Abstract
Background
Human papillomaviruses (HPVs) are causally associated with the tumorigenesis of several classes of cancers. However, the prevalence of HPV in gastric cancer (GC) has not yet been systematically reviewed. Hence, a meta-analysis was conducted to estimate the HPV prevalence in patients with GC, and its potential etiologic significance was assessed.
Methods
The pooled HPV prevalence and 95% confidence intervals (CIs) were estimated among all GC patients. Heterogeneity was described by using the I2 statistic. Sources of heterogeneity were explored by meta-regression and stratified analyses. The meta-influence was applied to evaluate the influence of a single study on the pooled estimates. Odds ratios (ORs) and 95% CIs were computed for case–control studies. For research providing clinicopathological parameters of age, sex, pathological, differentiated, and clinical stages, and HPV subtypes, the corresponding pooled ORs and 95% CIs were also calculated.
Results
Thirty studies were included in the current meta-analysis, involving 1,917 patients with GC and 576 controls. The pooled HPV prevalence was 28.0% (95% CI: 23.2%, 32.7%) among all the patients with GC, and the I2 was 96.9% (P<0.001). A pooled OR of 7.388 (95% CI: 3.876, 14.082) was achieved based on 15 case–control studies (I2=56.7%, P=0.004). Moreover, the HPV prevalence was significantly higher in patients from China than in those from non-Chinese regions (31% vs 9%, I2=95.0%, P<0.001). The pooled prevalence of HPV16 was 21% in GC tissues, and the pooled prevalence of HPV18 was 7% with an OR of 3.314 (95% CI =1.617, 6.792). HPV16 was 3 times more frequently detected than HPV18.
Conclusion
HPV could play a potential role in the pathogenesis of GC. A causal relationship can be confirmed only by detecting HPV in the cells of GC precursor lesions (gastric dysplasia or adenoma). In addition, this study might be beneficial for expounding the potential etiologic significance of molecular mechanism of gastric tumorigenesis and providing opinions regarding precautionary measures.
Keywords: gastric dysplasia, gastric adenoma, gastric tumorigenesis, odds ratios, prevalence, subtypes
Introduction
A growing amount of evidence has shown that virus infection, directly or indirectly, can result in numerous malignant tumors.1 Worldwide, annually, >550,000 new patients suffering from malignant tumors are associated with human papillomaviruses (HPVs) infection.2,3 Currently, >150 HPV subtypes with 15 species of high-risk types of HPV (HR-HPV) have been found. The HR-HPV family contains HPV16, HPV18, HPV31, HPV33, HPV35, HPV39, HPV45, HPV51, HPV52, HPV56, HPV58, HPV59, HPV66, and HPV68. HPV16 and HPV18 are the most common cancer-causing HPV subtypes on a global scale.2,3 Simultaneously, HPV16, HPV18, and HPV33 have been confirmed to be correlated with tumors in digestive system, such as oral cancer, esophageal cancer, and colorectal cancer.1,4,5 In 2012, 1 million new cases of gastric cancer (GC) occurred (952,000 cases, 6.8% of the total), leading to its rank as the fifth most common malignant tumor worldwide, following lung, breast, colorectum, and prostate cancers. More than 70% of cases (677,000 cases) occurred in the developing countries (456,000 in males and 221,000 in females), and worldwide, half of all the GCs occurred in Eastern Asia (mostly in China). The fatality rate of GC was ranked third, following only lung and liver cancers.6 In 2015, cancer statistics estimated that new cancer cases and deaths from GC numbered 24,590 and 10,720 (1.48% and 1.82% of the total, respectively) in the USA.7
Several meta-analyses have shown that HPV infection is a high-risk factor for carcinogenesis at some anatomic sites, including the skin, genital tract, respiratory tract, and disparate anatomic sites of the digestive tract.8–11 With the assumption that repeatedly persistent infection with HPV can cause dysplasia or adenocarcinoma in situ, as precursor lesions, it will eventually result in malignant transformation.12 Some studies have investigated HPV infection in gastrointestinal cancer.4,5,8,12–14 However, only sporadic research has reported the HPV prevalence in GC. Currently, whether there is a link between HPV infection and GC occurrence has not been systematically analyzed publicly.
In order to obtain insight into the HPV prevalence in GC tissues, the present meta-analysis was conducted, and this study facilitates and improves the understanding of the carcinogenicity of the virus in GC and provides new clues to GC etiology and prevention.
Materials and methods
Search strategy
The PubMed, EMBASE, Web of Science, Science Direct, Ovid, Wiley Online Library, and Cochrane Library databases, as well as the China National Knowledge Infrastructure, Chinese Chongqing VIP, Chinese Wan Fang, and China Biology Medicine databases, were searched to identify all the appropriate studies published on or before June 3, 2016, with the following search strategy: (HPV OR human papillomavirus) AND (gastric OR stomach OR cardia OR gastrointestinal) AND (adenocarcinoma OR carcinoma OR cancer OR neoplasm OR tumour OR tumor OR neoplasm* OR malignan*).
Study selection and inclusion criteria
The inclusion criteria for this meta-analysis are as follows: 1) studies had to estimate the prevalence of HPV in GC cases where the classification was unspecified or provide sufficient information; 2) data from case reports, review articles, meeting abstracts, unpublished reports, and letters were not eligible for this study; 3) studies had to investigate HPV DNA in human GC tissue; two cohort studies detecting HPV serum antibodies in patients with GC were excluded;13,15 4) only English- or Chinese-language studies could be included; and 5) the HPV detection methods used were polymerase chain reaction (PCR) and in situ hybridization (ISH).
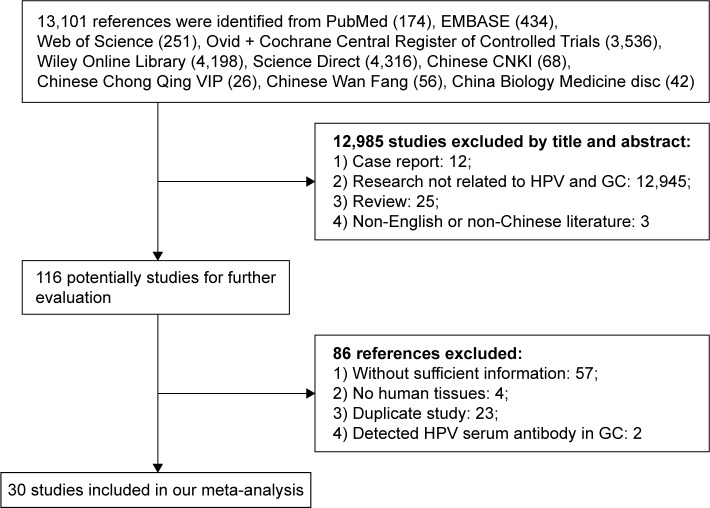
In addition, if two or more articles were published by the same group with the same case series, the one with larger sample size was selected. The research was conducted independently by two investigators, and disagreements were resolved by discussion or consulting with a third reviewer (Figure 1).
Figure 1.
The flow diagram of searching and screening process as well as the number of screened, excluded, and included publications.
Abbreviations: GC, gastric cancer; HPV, human papillomavirus.
Data extraction
The following data were extracted from the eligible studies: name of first author, published year, country, anatomic site, language, sample size (n), HPV DNA test method, materials, number of cases and controls; HPV subtype-specific, mean age, sex, pathological differentiation, and clinical stage (TNM) of GC.
Statistical analysis
Pooled HPV prevalence and pooled odds ratios (ORs) with their 95% confidence intervals (CIs) were used to evaluate the relation of HPV infection with the occurrence and development of GC. The STATA software, Version 12.0, was applied to analyze the eligible literature using the meta-module “meta” or “metan” command. Estimates, standard errors, and 95% CIs were used to calculate the HPV prevalence percentages in all the studies. ORs and 95% CIs were measured among 15 case–control studies, and pooled HPV prevalence and 95% CIs were computed among all the 30 studies. All the prevalence estimates were transformed logarithmically, which necessitated adding a correction factor of 0.5 to both the numerator and denominator for reported prevalence of 0. In these situations, inverse variance, Mantel–Haenszel (M–H) method, and DerSimonian and Laird (D+L) method required the addition of a small quantity (usually 0.5) to the cell counts in order to avoid division by zero errors. The pooled estimates were computed by using the Mantel–Haenszel method, assuming a fixed effects model or the random effects model of the D+L method. When significant heterogeneity occurred in the pooled estimates across studies, a random effects model was considered. In addition, heterogeneity was described by using the I2 statistic. Meta-regression models were estimated by the “metareg” command to analyze the heterogeneity of the pooled HPV prevalence in GC. Begg’s test (“metabias”) was accepted to draw funnel plots and describe the publication bias of funnel plot asymmetry (publication bias). The “metainf” command was selected to assess the influence of individual studies on the effect estimate. P<0.05 was considered to be statistically significant. The source of heterogeneity was explored by using the following approaches: sensitivity analysis, subgroup analysis, meta-regression, and the random effects model.
Results
Eligible studies
The flowchart shows a diagram of all the studies included in this meta-analysis (Figure 1). On the basis of the primary search strategy, a total of 13,101 related publications were identified. After browsing through the titles and abstracts, 116 references were deemed as eligible based on the selection and inclusion criteria. Through a strict review of the complete articles, 86 studies were excluded, including 57 studies without sufficient information, 4 studies conducted in mouse models, 23 reduplicated studies, and 2 studies detecting HPV serum antibody in GC. As a result, 30 eligible studies were included in this meta-analysis; of them, 10 were in English and 20 in Chinese, and they involved a total of 1,917 GC cases and 576 controls. Half of the 30 studies were case–control studies, and the remaining were case-only studies; the 15 case–control studies included 944 cases and corresponding 576 controls (Table 1).
Table 1.
The characteristics of all 30 eligible studies
| Author’s name | Year | Country | Anatomic site | Language | Sample size (N) | Method | Materials | Case (n)
|
Control (N)
|
HPV types | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HPV(+) | Total | HPV(+) | Total | |||||||||
| Saegusa et al34 | 1997 | Japan | GC | English | >50 | PCR | FFPE | 0 | 99 | NA | NA | HPV16 and HPV18 |
| Cândido et al48,a | 2013 | Brazil | GC | English | >50 | PCR | FFPE | 4 | 40 | 10 | 40 | HPV16 |
| Anwar et al16,a | 1995 | Japan | GC | English | >50 | PCR | FFPE | 23 | 51 | 2 | 12 | HPV16, HPV18, and HPV33 |
| Snietura et al35 | 2014 | Poland | GC | English | >50 | PCR | FFPE | 0 | 84 | NA | NA | 14 HPV subtypes |
| Sobti et al49 | 2001 | India | GC | English | <50 | PCR | FFPE | 4 | 9 | NA | NA | NA |
| Ding et al18 | 2010 | China | GCC | English | <50 | PCR | FFPE | 5 | 17 | NA | NA | HPV16 |
| Yuan et al31 | 2013 | China | GC | English | <50 | ISH | NA | 0 | 24 | NA | NA | 13 HR-HPV, 5 LR-HPV |
| Koshiol et al33 | 2010 | China | GCC | English | >50 | PCR | FFPE | 0 | 144 | NA | NA | HPV16 and HPV18 |
| Ma et al19,a | 2007 | China | GC | English | >50 | PCR | FFPE | 15 | 40 | 2 | 40 | HPV16 |
| Cai et al17 | 2006 | China | GC | English | <50 | PCR | FFPE | 19 | 46 | NA | NA | HPV16 |
| Guo et al36 | 2000 | China | GC | Chinese | <50 | PCR | FF | 6 | 41 | NA | NA | HPV16 and HPV18 |
| Sun et al24 | 2002 | China | GC | Chinese | >50 | PCR | NA | 28 | 64 | NA | NA | HPV16 and HPV18 |
| Huang et al37 | 2000 | China | GC | Chinese | >50 | ISH | FFPE | 39 | 96 | NA | NA | HPV16 and HPV18 |
| Dong et al20,a | 1999 | China | GC | Chinese | >50 | PCR | NA | 10 | 37 | 0 | 20 | HPV16 and HPV18 |
| Du et al21 | 2000 | China | GC | Chinese | <50 | PCR | NA | 9 | 29 | NA | NA | HPV16 and HPV18 |
| Zhu et al30,a | 2000 | China | GC | Chinese | >50 | PCR | FF | 11 | 42 | 0 | 42 | HPV16 and HPV18 |
| Wang et al25,a | 2013 | China | GC | Chinese | >50 | PCR | FFPE | 20 | 92 | 4 | 86 | HPV16 |
| Xu et al27,a | 2003 | China | GCC | Chinese | >50 | ISH | FFPE | 91 | 176 | 10 | 50 | HPV16 |
| Zhang et al39 | 2011 | China | GC | Chinese | >50 | PCR | FF | 45 | 62 | NA | NA | HPV16 and HPV18 |
| Liao et al40,a | 2001 | China | GC | Chinese | >50 | ISH | NA | 26 | 50 | 2 | 30 | HPV16 and HPV18(E6) |
| Wei et al26 | 2006 | China | GC | Chinese | <50 | PCR | FFPE | 19 | 46 | NA | NA | HPV16 |
| Zhang et al29,a | 2001 | China | GC | Chinese | >50 | PCR | FF | 15 | 40 | 0 | 10 | HPV16 and HPV18 |
| Cao et al51 | 2005 | China | GC | Chinese | <50 | PCR | FF | 0 | 47 | NA | NA | NA |
| Ma et al22,a | 2007 | China | GCC | Chinese | >50 | PCR | FFPE | 32 | 93 | 0 | 21 | HPV16 and HPV18 |
| Zhang et al52 | 2010 | China | GCC | Chinese | >50 | PCR | FF | 17 | 165 | NA | NA | NA |
| Zhou et al32,a | 1999 | China | GC | Chinese | >50 | PCR | FFPE | 19 | 50 | 0 | 20 | HPV16 |
| Rong et al23,a | 2007 | China | GCC | Chinese | <50 | PCR | FFPE | 16 | 21 | 2 | 21 | HPV16 |
| Yu et al28,a | 1999 | China | GC | Chinese | >50 | PCR | FFPE | 30 | 132 | 3 | 104 | HPV16 and HPV18 |
| Sha et al38,a | 1998 | China | GC | Chinese | >50 | PCR | FFPE | 27 | 65 | 4 | 65 | HPV16 |
| Su and He53,a | 2015 | China | GC | Chinese | <50 | PCR | NA | 1 | 15 | 0 | 15 | HPV16 and HPV18 |
Note:
Case–control study.
Abbreviations: FF, fresh-frozen; FFPE, formalin-fixed paraffin-embedded tissue; GC, gastric cancer; GCC, gastric cardia cancer; HPV, human papillomavirus; HR-HPV, high-risk types of HPV; ISH, in situ hybridization; LR-HPV, low-risk types of HPV; NA, not available; PCR, polymerase chain reaction.
Study characteristics
Among the 30 studies, 25 were conducted in China. The materials included fresh-frozen (FF) and formalin-fixed paraffin-embedded (FFPE) tissue. Moreover, a PCR-based technique was adopted to detect HPV DNA in 26 studies, whereas only 4 studies used ISH to detect HPV E6/E7 genes (Table 1). In addition, 15 studies provided the prevalence of HPV in different histological differentiations of patients with GC, which were divided into well-differentiated, moderately differentiated, poorly differentiated, and undifferentiated grades.16–30 Some studies provided concrete information about HPV prevalence in different clinical stages (TNM: I, II, III, and IV),16,19,27,31,32 the age and sex15,16,18,19,25,27,32 of the patients, and specific HPV types (HPV16 and HPV18).15,16,20–24,29–31,33–39 Table 1 provides the information about the studies. The sample sizes of the 30 eligible studies ranged from 30 (cases/controls: 15/15) to 236 (cases/controls: 132/104).
The overall HPV prevalence in GC
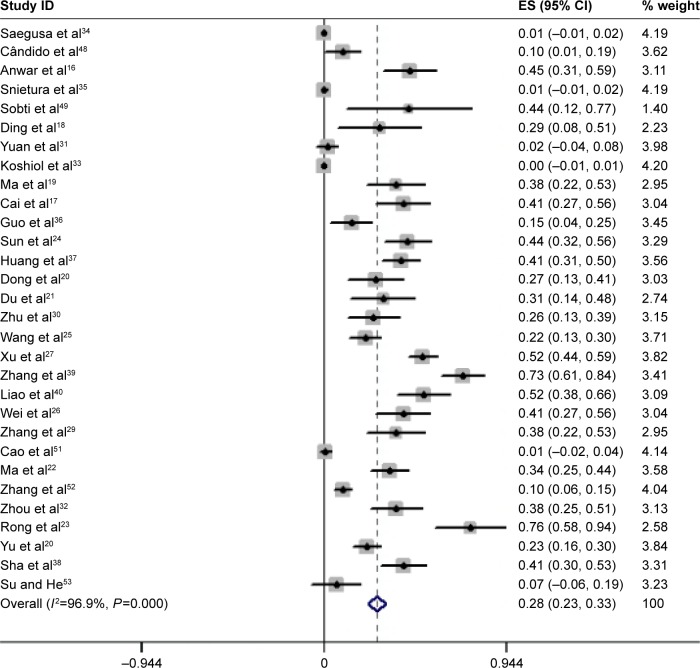
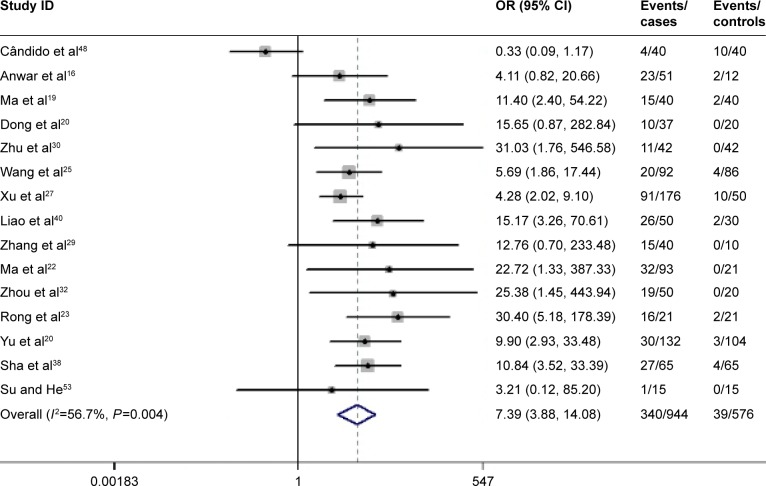
The HPV prevalence percentage was represented by decimals over forest plots. Figure 1 shows the HPV prevalence rates and 95% CI estimates from all the 1,917 GC cases, based on the D+L methods with a random effects model. The pooled HPV prevalence was 0.280 (95% CI: 0.232, 0.327), which equals a proportion of 28.0% (95% CI: 23.2%, 32.7%; z=11.63; P<0.01). The heterogeneity between the studies was obvious (I2=96.9%, P<0.01; Figure 2). The 15 case–control studies were generalized to a pooled OR of 7.388 (95% CI =3.876, 14.082), based on the D+L methods with random effects model, which was statistically significant (z=6.08, P<0.01; Figure 3). This result indicated that the HPV prevalence in GC cases was 7-fold more than that in the corresponding normal gastric tissues, but the heterogeneity between these studies could not be ignored (I2=56.7%, P=0.004).
Figure 2.
Overall association between HPV infection and GC risk.
Note: Forest plot (weights are from random effects analysis) of the pooled prevalence of HPV positivity in GC.
Abbreviations: GC, gastric cancer; HPV, human papillomavirus; ES, effect size.
Figure 3.
For 15 case–control studies, the estimates of ORs and their 95% CIs were plotted in a forest plot.
Note: Weights are from random effects analysis.
Abbreviations: CI, confidence interval; OR, odds ratio.
The HPV prevalence in subgroups stratified by country, sample size, HPV test method, and materials
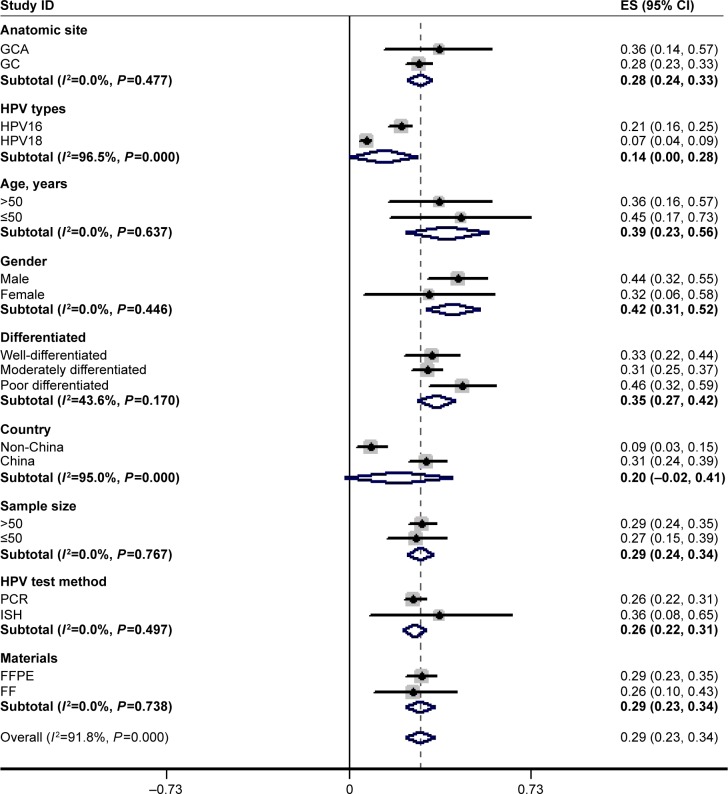
All research could be stratified by country, sample size, HPV test method, and materials (Table 1; Figure 4). The HPV prevalence was higher in cases from China than in those from non-Chinese regions (31% vs 9%). The HPV prevalence in the gastric cardia (36%) was more common than that in overall GC (28%), and the HPV prevalence was similar in cases from FFPE and FF tumor specimens (29% vs 26%). Oddly, the ISH method yielded a higher rate of HPV infection than did the PCR method (36% vs 26%), which was in contrast to the findings of many other studies.5,9–11 There was no distinct difference between large samples (≥50 cases) and small samples (<50 cases), with HPV prevalence rates of 29% and 27%, respectively.
Figure 4.
The estimates of HPV prevalence and their 95% CIs showed in decimals instead of percentages, plotted in a forest plot.
Notes: Weights are from random effects analysis. All searches were stratified by country, sample size, HPV test method, and materials. Five studies provided age and sex data, and 15 studies provided pathological differentiation; the 5 studies also provided clinical TNM stage information.
Abbreviations: CI, confidence interval; FF, fresh-frozen; FFPE, formalin-fixed paraffin-embedded tissue; GC, gastric cancer; GCA, gastric cardic cancer; HPV, human papillomavirus; PCR, polymerase chain reaction; ISH, in situ hybridization.
The HPV prevalence and its clinicopathological parameters in patients with GC
Age and sex
Information on age and sex was collected from 6 studies.16,18,19,25,27,32 HPV was positive in 75 of the 223 patients with GC aged >50 years, whereas it was positive in 45 of the 106 patients with GC aged <50 years. Then, a pooled OR of 0.993 (95% CI: 0.579, 1.700) was estimated by using the M-H method. There was no significant difference between HPV incidence and ages of patients with GC. Similarly, information on sex was available for 5 of the 30 studies. HPV was detected in 99 (44.4%) of the 223 male patients and in 33 (29.7%) of the 111 female patients with GC. Based on these findings, a significantly pooled OR of 1.698 (95% CI: 1.007, 2.862) was computed (z=1.99, P=0.047), which indicated that males were slightly more likely to have HPV-positive GC.
HPV subtypes
Sixteen studies provided data on specific HPV types (including HPV16 and HPV18).15,16,20–24,29–31,33–39 HPV16 was found in 210 (21%) of the 992 GC cases, whereas HPV18 was found in 90 (7%) of the 992 GC cases, with a significant OR of 3.314 (95% CI: 1.617, 6.792). It was found that HPV16 was 3 times more frequently detected than HPV18 (Figure 4).
Histological differentiation of GC
Fifteen studies provided the prevalence of HPV in GC of different histological differentiations, which were divided into well-differentiated, moderately differentiated, poorly differentiated, and undifferentiated GC.16–30 The HPV prevalence was 30.3% (142 of 468 patients) in well-differentiated/moderately differentiated GC, whereas the HPV prevalence was 43.0% (150 of 349 patients) in poorly differentiated/undifferentiated GC, with an OR of 1.569 (95% CI: 1.148, 2.143). Therefore, there were significant associations between HPV prevalence and histological grades of GC, and poorly differentiated/undifferentiated GC patients were more likely to be HPV-positive than well-differentiated/moderately differentiated cases (Figure 4).
Clinical stages (TNM)
Five studies provided concrete information about HPV prevalence in different clinical stages (TNM: I, II, III, IV).16,19,27,31,32 The HPV prevalence was 51.0% (49 of 96 patients) in TNM I/TNM II GC patients and 40.6% (58 of 143 patients) in TNM III/TNM IV GC patients. The statistically significant pooled OR was 0.414 (95% CI: 0.223, 0.770); therefore, it was uncertain whether HPV played a prognostic role in GC.
HPV prevalence and p53 gene mutation
Five studies in this meta-analysis16,22,24,37,40 with 354 cases provided information about the relationship between HPV prevalence and the p53 gene. However, the pooled OR of 1.534 (95% CI =0.752, 3.127) was insignificant.
Sensitivity analysis, meta-regression, and publication bias in case–control studies
Sensitivity analysis indicated that no individual study could influence the pooled effect estimate significantly. A meta-regression analysis was performed, and it was observed that the source of heterogeneity between the studies was from the item of country (China vs non-China, P=0.004). Begg’s test was implemented to screen for publication bias. As a result, with continuity correction (z=0.30, P>0.767), there was no evident publication bias (Figure 5).
Figure 5.
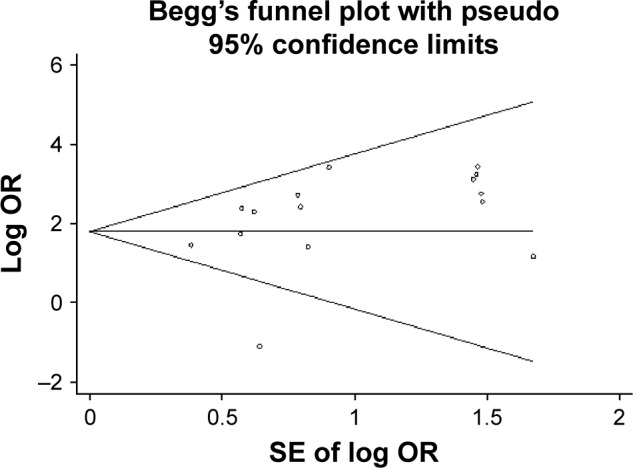
Begg’s test for publication bias (continuity-corrected: z=0.30, P>0.767) showing no evident publication bias.
Abbreviations: OR, odds ratio; SE, standard error.
Discussion
GC is one of the most frequent cancers worldwide. Almost two-thirds of the GC cases occurred in undeveloped regions. Moreover, in 2005, 0.3 million deaths and 0.4 million new cases of GC caused it to become the third most common cancer among Chinese people.41 Research conducted between 2005 and 2010 reported that the incidence and mortality of GC were 21.04% and 19.13% of all cancer-related cases and deaths, respectively, in Zhuanghe, China.42 It is generally believed that gastric tumorigenesis is correlated with Helicobacter pylori infection.43,44 However, in recent years, several studies have verified that gastric carcinogenesis is also associated with Epstein–Barr virus infection.43,44 In the present study, it was found that HPV prevalence increased GC risk.
To date, the present study was the first meta-analysis to systematically evaluate the association between HPV prevalence and GC as well as their clinicopathological features. In this meta-analysis, a pooled HPV prevalence of 28.0% (95% CI: 23.2%, 32.7%) in 1,917 GC cases among 30 included studies was estimated, and a pooled OR of 7.388 (95% CI =3.876, 14.082) was estimated among 15 case–control studies, which demonstrated that HPV prevalence was a high-risk factor in gastric carcinogenesis.
In addition, it was found that the pooled prevalence of HPV16 was 3-fold higher than that of HPV18 (21% vs 7%) in GC. Other HPV subtypes were infrequent in GC tissues. In addition, the prevalence of HPV in gastric cardia cancer was greater than that in overall GC (36% vs 28%). If HPV could enter the pathway via an oral route from the esophagus to the stomach, a higher HPV prevalence can be expected in oral cancers than in GC. Furthermore, 15 studies, with 817 cases in total, provided data on pathological grade of differentiation, and the HPV incidence in poorly differentiated/undifferentiated GC patients was greater than that in well-differentiated/moderately differentiated GC, with an OR of 1.569. In contrast, the HPV prevalence in clinically advanced stages (TNM III/TNM IV) was slightly less than that in early stages (TNM I/TNM II; OR =0.414; 95% CI: 0.223, 0.770). Therefore, the role of HPV in the prognosis of GC remains unclear.
However, the pathogenesis of GC is multifactorial, and HPV infection might be one of the infectious risk factors. With the assumption that repeatedly persistent infection with HPV can cause dysplasia or adenocarcinoma in situ, as precursor lesions, it will eventually result in malignant transformation. Therefore, it could be hypothesized that HPV might enter the anus and colorectum, causing infection from anogenital sites. Otherwise, HPV enters orally, with downward infection from the mouth to the esophagus and finally to the stomach. It has been systematically found in reviews that HPV prevalence was related to anal, colorectal, oral, pharyngeal, and esophageal carcinogenesis.1,4,5,8,14,45,46 The HPV prevalence was reported to be much more common in oral and anal cancers (58.0% and 80%) than in GC in the present study.45,46
Moreover, given insight into the molecular mechanism of gastric oncogenesis, the p53 gene is suppressor gene. It had been demonstrated that overexpression of p53 resulted in poor prognosis of GC patients.47 In the present study, it was found that the role of the p53 gene was unrelated with HPV prevalence in GC. Five studies included in the present meta-analysis,16,22,24,37,40 with 354 cases, provided information about the relationship between HPV prevalence and the p53 gene. The wild-type p53 gene is a tumor suppressor gene, and mutation of the p53 gene promoted malignant transformation of human epithelium cells. However, the pooled OR of 1.534 (95% CI =0.752, 3.127) was insignificant.16,22,24,37,40 Nevertheless, the result was restricted to sample sizes, which could be a source of instability.
The same relationship with the p53, p21,17,24,26,40 and p1618,35,37 genes was reported to found with HPV prevalence. Some studies have found that HPV could coinfect with H. pylori, leading to canceration,25 whereas others have found that HPV prevalence was unrelated to H. pylori.19,48 One study reported the prevalence of HPV in GC and also tested the telomerase activity.49 All of the aforementioned molecular mechanisms suggest that more studies should be conducted.
Finally, the quality of all the 15 case–control studies was assessed based on Newcastle–Ottawa Scale standard conditions. As a result, 13 studies were qualified, and 2 were of good quality.24,26 Another 15 case-only studies were not assessed yet. Generally, the research included in the present study was appraised as being up to the standard.
Limitations
There are some limitations of this study. First, the included studies were cross-sectional studies; therefore, it could not be affirmed that the prevalence of HPV in GC tissue was caused by the temporal infection or by contamination of tissue samples after GC tumorigenesis. Second, the present study was restricted such that only HPV test methods and research detecting HPV in human GC tissues were included. In addition, Kirkegård et al50 preformed an observational cohort study of HPV infection and GC risk in females with cervical conization. Kamangar et al13 and Van Doornum et al15 searched for HPV antibodies in serum samples from GC patients. None of these three studies were included in the present study, but they had insignificant consequences. Last but not least, among the 30 included suitable searches, 25 were performed in China, which is a substantial source of between-study heterogeneity for the estimated HPV prevalence in GC (a result of meta-regression). The pooled prevalence of HPV was significantly greater in China than in non-Chinese regions (31% vs 9%), which could be partly explained by the very high frequency of GC incidence in Chinese people. However, several studies have indicated that HPV-related cancers were more inclined to occur in the developing countries or economically and sanitationally underdeveloped areas.5
Conclusion
In conclusion, this meta-analysis indicated a high incidence of HPV infection in 1,917 GC patients, with a pooled prevalence of 28.0% and a significantly pooled OR of 7.388 between HPV prevalence and GC risk. Hence, HPV could play a vital role in the pathogenesis of GC, which would be conducive to expounding the potential etiologic significance of gastric tumorigenesis and could provide opinions regarding precautionary measures, but causal relationship can be confirmed only by detecting HPV in the cells of GC precursor lesions (gastric dysplasia or adenoma).
Acknowledgments
All authors have read and approved the final manuscript and consent to publish it. The authors thank Miss Xing-gu Lin for her supervision in the analysis process and paper correction.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chaturvedi AK, Anderson WF, Lortet-Tieulent J. World-wide trends in incidence rates for oral cavity and oropharyngeal cancer. Clin Oncol. 2013;31(36):4550–4559. doi: 10.1200/JCO.2013.50.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tommasino M. The human papilomavirus family and its role in carcinogenesis. Semin Cancer Biol. 2014;26(1):13–21. doi: 10.1016/j.semcancer.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 4.Hardefeldt HA, Cox MR, Eslick GD. Association between human papillomavirus (HPV) and oesophageal squamous cell carcinoma: a meta-analysis. Epidemiol Infect. 2014;142(6):1119–1137. doi: 10.1017/S0950268814000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baandrup L, Thomsen LT, Olesen TB, Andersen KK, Norrild B, Kjaer SK. The prevalence of human papillomavirus in colorectal adenomas and adenocarcinomas: a systematic review and meta-analysis. Eur J Cancer. 2014;50(8):1446–1461. doi: 10.1016/j.ejca.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 6.International Agency for Research on Cancer Globocan 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. [Accessed November 14, 2016]. Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
- 7.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 8.Stanley MA, Winder DM, Sterling JC, Goon PK. HPV infection, anal intra-epithelial neoplasia (AIN) and anal cancer: current issues. BMC Cancer. 2012;12(9):398. doi: 10.1186/1471-2407-12-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas LK, Bermejo JL, Vinokurova S, Jensen K, Bierkens M, Steenbergen R. Chromosomal gains and losses in human papillomavirus-associated neoplasia of the lower genital tract – a systematic review and meta-analysis. Eur J Cancer. 2014;50(1):85–98. doi: 10.1016/j.ejca.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Aldabagh B, Yu J, Arron ST. Role of human papillomavirus in cutaneous squamous cell carcinoma: a meta-analysis. J Am Acad Dermatol. 2014;70(4):621–629. doi: 10.1016/j.jaad.2014.01.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhai K, Ding J, Shi HZ. HPV and lung cancer risk: a meta-analysis. J Clin Virol. 2015;63:84–90. doi: 10.1016/j.jcv.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Rietbergen MM, Leemans CR, Bloemena E, et al. Increasing prevalence rates of HPV attributtable oropharyngeal squarnous cell carcinomas in the Netherlands as assesses by a validated test algorithm. Int J Cancer. 2013;132(7):1565–1571. doi: 10.1002/ijc.27821. [DOI] [PubMed] [Google Scholar]
- 13.Kamangar F, Qiao YL, Schiller JT, et al. Human papillomavirus serology and the risk of esophageal and gastric cancers: results from a cohort in a high-risk region in China. Int J Cancer. 2006;119(3):579–584. doi: 10.1002/ijc.21871. [DOI] [PubMed] [Google Scholar]
- 14.Ali YB, Faris ME, Mohamed SA. Screening for high risk human papillomavirus (HR-HPV) subtypes, among Sudanese patients with oral lesions. Int J Clin Exp Med. 2013;6(4):275–281. [PMC free article] [PubMed] [Google Scholar]
- 15.Van Doornum GJ, Korse CM, Buning-Kager JC, Bonfrer JM, Horenblas S. Reactivity to human papillomavirus type 16 L1 virus-like particles in sera from patients with genital cancer and patients with carcinomas at five different extragenital sites. Br J Cancer. 2003;88(7):1095–1100. doi: 10.1038/sj.bjc.6600870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anwar K, Nakakuki K, Imai H, Inuzuka M. Infection of human papillomavirus (hpv) and epstein-barr-virus (ebv) and p53 overexpression in human gastric-carcinoma. Int J Oncol. 1995;7(2):391–397. doi: 10.3892/ijo.7.2.391. [DOI] [PubMed] [Google Scholar]
- 17.Cai JL, Sun JZ, Yao F, Sun SG. Implication of HPV 16 infection and P21 gene mutation in the carcinogenesis and prognosis of gastric cancer. Chinese-German J Clin Oncol. 2006;5(2):99–100. [Google Scholar]
- 18.Ding GC, Ren JL, Chang FB, et al. Human papillomavirus DNA and P16INK4A expression in concurrent esophageal and gastric cardia cancers. World J Gastroenterol. 2010;16(46):5901–5906. doi: 10.3748/wjg.v16.i46.5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma TY, Liu WK, Chu YL, et al. Detection of human papillomavirus type 16 DNA in formalin-fixed, paraffin-embedded tissue specimens of gastric carcinoma. Eur J Gastroenterol Hepatol. 2007;19(12):1090–1096. doi: 10.1097/MEG.0b013e3282eeb4dc. [DOI] [PubMed] [Google Scholar]
- 20.Dong WG, Yu JP, Luo HS. Relationship between human papillomavirus infection and the development of gastric carcinoma. World Chinese J Digestol. 1999;7(1):50–52. Chinese. [Google Scholar]
- 21.Du GL, Li CP, Chen GD. The role HPV in the athogenesis of gastric adenocarcinoma. Chinese J Clin Gastroenterol. 2002;12(3):116–117. Chinese. [Google Scholar]
- 22.Ma YQ, Pu HW, Chen ZL. Biopathological significance of the abnormal expression of HPV16/18, p53 in cardiac cancer tissues in Xinjiang. J Xinjiang Med Univ. 2007;30(9):955–957. Chinese. [Google Scholar]
- 23.Rong XS, Chen J, Li M. A study of the relationship between Human papilloma virus and gastric cardia cancer. Acta Uni Med Nanjing (Natural Science) 2007;27(9):1023–1024. Chinese. [Google Scholar]
- 24.Sun SR, Yao F, Zhang AM. A research on the influence of HPV16, HPV18 infection and p53, p21 gene mutation in the prognosis of gastric cancer. Cancer Res Clin. 2002;12(2):84–85. Chinese. [Google Scholar]
- 25.Wang ZJ, Zhang YQ, Zhang YT. Analysis of relationship of the infection of human papillomavirus 16 H. pylori cagA gene and ureA gene in gastric carcinogenesis. J Pract Med Tech. 2013;20(10):1061–1064. Chinese. [Google Scholar]
- 26.Wei W, Sun SR, Yao F. Correlation of HPV16 infection and P21 gene mutation in gGastric cancer. Med J Wuhan Univ. 2006;27(1):42–43. Chinese. [Google Scholar]
- 27.Xu WG, Zhang LJ, Lu ZM. Detection of human papillomavirus type 16 E6 mRNA in carcinomas of upper digestivetract. Natl Med J China. 2003;83(21):74–78. Chinese. [PubMed] [Google Scholar]
- 28.Yu JP, Deng T, Yu HG. Study on association of human papillomavirus type 16 with gastric carcinoma. Chinese J Digest Endoscopy. 1999;16(1):29–31. Chinese. [Google Scholar]
- 29.Zhang WL, Li CH, Xiao B. Significance of human papillomavirus infection in gastric carcinoma. J Fourth Military Med Univ. 2001;22(17):1603–1606. Chinese. [Google Scholar]
- 30.Zhu GB, Zhang LF, Cheng J. Association of human papillomavirus 16 and its serum antibody in gastric carcinoma. Chinese J Gen Surg. 2000;15(12):50–52. Chinese. [Google Scholar]
- 31.Yuan XY, Wang MY, Wang XY, Chang AY, Li J. Non-detection of Epstein-Barr virus and human papillomavirus in a region of high gastric cancer risk indicates a lack of a role for these viruses in gastric carcinomas. Genet Mol Biol. 2013;36(2):183–184. doi: 10.1590/S1415-47572013005000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Y, Ye WT, Wu LD. Research of the relationship between HPV 16 infection and gastric cancer. World Chinese J Digestol. 1999;7(2):168. Chinese. [Google Scholar]
- 33.Koshiol J, Wei WQ, Kreimer AR, Ren JS, Gravitt P. The gastric cardia is not a target for human apillomavirus-induced carcinogenesis. Cancer Epidemiol Biomarkers Prev. 2010;19(4):1137–1139. doi: 10.1158/1055-9965.EPI-10-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saegusa M, Hashimura M, Takano Y, Ohbu M, Okayasu I. Absence of human papillomavirus genomic sequences detected by the polymerase chain reaction in oesophageal and gastric carcinomas in Japan. Clin Pathol Mol Pathol. 1997;50:101–104. doi: 10.1136/mp.50.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snietura M, Waniczek D, Piglowski W, et al. Potential role of human papilloma virus in the pathogenesis of gastric cancer. World J Gastroenterol. 2014;20(21):6632–6637. doi: 10.3748/wjg.v20.i21.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo L, Luo B, Mu YX. Study of infected HPV16/18 of the 41 case stomach neoplasm state. China J Cancer Prev Treat. 2000;7(2):141–142. Chinese. [Google Scholar]
- 37.Huang ZM, Li Q, Yan PS. Detection of high risk human papillomavirus, p16 and p53 proteins in gastric carcinoma and precancerous lesions. Chinese J Diagn Pathol. 2000;7(3):201–203. Chinese. [Google Scholar]
- 38.Sha Q, Cheng HZ, Xie XY. Detection of HPV16,18 in gastric adenocarcinoma and adjacent tissues and its clinical significance. Chinese J Digest. 1998;18(5):320. Chinese. [Google Scholar]
- 39.Zhang K, Yang YL, Ci YL. Detection of HPV prevalence in gastric cardia cancer tissues. Chinese J Gerontol. 2011;31(19):3835–3837. Chinese. [Google Scholar]
- 40.Liao ZL, Wei YJ, Yang YC. Researches on HPV E6 (16, 18), p53, p21WAF1 gene protein expression in gastric cancer tissues. J Guangxi Med Univ. 2001;18(4):489–490. Chinese. [Google Scholar]
- 41.Yang L. Incidence and mortality of gastric cancer in China. World J Gastroenterol. 2006;12(1):17–20. doi: 10.3748/wjg.v12.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jing JJ, Liu HY, Hao JK, et al. Gastric cancer incidence and mortality in Zhuanghe, China, between 2005 and 2010. World J Gastroenterol. 2012;18(11):1262–1269. doi: 10.3748/wjg.v18.i11.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy G, Pfeiffer R, Camargo MC, Rabkin CS. Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology. 2009;137(3):824–833. doi: 10.1053/j.gastro.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee JH, Kim SH, Han SH, An JS, Lee ES, Kim YS. Clinicopathological and molecular characteristics of Epstein-Barr virus-associated gastric carcinoma: a meta-analysis. J Gastroenterol Hepatol. 2009;24(3):354–365. doi: 10.1111/j.1440-1746.2009.05775.x. [DOI] [PubMed] [Google Scholar]
- 45.Zhu C, Ling Y, Dong C, Zhou X, Wang F. The relationship between oral squamous cell carcinoma and human papillomavirus: a meta-analysis of a Chinese population (1994–2011) PLoS One. 2012;7(5):e36294. doi: 10.1371/journal.pone.0036294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis. Int J Cancer. 2009;124(7):1626–1636. doi: 10.1002/ijc.24116. [DOI] [PubMed] [Google Scholar]
- 47.Serrano B, de Sanjose S, Tous S, et al. Human papillomavirus genotype attribution for HPVs 6, 11, 16, 18, 31, 33, 45, 52 and 58 in female anogenital lesions. Eur J Cancer. 2015;51(3):1732–1741. doi: 10.1016/j.ejca.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 48.Cândido AC, De Lima Filho JL, Martins DB, Mendes CM, Vieira JR, Ferraz AA. Association of human papillomavirus genomic sequences by polymerase chain reaction in gastric carcinomas in Brazil. Anal Quant Cytopathol Histopathol. 2013;35(1):1–6. [PubMed] [Google Scholar]
- 49.Sobti RC, Kochar J, Singh K, Bhasin D, Capalash N. Telomerase activation and incidence of HPV in human gastrointestinal tumors in North Indian population. Mol Cell Biochem. 2001;217(1–2):51–56. doi: 10.1023/a:1007224001047. [DOI] [PubMed] [Google Scholar]
- 50.Kirkegård J, Farkas DK, Søgaard M, Schmidt SA, Ostenfeld EB, Cronin-Fenton D. Conization as a marker of persistent cervical human papillomavirus (HPV) infection and risk of gastrointestinal cancer: a Danish 34-year nationwide cohort study. Cancer Causes Control. 2014;25(12):1677–1682. doi: 10.1007/s10552-014-0473-4. [DOI] [PubMed] [Google Scholar]
- 51.Cao HR, Wang JX, Yue XL. Searches on the relation between human papillomavirus (HPV) infection and gastric cancer in gastric cancer tissue. J Pract Oncol. 2005;19(6):422–424. Chinese. [Google Scholar]
- 52.Zhang J, Tian XY, Wu XJ, Zong XL, Wu J, Ji JF. Role of papillomavirus in adenocarcinoma of esophagogastric junction. Natl Med J China. 2010;90(32):2259–2262. Chinese. [PubMed] [Google Scholar]
- 53.Su LJ, He F. Analysis of the correlation between human papillomavirus (HPV) and epstein-barr virus (EBV) infection and upper gastrointestinal tumor patients. Int J Virol. 2015;22:159–161. Chinese. [Google Scholar]