Abstract
Recent clinical and preclinical research suggests that cannabidiol (CBD) and Δ9-tetrahydrocannabinol (Δ9-THC) have interactive effects on measures of cognition; however, the nature of these interactions is not yet fully characterized. To address this, the effects of Δ9-THC and CBD were investigated independently and in combination with proposed therapeutic dose ratios of 1:1 and 1:3 Δ9-THC:CBD in adult rhesus monkeys (n=6) performing a stop signal task (SST). Additionally, the development of tolerance to the effects of THC on SST performance was evaluated by determining the effects of acutely administered Δ9-THC (0.1-3.2 mg/kg), during a 24-day chronic Δ9-THC treatment period with Δ9-THC alone or with CBD. Results indicate that Δ9-THC (0.032 - 0.32 mg/kg) dose-dependently decreased ‘go’ success but did not alter ‘go’ reaction time or stop signal reaction time (SSRT); CBD (0.1-1.0 mg/kg) was without effect on all measures and, when co-administered in a 1:1 dose-ratio, did not exacerbate or attenuate the effects of Δ9-THC. When co-administered in a 1:3 dose-ratio, CBD (1.0 mg/kg) attenuated the disruptive effects of 0.32 mg/kg Δ9-THC but did not alter the effects of other Δ9-THC doses. Increases in ED50 values for the effects of Δ9-THC on SST performance were apparent during chronic Δ9-THC treatment, with little evidence for modification of changes in sensitivity by CBD. These results indicate that CBD, when combined with THC in clinically available dose-ratios does not exacerbate and, under restricted conditions, may even attenuate Δ9-THC’s behavioral effects.
Keywords: Δ9-Tetrahydrocannabinol (Δ9-THC), Cannabidiol (CBD), Stop Signal Reaction Time (SSRT), Stop Signal Task, Tolerance
INTRODUCTION
The therapeutic efficacy of medical marijuana is thought to be mediated by Δ9-tetrahydrocannabinol (Δ9-THC), the primary psychoactive component of cannabis. Δ9-THC exerts its appetite-stimulant, anti-nauseant, and anti-emetic effects (Pertwee & Thomas, 2009) by activating CB1 receptor-based mechanisms (Pertwee, 2008). The utility of Δ9-THC, however, is hindered by the development of tolerance and undesirable effects, including impaired motor coordination and disruptions in cognitive performance (Hall & Solowij, 1998; Karila et al., 2014; Solowij et al., 2002). Due to the growing legal acceptance of medical marijuana, identifying therapeutics that maintain efficacy but are devoid of the unwanted effects of Δ9-THC remains a goal of medications development.
Cannabidiol (CBD), the second most abundant constituent of marijuana, may also have medicinal value (Maa & Figi, 2014; Robson, 2005). However, the nature of CBD’s activity in the endocannabinoid system is unclear. Though its agonist/antagonist profile has not been well characterized at CB1 receptors (for which it has low affinity), CBD has been reported to have pharmacological effects as an inverse agonist at CB2 receptors, as well as agonist activity at the 5HT1a receptor with similar efficacy to 5-HT in increasing GTPYS binding (Pertwee, 2008; Russo, Burnett, Hall, and Parker, 2005).
Recent evidence suggests that CBD may modulate some of the medicinal and behavioral effects of Δ9-THC (see Russo & Guy, 2006, for review). In this regard, Sativex®, a nasal spray consisting of a 1:1 ratio of Δ9-THC: CBD has been developed for the treatment of muscle spasms induced by multiple sclerosis (MS). Anecdotal reports suggest that Sativex® decreases spasticity in MS patients with a relatively mild side effect profile and no sign of tolerance in patients undergoing a chronic treatment regimen (reviewed by Robson, 2005). Further, recent evidence in clinical and preclinical studies provide support that CBD may counteract some of the unwanted effects of Δ9-THC. In one naturalistic study, cannabis smokers who smoked high-CBD containing marijuana cigarettes had no memory impairments in a prose recall task whereas those who smoked low-CBD cigarettes showed marked task impairment (Morgan, Schafer, Freeman, and Curran, 2010). Other dose-controlled studies have yielded mixed results. In one study, administration of 600 mg oral CBD attenuated Δ9-THC (1.5 mg intravenous) induced paranoia and episodic memory deficits in humans (Englund et al., 2013), whereas recent evidence suggests that up to 800 mg oral CBD has no effect on the abuse-related subjective effects of smoked cannabis (Babalonis et al., 2015).
Studies in laboratory animals have also reported somewhat inconsistent interactions between Δ9-THC and CBD. In rhesus monkeys, CBD (0.5 mg/kg) has been shown to attenuate Δ9-THC-induced impairments in visuo-spatial memory (Wright Jr., Vandewater, and Taffe, 2013a) or, after a high dose of 30 mg/kg CBD, the rate-decreasing effects of 3.2 mg/kg Δ9-THC (Brady & Balster 1980). On the other hand, CBD did not alter Δ9-THC-induced deficits in motor coordination or progressive-ratio responding in rhesus monkeys (Wright et al. 2013a) and, in rats, did not affect Δ9-THC-induced hypolocomotion, hypothermia, or anxiety-like effects (Klein et al. 2011; Taffe et al. 2015). However, after 11 days of treatment with equal parts THC: CBD under an increasing chronic dosing regimen (1.0 - 3.0 mg/kg), rats showed larger decreases in social interaction compared to the social interaction decreases seen with Δ9-THC alone (Klein et al. 2011). Thus, the aggregate of previous research suggests that, although there is some suggestion that CBD interacts with selected effects of Δ9-THC, the interaction between these compounds seems to be dependent on both the assay and the length of time over which the compounds are being studied.
The SST measures the ability of the subject to withhold a prepotent response that has been initiated when a distinct ‘stop signal’ appears; the failure to inhibit a response is thought to reflect a cognitive deficit (motoric inhibition) common to many psychiatric disorders (Logan, 1994; Verbruggen & Logan, 2008). Based on prior studies in human subjects, it appears that Δ9-THC impairs motor inhibition in the SST, but that the effects of cannabis products on inhibitory control are still unclear (McDonald, Schleifer, Richards, & de Wit, 2003; Ramaekers et al., 2009; Wrege et al., 2014). The present study was conducted to further evaluate the effects of Δ9-THC on cognitive performance by determining whether THC-induced changes in SST performance in nonhuman primates are altered by acute or chronic administration of CBD. First, the acute effects of Δ9-THC, CBD, and their combination in two dose ratios (1:1 and 1:3) on SST performance were determined. Second, in view of the long term dosing regimens utilized for most applications of medicinal cannabis, studies were conducted to examine the effects of acute doses of Δ9-THC on SST performance during chronic treatment (24 days) with Δ9-THC, alone or in combination with CBD.
METHODS
Subjects
Adult male (n=3) and female (n=3) rhesus macaques (Maccaca mulatta) that weighed between 5-9 kg served as subjects in the present study. All subjects were gonadally intact. One subject was drug naïve; the other five subjects had a history of cocaine or nicotine self-administration and/or discrimination, and were drug-free for at least 2 months before drug testing. All monkeys were housed singly in stainless steel cages that provided olfactory, visual, and auditory interaction with conspecifics. A nutritionally balanced diet (5045 High Protein Monkey Diet, Purina Mills International INC., Brentwood, MO) supplemented with fresh fruit or vegetables and vitamins, was provided daily. Water was available ad libitum from an automatic watering system. Subjects also could earn 1-g banana-flavored pellets (Formula 4TUR banana flavor, grain-based pellet; Purina Mills Test Diet, Richmond, IN) during daily experimental sessions. A 12-hr light-dark cycle was in effect (lights on 0800 – 2000 hr) at all times.
Animal maintenance and research followed the guidelines provided by the Institute of Laboratory Animal Resources (ILAR-NRC, 2010) and the NIH Office of Laboratory Animal Welfare (OLAW). The Institutional Animal Care and Use Committee at McLean Hospital approved all experimental protocols. The research facility is licensed by the U.S. Department of Agriculture and consultant veterinarians monitored the health of the colony. Enrichment was provided through access to mirrors and toys in the home-cage, television or music, interaction with technical staff, and the opportunity to manipulate their environment during operant sessions (Line, 1989).
Apparatus and Task Procedure
Apparatus
Subjects were seated in standard nonhuman primate chairs (Model 515-SASR, PLAS Labs, Inc., Lansing, MI). Sessions were conducted in custom-built experimental chambers equipped with white noise and, facing the subject, a wall-mounted operant response panel. Operant panels contained three square translucent response keys (5.1cm×5.1cm) arranged 3.5 cm apart in a horizontal row 9 cm from the top of the operant panel. Each key could be transilluminated with red or green stimulus lights (SuperBright LEDs; Fairchild Semiconductor, San Jose, CA). Three circular translucent keys (1.9 cm in diameter) were located in a vertical column below the center response key and could also be transilluminated by yellow, red, or green stimulus lights. An externally mounted pellet dispenser delivered 1-g banana-flavored food pellets to a food tray directly below the response panel. Scheduling of stimulus presentation and recording of responses was accomplished with MED-PC IV (MED Associates, Georgia, VT) on a Hewlett-Packard (model 8100 Elite CMT PC) desktop PC.
Stop Signal Task
Stop signal task (SST) training procedures were based on previously published studies in rhesus macaques (Liu, Heitz, and Bradberry, 2009). Briefly, subjects were trained to respond within 1-sec on the center response key in the presence of a green (i.e., ‘go’ trial) stimulus light and withhold a response on the center response key in the presence of a red stimulus light (i.e., ‘stop’ stimulus) for food reinforcement. A correct trial initiated a 5-sec timeout (TO) period in which the green stimulus light was off and a yellow cue light below the center response key was illuminated, concurrent with the delivery of a banana flavored food pellet; the yellow cue light was turned off by the end of the TO. Incorrect trials resulted in a 10-sec TO during which all stimulus lights were extinguished and no food pellet was delivered. Responding during the TO periods reset the timer.
Once subjects responded reliably in the presence of the green stimulus light under the fixed-ratio 1 (FR1) condition, the response requirement was gradually increased over several sessions such that three successive correct ‘go’ trials (i.e., FR3) were required for food pellet delivery; incorrect ‘go’ trials reset the count to zero. ‘Stop’ trials were then randomly inserted and when task performance was stable under the separate ‘go’ and ‘stop’ stimulus conditions (‘go’ success ≥ 80% and ‘stop’ success ≥ 50% for three of four consecutive training sessions) a stop signal delay (SSD) contingency was introduced. Under this contingency, approximately 15% of ‘go’ trials resulted in the green (‘go’) stimulus light changing, within a specified time (<1-sec), to the red stimulus lights for 2-sec requiring the subject to withhold the response. During SSD trials, reinforcement was delivered if no response was made within the 2-sec red stimulus light presentation. A response during SSD trials resulted in a 10-sec TO, as described above. The SSD was systematically manipulated on an individual basis throughout each session using a 25-msec staircase procedure, (i.e., increased and decreased in 25-msec increments after, respectively, correct and incorrect SSD trials). Stable responding for SSD starting values was defined for individuals by an overall mean SSD success of 50 ± 10% for a block of three out of four consecutive training sessions; this SSD value was then used as the starting SSD for each individual throughout the remainder of the study.
Terminal conditions of the SST consisted of four cycles of interspersed FR3 ‘go’ and SSD trials within a single session. Sessions began between 0930 and 1130. Each cycle started with a 30-min TO period followed by an 8-min response period during which schedule contingencies were in effect and subjects could earn a maximum of 20 pellets (80 total pellets/session). The SSD was reset to each subject’s baseline at the beginning of each cycle. If 20 pellets were earned before the 8-min response period ended, all lights were extinguished for the remainder of the cycle and responding had no scheduled consequences.
Drug Testing Procedures
Pre-Chronic
During the pre-chronic phase, acute drug tests were separated by at least four days; an interval that has previously been used to assess the effects of cannabinoids on cognitive performance in rhesus monkeys (Wright et al., 2013a). Drug tests were only performed following a control session in which a subject met a stability criterion of over 80% go accuracy for all cycles. All drug tests were performed once in each subject (i.e. four tests/subject). Otherwise, training, or as relevant, control test sessions continued once daily (5-6 days per week). Δ9-THC was studied over a range of doses selected on the basis of previous studies (McMahon, 2011; Winsauer, Lambert, and Moerschbaecher, 1999). Doses of CBD were selected on the basis of clinically available or proposed preparations of CBD combined with Δ9-THC. A vehicle injection preceded the first cycle of each test session, followed by cumulative doses of each drug or drug combination during subsequent TO periods. If responding was suppressed (i.e., < 4 correct ‘go’ trials) during any cycle, the next cumulative dose was not administered but the session continued until the four cycles were completed.
Chronic
Insofar as was possible, subjects were divided into two matched groups for chronic studies (Δ9-THC group and Δ9-THC+CBD group) based on the results of pre-chronic drug testing for the dependent variables of ‘go’ success percentage, ‘go’ reaction time, and SSRT. Chronic treatment consisted of three successive phases of progressively increasing daily drug dosage without washout, separated by one day during which a Δ9-THC dose-effect curve was determined (see below). All subjects received treatments at 0600 hours in Phase 1 (Days 1-4), and in divided treatments at 0600 hours & 1800 hours in Phases 2 (Days 6-11) and 3 (Days 13-23). The interval between the daily dose administration and training sessions was approximately 3-5 hours which limited the direct effects of Δ9-THC on training task performance (based on the reported THC half-life of 120 min in rhesus monkeys; Ginsburg et al., 2014).
On test days no chronic dose was administered and, instead, the effects of cumulative doses of Δ9-THC (0.1-3.2 mg/kg) in the SST were determined. The interval between the chronic dose administration and test sessions was approximately 15.5-17.5 hours. The limited hold on ‘go’ trials was increased to 2 sec for two subjects during Phase 3 of the experiment because, during Phase 2, one subject in each group (Mm 066 ♀ & 8189 ♂) failed to respond during training sessions.
Data Analysis
The principle dependent variables were ‘go’ trial success, ‘go’ reaction time, and SSRT. ‘Go’ trial success was calculated as the number of correct ‘go’ trials divided by the total ‘go’ trials presented for a given cycle. A success of 0% was used for instances in which a subject did not respond. Average ‘go’ trial reaction time was calculated as the latency to respond following the onset of the ‘go’ stimulus during correct ‘go’ trials and transformed to change from control for each subject. This method of reaction time analysis corresponds with those used in previous studies in rhesus monkeys under similar SST contingencies (Liu et al., 2009). SSRT was calculated by subtracting the mean ‘go’ trial reaction time from the mean SSD value.
Trials in which subjects did not respond within the limited hold were excluded from ‘go’ reaction time analysis. Likewise, drug testing cycles in which a subject displayed 100% ‘stop’ trial success, i.e. when subjects did not respond, were excluded from SSRT analysis. The exclusion criteria were used because, in addition to decreasing reaction speed, high doses of Δ9-THC produce other behavioral effects that may impede task performance (e.g. effects on motivation or attention) that may artefactually increase ‘go’ reaction time and SSRT if a maximal value (i.e., 1 sec) was used.
Analysis
Average values for ‘go’ trial success, ‘go’ reaction time, and SSRT during each cycle on sessions prior to drug tests were used as control values. To assess the effects of Δ9-THC and CBD alone on SST performance during the pre-chronic treatment period of the study, one-way repeated measures ANOVA with Dunnett’s post hoc comparisons were used to determine the statistical significance of drug effects from control (cycle 1 of test session). To assess the effects of CBD and THC combinations (i.e., 1:1 and 1:3) compared to THC alone and control sessions, a two-way repeated measures ANOVA with factors of Treatment and Cycle (i.e., dose) was used, and Holm-Sidak post hoc tests were performed where appropriate. For data sets in which data points were excluded because subjects did not respond (i.e. pre-chronic ‘go’ reaction time change and SSRT; see above), linear regression was performed on dose effect curves with individual data points. Significance was set at p < .05 and all statistical tests were performed using GraphPad Prism (Version 6.0).
Data obtained during the chronic treatment period of the study for ’go’ success percentage, ‘go’ reaction time change from control, and SSRT are presented as individual and/or grouped data. While small sample size precluded ANOVA analysis, ED50 values were calculated to determine the dose of test drugs that disrupted group mean ‘go’ trial success to 50% in Δ9-THC test sessions using log-linear interpolation and then converted to linear values for data presentation and comparison to pre-chronic Δ9-THC ED50 values. ED50 values were not determined when group responding did not decrease below 50% success after the highest Δ9-THC dose; in these cases a conservative estimate of the highest Δ9-THC dose tested (3.2 mg/kg) was used as the ED50 estimate and changes from pre-chronic performance are presented as greater than (>).
Drugs
Δ9-THC was generously supplied by the NIDA Drug Supply Program. Cannabidiol (CBD) was synthesized by S. Nikas at the Center for Drug Discovery, Northeastern University. Both drugs were prepared in a vehicle of 95% ethanol, Alkamuls® EL 620, and saline in a 1:1:18 ratio. All testing session drug injections were administered intramuscularly (i.m.) and administered 30-min before a cycle.
RESULTS
Control SST Performance
All subjects achieved criterion levels of SST performance during the training phase (range 11 – 19 sessions). ’Go’ success percentage and SSRT performance was similar across cycles and between control test sessions (all F’s < (1.47), p’s > .18); however, ‘go’ reaction time slightly slowed by about 35 msec in Cycle 4 compared to Cycle 1 (F(3,15)=4.08, p = .03). Overall mean performance during control was 94 ± 1.8% for the ‘go’ trial success percentage, 636 ± 50 msec for the ‘go’ trial reaction time, and 245 ± 28 msec for the SSRT.
Pre-chronic Drug Tests
Δ9-Tetrahydrocannabinol (Δ9-THC)
As shown in Figure 1 (left panels), Δ9-THC produced dose-related decreases in ‘go’ trial success percentage (F(3,15) = 7.59; p<0.01, RM ANOVA; top panel) while reaction time was not changed (F(1,12)=0.78, p = .39, linear regression; bottom panel). The highest cumulative dose of Δ9-THC, 0.32 mg/kg, decreased ‘go’ trial success to approximately 25% from 87% (p = 0.001, Dunnett), whereas doses of Δ9-THC below 0.32 mg/kg did not significantly alter ’go’ success. Similarly, doses of Δ9-THC below 0.32 mg/kg did not significantly alter SSRT (F(1,9)=0.01, p = .94, linear regression; Table 1). The 0.32 mg/kg Δ9-THC dosage suppressed responding to below 50% in five of the six subjects, precluding quantification of a reliable value for SSRT.
Figure 1. ‘Go’ trial success (±SEM) and reaction time change (±SEM) during pre-chronic tests with Δ9-THC and CBD (Left Panels), or 1 Δ9-THC: 1 CBD, and 1 Δ9-THC: 3 CBD (Right Panels).
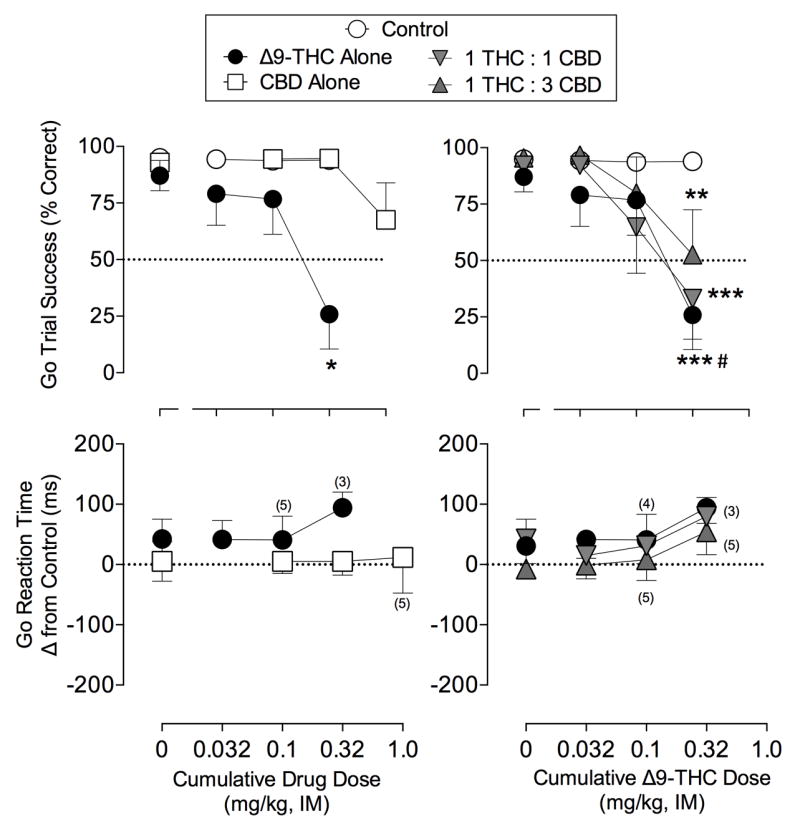
Abscissa: cumulative drug dose in mg/kg. Top ordinates: ‘Go’ trial success percentage for all ‘go’ trials; Bottom ordinates: ‘Go’ trial reaction time for responding completed within 1 second of stimulus onset as change from control. As indicated, some subjects were excluded in go reaction time analysis with numbers in parentheses indicating sample size when data was excluded, otherwise n=6. *p<0.05 vs. vehicle. *p<0.05, **p<0.01, ***p<0.001 from control tests; #p<0.05 THC alone vs THC 1:3
Table 1. Effects of Δ9-THC and CBD on SSRT (msec) during pre-chronic tests.
| Δ9-THC or CBD Dose (mg/kg) | Δ9-THC | 1:1 Δ9-THC:CBD | 1:3 Δ9-THC:CBD | CBD |
|---|---|---|---|---|
| 0.0 | 252 (±23) | 280 (±27) | 233 (±34) | 252 (±35) |
| 69% (8%) | 60%(8%) | 53%(6%) | 57%(9%) | |
|
| ||||
| 0.032 | 268 (±37) | 231 (±42) | 254 (±35) | - |
| 60%(7%) | 61%(6%) | 52%(7%) | ||
|
| ||||
| 0.1 | 264 (±35) # | 230 (±53)### | 258 (±45) # | 243 (±52) |
| 60%(7%) | 63%(13%) | 49%(11%) | 56%(4%) | |
|
| ||||
| 0.32 | 310 (n=1) | 265 (±48) ### | 246 (±47) ### | 244 (±43) |
| 83% | 76%(5%) | 59%(8%) | 57%(8%) | |
|
| ||||
| 1.0 | 247 (±46)## | |||
| 47%(10%) | ||||
Data shown are mean (±SEM) values for SSRTs in pre-chronic drug tests with corresponding stop trial accuracies (±SEM) below in italics. Subjects with 100% stop accuracy in drug cycles were excluded from analysis (see methods).
n=5,
n=4,
n=3,
else n=6.
Cannabidiol (CBD)
CBD administration did not significantly alter ‘go’ trial success percentage (F(3,15)=2.20; p=.13, RM ANOVA) or ‘go’ reaction time (F(1,15)=0.02, p = .90, linear regression) compared to vehicle treatment. It should be noted, however, that 1.0 mg/kg CBD decreased ‘go’ trial success to under 50% in two of the six subjects, contributing to the non-significant effect seen in the top left panel of Figure 1. Doses of CBD up to 1.0 mg/kg also had no significant effect on the SSRT (F(1,14)=0.002, p =.95, linear regression; Table 1).
Δ9-THC + CBD Combinations
The right panels of Figure 1 show the effect of CBD administered in combination with Δ9-THC on ‘go’ trial success and ‘go’ reaction time. A significant interaction (cycle × treatment: F (9,45)=3.23, p < 0.01; RM ANOVA) was present for ‘go’ accuracy. Similar to Δ9-THC alone, the highest doses of each Δ9-THC:CBD combination significantly disrupted ‘go’ trial success (p < .01, both cases; Holm-Sidak), whereas lower doses had no effect on task performance. Notably, the 1:3 combination of Δ9-THC:CBD significantly attenuated the disruptive effects of 0.32 mg/kg THC alone, yielding a 27% increase in ‘go’ trial success percentage (p = .049, Holm-sidak). Combinations of Δ9-THC:CBD did not significantly change ‘go’ reaction time (F < 1.7, p > .22, all cases, linear regression; Figure 1, bottom right) or SSRT (Table 1; all Fs<0.23, p>.64, linear regression).
Effects of Chronic Cannabinoid Treatment
Training Session Performance
As seen in Figure 2, both treatment groups (i.e., THC alone and THC+CBD) showed disruptions in ‘go’ success percentage during the 4 days of treatment with 0.32 mg/kg/day Δ9-THC in Phase 1. Inspection of the data revealed that in all subjects, go success was decreased to <35% in the THC alone group on the first day after treatment; however, go success was decreased to <35% in only one subject in the THC/CBD group. By the fourth day of THC treatment, go success was >80% in two subjects in each group and <30% in the remaining subjects in each group. Thus, mean ‘go’ success was 66% and 63% for THC alone and THC+CBD groups, respectively, prior to the first test with cumulative doses of Δ9-THC. ‘Go’ success was maintained at this or a higher level throughout Phase 2 and into Phase 3 of chronic treatment. In Phase 3 however, when the ‘go’ hold was increased for the two unresponsive subjects (see methods), ‘go’ success improved and returned to pre-chronic control levels of performance (>80% success) by the end of Phase 3.
Figure 2. ‘Go’ trial success (±SEM) in training sessions before and during chronic treatment with and corresponding drug treatment regimens.
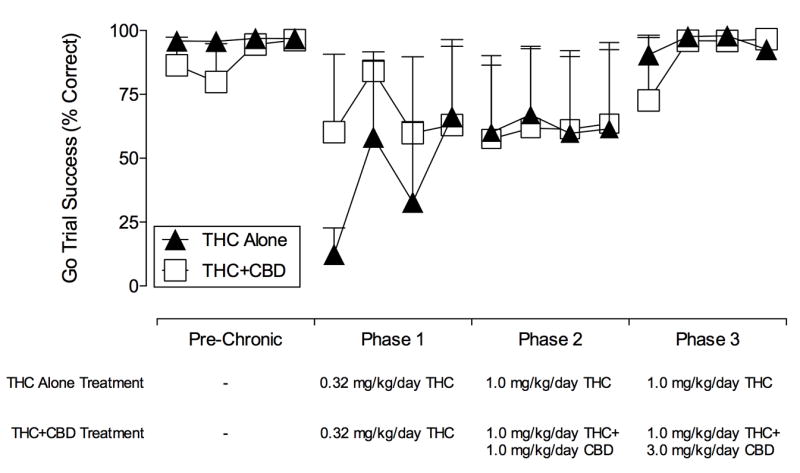
Data are presented for the last 4 consecutive training sessions for each Phase per group. Abscissa: Corresponding Phase. Ordinates: ‘Go’ trial success percentage for all ‘go’ trials. Bottom: Daily drug treatment administered. All subjects received treatments at 0600 in Phase 1 (Days 1-4), and at 0600 & 1800 in Phases 2 (Days 6-11) and 3 (Days 13-23). All Phases were separated by one day, during which the effects of cumulative doses of Δ9-THC (0.1-3.2 mg/kg) in the SST were determined. In Phase 3 of chronic treatment, the limited hold was increased for two subjects (see methods), after which group ‘go’ success returned to pre-chronic levels.
Chronic Treatment with Δ9-THC Alone
The effects of chronic Δ9-THC treatment on ‘go’ success during Δ9-THC tests are shown for both individual subjects and the group in Figure 3, top panels. Four days of treatment with 0.32 mg/kg Δ9-THC produced a 9-fold increase in group ED50 values for Δ9-THC-induced disruptions in ‘go’ trial success (Chronic Test 1; ED50 =1.18 mg/kg) compared to pre-chronic test performance (ED50 = 0.13 mg/kg). ‘Go’ trial success on Chronic Test 1 after 0.32 mg/kg Δ9-THC increased from 21.0 ± 14% during pre-chronic testing to 95.6 ± 2% after Phase 1 of chronic Δ9-THC administration. Two of the three subjects continued to respond at 1.0 mg/kg Δ9-THC (over 50% success) and performance was near 100% after 3.2 mg/kg Δ9-THC in one subject. Six days of treatment with 1.0 mg/kg Δ9-THC did not further alter performance (10-fold increase in group ED50 compared to pre-chronic test; ED50 = 1.31 mg/kg Chronic Test 2). The effects of 0.1 mg/kg Δ9-THC in Mm 066 were excluded from analysis due to the absence of responding which resumed following the next Δ9-THC injection. In Chronic Test 3, the Δ9-THC dose-effect function was shifted to the right compared to Chronic Test 2 in all subjects and the group ED50 increased 22-fold (ED50 = 2.86) compared to pre-chronic testing. Finally, measures of ‘go’ reaction time and SSRT were not appreciably altered from acute testing (Table 2).
Figure 3. Individual and Group ‘go’ trial success during pre-chronic Δ9-THC tests and Δ9-THC tests after chronic dosing with Δ9-THC (Top Panels) or Δ9-THC + CBD (Bottom Panels).

Abscissa: cumulative drug dose in mg/kg. Ordinates: ‘Go’ trial success percentage for all ‘go’ trials. Chronic Test 1 – 0.32 mg/kg/day Δ9-THC both groups; Chronic Test 2 – 1.0 mg/kg/day Δ9-THC both groups and 1.0 mg/kg/day CBD for Δ9-THC:CBD group (n=2); Chronic Test 3 - Chronic Test 2 – 1.0 mg/kg/day Δ9-THC both groups and 3.0 mg/kg/day CBD for Δ9-THC:CBD group. Subject Mm 066 (Top Middle, Right) did not respond after 0.1 mg/kg during Chronic Test 2. Subject Mm 8189 (Bottom Middle, Right) did not respond during Chronic Test 2 (see text for details).
Table 2. ‘Go’ Reaction Time Change (ms) and SSRT (ms) for Each Subject During Pre-chronic and Chronic Treatment Δ9-THC Tests; ‘-’ = No responding or indeterminable.
| Pre Chronic | Test 1 | Test 2 | Test 3 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||
| Δ9-THC Dose (mg/kg) | 0.0 | 0.032 | 0.1 | 0.32 | 0.1 | 0.32 | 1.0 | 3.2 | 0.1 | 0.32 | 1.0 | 3.2 | 0.1 | 0.32 | 1.0 | 3.2 | |
| Go Reaction Time Change: | |||||||||||||||||
|
| |||||||||||||||||
| Δ9-THC Alone | Mm 8827 | 28.3 | 93.1 | 177 | 59.4 | 31.7 | 52.1 | 112 | 57.2 | 43.7 | 74.5 | 111 | 2.84 | 12.9 | 30.7 | 27.1 | -44.6 |
| Mm 070 | 45.3 | 151 | - | - | 70.5 | 76.1 | 113 | - | 35.4 | 47.0 | - | - | 37.9 | 28.3 | 59.3 | 49.5 | |
| Mm 066 | -25.0 | -47.4 | -25.1 | 78.6 | 105 | -4.85 | - | - | - | 65.3 | 253 | - | 105 | 92.6 | 123 | 390 | |
|
|
|||||||||||||||||
| Mean | 16.2 | 65.6 | 75.9 | 69 | 69.2 | 41.1 | 113 | 57.2 | 39.6 | 62.2 | 182 | 2.8 | 52.0 | 50.6 | 70.0 | 132 | |
|
| |||||||||||||||||
| Δ9-THC and CBD | Mm 665 | 106 | 74.5 | 70.2 | 145 | -15.4 | 69.3 | 197 | 253 | -48.8 | -37.4 | 16.0 | 147 | 1.56 | 113 | 206 | 186 |
| Mm 113 | 158 | 14.1 | 30.1 | - | 57.4 | -23.1 | -59.7 | -59.9 | -62.2 | -61.1 | -66.1 | -53.7 | -59.6 | 40.3 | 37.3 | 19.6 | |
| Mm 8189 | -60.8 | -34.3 | -47.3 | - | -34.1 | 49.8 | - | - | - | - | - | - | 169 | 51.2 | 65.6 | 152 | |
|
|
|||||||||||||||||
| Mean | 67.7 | 18.1 | 17.7 | 145 | 2.61 | 32.0 | 69.1 | 96.4 | -55.5 | -49.2 | -25.1 | 46.5 | 31.6 | 54.1 | 82.1 | 106 | |
|
| |||||||||||||||||
| Stop Signal Reaction Time: | |||||||||||||||||
|
| |||||||||||||||||
| Δ9-THC Alone | Mm 8827 | 215 | 266 | 326 | 310 | 343 | 273 | 397 | 357 | 305 | 283 | 258 | 365 | 241 | 296 | 217 | 303 |
| Mm 070 | 286 | 415 | - | - | 308 | 291 | - | - | 306 | 235 | - | - | 309 | - | 334 | - | |
| Mm 066 | 325 | 311 | 359 | - | 405 | 342 | - | - | - | 372 | - | - | 417 | 437 | 455 | - | |
|
|
|||||||||||||||||
| Mean | 275 | 331 | 343 | 310 | 352 | 302 | 397 | 357 | 306 | 297 | 258 | 365 | 323 | 267 | 335 | 303 | |
|
| |||||||||||||||||
| Δ9-THC and CBD | Mm 665 | 248 | 261 | 256 | - | 203 | 226 | 332 | - | 192 | 201 | 230 | 329 | 180 | 249 | - | 302 |
| Mm 113 | - | 198 | 208 | - | 258 | 147 | 188 | 173 | 149 | 121 | 169 | 189 | 123 | - | 210 | 213 | |
| Mm 8189 | 163 | 160 | 174 | - | 183 | 182 | - | - | - | - | - | - | 344 | 249 | 278 | 289 | |
|
|
|||||||||||||||||
| Mean | 206 | 206 | 213 | - | 215 | 185 | 261 | 173 | 171 | 161 | 200 | 259 | 216 | 249 | 259 | 268 | |
Chronic Treatment with Δ9-THC + Cannabidiol
The effects of chronic treatment in the Δ9-THC + CBD group are shown in Figure 3, bottom panels. Similar to the THC-only group, four days of 0.32 mg/kg Δ9-THC administration produced a 9-fold increase in the group ED50 value (ED50 = 2.01 mg/kg) for Δ9-THC-induced disruptions in ‘go’ trial success compared to pre-chronic testing (ED50=0.23 mg/kg). ‘Go’ trial success following 0.32 mg/kg Δ9-THC was 30.8 ± 30.8% during pre-chronic testing and increased to 77.8 ± 9.2% during Chronic Test 1. Additionally two of the three subjects showed little disruption after 1.0 mg/kg Δ9-THC, whereas two subjects (Mm113 & Mm 665) responded at about 60% ‘go’ success after 3.2 mg/kg Δ9-THC.
When CBD was added to Δ9-THC at a 1:1 ratio during chronic treatment (Phase 2), success was >80% for two subjects through the highest dose of Δ9-THC tested in Chronic Test 2. The third subject (Mm8189) failed to respond during all cycles (data not shown), precluding a reliable ED50 determination. The lack of responding in Mm8189 was likely related to the complete cessation of responding in the previous training session; though reaction time was slowed, responding resumed when the limited hold was increased to 2-sec after Chronic Test 2. By the end of chronic treatment with the 1:3 ratio of Δ9-THC:CBD (Chronic Test 3), performance was at control levels (i.e., >80% ‘go’ success) after administration of all doses (0.1-3.2 mg/kg) of Δ9-THC and the estimated >14 fold increase in the group ED50 value (ED50 >3.2mg/kg) was likely much greater. In contrast to the Δ9-THC only group in which performance in two of three subjects was disrupted following 3.2 mg/kg Δ9-THC, all three subjects treated with Δ9-THC + CBD responded at >88% success after the same dose of Δ9-THC (Figure 3, bottom panels). Similar to the Δ9-THC only group, there was no consistent change in reaction time and SSRT performance in tests during chronic treatment (Table 2).
DISCUSSION
The present study assessed the effects of Δ9-THC, CBD, and their combinations on SST performance in rhesus macaques. Notwithstanding some methodological modifications to previously reported SST procedures (FR3 ‘go’ reinforcement schedule, multiple cycle test sessions), control measures of ‘Go’ trial performance, reaction time, and SSRT were similar to previous studies in rhesus monkeys and human subjects (c.f., Fillmore & Rush, 2002; Liu et al., 2009). Overall, the present findings show that a high dose of Δ9-THC disrupted task performance, whereas CBD had no consistent effect within the dose-range studied. Co-administration of Δ9-THC and CBD in dose-ratios that have been proposed for clinical use did not produce effects that differed markedly from those of Δ9-THC alone.
Effects of Δ9-THC and CBD on Pre-Chronic SST Performance
Δ9-THC produced dose-dependent disruptions in SST performance, evidenced by marked reductions in ‘go’ success percentage that are consistent with its effects on other types of complex behavioral and cognitive tasks in nonhuman primates. The highest doses of Δ9-THC used in the pre-chronic phase of the present study (0.1-0.32 mg/kg) have previously been shown to impair performance in conditional discrimination acquisition, visuo-spatial paired associated learning, and reversal learning tasks in monkeys (Winsauer et al., 1999; Winsauer et al., 2011; Wright et al., 2013a, Wright Jr,, Vandewater, Parsons, and Taffe, 2013b). These doses of Δ9-THC also can produce other behavioral effects, including response rate decreasing and discriminative-stimulus effects in rhesus macaques that may be related to its motoric or subjective effects (Gold et al., 1992; Hruba, Ginsberg, and McMahon, 2012; Kangas et al., 2016; Wiley, Huffman, Balster, and Martin, 1995). Pharmacokinetic data in rhesus monkeys has shown that acute administration of 0.2 mg/kg Δ9-THC, IM yields peak plasma levels at 30-min of approximately 69.3 ng/ml Δ9-THC (Wright Jr. et al., 2013b), which is similar to peak plasma levels of Δ9-THC (57.3 ng/ml) in recreational users after inhalation of moderate potency cannabis (i.e., 0.25 mg/kg Δ9-THC; Ramaekers et al., 2006). Consequently, the dose range of Δ9-THC in the present study likely encompasses levels of Δ9-THC that are achieved through self-administration in the human population.
Doses of Δ9-THC up to 0.32 mg/kg did not significantly increase ‘go’ reaction time in the stop signal task when responding was present within 1-sec of stimulus onset. Though this may be due to a lack of sensitivity of the SST to detect reaction time changes, it is unlikely that task disruption was due to slower reaction times as Δ9-THC has documented effects on motivation for food reinforcement and attention (Kangas et al., 2016; Wright et al., 2013a). In relation to the former, the present study observed that some subjects did not consume food pellets following administration of Δ9-THC doses that disrupted responding. Nonetheless, reaction time findings were concordant with other studies in human subjects, which have not observed significant increases in reaction time in the stop signal task or the related go/no-go task after Δ9-THC administration ( Borgwardt et al., 2008; McDonald et al., 2003; van Wel et al., 2013).
In the present study, Δ9-THC up to a dose of 0.1 mg/kg had no effect on SSRT. In previous studies of Δ9-THC’s effects on SSRT performance in recreational cannabis users, 0.5 mg/kg Δ9-THC increased stop signal reaction time whereas 0.25 mg/kg Δ9-THC had no significant effect (Ramaekers et al., 2006). Thus, it remains possible that high doses of Δ9-THC (>0.1 mg/kg for the present study) are required to influence motoric inhibition, while low to moderate doses (<0.1 mg/kg) are without effect (also see McDonald et al., 2003). Importantly, doses of Δ9-THC that alter SSRT also appear to affect other measures of SST performance such as omission errors and response accuracy (Ramaekers et al., 2006, Ramaekers, Kauert, van Ruitenbeek, Toennes, and Moeller, 2009). Combined with the finding that 0.32-mg/kg Δ9-THC disrupted general task performance in the present study, the effects of Δ9-THC on SSRT performance do not appear to be consistent with a selective effect on response inhibition.
CBD did not consistently alter stop signal task performance in the dose range tested. The highest CBD dose (1.0 mg/kg) slightly lowered ‘go’ success percentage for the group; however, this decrease reflects data from two of the six subjects. Nevertheless, this result may suggest higher CBD doses could disrupt behavior in this task as previous studies have found that 30 mg/kg CBD decreases operant performance in rhesus monkeys (Brady & Balster. 1980).
These results add to previous work in humans in which 600 mg of CBD had no effect on the go/no-go task and did not modulate brain activity in regions associated with response inhibition, i.e., the right inferior frontal and anterior cingulate gyrus (Borgwardt et al., 2008). They also are consistent with data showing that 0.5 mg/kg CBD had no effect on visuo-spatial associated memory in rhesus macaques (Wright et al., 2013a). Taken together, these findings suggest CBD is well tolerated at doses up to 1.0 mg/kg and does not affect motoric inhibition.
Although CBD did not alter the effects of Δ9-THC when administered in a 1:1 dose ratio, the 1:3 Δ9-THC:CBD ratio did attenuate the impairment of task performance produced by 0.32 mg/kg Δ9-THC. This finding is consistent with a previous report that CBD attenuated Δ9-THC-induced deficits in a visuo-spatial paired associate learning task in rhesus macaques, although that attenuation was observed following treatment with an 1:1 and 1:2.5 Δ9-THC:CBD ratio (Wright et al., 2013a). While the biological significance of the mild attenuation is unclear, our results suggest that co-administration of CBD at therapeutic dose-ratios may reduce some of the behavioral effects of Δ9-THC.
Effects of Chronic Δ9-THC and CBD
Daily administration of Δ9-THC during Phase 1 (0.32 mg/kg/day of Δ9-THC) of chronic treatment produced effects on SST performance that were similar to those in pre-chronic determinations—but with considerable tolerance. That is, a ten-fold greater Δ9-THC dose than in pre-chronic treatment was needed to suppress performance to below 50% ‘go’ success. Subsequent treatment with 1.0 mg/kg for 6 additional days did not further shift the dose-effect curve. The shift is similar to ~9-fold changes in ED50 values for monkeys discriminating Δ9-THC from vehicle after 14 days of 1.0 mg/kg/day Δ9-THC (Hruba et al., 2012). Further, the over 14-fold increases in ED50 values for ‘go’ success in both groups at the end of chronic treatment (24 days) is consistent with previous studies in which 0.32 mg/kg/day of Δ9-THC for 28 days produced an approximately 9-fold increase in ED50 values for response acquisition and performance (Winsauer et al. 2011) or in which 24 days of 2.0 mg/kg/day Δ9-THC in rhesus monkeys engendered 23- and 160-fold increases in ED50 values for behavioral disruption under schedules of food reinforcement and stimulus termination, respectively (McMahon, 2011). In sum, these findings indicate that the magnitude of tolerance likely is related to daily dosage.
The addition of CBD to THC has been suggested as one approach to eliminating some of the unwanted behavioral effects of medicinal THC (Russo & Guy, 2006), and Δ9-THC:CBD combinations are currently used clinically to ameliorate symptoms of multiple sclerosis (Fernández, 2014). Despite the heightened interest in CBD’s current and potential clinical benefits the interactive effects of Δ9-THC and CBD chronically are poorly defined. In rodents, CBD was found to significantly enhance the effects of chronic treatment with Δ9-THC on inhibition of body weight gain and decreased social interaction (Klein et al., 2011). In the present study, Δ9-THC:CBD-treated monkeys appeared to perform similarly to the Δ9-THC-treated group in the first two phases of treatment but the disruptive effects of high Δ9-THC doses were less apparent after treatment with the 1:3 Δ9-THC:CBD combination. Thus, the Δ9-THC alone group completed <50% of ‘go’ trials successfully after the highest cumulative dose of Δ9-THC (3.2 mg/kg) whereas all subjects in the Δ9-THC+CBD group performed at control levels of performance by the end of treatment. These data suggest that concurrent exposure to CBD enhanced tolerance to Δ9-THC’s task disruptive effects during chronic treatment, and that the interaction between these compounds may be most apparent during chronic or repeated administration. However, in the absence of additional data, this possibility must remain speculative.
Possible mechanisms of THC/CBD Interactions
The mechanisms through with CBD may interact with Δ9-THC are unclear. Available evidence raises the possibility that effects of Δ9-THC and CBD are commonly mediated, directly or indirectly, through CB1 receptor mechanisms (Hayakawa et al., 2008; Pertwee, 2008). It is also possible that some of CBD’s effects, especially during chronic treatment, may reflect its influence on Δ9-THC pharmacokinetics (Klein et al., 2011, but see Karschner, Darwin, Goodwin, Wright, and Huestis, 2010; Nadulski et al., 2005) or, alternatively, its inhibition of hydrolytic degradation of the endocannabinoid anandamide (Bisogno et al., 2001). In the latter regard, 14-28 days of daily treatment with CBD has been reported to increase anandamide levels in humans (Leweke et al. 2012). Notwithstanding these indications of the involvement of common cannabinergic mechanisms, other studies suggest that Δ9-THC and CBD act through dissimilar mechanisms. For example, tolerance to the neuroprotective effects of cannabinoids has been reported after chronic treatment with Δ9-THC but not CBD. Additionally, neuroprotective effects of CBD during chronic treatment are not blocked by the CB1 antagonist SR141716 but can be inhibited by the 5HT1a receptor antagonist WAY100135 (Hayakawa et al., 2007). The latter findings are consistent with the reported agonist actions of CBD at the 5HT1a receptor (Espejo-Porras, Fernández-Ruiz, Pertwee, Mechoulam, and García, 2013; Russo et al., 2005), and suggest a role for this neurochemical mechanism in at least the neuroprotective effects of CBD. In summary, notwithstanding some intriguing findings, the neurochemical mechanism of action for CBD is currently poorly understood and deserves further study.
Limitations
A potential limitation of the present study is that most subjects had a history of drug (i.e., stimulants) exposure, which may detrimentally influence performance on cognitive tasks requiring response inhibition (Kromrey, Gould, Nader, and Czoty, 2015; Liu et al., 2009). However, cocaine-induced deficits have been shown to dissipate within two months of abstinence (Kromrey et al., 2015) and subjects used in the present study had at least two months of drug abstinence prior to testing. Further, baseline performance was comparable to that of drug-naive monkeys (Liu et al., 2009) suggesting that previous drug history was not a critical factor in the present studies.
Another limitation of the present study was the small size of the groups in the chronic study (n=3 per group). Though this raises issues with between group comparisons, results were consistent as all six subjects displayed tolerance to Δ9-THC and all subjects in the THC+CBD group displayed no task disruption at the highest Δ9-THC dose tested in Phase 3. Only one study has systematically investigated chronic co-administration of these compounds in laboratory subjects (Klein et al., 2011), finding that CBD may potentiate some behavioral effects of Δ9-THC. Despite small sample size, our findings yielded a similar trend, where CBD appeared to augment tolerance to Δ9-THC’s task disruptive effects. Thus, the current study provides important initial insight into the effects of chronic Δ9-THC/CBD co-administration and highlights the necessity for further characterization of the effects of chronic treatment with these cannabis products.
Lastly, the use of gonadally intact females may have added an additional source of variability to the study. Inspection of individual data suggests no clear differences between male and female subjects during cannabinoid administration in the pre-chronic or chronic phases of the present study. Nevertheless, future studies to systematically investigate the role of sex hormones in the effects of cannabinoids on SST performance are needed to confirm these initial observations (see Crane, Schuster, Fusar-Poli, & Gonzalez, 2013 for review).
Conclusions
The results of the present study indicate that Δ9-THC and CBD had no effect on measures of response inhibition but that Δ9-THC dose-dependently disrupted overall SST performance. Further, CBD did not exacerbate Δ9-THC -induced deficits in task performance when combined with Δ9-THC at clinically meaningful dose ratios; consistent with the view that CBD does not accentuate (and may attenuate) THC’s behaviorally disruptive effects. Though CBD modestly attenuated the disruptive effects of THC in acute studies, this effect may have been greater when the two constituents were administered repeatedly. Additional research to examine a wider range of conditions under which CBD may attenuate the behavioral effects of Δ9-THC or modulate tolerance development is needed to fully understand the interaction of these two cannabis constituents.
Public Significance Statements.
This study found that cannabidiol, the second most abundant constituent of marijuana, does not exacerbate and, under restricted conditions, may even attenuate the behaviorally disruptive effects of Δ9-tetrahydrocannabinol (Δ9-THC). These findings highlight the complex interaction of these two cannabinoids, understanding of which may yield safer and more tolerable cannabis-based therapeutics.
Acknowledgments
This work was funded in part by NIDA grant R01-DA026892 (Bergman, P.I.) and K01-DA039306 (Kohut, P.I.) from the National institute on Drug Abuse, NIH. The funding source had no other role other than financial support.
All authors contributed to and have approved the final manuscript.
The authors would like to thank Dr. Roger D. Spealman for comments on an earlier version of the manuscript as well as Claire E. Barkin, Timothy Gillis, Olga Smirnova, and Nate Osiris for technical assistance throughout the study.
Footnotes
DISCLOSURES The authors declare no conflicts of interest.
References
- Babalonis S, Lofwall MR, Nuzzo PA, Elayi C, Malcolm RJ, Haney M, Walsh SL. Examination of the behavioral effects of oral cannabidiol alone and in combination with smoked marijuana. Drug and Alcohol Dependence. 2015 http://doi.org/10.1016/j.drugalcdep.2015.07.953.
- Bisogno T, Hanuš L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. British Journal of Pharmacology. 2001;134(4):845–852. doi: 10.1038/sj.bjp.0704327. http://doi.org/10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgwardt SJ, Allen P, Bhattacharyya S, Fusar-Poli P, Crippa JA, Seal ML, et al. Neural Basis of Δ-9-Tetrahydrocannabinol and Cannabidiol: Effects During Response Inhibition. Biological Psychiatry. 2008;64(11):966–973. doi: 10.1016/j.biopsych.2008.05.011. http://doi.org/10.1016/j.biopsych.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Brady KT, Balster RL. The effects of Δ9-tetrahydrocannabinol alone and in combination with cannabidiol on fixed-interval performance in rhesus monkeys. Psychopharmacology. 1980;72(1):21–26. doi: 10.1007/BF00433803. http://doi.org/10.1007/BF00433803. [DOI] [PubMed] [Google Scholar]
- Crane NA, Schuster RM, Fusar-Poli P, Gonzalez R. Effects of Cannabis on Neurocognitive Functioning: Recent Advances, Neurodevelopmental Influences, and Sex Differences. Neuropsychology Review. 2013;23(2):117–137. doi: 10.1007/s11065-012-9222-1. http//:doi.org/10.1007/s11065-012-9222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund A, Morrison PD, Nottage J, Hague D, Kane F, Bonaccorso S, et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. Journal of Psychopharmacology (Oxford, England) 2013;27(1):19–27. doi: 10.1177/0269881112460109. http://doi.org/10.1177/0269881112460109. [DOI] [PubMed] [Google Scholar]
- Espejo-Porras F, Fernández-Ruiz J, Pertwee RG, Mechoulam R, García C. Motor effects of the non-psychotropic phytocannabinoid cannabidiol that are mediated by 5-HT1A receptors. Neuropharmacology. 2013;75:155–163. doi: 10.1016/j.neuropharm.2013.07.024. http://doi.org/10.1016/j.neuropharm.2013.07.024. [DOI] [PubMed] [Google Scholar]
- Fernández O. Advances in the management of multiple sclerosis spasticity: recent clinical trials. European Neurology. 2014;72(Suppl 1):9–11. doi: 10.1159/000367616. http://doi.org/10.1159/000367616. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug and Alcohol Dependence. 2002;66(3):265–273. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- Ginsburg BC, Hruba L, Zaki A, Javors M, McMahon LR. Blood levels do not predict behavioral or physiological effects of delta9-tetrahydrocannabinol in rhesus monkeys with different patterns of exposure. Drug and Alcohol Dependence. 2014;139:1–8. doi: 10.1016/j.drugalcdep.2014.02.696. http://doi.org/10.1016/j.drugalcde[.2014.02.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold LH, Balster RL, Barrett RL, Britt DT, Martin BR. A comparison of the discriminative stimulus properties of delta 9-tetrahydrocannabinol and CP 55,940 in rats and rhesus monkeys. The Journal of Pharmacology and Experimental Therapeutics. 1992;262(2):479–486. [PubMed] [Google Scholar]
- Hall W, Solowij N. Adverse effects of cannabis. The Lancet. 1998;352(9140):1611–1616. doi: 10.1016/S0140-6736(98)05021-1. http://doi.org/10.1016/S0140-6736(98)05021-1. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Mishima K, Hazekawa M, Sano K, Irie K, Orito K, et al. Cannabidiol potentiates pharmacological effects of Δ9-tetrahydrocannabinol via CB1 receptor-dependent mechanism. Brain Research. 2008;1188:157–164. doi: 10.1016/j.brainres.2007.09.090. http://doi.org/10.1016/j.brainres.2007.09.090. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Mishima K, Nozako M, Ogata A, Hazekawa M, Liu A-X, et al. Repeated treatment with cannabidiol but not Δ9-tetrahydrocannabinol has a neuroprotective effect without the development of tolerance. Neuropharmacology. 2007;52(4):1079–1087. doi: 10.1016/j.neuropharm.2006.11.005. http://doi.org/10.1016/j.neuropharm.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Hruba L, Ginsburg BC, McMahon LR. Apparent Inverse Relationship between Cannabinoid Agonist Efficacy and Tolerance/Cross-Tolerance Produced by 9-Tetrahydrocannabinol Treatment in Rhesus Monkeys. Journal of Pharmacology and Experimental Therapeutics. 2012;342(3):843–849. doi: 10.1124/jpet.112.196444. http://doi.org/10.1124/jpet.112.196444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas BD, Leonard MZ, Shukla VG, Alapafuja SO, Nikas SP, Makriyannis A, Bergman J. Comparisons of Δ9-tetrahydrocannabinol and Anandamide on a Battery of Cognition-related Behavior in Nonhuman Primates. Journal of Pharmacology and Experimental Therapeutics. 2016;357:125–133. doi: 10.1124/jpet.115.228189. http://doi.org/10.1124/jpet.115.228189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karila L, Roux P, Rolland B, Benyamina A, Reynaud M, Aubin H, Lancon C. Acute and Long-Term Effects of Cannabis Use: A Review. Current Pharmaceutical Design. 2014;20(25):4112–4118. doi: 10.2174/13816128113199990620. http://doi.org/10.2174/13816128113199990620. [DOI] [PubMed] [Google Scholar]
- Karschner EL, Darwin WD, Goodwin RS, Wright S, Huestis MA. Plasma Cannabinoid Pharmacokinetics following Controlled Oral 9-Tetrahydrocannabinol and Oromucosal Cannabis Extract Administration. Clinical Chemistry. 2010;57(1):66–75. doi: 10.1373/clinchem.2010.152439. http://doi.org/10.1373/clinchem.2010.152439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Karanges E, Spiro A, Wong A, Spencer J, Huynh T, et al. Cannabidiol potentiates Δ9-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats. Psychopharmacology. 2011;218(2):443–457. doi: 10.1007/s00213-011-2342-0. http://doi.org/10.1007/s00213-011-2342-0. [DOI] [PubMed] [Google Scholar]
- Kromrey SA, Gould RW, Nader MA, Czoty PW. Effects of prior cocaine self- administration on cognitive performance in female cynomolgus monkeys. Psychopharmacology. 2015;232(11):2007–2016. doi: 10.1007/s00213-015-3865-6. http://doi.org/10.1007/s00213-015-3865-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Translational Psychiatry. 2012;2(e94) doi: 10.1038/tp.2012.15. http://doi.org/10.1038/tp.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Line SW. Environmental enrichment for laboratory primates. Journal of American Veterinary Medical Association. 1989;190(7):854–859. [PubMed] [Google Scholar]
- Liu S, Heitz RP, Bradberry CW. A touch screen based Stop Signal Response Task in rhesus monkeys for studying impulsivity associated with chronic cocaine self-administration. Journal of Neuroscience Methods. 2009;177(1):67–72. doi: 10.1016/j.jneumeth.2008.09.020. http://doi.org/10.1016/j.jneumeth.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD. On the ability to inhibit thought and action: a users’ guide to the stop signal paradigm. In: Dagenbach TH, Carr D, editors. Inhibitory processes in attention, memory, and language. San Diego, CA: Academic Press; 1994. pp. 189–239. [Google Scholar]
- Maa E, Figi P. The case for medical marijuana in epilepsy. Epilepsia. 2014;55(6):783–786. doi: 10.1111/epi.12610. http://doi.org/10.1111/epi.12610. [DOI] [PubMed] [Google Scholar]
- McDonald J, Schleifer L, Richards JB, de Wit H. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology. 2003;28:1356–1365. doi: 10.1038/sj.npp.1300176. http://doi.org/10.1038/sj.npp.1300176. [DOI] [PubMed] [Google Scholar]
- McMahon LR. Chronic Δ9 -tetrahydrocannabinol treatment in rhesus monkeys: differential tolerance and cross-tolerance among cannabinoids. British Journal of Pharmacology. 2011;162(5):1060–1073. doi: 10.1111/j.1476-5381.2010.01116.x. http://doi.org/10.1111/j.1476-5381.2010.01116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CJA, Schafer G, Freeman TP, Curran HV. Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: naturalistic study. The British Journal of Psychiatry. 2010;197(4):285–290. doi: 10.1192/bjp.bp.110.077503. http://doi.org/10.1192/bjp.bp.110.077503. [DOI] [PubMed] [Google Scholar]
- Nadulski T, Pragst F, Weinberg G, Roser P, Schnelle M, Fronk EM, Stadelmann AM. Randomized, double-blind, placebo-controlled study about the effects of cannabidiol (CBD) on the pharmacokinetics of Delta9-tetrahydrocannabinol (THC) after oral application of THC verses standardized cannabis extract. Therapeutic Drug Monitoring. 2005;27(6):799–810. doi: 10.1097/01.ftd.0000177223.19294.5c. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. The diverse CB 1and CB 2receptor pharmacology of three plant cannabinoids: Δ 9- tetrahydrocannabinol, cannabidiol and Δ 9-tetrahydrocannabivarin. British Journal of Pharmacology. 2008;153(2):199–215. doi: 10.1038/sj.bjp.0707442. http://doi.org/10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Thomas A. Therapeutic Applications for Agents that Act at CB1 and CB2 Receptors. In: Reggio PH, editor. The Cannabinoid Receptors. New York, NY: Humana Press; 2009. pp. 361–392. [Google Scholar]
- Ramaekers JG, Kauert G, van Ruitenbeek P, Theunissen EL, Schneider E, Moeller MR. High-Potency Marijuana Impairs Executive Function and Inhibitory Motor Control. Neuropsychopharmacology. 2006;31:2296–2303. doi: 10.1038/sj.npp.1301068. http://doi.org/10.1038/sj.npp.1301068. [DOI] [PubMed] [Google Scholar]
- Ramaekers J, Kauert G, Theunissen E, Toennes S, Moeller M. Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. Journal of Psychopharmacology. 2009;23(3):266–277. doi: 10.1177/0269881108092393. http://doi.org/10.1177/0269881108092393. [DOI] [PubMed] [Google Scholar]
- Robson P. Human studies of cannabinoids and medicinal cannabis. Handbook of Experimental Pharmacology. 2005;168:719–756. doi: 10.1007/3-540-26573-2_25. [DOI] [PubMed] [Google Scholar]
- Russo EB, Burnett A, Hall B, Parker KK. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochemical Research. 2005;30(8):1037–1043. doi: 10.1007/s11064-005-6978-1. http://doi.org/10.1007/s11064-005-6978-1. [DOI] [PubMed] [Google Scholar]
- Russo E, Guy GW. A tale of two cannabinoids: The therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Medical Hypotheses. 2006;66(2):234–246. doi: 10.1016/j.mehy.2005.08.026. http://doi.org/10.1016/j.mehy.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, et al. Cognitive functioning of long-term heavy cannabis users seeking treatment. Jama. 2002;287(9):1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- van Wel JHP, Kuypers KPC, Theunissen EL, Toennes SW, Spronk DB, Verkes RJ, Ramaekers JG. Single doses of THC and cocaine decrease proficiency of impulse control in heavy cannabis users. British Journal of Pharmacology. 2013;170(7):1410–1420. doi: 10.1111/bph.12425. http://doi.org/10.1111/bph.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbuggen F, Logan GD. Response inhibition in the stop-signal paradigm. Trends in Cognitive Sciences. 2008;12(11):418–424. doi: 10.1016/j.tics.2008.07.005. http://doi:10.1016/j.tics.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Huffman JW, Balster RL, Martin BR. Pharmacological specificity of the discriminative stimulus effects of Δ 9-tetrahydrocannabinol in rhesus monkeys. Drug and Alcohol Dependence. 1995;40(1):81–86. doi: 10.1016/0376-8716(95)01193-5. http://doi.org/10.1016/0376-8716(95)01193-5. [DOI] [PubMed] [Google Scholar]
- Winsauer PJ, Lambert P, Moerschbaecher JM. Cannabinoid ligands and their effects on learning and performance in rhesus monkeys. Behavioural Pharmacology. 1999;10(5):497–511. doi: 10.1097/00008877-199909000-00008. [DOI] [PubMed] [Google Scholar]
- Winsauer PJ, Molina PE, Amedee AM, Filipeanu CM, McGoey RR, Troxclair DA, et al. Tolerance to chronic delta-9-tetrahydrocannabinol (Δ9 -THC) in rhesus macaques infected with simian immunodeficiency virus. Experimental and Clinical Psychopharmacology. 2011;19(2):154–172. doi: 10.1037/a0023000. http://doi.org/10.1037/a0023000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrege J, Schmidt A, Walter A, Smieskova R, Bendfeldt K, Radue E-W, et al. Effects of cannabis on impulsivity: a systematic review of neuroimaging findings. Current Pharmaceutical Design. 2014;20(13):2126–2137. doi: 10.2174/13816128113199990428. http://doi.org/10.2174/13816128113199990428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MJ, Jr, Vandewater SA, Taffe MA. Cannabidiol attenuates deficits of visuospatial associative memory induced by Δ 9tetrahydrocannabinol. British Journal of Pharmacology. 2013a;170(7):1365–1373. doi: 10.1111/bph.12199. http://doi.org/10.1111/bph.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MJ, Vandewater SA, Parsons LH, Taffe MA. Δ(9)Tetrahydrocannabinol impairs reversal learning but not extra-dimensional shifts in rhesus macaques. Neuroscience. 2013b;235:51–58. doi: 10.1016/j.neuroscience.2013.01.018. http://doi.org/10.1016/j.neuroscience.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


