Abstract
ABO, H, secretor and Lewis histo-blood system genes control the expression of part of the carbohydrate repertoire present in areas of the body occupied by microorganisms. These carbohydrates, besides having great structural diversity, act as potential receptors for pathogenic and non-pathogenic microorganisms influencing susceptibility and resistance to infection and illness. Despite the knowledge of some structural variability of these carbohydrate antigens and their polymorphic levels of expression in tissue and exocrine secretions, little is known about their biological importance and potential applications in medicine. This review highlights the structural diversity, the biological importance and potential applications of ABO, H, Lewis and secretor histo-blood carbohydrates.
Keywords: Histo-blood groups, ABO system, Lewis system, Carbohydrate antigen, Glycosyltransferases
Introduction
The relationships between humans and microorganisms that colonize their body surface, cavities and mucous membranes begin at birth and continue throughout life. These relationships include a continuum of mutually beneficial conditions or otherwise (symbiosis, commensalism) as well as injury to one of the parties (parasitism).1 Some of the carbohydrates expressed by the epithelial cells of the body surface as well as in the gastrointestinal, respiratory and genitourinary systems are closely involved in these relationships and play a critical role in the symbiosis, commensalism and parasitism continuum.2, 3
ABO (ABO, 9q34.1), H (FUT1, 19q13.3), secretor (FUT2, 19q.13.3) and Lewis (FUT3: 19p13.3) histo-blood system genes control the expression of part of the carbohydrate repertoire present in areas occupied by microorganisms.2, 4 These carbohydrates besides having great structural diversity, act as potential receptors for pathogenic and non-pathogenic microorganisms influencing susceptibility and resistance to infection and illness. It is believed that this structural diversity results from environment pressure and plays an important role in the symbiosis, commensalism and parasitism continuum.2, 5
Evidence supports the proposition that ABO, H, Lewis and secretor histo-blood group carbohydrates are related to susceptibility and resistance to infections and infectious diseases.5, 6, 7, 8, 9 Experimental analyzes have clarified the biochemical and molecular basis underlying some of these relationships.10, 11, 12 This review highlights the structural diversity and biological importance of ABO, H, Lewis and secretor histo-blood carbohydrates.
Histo-blood group systems
The expression “histo-blood system” was first proposed for the ABO system.13 Later, it was extended to H, Lewis and secretor systems as their carbohydrates are also expressed in other tissues and exocrine secretions.14 These histo-blood group systems have strong relationships at genomic, enzymatic, biochemical, tissue and immune levels. Knowing the structural variability of their carbohydrate antigens allows us to understand the evolutionary importance and biological role especially in terms of diseases.2
The International Society for Blood Transfusion (ISBT) Working Party on Red Cell Immunogenetics and Blood Group Terminology identifies each blood group system according to its discovery (http://www.isbtweb.org/working-parties/red-cell-immunogenetics-and-blood-group-terminology/). ABO, Lewis and H histo-blood group systems are identified by the numbers 001, 007 and 018, respectively. As secretor is not a true blood group system, it was not included among those recognized by the ISBT Working Party. However, as it controls the expression of ABO and Lewis carbohydrates in tissues and exocrine secretions it must be considered an alloantigen system.15
Phenotyping of histo-blood group systems
Histo-blood group systems are commonly identified by or inferred from red blood cell phenotyping. Different phenotypes are known including ABO [A, B, AB, O], H [H, H-deficient], secretor [secretor, non-secretor] and Lewis [Le(a+b−), Le(a−b+), Le(a+b+), Le(a−b−)].16 The ABO system is characterized by the presence or absence of two carbohydrate antigens (A and B) on the red blood cell membrane and three regular antibodies (Anti-A, anti-B, anti-A,B) in the blood plasma.15 The H system is characterized by the presence of only one carbohydrate antigen (H) on the red blood cell membrane. H-deficient individuals contain one regular antibody (anti-H) in the blood plasma. Three rare types of H-deficient phenotypes have been described: the classical Bombay phenotype which is non-secretor, the H-partially deficient phenotype which is non-secretor and the Para-Bombay phenotype which is secretor.15, 16
Secretors express the ABO carbohydrates in exocrine secretions in addition to in red blood cells. On the other hand, non-secretors express ABO carbohydrates only in red blood cells. Le(a+b−) phenotype correlates to a non-secretor phenotype while Le(a−b+) correlates to a secretor phenotype and Le(a+b+) correlates to a weak secretor phenotype. Le(a−b−) can be secretor or non-secretor. However, due to potential cross-reactivity between anti-Lea and anti-Leb antisera and even the weak adsorption of Lea and Leb carbohydrates in the red blood cell membrane, this correlation is not always true, particularly in diseases.17, 18
The identification of red blood cell phenotypes is commonly based on an agglutination reaction with tests performed with slides, tubes, microplates, gel column, and automation.19 However, the results are affected by the method used and the quality of antisera and can be inaccurate especially in relation to the Lewis histo-blood group system.15, 17 Therefore, red cell serology techniques alone are not sufficient to characterize the structural diversity of histo-blood group carbohydrates in tissues and exocrine secretions. As the genes of these histo-blood group systems all interact, the combination of serology and genotyping is a good strategy to predict the diversity of carbohydrates expressed in tissues and exocrine secretions.17 Additionally, immunochemistry, proton nuclear magnetic resonance (NMR) imaging and mass spectrometry (MALDI and Q-TofMS/MS) are useful tools to resolve the carbohydrate structure and diversity; however, these techniques and some of the reagents required are not routinely available.2
Histo-blood group phenotype frequencies in populations
Since the beginning of the last century, studies on population variability demonstrated that the histo-blood group phenotypes occur in all populations but their frequencies vary widely from one ethnicity to another.20 The O phenotype is common among Africans and South American natives while A and B are common in North European and Asian countries, respectively. H-deficient phenotypes are rare in all populations but they are more frequent in India and Reunion Island, located in the Indian Ocean. Although present in all populations, the secretor phenotype occurs in 80% of Caucasians. The Le(a+b−) phenotype is found in more than 20% of Caucasians and Blacks but it is rare in Asians. Le(a−b+) is frequent in all populations but Le(a+b+) is more common in Asians and Polynesians than populations from the Western world. Le(a−b−) is rare in Caucasians but is common among Blacks.16, 21 The reasons why the differential distribution of histo-blood phenotypes occurs at population level are not fully understood but it is believed that selective pressure imposed by disease-causing microorganisms contributed to this process.9
Biochemical basis of histo-blood group carbohydrates
ABO, H, secretor and Lewis histo-blood group carbohydrates are not primary gene products. They are synthesized by specific glycosyltransferases encoded by the ABO, FUT1, FUT2 and FUT3 genes.15 These enzymes incorporate sequentially, monosaccharide units to linear or branched precursor oligosaccharide chains, modifying and creating new antigenic specificities.16
The biosynthetic pathways and the biochemical basis of these histo-blood group systems were established from glycoproteins in ovarian cyst fluids. It was established that their carbohydrate antigens are oligosaccharide chains present as free forms or bound to sphingolipids (glycolipids) or proteins (glycoproteins). Pioneer studies revealed that the glycosyltransferases have functional similarities and require precursor oligosaccharides, nucleotide sugar donors and divalent ions.22, 23
Carbohydrate units and precursor oligosaccharide chains
Six types of monosaccharides are found in the ABO, H, Lewis and secretor histo-blood group carbohydrates: β-d-glucose (Glc), β-d-N-acetylglucosamine (GlcNAc), β-d-galactose (Gal), β-d-N-acetylgalactosamine (GalNAc), α-fucose (Fuc) and d-mannose (Man). Another monosaccharide carrying nine carbon atoms, α-d-N-acetylneuraminic acid (NeuAc) may also be found in these carbohydrates. l-Fucose is the immunodominant monosaccharide present in H, Lea and Leb carbohydrates while N-acetylgalactosamine (GalNAc) and galactose (Gal) are the immunodominant monosaccharides present in A and ALeb, and B and BLeb carbohydrates, respectively.23
Six types of precursor oligosaccharide chains have been characterized. Type 1 is found in the epithelium of the gastrointestinal, respiratory, genitourinary tracts and in exocrine secretions. Type 2 is predominant in hematopoietic tissue and vascular endothelium. Type 3 can be found linked to mucins or as an extension of the A Type 2 carbohydrate due to the addition of a Galactose to the terminal N-acetylgalactosamine. Type 4 is abundant in renal tissue. Type 5 is synthetic and has not yet been isolated from human tissues. Type 6 is found in human intestinal cells. The glycosylation of any of these precursor oligosaccharide chains give rise to different carbohydrate antigens, which may differ in the spatial conformation and affinity to monoclonal antibodies.13, 16, 18, 23, 24 Table 1 shows the linear structures of the six precursor oligosaccharide chains.
Table 1.
Linear structure of precursor oligosaccharide chains of the ABO, H, Lewis and secretor histo-blood group systems.
| Types | Terminal structures | Expressed forms |
|---|---|---|
| 1 | Galβ1→3GlcNAcβ1-R | Glycoproteins, glycolipids |
| 2 | Galβ1→4GlcNAcβ1-R | Glycoproteins, glycolipids |
| 3 | Galβ1→3GalNAcα1-R | Glycolipids |
| 4 | Galβ1→3GalNAcβ1-R | Glycolipids |
| 5 | Galβ1→3Galβ1-R | Synthetic structure |
| 6 | Galβ1→4Glcβ1-R | Glycoproteins, glycolipids |
Source: Adapted from Oriol.16
The structural diversity of the ABO, H, Lewis and secretor histo-blood group carbohydrates is enormous and influenced by a range of factors. The type of monosaccharide and the glycosidic bond between them, the ionic charge, the carbon ring size, the linearity and branching, the chain extension, and α and β anomeric conformations, result in more than 1.05×1012 possible structural combinations that can be obtained from the combinations of these six monosaccharides.25 Additionally, the structural differences in these precursor oligosaccharides favor structural variability and raise the complexity of the carbohydrate repertoire in tissues and exocrine secretions.2
Histo-blood group glycosyltransferases
The glycosyltransferases acting on the biosynthesis of ABO, H, secretor and Lewis histo-blood group carbohydrates are structurally related to type II transmembrane glycosyltransferases and have some common characteristics.23 They require uridine diphosphate (UDP) and guanidine diphosphate (GDP) as nucleotide sugar donors as well as divalent ions to sequentially incorporate monosaccharide units into the precursor oligosaccharide chains.26 To modify and create new ABO, H, Lewis and secretor histo-blood group carbohydrate structures, some of these enzymes compete for the same precursor. Therefore the presence, absence or combination of these glycosyltransferases will determine the quality and the quantity of carbohydrates expressed by individuals.2, 15 Table 2 presents general characteristics of the histo-blood group glycosyltransferases and the carbohydrate antigens synthesized.
Table 2.
ABO, H, Lewis and secretor histo-blood group systems, gene location, glycosyltransferases and synthesized carbohydrate structures of type 1 and type 2 precursor oligosaccharides.
| Systems | Genea | Chromosome location | Enzyme | Abbreviation | ECb | Nucleotide donor | Synthesized carbohydrate |
|---|---|---|---|---|---|---|---|
| H | FUT1 | 19q13.3 | α1,2-Fucosyltransferase | FUTI | 2.4.1.69 | GDP | H type 2 |
| Secretor | FUT2 | 19q13.3 | α1,2-Fucosyltransferase | FUTII | 2.4.1.69 | GDP | H type 1 |
| Lewis | FUT3 | 19p13.3 | α1,3/4-Fucosyltransferase | FUTIII | 2.4.1.65 | GDP | Lea, Leb, ALeb, BLeb |
| ABO | ABO | 9q.34.1 | α1,3-N-Acetylgalactosaminiltransferase | GTA | 2.4.1.40 | UDP | A type 1, A type 2 |
| α1,3-D-Galactosyltransferase | GTB | 2.4.1.37 | UDP | B type 1, B type 2 |
EC: Enzyme Commission Number; GDP: guanidine diphosphate; UDP: uridine diphosphate.
As stated by Human Genome Committee.
Source: Adapted from Schenkel-Brunner.23
Some glycosyltransferases acting on the histo-blood group carbohydrate biosynthesis have redundancy and degeneration. Redundancy is observed when two separate enzymes synthesize the same antigen. For example, FUT1 gene-defined fucosyltransferase and FUT2 gene-defined fucosyltransferase are capable of synthesizing the H Type 2 carbohydrate from the same precursor oligosaccharide (Type 2). Degeneration occurs when the same enzyme synthesizes different carbohydrate structures. For example, FUT2 gene-defined fucosyltransferase is capable of synthesizing H Type 1 and H Type 2 carbohydrates from their respective Type 1 and Type 2 precursor oligosaccharides. The rare phenotypes B(A) and A(B) which illustrate the synthesis of small amounts of A carbohydrate by the group B galactosyltransferase (GTB) and vice versa is another example of degeneration in the ABO histo-blood group system. Additionally, FUT3 gene-defined fucosyltransferase is capable of synthesizing at least four different histo-blood group carbohydrates (Lea, Leb, ALea and ALeb) derived from the type 1 precursor oligosaccharide.27 Redundancy and degeneration create additional levels of complexity in these histo-blood group systems.16 The level of expression as well as the location of these enzymes in the Golgi compartments influence their competition for monosaccharide donors and acceptors, determining variations in the type, size and amount of each synthesized carbohydrate structure.15
Biosynthesis of histo-blood group carbohydrates
The histo-blood group carbohydrates synthesized from type 2 precursor oligosaccharides are intrinsic to the red blood cell membrane as they are expressed by red blood cell precursor cells. However, those synthesized from type 1 precursor oligosaccharide (A, B, H, Lea and Leb) are extrinsic since they originate in the liver, pancreas, kidney and small intestine, are transported from their place of synthesis to the blood plasma and then adsorbed into the red blood cell membrane.27, 28
The biosynthesis of ABO, H, Lewis and secretor histo-blood carbohydrates is a complex event and dependent on interactions between FUTI, FUTII, FUTIII, group A N-acetylglucosaminyltransferase (GTA) and GTB proteins.27 FUTI, GTA and GTB allow the synthesis of H, A and B carbohydrates from type 2 oligosaccharides in mesodermal and hematopoietic tissues and vascular endothelium.29 FUTII, FUTIII, GTA and GTB allow the synthesis of H, A, B, Lea, Leb, ALeb and BLeb carbohydrates from the type 1 oligosaccharides in ectodermal tissues such as the gut, respiratory and urinary mucosae and exocrine secretions.16 Therefore, these interactions result in a different tissue profile of carbohydrates to that found on the red blood cell membrane.2, 15
Additional complexities occur at a tissue level. For example, the expression of ABO and Lewis carbohydrates on pyloric and duodenal mucosae is related to the migration of cells from Brunner glands.30 Cells migrating to the surface of the gastric and duodenal epithelium express ABO and Lewis carbohydrates under the control of FUTII, FUTIII, GTA and GTB. Those migrating to deep areas of the Brunner glands express these carbohydrates under the control of FUTI, GTA and GTB.16
The synthesis of ABO carbohydrates is similar in mesodermal tissues as in ectodermal tissues but under distinct genetic control. FUTI acts like FUTII but it generally uses type 2 precursor oligosaccharides to form the H type 2 carbohydrates. H type 2 carbohydrates can be converted into A type 2 and B type 2 carbohydrates by GTA and GTB, respectively.15, 16 Despite the reduced diversity, these type 2 carbohydrates are crucial in transfusion procedures and solid organ transplantation.
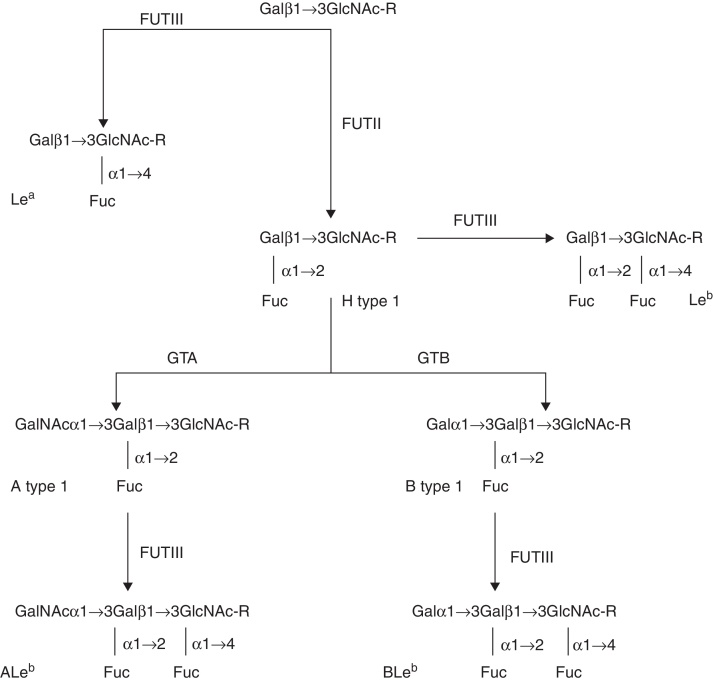
In ectodermal tissues, FUTII transfers a Fuc unit through a α1→2 glycosidic bond to the carbon 2 of the terminal Gal of the type 1 precursor oligosaccharide building up the H type 1 carbohydrate. This structure can be converted into A type 1 or B type 1 by GTA or GTB, respectively. GTA adds a Gal-NAc unit through a α1→3 glycosidic bond to carbon 3 of the terminal Gal of the H type 1 carbohydrate. GTB adds a Gal monosaccharide through a α1→3 glycosidic bond to carbon 3 of the terminal Gal of the H type 1 carbohydrate.31
The synthesis of Lewis antigens occurs in different organs increasing the complexity of histo-blood group systems. FUTIII creates new antigenic specificity and diversifies the previous carbohydrates by the synthesis of Lea, Leb, ALeb and BLeb. It incorporates a Fuc molecule by a α1→4 glycosidic bond to carbon 4 of the subterminal Glc-NAc of the type 1 precursor oligosaccharide to form the Lea carbohydrate. By the same reaction, FTUIII forms the Leb carbohydrate from the H type 1 antigen. FUTIII can add a second Fuc unit by a α1→4 glycosidic bond to the carbon 4 of the N-acetylglucosamine subterminal antigens A and B, converting them into ALeb and BLeb carbohydrates, respectively.16
The role of the functional FUTII is crucial to the action of other histo-blood group glycosyltransferases in diversifying the histo-blood group carbohydrates in secretors. It synthesizes the H type 1 carbohydrate, the common substrate for GTA, GTB and FUTIII. Thus, these enzymes compete for the same substrate and their efficiency will determine the level of expression of each carbohydrate structure. For example, due to the absence of GTA and GTB, the O phenotype expresses a high level of the Leb carbohydrate. On the other hand, the A, B and AB phenotypes express more ALeb and BLeb than Leb carbohydrates. Since non-secretors do not express an active FUTII they will form the Lea carbohydrate from the type 1 precursor oligosaccharide if they carry a functional FUTIII independent of their ABO phenotype.15, 16, 21 For example, this set of glycosylation processes seems to modulate innate immunity responses in the mucosa and may contribute to the risk of gastric disease by reducing the bacterial density and associated inflammation in Helicobacter pylori infection.32
Alternative nomenclature for histo-blood group carbohydrates
ABO, secretor and Lewis carbohydrates can be named by the number of monosaccharide units present in their structure and by the type of precursor oligosaccharide. For instance, A-4-6 refers to A carbohydrate antigen carrying four monosaccharide units derived from the type 6 precursor oligosaccharide while H-5-2 refers to H carbohydrate specificity with five monosaccharide units derived from type 2 precursor oligosaccharide.31 All these carbohydrates react with commercial polyclonal and monoclonal antibodies but they present distinct spatial conformations which can be recognized by some specific monoclonal antibodies as well as microbial adhesins.18, 33, 34 Figure 1 illustrates the biosynthesis of histo-blood group carbohydrates from the type 1 precursor oligosaccharide. Table 3 contains the combinations of histo-blood group glycosyltransferases, the carbohydrates expressed, and the mucosa and red blood cell phenotypes.
Figure 1.
Biosynthesis of ABH-Lewis from type 1 precursor oligosaccharides. Guanidine diphosphate and uridine diphosphate indicate the monosaccharide donors.
Table 3.
Histo-blood group glycosyltransferases, carbohydrates and mucosa and red blood cell phenotypes.
| ABO | FUTII | FUTIII | Carbohydrates | Mucosa phenotypes | Red blood cell phenotypes |
|---|---|---|---|---|---|
| A, B, AB, O | Active | Active | H, A and/or B Lea, Leb, ALeb, BLeb |
Secretor Lewis positive |
A, B, AB, O Le(a−b+) |
| A, B, AB, O | Active | Inactive | H, A and/or B | Secretor Lewis negative |
A, B, AB, O Le(a−b−) |
| A, B, AB, O | Inactive | Active | Lea | Non-secretor Lewis positive |
A, B, AB, O Le(a+b−) |
| A, B, AB, O | Inactive | Inactive | POa | Non-secretor Lewis negative |
A, B, AB, O Le(a−b−) |
Type 1 precursor oligosaccharyde.
Carbohydrate structural variations in common and rare histo-blood groups
A and B carbohydrates present a simple structural difference on carbon 2 of the terminal GalNAc and Gal, respectively. GalNAc has an N-acetyl group linked to carbon 2 whereas a hydroxyl group is linked to the same carbon in Gal. This small difference changes the tridimensional conformation of the terminal A and B carbohydrate portions even if other parts remain unaltered. This distinct portion contains epitopes that selectively react with anti-A and anti-B antibodies. Anti-A,B antibodies can react with the common portions of these carbohydrates.25
A1, A2 and some rare ABO subgroups also present structural differences in their carbohydrates as revealed by an analysis of glycolipids. Immunochemical studies by thin layer chromatography and monoclonal antibodies demonstrated a predominance of the A type 4 carbohydrate in the A1 compared to the A2 subgroup.34 Additionally, novel carbohydrate structural variations were observed in weak A subgroups.33 These studies suggest that mutations in the A gene allow the expression of variant GTA which seems to be potentially able to synthesize a novel ABO carbohydrate structure.
Lea and Leb differ not only by the number of Fuc units, but also by the length of the oligosaccharide chains. The Lea carbohydrate is monofucosylated and Leb is difucosylated. While these chains are short in Leb they are elongated in most carbohydrates carrying Lea specificity.24, 35 These differences are coincident, respectively, with the presence and absence of a functional FUT2 gene-defined fucosyltransferase. The reasons for these differences are not totally understood from the evolutionary perspective but it is possible that they result from biological pressure by microorganisms that colonize areas of the body where these carbohydrates are expressed.2 Apparently, the epitopes of Lea extended carbohydrates offer better access to anti-Lea antibodies, reducing cross reactivity compared to anti-Leb.18
Variability and biological importance of histo-blood group carbohydrates
The environment exerts constant pressure on living beings and drives them to create diversity in order to evolve and be perpetuated in nature with the maintenance of diversity being regulated by the selection of the best adaptation. Since diversity results directly from genetic variations, microorganisms and diseases are essential factors that act in the selection and maintenance of species diversity.36, 37, 38, 39
The activity of FUTI, FUTII, FUTIII, GTA and GTB in the glycosylation of precursor oligosaccharides, besides creating new antigenic structures, diversifies the pre-existent structures allowing a high degree of variability.2 The variability of histo-blood group carbohydrates extends beyond the boundaries of gene and glycosyltransferase polymorphisms.40 Distinct levels of glycosylation in precursor oligosaccharides are responsible for the carbohydrate structural diversity and the polymorphisms seen in these antigenic systems. The first level, controlled by FUTIII, results in the synthesis of the Lea carbohydrate. The second level, controlled by FUTI and FUTII, results in the expression of H type 2 and H type 1 carbohydrates, respectively. At the third level, controlled by FUTIII, the H type 1 antigen is converted into Leb. GTA and GTB act at the fourth level allowing the synthesis of A type 1 and B type 1, and A type 2 and B type 2 carbohydrates from H type 1 and type 2 carbohydrates, respectively. Finally, the fifth level, also controlled by the FUTIII enzyme, results in the conversion of A type 1 into ALeb and B type 1 into BLeb.16
FUTI, FUTII, FUTIII, GTA and GTB create α-glycosidic bonds to incorporate each monosaccharide unit in precursor oligosaccharides. However, the inner core of these precursors contains β-glycosidic bonds.23 Maybe this feature stops pathogenic microorganisms able to produce β-glucosidase to use histo-blood group carbohydrates as receptors by breaking glycoside bonds of β-glycosylated oligosaccharide chains. This suggests a potential reason for the abundance of α-carbohydrate structures in the gastrointestinal, respiratory, and genitourinary tracts and exocrine secretions. These sites are inhabited by great diversity of microorganisms and it is possible that the α-glycosidic bonds protect the inner core of the precursor oligosaccharides from microbial exoglycosidases attack.2
Currently, there is strong evidence that these histo-blood group carbohydrates and the microbial adhesins that recognize them are important links in the relationship that humans have with the microorganisms that colonize their body surface, cavities and mucosa.5, 8, 41, 42 The nature of the interactions between parasites and their hosts is complex, but it is also possible that the ABO, secretor and Lewis histo-blood group carbohydrates represent important pieces in this process. Maybe the histo-blood group glycosyltransferases have evolved to control the part of synthesis of oligosaccharides expressed in the gastrointestinal, respiratory and genitourinary tracts through glycosylation and structural diversification. This strategy may represent an important biological event in the change of potential receptors for pathogenic microorganisms. Therefore, the contribution of ABO, H, secretor and Lewis histo-blood group systems in the diversity of populations may be associated with greater chance of success of our species in epidemic events.2
In this scenario, ABO, H, secretor and Lewis histo-blood group systems contribute with their different polymorphic levels to the ethnic diversity of the human species acting through mechanisms of evolution such as gene flow, genetic drift, founder effect, and natural selection.36, 43 Through glycosylation and structural diversification of precursor oligosaccharides, these systems influence the glycoconjugate repertoire expressed in mucosae and exocrine secretions.40, 42 Consequently, they affect the innate immune response, susceptibility to infections and the parasitism, symbiosis and commensalism continuum.1, 42, 44
Medical importance of histo-blood group carbohydrates
The carbohydrate variability resulting from these histo-blood group systems has important implications in susceptibility to infections, innate and adaptive immune responses, cancer, solid organ transplantation as well as new technologies applied to blood transfusion. Due to the simplicity and low cost of identifying histo-blood group phenotypes in red blood cells, a large number of independent, quick, simple studies explored them as potential markers for diseases. Many of them did not consider the diversity of carbohydrates in the tissues infected by microorganisms as well as the strains or serotypes of the same microorganism among other possible confounding factors.44 However, some well-designed studies provided a better understanding of the potential relationships between histo-blood group carbohydrates and microorganisms as well as their ethnic distribution worldwide.
Helicobacter pylori strains expressing the blood group binding adhesin (BAbA) are able to bind to the Leb carbohydrate that is highly expressed in gastric epithelial cells related to O and secretor histo-blood groups.45 Additionally, the observation that South American specialist strains of H. pylori are more able to bind to H type 1 and Leb carbohydrates than generalist strains that are able to bind to other histo-blood group carbohydrates is coincident with the predominance of the O blood group in Amerindians.46 These studies offered one explanation for an old enigma: Why individuals with the O blood group are more prone to gastroduodenal diseases such as gastritis and peptic ulcers.
It is believed that the low and high frequencies of O and B blood groups, respectively, in some areas of Bangladesh are related to selective pressure imposed by the severity of cholera.47 The severity of this disease is related to the cholera toxin secreted by Vibrio cholera that binds more strongly to the H type 1 carbohydrate than the B carbohydrate.48 Another suggestion is that the high frequency of the O blood group in endemic areas of malaria results from selective pressure related to the severity of this disease caused by Plasmodium falciparum.49 These authors hypothesize that individuals carrying this phenotype have reduced cytoadherence of P. falciparum to red blood cells and consequently have some survival advantage compared to individuals with non-O and non-Le(a−b−) phenotypes who tend to develop severe malaria.
Cholera, malaria and H. pylori infection affect individuals at any age. However, the first two tend to be more severe than the gastric diseases caused by H. pylori. Therefore, it is possible that cholera and malaria exert greater selective pressure by death before reproductive age than H. pylori infection, thus contributing to the low and high frequencies of O blood group individuals in endemic areas of these diseases.
There is convincing evidence that the H carbohydrate in human milk contributes to the protection of infants against Campylobacter jejuni and other enteric pathogens. By binding to specific ligands, the H type 2 carbohydrate inhibits the attachment of these microorganisms to intestinal cells thereby protecting breastfeeding babies.50 Non-secretors are relatively resistant to infection by norovirus and secretors present a variable degree of susceptibility since this virus uses some histo-blood group carbohydrates in the gastrointestinal tract as receptors. About half of secretors are susceptible to infection, develop an early mucosal immune response with specific IgA and become protected. The rest of these individuals develop a late mucosal immune response with specific IgG an IgA antibodies.51 Histological analysis of gastric mucosa from O, non-secretor histo-blood group patients infected by H. pylori revealed a higher level of lymphocyte infiltration compared to other phenotypes.52 Higher levels of interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α) and nitric oxide (NO) are produced by monocytes of O blood group individuals compared to non-O blood groups.53 Taking together these data demonstrate that the diversity of histo-blood group carbohydrates can modulate, at least in part, the innate and adaptive immune responses. Table 4 presents some examples of associations between histo-blood group phenotypes and microorganisms.
Table 4.
Examples of associations between some histo-blood group phenotypes and microorganisms.
| Microorganisms | Histo-blood group phenotypes | Biological effects |
|---|---|---|
| Gastrointestinal tract | ||
| Helicobacter pylori | O, Le(a−b+) | Peptic ulcers, Gastritis |
| Escherichia coli | B | Gastrointestinal infection |
| Giardia lamblia | A | Re-infestation |
| Toxoplasma gondii | B | Not determined |
| Candida albicans | O, non-secretor | Gastric inflammation |
| Vibrio cholera | O, Le(a+b−) | Severe diarrhea |
| Salmonella typhimurium | B | Typhoid fever |
| Norovirus | Non-secretor | Gastroenteritis |
| Respiratory tract | ||
| Streptococcus pneumoniae | A and O | Pneumonia |
| Streptococcus pyogenes | A, non-secretor | Rheumatic fever |
| Influenza A | O | Influenza |
| Mycobacterium tuberculosis | O | Tuberculosis |
| Genitourinary tract | ||
| Escherichia coli | A, B, Le(a+b−) | Urinary infection |
| Pseudomonas aeruginosa | B | Upper urinary infection |
| Neisseria gonorrhoeae | B | Urethral infection |
Source: Adapted from Blackwell et al.44
Associations of histo-blood groups systems with cancer have been published in the past but many of them found low relative risk. An old study carried out by Aird et al. with a large sample size found that A blood group individuals have 20% higher risk of developing stomach carcinoma than O blood group subjects.54 More recently, two large, independent, and prospective cohorts showed that people with A, B and AB blood groups are more likely to develop pancreatic cancer than those with O blood groups.55
Some studies have reported loss and aberrant expressions of histo-blood group carbohydrates at different stages of cancer. Loss of usual histo-blood group carbohydrates seems to correlate with a poor prognosis. Lee et al. demonstrated that the loss of the A carbohydrate in the tumor has a poor prognosis in non-small cell lung cancer.56 It has been demonstrated that the expression of H type 1 carbohydrates in the normal colon is under the control of FUT2 gene-encoded fucosyltransferase. However the aberrant expressions of H type 2 and H type 3/4 carbohydrates in colon cancer tissues of secretors is regulated by the same enzyme.57 The reasons underlying these changes have not been clarified. It has been pointed out that a relative down-regulation of glycosyltransferases, the loss of heterozygosity as well as hypermethylation of gene promoters are possible events involved in this process.58
Some of the histo-blood group carbohydrates have high immunogenicity and play an important role in histocompatibility. A and B carbohydrates from ABO histo-blood group system present in the vascular endothelium react with the potent natural anti-A, anti-B, and anti-A,B antibodies activating the complement system and increasing the risk of antibody-mediated rejection of solid organ transplantations.59 However, transplantation of solid organs from ABO incompatible donors has provided promising results. It has been suggested that distinct structural differences and antigenicity of the carbohydrates present in the vascular endothelium compared to red blood cells can modulate the immune response of the recipient thus affecting engraftment.60 However, these structural differences, as well as immune modulator mechanisms of accommodation, have not been clarified.
The increasing knowledge about the structural diversity of histo-blood group carbohydrates has contributed to the development of new technologies applied to transfusion medicine, cancer and therapy. The insertion of function spacer lipid constructs allows the creation of red blood cells with a controlled amount of carbohydrate for use in laboratory quality control of common and rare ABO and Lewis histo-blood group phenotypes.61 This technology facilitates the evaluation of monoclonal antibody performance in routine procedures.18 Additionally, it improves our knowledge of many basic aspects of hemolytic transfusions in animal models.62
Knowing the biological implications of ABO, H, secretor and Lewis histo-blood group systems in diseases can provide the basis for new therapeutic applications. Anti-adhesion therapy provides an opportunity to use histo-blood group carbohydrates in the treatment of infections; blocking adhesion to cells expressing these carbohydrates is an alternative strategy to antibiotics. These strategies may be useful in cases of microbial resistance to antibiotics and chemotherapy especially in patients being treated for long periods. This form of therapy can have desirable effects at a lower cost than the production of specific antibiotics and vaccines, including in cases where vaccination is still not satisfactory.63, 64 Additionally, the use of carbohydrate microarrays is one of the strategies used to explore potential natural ligands of antitumor monoclonal antibodies which allow cancer subtyping toward identifying targets for immunotherapy.65
Concluding remarks
The expression of ABO, H, Lewis and secretor histo-blood group carbohydrates is capable of producing at least three biological effects of medical importance: structural modification of precursor oligosaccharides, expression of a distinct carbohydrate tissue profile and potent natural antibodies.15 These effects influence susceptibility to infections, since these carbohydrates act as receptors for microorganisms or other substances (toxins, allergens, etc.).9 Therefore these biological events represent a vast field for medical research and technologies.
The tissue expression of ABH-Lewis antigens is more complex than it appears when strictly analyzed from red blood cell phenotypes. Genes encoding FUTI, FUTII, FUTIII, GTA and GTB are responsible for the qualitative and quantitative variability of these antigens in mucosal and exocrine secretions.15, 16 Therefore, the different polymorphic levels of ABO, H, secretor and Lewis histo-blood group systems may have greater biological importance than it seems from the mere presence of their antigens in the red blood cell membrane.2, 15
There has been growing evidence that ABO, H, secretor and Lewis histo-blood group systems are not neutral polymorphisms as they influence susceptibility to infections, disease progression and innate immune response.43, 52 Despite the knowledge of some structural variability in these carbohydrate antigens, their polymorphic levels of expression and potential applications in modern and personalized medicine in the “-omics” era, little is known about their biological importance programmed in nature. New studies to understand the relationship of these systems with microorganisms and the environment may contribute to our understanding of the evolutionary pressure that created and maintains the high variability of polymorphisms in human beings.
Financial support
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES (Grant 1542-03-6) and São Paulo Research Foundation – FAPESP (Grants: 2009/17540-2; 2011/08075-4).
Conflicts of interest
The author declares no conflict of interest.
References
- 1.Chow J., Lee S.M., Shen Y., Khosravi A., Mazmanian S.K. Host-bacterial symbiosis in health and disease. Adv Immunol. 2010;107:243–274. doi: 10.1016/B978-0-12-381300-8.00008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henry S.M. Molecular diversity in the biosynthesis of GI tract glycoconjugates. A blood group related chart microorganism receptors. Transfus Clin Biol. 2001;8:226–230. doi: 10.1016/s1246-7820(01)00112-4. [DOI] [PubMed] [Google Scholar]
- 3.Cash H.L., Whitham C.V., Behrendt C.L., Hooper L.V. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maynard C.L., Elson C.O., Hatton R.D., Weaver C.T. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489:231–241. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imberty A., Varrot A. Microbial recognition of human cell surface glycoconjugates. Curr Opin Struct Biol. 2008;18:567–576. doi: 10.1016/j.sbi.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Anstee D.J. The relationship between blood groups and disease. Blood. 2010;115:4635–4643. doi: 10.1182/blood-2010-01-261859. [DOI] [PubMed] [Google Scholar]
- 7.Varki A., Gagneux P. Multifarious roles of sialic acids in immunity. Ann N Y Acad Sci. 2012;1253:16–36. doi: 10.1111/j.1749-6632.2012.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kato K., Ishiwa A. The role of carbohydrates in infection strategies of enteric pathogens. Trop Med Health. 2015;43:41–52. doi: 10.2149/tmh.2014-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooling L. Blood groups in infection and host susceptibility. Clin Microbiol Rev. 2015;28:801–870. doi: 10.1128/CMR.00109-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gowda A.S., Madhunapantula S.V., Achur R.N., Valiyaveettil M., Bhavanandan V.P., Gowda D.C. Structural basis for the adherence of Plasmodium falciparum-infected erythrocytes to chondroitin 4-sulfate and design of novel photoactivable reagents for the identification of parasite adhesive proteins. J Biol Chem. 2007;282:916–928. doi: 10.1074/jbc.M604741200. [DOI] [PubMed] [Google Scholar]
- 11.Björnham O., Bugaytsova J., Borén T., Schedin S. Dynamic force spectroscopy of the Helicobacter pylori BabA-Lewis b binding. Biophys Chem. 2009;143(1–2):102–105. doi: 10.1016/j.bpc.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Parra G.I., Abente E.J., Sandoval-Jaime C., Sosnovtsev S.V., Bok K., Green K.Y. Multiple antigenic sites are involved in blocking the interaction of GII.4 norovirus capsid with ABH histo-blood group antigens. J Virol. 2012;86:7414–7426. doi: 10.1128/JVI.06729-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clausen H., Hakomori S.I. ABH and related histoblood group antigens; immunochemical differences in carrier isotypes and their distribution. Vox Sang. 1989;56:1–20. doi: 10.1111/j.1423-0410.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 14.Nydegger U.E., Tevaearai H., Berdat P., Rieben R., Carrel T., Mohacsi P. Histo-blood group antigens as allo-and autoantigens. An N Y Ac Sci. 2005;1050:40–51. doi: 10.1196/annals.1313.006. [DOI] [PubMed] [Google Scholar]
- 15.Henry S.M., Samuelsson B. ABO polymorphisms and their putative biological relationships with disease. In: King M.-J., editor. Human blood cells. Consequences of genetic polymorphisms and variations. Imperial College Press; 2000. pp. 15–103. [Google Scholar]
- 16.Oriol R., Abo H.h. Lewis and secretion: serology, genetics and tissue distribution. In: Cartron J.P., Rouger P., editors. Blood cell biochemistry: molecular basis of human blood group antigens. Plenum; New York: 1995. pp. 37–73. [Google Scholar]
- 17.Previato M., Borim M.P., Liberatore R.D., Jr., Pires A.C., Dias M.A., Brandão de Mattos C.C. Lewis histo-blood group system phenotyping and genotyping reveal divergence in the association of Le(a−b−) phenotype and type 1 diabetes. Vox Sang. 2015;108:281–286. doi: 10.1111/vox.12211. [DOI] [PubMed] [Google Scholar]
- 18.Williams E., Korchagina E., Frame T., Ryzhov I., Bovin N., Henry S. Glycomapping the fine specificity of monoclonal and polyclonal Lewis antibodies with type-specific Lewis kodecytes and function-spacer-lipid constructs printed on paper. Transfusion (Paris) 2016;56:325–333. doi: 10.1111/trf.13384. [DOI] [PubMed] [Google Scholar]
- 19.Mujahid A., Dickert F.L. Blood group typing: from classical strategies to the application of synthetic antibodies generated by molecular imprinting. Sensors (Basel) 2015;16 doi: 10.3390/s16010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mourant A.E. Oxford; London: 1983. Blood relations: blood groups and anthropology; pp. 13–20. [Google Scholar]
- 21.Henry S., Oriol R., Samuelsson B. Lewis histo-blood group system and associated secretory phenotypes. Vox Sang. 1995;69:166–182. doi: 10.1111/j.1423-0410.1995.tb02591.x. [DOI] [PubMed] [Google Scholar]
- 22.Morgan W.T.J., Watkins W.M. Unravelling the biochemical basis of blood group ABO and Lewis antigenic substances. Glycoconj J. 2000;17(7–9):501–530. doi: 10.1023/a:1011014307683. [DOI] [PubMed] [Google Scholar]
- 23.Schenkel-Brunner H. Springer; Wien: 2000. Human blood groups: chemical and biochemical basis of antigen specificity; pp. 54–248. [Google Scholar]
- 24.Angstrom J., Larsson T., Hansson G.C., Karlsson K., Henry S. Default biosynthesis pathway for blood group-related glycolipids in human small intestine as defined by structural identification of linear and branched glycosilceramides in a group O Le(a−b−) nonsecretor. Glycobiology. 2004;14:1–12. doi: 10.1093/glycob/cwh003. [DOI] [PubMed] [Google Scholar]
- 25.Gillver L.G., Henry S.M. Review: biochemistry of carbohydrate blood group antigens. Immunohematology. 2003;19:33–42. [PubMed] [Google Scholar]
- 26.Gagnon S.M., Meloncelli P.J., Zheng R.B., Haji-Ghassemi O., Johal A.R., Borisova S.N. High resolution structures of the human ABO(H) blood group enzymes in complex with donor analogs reveal that the enzymes utilize multiple donor conformations to bind substrates in a stepwise manner. J Biol Chem. 2015;290:27040–27052. doi: 10.1074/jbc.M115.682401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oriol R., Le Pendu L., Mollicone R. Genetics of ABO, H, Lewis, X and related antigens. Vox Sang. 1986;51:161–171. doi: 10.1111/j.1423-0410.1986.tb01946.x. [DOI] [PubMed] [Google Scholar]
- 28.Henry S.M. Review: phenotyping for Lewis and secretor histo-blood group antigens. Immunohematology. 1999;12:51–61. [PubMed] [Google Scholar]
- 29.Oriol R. Genetic control of the fucosylation of ABH precursor chains. Evidence for new epistatic interactions in different cells and tissues. J Immunogenet. 1990;17(4–5):235–245. doi: 10.1111/j.1744-313x.1990.tb00877.x. [DOI] [PubMed] [Google Scholar]
- 30.Mollicone R., Le Pendu J., Bara J., Oriol R. Heterogeneity of the ABH antigens determinants expressed in human pyloric and duodenal mucosae. Glycoconjugate J. 1986;3:187–202. [Google Scholar]
- 31.Holgersson J., Breimer B.E., Samuelsson B. Basic biochemistry of cell surface carbohydrates and aspects of the tissue distribution of histo-blood ABH and related glycosphingolipids. APMIS Suppl. 1992;27:18–27. [PubMed] [Google Scholar]
- 32.Lindén S., Mahdavi J., Semino-Mora C., Olsen C., Carlstedt I., Borén T. Role of ABO secretor status in mucosal innate immunity and H. pylori infection. PLoS Pathog. 2008;4:e2. doi: 10.1371/journal.ppat.0040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Svensson L., Rydberg L., Hellberg A., Gilliver L.G., Olsson M.L., Henry S.M. A novel glycolipid variations revealed by monoclonal antibody immunochemical analysis of weak ABO subgroups of A. Vox Sang. 2005;89:27–38. doi: 10.1111/j.1423-0410.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- 34.Svensson L., Rydberg L., de Mattos L.C., Henry S.M. Blood group A1 and A2 revisited: an immunochemical analysis. Vox Sang. 2009;96:56–61. doi: 10.1111/j.1423-0410.2008.01112.x. [DOI] [PubMed] [Google Scholar]
- 35.Henry S., Jovall P.A., Ghardashkhani S., Elmgren A., Martinsson T., Larson G. Structural and immunochemical identification of Le(a), Le(b), H type 1, and related glycolipids in small intestinal mucosa of a group O Le(a−b−) nonsecretor. Glycoconj J. 1997;14:209–223. doi: 10.1023/a:1018541821819. [DOI] [PubMed] [Google Scholar]
- 36.Mielke J.H., Konigsberg L.W., Relethford J.H. Oxford University Press; New York: 2006. Human biological variation. [Google Scholar]
- 37.Haldane J.B.S. Disease and evolution. La Ricerca Scientifica. 1949;19(suppl A19):68–76. [Google Scholar]
- 38.Pfennig K.S. Evolution of pathogen virulence: the role of variation in host phenotype. Proc Biol Sci. 2001;268:755–760. doi: 10.1098/rspb.2000.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahim N.G., Harismendy O., Topol E.J., Frazer K.A. Genetic determinants of phenotypic diversity in humans. Genome Biol. 2008;9:215. doi: 10.1186/gb-2008-9-4-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oriol R., Mollicone R., Couillin P., Dalix A.M., Candelier J.J. Genetic regulation of the expression of ABH and Lewis antigens in tissues. APMIS. 1992;27:28–38. [PubMed] [Google Scholar]
- 41.Karlsson K.A. Microbial recognition of target-cell glycoconjugates. Curr Opin Struct Biol. 1995;5:622–635. doi: 10.1016/0959-440x(95)80054-9. [DOI] [PubMed] [Google Scholar]
- 42.Hooper L.V., Gordon J.I. Glycans as legislator of host–microbial interaction: spanning the spectrum from symbiosis to pathogenicity. Glycobiology. 2001;11:1R–10R. doi: 10.1093/glycob/11.2.1r. [DOI] [PubMed] [Google Scholar]
- 43.Fumagalli M., Cagliani R., Pozzoli U., Riva S., Comi G.P., Menozzi G. Widespread balancing selection and pathogen-driven selection at blood group antigen genes. Genome Res. 2009;19:199–212. doi: 10.1101/gr.082768.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blackwell C.C., Wier D.M., Braum J.M., Almadani O.M., Busuttil A. Blood group phenotypes and infectious diseases. In: Belamy R., editor. Susceptibility to infectious diseases: the importance of the host genetics. Cambridge University Press; New York: 2004 p. pp. 309–336. [Google Scholar]
- 45.Ilver D., Arnqvist A., Ogren J., Frick I.M., Kersulyte D., Incecik E.T. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279:373–377. doi: 10.1126/science.279.5349.373. [DOI] [PubMed] [Google Scholar]
- 46.Aspholm-Hurtig M., Dailide G., Lahmann M., Kalia A., Ilver D., Roche N. Functional adaptation of BabA, the H. pylori ABO blood group antigen binding adhesin. Science. 2004;305:519–522. doi: 10.1126/science.1098801. [DOI] [PubMed] [Google Scholar]
- 47.Glass R.I., Holmgren J., Haley C.E., Khan M.R., Svennerholm A.M., Stoll B.J. Predisposition for cholera of individuals with O blood group. Possible evolutionary significance. Am J Epidemiol. 1985;121:791–796. doi: 10.1093/oxfordjournals.aje.a114050. [DOI] [PubMed] [Google Scholar]
- 48.Vasile F., Reina J.J., Potenza D., Heggelund J.E., Mackenzie A., Krengel U. Comprehensive analysis of blood group antigen binding to classical and El Tor cholera toxin B-pentamers by NMR. Glycobiology. 2014;24:766–778. doi: 10.1093/glycob/cwu040. [DOI] [PubMed] [Google Scholar]
- 49.Cserti C.M., Dzik W.H. The ABO blood group system and Plasmodium falciparum malaria. Blood. 2007;110:2250–2258. doi: 10.1182/blood-2007-03-077602. [DOI] [PubMed] [Google Scholar]
- 50.Ruiz-Palacios G.M., Cervantes L.E., Ramos P., Chavez-Munguia B., Newburg D.S. Campylobacter jejuni binds intestinal H(O) antigen (Fuc alpha 1, 2 Gal beta 1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J Biol Chem. 2003;278:14112–14120. doi: 10.1074/jbc.M207744200. [DOI] [PubMed] [Google Scholar]
- 51.Lindesmith L., Moe C., Marionneau S., Ruvoen N., Jiang X., Lindblad L. Human susceptibility and resistance to Norwalk virus infection. Nat Med. 2003;9:548–553. doi: 10.1038/nm860. [DOI] [PubMed] [Google Scholar]
- 52.Heneghan M.A., Moran A.P., Feeley K.M., Egan E.L., Goulding J., Connolly C.E. Effect of host Lewis and ABO blood group antigen expression on Helicobacter pylori colonisation density and the consequent inflammatory response. FEMS Immunol Med Microbiol. 1998;20:257–266. doi: 10.1111/j.1574-695X.1998.tb01135.x. [DOI] [PubMed] [Google Scholar]
- 53.Alkout A.M., Blackwell C.C., Weir D.M. Increased inflammatory responses of persons of blood group O to Helicobacter pylori. J Infect Dis. 2000;181:1364–1369. doi: 10.1086/315375. [DOI] [PubMed] [Google Scholar]
- 54.Aird I., Bentall H.H., Roberts J.A. A relationship between cancer of stomach and the ABO blood groups. Br Med J. 1953;1:799–801. doi: 10.1136/bmj.1.4814.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolpin B.M., Chan A.T., Hartge P., Chanock S.J., Kraft P., Hunter D.J. ABO blood group and the risk of pancreatic cancer. J Natl Cancer Inst. 2009;101:424–431. doi: 10.1093/jnci/djp020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee S.J., Ro J.Y., Sahin A.A., Hong W.K., Brown B.W., Mountain C.F. Expression of blood-group antigen A — a favorable prognostic factor in non-small-cell lung cancer. N Engl J Med. 1991;324:1084–1090. doi: 10.1056/NEJM199104183241603. [DOI] [PubMed] [Google Scholar]
- 57.Fujitani N., Liu Y., Toda S., Shirouzu K., Okamura T., Kimura H. Expression of H type 1 antigen of ABO histo-blood group in normal colon and aberrant expressions of H type 2 and H type 3/4 antigens in colon cancer. Glycoconj J. 2000;17:331–338. doi: 10.1023/a:1007173722426. [DOI] [PubMed] [Google Scholar]
- 58.Dabelsteen E., Gao S. ABO blood-group antigens in oral cancer. J Dent Res. 2005;84:21–28. doi: 10.1177/154405910508400103. [DOI] [PubMed] [Google Scholar]
- 59.Mengel M., Husain S., Hidalgo L., Sis B. Phenotypes of antibody-mediated rejection in organ transplants. Transpl Int. 2012;25:611–622. doi: 10.1111/j.1432-2277.2012.01484.x. [DOI] [PubMed] [Google Scholar]
- 60.Aikawa A., Saito K., Takahashi K. Trends in ABO-incompatible kidney transplantation. Exp Clin Transplant. 2015;13(Suppl 1):18–22. [PubMed] [Google Scholar]
- 61.Henry S.M. Modification of red blood cells for laboratory quality control use. Curr Opin Hematol. 2009;16:467–472. doi: 10.1097/MOH.0b013e328331257e. [DOI] [PubMed] [Google Scholar]
- 62.Oliver C., Blake D., Henry S. Modeling transfusion reactions and predicting in vivo cell survival with kodecytes. Transfusion (Paris) 2011;51:1723–1730. doi: 10.1111/j.1537-2995.2010.03034.x. [DOI] [PubMed] [Google Scholar]
- 63.Sunasee R., Adokoh C.K., Darkwa J., Narain R. Therapeutic potential of carbohydrate-based polymeric and nanoparticle systems. Expert Opin Drug Deliv. 2014;11:867–884. doi: 10.1517/17425247.2014.902048. [DOI] [PubMed] [Google Scholar]
- 64.Wang Q., Ling C.C. Addressing the global need to combat multidrug resistance: carbohydrates may hold the key. Future Med Chem. 2014;6:1539–1543. doi: 10.4155/fmc.14.109. [DOI] [PubMed] [Google Scholar]
- 65.Wang D., Tang J., Liu S., Huang J. Carbohydrate microarrays identify blood group precursor cryptic epitopes as potential immunological targets of breast cancer. J Immunol Res. 2015;2015:510810. doi: 10.1155/2015/510810. [DOI] [PMC free article] [PubMed] [Google Scholar]