual observations and error bars represent one standard deviation from the averages. The K d values are derived from the best fit of data (solid lines) to the equilibrium binding equation described previously (Origin 6.0).24, 25
Table 1.
Binding and Turnover of I‐Tyr, Br‐Tyr and Cl‐Tyr by dmIYD
| Substrate | K d a (µM) | K m b (µM) | k cat (s−1)b (× 10−2) | k cat/K m (s−1 M−1)b (× 103) |
|---|---|---|---|---|
| I‐Tyr | 0.62 ± 0.08 | 14 ± 4 | 12 ± 1 | 9 ± 3 |
| Br‐Tyr | 0.47 ± 0.06 | 8 ± 2 | 6.9 ± 0.5 | 9 ± 2 |
| Cl‐Tyr | 0.46 ± 0.08 | 21 ± 6 | 5.0 ± 0.6 | 2.4 ± 0.7 |
Determined from the data illustrated in Figure 2.
Determined from the data illustrated in Supporting Information Figure S5. Errors derive from least square fitting.
Dehalogenation of the halotyrosines was examined under similar conditions and was initiated as usual with the reductant dithionite since the reducing power of NADPH is not utilized directly by the enzyme.2 The product tyrosine was detected and quantified by reverse‐phase HPLC (Fig. 3, Supporting Information S4) and the initial rates of reaction exhibited a substrate dependence that was consistent with Michaelis‐Menten kinetics (Supporting Information Fig. S5). Dechlorination of Cl‐Tyr was least efficient based on its relatively large K m and small k cat values (Table 1). Compensatory differences in the K m and k cat values for deiodination and debromination of I‐Tyr and Br‐Tyr yielded equivalent k cat/K m values that were approximately 3.5‐fold larger than that of Cl‐Tyr. No detectable signal for tyrosine was observed after F‐Tyr was used as a substrate even after increasing the concentration of dmIYD by 25‐fold and the incubation time by 6‐fold (Supporting Information Fig. S6).
Figure 3.
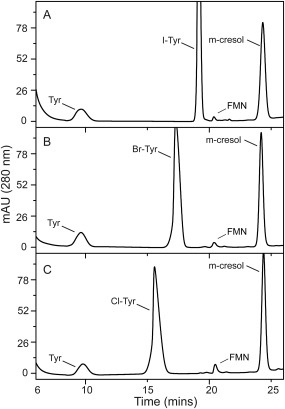
Detection of tyrosine after halotyrosine turnover by dmIYD using reverse phase HPLC. (A) I‐Tyr (40 µM) was incubated with dmIYD (0.08 µM) for 20 mins. (B) Br‐Tyr (40 µM) incubated with dmIYD (0.12 µM) for 20 mins. (C) Cl‐Tyr (40 µM) incubated with dmIYD (0.2 µM) for 20 mins. m‐Cresol was added as an internal standard. For complete conditions refer to the Materials and Methods section. The signal for FMN derives from dmIYD.
Relative contribution of active site residues for substrate binding and dehalogenation
The co‐crystal structure of mouse IYD·I2‐Tyr identified three critical active site residues (E153, Y157 and K178) that coordinate the substrate zwitterion with their side chains [Fig. 4(A)].26 These residues are highly conserved in dmIYD (E154, Y158 and K179) [Fig. 4(B)] and many other IYDs predicted by genomic data.10 Their high degree of conservation through evolution suggests that these active site residues are critical for the dehalogenation activity or at least halotyrosine recognition. Information regarding their relative importance was first attempted by mutagenesis of mouse IYD but these studies were limited by the insolubility of its Lys mutation and the presence of Cys to Ala mutants required for its expression system.28 Interest in these residues has been renewed after IYD was found to be pervasive and well represented in the nitro‐FMN reductase superfamily. Identifying the relative contribution of each residue will help to define the origins of this enzyme and its possible substrate specificity throughout metazoa.
Figure 4.
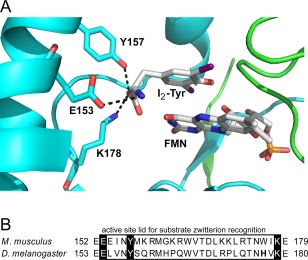
Residues of mouse IYD that coordinate the substrate zwitterion. (A) The two identical polypeptides comprising native IYD are indicated in blue and green and the active site contains diiodotyrosine (I2‐Tyr) stacked above the cofactor FMN as observed in the co‐crystal structure (PDB ID 3GH8).26 (B) Sequence alignment of the active site lid region of IYD from Mus musculus (mouse) and Drosophila IYD was generated by MUSCLE.27 Conserved residues binding to the substrate zwitterion are highlighted in black. Corresponding amino acid residue numbers are indicated on the left and right of each sequence.
The enzyme dmIYD exhibits a very strong affinity for I2‐Tyr with a K d of less than 1 µM. [Fig. 5(A), Table 2]. In analogy to IYD from mouse, dmIYD likely provides hydrogen bonding to the substrate carboxylate through the phenolic OH of Y158. Loss of this group in the Y158F mutant resulted in a 7‐fold decrease in the enzyme's affinity for I2‐Tyr. Likewise, loss of an active site carboxylate responsible for recognition of the substrate ammonium ion by an E154Q mutation diminished affinity for I2‐Tyr by 30‐fold. The most deleterious mutation K179Q for binding eliminates the potential association between a sidechain ammonium group and the substrate carboxylate and consequently weakened the K d for I2‐Tyr by 46‐fold. Although dmIYD exhibits a similar affinity for I2‐Tyr and I‐Tyr, all of the mutant enzymes bind I‐Tyr more weakly than I2‐Tyr by 7‐ to 10‐fold [Fig. 5(B), Table 2].
Figure 5.
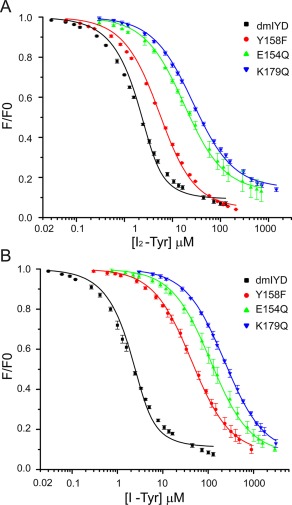
Loss of binding affinity for active site mutants of dmIYD. Quenching of FMN fluorescence was monitored during titration of dmIYD (3 µM) and its mutants with (A) I2‐Tyr and (B) I‐Tyr in 200 mM KCl and 100 mM potassium phosphate pH 7.4. Fluorescence emission was monitored at 520 nm with excitation at 450 nm. Each data point represents an average of three individual observations and error bars represent one standard deviation from the averages. The K d values are derived from the best fit of the data (solid lines) to the equilibrium binding equation described previously (Origin 6.0).24, 25
Table 2.
Effects of Active Site Mutation on Substrate Binding and Turnover of dmIYD
| K D (µM)a | I2‐Tyr | ||||
|---|---|---|---|---|---|
| enzyme | I‐Tyr | I2‐Tyr | K M (μM)b |
k
cat (s−1)b
(× 10−2) |
k
cat/K
m(M−1 s−1)b
(× 103) |
| dmIYD | 0.62 ± 0.08c | 0.54 ± 0.05 | 11 ± 2 | 10.8 ± 0.7 | 10 ± 2 |
| Y158F | 42 ± 2 | 3.8 ± 0.2 | 90 ± 20 | 25 ± 3 | 2.8 ± 0.7 |
| E154Q | 112 ± 5 | 16 ± 0.8 | 3400 ± 500 | 4.1 ± 0.3 | 0.012 ± 0.002 |
| K179Q | 230 ± 10 | 25 ± 1 | 700 ± 100 | 13.1 ± 0.8 | 0.19 ± 0.03 |
Catalytic efficiencies of dehalogenation by dmIYD and its mutants were compared using a standard assay measuring [125I]‐iodide release from [125I]‐I2‐Tyr in the presence of dithionite.29, 30 The Y158F mutation produced an 8‐fold increase in K m while k cat increased by little over 2‐fold (Table 2). The overall impact on k cat/K m was a modest 3.5‐fold decrease from that of dmIYD. The K179Q mutation produced a large 64‐fold increase in K m while k cat was largely unaffected. Together, this resulted in a decrease in catalytic efficiency by 53‐fold relative to that of dmIYD. The E154Q affected K m to the greatest extent with an increase of more than 300‐fold and yet k cat was only modestly reduced by almost 3‐fold. Still, these effects together suppressed the catalytic efficiency of E154Q by 830‐fold relative to that of dmIYD.
Discussion
Substrate selectivity of dmIYD
Based on the precedence set by mammalian IYD, I‐Tyr, Br‐Tyr and Cl‐Tyr were all possible substrates for dmIYD and this study confirmed that all were recognized and processed by dmIYD. No substantial preference was evident for these halotyrosines. Each exhibited nearly identical binding affinities with sub‐μM K d values and similar catalytic efficiencies (k cat/K m) for dehalogenation (Table 1). The individual k cat values decrease as the strength of the aryl‐halogen bond increases. However, the response of k cat is relatively small (∼ 2‐fold) for the significant difference of 30 kcal/mol for the aryl‐I to aryl‐Cl bond dissociation energies.31 The k cat/K m of dmIYD for I‐Tyr and Br‐Tyr are nearly identical to the pre‐steady state rate constants for turnover of I‐Tyr and Br‐Tyr by human IYD (k ox, 8.6 × 103 M−1 s−1 and 7.3 × 103 M−1 s−1).14 However, the k cat/K m of dmIYD for Cl‐Tyr is 5‐fold higher than the k ox of human IYD (0.4 × 103 M−1 s−1)14 indicating that the Drosophila enzyme is less discriminating than its human counterpart. One exception to this trend is the relative affinity for F‐Tyr versus I‐Tyr, Br‐Tyr and Cl‐Tyr. For dmIYD, the K d value for F‐Tyr is 25‐fold higher than those for the other monohalotyrosines whereas human IYD exhibits a corresponding difference of only 10‐fold.24 No defluorination has yet been observed with F‐Tyr even after substantial increases of enzyme concentration and reaction time. The upper limit for turnover is at least three orders of magnitude less than that for Cl‐Tyr (k cat < 3 × 10−5 s−1). This inability to reduce F‐Tyr is not surprising due to the very high bond dissociation energy of aryl‐F bonds and the lack of naturally occurring fluorinated tyrosine derivatives.32
The steady‐state parameters of dmIYD suggest that a number of halotyrosines could be physiologically relevant but insufficient data is available on which may by present in Drosophila. Little is known about an iodine requirement in insects and the proteins that are crucial for TH metabolism in mammals are not apparent in the Drosophila genome. This includes a lack of homologous enzymes responsible for iodinating tyrosyl residues in TH biosynthesis (thyroid peroxidase) and deiodinating TH (iodothyronine deiodinases) and the transporter associated with iodide uptake in the thyroid (sodium/iodide symporter).12 If I‐Tyr is generated within Drosophila, other pathways are likely responsible such as the peroxidase‐dependent sclerotization of cuticle.18, 33 In this case, IYD could provide protection against release of I‐Tyr. This halotyrosine is known to be toxic to Drosophila based on its inhibition of tyrosine hydroxylase and perturbation of dopamine biosynthesis.34
A possible need for dehalogenating Br‐Tyr and Cl‐Tyr in Drosophila is less apparent since neither has been reported to be toxic. Bromide has recently been shown essential for Drosophila and exclusion of bromide from the diet can be lethal.35 This halide is essential for collagen crosslinking and acts through the generation of hypobromous acid from a peroxidase‐like activity of peroxidasin.36 Hypobromous acid has the potential to brominate tyrosine as well but this process has not yet been identified in vivo. In contrast, Cl‐Tyr has been reported in the sclerotized cuticle of locusts and other insects.37 Thus, dmIYD may also support catabolism of these halotyrosines in a manner reminiscent of IYD's multiple functions in mammals.
Active site coordination of the halotyrosine zwitterion
The three active site residues Glu, Tyr and Lys that coordinate to the substrate zwitterion are highly conserved in IYDs from mammals to prokaryotes and are thought to be general markers for halotyrosine processing within the IYD subgroup of the nitro‐FMN reductase superfamily.10 Initial attempts to measure their relative importance were based on mouse IYD but heterologous expression of this gene required additional Cys to Ala mutations to produce soluble protein.28 The information gleaned from this system was further limited by an inability to measure the catalytic activity of the Glu to Gln mutant accurately or even characterize the Lys to Gln mutant since it could not be expressed in a soluble form.28 In contrast, the corresponding mutations E154Q, Y158F and K179Q of dmIYD were well tolerated within the native sequence and in each case yielded soluble proteins. The active site E154 was found to be the most significant of the three residues for dehalogenation. The conservative change of E154Q that essentially removed only the negative charge of the carboxylate decreased the catalytic efficiency by three orders of magnitude (Table 2). This result cannot be attributed entirely to a decrease in substrate affinity as previously suggested for the equivalent mutant of mouse IYD28 since an alternative K179Q mutation in dmIYD suffered from a greater loss of binding affinity with I2‐Tyr (46‐ versus 30‐fold) yet maintained a higher efficiency of catalysis relative to the E154Q mutant. Binding and catalysis are least affected by the Y158F mutation that eliminated a potential hydrogen bond between a phenolic OH and the substrate zwitterion. The overall effect of these mutations on catalysis are all likely related to the ability of their enzyme‐substrate complexes to move an active site Thr into position for hydrogen bonding to the FMN N5. The importance of such a substrate‐dependent activation of FMN was suggested previously from both structural and catalytic studies of the homologous human IYD as well as catalytic studies of a homologous bacterial IYD.24, 38
Additional investigations on human IYD revealed preferential binding of the deprotonated phenolate form of the halotyrosine substrate rather than its neutral phenol form.24 This result is consistent with a coordination of the phenolate to both the 2′‐hydroxy group of FMN and an amide backbone hydrogen as observed in all co‐crystal structures of IYD·I‐Tyr.24, 26 However, the K d values of I‐Tyr and I2‐Tyr do not reflect the greater fraction of the phenolate form of I2‐Tyr (91%, pK a 6.4)39 relative to I‐Tyr (7%, pK a 8.5)40 under conditions of the assays (pH 7.4). Instead, the K d value of human IYD is smaller for I‐Tyr than I2‐Tyr.24 This suggests that the structural changes required for accommodating the added steric bulk of a second iodo substituent likely dominates the relative affinity of these substrates.26 For dmIYD, these opposing properties of steric bulk and phenolate population may balance each other since the K d values for I‐Tyr and I2‐Tyr are experimentally indistinguishable (Table 2). This balance does not extend to the three active site mutants. Although the mutated residues most directly affect coordination of the zwitterion that is common to both I‐Tyr and I2‐Tyr, their impact is 7‐ to 10‐fold greater on the affinity of I‐Tyr than I2‐Tyr (Table 2). For the first time, the bulky substrate exhibits a lower K d value and may reflect a greater sensitivity to the relatively high fractional population of its phenolate form in solution.
Material and methods
Materials
All oligonucleotide primers were obtained from Integrated DNA Technologies. 3‐Bromo‐L‐tyrosine (95% pure) was purchased from AEchem Scientific Corporation. 3‐Fluoro‐L‐tyrosine (97% pure) was purchased from Astatech Inc. 3‐Iodo‐L‐tyrosine (97% pure) and 3, 5‐diiodo‐L‐tyrosine (97% pure) were purchased from Acros Organics and Sigma‐Aldrich, respectively. DNA sequencing was performed by Genewiz.
General methods
The extinction coefficient for Drosophila IYD (expressed sequence, dmIYD) was determined using ExPASy Protparam (ε280 of dmIYD and its mutants E154Q and K179Q, 25.9 mM−1 cm−1 and the Y157Q mutant, 24.4 mM−1 cm−1).41 The concentration of the FMN associated with dmIYD was estimated by A450 and the extinction coefficient of free FMN in solution (12.5 mM−1 cm−1).42 Protein concentration was measured by A280 after correcting for the contribution by FMN estimated from its A280/A450 of 1.57 derived from free FMN. Quenching of FMN fluorescence upon ligand binding was used to measure equilibrium dissociation constants (K d) for halotyrosines as previously described [Eq. (1)] and based on measured fluorescence (F), initial fluorescence intensity (Fo), net change in fluorescence intensity (ΔF), total enzyme concentration ([E]t) and ligand concentration ([L]).24, 25 The initial rate of deiodination was monitored by the release of [125I]‐iodide from [125I]‐I2‐Tyr as previously described.10, 29, 30
| (1) |
Cloning of iodotyrosine deiodinase from Drosophila melanogaster
This homolog identified as dmIYD (isoform B, Accession NP_001163414.1, Flybase CG6279‐PB) was truncated to remove an N‐terminal transmembrane domain (amino acids 2‐40) predicted by TMHMM.43 The resulting gene sequence with addition of a C‐terminal His6 tag was synthesized by Blue Heron Biotechnology for optimized expression in Escherichia coli (Supporting Information Fig. S1) and subcloned to generate an N‐terminal SUMO fusion construct using the pSMT3 vector (a gift from Dr C. Lima).44 A forward primer 5′‐AAACAAACATATGAAAACTTATAATTTAGATGAAC‐3′ and a reverse primer 5′‐AATTAATCTCGAGTTAGTGGTGATGGT‐3' containing NdeI and XhoI restriction sites respectively (underlined) were used to amplify and subclone the dmIYD gene above into a pET24a vector (Novagen) for subsequent protein expression lacking the SUMO tag.
Site‐directed mutagenesis
The pET24a‐dmIYD vector and Pfu turbo DNA polymerase (Agilent) were used for generating the enzyme mutations. The E154Q mutation was introduced with the primer 5′‐GTAGAACAAGAACAGTTAGTCAATTAC‐3′ and its reverse complement. Similarly, the Y158F mutation was introduced with the primer 5′‐AAATTGTAGAACAAGAAGAATTAGTCAATTTCTCCCAACGTATGCAT‐3′ and its reverse complement and the K179Q mutation was introduced with the primer 5′‐ACCAATCATGTACAGGAATACTTAACC‐3′ and its reverse complement. In each case, the mutated codons are underlined. Procedures followed those recommended by the Quikchange site‐directed mutagenesis protocol (Agilent). Proper amplification of the mutant‐containing plasmids was confirmed by 1% agarose gel electrophoresis prior to their transformation into electro‐competent Genehogs E. coli cells (Invitrogen). Plasmids were purified from cultures grown from single colonies and the desired mutations were confirmed by DNA sequencing.
Protein expression and purification
The enzyme dmIYD and its mutants were expressed and purified as described previously.10 Briefly, cell cultures were incubated with isopropyl‐β‐D‐1‐thiogalactopyranoside (25 µM) at 16ºC for 12‐14 h. Purification of protein containing the SUMO fusion followed a published procedure10 except for the addition of 25 mM imidazole in the lysis buffer to reduce non‐specific binding during Ni2+ affinity chromatography. Protein lacking the SUMO fusion was purified equivalently using Ni2+ affinity chromatography followed by size exclusion chromatography.
Dehalogenation of 3‐halotyrosine
Dehalogenation of the 3‐halotyrosines was monitored by the formation of tyrosine as detected during reverse‐phase (C‐18) HPLC. The indicated concentration of dmIYD, m‐cresol (30 µM) as an internal standard and varying concentrations of halotyrosine were combined in 200 mM potassium chloride and 100 mM potassium phosphate pH 7.4. Reaction of this mixture was initiated by addition of 5% sodium dithionite in 5% sodium bicarbonate (100 µl) to a final volume of 1 ml and incubation was maintained for the indicated time at 25°C. Reaction was quenched by addition of 88% formic acid (50 µl). The entire mixture was applied to a Microsorb MV 300‐5 C18 analytical column (Varian) using solvents A (0.44% aq. formic acid) and B (0.44% formic acid in acetonitrile). Products were separated with a linear gradient from 0% to 5% B over 10 mins followed by 5% to 60% B over the next 15 mins (1 ml/min). A brief linear drop from 60% to 30% B over the next 2 mins was added to prevent baseline drifting near the internal standard. Finally, the column was washed with 95% B and re‐equilibrated with 100% A for subsequent analyses. Detection of tyrosine was calibrated from a standard curve generated by the response to a series of known concentrations of tyrosine in analogous reaction mixtures lacking substrate. The commercial source of Br‐Tyr contained a trace amount of tyrosine (5%) and its contribution to the product signal was subtracted prior to analysis (Supporting Information Fig. S4).
Conclusions
IYD from Drosophila promotes dehalogenation of I‐Tyr, Br‐Tyr and Cl‐Tyr with nearly equivalent efficiencies and has the potential to act broadly on all of these substrates in vivo in analogy to human IYD. Detailed studies on the possible generation of each halotyrosine in Drosophila will be necessary before the physiological significance of the individual dehalogenation reactions can be determined unambiguously. At least for deiodination, the active site E154 may be used as the most significant marker for future annotation of IYD homologs that process halotyrosines. Mutation of this residue suppresses catalysis more than mutation of two alternative residues (Y158 and K179) that also provide coordination to the substrate zwitterion. Such information helps to build confidence that genes labeled as putative NADH oxidases from Hyperthermus butylicus (Accession WP_011822328.1) and Staphylothermus marinus (Accession WP_011839241.1), for example, likely function instead as deiodinases based on the presence of the crucial Glu and Lys in the active sites. Only the most dispensable Tyr is missing from this region.
Supporting information
Supporting Information
Acknowledgments
The authors thank Dr. C. Lima for providing the pSMT3 plasmid and the SUMO specific protease ULP1 and Drs. S. Eun and X. Chen for an introduction to Drosophila biology. The authors have no conflict of interest to report.
References
- 1. Roche J, Michel R, Michel O, Lissitzky S (1952) Sur la déshalogénation enzymatique des iodotyrosines par le corps thyroïde et sur son rôle physiologique. Biochim Biophys Acta 9:161–169. [DOI] [PubMed] [Google Scholar]
- 2. Goswami A, Rosenberg IN (1977) Studies on a soluble thyroid iodotyrosine deiodinase: Activation by NADPH and electron carriers. Endocrinology 101:331–341. [DOI] [PubMed] [Google Scholar]
- 3. Rosenberg IN, Goswami A (1979) Purification and characterization of a flavoprotein from bovine thyroid with iodotyrosine deiodinase activity. J Biol Chem 254:12318–12325. [PubMed] [Google Scholar]
- 4. Rokita SE Flavoprotein dehalogenases In Hille R, Miller SM, Palfey B, Eds. (2013) Handbook of flavoproteins. Berlin: DeGruyter, pp 337–350. [Google Scholar]
- 5. Rokita SE, Adler JM, McTamney PM, Watson JA, Jr (2010) Efficient use and recycling of the micronutrient iodide in mammals. Biochimie 92:1227–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moreno JC, Klootwijk W, van Toor H, Pinto G, D'Alessandro M, Lèger A, Goudie D, Polak M, Grüters A, Visser TJ (2008) Mutations in the iodotyrosine deiodinase gene and hypothyroidism. N Engl J Med 358:1811–1818. [DOI] [PubMed] [Google Scholar]
- 7. Hulbert AJ (2000) Thyroid hormones and their effects: a new perspective. Biol Rev 75:519–631. [DOI] [PubMed] [Google Scholar]
- 8. Paris M, Brunet F, Markov G, Schubert M, Laudet V (2008) The amphioxus genome enlightens the evolution of the thyroid hormone signaling pathway. Dev Genes Evol 218:667–680. [DOI] [PubMed] [Google Scholar]
- 9. Paris M, Escriva H, Schubert M, Brunet F, Brtko J, Ciesielski F, Roecklin D, Vivat‐Hannah V, Jamin EL, Cravedi J‐P, Scanlan TS, Renaud J‐P, Holland ND, Laudet V (2008) Amphioxus postembryonic development reveals the homology of chordate metamorphosis. Curr Biol 18:825–830. [DOI] [PubMed] [Google Scholar]
- 10. Phatarphekar A, Buss JM, Rokita SE (2014) Iodotyrosine deiodinase: a unique flavoprotein present in organisms of diverse phyla. Mol Biosyst 10:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eales JG (1997) Iodine metabolism and thyroid‐related functions in organisms lacking thyroid follicles: are thyroid hormones also vitamins? Proc Soc Exp Biol Med 214:302–317. [DOI] [PubMed] [Google Scholar]
- 12. Flatt T, Moroz LL, Tatar M, Heyland A (2006) Comparing thyroid and insect hormone signaling. Integr Comp Biol 46:777–794. [DOI] [PubMed] [Google Scholar]
- 13. McTamney PM, Rokita SE (2009) A mammalian reductive deiodinase has broad power to dehalogenate chlorinated and brominated substrates. J Am Chem Soc 131:14212–14213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bobyk KD, Ballou DP, Rokita SE (2015) Rapid kinetics of dehalogenation promoted by iodotyrosine deiodinase from human thyroid. Biochemistry 54:4487–4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buss IH, Senthilmohan R, Darlow BA, Mogridge N, Kettle AJ, Winterbourn CC (2003) 3‐Chlorotyrosine as a marker of protein damage by myeloperoxidase in tracheal aspirates from preterm infants: association with adverse respiratory outcome. Pediatr Res 53:455–462. [DOI] [PubMed] [Google Scholar]
- 16. Wu W, Samoszuk MK, Comhair SAA, Thomassen MJ, Farver CF, Dweik RA, Kavuru MS, Erzurum SC, Hazen SL (2000) Eosinophils generate brominating oxidants in allergen‐induced asthma. J Clin Invest 105:1455–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mani AR, Moreno JC, Visser TJ, Moore KP (2016) The metabolism and de‐bromination of bromotyrosine in vivo. Free Radic Biol Med 90:243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hunt S (1984) Halogenated tyrosine derivatives in invertebrate scleroproteins: isolation and identification. Methods Enzymol 107:413–438. [DOI] [PubMed] [Google Scholar]
- 19. Birkedal H, Khan RK, Slack N, Broomell C, Lichtenegger HC, Zok F, Stucky GD, Waite JH (2006) Halogenated veneers: protein cross‐linking and halogenation in the jaws of Nereis, a marine polychaete worm. ChemBioChem 7:1392–1399. [DOI] [PubMed] [Google Scholar]
- 20. Hall HG (1978) Hardening of the sea urchin fertilization envelope by peroxidase‐catalyzed phenolic coupling of tyrosines. Cell 15:343–355. [DOI] [PubMed] [Google Scholar]
- 21. Attrill H, Falls K, Goodman JL, Millburn GH, Antonazzo G, Rey AJ, Marygold SJ (2016) FlyBase: establishing a gene group resource for Drosophila melanogaster . Nucleic Acids Res 44:D786–D792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wheeler BM (1950) Halogen metabolism of Drosophila gibberosa. I. Iodine metabolism studied by means of I131 . J Exp Zool 115:83–107. [Google Scholar]
- 23. Häggblom MM, Bossert ID Halogenated organic compounds—a global perspective In: Häggblom MM, Bossert ID, Eds. (2003) Dehalogenation: microbial processes and environmental applications. Boston: Kluwer Academic Publishers, pp 3–32. [Google Scholar]
- 24. Hu J, Chuenchor W, Rokita SE (2015) A Switch between one‐ and two‐electron chemistry of the Human flavoprotein iodotyrosine deiodinase is controlled by substrate. J Biol Chem 290:590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Warner JR, Copley SD (2007) Pre‐steady‐state kinetic studies of the reductive dehalogenation catalyzed by tetrachlorohydroquinone dehalogenase. Biochemistry 46:13211–13222. [DOI] [PubMed] [Google Scholar]
- 26. Thomas SR, McTamney PM, Adler JM, LaRonde‐LeBlanc N, Rokita SE (2009) Crystal structure of iodotyrosine deiodinase, a novel flavoprotein responsible for iodide salvage in thyroid glands. J Biol Chem 284:19659–19667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Buss JM, McTamney PM, Rokita SE (2012) Expression of a soluble form of iodotyrosine deiodinase for active site characterization by engineering the native membrane protein from Mus musculus . Protein Sci 21:351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rosenberg IN, Goswami A (1984) Iodotyrosine deiodinase from bovine thyroid. Methods Enzymol 107:488–500. [DOI] [PubMed] [Google Scholar]
- 30. Friedman JE, Watson JA, Lam DWH, Rokita SE (2006) Iodotyrosine deiodinase is the first mammalian member of the NADH oxidase/flavin reductase superfamily. J Biol Chem 281:2812–2819. [DOI] [PubMed] [Google Scholar]
- 31. McMillen DF, Golden DM (1982) Hydrocarbon bond dissociation energies. Ann Rev Phys Chem 33:493–532. [Google Scholar]
- 32. Odar C, Winkler M, Wiltschi B (2015) Fluoro amino acids: A rarity in nature, yet a prospect for protein engineering. Biotechnol J 10:427–446. [DOI] [PubMed] [Google Scholar]
- 33. Hopkins TL, Kramer KJ (1992) Insect cuticle sclerotization. Annu Rev Entomol 37:273–302. [Google Scholar]
- 34. Neckameyer WS (1996) Multiple roles for dopamine in Drosophila development. Dev Biol 176:209–219. [DOI] [PubMed] [Google Scholar]
- 35. McCall SA, Cummings CF, Bhave G, Vanacore R, Page‐McCaw A, Hudson BG (2014) Bromine Is an essential trace element for assembly of collagen IV scaffolds in tissue development and architecture. Cell 157:1380–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bhave G, Cummings CF, Vanacore RM, Kumagai‐Cresse C, Ero‐Tolliver IA, Rafi M, Kang J‐S, Pedchenko V, Fessler LI, Fessler JH, Hudson BG (2012) Peroxidasin forms sulfilimine chemical bonds using hypohalous acids in tissue genesis. Nat Chem Biol 8:784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Andersen SO (2004) Chlorinated tyrosine derivatives in insect cuticle. Insect Biochem Mol Biol 34:1079–1087. [DOI] [PubMed] [Google Scholar]
- 38. Mukherjee A, Rokita SE (2015) Single amino acid switch between a flavin‐dependent dehalogenase and nitroreductase. J Am Chem Soc 137:15342–15345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ralston IM, Dunford HB (1980) Horseradish peroxidase. XLII. Oxidations of L‐tyrosine and 3,5‐diiodo‐L‐tyrosine by compound II. Can J Biochem 58:1270–1276. [DOI] [PubMed] [Google Scholar]
- 40. Das TN (1998) Redox chemistry of 3‐iodotyrosine in aqueous medium. J Phys Chem A 102:426–433. [Google Scholar]
- 41. Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A, Protein identification and analysis tools on the ExPASy server In: Walker JM, Ed. (2006) The proteomics protocols handbook. New York: Humana Press, pp 571–607. [Google Scholar]
- 42. Koziol J (1971) Fluorometric analyses of riboflavin and its coenzymes. Methods Enzymol 18:253–285. [Google Scholar]
- 43. Krogh A, Larsson B, von Heijne G, Sonnhammer ELL (2001) Predicting transmembrane protein topology with a hidden markov model: application to complete genomes. J Mol Biol 305:567–580. [DOI] [PubMed] [Google Scholar]
- 44. Mossessova E, Lima CD (2000) Ulp1‐SUMO crystal structure and genetic analysis reveal conserved interactions and a regulatory element essential for cell growth in yeast. Mol Cell 5:865–876. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


