Abstract
Toll-like receptors (TLRs) are an essential component of the innate immune system. While a number of studies have described TLR expression in the female reproductive tract, few have examined the temporal expression of TLRs within the human placenta. We hypothesized that the pattern of TLR expression in the placenta changes throughout the first and second trimester, coincident with physiological changes in placental function and the demands of innate immunity. We collected first and second trimester placental tissue and conducted quantitative PCR analysis for TLRs 1-10, followed by immunohistochemistry to define the cell specific expression pattern of a subset of these receptors. Except for the very earliest time points, RNA expression for TLRs 1-10 was stable out to 20 weeks gestation. However, the pattern of protein expression evolved over time. Early first trimester placenta demonstrated a strong, uniform pattern predominantly in the inner villous cytotrophoblast layer. As the placenta matured through the second trimester, both the villous cytotrophoblasts and the pattern of TLR expression within them became disorganized and patchy, with Hofbauer cells now identifiable in the tissue also staining positive. We conclude from this data that placental TLR expression changes over the course of gestation, with a tight barrier of TLRs forming a wall of defense along the cytotrophoblast layer in the early first trimester that breaks down as pregnancy progresses. These data are relevant to understanding placental immunity against pathogen exposure throughout pregnancy and may aid in our understanding of the vulnerable period for fetal exposure to pathogens.
Keywords: innate immunity, reproductive immunology, pregnancy, Toll-like receptors, placenta
INTRODUCTION
The mammalian placenta is a highly specialized organ composed of both maternal and fetal cells that performs vital functions for the developing fetus during gestation. In addition to transfer of nutrients, gas exchange, elimination of waste, and hormone production, the placenta is an important site of immune defense for the fetus against maternal rejection as well as exogenous microbial insults. During pregnancy, the maternal immune system adapts to permit tolerance of the fetal allograft while maintaining defenses against harmful pathogens [reviewed in (1, 2)]. Any disturbance in this tightly regulated balance may lead to a breach in immune defenses, intrauterine infection by bacteria or viruses, and obstetrical complications such as miscarriage, preterm labor, intrauterine growth restriction, and preeclampsia. Elucidating the etiology of intrapartum infection requires identifying not only the infectious agent but also the route of entry into the amniotic cavity.
In contrast to the formerly held belief that the amniotic tissues are sterile, a number of studies over the past two years suggest the placenta harbors a unique, low-level microbiome that may be linked to birth outcome (3–7). Surprisingly, these studies demonstrate that the taxonomic profile of the gravid vaginal microbiome was quite dissimilar to that of the placental microbiome, favoring hematogenous dissemination over intrauterine ascension as the more common route of entry of infectious agents. It is therefore likely that immune defenses contained within the placenta, the barrier between maternal and fetal circulation, are critical in preventing the transmission of both commensals and pathogens.
Toll-like receptors (TLRs) are a fundamental component of the innate immune system (8) and constitute an important host defense in the placenta against intrapartum infection. This family of transmembrane receptors plays a pivotal role in the recognition of microbial ligands by the innate immune system (9, 10). Toll, initially identified as a receptor involved in embryonic development in Drosophila (11), was found to regulate important antimicrobial responses against fungi in the adult fly (12), an observation that set off an explosion of research to identify similar receptors in humans and mice. At least ten mammalian orthologues of Toll have been identified, and most have been implicated in cellular responses to microbial pathogens [reviewed in (13–17). For example, TLR4 and the secreted protein myeloid differentiation factor 2 (MD-2; also known as lymphocyte antigen 96 or Ly96) are required for recognition of lipopolysaccharide (LPS); TLR2, paired with TLR1 or TLR6, recognizes bacterial lipoproteins and lipoteichoic acid; TLR5 recognizes bacterial flagellin; TLR9 recognizes CpG enriched double-stranded DNA; TLR7 and TLR8 recognize single-stranded RNA (ssRNA); and TLR3 recognizes double stranded RNA (dsRNA) [reviewed (14)]. The TLRs can be sub-divided into surface expressed TLRs (TLR1, -2, -4 and -5) and endosomal expressed TLRs (TLR3, -7, -8, and -9). Upon recognition of pathogen-associated ligands, the TLRs dimerize, initiating a signaling cascade that leads to the secretion of proinflammatory cytokines and antimicrobial peptides, induction of interferon stimulated genes (primarily from the endosomal TLRs and TLR4), as well as activation of the adaptive immune response.
In contrast to the abundant data on TLR function, the temporal expression of toll-like receptors (TLRs) throughout pregnancy has not been as well studied. It is known that TLRs 1-10 are expressed in term human placenta, and that TLR2 and TLR5 transcripts appear to rise in association with labor (18). A recent survey of expression and function of TLRs in first trimester cytotrophoblasts suggests that TLRs are broadly expressed at 6–12 weeks gestation (19), although a comparison to second and third trimester placental tissue was not done. Knowledge of temporal shifts in TLR expression at the maternal-fetal interface may be important clinically to establish periods of increased maternal susceptibility to transmit infections such as CMV and Zika virus or optimal windows for treatment of infections that could impact the fetus. In this report, we describe the expression of TLRs in the human placenta at the level of gene expression and protein, reviewing the relevant details of placental histology over the first and second trimester.
MATERIALS AND METHODS
Study samples
First and second trimester placentas were obtained from elective termination of pregnancies under a protocol approved by the Institutional Review Board (IRB) for the Boston University Medical Campus and in accordance with the principles expressed in the Declaration of Helsinki. Criteria for exclusion of placental samples included known current maternal viral or pelvic infection, preexisting fetal demise, and known abnormal fetal karyotype. Termination of pregnancy was performed by suction dilation and evacuation. Samples were divided according to trimester of collection as follows: first trimester, up to 12 weeks; and second trimester, 12–24 weeks gestation. Gestational age was dated according to the subject’s last reported menstrual period and/or ultrasound dating as well as foot measurement when possible. At least 2 placental specimens representing each week of gestation were obtained during the first trimester of pregnancy from 6–12 weeks and during the second trimester from 13–22 weeks. More specific numbers of samples per analysis are provided below. Placental tissue utilized for RNA analysis was stored in RNAlater (Qiagen) for later processing for RNA extraction. The remaining samples for histology were fixed in 10% unbuffered methanol-free formaldehyde and processed for embedding in wax; 5 μm thick sections were cut from each sample and placed on glass slides for histological analysis.
RNA isolation
Placental tissue was homogenized by using a Tissue Homogenizer LT (Qiagen) and TRIzol (Life Technologies). After pelleting tissue debris, tissue homogenates were transferred to a fresh tube and chloroform was added. After precipitation of protein, the RNA-rich upper aqueous phase was transferred, mixed with an equal volume of 70% ethanol, and then loaded onto RNeasy Mini Kit columns (Qiagen). All RNA was treated with RNase-free DNase (Qiagen) prior to cDNA synthesis.
Quantitative reverse transcriptase PCR analysis
A total of 11 samples were available for PCR analysis (first trimester, n=6 samples; second trimester, n=5 samples). Quantitative reverse transcription PCR analysis (qRT-PCR) for TLR expression was performed with the TaqMan Expression Assay system (Life Technologies), which utilizes FAM-MGB probes for each target. Primer IDs for the target genes [TLRs 1-10, MD-2, GAPDH, and N-myc downstream-regulated gene 1 (NDRG1)] are shown in Table 1. cDNA preparation was carried out using High Capacity RNA-to-cDNA kit (Life Technologies). The final 20 μl PCR reaction mixture consisted of 10 μl of 2x TaqMan Gene Expression Master mix, 1 μl of cDNA, 8 μl of RNase-free water, and 1 μl of TaqMan Gene Expression Assay mixture containing the target primer. Reactions were performed in a 96-well plate and the housekeeping gene GAPDH was used as internal control. Real-Time PCR was run on an Applied Biosystems StepOnePlus™ Real-Time PCR System using a standard program (50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15s and 60°C for 1 min).
Table 1.
RT-qPCR primer ID, TaqMan® Gene Expression Assay
| Target | Assay ID |
|---|---|
| TLR1 | Hs00413978_m1 |
| TLR2 | Hs01014511_m1 |
| TLR3 | Hs00152933_m1 |
| TLR4 | Hs00152939_m1 |
| TLR5 | Hs01019558_m1 |
| TLR6 | Hs01039989_s1 |
| TLR7 | Hs00152971_m1 |
| TLR8 | Hs00152972_m1 |
| TLR9 | Hs00152973_m1 |
| TLR10 | Hs01675179_m1 |
| NDRG1 | Hs00608387_m1 |
| GAPDH | Hs99999905_m1 |
PCR data was analyzed using StepOne Software v2.3, and the comparative Ct method to calculate relative quantitation. The Ct from the target genes of each sample was normalized to the Ct of the reference gene GAPDH, to generate the sample delta Ct (ΔCt sample = Ct reference gene GAPDH − Ct target). To compare the gene expression levels between samples of different gestational age, the target genes were normalized to sample 1, which we defined as the earliest gestational age (sample ΔΔCt = sample ΔCt − sample 1ΔCt). To compare the different TLR gene expression levels, the TLR genes were normalized to the expression of TLR10 sample 1, which was the lowest expressed gene by both semi-quantitative PCR and real-time PCR. The Relative Quantitation (RQ) was calculated as: RQ = log2(−ΔΔCt). Graphpad Prism software 6.0 was used for statistical analysis of the data. For statistical comparison of a given target between the first and second trimester, a student’s t-test was used. A p value < 0.05 was considered statistically significant. The R-squared (R2) value for RQ vs. gestational age was calculated using a simple linear regression model.
Histology and immunohistochemistry
Two placental samples per gestational week studied were analyzed for TLR expression except as noted here: week 7 utilized 4 samples, and weeks 18 and 19 utilized 3 samples. For general histological analysis, sections from each gestational week studied were stained with routine hematoxylin and eosin (H&E). A trained pathologist in Obstetrics and Gynecology routinely screened these slides for the presence of inflammation, infection or abnormal placental development. No morphological evidence for any pathological conditions or changes were detected in any of the placental tissues examined. All placental samples that were studied for expression of the TLRs were therefor judged to be normal.
The immunohistochemical analysis of TLR expression in placental tissue was carried out as previously described (20). Briefly, an antigen retrieval step was carried out by immersing the sections in a citrate buffer (pH 6.0), and heating them to 125°C for 30 seconds in a pressure cooker (Biocare Medical, Concord, CA, USA). The sections were rinsed thoroughly in distilled water and incubated with a blocking reagent (Background Sniper, Biocare Medical) prior to the application of primary antibodies directed against TLR2 (Novus Biologicals, Littleton, CO, USA) at 1:100 dilution; TLR3 (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) at 1:100 dilution; TLR4 (Novus Biologicals) at 1:120 dilution; TLR5 (Santa Cruz Biotechnology) at 1:80 dilution; and TLR9 (Santa Cruz Biotechnology) at 1:110 dilution for 60 minutes at room temperature. This was followed by washing in Tris buffer containing 0.1% Tween 20 (TBST). The antibodies were detected with a proprietary secondary reagent (MACH 4 Universal Alkaline Phosphatase, Biocare Medical) that detects both mouse and rabbit primary antibodies. The sections were then washed in TBST, and the antibodies visualized by incubating with a substrate for alkaline phosphatase (Vulcan Fast Red Chromogen, Biocare Medical) that stains positive cells a bright red. Development of the staining was monitored by light microscopy. Following a final wash, the sections were counterstained in aqueous hematoxylin mounted in a glycerin-based medium and mounted on a coverslip. Sections were analyzed under an Olympus microscope (Olympus America Inc., Center Valley, PA, USA) and images captured with a DP70 digital camera.
Negative controls for the immunohistochemistry were processed in the absence of the primary antibodies. These were replaced with either the antibody diluent or a mouse or rabbit control antibody (Vector Laboratories, Burlingame, CA, USA) at the same concentration.
RESULTS
Expression of TLR genes across the first and second trimester is stable
In order to quantify the changes in TLR gene expression in the placenta during the first and second trimester of pregnancy, we conducted a quantitative analysis for placental expression of the genes for TLRs 1-10 and the TLR4-associated protein, MD2. Comparisons were made to the housekeeping gene GAPDH, as well as the placental gene NDRG1, which has been shown to be expressed throughout the placenta in a relatively stable manner (21). We found mRNA for all 10 TLRs and MD2 could be detected, and for the majority of the TLRs, expression increased with gestational age. This is illustrated in Table 2, where raw Ct values are shown for each of the TLR targets tested, and Fig. 1, where relative gene expression (RQ) is graphed against trimester and gestational age. We found expression of TLR3 was the highest compared to the other TLRs in both the first and second trimester without a significant change in expression over time. We found a statistically significant increase in the RQ value in the second trimester compared to the first trimester samples for TLR1, TLR4, TLR7 and TLR8, with a non-significant trend towards increased RQ for TLR2 and TLR10 (Fig. 1A). For some of the TLRs (TLR1, TLR4, TLR7, TLR8), the increase in gene expression could be predicted by the gestational age, as shown by the calculated R squared value (Fig. 1B). In comparison, expression of the placental gene NDRG1 is stable across gestation. The limitation of this data is that it reflects gene expression not protein expression within a tissue of mixed cellular composition. To further examine cell specific expression in polarized cells within a tissue, we turned to immunohistochemistry.
Table 2.
Raw Ct values
| First Trimester | Second Trimester | p-value | |||
|---|---|---|---|---|---|
| Raw Ct (Mean ± SEM) | n | Raw Ct (Mean ± SEM) | n | ||
| TLR1 | 30.96 ± 0.6694 | 6 | 27.21 ± 0.5113** | 5 | 0.0020 |
| TLR2 | 32.56 ± 0.9181 | 6 | 28.13 ± 0.7483** | 5 | 0.0055 |
| TLR3 | 26.07 ± 0.3476 | 6 | 25.27 ± 0.2160 | 5 | 0.0947 |
| TLR4 | 29.91 ± 0.4826 | 6 | 26.44 ± 0.2186*** | 5 | 0.0002 |
| TLR5 | 30.26 ± 0.5319 | 5 | 28.60 ± 0.1483* | 5 | 0.0168 |
| TLR6 | 30.62 ± 0.4225 | 6 | 28.24 ± 0.3195** | 5 | 0.0019 |
| TLR7 | 30.48 ± 0.7499 | 6 | 27.13 ± 0.2657** | 5 | 0.0037 |
| TLR8 | 35.47 ± 0.5801 | 5 | 31.89 ± 0.5597** | 5 | 0.0022 |
| TLR9 | 35.79 ± 0.1746 | 6 | 35.02 ± 1.045 | 5 | 0.445 |
| TLR10 | 37.64 ± 0.2855 | 5 | 35.44 ± 0.6617* | 5 | 0.0157 |
| MD2 | 28.36 ± 0.3990 | 6 | 25.86 ± 0.1996*** | 5 | 0.0005 |
| NDRG1 | 24.70 ± 0.2163 | 6 | 24.63 ± 0.5569 | 5 | 0.9056 |
| GAPDH | 20.63 ± 0.5354 | 6 | 20.05 ± 0.3347 | 5 | 0.4127 |
p<0.05;
p<0.005;
p<0.001 compared to first trimester.
Fig. 1.
Real time PCR analysis of TLR expression in placental tissue is shown. RNA isolated from first and second trimester placental tissue was subjected to RT-qPCR and normalized as described in the Methods section. Each data point represents an individual placenta, with n = 6 samples analyzed for first trimester, and n = 5 samples analyzed for second trimester except as follows: n = 5 for TLR5, TLR8 and TLR10 in the first trimester as no product was detected for TLR8 and TLR10 in a 6 week sample and TLR5 in a 7 week sample. (A) RQ is graphed on the y-axis according to trimester, and data shown is the mean with the SEM. PCR for each sample was repeated twice. Statistical significance (p-value) was calculated using a t-test: *, p < 0.05; **, p < 0.01; ***, p < 0.001. (B) Scatter plot analysis of TLR expression over 6–19 weeks gestation is depicted. RNA isolated from placentas of 6–19 weeks of gestation was subjected to Real-Time PCR for TLRs 1-10, MD2 and the control gene NDRG1, as described in the Methods. Each data point represents an individual placenta. Data shown above is the relative quantitation (RQ) of each gene vs. gestational age in the first and second trimester. The R2 values were calculated using a simple linear regression model.
Histological changes within the placental villi during the first and second trimester
We began by characterizing the histological changes that occur within the chorionic villi of the placenta over the first and second trimester using routine H&E staining of tissues, analyzing at least two tissues obtained at each time point. As shown in Fig. 2, there was considerable variation in the morphological composition of the placental villi with progression of gestation. As has been described, during the earliest weeks of gestation that we could evaluate (6–8 weeks), the inner villous cytotrophoblast layer was essentially continuous, consisting of small, regularly shaped cuboidal cells with round to oval nuclei (Fig. 2A). With further progression (9–12 weeks), the villous cytotrophoblast layer of some villi was noted to be more disorganized, whereas in some others it appeared as an attenuated layer of cells that were more flattened (Fig. 2B, 2C). Towards the end of the first trimester, a number of villi could be seen with scattered, large, cells within the cytotrophoblast layer (Fig. 2D).
Fig. 2.
Variations in the morphological appearance of the cytotrophoblast in villi during gestation. Cytotrophoblasts are seen as (A) a competent layer of small cells at 6 weeks; (B) a disorganized layer of cells at 12 weeks; (C) an attenuated layer of cells at 12 weeks; (D) large cells present in some villi at 12 weeks; (E) a loosely organized layer of cells at 13 weeks; (F) very large and irregular shaped cells now present in many villi at 13 weeks; and (G) mostly absent from villi that appear now to be surrounded by only the syncytiotrophoblast at 22 weeks. Original magnification: A, 10x; B–E, 20x; F, 40x; G 20x.
As gestation proceeded into the second trimester, the morphological changes observed to be taking place in the first trimester progressed such that few villi now possessed an intact layer of villous cytotrophoblasts. Although an attenuated cytotrophoblast layer could still be detected in some villi, in the majority of cases, cell-cell contact between cytotrophoblasts was lost leaving a loosely organized cellular layer beneath the outer syncytiotrophoblast (Fig. 2E). This resulted in a greater level of structural diversity between the individual villi for each placental sample compared to that observed in the first trimester. Moreover, it appeared that the few identifiable cytotrophoblasts were now increased in size and irregular in shape when compared to those present in the first trimester, perhaps suggesting increased differentiation towards a syncytiotrophoblast phenotype (Fig. 2F).
With further maturation of the placenta during the last weeks of the second trimester, it was observed that many villi still possessed small but varying numbers of large villous cytotrophoblast cells scattered at the periphery. For many other villi, however, it was difficult to precisely identify the presence of any villous cytotrophoblast layer, and the villi instead appeared to be surrounded by a single layer of syncytiotrophoblast cells (Fig. 2G).
Expression of TLRs within the placental villi during the first trimester of pregnancy
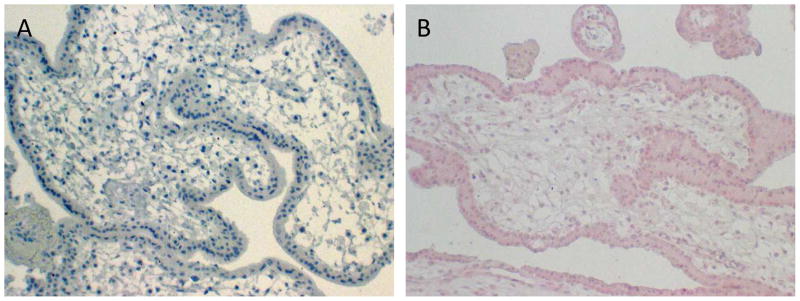
Using immunohistochemistry protocols developed by our laboratory we examined the expression of TLR2, TLR3, TLR4, TLR5, and TLR9 in the available placental tissues. Negative controls of placental tissue samples observed at any stage of gestation showed no to little evidence of background or non-specific staining when the primary antibody was replaced with the antibody diluent (Fig. 3A). There was however a slight increase in background staining in placental tissues when incubated with either the rabbit IgG or mouse IgG negative controls (Fig. 3B).
Fig 3.
Representative examples of negative controls. (A) Primary antibody was replaced with the antibody diluent (9 week sample). (B) Primary antibody was replaced with a negative control rabbit IgG (7 week sample). Original magnification: A–B, 10x.
We found that in the early first trimester, at 6–7 weeks of gestation, the inner villous cytotrophoblast layer expresses all the TLRs that were studied (Fig. 4). Staining for TLR2 appeared especially intense on the cell membrane of the villous cytotrophoblast adjacent to the syncytiotrophoblast layer (Fig. 4A). This often gave the appearance of an apparent solid band of TLR2-positive staining dividing these two layers of cells (Fig. 4B). Staining for TLR4 and TLR5 was also membrane associated, but in contrast to TLR2, expression was not polarized (Fig. 4D and 4E). As expected for the endosomal TLRs, expression of TLR3 and TLR9 was observed as positive cytoplasmic staining within cytotrophoblasts (Figs. 4C and 4F), although unlike the surface expressed TLRs, the positive cells were discontinuous in some areas.
Fig 4.
TLR expression by the villous cytotrophoblasts during early (6–7 weeks) first trimester of pregnancy is depicted. (A) Intense staining for TLR2 at 6 weeks with (B) polarized expression of TLR2 at the outer (apical) plasma membrane adjacent the syncytiotrophoblast layer (7 weeks). (C) Cytoplasmic staining of TLR3 (7 weeks). Membrane staining of (D) TLR4 (7 weeks) and (E) TLR5 (7 weeks). (F) Cytoplasmic staining of TLR9 (7 weeks). Original magnification: A, 10x; B, 40x; C–E, 20x; F, 10x.
The expression of TLR2, TLR3, TLR4, and TLR5, as evidenced by the intensity and pattern of positive staining in the cytotrophoblasts for all these TLRs, appeared to be fairly consistent, not only between the different placental samples studied at this stage, but also between the individual villi present within the same sample. The exception was TLR9, for which some villi, even from the same placenta, demonstrated abundant positive cytoplasmic granules throughout the sample, while others showed few or no cytoplasmic granules within the cells of the cytotrophoblast layer.
Later in the first trimester (8–12 weeks), there was more variability in the intensity and distribution of the staining (Fig. 5). Villi that still possessed an intact cytotrophoblast layer consisting of conspicuous, well-developed, round cells were found to stain positive for the TLRs 2, 3, 4, 5 and 9 in a manner resembling that observed at 6–7 weeks gestation. The flattened, discontinuous cytotrophoblasts present in other villi also expressed TLR2 in a similar fashion to the earlier stages of gestation, with what appeared to be a continuous band of intense staining along the outer membrane bordering the syncytiotrophoblast (Fig. 5A). However, staining for TLR3 in many areas was more intense on the outer villous cytotrophoblast cell membrane adjacent to the syncytiotrophoblast layer, similar to what we had observed with TLR2, with some cytosolic staining as well (Fig. 5B). In contrast, the staining for both TLR4 and TLR5 appeared to be more uniform on the cell membrane of these cells, with some staining visible in the cytosol (Fig. 5C and 5D). The villous cytotrophoblasts, either as a discontinuous or flattened layer of cells, also stained positive for TLR9 with some variability in the expression similar to what we had observed earlier in gestation (Fig. 5E and 5F).
Fig 5.
TLR expression by the villous cytotrophoblasts during late (8–12 weeks) first trimester of pregnancy is depicted. Attenuated layer of cells expressing (A) TLR2 (9 weeks) and (B) TLR3 (8 weeks) on the apical cell membrane adjacent the syncytiotrophoblast layer. Putative Hofbauer cell with positive staining for TLR3 (arrow). Expression of (C) TLR4 (12 weeks) and (D) TLR5 (12 weeks) by disorganized round cells. Expression of TLR9 by either (E) disorganized (12 weeks) or (F) attenuated cells (9 weeks). Original magnification: A, 10x; B–F, 20x.
Expression of TLRs within the placental villi during the second trimester of pregnancy
During the early stages of the second trimester (13–17 weeks), the villous cytotrophoblasts in most villi were present as a loosely organized layer of large, often irregularly shaped cells (Fig. 6). It was observed that only a small number of these isolated cells were now expressing TLR2, although the pattern still resembled that observed in the first trimester, with intense expression on the membrane adjacent to the syncytiotrophoblasts (Fig. 6A). Other cytotrophoblast cells showed no TLR2 staining (Fig. 6B). TLR3 also appeared to be expressed by the villous cytotrophoblasts at this stage with both cytosolic and membrane staining detected (Fig. 6C). Intense staining was detected for TLR4 in the villous cytotrophoblasts, whether they were present as a disorganized layer or as individual cells scattered around the villi (Figs. 6D and 6E). Strong expression of TLR5 was also observed (Fig. 6F). As in the earlier time points, there appeared to be variability in the expression of TLR9 by the villous cytotrophoblasts. Whereas in some villi only a few cells stained positive for TLR9, in others it was apparent that most if not all of the cells contained intensely TLR9 positive granules (Fig. 6G).
Fig 6.
TLR expression by the villous cytotrophoblasts during early (13–17 weeks) second trimester of pregnancy is depicted. At this stage the cytotrophoblasts appear as a disorganized layer of large irregularly shaped cells. (A) TLR2 is expressed by a small number of cells (13 weeks), and some villi (B) stain negative for TLR2 (15 weeks; small arrow). Note adjacent putative Hofbauer cell that stained positive for TLR2 (large arrow). Expression of (C) TLR3 (13 weeks); TLR4 (D, 13 weeks; E, 15 weeks); (F) TLR5 (13 weeks); and (G) TLR9 (15 weeks). Original magnification: A–B, 40x; C–D, 20x; E, 10x; F–G, 20x.
As the placenta developed during the last weeks of the second trimester (18–22 weeks), it was observed that some of the disorganized and often isolated cells of the villous cytotrophoblast layer still maintained the polarized staining for TLR2 with greater expression present on the cell membrane adjacent to the syncytiotrophoblast (Fig. 7A). Interestingly, the staining for TLR3 by the villous cytotrophoblasts was found to vary. For many villi, the remnant villous cytotrophoblast layer of large cells displayed uniform cytoplasmic expression of TLR3 (Fig. 7B), while in other villi, cells displayed TLR3 expression in a polarized fashion, similar to TLR2, with more intense staining of the cell membrane adjacent to the syncytiotrophoblast (Fig. 7C). Expression of TLR4 was consistently detected on all villous cytotrophoblast cells (Fig. 7D), while expression of TLR5 and TLR9 was not regularly detected (Fig. 7E and 7F).
Fig. 7.
TLR expression by the villous cytotrophoblasts during late (18–22 weeks) second trimester of pregnancy is depicted. (A) Remnant cells still expressed TLR2 with often intense staining of the cell membrane adjacent the syncytiotrophoblast (18 weeks). (B) TLR3 was expressed either as distinct positive cytoplasmic staining (22 weeks) or (C) as polarized staining with higher expression by the cell membrane next to the syncytiotrophoblast (21 weeks). Isolated cytotrophoblast cells expressing (D) TLR4 (20 weeks) and (E) TLR5 (22 weeks) and (F) TLR9 (18 weeks). Original magnification: A–F, 20x.
Hofbauer cells
Cells that morphologically resembled Hofbauer cells were detected in villi during the early weeks (6–7) of gestation. As pregnancy proceeded however increasing numbers of putative Hofbauer cells appeared to be present in placental villi. These cells occurred in varying numbers in villi and many were found to stain positive for TLR2, 3, 4, 5 and 9. Examples of this are TLR2 (Fig. 6B arrow), TLR3 (Fig. 5B arrow) and TLR5 (Fig. 6F arrow).
DISCUSSION
Pregnancy is often perceived as an immune suppressed state that is necessary for the mother to tolerate the developing fetus. However, this over-simplified view underestimates the complex immunological exchange that occurs at the maternal-fetal interface within the placenta, particularly when microbial pathogens or commensals could be encountered. An inadequate immune response would allow the infection to spread to the fetus, while an excessive inflammatory response could lead to miscarriage, preterm labor, or other consequences of placental dysfunction. The innate immune system plays an essential role in balancing these often conflicting priorities with the goal of maintaining an appropriate environment for the developing fetus while protecting the health of the mother.
The temporal regulation of TLRs in the placenta has not been widely studied. Ours is the first study to examine placental expression of the TLRs across the first and second trimester by both mRNA and protein. TLR gene expression was surprisingly consistent between individuals in the second trimester, although there was some variability within the first trimester for a subset of the TLRs. For example, placental TLR2 expression in the latter half of the first trimester was variable between individuals, as was TLR7 and TLR8. This coincides with the histological changes we observed in the first trimester, where the villous cytotrophoblast layer becomes more disorganized, suggesting a very dynamic tissue at this early stage. Of the TLRs studied, TLR3 was most highly expressed at the level of mRNA. In contrast, TLR9 and TLR10 mRNA expression was the lowest among the TLRs studied. Because our gene expression studies reflect mRNA levels throughout a tissue of mixed cellularity, it is impossible to determine the relative contribution of the individual cell types, such as villous cytotrophoblasts vs. Hofbauer cells.
Clarification comes from the immunohistochemistry studies, where protein expression can be determined in situ. The most striking expression pattern was the band of TLR2 staining at the outer plasma membrane of the intact villous cytotrophoblast layer in the very early first trimester placenta. In fact, all the surface-expressed TLRs we examined (TLR2, TLR4, TLR5) demonstrated a similarly contiguous pattern of staining in this early stage placenta. TLR3 was generally detected in the cytosol; however, there were situations where TLR3 appeared to be expressed at the plasma membrane. While TLR3 is commonly considered an endosomal TLR, its expression has been described outside this compartment. For example, fibroblasts (but not dendritic cells) were shown to express TLR3 at the cell surface (22, 23), and it has been hypothesized that cell surface TLR3 binds dsRNA and becomes activated upon internalization (24). TLR9 staining was also localized to the cytosol, although by RT-PCR its expression was relatively lower than that seen for the other TLRs.
The strong TLR3 expression by both RT-PCR and IHC is worth noting as TLR3 is a receptor for dsRNA and particularly important for danger signals. In addition to dsRNA viruses, dsRNA is formed as an intermediate during replication of some ssRNA viruses (such as respiratory syncytial virus, West Nile, influenza A, coxsackievirus, etc.) as well as DNA viruses (25). Cellular dsRNA can also be released during necrotic cell death [reviewed in (26)]. TLR3 activation is a strong inducer of type I IFNs and an antiviral immune response through the activation of IRF3 and NF-κB [reviewed in (27, 28)], and mouse models suggest that TLR3 activation can lead to preterm birth (29, 30). It was recently reported that placental IFN-β regulates inflammation by inhibiting responses to LPS, suggesting that inhibition of type I IFNs could remove the brake from inflammation induced by bacterial commensals and pathogens and lead to placental dysfunction and pregnancy loss (31). Thus, the presence of TLR3 could play a regulatory role in modulating inflammation through the induction of type I IFN.
Our data is consistent with what others have reported. For example, Abrahams et al. demonstrated that TLR2 and TLR4 are expressed by villous cytotrophoblasts and extravillous trophoblasts, but not by syncytiotrophoblasts in first trimester placenta (32). Tangerås et al reported expression of functional TLRs 2–5 and TLR9 in isolated first trimester cytotrophoblasts (19). Ours is the first study to correlate expression of TLRs with histological changes in the placenta. It is interesting to speculate on the evolutionary adaptations that would shape the temporal and spatial patterns of TLR gene expression in the placenta. Early in the first trimester when the fetus is most vulnerable, the villous cytotrophoblasts appear to provide a firm line of defense, demonstrating the active role played by these cells as an arm of the innate immune system and countering the argument that pregnancy is a state of immune suppression. The presence of the full complement of TLRs in this tissue reflects the pressure to protect the fetus against invading pathogens as it weathers the first-trimester tests of chromosomal fitness, maternal antibody attack, endocrine imbalances, and defective implantation. As pregnancy progresses, however, breaches in this line of defense appear, perhaps reflecting improved fetal resilience as well as the need to temper an excessive inflammatory response that can be both harmful and beneficial to mother and fetus, leading to consequences such as miscarriage or preeclampsia.
An improved understanding of the temporal and spatial regulation of TLRs in the placenta and the role they play in maintaining homeostasis could be exploited clinically. Much to the disappointment of clinicians, treatment of infections such as vaginitis (33, 34) or periodontal disease (35–38) during pregnancy has not reduced the incidence of preterm birth. Selective use of TLR antagonists in conjunction with more traditional antimicrobial treatments could prove useful as an approach to managing pregnancy-associated infections.
Highlights.
Cytotrophoblasts express multiple TLRs.
TLR expression across early gestation is dynamic, especially in the first trimester.
The villous cytotrophoblast layer becomes more disorganized as the placenta matures.
Acknowledgments
The authors would like to thank the patients and clinical staff of the Obstetrics and Gynecology Department at Boston Medical Center for their support of this project. We thank Dr. Joe Politch for assistance with formatting figures, Dr. Deborah J. Anderson for insightful discussions, and the BUMC Analytical Instrumentation Core (AIC) for access to core instruments. This work was supported by funding from the NIH/NIAID through grant number AI101088.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Land WG. How evolution tells us to induce allotolerance. Exp Clin Transplant. 2015;13(Suppl 1):46–54. [PubMed] [Google Scholar]
- 2.Svensson-Arvelund J, Ernerudh J. The Role of Macrophages in Promoting and Maintaining Homeostasis at the Fetal-Maternal Interface. Am J Reprod Immunol. 2015;74(2):100–9. doi: 10.1111/aji.12357. [DOI] [PubMed] [Google Scholar]
- 3.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6(237):237ra65. doi: 10.1126/scitranslmed.3008599. 6/237/237ra65 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao B, Stout MJ, Lee I, Mysorekar IU. Placental Microbiome and Its Role in Preterm Birth. Neoreviews. 2014;15(12):e537–e545. doi: 10.1542/neo.15-12-e537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collado MC, Rautava S, Aakko J, Isolauri E, Salminen S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci Rep. 2016;6:23129. doi: 10.1038/srep23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong XD, Li XR, Luan JJ, Liu XF, Peng J, Luo YY, Liu CJ. Bacterial communities in neonatal feces are similar to mothers’ placentae. Can J Infect Dis Med Microbiol. 2015;26(2):90–4. doi: 10.1155/2015/737294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng J, Xiao X, Zhang Q, Mao L, Yu M, Xu J. The Placental Microbiome Varies in Association with Low Birth Weight in Full-Term Neonates. Nutrients. 2015;7(8):6924–37. doi: 10.3390/nu7085315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medzhitov R, Janeway C., Jr Innate immunity. N Engl J Med. 2000;343(5):338–44. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 9.Medzhitov R, Janeway CA., Jr Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91(3):295–8. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 10.Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci U S A. 1998;95(2):588–93. doi: 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morisato D, Anderson KV. Signaling pathways that establish the dorsal-ventral pattern of the Drosophila embryo. Annu Rev Genet. 1995;29:371–99. doi: 10.1146/annurev.ge.29.120195.002103. [DOI] [PubMed] [Google Scholar]
- 12.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86(6):973–83. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 13.Akira S. Mammalian Toll-like receptors. Curr Opin Immunol. 2003;15(2):238. doi: 10.1016/s0952-7915(02)00013-4. [DOI] [PubMed] [Google Scholar]
- 14.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11(5):373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 16.Lien E, Ingalls RR. Toll-like receptors. Crit Care Med. 2002;30(1 Suppl):S1–11. [PubMed] [Google Scholar]
- 17.O’Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci STKE. 2000;2000(44):RE1. doi: 10.1126/stke.442000re1. [DOI] [PubMed] [Google Scholar]
- 18.Patni S, Wynen LP, Seager AL, Morgan G, White JO, Thornton CA. Expression and activity of Toll-like receptors 1-9 in the human term placenta and changes associated with labor at term. Biol Reprod. 2009;80(2):243–8. doi: 10.1095/biolreprod.108.069252. biolreprod.108.069252 [pii] [DOI] [PubMed] [Google Scholar]
- 19.Tangerås LH, Stodle GS, Olsen GD, Leknes AH, Gundersen AS, Skei B, Vikdal AJ, Ryan L, Steinkjer B, Myklebost MF, Langaas M, Austgulen R, Iversen AC. Functional Toll-like receptors in primary first-trimester trophoblasts. J Reprod Immunol. 2014 doi: 10.1016/j.jri.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Pudney J, Anderson DJ. Expression of toll-like receptors in genital tract tissues from normal and HIV-infected men. Am J Reprod Immunol. 2011;65(1):28–43. doi: 10.1111/j.1600-0897.2010.00877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wyatt SM, Kraus FT, Roh CR, Elchalal U, Nelson DM, Sadovsky Y. The correlation between sampling site and gene expression in the term human placenta. Placenta. 2005;26(5):372–9. doi: 10.1016/j.placenta.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto M, Funami K, Tanabe M, Oshiumi H, Shingai M, Seto Y, Yamamoto A, Seya T. Subcellular localization of Toll-like receptor 3 in human dendritic cells. J Immunol. 2003;171(6):3154–62. doi: 10.4049/jimmunol.171.6.3154. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto M, Kikkawa S, Kohase M, Miyake K, Seya T. Establishment of a monoclonal antibody against human Toll-like receptor 3 that blocks double-stranded RNA-mediated signaling. Biochem Biophys Res Commun. 2002;293(5):1364–9. doi: 10.1016/S0006-291X(02)00380-7. [DOI] [PubMed] [Google Scholar]
- 24.Murakami Y, Fukui R, Motoi Y, Kanno A, Shibata T, Tanimura N, Saitoh S, Miyake K. Roles of the cleaved N-terminal TLR3 fragment and cell surface TLR3 in double-stranded RNA sensing. J Immunol. 2014;193(10):5208–17. doi: 10.4049/jimmunol.1400386. [DOI] [PubMed] [Google Scholar]
- 25.Jensen S, Thomsen AR. Sensing of RNA viruses: a review of innate immune receptors involved in recognizing RNA virus invasion. J Virol. 2012;86(6):2900–10. doi: 10.1128/JVI.05738-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chattopadhyay S, Sen GC. dsRNA-Activation of TLR3 and RLR Signaling Gene Induction-Dependent and Independent Effects. J Interferon Cytokine Res. 2014;34(6):427–36. doi: 10.1089/jir.2014.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsumoto M, Funami K, Oshiumi H, Seya T. Toll-like receptor 3: a link between toll-like receptor, interferon and viruses. Microbiol Immunol. 2004;48(3):147–54. doi: 10.1111/j.1348-0421.2004.tb03500.x. [DOI] [PubMed] [Google Scholar]
- 28.Schroder M, Bowie AG. TLR3 in antiviral immunity: key player or bystander? Trends Immunol. 2005;26(9):462–8. doi: 10.1016/j.it.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Cardenas I, Means RE, Aldo P, Koga K, Lang SM, Booth CJ, Manzur A, Oyarzun E, Romero R, Mor G. Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J Immunol. 2010;185(2):1248–57. doi: 10.4049/jimmunol.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koga K, Cardenas I, Aldo P, Abrahams VM, Peng B, Fill S, Romero R, Mor G. Activation of TLR3 in the trophoblast is associated with preterm delivery. Am J Reprod Immunol. 2009;61(3):196–212. doi: 10.1111/j.1600-0897.2008.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Racicot K, Kwon JY, Aldo P, Abrahams V, El-Guindy A, Romero R, Mor G. Type I Interferon Regulates the Placental Inflammatory Response to Bacteria and is Targeted by Virus: Mechanism of Polymicrobial Infection-Induced Preterm Birth. Am J Reprod Immunol. 2016;75(4):451–60. doi: 10.1111/aji.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abrahams VM, Bole-Aldo P, Kim YM, Straszewski-Chavez SL, Chaiworapongsa T, Romero R, Mor G. Divergent trophoblast responses to bacterial products mediated by TLRs. J Immunol. 2004;173(7):4286–96. doi: 10.4049/jimmunol.173.7.4286. [DOI] [PubMed] [Google Scholar]
- 33.Carey JC, Klebanoff MA, Hauth JC, Hillier SL, Thom EA, Ernest JM, Heine RP, Nugent RP, Fischer ML, Leveno KJ, Wapner R, Varner M. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 2000;342(8):534–40. doi: 10.1056/NEJM200002243420802. [DOI] [PubMed] [Google Scholar]
- 34.Klebanoff MA, Carey JC, Hauth JC, Hillier SL, Nugent RP, Thom EA, Ernest JM, Heine RP, Wapner RJ, Trout W, Moawad A, Leveno KJ, Miodovnik M, Sibai BM, Van Dorsten JP, Dombrowski MP, O’Sullivan MJ, Varner M, Langer O, McNellis D, Roberts JM. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med. 2001;345(7):487–93. doi: 10.1056/NEJMoa003329. [DOI] [PubMed] [Google Scholar]
- 35.Macones GA, Parry S, Nelson DB, Strauss JF, Ludmir J, Cohen AW, Stamilio DM, Appleby D, Clothier B, Sammel MD, Jeffcoat M. Treatment of localized periodontal disease in pregnancy does not reduce the occurrence of preterm birth: results from the Periodontal Infections and Prematurity Study (PIPS) Am J Obstet Gynecol. 2010;202(2):147 e1–8. doi: 10.1016/j.ajog.2009.10.892. S0002-9378(09)02118-8 [pii] [DOI] [PubMed] [Google Scholar]
- 36.Michalowicz BS, Hodges JS, DiAngelis AJ, Lupo VR, Novak MJ, Ferguson JE, Buchanan W, Bofill J, Papapanou PN, Mitchell DA, Matseoane S, Tschida PA. Treatment of periodontal disease and the risk of preterm birth. N Engl J Med. 2006;355(18):1885–94. doi: 10.1056/NEJMoa062249. 355/18/1885 [pii] [DOI] [PubMed] [Google Scholar]
- 37.Newnham JP, Newnham IA, Ball CM, Wright M, Pennell CE, Swain J, Doherty DA. Treatment of periodontal disease during pregnancy: a randomized controlled trial. Obstet Gynecol. 2009;114(6):1239–48. doi: 10.1097/AOG.0b013e3181c15b40. 00006250-200912000-00013 [pii] [DOI] [PubMed] [Google Scholar]
- 38.Offenbacher S, Beck JD, Jared HL, Mauriello SM, Mendoza LC, Couper DJ, Stewart DD, Murtha AP, Cochran DL, Dudley DJ, Reddy MS, Geurs NC, Hauth JC. Effects of periodontal therapy on rate of preterm delivery: a randomized controlled trial. Obstet Gynecol. 2009;114(3):551–9. doi: 10.1097/AOG.0b013e3181b1341f. 00006250-200909000-00011 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]