Abstract
Hydrogen sulfide (H2S) has long been known as a toxic gas. However, recently accumulated evidence suggests that H2S contributes to a variety of physiologic and pathologic processes. Endogenous H2S production is regulated by multiple enzymes that are differentially expressed in the cardiovascular, neuronal, immune, renal, respiratory, gastrointestinal, reproductive, liver, and endocrine systems. Alteration of H2S metabolism may affect multiple signaling pathways and tissue homeostasis. The growing number of diverse targets for which H2S serves as a gasotransmitter has been extensively reviewed elsewhere. In this review, the authors discuss current emerging evidence that H2S regulates mesenchymal stem cell and T-cell functions.
Keywords: treg cells, molecular mechanism, stemness, immune homeostasis, gasotransmitter, regeneration
Introduction
Hydrogen sulfide (H2S) is a colorless, flammable gas with a characteristic odor of rotten eggs that has long been considered to be a toxic environmental pollutant. However, accumulating experimental evidence shows that this gas naturally exists in many biological systems and likely joins nitric oxide and carbon monoxide to serve as the third gasotransmitter in mammalian biology (Wang 2012). H2S freely diffuses through cell membranes to elicit various responses and modulate a variety of cellular events independently of membrane receptor or second messenger systems. H2S is endogenously synthesized in mammalian tissues by 3 enzymes—namely, cystathionine beta-synthase (CBS), cystathionine gamma-lyase (CSE), and cysteine aminotransferase. Cysteine aminotransferase acts in concert with 3-mercaptopyruvate sulfurtransferase to release H2S from L-cysteine and ketoacids. The distribution of H2S-synthesizing enzymes has a tissue-specific pattern: CBS is highly expressed in the brain and nervous systems, and CSE is the predominant enzyme in the vasculature and heart (Li et al. 2011; Vandiver and Snyder 2012). Alternations to H2S metabolism lead to an array of pathologic disturbances in the form of hypertension, atherosclerosis, heart failure, diabetes, cirrhosis, sepsis, neurodegenerative disease, and asthma in animal models and humans (Li et al. 2011). The effects of H2S on various systems have been extensively studied and summarized (Wang 2012). Recently emerging evidence suggests that H2S regulates the bone metabolism equilibrium via mesenchymal stem cells (MSCs) and also regulates immune homeostasis, especially in T cells (Liu, Yang, et al. 2014; Oh and Li 2015; Yang et al. 2015). Despite the large number of biological effects that have been observed both in vitro and in vivo upon alteration of H2S metabolism, the detailed molecular mechanisms underlying it are not fully understood. Here, we review recent progress in understanding H2S-regulated MSC stemness and Treg-cell homeostasis.
H2S Regulates MSC Stemness
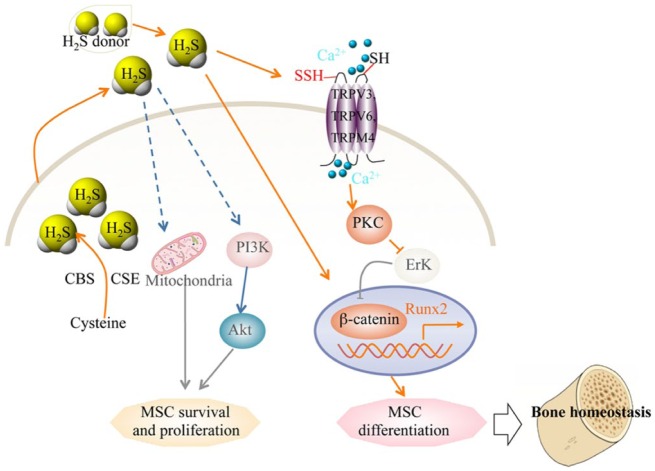
Patients with CBS deficiency may have disorders affecting multiple systems, including dislocated lenses, intellectual disability, premature arteriosclerosis, and thrombosis. Epidemiologic and clinical studies suggest that these patients have an increased risk of fracture and osteoporosis (McLean et al. 2004; van Meurs et al. 2004; Dhonukshe-Rutten et al. 2005; Gjesdal et al. 2007). MSCs—progenitor cells with the capacities for self-renewal and multilineage differentiation into osteoblasts, chondrocytes, myocytes and adipocytes—play crucial roles in the maintenance of tissue homeostasis. Several recent studies suggest that H2S governs MSC behavior. Endogenous H2S is required to maintain proliferation and differentiation of neural stem cells through activation of Erk1/2, to augment the proliferation and survival of human-induced pluripotent stem cell–derived MSCs by activation of the PI3K/Akt pathway, and to restore endothelial progenitor cell function (Wang et al. 2013; Zhao et al. 2013; Liu, Chen, et al. 2014; Liu, Yang, et al. 2014). A recent study showed that MSCs produce H2S and that endogenous H2S plays a crucial role in the maintenance of MSC function to ensure bone homeostasis (Liu, Yang, et al. 2014). H2S deficiency results in impaired osteogenic differentiation and proliferation in bone marrow MSCs (BMMSCs). H2S deficiency in CBS knockout mice leads to a consistently osteoporotic phenotype, which can be rescued by treatment with GYY4137, which slowly releases H2S. Mechanically, H2S deficiency causes aberrant intracellular Ca2+ influx because of reduced sulfhydration of cysteine residues on combined Ca2+ transient receptor potential (TRP) channels (TRPV3, TRPV6, and TRPM4). The decreased Ca2+ flux downregulates PKC/Erk-mediated Wnt/β-catenin signaling, resulting in attenuated osteogenic differentiation of BMMSCs (Liu, Yang, et al. 2014). H2S donor GYY4137 treatment increases bone formation and prevents trabecular bone loss induced by ovariectomy via increasing BMMSC osteogenic differentiation. H2S activates the Wnt signaling of BMMSCs through increased production of the Wnt ligands Wnt16, Wnt2b, Wnt6, and Wnt10b in the bone marrow. These studies imply that restoration of H2S levels is a potential novel therapeutic approach for postmenopausal osteoporosis (Grassi et al. 2015).
Additionally, MSCs have great potential for tissue regeneration. Apoptosis of transplanted cells represents a major challenge in MSC-based regenerative medicine. H2S has recently been proposed as an endogenous mediator or inhibitor of cell apoptosis in various systems. H2S treatment could increase MSC proliferation and survival and attenuate hypoxia-, oxidant-, or serum deprivation–induced apoptosis (Fox et al. 2012; Xie et al. 2012; Zhao et al. 2013; Li et al. 2014; Aykan et al. 2015; Guo et al. 2015). Mechanically, H2S activates PI3K/Akt, Erk1/2, and GSK-3β (glycogen synthase kinase-3β) pathways to decrease hypoxia-induced MSC apoptosis. It was reported that H2S treatment activates Erk and decreases phosphorylation of Akt in MSCs (Aykan et al. 2015). In addition, H2S may regulate MSC function through increasing antiapoptosis gene Bcl-2 expression to attenuate mitochondrial injury and endoplasmic reticulum (ER) stress induced by hypoxia (Li et al. 2014; Aykan et al. 2015; Guo et al. 2015). Moreover, proinflammatory cytokines could induce CSE expression and H2S synthesis to interfere with the chronic inflammatory response in rheumatoid arthritis (Fox et al. 2012). These findings suggest that modulation of H2S metabolism may serve as a therapeutic approach to promote the viability of transplanted MSCs and facilitate MSC-based regeneration. Consistent with this, it was reported that H2S improves transplanted MSC survival in infarcted myocardium and aids in cardiac repair (Xie et al. 2012). To further understand the role of H2S on transplanted MSCs and translate these findings from the bench top to the clinic, more studies of preclinical animal models are needed.
Since the first isolation of dental pulp stem cells (DPSCs) from tooth pulp in 2000, several types of MSCs have been identified in specialized craniofacial tissues—including stem cells from human exfoliated deciduous teeth, periodontal ligament stem cells, dental follicle precursor cells, stem cells from the apical papilla, and stem cells derived from gingiva (Gronthos et al. 2000; Miura et al. 2003; Seo et al. 2004; Morsczeck et al. 2005; Sonoyama et al. 2008; Zhang et al. 2009). These dental stem cells display self-renewal and multilineage differentiation potential as observed in BMMSCs. Differences have been noted between these dental stem cell populations and BMMSCs; for example, dental stem cells appear to be more apt to undergo odontogenic rather than osteogenic differentiation (Huang et al. 2009). The oral cavity has a plethora of bacteria residing in biofilms. When the dynamic ecologic equilibrium in the biofilm is disturbed, some of the bacteria contribute to oral diseases such as caries, gingivitis, and periodontitis (Aas et al. 2005). Some bacteria are known to produce considerable amounts of H2S, which may cause cell toxicity by inducing apoptosis or facilitating bacterial invasion. Despite the obvious toxic activity of exogenous H2S, several studies recently reported a novel role of H2S in the physical functions of dental stem cells (Zhang et al. 2010). H2S is expressed in periodontal ligament stem cells and plays a critical role in cell proliferation and osteogenic and adipogenic differentiation, while a high concentration of H2S donor significantly inhibits osteogenic differentiation of periodontal ligament stem cells, implying that a physiologic concentration of H2S is needed for periodontal tissue homeostasis (Su et al. 2015). It has been suggested that H2S is involved in physiologic and pathologic effects on the liver. Recently, studies showed that H2S induces human BMMSC and DPSC hepatic differentiation with higher expression of hepatic markers α-fetoprotein, albumin, and carbamoyl phosphate synthetase and increases urea concentrations and glycogen synthesis (Ishkitiev et al. 2012; Okada et al. 2014). Exogenous H2S donor treatment increases human DPSC apoptosis by activating a mitochondrial pathway, implying that a high concentration of H2S might be one of the factors modifying the pathogenesis of pulpitis by causing loss of viability of DPSCs through apoptosis (Kobayashi et al. 2011). Exogenous H2S is a major cause of halitosis or bad breath, and a high concentration of H2S in gingival fluid has been reported to be highly toxic for oral tissues and to be involved in the etiology and progression of periodontitis (Calenic et al. 2010; Fig. 1). These studies indicate that H2S may be a double-edged sword in oral health.
Figure 1.
Schematic diagram of hydrogen sulfide (H2S) regulating mesenchymal stem cell (MSC) function. H2S is physiologically generated by cystathionine beta-synthase (CBS) and cystathionine gamma-lyase (CSE) in MSCs. The levels of endogenous or exogenous H2S affect sulfhydration of calcium channels to regulate WNT/β-catenin-mediated osteogenic mast gene Runx2, controlling MSC differentiation and bone homeostasis. H2S also regulates MSC survival and proliferation via alteration of mitochondrial function and the PI3K/Akt pathway. TRP, transient receptor potential.
H2S Governs T-cell Homeostasis
The role of H2S in the immune system and inflammatory processes has long been debated. H2S has been reported to exert both proinflammatory and anti-inflammatory effects via regulating various immune cell functions, such as T-cell activation and proliferation, monocyte apoptosis, polymorphonuclear cell apoptosis, leukocyte adhesion and infiltration, and inflammatory cytokine release by immune cells. The effects of H2S-regulated inflammation are implicated in a plethora of human diseases, including autoimmune diseases, Alzheimer’s disease, Parkinson’s disease, asthma, hind- paw edema, acute pancreatitis, lipopolysaccharide-induced endotoxemia, sepsis, and rheumatoid arthritis (Predmore et al. 2012; Wang 2012).
A number of factors are involved in determining the anti-inflammatory or proinflammatory effects of H2S. The wide range of H2S concentrations (0.05 to 0.5 mg/kg) and administration routes (continuous intravenous infusion vs. a bolus administration) for H2S may yield different effects on inflammation. Even at the same dosage, H2S may cause opposite effects depending on its release rate (novel slow-releasing donor GYY4137 vs. fast-releasing donor NaHS). The effects of H2S in inflammation may also vary by species (rats vs. mice and others), the model of inflammation (regional vs. systemic inflammation), and the organ from which the H2S originates (brain vs. pancreas, etc.; Wang 2012). Most studies to date have used exogenously applied H2S donors, which may be useful in determining the therapeutic value of H2S in inflammation but do not illustrate the role of endogenous H2S. Direct and solid evidence should be derived from animal models that regulate H2S metabolism in vivo, such as CBS- or CSE-knockout mice, or the factors regulating the expression of CSE, CBS, or both. Recent progress in probing the effects of H2S on T-cell activation and differentiation using CBS- or CSE-knockout mice may shed light on this controversial subject. T-helper (Th) cells play critical roles in mediating adaptive immunity and are involved in autoimmunity, asthma, and allergic responses as well as in tumor immunity. During T-cell receptor (TCR) activation in a particular cytokine milieu, naive CD4+ T cells may differentiate into one of several lineages of Th cells, including Th1, Th2, Th17, and regulatory T (Treg) cells. The distinctive differentiated states of various CD4+ effector/regulatory T-cell subpopulations are largely triggered by particular cytokines and determined by the set of transcription factors (Zhu et al. 2010). The gasotransmitter may serve as another factor to regulate T-cell lineage determination.
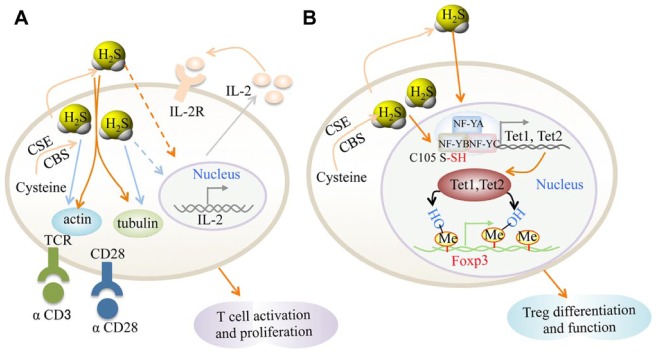
H2S can enhance TCR-dependent T-cell activation and interleukin 2 (IL-2) expression, as assessed by CD69 expression, IL-2 expression, and CD25 levels. H2S also enhances T-cell proliferation, suggesting that H2S represents a novel immunomodulatory molecule in T cells (Miller et al. 2012; Fig. 2A). Mechanically, H2S at physiologic concentrations increases T-cell capacity to form an immunologic synapse by altering cytoskeletal actin dynamics and increasing the reorientation of the microtubule-organizing center or stimulating T-cell activation. It was suggested that binding of secreted protein thrombospondin-1 to CD47 elicits signals that block the stimulatory activity of H2S on T-cell activation and limit the induction of CSE and CBS gene expression, thereby limiting the autocrine role of H2S in T-cell activation (Kaur et al. 2015). Additionally, H2S is able to induce pH- and caspase-dependent apoptosis of human T cells (Jurkat cells). H2S also induces T-cell blebbing by activating the Rho pathway (ROCK-1 and ROCK-2; Kanno et al. 2013).
Figure 2.
Schematic diagram of hydrogen sulfide (H2S) signaling in T cells. (A) H2S signaling in T-cell activation. H2S enhances T-cell receptor (TCR) stimulated T-cell activation and interleukin 2 (IL-2) expression. The targets of H2S may be actin and tubulin cytoskeleton, and the endogenous H2S level is increased by T-cell activation. The production of H2S by activated T cells may act as an autocrine or paracrine enhancer of T-cell activation. (B) H2S signaling in Treg cell differentiation. H2S sulfhydrates NF-YB to regulate Tet1 and Tet2 expression. Tet1 and Tet2 directly bind to the Foxp3 locus to convert 5-methylcytosine to 5-hydroxymethylcytosine, leading to increased Foxp3 demethylation and increased Treg-cell differentiation and stability. CBS, cystathionine beta-synthase; CSE, cystathionine gamma-lyase.
In CSE-knockout mice, CSE deficiency and reduced H2S production in the lungs cause aggravated allergen-induced airway hyperresponsiveness, acute asthma, profound airway inflammation, and elevated levels of Th2 cytokines (e.g., IL-5, IL-13, and eotaxin-1) in bronchoalveolar lavage fluid after ovalbumin challenge. More important, NaHS supplement rescued CSE-knockout mice from the aggravated pathologic process of asthma, indicating that the CSE/H2S system plays a critical protective role in the development of asthma. In addition, NaHS at 50 µmol/L significantly inhibits the viability of CSE-knockout lymphocytes and the secretion of IL-5 and IL-13 in these cells, but these effects are not seen in wild-type lymphocytes. This study implies that H2S is a potential regulator for Th2-cell lineage determination (Gao et al. 2013). Exogenous H2S induces caspase-independent death of peripheral CD8+ T cells that depends on their intracellular glutathione levels. H2S also reduces the cytotoxic response of peripheral blood lymphocytes as well as their production of IL-2, thereby decreasing local inflammatory responses (Mirandola et al. 2007). Taken together, these studies provide new basic knowledge of the clinically relevant anti-inflammatory effects of sulfur compounds.
Treg cells, which express the forkhead-box transcription factor Foxp3, are essential for the maintenance of immune tolerance and homeostasis. Treg cells suppress a variety of immune responses (e.g., Th1, Th2, and Th17 cells) and unwanted immunity against a diverse of antigens, including self-antigens, commensal bacteria-derived antigens, and environmental allergens. For this reason, a lack of Treg cells may cause severe systemic inflammatory diseases manifested by autoimmunity, colitis, and allergies (Sakaguchi et al. 2008). The establishment and maintenance of a stable Treg-cell lineage requires synergistic Foxp3 expression and the acquisition of Treg cell–specific epigenetic modifications (Ohkura et al. 2012). Recently, a study showed that T cells express the enzymes CBS and CSE, which catalyze H2S synthesis. H2S is required for Foxp3+ Treg cell differentiation, and loss of Treg cells in CBS-deficient mice resulted in early lethality, autoantibody production, and immune cell infiltration into various tissues. The provision of either exogenous Treg cells or H2S donor attenuated the disease phenotype. Mechanically, H2S maintains expression of methylcytosine dioxygenases Tet proteins (Tet1 and Tet2), which is the key catalyzed enzyme involved in the conversion of 5-methylcytosine to 5-hydroxymethylcytosine, through sulfhydrating NFYB (nuclear transcription factor Y subunit beta). H2S increases Tet-mediated active DNA demethylation to facilitate Treg cell–specific hypomethylation establishment and enhance Treg-cell stability. Furthermore, transforming growth factor β activates Smad3, and IL-2 activates Stat5 to facilitate Tet1 and Tet2 binding to Foxp3, thereby enhancing DNA demethylation (Feng et al. 2014; Oh and Li 2015; Yang et al. 2015; Fig. 2B). H2S serves as a costimulator to govern T-cell activation, proliferation, and lineage determination (Miller et al. 2012). It may be a novel therapeutic approach for inflammation and autoimmune disease treatment via targeting H2S metabolism. A recent study reported that T cells are required for orthodontic tooth movement (OTM), indicating that H2S metabolism may participate in OTM via regulating T-cell activity (Yan et al. 2015). T cell–mediated immune response may be one alternative target through which H2S regulates oral health.
Molecular Targets of H2S
The mechanisms through which H2S exerts its physiologic and pathologic effects are not fully understood. A variety of cellular events—such as intracellular cell signaling, iron channels, cell metabolism, and protein sulfhydration—may contribute to H2S-mediated molecular and cellular responses (Li et al. 2011; Wang 2012).
Intracellular Cell Signal Transduction Pathways
A number of signal transduction pathways may be recruited by H2S to fine-tune its effects on different tissues and cells. H2S may inhibit or activate NF-κB nuclear translocation and affect the activity of numerous kinases, including p38 mitogen-activated protein kinase (MAPK), extracellular signal–regulated kinase (ERK), and Akt signaling. MAPK signaling, which involves SAPK/JNK, P38-MAPK, and ERK, can be differentially regulated by H2S, leading to various cellular reactions, including proliferation, apoptosis, differentiation, and cell cycle progression. For instance, H2S activates ERK to induce the apoptosis of human aortic smooth muscle cells and inhibit their proliferation (Yang et al. 2004), whereas activation of P38-MAPK by H2S induces apoptosis of INS-1E cells and affects survival of human polymorphonuclear cells (Rinaldi et al. 2006). H2S is able to promote angiogenesis and vascular remodeling via the phosphatidylinositol 3-kinase (PI3K) / Akt / survivin axis in endothelial cells by augmenting phosphorylation of ERK and p38 in vascular smooth muscle cells (VSMCs). H2S downregulates a number of proinflammatory genes involved in cardiac ischemic/reperfusion injury by preventing the nuclear translocation of NF-κB (Miller et al. 2012). In addition, administration of GYY4137 to lipopolysaccharide-injected rats results in the activation of signal transducer and activator of transcription 3 (STAT3; Aggarwal et al. 2009; Li et al. 2009). NaHS also induces the nuclear localization of the transcription factor Nrf-2 (NF-E2-related factor 2) in experimental myocardial ischemia (Calvert et al. 2009). H2S-activated signaling transduction pathways were also reported to be essential in maintaining MSC proliferation and differentiation. For instance, H2S activates PKC/Erk-mediated Wnt/β-catenin signaling to promote osteogenic differentiation of MSCs (Liu, Yang, et al. 2014; Grassi et al. 2015). Furthermore, H2S activates the PI3K/Akt, Erk1/2, and GSK-3β pathways, thereby decreasing hypoxia-induced MSC apoptosis (Fox et al. 2012; Xie et al. 2012; Li et al. 2014; Guo et al. 2015).
Protein Sulfhydration
H2S converts cysteine residue -SH groups in target proteins to hydropersulfide (-SSH), a modification known as protein sulfhydration (Mustafa et al. 2009). Compared with the well-studied protein posttranslational modification called nitrosylation by nitric oxide, sulfhydration is more widespread: 10% to 25% of proteins are sulfhydrated in vivo, whereas around 1% to 2% of proteins are nitrosylated. Sulfhydration is more stable than nitrosylation, which makes it is easily detected and explored by mass spectrometry. Furthermore, sulfhydration usually increases activity of modified proteins, while nitrosylation seems to decrease most proteins’ activity (Wang 2012). Recent studies showed that GAPDH, KATP channel (Mustafa et al. 2009), p65 subunit of NF-κB (Sen et al. 2012), TRP calcium channel (Liu, Yang, et al. 2014), and NFYB proteins (Yang et al. 2015) are activated by H2S-mediated sulfhydration. Sulfhydration may contribute to the regulation of inflammation, ER stress, and vascular tension (Mustafa et al. 2009; Paul and Snyder 2012). For instance, recent studies showed that the cell survival promotion action of NF-κΒ is mediated through sulfhydration of the p65 in macrophages, whereas in cancer, diminishing sulfhydration of NF-κB may have beneficial therapeutic effects (Paul and Snyder 2012; Sen et al. 2012).
Ion Channels
A growing number of studies are attempting to elucidate the involvement of ion channels (K+, Cl-, and Ca2+) in H2S-related signaling and the associated regulatory pathways. By targeting KATP channels, H2S regulates the inflammatory process, pain, and cell death and exerts its beneficial protective effects against ischemia damage, hypertension, and apoptosis (Wang 2012). For example, H2S-induced dilation of blood vessels relies, at least partially, on its ability to open vascular smooth muscle KATP channels (Zhao et al. 2001). H2S also stimulates BKCa (big conductance Ca2+-sensitive K+) channels in rat pituitary tumor cells (Sitdikova et al. 2010). The cystic fibrosis transmembrane conductance regulator Cl- channel was reported to be involved in cell protection by H2S against oxidative stress (Kimura et al. 2006). Based on their electrophysiologic features, Ca2+ channels are classified as high voltage activated (including L-, N-, P-/Q-, and R-type) and low voltage activated (T-type). In addition, there are transmitter-gated Ca2+-permeable ion channels, TRP ion channels, and Ca2+ pumps located in the plasma membrane (Ichiyama et al. 2015). The previous data suggest that L- and T-type Ca2+ channels and TRP channels are the main targets of H2S. For instance, NaHS negatively modulates L-type Ca2+ channels composed by the CaV1.2 subunits to exert cardioprotective effects. H2S may act as a nociceptive messenger via activation of T-type Ca2+ channels, especially during inflammatory processes (Munaron et al. 2013). TRPV1-mediated Ca2+ entry enhances substance P release to excite cholinergic secretomotor neurons (Tang et al. 2008). H2S activates Wnt/β-catenin signaling via stimulating TRPV3, TRPV6, and TRPM4 Ca2+ channels in MSCs to promote osteogenic differentiation (Liu, Yang, et al. 2014). Despite the focus of most studies to date on KATP and Ca2+ channels, it is worth of noting that preliminary data imply that additional Na+ and other channels may serve as novel targets of H2S.
Cell Metabolism
It has been known for decades that H2S inhibits cytochrome c oxidase and reduced cell energy production (Li et al. 2011; Wang 2012). Emerging evidence implies that cell metabolism status alteration may be among the H2S-targeted cellular events. For instance, H2S protects the vascular endothelium in hyperglycemia by preserving mitochondrial function to improve endothelial metabolic state and maintain endothelial function (Suzuki et al. 2011). It was also reported that H2S exposure reduces cell oxidative phosphorylation and ATP biosynthesis (Cooper and Brown 2008; Kiss et al. 2008). H2S also affects cell proliferation or apoptosis by altering cell fate during the course of the cell cycle. For instance, in oral epithelial-like cells, H2S decreases DNA synthesis, decreases Rb phosphorylation, and increases p21Cip1, indicating that H2S inhibits epithelial-like cell proliferation due to cell cycle arrest via expression of p21Cip1 (Takeuchi et al. 2008). H2S is a reducing agent and possesses antioxidant effects to offer cytoprotection—for example, reducing the cytotoxic effects of hydrogen peroxide and oxidized low-density lipoprotein (oxLDL) on cultured human umbilical vein endothelial cells (HUVECs) (Jeney et al. 2009). H2S also regulates ER stress in a dose-dependent manner. For instance, H2S attenuates high-fat diet–induced cardiac dysfunction via suppression of ER stress (Barr et al. 2015), while a high level of H2S results in ER stress in cultured INS-1E cells (Yang et al. 2007). These studies suggest that H2S is synthesized naturally and elicits fine control over cellular metabolic processes via different processes.
The known molecular targets of H2S can be grouped broadly into effects on protein modification, cellular signaling, ion channel, and cell metabolism, exerted individually or jointly. For instance, KATP channel and TRP Ca2+ channels have been reported to be sulfhydrated by H2S. Furthermore, H2S could open Ca2+ channels to activate Wnt-β catenin signaling in MSCs (Mustafa et al. 2009; Liu, Yang, et al. 2014; Fig. 3). These studies indicate that the overall effects of H2S in a particular cell or tissue may depend on the balance or synergism of different targets. Greater insights into the interaction of different molecular targets of H2S are needed to illustrate the precise mechanism how H2S exerts biological effects, which will be beneficial for the treatment of diseases related to abnormal H2S metabolism.
Figure 3.
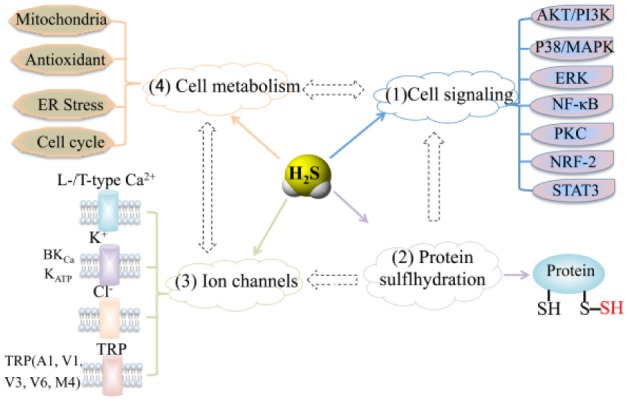
Molecular targets of hydrogen sulfide (H2S). These can be grouped into effects on (1) intracellular signaling and transcription factors, (2) protein modifications, (3) ion channels, and (4) cell metabolism. ER, endoplasmic reticulum; -SH, thiol; -SSH, hydropersulfide; TRP, transient receptor potential.
Conclusions and Perspectives
Accumulating evidence suggests that H2S regulates a variety of physiologic and pathologic processes. H2S is required to maintain MSC stemness and function, ensuring bone and connective tissue homeostasis. Setting aside its toxic effects on oral health, H2S also plays crucial roles in the survival, proliferation, and differentiation of dental stem cells. Moreover, H2S promotes T-cell activation and Th-cell (Treg cell) differentiation to control immune system homeostasis. H2S regulates cellular signaling, K+ and Ca2+ channels, and protein sulfhydration in a diverse set of molecular targets. Additionally, H2S contributes to regulation of cellular metabolism. H2S donor may therefore serve as an alternative therapeutic intervention for various diseases, such as osteoporosis, immune disorders, and inflammation, which may be mainly caused by H2S deficiency. Despite these advances, there are still many uncertainties to translate bench-top studies into clinical applications. A better understanding of the precise cellular and intracellular sites of these effects could be beneficial for developing H2S-based therapies for specific diseases. A thorough evaluation of the physiologic and pathophysiologic roles of H2S using genetically modified animals is needed. It will be important to determine the contributions of various molecular mechanisms and how they are related. When progress is made in these areas, novel agents that modulate H2S bioavailability might be efficacious for diseases related to aberrant H2S metabolism, such as acute myocardial infarction, diabetes, arthritis, metabolic syndrome, complications of organ transplantation, inflammatory disease, and hypertension.
Author Contributions
R. Yang, contributed to conception, design, and data analysis, drafted the manuscript; Y. Liu, contributed to conception and design, critically revised the manuscript; S. Shi, contributed to conception and design, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
This work was supported by grants from the National Institute of Dental and Craniofacial Research, National Institutes of Health, U.S. Department of Health and Human Services (R01DE017449 to S.S.).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. 2005. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 43(11):5721–5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal BB, Kunnumakkara AB, Harikumar KB, Gupta SR, Tharakan ST, Koca C, Dey S, Sung B. 2009. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann N Y Acad Sci. 1171:59–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aykan A, Ozturk S, Sahin I, Avcu F, Sagkan RI, Isik S. 2015. The effects of hydrogen sulfide on adipocyte viability in human adipocyte and adipocyte-derived mesenchymal stem cell cultures under ischemic conditions. Ann Plast Surg. 75(6):657–665. [DOI] [PubMed] [Google Scholar]
- Barr LA, Shimizu Y, Lambert JP, Nicholson CK, Calvert JW. 2015. Hydrogen sulfide attenuates high fat diet-induced cardiac dysfunction via the suppression of endoplasmic reticulum stress. Nitric Oxide. 46:145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calenic B, Yaegaki K, Murata T, Imai T, Aoyama I, Sato T, Ii H. 2010. Oral malodorous compound triggers mitochondrial-dependent apoptosis and causes genomic DNA damage in human gingival epithelial cells. J Periodontal Res. 45(1):31–37. [DOI] [PubMed] [Google Scholar]
- Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ. 2009. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res. 105(4):365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper CE, Brown GC. 2008. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J Bioenerg Biomembr. 40(5):533–539. [DOI] [PubMed] [Google Scholar]
- Dhonukshe-Rutten RA, Pluijm SM, de Groot LC, Lips P, Smit JH, van Staveren WA. 2005. Homocysteine and vitamin B12 status relate to bone turnover markers, broadband ultrasound attenuation, and fractures in healthy elderly people. J Bone Miner Res. 20(6):921–929. [DOI] [PubMed] [Google Scholar]
- Feng Y, Arvey A, Chinen T, van der Veeken J, Gasteiger G, Rudensky AY. 2014. Control of the inheritance of regulatory t cell identity by a cis element in the Foxp3 locus. Cell. 158(4):749–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox B, Schantz JT, Haigh R, Wood ME, Moore PK, Viner N, Spencer JP, Winyard PG, Whiteman M. 2012. Inducible hydrogen sulfide synthesis in chondrocytes and mesenchymal progenitor cells: is H2S a novel cytoprotective mediator in the inflamed joint? J Cell Mol Med. 16(4):896–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Chen J, Li K, Wu T, Huang B, Liu W, Kou X, Zhang Y, Huang H, Jiang Y, et al. 2013. Replacement of Oct4 by Tet1 during iPSC induction reveals an important role of DNA methylation and hydroxymethylation in reprogramming. Cell Stem Cell. 12(4):453–469. [DOI] [PubMed] [Google Scholar]
- Gjesdal CG, Vollset SE, Ueland PM, Refsum H, Meyer HE, Tell GS. 2007. Plasma homocysteine, folate, and vitamin B12 and the risk of hip fracture: The hordaland homocysteine study. JJ Bone Miner Res. 22(5):747–756. [DOI] [PubMed] [Google Scholar]
- Grassi F, Tyagi AM, Calvert JW, Gambari L, Walker LD, Yu M, Robinson J, Li JY, Lisignoli G, Vaccaro C, et al. 2015. Hydrogen sulfide is a novel regulator of bone formation implicated in the bone loss induced by estrogen deficiency. J Bone Miner Res. 31(5):949–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. 2000. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 97(25):13625–13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Li CS, Wang CM, Xie YJ, Wang AL. 2015. CSE/H2S system protects mesenchymal stem cells from hypoxia and serum deprivationinduced apoptosis via mitochondrial injury, endoplasmic reticulum stress and PI3K/Akt activation pathways. Mol Med Rep. 12(2):2128–2134. [DOI] [PubMed] [Google Scholar]
- Huang GT, Gronthos S, Shi S. 2009. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 88(9):792–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama K, Chen T, Wang X, Yan X, Kim BS, Tanaka S, Ndiaye-Lobry D, Deng Y, Zou Y, Zheng P, et al. 2015. The methylcytosine dioxygenase Tet2 promotes DNA demethylation and activation of cytokine gene expression in T cells. Immunity. 42(4):613–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishkitiev N, Calenic B, Aoyama I, Ii H, Yaegaki K, Imai T. 2012. Hydrogen sulfide increases hepatic differentiation in tooth-pulp stem cells. J Breath Res. 6(1):017103. [DOI] [PubMed] [Google Scholar]
- Jeney V, Komodi E, Nagy E, Zarjou A, Vercellotti GM, Eaton JW, Balla G, Balla J. 2009. Supression of hemin-mediated oxidation of low-density lipoprotein and subsequent endothelial reactions by hydrogen sulfide (H(2)S). Free Radic Biol Med. 46(5):616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno S, Hirano S, Sagi M, Chiba S, Takeshita H, Ikawa T, Ichiba K, Nagai T, Takada M, Sakamoto K, et al. 2013. Sulfide induces apoptosis and Rho kinase-dependent cell blebbing in Jurkat cells. Arch Toxicol. 87(7):1245–1256. [DOI] [PubMed] [Google Scholar]
- Kaur S, Schwartz AL, Miller TW, Roberts DD. 2015. Cd47-dependent regulation of H(2)S biosynthesis and signaling in T cells. Methods Enzymol. 555:145–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Dargusch R, Schubert D, Kimura H. 2006. Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid Redox Signal. 8(3–4):661–670. [DOI] [PubMed] [Google Scholar]
- Kiss L, Deitch EA, Szabo C. 2008. Hydrogen sulfide decreases adenosine triphosphate levels in aortic rings and leads to vasorelaxation via metabolic inhibition. Life Sci. 83(17–18):589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi C, Yaegaki K, Calenic B, Ishkitiev N, Imai T, Ii H, Aoyama I, Kobayashi H, Izumi Y, Haapasalo M. 2011. Hydrogen sulfide causes apoptosis in human pulp stem cells. J Endod. 37(4):479–484. [DOI] [PubMed] [Google Scholar]
- Li C, Guo Z, Guo B, Xie Y, Yang J, Wang A. 2014. Inhibition of the endogenous CSE/H(2)S system contributes to hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells. Mol Med Rep. 9(6):2467–2472. [DOI] [PubMed] [Google Scholar]
- Li L, Rose P, Moore PK. 2011. Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol. 51:169–187. [DOI] [PubMed] [Google Scholar]
- Li L, Salto-Tellez M, Tan CH, Whiteman M, Moore PK. 2009. Gyy4137, a novel hydrogen sulfide-releasing molecule, protects against endotoxic shock in the rat. Free Radic Biol Med. 47(1):103–113. [DOI] [PubMed] [Google Scholar]
- Liu F, Chen DD, Sun X, Xie HH, Yuan H, Jia W, Chen AF. 2014. Hydrogen sulfide improves wound healing via restoration of endothelial progenitor cell functions and activation of angiopoietin-1 in type 2 diabetes. Diabetes. 63(5):1763–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Yang R, Liu X, Zhou Y, Qu C, Kikuiri T, Wang S, Zandi E, Du J, Ambudkar IS, et al. 2014. Hydrogen sulfide maintains mesenchymal stem cell function and bone homeostasis via regulation Of Ca(2+) channel sulfhydration. Cell Stem Cell. 15(1):66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean RR, Jacques PF, Selhub J, Tucker KL, Samelson EJ, Broe KE, Hannan MT, Cupples LA, Kiel DP. 2004. Homocysteine as a predictive factor for hip fracture in older persons. N Engl J Med. 350(20):2042–2049. [DOI] [PubMed] [Google Scholar]
- Miller TW, Wang EA, Gould S, Stein EV, Kaur S, Lim L, Amarnath S, Fowler DH, Roberts DD. 2012. Hydrogen sulfide is an endogenous potentiator of T cell activation. J Biol Chem. 287(6):4211–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirandola P, Gobbi G, Sponzilli I, Pambianco M, Malinverno C, Cacchioli A, De Panfilis G, Vitale M. 2007. Exogenous hydrogen sulfide induces functional inhibition and cell death of cytotoxic lymphocytes subsets. J Cell Physiol. 213(3):826–833. [DOI] [PubMed] [Google Scholar]
- Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. 2003. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 100(10):5807–5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsczeck C, Gotz W, Schierholz J, Zeilhofer F, Kuhn U, Mohl C, Sippel C, Hoffmann KH. 2005. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 24(2):155–165. [DOI] [PubMed] [Google Scholar]
- Munaron L, Avanzato D, Moccia F, Mancardi D. 2013. Hydrogen sulfide as a regulator of calcium channels. Cell Calcium. 53(2):77–84. [DOI] [PubMed] [Google Scholar]
- Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, Snyder SH. 2009. H2S signals through protein S-sulfhydration. Sci Signal. 2(96):ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SA, Li MO. 2015. TETs link hydrogen sulfide to immune tolerance. Immunity. 43(2):211–213. [DOI] [PubMed] [Google Scholar]
- Ohkura N, Hamaguchi M, Morikawa H, Sugimura K, Tanaka A, Ito Y, Osaki M, Tanaka Y, Yamashita R, Nakano N, et al. 2012. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity. 37(5):785–799. [DOI] [PubMed] [Google Scholar]
- Okada M, Ishkitiev N, Yaegaki K, Imai T, Tanaka T, Fukuda M, Ono S, Haapasalo M. 2014. Hydrogen sulphide increases hepatic differentiation of human tooth pulp stem cells compared with human bone marrow stem cells. Int Endod J. 47(12):1142–1150. [DOI] [PubMed] [Google Scholar]
- Paul BD, Snyder SH. 2012. H(2)S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol. 13(8):499–507. [DOI] [PubMed] [Google Scholar]
- Predmore BL, Lefer DJ, Gojon G. 2012. Hydrogen sulfide in biochemistry and medicine. Antioxid Redox Signal. 17(1):119–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi L, Gobbi G, Pambianco M, Micheloni C, Mirandola P, Vitale M. 2006. Hydrogen sulfide prevents apoptosis of human PMN via inhibition of p38 and caspase 3. Lab Invest. 86(4):391–397. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. 2008. Regulatory T cells and immune tolerance. Cell. 133(5):775–787. [DOI] [PubMed] [Google Scholar]
- Sen N, Paul BD, Gadalla MM, Mustafa AK, Sen T, Xu R, Kim S, Snyder SH. 2012. Hydrogen sulfide-linked sulfhydration of NF-kappaB mediates its antiapoptotic actions. Mol Cell. 45(1):13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo B-M, Miura M, Gronthos S, Mark Bartold P, Batouli S, Brahim J, Young M, Gehron Robey P, Wang CY, Shi S. 2004. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 364(9429):149–155. [DOI] [PubMed] [Google Scholar]
- Sitdikova GF, Weiger TM, Hermann A. 2010. Hydrogen sulfide increases calcium-activated potassium (BK) channel activity of rat pituitary tumor cells. Pflugers Arch. 459(3):389–397. [DOI] [PubMed] [Google Scholar]
- Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, Huang GT. 2008. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 34(2):166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Liu D, Liu Y, Zhang C, Wang J, Wang S. 2015. Physiologic levels of endogenous hydrogen sulfide maintain the proliferation and differentiation capacity of periodontal ligament stem cells. J Periodontol. 86(11):1276–1286. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Olah G, Modis K, Coletta C, Kulp G, Gero D, Szoleczky P, Chang T, Zhou Z, Wu L, et al. 2011. Hydrogen sulfide replacement therapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proc Natl Acad Sci U S A. 108(33):13829–13834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Setoguchi T, Machigashira M, Kanbara K, Izumi Y. 2008. Hydrogen sulfide inhibits cell proliferation and induces cell cycle arrest via an elevated p21 Cip1 level in Ca9-22 cells. J Periodontal Res. 43(1):90–95. [DOI] [PubMed] [Google Scholar]
- Tang HB, Li YS, Miyano K, Nakata Y. 2008. Phosphorylation of TRPV1 by neurokinin-1 receptor agonist exaggerates the capsaicin-mediated substance P release from cultured rat dorsal root ganglion neurons. Neuropharmacology. 55(8):1405–1411. [DOI] [PubMed] [Google Scholar]
- Vandiver M, Snyder SH. 2012. Hydrogen sulfide: a gasotransmitter of clinical relevance. J Mol Med (Berl). 90(3):255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meurs JB, Dhonukshe-Rutten RA, Pluijm SM, van der Klift M, de Jonge R, Lindemans J, de Groot LC, Hofman A, Witteman JC, van Leeuwen JP, et al. 2004. Homocysteine levels and the risk of osteoporotic fracture. N Engl J Med. 350(20):2033–2041. [DOI] [PubMed] [Google Scholar]
- Wang R. 2012. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev. 92(2):791–896. [DOI] [PubMed] [Google Scholar]
- Wang Z, Liu DX, Wang FW, Zhang Q, Du ZX, Zhan JM, Yuan QH, Ling EA, Hao AJ. 2013. L-Cysteine promotes the proliferation and differentiation of neural stem cells via the CBS/H2S pathway. Neuroscience. 237:106–117. [DOI] [PubMed] [Google Scholar]
- Xie X, Sun A, Zhu W, Huang Z, Hu X, Jia J, Zou Y, Ge J. 2012. Transplantation of mesenchymal stem cells preconditioned with hydrogen sulfide enhances repair of myocardial infarction in rats. Tohoku J Exp Med. 226(1):29–36. [DOI] [PubMed] [Google Scholar]
- Yan Y, Liu F, Kou X, Liu D, Yang R, Wang X, Song Y, He D, Gan Y, Zhou Y. 2015. T cells are required for orthodontic tooth movement. J Dent Res. 94(10):1463–1470. [DOI] [PubMed] [Google Scholar]
- Yang G, Sun X, Wang R. 2004. Hydrogen sulfide-induced apoptosis of human aorta smooth muscle cells via the activation of mitogen-activated protein kinases and caspase-3. FASEB J. 18(14):1782–1784. [DOI] [PubMed] [Google Scholar]
- Yang G, Yang W, Wu L, Wang R. 2007. H2S, endoplasmic reticulum stress, and apoptosis of insulin-secreting beta cells. J Biol Chem. 282(22):16567–16576. [DOI] [PubMed] [Google Scholar]
- Yang R, Qu C, Zhou Y, Konkel JE, Shi S, Liu Y, Chen C, Liu S, Liu D, Chen Y, et al. 2015. Hydrogen sulfide promotes Tet1- and Tet2-mediated Foxp3 demethylation to drive regulatory T cell differentiation and maintain immune homeostasis. Immunity. 43(2):251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JH, Dong Z, Chu L. 2010. Hydrogen sulfide induces apoptosis in human periodontium cells. J Periodontal Res. 45(1):71–78. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Shi S, Le AD. 2009. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 183(12):7787–7798. Erratum in J Immunol. 2010;184(3):1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Zhang J, Lu Y, Wang R. 2001. The vasorelaxant effect of H(2)S as a novel endogenous gaseous k(atp) channel opener. EMBO J. 20(21):6008–6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Wei H, Kong G, Shim W, Zhang G. 2013. Hydrogen sulfide augments the proliferation and survival of human induced pluripotent stem cell-derived mesenchymal stromal cells through inhibition of BKCa. Cytotherapy. 15(11):1395–1405. [DOI] [PubMed] [Google Scholar]
- Zhu J, Yamane H, Paul WE. 2010. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 28:445–489. [DOI] [PMC free article] [PubMed] [Google Scholar]