Abstract
Objectives
Talimogene laherparepvec is the first oncolytic immunotherapy to receive approval in Europe, the USA and Australia. In the randomized, open-label Phase III OPTiM trial (NCT00769704), talimogene laherparepvec significantly improved durable response rate (DRR) versus granulocyte-macrophage colony-stimulating factor (GM-CSF) in 436 patients with unresectable stage IIIB–IVM1c melanoma. The median overall survival (OS) was longer versus GM-CSF in patients with earlier-stage melanoma (IIIB–IVM1a). Here, we report a detailed subgroup analysis of the OPTiM study in patients with IIIB–IVM1a disease.
Patients and methods
The patients were randomized (2:1 ratio) to intralesional talimogene laherparepvec or subcutaneous GM-CSF and were evaluated for DRR, overall response rate (ORR), OS, safety, benefit–risk and numbers needed to treat. Descriptive statistics were used for subgroup comparisons.
Results
Among 249 evaluated patients with stage IIIB–IVM1a melanoma, DRR was higher with talimogene laherparepvec compared with GM-CSF (25.2% versus 1.2%; P<0.0001). ORR was also higher in the talimogene laherparepvec arm (40.5% versus 2.3%; P<0.0001), and 27 patients in the talimogene laherparepvec arm had a complete response, compared with none in GM-CSF-treated patients. The incidence rates of exposure-adjusted adverse events (AE) and serious AEs were similar with both treatments.
Conclusion
The subgroup of patients with stage IIIB, IIIC and IVM1a melanoma (57.1% of the OPTiM intent-to-treat population) derived greater benefit in DRR and ORR from talimogene laherparepvec compared with GM-CSF. Talimogene laherparepvec was well tolerated.
Keywords: benefit–risk, clinical trial, durable response rate, immunotherapy, oncolytic virus, talimogene laherparepvec
Introduction
Oncolytic viruses, with natural or engineered effects on the immune system, are novel anti-cancer therapies.1 These oncolytic immunotherapies are designed to replicate in and kill tumor cells without harming normal tissues and increase host immune cell recognition of tumor and viral antigens exposed during oncolysis.2 Talimogene laherparepvec is the first oncolytic immunotherapy to show an improved response in a Phase III randomized clinical trial and recently became the first oncolytic immunotherapy to be approved by the European Commission following approval of the product by the US Food and Drug Administration (FDA).3–6 Talimogene laherparepvec is a herpes simplex virus (HSV) type 1 (HSV-1)-based intralesional oncolytic immunotherapy, modified to attenuate viral pathogenicity, increase antigen presentation and induce selective tumor lysis.7–9 An enhanced anti-tumor immune response is achieved though the insertion and expression of the gene encoding human granulocyte-macrophage colony-stimulating factor (GM-CSF), which results in local GM-CSF production and the subsequent induction of tumor-specific T-cell responses.7–9
The FDA approval for talimogene laherparepvec was based on the overall, intent-to-treat (ITT) population of the randomized, multicenter, open-label Phase III OPTiM study (NCT00769704), which included 436 patients with unresectable stage IIIB–IVM1c melanoma. In this ITT population, intralesional talimogene laherparepvec significantly improved durable response rate (DRR; a continuous response for ≥6 months, primary endpoint) compared with subcutaneous GM-CSF (16.3% versus 2.1%, respectively; P<0.001) and was well tolerated.6 Secondary endpoint analyses included overall survival (OS), and a median survival difference of 23.3 versus 18.9 months was observed with talimogene laherparepvec versus GM-CSF (hazard ratio [HR]: 0.79; 95% confidence interval [CI]: 0.62, 1.00; P=0.051).6 Further analysis showed talimogene laherparepvec efficacy to be greater in patients with unresectable melanoma that was regionally or distantly metastatic with no visceral disease.6 In patients with stage IIIB–IVM1a melanoma, median OS was longer in the talimogene laherparepvec arm (41.1 months; 95% CI: 30.6 months, not evaluable) compared with the GM-CSF arm (21.5 months; 95% CI: 17.4, 29.6 months). The HR for the difference between the arms was 0.57 (95% CI: 0.40, 0.80; P<0.001 [descriptive]). Based on these results, as well as the DRR findings reported herein, talimogene laherparepvec was approved in Europe for adults with unresectable stage IIIB, IIIC or IVM1a melanoma with no bone, brain, lung or other visceral disease.4 Consequently, we report here a detailed analysis of the efficacy and safety of talimogene laherparepvec in patients with stage IIIB/C or IVM1a melanoma in the OPTiM study (reflecting the European Union indication), including analysis of benefit–risk and the numbers needed to treat for either benefit or harm.
Patients and methods
Patients
The full inclusion/exclusion criteria of the OPTiM study have been described in detail previously6 and are reported briefly here. Eligible patients were ≥18 years of age, with histologically/cytologically confirmed, unresectable, bidimensionally measurable stage IIIB–IV melanoma, which was suitable for direct or ultrasound-guided injection. Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≤1, adequate organ function and serum lactate dehydrogenase ≤1.5× upper limit of normal were also required. Patients with bone metastases, ≥3 visceral metastases (except lung or nodal metastases associated with visceral organs), any visceral metastasis >3 cm, or active cerebral metastases were excluded; liver metastases had to be stable for ≥1 month before random assignment. Patients in need of intermittent or chronic treatment with an antiviral agent (for example, acyclovir) or high-dose steroids were also excluded. Both first-line and previously treated patients were enrolled in the OPTiM study.
The patients provided written informed consent, and the protocol and study procedures received approval from all participating study centers (Table S1). Amgen Inc, the sponsor of the study, was responsible for the development of the protocol. The trial is registered on ClinicalTrials.gov (identifier: NCT00769704).
Study design and treatment
OPTiM was an open-label, Phase III clinical trial conducted at 64 centers in Canada, South Africa, the United Kingdom and the United States. Patients were randomized 2:1 to receive intralesional talimogene laherparepvec or subcutaneous recombinant GM-CSF. Random assignment was stratified by site of first recurrence, presence of liver metastases, disease stage and prior nonadjuvant systemic treatment. Talimogene laherparepvec was administered by intralesional injection at an initial concentration of 106 pfu/mL on Day 1, followed by a concentration of 108 pfu/mL on Day 22 (cycle 1) and then once every 2 weeks in 28-day cycles for 12 months or up to 18 months in patients with stable or responding disease at 12 months. Discontinuation of treatment due to progressive disease per response assessment criteria was not required before 24 weeks, unless alternate therapy was clinically indicated or the patient was intolerant to treatment. Injected volume per lesion ranged from 0.1 mL for lesions <0.5 cm to 4.0 mL for lesions >5 cm in the longest diameter (Table 1). GM-CSF 125 µg/m2 was administered subcutaneously once daily for 14 days in 28-day cycles for 12 months or up to 18 months in patients with stable or responding disease at 12 months. Further details related to dosing in this study have been reported previously.6
Table 1.
Talimogene laherparepvec injection volume by lesion size
| Lesion size (longest diameter) | T-VEC injection volume |
|---|---|
| >5 cm | Up to 4 mL |
| >2.5–5 cm | Up to 2 mL |
| >1.5–2.5 cm | Up to 1 mL |
| >0.5–1.5 cm | Up to 0.5 mL |
| ≤0.5 cm | Up to 0.1 mL |
Abbreviation: T-VEC, talimogene laherparepvec.
Endpoints and assessments
We report exploratory analyses for the subgroup of patients with stage IIIB/IIIC or IVM1a melanoma. The primary endpoint of OPTiM was DRR, defined as the rate of objective response (complete response [CR] or partial response [PR]) lasting ≥6 months continuously and with onset beginning within the first 12 months. Additional efficacy assessments included overall response rate (ORR), OS (time from randomization to death), time to treatment failure (TTF), duration of response, incidence and time to the use of subsequent anti-cancer treatment (defined as the time from randomization to the first dose) and health-related quality of life (HRQoL). Furthermore, a number needed to treat to achieve a benefit (NNTB) analysis and a number needed to treat to experience a harm (NNTH) analysis were performed.10,11
For the purposes of evaluating response, visible or palpable lesions were clinically assessed with a caliper or ruler every 4 weeks. Whole-body computed tomography (CT), positron emission tomography (PET) or PET–CT was used to assess deeper palpable lesions and nonpalpable subcutaneous and distant metastatic lesions every 3 months. Response was evaluated per modified World Health Organization criteria.12 Patients with a best response per investigator of CR or PR or receiving treatment for ≥9 months were evaluated by a blinded endpoint assessment committee (EAC).
The Functional Assessment of Cancer Therapy-Biologic Response Modifier (FACT-BRM) questionnaire was used to assess improvements in HRQoL.13 The trial outcome index (TOI; sum of scores for physical well-being, functional well-being and two treatment-specific subscales: additional concerns [physical] and additional concerns [mental]), FACT-BRM total score and the total score in each of the six FACT-BRM subdomains as well as in three individual items (overall HRQoL, pain and ability to work) were assessed. Patients were required to complete the questionnaire on Day 1 of each treatment cycle (before any treatment-related study procedures, including administration of investigational product) and at the final (end of treatment) visit. Improvement was defined as an increase from baseline sustained for ≥1 cycle. Clinically relevant improvements in TOI and FACT-BRM total score were defined as increases of ≥5 points, improvements in individual domains were defined as increases of ≥2 points and improvements in individual items were defined as increases of ≥1 point.14 Patients with baseline scores that allowed for the required absolute improvement and who had at least one post-baseline score were evaluable.
For the NNTB analysis, the benefits included DRR, ORR, probability of being alive, probability of treatment failure and probability of no new therapy. For the NNTH analysis, harms (risks) were defined based on the mechanism of action of talimogene laherparepvec and included immune-mediated events, oral herpes and bacterial cellulitis. Confirmation of bacterial cellulitis or cause of herpes (HSV or talimogene laherparepvec) was not required. A fixed follow-up period of 18 months was selected for the analysis (the maximum duration of treatment in the study). The incidence, severity and type of adverse events (AEs) occurring between the first administration of study treatment and 30 days after last treatment were evaluated using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0).
Statistical analysis
Sample size calculations for the OPTiM study have been reported elsewhere.6
In this subgroup analysis, efficacy was evaluated in all patients with stage IIIB/IIIC or IVM1a melanoma from the ITT population (not all treated). The safety analysis included patients with stage IIIB/IIIC or IVM1a melanoma who received at least one dose of talimogene laherparepvec or GM-CSF.
Disease stage was prespecified as a randomization stratification factor in the OPTiM study design and for subgroup analysis in the statistical analysis plan. All subgroup comparisons are descriptive with statistical significance assessed at a nominal two-sided 5% level without multiplicity adjustment. An unadjusted Fisher’s exact test was used to evaluate the difference in DRR and ORR between treatment arms. Unadjusted log-rank tests and Cox proportional hazards models were used to evaluate OS, TTF, time to response and duration of response. All analyses were performed using SAS software (version 9.2; SAS Institute Inc., Cary, NC, USA).
An analysis was performed to examine the relationship between durable response and OS. In a landmark analysis, OS was compared for patients who had achieved a durable response and were alive at 12 months after randomization with those who had not achieved a durable response and were alive at 12 months, mitigating the potential for lead-time bias.15 Patients who died or who were censored before the landmark time were not included in this analysis. The association between durable response and the time to the use of subsequent systemic anti-melanoma treatment was also evaluated in a landmark analysis. These analyses were performed using a Cox proportional hazards model and log-rank test.
NNTB and NNTH were calculated as the reciprocal of the difference in the proportion of patients with the specified benefit or risk between the active treatment (talimogene laherparepvec) and the control group (GM-CSF) by 18 months.
Results
Patient characteristics, disposition and treatment
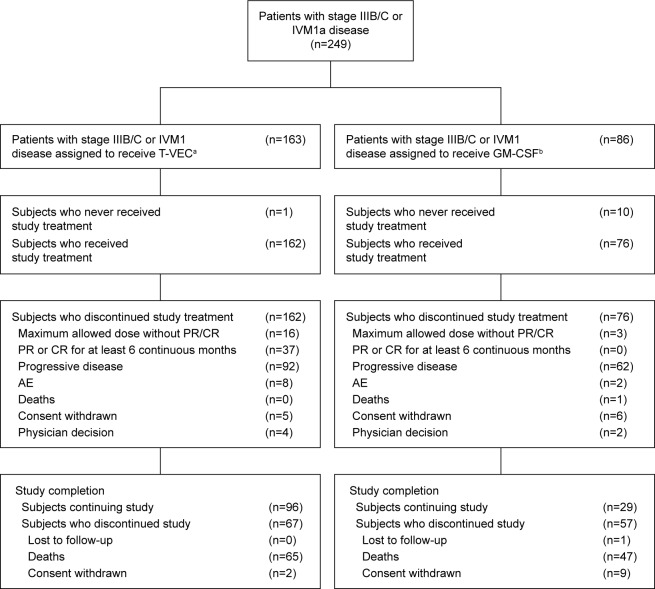
In total, 249 patients with stage IIIB (n=34), IIIC (n=97), or IVM1a (n=118) disease (57.1% of the OPTiM ITT population) were randomized to receive talimogene laherparepvec (n=163) or GM-CSF (n=86). Patient disposition is shown in Figure 1. Baseline demographic and clinical characteristics for these patients are shown in Table 2. Overall, baseline characteristics were generally balanced between the talimogene laherparepvec and GM-CSF arms. More patients in the talimogene laherparepvec versus the GM-CSF arm had an ECOG PS of 0 (73.6% versus 62.8%, respectively). Details relating to sites of disease are captured in Table 2. Additionally, baseline HSV-1 seropositive status was higher in the talimogene laherparepvec arm (62.0% versus 54.7%). BRAF status was known in 32% of patients and was equally distributed between the two treatment groups. More than half (55.0%) of the patients had not received prior therapy at baseline.
Figure 1.
Patient disposition for the stage IIIB/C, IVM1a OPTiM subpopulation.
Notes: aTalimogene laherparepvec was administered intralesionally ≤4 mL ×106 pfu/mL once and, after 3 weeks, ≥4 mL ×108 pfu/mL every 2 weeks. bGM-CSF was administered 125 µg/m2 subcutaneously for 14 days in 4-week cycles.
Abbreviations: AE, adverse event; CI, confidence interval; CR, complete response; GM-CSF, granulocyte-macrophage colony-stimulating factor; NNTB, number needed to treat to achieve a benefit; NNTH, number needed to treat to experience a harm; PR, partial response; T-VEC, talimogene laherparepvec.
Table 2.
Baseline demographic and clinical characteristics for the stage IIIB/C or IVM1a OPTiM subpopulation
| T-VEC (n=163) |
GM-CSF (n=86) |
|
|---|---|---|
| Age, years | ||
| Median (range) | 63 (24–94) | 63 (28–91) |
| Sex, n (%) | ||
| Male | 92 (56.4) | 47 (54.7) |
| Female | 71 (43.6) | 39 (45.3) |
| Disease stage, n (%) | ||
| IIIB | 22 (13.5) | 12 (14.0) |
| IIIC | 66 (40.5) | 31 (36.0) |
| IVM1a | 75 (46.0) | 43 (50.0) |
| ECOG PS, n (%) | ||
| 0 | 120 (73.6) | 54 (62.8) |
| 1 | 42 (25.8) | 24 (27.9) |
| Missing | 1 (0.6) | 8 (9.3) |
| Sites of disease,a n (%) | ||
| Skin | 110 (67.5) | 49 (57.0) |
| Lymph nodes | 70 (42.9) | 32 (37.2) |
| Soft tissue | 79 (48.5) | 35 (40.7) |
| Lung | 0 (0.0) | 0 (0.0) |
| Liver | 0 (0.0) | 0 (0.0) |
| Brain | 0 (0.0) | 0 (0.0) |
| Other metastases | 4 (2.5)b | 0 (0.0) |
| Other | 19 (11.7) | 15 (17.4) |
| Missing | 1 (0.6) | 8 (9.3) |
| Lactate dehydrogenase, n (%) | ||
| ≤ULN | 154 (94.5) | 75 (87.2) |
| >ULN | 2 (1.2) | 2 (2.3) |
| Unknown | 1 (0.6) | 1 (1.2) |
| HSV-1 serostatus, n (%) | ||
| Negative | 51 (31.3) | 25 (29.1) |
| Positive | 101 (62.0) | 47 (54.7) |
| Unknown | 11 (6.7) | 14 (16.3) |
| BRAF status, n (%) | ||
| Mutated | 27 (16.6) | 14 (16.3) |
| Wild type | 24 (14.7) | 14 (16.3) |
| Unknown or missing | 112 (68.7) | 58 (67.4) |
| Line of therapy, n (%) | ||
| First | 92 (56.4) | 45 (52.3) |
| Second or later | 71 (43.6) | 41 (47.7) |
|
| ||
|
T-VEC (n=71) |
GM-CSF (n=41) |
|
|
| ||
| Previous therapy, n (%)c | ||
| Chemotherapy | 28 (39.4) | 14 (34.1) |
| Biological/targeted therapy (investigational)d | 11 (15.5) | 7 (17.1) |
| Vemurafenib | 1 (1.4) | 0 (0.0) |
| Immunotherapy | 36 (50.7) | 22 (53.7) |
| Interleukin-2 | 13 (18.3) | 9 (22.0) |
| Interferon alpha | 24 (33.8) | 18 (43.9) |
| GM-CSF | 0 (0.0) | 0 (0.0) |
| Anti-CTLA4 | 3 (4.2) | 2 (4.9) |
| Anti-PD1 | 0 (0.0) | 0 (0.0) |
| Isolated limb perfusion or infusion | 17 (23.9) | 8 (19.5) |
| Radiotherapy | 19 (26.8) | 9 (22.0) |
| Other | 11 (15.5) | 5 (12.2) |
Notes:
Lymph nodes: axillary lymph nodes, cervical lymph nodes, thoracic lymph nodes, intra-abdominal lymph nodes and inguinal lymph nodes. Other metastases: locally advanced disease involving a visceral organ or impacting other non-skin and non-lymphatic organs, including thyroid gland, heart/pericardium, gastrointestinal tract, pancreas, gallbladder, kidney, uterus, ovary, adrenal gland and peritoneum. Soft tissues: soft tissue of arm, leg and trunk/back. Others: pleural effusion, ascites and others (the locations already included as “other metastases” were excluded).
Metastases in these patients were reported in the case report form at baseline and may have appeared at additional sites after this point.
Subset of patients reported to have ≥1 line of prior therapy (patients could be treated by multiple agents belonging to different categories).
Biological therapy included sorafenib, bevacizumab, tamoxifen, thalidomide and others.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; GM-CSF, granulocyte-macrophage colony-stimulating factor; HSV-1, herpes simplex virus type 1; PS, performance status; T-VEC, talimogene laherparepvec; ULN, upper limit of normal.
The median duration of treatment in the talimogene laherparepvec and GM-CSF groups was 25.7 (range 3.6–78.9) and 10.0 weeks (range 0.6–58.3), respectively. The patients in the talimogene laherparepvec arm received a median of 14 injections (range 2–38).
Durable and overall response
DRR per EAC assessment was higher in patients treated with talimogene laherparepvec compared with the GM-CSF group in the stage IIIB/C or IVM1a subpopulation (25.2% versus 1.2%, respectively; treatment difference 24% [95% CI: 17.0–31.0]; P<0.0001; Table 3). The onset of durable response prior to 12 months in either treatment arm was associated with a 94% decrease in the risk of death (HR 0.06; [95% CI: 0.01, 0.45]; P=0.0002). Similarly, regardless of treatment arm, the onset of durable response prior to 9 or 18 months was associated with a 90% or 85% decrease in the risk of death, respectively. Among the 41 patients with a durable response to talimogene laherparepvec, 20 (49%) experienced progression prior to response (defined as the appearance of a new lesion or >25% increase in total baseline tumor area).
Table 3.
Efficacy for the stage IIIB/C or IVM1a OPTiM sub-population
| T-VEC (n=163) | GM-CSF (n=86) | |
|---|---|---|
| DRR, n (%)* | 41 (25.2) | 1 (1.2) |
| 95% CIa | 18.5, 31.8 | 0.0, 3.4 |
| Best response, n (%) | ||
| Complete response | 27 (16.6) | 0 (0.0) |
| Partial response | 39 (23.9) | 2 (2.3) |
| Not in response | 22 (13.5) | 9 (10.5) |
| Not reviewed by EACb | 75 (46.0) | 75 (87.2) |
| ORR, n (%)** | 66 (40.5) | 2 (2.3) |
| 95% CIa | 32.9, 48.4 | 0.3, 8.1 |
| Estimated OS probability, % (95% CI) | ||
| At 12 months | 87.0 (80.8, 91.3) | 76.8 (66.1, 84.6) |
| At 24 months | 64.8 (56.9, 71.6) | 46.2 (35.1, 56.5) |
| At 36 months | 54.7 (46.6, 62.0) | 34.3 (24.0, 44.7) |
| At 48 months | 45.6 (35.9, 54.8) | 23.4 (12.4, 36.3) |
| TTF per investigator (median), months (95% CI) | 13.1 (8.3, NE) | 3.3 (2.8, 4.3) |
| HR (95% CI) | 0.27 (0.19, 0.39) | |
|
| ||
| T-VEC (n=66)c | GM-CSF (n=2)c | |
|
| ||
| In response and censored, | 46 (69.7) | 1 (50.0) |
| n (%) | ||
| Duration of response (median), monthsd | NE | NE |
| Time to response per EAC (median), months (95% CI) | 4.0 (3.2, 5.0) | 3.8 (1.9, 5.6) |
|
| ||
| T-VEC (n=67) | GM-CSF (n=43) | |
|
| ||
| Subsequent anti-cancer therapy incidence, n (%)e | ||
| Ipilimumab, vemurafenib, dabrafenib, trametinib or anti-PD1 antibody | 67 (41.1) | 43 (50.0) |
| Ipilimumab | 61 (37.4) | 32 (37.2) |
| Vemurafenib | 15 (9.2) | 13 (15.1) |
| Dabrafenib | 6 (3.7) | 2 (2.3) |
| Trametinib | 3 (1.8) | 0 (0.0) |
| Anti-PD1 antibody | 2 (1.2) | 4 (4.7) |
| Time to use, months – median (range) | ||
| Ipilimumab, vemurafenib, dabrafenib, trametinib or anti-PD1 antibody | 9.7 (2.2–52.7) | 8.8 (1.4–41.4) |
| Ipilimumab | 9.7 (2.2–52.7) | 9.0 (1.4–27.2) |
| Vemurafenib | 16.2 (3.4–39.4) | 13.1 (2.1–43.9) |
| Dabrafenib | 19.4 (7.0–41.8) | 7.1 (3.7–10.5) |
| Trametinib | 18.0 (13.4–41.8) | N/A |
| Anti-PD1 antibody | 19.3 (11.1–27.5) | 18.7 (6.2–41.4) |
Notes:
Binomial proportion with asymptotic 95% CI.
Only patients with a best response per investigator of CR or PR or receiving treatment for ≥9 months were evaluated by EAC.
Responders only.
The duration of response is defined as the longest individual period from entering response (PR or CR) to the first documented evidence of the patient no longer meeting the criteria for being in response or death, whichever is earlier.
Data from OPTiM final analysis.
Unadjusted P-value <0.0001; P-value was calculated using Fisher’s exact test and is descriptive for the subpopulation analyses.
P-value <0.0001; P-value was calculated using Fisher’s exact test and is descriptive for the subpopulation analyses.
Abbreviations: CI, confidence interval; CR, complete response; DRR, durable response rate; EAC, endpoint assessment committee; GM-CSF, granulocyte-macrophage colony-stimulating factor; HR, hazard ratio; N/A, not applicable; NE, not estimable; ORR, overall response rate; OS, overall survival; PR, partial response; TTF, time to treatment failure; T-VEC, talimogene laherparepvec.
ORR was also higher in the talimogene laherparepvec arm (40.5% versus 2.3%, respectively; P<0.0001). In total, 27 patients (16.6%) in the talimogene laherparepvec arm had a CR compared with no GM-CSF-treated patients (0.0%; Table 3). The estimated 5-year survival rate for patients with an overall response was 78%.
Among responding patients in the talimogene laherparepvec arm (n=66), the median time to response was 4.0 months (95% CI: 3.2, 5.0) compared with 3.8 months (95% CI: 1.9, 5.6) for the two responding patients in the GM-CSF group. The median duration of response was not reached in the talimogene laherparepvec responders; 46 patients (69.7%) were still in response at the last tumor assessment for the primary analysis. The estimated probability of being in response at 15 months from the response onset was 64% (95% CI: 49%, 75%) among talimogene laherparepvec responders.
Time to treatment failure
The median TTF was 13.1 months (95% CI: 8.3 months to not estimable) in the talimogene laherparepvec arm versus 3.3 months (95% CI: 2.8–4.3 months) in the GM-CSF arm (HR 0.27; 95% CI: 0.19, 0.39; Table 3).
Overall survival
The median OS and Kaplan–Meier survival plot for the stage IIIB/C or IVM1a subpopulation have been published previously.6 At the time of the primary OS analysis, 137 patients had died in this subpopulation, including 80 (49.1%) receiving talimogene laherparepvec and 57 (66.3%) of those in the GM-CSF arm (median OS 41.1 versus 21.5 months). The estimated 12-, 24-, 36- and 48-month survival rates were all higher in the talimogene laherparepvec group compared with the GM-CSF group (Table 3).
Incidence of selected systemic anticancer treatment and time to first use
Following talimogene laherparepvec/GM-CSF therapy, systemic anti-cancer treatment (including ipilimumab, vemurafenib, dabrafenib, trametinib or a PD1 antibody) was used in 41.1% of talimogene laherparepvec-treated patients compared with 50.0% of patients who received GM-CSF (Table 3). Subsequent anti-cancer therapy was given at a median of 9.7 and 8.8 months after randomization to talimogene laherparepvec and GM-CSF treatment, respectively (Table 3). Among patients with tumor assessments for ≥9 months, durable response in either treatment arm was associated with a 67% reduction in the risk of initiating subsequent therapy (HR 0.33; 95% CI: 0.14, 0.76).
Health-related quality of life
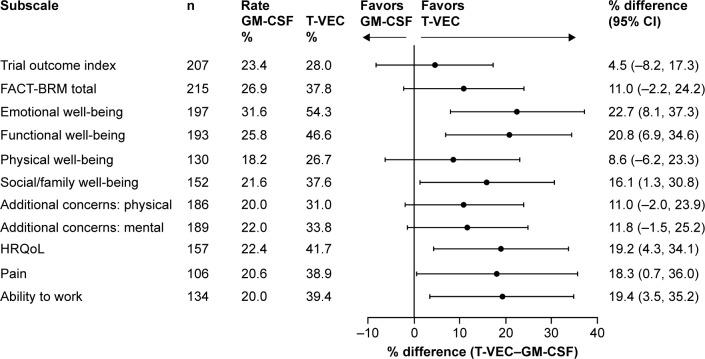
In total, 215 patients were evaluable for HRQoL assessments. More patients treated with talimogene laherparepvec versus GM-CSF had improvements in each of the 11 FACT-BRM-based measures of HRQoL assessed (Figure 2). The TOI score improved in 28.0% of patients with talimogene laherparepvec compared with 23.4% of patients with GM-CSF (difference 4.5%; 95% CI: −8.2%, 17.3%). The FACT-BRM total score improved in 37.8% of patients treated with talimogene laherparepvec compared with 26.9% of patients treated with GM-CSF (difference 11.0%; 95% CI: −2.2%, 24.2%). Differences in six of 11 measures reached statistical significance: emotional well-being, 22.7% (95% CI: 8.1%, 37.3%); functional well-being, 20.8% (95% CI: 6.9%, 34.6%); social/family well-being, 16.1% (95% CI: 1.3%, 30.8%); overall HRQoL, 19.2% (95% CI: 4.3%, 34.1%); pain, 18.3% (95% CI: 0.7%, 36.0%); and ability to work, 19.4% (95% CI: 3.5%, 35.2%).
Figure 2.
Improvement in HRQoL assessed by FACT-BRM during treatment with talimogene laherparepvec and GM-CSF in the stage IIIB/C or IVM1a OPTiM subpopulation (ITT).a
Notes: aScores from unscheduled visits were not included. A patient is considered evaluable for a domain if baseline score is not the best score, there is room for evaluable improvement and there is at least one post-baseline score. TOI and total improvements are defined as ≥5-point score increase from baseline with a ≥1 cycle duration. HRQoL, pain and work improvements are defined as ≥1-point score increase from baseline with a ≥1 cycle duration. All other subscales are based on a ≥2-point score increase from baseline with a ≥1 cycle duration.
Abbreviations: CI, confidence interval; FACT-BRM, Functional Assessment of Cancer Therapy-Biologic Response Modifier; GM-CSF, granulocyte-macrophage colony-stimulating factor; HRQoL, health-related quality of life; TOI, trial outcome index; T-VEC, talimogene laherparepvec.
Safety
The incidence of any grade AE was similar in the two study arms: 98.8% versus 93.4% for talimogene laherparepvec and GM-CSF, respectively (Table 4). The exposure-adjusted AE incidence rates (per one patient-year) were 20.9 versus 18.5 in the talimogene laherparepvec and GM-CSF arms, respectively. The most commonly reported AEs in either the talimogene laherparepvec or GM-CSF arm, respectively, were fatigue (50.9% versus 36.8%), chills (49.7% versus 7.9%), pyrexia (39.9% versus 10.5%), influenza-like illness (33.7% versus 9.2%) and nausea (33.7% versus 21.1%; Table 4). Among talimogene laherparepvec-treated patients, the incidence of these most commonly reported AEs was highest during the first cycle (defined as two consecutive treatments with talimogene laherparepvec; fatigue 24.5%, chills 30.7%, pyrexia 27.6%, influenza-like illness 26.4% and nausea 17.8%) and decreased over time. In cycle 5, the incidence of fatigue, chills, pyrexia, influenza-like illness and nausea were 10.3%, 8.4%, 8.4%, 4.7% and 6.5%, respectively. Aside from influenza-like illness (which occurred after a median time of 3 days), the first occurrence of the most common AEs coincided with the second injection of talimogene laherparepvec.
Table 4.
Summary of AEs for the stage IIIB/C or IVM1a OPTiM subpopulation
| T-VEC (n=163) |
GM-CSF (n=76) |
|
|---|---|---|
| Grade 1–5 AEs, n (%) | 161 (98.8) | 71 (93.4) |
| Grade 1–5 AEs occurring with ≥10% frequency in ≥1 arm | ||
| Fatigue | 83 (50.9) | 28 (36.8) |
| Chills | 81 (49.7) | 6 (7.9) |
| Pyrexia | 65 (39.9) | 8 (10.5) |
| Influenza-like illness | 55 (33.7) | 7 (9.2) |
| Nausea | 55 (33.7) | 16 (21.1) |
| Injection site pain | 49 (30.1) | 5 (6.6) |
| Diarrhea | 35 (21.5) | 7 (9.2) |
| Myalgia | 30 (18.4) | 4 (5.3) |
| Vomiting | 30 (18.4) | 7 (9.2) |
| Pain in extremity | 29 (17.8) | 6 (7.9) |
| Pain | 28 (17.2) | 9 (11.8) |
| Arthralgia | 27 (16.6) | 5 (6.6) |
| Headache | 27 (16.6) | 7 (9.2) |
| Constipation | 20 (12.3) | 1 (1.3) |
| Rash | 20 (12.3) | 4 (5.3) |
| Dizziness | 19 (11.7) | 1 (1.3) |
| Upper respiratory tract infection | 19 (11.7) | 6 (7.9) |
| Edema peripheral | 14 (8.6) | 8 (10.5) |
| Pruritus | 14 (8.6) | 12 (15.8) |
| Decreased appetite | 12 (7.4) | 9 (11.8) |
| Injection site erythema | 10 (6.1) | 16 (21.1) |
| Injection site reaction | 6 (3.7) | 10 (13.2) |
| Grade ≥3 AEs, n (%) | 53 (32.5) | 18 (23.7) |
| Grade ≥3 AEs occurring in ≥3 patients in ≥1 arm | ||
| Pain in extremities | 4 (2.5) | 0 (0.0) |
| Fatigue | 3 (1.8) | 1 (1.3) |
| Hypokalemia | 3 (1.8) | 1 (1.3) |
| Cellulitis | 3 (1.8) | 0 (0.0) |
| Deep vein thrombosis | 3 (1.8) | 0 (0.0) |
| Dehydration | 3 (1.8) | 0 (0.0) |
| Infected neoplasm | 3 (1.8) | 0 (0.0) |
| Injection site pain | 3 (1.8) | 0 (0.0) |
| Serious AEs, n (%) | ||
| Serious AEs (grade 1–5) | 33 (20.2) | 10 (13.2) |
| Serious AEs (grade 1–5) in ≥2 patients | ||
| Cellulitis | 4 (2.5) | 0 (0.0) |
| Infected neoplasm | 3 (1.8) | 0 (0.0) |
| Anemia | 2 (1.2) | 0 (0.0) |
| Disease progression | 2 (1.2) | 1 (1.3) |
| Metastasis to central nervous system | 2 (1.2) | 0 (0.0) |
| Pyrexia | 2 (1.2) | 0 (0.0) |
| Rib fracture | 2 (1.2) | 0 (0.0) |
| Tumor pain | 2 (1.2) | 0 (0.0) |
| Serious AEs of grade ≥3 | 28 (17.2) | 8 (10.5) |
| Treatment-related serious AEs | 10 (6.1) | 0 (0.0) |
| AEs leading to discontinuation of study treatment, n (%) | ||
| All | 14 (8.6) | 5 (6.6) |
| Serious | 8 (4.9) | 3 (3.9) |
| Non-serious | 6 (3.7) | 2 (2.6) |
| Fatal AEs – on study, n (%) | 1 (0.6)a | 0 (0.0) |
Notes: Safety population included all randomized and treated subjects. Subjects were analyzed using the treatment received. AEs include all those that began between the first administration of study treatment and 30 days after the last administration of study treatment. AEs were coded using MedDRA version 15.1.
Not attributed to treatment.
Abbreviations: AE, adverse event; GM-CSF, granulocyte-macrophage colony-stimulating factor; T-VEC, talimogene laherparepvec.
AEs of grade ≥3 and serious AEs are listed in Table 4. Rates of AE of grade ≥3 were higher in the talimogene laherparepvec arm versus the GM-CSF arm (32.5% versus 23.7%, respectively; Table 4). The only serious AE occurring in ≥2% of patients was cellulitis (talimogene laherparepvec, n=4 [2.5%]; GM-CSF, n=0 [0.0%]). Exposure-adjusted serious AE incidence rates (per one patient-year) were 0.34 versus 0.39 in the talimogene laherparepvec and GM-CSF arms, respectively.
The rate of discontinuation as a result of AEs was 8.6% and 6.6% with talimogene laherparepvec and GM-CSF, respectively. Respiratory, thoracic and mediastinal disorders (specifically, bronchial hyperreactivity, obstructive airways disorder, pleuritic pain and pneumonitis) most commonly resulted in treatment discontinuation in the talimogene laherparepvec arm (n=4).
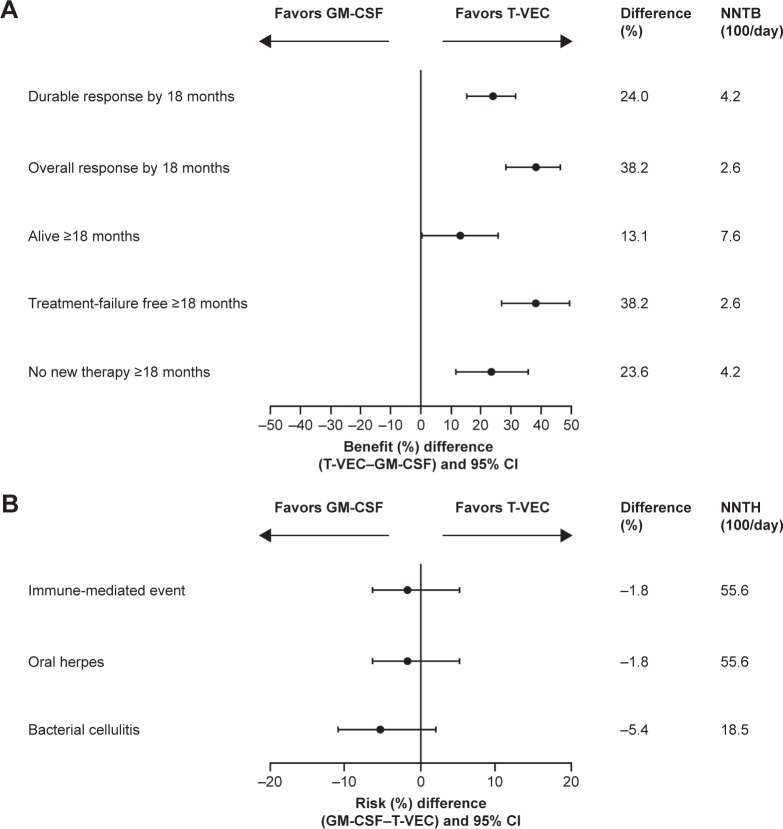
Benefit–risk analyses
For every 100 patients receiving talimogene laherparepvec, an average of 24 additional patients achieved a durable response by 18 months versus GM-CSF (Figure 3A). Therefore, approximately four patients (100÷24) would need to be treated in order to experience the benefit of DRR with talimogene laherparepvec relative to GM-CSF by 18 months (Figure 3A). Over the same time period, for every 100 patients receiving talimogene laherparepvec, an average of five additional patients experienced cellulitis and two additional patients had immune-mediated or oral herpes events versus GM-CSF (Figure 3B).
Figure 3.
Benefit–risk analysis. (A) Benefits by 18 months; (B) Risks by 18 months for the stage IIIB/C or IVM1a OPTiM subpopulation.
Notes: Immune-mediated events occurring with talimogene laherparepvec in the stage IIIB/C or IVM1a OPTiM subpopulation included glomerulonephritis/renal papillary necrosis (grade 2), glomerulonephritis/renal failure (grade 3), vasculitis (grade 2), pneumonitis (two episodes in one patient; grades 2 and 3) and psoriasis (two episodes in one patient; grades 1 and 3). Vitiligo also occurred in 12 (7%) patients; all of these events were non-serious.
Abbreviations: CI, confidence interval; GM-CSF, granulocyte-macrophage colony-stimulating factor; NNTB, number needed to treat to achieve a benefit; NNTH, number needed to treat to experience a harm; T-VEC, talimogene laherparepvec.
Among the talimogene laherparepvec-treated patients with a DRR by 18 months, ~95% did not experience any specified risks (Table 5). Among talimogene laherparepvec-treated patients without durable responses by 18 months, 14% experienced the specified risks (Table 5).
Table 5.
Durable response by risk by 18 months for the stage IIIB/C or IVM1a OPTiM subpopulation
| T-VEC | GM-CSF | |
|---|---|---|
| Patients with durable response | n=41 | n=1 |
| Patients with risks,a n (%)b | 2 (4.9) | 0 (0.0) |
| Patients without risks,a n (%)b | 39 (95.1) | 1 (100.0) |
| Patients without durable response | n=121 | n=75 |
| Patients with risks,a n (%)c | 17 (14.0) | 3 (4.0) |
| Patients without risks,a n (%)c | 104 (86.0) | 72 (96.0) |
Notes: Subjects who were part of both the ITT and safety population have been included in this summary.
Risks include immune-related events, oral herpes and bacterial cellulitis.
Percentages were calculated using the number of patients with a durable response as denominator.
Percentages were calculated using the number of patients without a durable response as denominator.
Abbreviations: GM-CSF, granulocyte-macrophage colony-stimulating factor; T-VEC, talimogene laherparepvec.
Discussion
OPTiM was the first randomized, controlled Phase III study evaluating an oncolytic immunotherapy in patients with advanced melanoma. The study, which included both first-line and previously treated patients (>40% received prior therapy), met its primary endpoint: talimogene laherparepvec significantly improved DRR in the unresected stage IIIB–IV melanoma compared with subcutaneous GM-CSF.6 A previously reported subgroup analysis of the OPTiM study suggested particular benefit in patients with stage IIIB–IVM1a disease, with a median OS of 41.1 months for patients treated with talimogene laherparepvec compared with 21.5 months for those treated with GM-CSF (19.6-month improvement).6 In comparison, in the OPTiM overall ITT population, a 4.4-month improvement in median OS was observed for patients receiving talimogene laherparepvec versus GM-CSF.
The earlier-stage population in OPTiM is the largest Phase III dataset reported for patients with stage IIIB–IVM1a melanoma. Given the recent European Union indication approving talimogene laherparepvec as a treatment option in unresectable stage IIIB–IVM1a disease with no bone, brain, lung or other visceral disease, we comprehensively evaluated the efficacy and safety of talimogene laherparepvec compared with GM-CSF in this subgroup. In the stage IIIB–IVM1a subgroup, compared with GM-CSF, talimogene laherparepvec improved DRR and ORR by 24% and 38%, respectively. This is higher than observed in the OPTiM overall ITT population (14% and 21%, respectively).6 This is also important since durable response before 1 year was associated with >90% decrease in the risk of death, and the estimated 5-year survival rate was almost 80% for patients with an overall response. This suggests that talimogene laherparepvec could delay or prevent relapses or preclude progression to later stage disease in this patient population. Indeed, a recent multivariate analysis found that talimogene laherparepvec-treated patients with stage IIIB–IVM1a melanoma had a 59% lower risk of developing visceral and bone metastases.16 Additionally, estimated OS probability in patients with stage IIIB/C or IVM1a disease indicates that nearly twice as many patients treated with talimogene laherparepvec would be alive at 4 years, compared with GM-CSF-treated patients.
It is difficult to make comparisons between talimogene laherparepvec and other melanoma immunotherapies, as the distribution of patients across the disease stages differs among various other Phase III trials and total numbers of evaluable patients in the stage IIIB/C or IVM1a melanoma subpopulation are small. Specifically, the design of the trials for ipilimumab, vemurafenib, nivolumab and pembrolizumab with different eligibility and assessment criteria,17–21 and including fewer earlier stage metastatic patients, makes direct comparisons challenging. Nevertheless, the numerically higher CR rate with talimogene laherparepvec in stage IIIB/C or IVM1a (17%) compared with other therapies in more advanced disease,17–21 as well as compared with the overall OPTiM population, which included stage IVM1b/c patients (overall CR of 10.8%), highlights the importance of identifying and treating patients with melanoma early. In this regard, CR is strongly associated with long-term survival.22 Indeed, more than half of patients with no visceral disease treated with talimogene laherparepvec were alive at 3 years. Published data with other melanoma agents in earlier stage metastatic melanoma are limited.
As was seen in the overall OPTiM population, talimogene laherparepvec was well tolerated in patients with stage IIIB/C or IVM1a melanoma. The majority of AEs were of grade 1–2, transient and diminished over time with subsequent injections. Rates of AE of grade ≥3 were higher with talimogene laherparepvec compared with GM-CSF. However, exposure-adjusted AE rates and serious AE rates were similar between the two groups (duration of treatment was >15 weeks longer with talimogene laherparepvec versus GM-CSF). The most frequent AE of grade ≥3 observed with talimogene laherparepvec was pain in the extremities (2.5%), and the remaining AEs of grade ≥3 occurred in <2% of patients. AE-related study discontinuation was low. The NNTB and NNTH analyses indicated that the benefit–risk balance is in favor of talimogene laherparepvec. Specifically, for patients who responded to treatment, ~95% experienced an unqualified success (benefit with no harm). In contrast, for patients who failed to respond, only 14% represented an unmitigated failure (harm with no benefit). This safety profile is favorable compared with other approved melanoma treatments, which can result in higher levels of grade ≥3 treatment-related AEs or treatment-related deaths, and with which dose interruptions and modifications may be required.17,23,24 While the safety profile of talimogene laherparepvec is favorable, it is a live, attenuated virus and appropriate precautions should be used during handling and administration to avoid accidental exposure, such as those outlined in institutional guidelines. In the event of accidental infection or exposure, talimogene laherparepvec is susceptible to antiviral agents such as acyclovir and famciclovir, although no cases of household transmission have been reported to date. Talimogene laherparepvec is also susceptible to common disinfectants and virucidal agents.
The reasons for the more pronounced efficacy with talimogene laherparepvec therapy in patients with earlier-stage disease are not fully understood. One explanation is that heterogeneity in metastatic cells may increase treatment resistance, reducing the response to a single-agent therapeutic.25,26 Another explanation is that disease control with talimogene laherparepvec in patients with stage IIIB/C or IVM1a melanoma may be achieved through direct locoregional lytic effects of the virus, as well as immune effects, whereas responses in visceral lesions may be more reliant on systemic immune effects alone.9 In evaluation of melanoma patients treated with talimogene laherparepvec in a Phase II trial, MART-1-specific CD8+ T cells were identified in both injected and un-injected lesions, but the number of such cells was greater in the injected lesions.27 Other analyses have shown that talimogene laherparepvec injection may activate T cells that preferentially traffic to metastases in similar anatomic sites.6,28 Thus, combining talimogene laherparepvec with agents that can help expand tumor-reactive T cells is of significant interest for enhancing regression of un-injected lesions. An additional explanation is that patients with earlier stage melanoma have a smaller tumor burden, and as seen with other therapies, a smaller tumor burden has previously been shown to be associated with improved clinical outcomes in patients receiving talimogene laherparepvec.29 Finally, it has been suggested that some patients with visceral disease may have had insufficient time to derive benefit from the talimogene laherparepvec-initiated systemic anti-tumor activity in the OPTiM trial.6,25,26
The limitation of this study is that the data in this exploratory subanalysis are inherently less robust than those of the OPTiM ITT population, as the study was not specifically designed to detect significant differences between subgroups. Nevertheless, the subgroup of patients with earlier-stage disease did represent over half of the overall population, and the pronounced efficacy and favorable safety profile indicate the utility of talimogene laherparepvec as a single agent in patients with earlier-stage melanoma, particularly in the control of locoregional disease involving skin, soft tissue and lymph node locations (which is often disfiguring, painful and psychologically disturbing).
Several unanswered questions remain from the OPTiM study that could help to guide the role of talimogene laherparepvec alongside other therapeutics in clinical practice. The first unanswered question is whether there are predictive molecular markers associated with talimogene laherparepvec efficacy. In this respect, biomarkers were not evaluated in the OPTiM study. However, studies are underway with talimogene laherparepvec to evaluate immune response and to help identify potential predictive immune markers, such as intratumoral CD8+ T-cell density, peripheral blood levels of activated CD8+ T cells and total peripheral blood level of T cells (eg, ClinicalTrial.gov identifier NCT02366195). Preliminary data suggest that talimogene laherparepvec monotherapy leads to an increase in circulating cytotoxic T cells (CD3+/CD8+) and an upregulation of PD-1 and TIM-3 on these cells, providing some evidence that immune markers may ultimately be useful for evaluating efficacy with this therapeutic.30
A second unanswered question from the OPTiM study relates to whether talimogene laherparepvec should be used prior to checkpoint inhibitors and targeted (BRAF/MEK) therapies. In this regard, the recent guidelines from the National Comprehensive Cancer Network recommend the use of talimogene laherparepvec as a primary treatment for stage III in-transit melanoma (based on category 1 evidence) and suggest that checkpoint inhibitors/targeted therapies should be used in more advanced disease.31 Additionally, since talimogene laherparepvec appears to be priming the immune system, it may be beneficial if used prior to administration of checkpoint inhibitors as a mechanism for expanding the neoantigen repertoire; data from clinical trials are warranted to confirm this clinically. Furthermore, as talimogene laherparepvec may augment the immune and antitumor responses, those patients who do not have a CR with monotherapy and can tolerate a greater toxicity burden may benefit from combination treatments. Indeed, the relatively low rate of grade 3 or 4 AEs and relative lack of immune-related AEs with talimogene laherparepvec – and its potentially complementary mechanism of action with other approved melanoma agents – support such an investigational approach. Trials investigating talimogene laherparepvec in combination with other immunotherapies, in stage IIIB–IVM1c disease, including ipilimumab (NCT01740297) and pembrolizumab (NCT02263508) in melanoma are underway, with promising early results.30,32 Findings to date suggest that these combinations have greater efficacy than either therapy alone, but with no additional safety concerns above those expected for each monotherapy.30,32
A third unanswered question from the OPTiM study relates to the sequencing of talimogene laherparepvec following other treatments. While response to talimogene laherparepvec in the OPTiM study has been reported to be improved in patients with treatment-naïve metastatic melanoma,6 line of therapy was not retained as an independent predictor for durable response in a multivariate analysis considering disease stage (P=0.0763).33 Therefore, the results suggest that regardless of previous treatment, talimogene laherparepvec could be considered for patients with stage IIIB, IIIC or IVM1a melanoma, especially those who are older, given the manageable side-effect profile and positive benefit–risk balance. However, the OPTiM study was initiated in 2009 before the availability of checkpoint inhibitors and targeted (BRAF/MEK) therapies for melanoma. Therefore, from the OPTiM data set, it is not possible to ascertain the efficacy of talimogene laherparepvec following these now commonly used treatments.
In terms of when to consider changing treatment in patients receiving talimogene laherparepvec monotherapy, participants in the OPTiM trial were treated for a minimum of 6 months to allow for delayed immune-mediated anti-tumor effects to occur. Furthermore, the current analysis indicates that the median time to response with talimogene laherparepvec monotherapy is 4 months. Additionally, as observed with other immunotherapies, it is important to note that progression prior to response can occur in a large number of patients (~50% with talimogene laherparepvec). Therefore, continued dosing in the presence of enlarging or new lesions in clinically stable patients could be considered.
Conclusion
This subgroup analysis demonstrated that treatment with talimogene laherparepvec, an oncolytic immunotherapy, improved DRR and ORR compared with GM-CSF in patients with earlier stage metastatic melanoma (stage IIIB, IIIC or IVM1a) and was well tolerated. These results support the approval for talimogene laherparepvec in Europe, where it is indicated for patients with regional or distantly metastatic (stage IIIB–IVM1a) unresectable melanoma with no bone, brain, lung or other visceral disease.4 Talimogene laherparepvec is the first treatment of its kind, and with European Commission and FDA approval, it represents a novel treatment option for patients with melanoma.
Supplementary material
Table S1.
List of institutions involved in the design, conduct, analysis, and reporting of the OPTiM study
| Addenbrookes Hospital, Cambridge, UK |
| Baptist Cancer Institute, Jacksonville, FL, USA |
| Barrett Cancer Center, Cincinnati, OH, USA |
| Boemfontein Medi-Clinic, GVI Oncology, Kraaifontein, South Africa |
| California Pacific Medical Center, San Francisco, CA, USA |
| Cancer Research UK Clinical Centre, St James’ University Teaching Hospital, Leeds, UK |
| Cancer Sciences Division, Southampton University Hospitals, Southampton, UK |
| Churchill Hospital, Medical Oncology Unit, Oxford, UK |
| Cleveland Clinic Foundation, Taussig Cancer Center, Cleveland, OH, USA |
| Clinical Research Unit, Jewish General Hospital, Montreal, QC, Canada |
| Columbia University Medical Center, New York, NY, USA |
| Duke University Medical Center, Durham, NC, USA |
| Earle A Chiles Research Institute, Portland, OR, USA |
| Emory University Hospital, Winship Cancer Institute, Atlanta, GA, USA |
| Gabrail Cancer Center, Canton, OH, USA |
| Greenville Hospital Systems, Greenville, SC, USA |
| GVI Oncology Clinical Trials Unit, GVI Oncology, Port Elizabeth, South Africa |
| GVI Oncology, Rondebosch Medical Centre, Rondebosch, Cape Town, South Africa |
| H Lee Moffitt Cancer Center, Tampa, FL, USA |
| Hopelands Cancer Centre, Pietermaritzburg, South Africa |
| Hubert Humphrey Cancer Center, Robbinsdale, MN, USA |
| Indiana University, Indianapolis, IN, USA |
| Investigative Clinical Research of Indiana, Indianapolis, IN, USA |
| Jon and Karen Huntsman Cancer, Murray, UT, USA |
| Kansas City Cancer Center, Kansas City, MO, USA |
| Lakeland Regional Cancer Center, Lakeland, FL, USA |
| Leicester Royal Infirmary, Department of Oncology, Leicester, UK |
| Mary Crowley Medical Research Center, Dallas, TX, USA |
| Mary Potter Oncology Centre, Little Company of Mary Hospital, Pretoria, South Africa |
| Mayo Clinic, Rochester, MN, USA |
| MD Anderson Cancer Ctr Orlando, Orlando, FL, USA |
| Medical University of South Carolina, Charleston, SC, USA |
| Midland Allison Cancer Center, Midland, TX, USA |
| Morristown Memorial Hospital, Morristown, NY, USA |
| Mount Sinai CCOP, Miami Beach, FL, USA |
| Mt Sinai Medical Center, Department of Surgery, New York, NY, USA |
| New Mexico Cancer Care Alliance, Albuquerque, NM, USA |
| Onc and Hem Associates of Southwest Virginia, Salem, VA, USA |
| Oncology Specialists, Park Ridge, IL, USA |
| Palm Beach Cancer Center, West Palm Beach, FL, USA |
| Princess Margaret Hospital, University Health Network, Toronto, ON, Canada |
| Redwood Regional Medical Group, Santa Rosa, CA, USA |
| Rhode Island Hospital, Providence, RI, USA |
| Rosebank Oncology Centre, Johannesburg, South Africa |
| Roswell Park Cancer Institute, Buffalo, NY, USA |
| Royal Free Hospital, Academic Department of Oncology, London, UK |
| Rush University Medical Center, Chicago, IL, USA |
| St George’s University of London, London, UK |
| St Louis University Hospital, Department of Surgery, St Louis, MO, USA |
| St Lukes Cancer Center, Bethlehem, PA, USA |
| St Mary’s Medical Center, San Francisco, CA, USA |
| Texas Cancer Center – Abilene, Abilene, TX, USA |
| The Royal Marsden NHS Trust, Head & Neck Unit, London, UK |
| Thomas Jefferson University, Philadelphia, PA, USA |
| Univ of Louisville, James Graham Brown Cancer Center, Louisville, KY, USA |
| University of Arizona Cancer Center, Tucson, AZ, USA |
| University of Arkansas for Medical Sciences, Little Rock, AR, USA |
| University of California, San Diego Moores Cancer Center, La Jolla, CA, USA |
| University of Colorado Cancer Center, Aurora, CO, USA |
| University of Iowa Holden Comprehensive Cancer Center, Iowa City, IA, USA |
| University of Kansas Medical Center, Kansas City, KS, USA |
| University of North Carolina at Chapel Hill, Chapel Hill, NC, USA |
| University of Pretoria & Steve Biko Academic Hospital Complex, Pretoria, South Africa |
| University of Texas – MD Anderson Cancer Center, Houston, TX, USA |
| University of Utah, Huntsman Cancer Institute, Salt Lake City, UT, USA |
| Vanderbilt Ingram Cancer Center, Nashville, TN, USA |
| Wake Forest University, Department of Surgical Oncology Medical Center, Salem, NC, USA |
| Washington University School of Medicine Division of Oncology, St Louis, MO, USA |
| Wellcome Trust Clinical Research Facility, Queen Elizabeth Hospital, Birmingham, UK |
| Wilgers Oncology Centre, Pretoria, South Africa |
Acknowledgments
The authors wish to acknowledge the patients who participated in the OPTiM study (NCT00769704), OPTiM study investigators and their staff and the study team at Amgen. Medical writing support was provided by Louise Niven, DPhil, of Aspire Scientific Limited (Bollington, UK) and was funded by Amgen.
Footnotes
Disclosure
Kevin J Harrington discloses membership of scientific advisory boards for Amgen, AstraZeneca, Bristol Myers Squibb, Merck Sharp & Dohme, Pfizer and Viralytics and discloses a consulting role with Amgen, AstraZeneca, Bristol Myers Squibb, Merck Sharp & Dohme, Pfizer and Viralytics. Kevin J Harrington acknowledges support from the The Institute of Cancer Research/The Royal Marsden Hospital NIHR Biomedical Research Centre. Robert HI Andtbacka discloses a consulting role with Amgen. Frances Collichio discloses a consulting role with Amgen; the University of North Carolina School of Medicine receives clinical trials funding from Amgen. Gerald Downey, Lisa Chen and Zsolt Szabo are employees of Amgen. Howard L Kaufman discloses membership of scientific advisory boards for Alkermes, Amgen, EMD Serono, Merck, Prometheus and Sanofi and involvement in a speaker’s bureau for Merck. The authors report no other conflicts of interest in this work.
References
- 1.Workenhe ST, Mossman KL. Oncolytic virotherapy and immunogenic cancer cell death: sharpening the sword for improved cancer treatment strategies. Mol Ther. 2014;22(2):251–256. doi: 10.1038/mt.2013.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiocca EA, Rabkin SD. Oncolytic viruses and their application to cancer immunotherapy. Cancer Immunol Res. 2014;2(4):295–300. doi: 10.1158/2326-6066.CIR-14-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United States Food and Drug Administration [webpage on the Internet] Food and Drug Administration (FDA) Approves First-of-Its-Kind Product for the Treatment of Melanoma. 2015. [Accessed December 1, 2015]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm469571.htm.
- 4.European Medicines Agency The Committee for Medicinal Products for Human Use (CHMP) Summary of Opinion for Imlygic. 2015. [Accessed December 1, 2015]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/002771/WC500195907.pdf.
- 5.Ledford H. Cancer-fighting viruses win approval. Nature. 2015;526(7575):622–623. doi: 10.1038/526622a. [DOI] [PubMed] [Google Scholar]
- 6.Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 7.Liu BL, Robinson M, Han ZQ, et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 2003;10(4):292–303. doi: 10.1038/sj.gt.3301885. [DOI] [PubMed] [Google Scholar]
- 8.Hu JC, Coffin RS, Davis CJ, et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 2006;12(22):6737–6747. doi: 10.1158/1078-0432.CCR-06-0759. [DOI] [PubMed] [Google Scholar]
- 9.Senzer NN, Kaufman HL, Amatruda T, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol. 2009;27(34):5763–5771. doi: 10.1200/JCO.2009.24.3675. [DOI] [PubMed] [Google Scholar]
- 10.Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ. 1995;310(6977):452–454. doi: 10.1136/bmj.310.6977.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulzer M, Mancini GB. ‘Unqualified success’ and ‘unmitigated failure’: number-needed-to-treat-related concepts for assessing treatment efficacy in the presence of treatment-induced adverse events. Int J Epidemiol. 1996;25(4):704–712. doi: 10.1093/ije/25.4.704. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization . World Health Organization (WHO) Handbook for Reporting Results of Cancer Treatment. Geneva: World Health Organization; 1979. [Google Scholar]
- 13.Bacik J, Mazumdar M, Murphy BA, et al. The functional assessment of cancer therapy-BRM (FACT-BRM): a new tool for the assessment of quality of life in patients treated with biologic response modifiers. Qual Life Res. 2004;13(1):137–154. doi: 10.1023/B:QURE.0000015297.91158.01. [DOI] [PubMed] [Google Scholar]
- 14.Yost KJ, Sorensen MV, Hahn EA, Glendenning GA, Gnanasakthy A, Cella D. Using multiple anchor- and distribution-based estimates to evaluate clinically meaningful change on the functional assessment of cancer therapy-biologic response modifiers (FACT-BRM) instrument. Value Health. 2005;8(2):117–127. doi: 10.1111/j.1524-4733.2005.08202.x. [DOI] [PubMed] [Google Scholar]
- 15.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response and other comparisons of time-to-event by outcome variables. J Clin Oncol. 2008;26(24):3913–3915. doi: 10.1200/JCO.2008.16.1000. [DOI] [PubMed] [Google Scholar]
- 16.Andtbacka RHI, Kaufman HL, Collichio FA, et al. Reduced risk of developing visceral or bone metastasis in patients with stages IIIB, IIIC, and IVM1a melanoma treated with talimogene laherparepvec versus GM-CSF; Poster presented at: Society for Melanoma Research Congress; San Francisco, CA: 2015. [Google Scholar]
- 17.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 21.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 22.Bedikian AY, Johnson MM, Warneke CL, et al. Does complete response to systemic therapy in patients with stage IV melanoma translate into long-term survival? Melanoma Res. 2011;21(1):84–90. doi: 10.1097/CMR.0b013e328341445f. [DOI] [PubMed] [Google Scholar]
- 23.Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380(9839):358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 24.Larkin J, Del Vecchio M, Ascierto PA, et al. Vemurafenib in patients with BRAF(V600) mutated metastatic melanoma: an open-label, multicentre, safety study. Lancet Oncol. 2014;15(4):436–444. doi: 10.1016/S1470-2045(14)70051-8. [DOI] [PubMed] [Google Scholar]
- 25.Allison KH, Sledge GW. Heterogeneity and cancer. Oncology (Williston Park) 2014;28(9):772–778. [PubMed] [Google Scholar]
- 26.Alsaggar M, Yao Q, Cai H, Liu D. Differential growth and responsiveness to cancer therapy of tumor cells in different environments. Clin Exp Metastasis. 2016;33(2):115–124. doi: 10.1007/s10585-015-9761-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufman HL, Kim DW, DeRaffele G, Mitcham J, Coffin RS, Kim-Schulze S. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann Surg Oncol. 2010;17(3):718–730. doi: 10.1245/s10434-009-0809-6. [DOI] [PubMed] [Google Scholar]
- 28.Chang CJ, Tai KF, Roffler S, Hwang LH. The immunization site of cytokine-secreting tumor cell vaccines influences the trafficking of tumor-specific T lymphocytes and antitumor efficacy against regional tumors. J Immunol. 2004;173(10):6025–6032. doi: 10.4049/jimmunol.173.10.6025. [DOI] [PubMed] [Google Scholar]
- 29.Kaufman H, Amatruda T, Nemunaitis JJ, et al. Tumor size and clinical outcomes in melanoma patients (MEL pts) treated with talimogene laherparepvec (T-VEC); Poster presented at: American Society of Clinical Oncology Annual Meeting; Chicago, IL: 2015. Chap. [Google Scholar]
- 30.Long GV, Dummer R, Ribas A, et al. Efficacy analysis of MASTER-KEY-265 phase 1b study of talimogene laherparepvec (T-VEC) and pembrolizumab (pembro) for unresectable stage IIIB-IV melanoma; Poster presented at: American Society of Clinical Oncology Annual Meeting; Chicago, IL: 2016. [Google Scholar]
- 31.Coit DG, Thompson JA, Algazi A, et al. Melanoma, version 2. 2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14(4):450–473. doi: 10.6004/jnccn.2016.0051. [DOI] [PubMed] [Google Scholar]
- 32.Puzanov I, Milhem MM, Minor D, et al. Talimogene laherparepvec in combination with ipilimumab in previously untreated, unresectable stage IIIB-IV melanoma. J Clin Oncol. 2016;34(22):2619–2626. doi: 10.1200/JCO.2016.67.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.European Medicines Agency The Committee for Medicinal Products for Human Use (CHMP) Assessment Report for Imlygic. 2015. [Accessed March 1, 2016]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002771/WC500201082.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
List of institutions involved in the design, conduct, analysis, and reporting of the OPTiM study
| Addenbrookes Hospital, Cambridge, UK |
| Baptist Cancer Institute, Jacksonville, FL, USA |
| Barrett Cancer Center, Cincinnati, OH, USA |
| Boemfontein Medi-Clinic, GVI Oncology, Kraaifontein, South Africa |
| California Pacific Medical Center, San Francisco, CA, USA |
| Cancer Research UK Clinical Centre, St James’ University Teaching Hospital, Leeds, UK |
| Cancer Sciences Division, Southampton University Hospitals, Southampton, UK |
| Churchill Hospital, Medical Oncology Unit, Oxford, UK |
| Cleveland Clinic Foundation, Taussig Cancer Center, Cleveland, OH, USA |
| Clinical Research Unit, Jewish General Hospital, Montreal, QC, Canada |
| Columbia University Medical Center, New York, NY, USA |
| Duke University Medical Center, Durham, NC, USA |
| Earle A Chiles Research Institute, Portland, OR, USA |
| Emory University Hospital, Winship Cancer Institute, Atlanta, GA, USA |
| Gabrail Cancer Center, Canton, OH, USA |
| Greenville Hospital Systems, Greenville, SC, USA |
| GVI Oncology Clinical Trials Unit, GVI Oncology, Port Elizabeth, South Africa |
| GVI Oncology, Rondebosch Medical Centre, Rondebosch, Cape Town, South Africa |
| H Lee Moffitt Cancer Center, Tampa, FL, USA |
| Hopelands Cancer Centre, Pietermaritzburg, South Africa |
| Hubert Humphrey Cancer Center, Robbinsdale, MN, USA |
| Indiana University, Indianapolis, IN, USA |
| Investigative Clinical Research of Indiana, Indianapolis, IN, USA |
| Jon and Karen Huntsman Cancer, Murray, UT, USA |
| Kansas City Cancer Center, Kansas City, MO, USA |
| Lakeland Regional Cancer Center, Lakeland, FL, USA |
| Leicester Royal Infirmary, Department of Oncology, Leicester, UK |
| Mary Crowley Medical Research Center, Dallas, TX, USA |
| Mary Potter Oncology Centre, Little Company of Mary Hospital, Pretoria, South Africa |
| Mayo Clinic, Rochester, MN, USA |
| MD Anderson Cancer Ctr Orlando, Orlando, FL, USA |
| Medical University of South Carolina, Charleston, SC, USA |
| Midland Allison Cancer Center, Midland, TX, USA |
| Morristown Memorial Hospital, Morristown, NY, USA |
| Mount Sinai CCOP, Miami Beach, FL, USA |
| Mt Sinai Medical Center, Department of Surgery, New York, NY, USA |
| New Mexico Cancer Care Alliance, Albuquerque, NM, USA |
| Onc and Hem Associates of Southwest Virginia, Salem, VA, USA |
| Oncology Specialists, Park Ridge, IL, USA |
| Palm Beach Cancer Center, West Palm Beach, FL, USA |
| Princess Margaret Hospital, University Health Network, Toronto, ON, Canada |
| Redwood Regional Medical Group, Santa Rosa, CA, USA |
| Rhode Island Hospital, Providence, RI, USA |
| Rosebank Oncology Centre, Johannesburg, South Africa |
| Roswell Park Cancer Institute, Buffalo, NY, USA |
| Royal Free Hospital, Academic Department of Oncology, London, UK |
| Rush University Medical Center, Chicago, IL, USA |
| St George’s University of London, London, UK |
| St Louis University Hospital, Department of Surgery, St Louis, MO, USA |
| St Lukes Cancer Center, Bethlehem, PA, USA |
| St Mary’s Medical Center, San Francisco, CA, USA |
| Texas Cancer Center – Abilene, Abilene, TX, USA |
| The Royal Marsden NHS Trust, Head & Neck Unit, London, UK |
| Thomas Jefferson University, Philadelphia, PA, USA |
| Univ of Louisville, James Graham Brown Cancer Center, Louisville, KY, USA |
| University of Arizona Cancer Center, Tucson, AZ, USA |
| University of Arkansas for Medical Sciences, Little Rock, AR, USA |
| University of California, San Diego Moores Cancer Center, La Jolla, CA, USA |
| University of Colorado Cancer Center, Aurora, CO, USA |
| University of Iowa Holden Comprehensive Cancer Center, Iowa City, IA, USA |
| University of Kansas Medical Center, Kansas City, KS, USA |
| University of North Carolina at Chapel Hill, Chapel Hill, NC, USA |
| University of Pretoria & Steve Biko Academic Hospital Complex, Pretoria, South Africa |
| University of Texas – MD Anderson Cancer Center, Houston, TX, USA |
| University of Utah, Huntsman Cancer Institute, Salt Lake City, UT, USA |
| Vanderbilt Ingram Cancer Center, Nashville, TN, USA |
| Wake Forest University, Department of Surgical Oncology Medical Center, Salem, NC, USA |
| Washington University School of Medicine Division of Oncology, St Louis, MO, USA |
| Wellcome Trust Clinical Research Facility, Queen Elizabeth Hospital, Birmingham, UK |
| Wilgers Oncology Centre, Pretoria, South Africa |