Abstract
Recent studies have shown that native phosphorylated full-length porcine amelogenin (P173) and its predominant cleavage product (P148) can inhibit spontaneous calcium phosphate formation in vitro by stabilizing an amorphous calcium phosphate (ACP) precursor phase. Since full-length amelogenin undergoes proteolysis by matrix metalloproteinase 20 (MMP20, enamelysin) soon after secretion, the present study was conducted to assess the effect of amelogenin proteolysis on calcium phosphate formation. Calcium and phosphate were sequentially added to protein solutions without and with added MMP20 (ratio = 200:1) under physiological-like conditions of ionic strength (163 mM) in 50 mM Tris-HCl (pH 7.4) at 37 °C. Protein degradation with time was assessed by gel-electrophoresis, and mineral products formed were characterized by transmission electron microscopy (TEM). MMP20 was found to cleave P173 to primarily generate P148, along with P162, P46-148, and P63/64-148. In sharp contrast, MMP20 did not cleave P148. In addition, the formation of well-aligned bundles of enamel-like hydroxyapatite (HA) crystals was promoted in the presence of P173 with added MMP20, while only ACP particles were seen in the absence of MMP20. Although P148 was found to have a somewhat lower capacity to stabilize ACP and prevent HA formation compared with P173 in the absence of MMP20, essentially no HA formation was observed in the presence of somewhat higher concentrations of P148 regardless of MMP20 addition, due to the lack of observed protein proteolysis. Present findings suggest that ACP transformation to ordered arrays of enamel crystals may be regulated in part by the proteolysis of full-length native amelogenin, while the predominant amelogenin degradation product in developing enamel (e.g., P148) primarily serves to prevent uncontrolled mineral formation during the secretory stage of amelogenesis.
Keywords: amelogenesis, amorphous calcium phosphate, dental enamel, enamel matrix proteins, hydroxyapatite, crystal growth
Introduction
Dental enamel, the most highly mineralized tissue of the body (>97% mineral), comprises an organized assembly of enamel rods. Each rod is composed of densely packed and extremely long coaligned carbonatoapatite crystals that extend almost the full thickness of the enamel layer. During the secretory stage of enamel development, matrix proteins secreted by ameloblasts control the appositional growth of elongating but very thin ribbons of apatitic mineral (Smith 1998). These proteins are subsequently removed prior to the volumetric growth of initially formed mineral ribbons during the maturation stage of amelogenesis. The amount and nature of proteins present in the local environment are believed to affect this volumetric growth process (Smith et al. 2011). Amelogenin, the major constituent (~90%) of the enamel matrix, has been shown to be essential for enamel formation (Gibson et al. 2001). During enamel development, however, full-length amelogenin is processed by proteinases soon after secretion. Full-length amelogenins exhibit a capacity to guide the formation of ordered bundles of hydroxyapatite (HA) crystals in vitro, unlike amelogenin cleavage products that lack the hydrophilic C-terminus (Beniash et al. 2005; Kwak et al. 2009; Le Norcy et al. 2011a, 2011b; Wiedemann-Bidlack et al. 2011; Kwak et al. 2014). In pig, the parent full-length amelogenin, P173, undergoes degradation to produce smaller protein fragments, including P148, which is the most abundant protein in the enamel matrix. Matrix metalloproteinase 20 (MMP20), expressed by ameloblasts during secretory and transition stages of amelogenesis (Bartlett et al. 1996; Bartlett et al. 1998; Bègue-Kirn et al. 1998), has been reported to process amelogenins to yield all cleavage products found in secretory-stage porcine enamel (Nagano et al. 2009). MMP20 is also essential for proper enamel formation. MMP20-deficient mice (Caterina et al. 2002), like amelogenin-null mice (Gibson et al. 2001), exhibit dramatic enamel phenotypes.
The influences of amelogenin proteolysis on factors that affect mineralization are not fully understood, although in vitro studies have provided some insight into this process. Studies have addressed the coassembly between amelogenin and its proteolytic products during MMP20 digestion (He et al. 2008; Uskokovic et al. 2008; Yang et al. 2011), the effect of mineral ions on MMP20 kinetics of amelogenin hydrolysis (Khan et al. 2012), the apatite binding affinities of amelogenin cleavage products (Sun et al. 2008), the effect of apatite on the rate of amelogenin proteolysis by MMP20 (Sun et al. 2010), and the effect of MMP20-induced hydrolysis of amelogenin on the rate of mineral growth on crystalline substrates (Uskokovic et al. 2011). Of note, Sun et al. (2010) reported that MMP20 hydrolyzed a recombinant version of P148 with lower efficiency compared with full-length amelogenin. These prior studies, however, used recombinant amelogenins, which primarily differ from native amelogenins by lacking a single phosphate group on serine 16 (Appendix Fig. 1). Recent studies in our laboratory using native phosphorylated porcine amelogenins have shown that this single phosphate group significantly affects protein-mediated mineralization in vitro. In particular, we have shown that phosphorylated full-length porcine amelogenin P173 (Wiedemann-Bidlack et al. 2011) and its predominant proteolytic degradation product P148 (Kwak et al. 2009), as well as the phosphorylated leucine-rich amelogenin peptide (LRAP; Le Norcy et al. 2011a), have an enhanced capacity to stabilize amorphous calcium phosphate (ACP) precursors and inhibit the transformation of ACP nanoparticles to HA crystals under conditions that support spontaneous calcium phosphate formation, in comparison to that seen using nonphosphorylated amelogenins. ACP stabilization and transformation to HA have been found to occur during the initial formation of ordered bundles of apatitic enamel crystals in the secretory stage of developing enamel (Beniash et al. 2009). These latter findings, as well as those noted in the preceding paragraph, have led us to hypothesize that this biologically relevant mineral phase transformation is an active process induced by MMP20 proteolysis of full-length native amelogenin. The present study was conducted to test this hypothesis by investigating the effect of MMP20 proteolysis on the capacity of full-length (P173) and cleaved (P148) native porcine amelogenins to guide the ordered formation of enamel-like crystals in vitro.
Materials and Methods
Preparation of Porcine Amelogenins
Full-length native porcine amelogenin, P173, and its predominant cleavage product, P148, were isolated from developing teeth and purified, as reported (Yamakoshi et al. 1994). Based on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis, the purity of P173 and P148 was assessed to be >90% and 86% to 90%, respectively. Native amelogenins contain a single phosphate group at serine 16 and an N-terminal methionine, which are absent in their respective recombinant counterparts, rP172 and rP147. P148 lacks 25 C-terminal amino acids found in P173 (Appendix Fig. 1). Lyophilized proteins were weighed and dissolved in distilled deionized water (DDW) to yield 5-mg/mL stock solutions. Protein solutions had pH values ~3 and were maintained at 4 °C for at least 24 h to ensure complete dissolution, as confirmed using dynamic light scattering (Wiedemann-Bidlack et al. 2007). Protein stock solutions were centrifuged (11,340 × g, Eppendorf Centrifuge 5403, Eppendorf North America) at 4 °C for 20 min, just prior to use, to remove dust and any particulate matter.
Preparation of Porcine MMP20
Soft enamel from 5-mo-old pig second molars was removed by scraping and sequentially extracted using phosphate (pH 7.4) and carbonate (pH 10.8) buffers. The latter alkaline extract was fractionated by ion-exchange chromatography. Four fractions were then further fractionated by a step gradient with 0, 0.02, 0.05, and 0.5 M NaCl. MMP20 was eluted in the second peak (= 0.02 M NaCl). This fraction was concentrated and stored at −80 °C. Proteinase activity was confirmed by zymography and SDS-PAGE, as previously reported (Yamakoshi et al. 2013). Based on SDS-PAGE, its purity was estimated to be 70% to 80%. In selected experiments, the catalytic domain of human recombinant MMP20 (Giotto Biotech S.r.l.) was also used.
Mineralization Studies with and without Added MMP20
Calcium and pH-adjusted phosphate (pH 10.9) solutions were sequentially added to protein solutions, as previously described (Kwak et al. 2009; Kwak et al. 2011). The final solutions contained 2.5 mM calcium (CaCl2), 1.5 mM phosphate (KH2PO4), and 1 mg/mL amelogenin (final volume = 0.03 mL). Unlike previous studies (Kwak et al. 2009), the present study was carried out at a physiological ionic strength (IS) of 163 mM, adjusted by the addition of KCl and 50 mM Tris-HCl buffer (pH 7.4 at 37 °C). Tris-HCl buffer was included in the calcium and phosphate solutions prior to mixing. This solution was designed to mimic the IS of secretory enamel fluid (Aoba and Moreno 1987) and to optimize MMP20 activity by maintaining solution pH during the 48-h experimental time period. Protein degradation was initiated by adding 5 µg/mL MMP20 (MMP20/protein [wt:wt] = 1:200). Sample tubes were then placed in a water bath at 37 °C. To minimize evaporation, tubes were sealed with caps or parafilm (America National Can). Each experiment included 7 identically prepared sample tubes, which were used subsequently for transmission electron microscopy (TEM) and SDS-PAGE analyses at preselected times: 0.5, 4, 8, 12, 18, 24, and 48 h. After removing two 5-µL aliquots for TEM analyses at each time, the remaining sample tube content was used for SDS-PAGE analyses to assess proteolysis, as described below. Each experiment was repeated 6 to 10 times. Control mineralization experiments were performed without MMP20, using DDW instead of MMP20 solution. To confirm effects of P148 on HA formation (with and without MMP20), P148 concentration was varied using 1, 2, and 3 mg/mL, while the P148 to MMP20 ratio remained fixed at 200:1. MMP20-induced proteolysis of amelogenins was also assessed under nonmineralizing conditions, as described above for the mineralization studies, but using DDW instead of KH2PO4 solution. Experiments were repeated 4 to 5 times.
Gel Electrophoresis
At noted times, after the removal of two 5-µL aliquots for TEM, equal volumes of sample buffer (Novex Tris-Glycine SDS Sample Buffer; Life Technologies) were added to each sample tube to quench the reaction and to ensure complete protein recovery, including that which adheres to test tube surfaces. The mixtures were vortexed and boiled at 80 °C for 2 min and then immediately frozen (−20 °C). SDS-PAGE was performed using Novex 18% Tris-Glycine Gels (Life Technologies) that were subsequently stained with Coomassie blue (SimplyBlue Safe Stain; Life Technologies). Molecular weights of migrating bands were estimated using a standard curve of the log values of known molecular weight markers (Novex Sharp Prestained Protein Standard and BenchMark Prestained Protein Ladder; Life Technologies) plotted versus migration distances using SigmaPlot 10.0 (Systat Software). To assess the extent of proteolysis, gel band densities were estimated using Image J 1.44o (National Institutes of Health). Band densities for each SDS gel were normalized to the intensity of 4 marker bands (30, 20, 15, and 10 KDa) used in each gel.
TEM and Selected-Area ElectronDiffraction Analyses
Two 5-µL aliquots of each solution taken at specified times during the mineralization experiments were used to prepare duplicate TEM grids. Each aliquot was placed on a carbon-coated Cu grid (Electron Microscopy Sciences) for 0.5 to 1 min, blotted vertically against filter paper, quickly rinsed with DDW, blotted again, and then air-dried. Images were then obtained in bright-field and selected-area electron diffraction (SAED) modes using a JEOL 1200 TEM at 100 KV and captured by an AMT CCD camera (AMT). TEM was used to assess mineral morphology and orientation, while SAED was used to assess mineral phase and crystal orientation.
Results
In the absence of the native amelogenins and MMP20, ACP nanoparticles were predominantly seen at 24 h (Fig. 1A). These particles were found to almost completely transform into randomly arranged HA plate-like crystals at 48 h (Fig. 1B). SAED analyses (Fig. 1B, inset) showed ring patterns for 002 and 004 reflections, along with reflections for 121, 112, 300 crystal planes, indicating that formed crystals are not oriented. With full-length P173, however, ACP particles were solely observed at both 24 and 48 h (Fig. 1C, D). Under the same conditions, ACP particles were similarly found in the presence of truncated P148 at 24 h (Fig. 1E) and 48 h (Fig. 1F), although some plate-like HA crystals were observed at the latter time (Fig. 1G). This slightly reduced capacity of P148 to stabilize ACP at higher IS, in comparison to P173, may be due to a tendency for the more hydrophobic P148 to aggregate (Kwak et al. 2011). At higher P148 concentrations of 2 mg/mL (Fig. 1H) and 3 mg/mL (Appendix Fig. 2A), ACP was almost exclusively observed. In the presence of P173 with added MMP20, spherical particles of ACP were again exclusively observed throughout the first 24 h, as confirmed by SAED (Fig. 2A–C, insets), although dense bundles of HA crystals began to appear at 24 h (data not shown), along with the predominance of ACP nanoparticles (Fig. 2C). By 48 h (Fig. 2D), however, large thick bundles of well-aligned needle-like HA crystals were readily observed, along with remaining ACP particles (Fig. 2E). Extended networks of dense and well-aligned HA crystals could also be seen at 48 h (Fig. 2F). SAED analyses (Fig. 2D, F, insets) are consistent with the formation of HA crystals coaligned along their c-axes, as indicated by the narrow arc patterns at 002 and 004 positions. These patterns are clearly different in comparison to the SAED patterns of randomly aggregated plate-like crystals seen in the control (Fig. 1B, inset).
Figure 1.
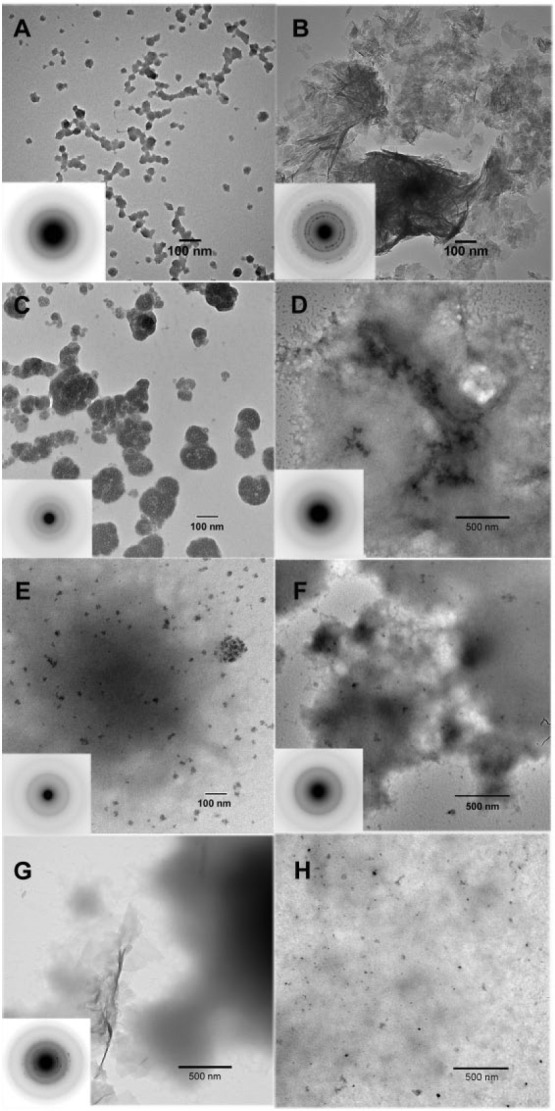
Transmission electron microscopy (TEM) and selected area electron diffraction (SAED) (insets) analyses of calcium phosphate mineral products formed. As a control, in the absence of protein, amorphous calcium phosphate (ACP) particles were predominantly observed at 24 h (A). Subsequently, these initially formed ACP nanoparticles were found to almost completely transform into large agglomerations of randomly arranged plate-like hydroxyapatite (HA) crystals at 48 h (B). In the presence of 1 mg/mL P173 alone, only ACP particles were observed at 24 h (C) and 48 h (D). In the presence of 1 mg/mL P148 (E–G) without MMP20, ACP was observed at both 24 h (E) and 48 h (F), although some randomly disordered plate-like HA crystals were also found at 48 h (G). In the presence of 2 mg/mL P148 without MMP20, however, ACP particles were almost exclusively found at 48 h (H), along with protein aggregates that tended to exhibit greater contrast.
Figure 2.
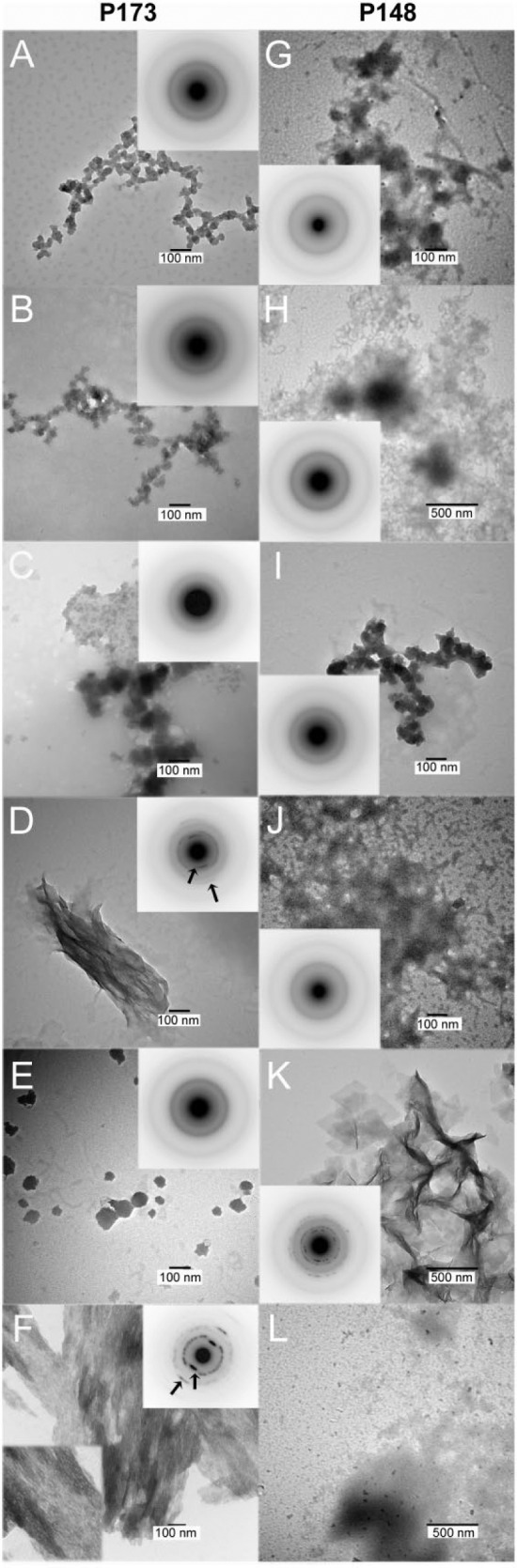
Transmission electron microscopy (TEM) and selected area electron diffraction (SAED) (insets) analyses of mineral phases formed in the presence of 1 mg/mL P173 and P148 with added MMP20 (200:1), respectively, examined at selected times: 4 h (A, G), 12 h (B, H), 24 h (C, I), and 48 h (D, E, F, J, K), as described in Experimental Procedures. In the presence of P173 with added MMP20, as shown in A–C, amorphous calcium phosphate (ACP) was initially formed and predominately observed up to 24 h based on the SAED patterns (insets), although hydroxyapatite (HA) crystals started to appear at 24 h (data not shown). ACP particle size was found to vary somewhat due to growth and/or aggregation (Kwak et al. 2009). After 48 h, however, well-aligned bundles of needle-like HA crystals were readily observed (D), along with remaining ACP particles (E). Extended networks of dense and well-aligned HA crystals could also be seen at 48 h (F), in the presence of P173 with added MMP20. SAED analyses (D and F, insets) are consistent with the formation of HA crystals coaligned along their c-axes, as indicated by the narrow arc patterns at 002 and 004 reflections (arrows). In the presence of P148 with added MMP20, as shown in G–J (insets), ACP nanoparticles were observed throughout the first 24 h and again at 48 h, along with predominant protein aggregates with dark contrast. At 48 h, some agglomerates of disordered plate-like HA crystals were also observed (K, inset). In the presence of 2 mg/mL P148 with MMP20 (200:1) at 48 h (L), however, only ACP particles were found. The capacity of P148 to stabilize ACP in the presence of MMP20 was also examined using a higher P148 concentration (Appendix Fig. 2B).
In the presence of P148 with added MMP20, ACP nanoparticles were observed throughout the first 24 h and again at 48 h (Fig. 2G–J, insets). At 48 h, as in the absence of MMP20, some agglomerates of plate-like HA crystals were also observed, along with protein aggregates (Fig. 2K, inset). The HA crystals were disordered and exhibited morphology similar to those seen in controls that lacked added protein or MMP20 (Fig. 1B). However, at higher P148 concentrations of 2 mg/mL (Fig. 2L) and 3 mg/mL (Appendix Fig. 2B) in the presence of MMP20, ACP was found at 48 h with little to no crystalline HA present, as was also seen in the absence of MMP20 (Fig. 1H, Appendix Fig. 2A).
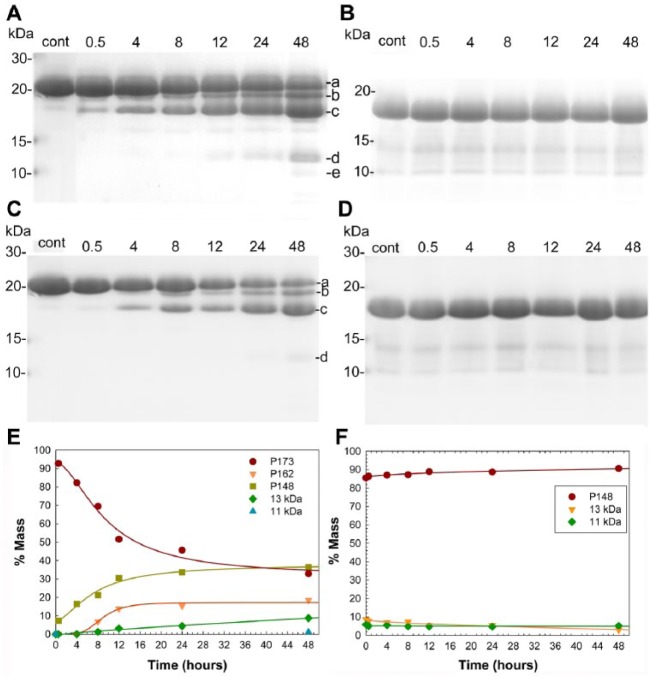
Under mineralizing conditions without MMP20, SDS-PAGE showed that P173 and P148 bands remained unchanged over the 48-h period (Appendix Fig. 3), as expected. As shown in Figure 3A, a gradual decrease in P173 content with time was induced by added MMP20 to primarily yield a steady and significant increase in P148 content, along with smaller amounts of lower molecular weight species, as illustrated, including P162, P46-148, and P63/64-148. The increase in P148 began to level off at ~24 h, as the smaller amounts of lower molecular weight bands emerged. At 48 h, the P173 content was ~33%, while that of P148 grew to ~37% of total protein (Fig. 3E). As shown in Figure 3B, MMP20 did not degrade P148 during the 48-h period. Essentially no change was observed in the protein profile or relative amounts of P148 and small amounts of lower molecular weight materials contained in the native starting material (Fig. 3F). Consistent with this latter observation, P148 was found to accumulate during MMP20-catalyzed hydrolysis of P173 (Fig. 3A, E). Under nonmineralization conditions (Fig. 3C, D), the proteolysis behaviors of P173 and P148 were similar to those observed under mineralizing conditions. The observed difference in MMP20 substrate specificity for P173 and P148 was further confirmed using a commercially available human recombinant catalytic domain of MMP20 under mineralizing and nonmineralizing conditions (Appendix Fig. 4).
Figure 3.
Proteolytic processing of P173 (A, C) and P148 (B, D) by native MMP20 (200:1) under mineralization (A, B) and nonmineralization (C, D) conditions, based on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analyses of whole-tube samples taken at selected time points over the 48-h experimental period. SDS-PAGE analyses showed that these starting native protein preparations (control, cont) contained relatively small amounts of lower molecular weight species. P173 was found to undergo progressive proteolysis in the presence of MMP20 to primarily yield P148, along with smaller amounts of lower molecular weight species, as illustrated: a, P173; b, P162; c, P148; d, P46-148; and e, P63/64-148. In contrast, essentially no change was observed in the P148 sample profile or in the size of the P148 band under the same experimental conditions. Quantitative assessments of the proteolytic processing of P173 (E) and P148 (F) by native MMP20 (200:1) under mineralization conditions were carried out based on SDS-PAGE results (A, B). As shown (E), by 48 h, P173 content was progressively reduced to around 33%, while that of P148 primarily grew to about 37% of total protein, along with lesser increases in lower molecular weight species. In contrast, the content of P148 did not change following exposure to MMP20 (F). In a comparative study, proteolytic processing of P173 (C) and P148 (D) by native MMP20 (200:1) under nonmineralization conditions was assessed based on SDS-PAGE analyses using the same sampling method. Under these conditions, P173 was found to undergo progressive degradation, resulting primarily in the accumulation of P148 and lower levels of smaller cleavage products (C): a, P173; b, P162; c, P148; and d, P46-148. In contrast, almost no change in the P148 sample profile was observed over the 48-h experimental period (D).
Discussion
Full-length porcine amelogenin, P173, undergoes proteolytic degradation soon after secretion (Uchida et al. 1991). P148 has been found to be the predominant cleavage product and amelogenin in developing porcine enamel (Nagano et al. 2009), representing 49.5% of total amelogenin, while the secreted full-length amelogenin P173 is reduced to only 7.4% of amelogenin present (Wen et al. 1999). These in vivo findings might suggest that MMP20 has a lower specificity toward the more hydrophobic P148 in comparison to full-length P173. Our current in vitro findings support this conclusion. Both in the absence and presence of mineralizing conditions, MMP20 was found to cleave P173 to primarily generate P148 and smaller amounts of lower molecular weight fragments (Fig. 3A, C). In contrast, P148 digestion by MMP20 was not observed under the same conditions (Fig. 3B, D). This observed lower efficiency of P148 proteolysis by MMP20, in comparison to P173, is consistent with a prior report on the MMP20-induced degradation of recombinant nonphosphorylated truncated amelogenin rP148 compared with the full-length recombinant nonphosphorylated amelogenin rP172 (Sun et al. 2010). Although the basis for observed differences in P173 and P148 degradation by MMP20 is not known, it may be related to differences in their self-assembly behaviors. P173 has been found to form rather isolated chain-like assemblies in the presence of calcium at high ionic strength (i.e., 163 mM), while P148 was found to be highly aggregated and form relatively large globular structures (Kwak et al. 2011; Wiedemann-Bidlack et al. 2007; Wiedemann-Bidlack et al. 2011).
Throughout the secretory stage, while MMP20 is being secreted, enamel crystals grow primarily in length as the enamel layer thickens (Hu et al. 2007). As discussed in the Introduction, we propose that initial enamel crystal formation requires the active degradation of full-length amelogenin by MMP20. In the current study, in the presence of P173 and added MMP20, well-aligned dense bundles of HA crystals are generated, while in the absence of MMP20, only ACP nanoparticles are found in support of our hypothesis. These results demonstrate that the transformation of stabilized ACP into ordered bundles of HA crystals, as seen in the secretory stage of enamel formation (Beniash et al. 2009), can be brought about via P173 proteolysis. As expected, the P173 inhibitory effect is concentration dependent (Kwak et al. 2014). Using a similar approach (albeit at lower ionic strength), at a much reduced protein concentration (0.4 mg/mL or 40% of the concentration used in the present study), P173 did not prevent crystal growth but rather guided the formation of well-aligned bundles of apatitic crystals, as seen in the present study. Although the inhibitory effect of truncated P148 on in vitro mineralization was also found to be concentration dependent, P148 did not similarly guide ordered mineral formation when present at lower concentrations, like full-length P173 (Kwak et al. 2014). This difference in effects on mineralization may again be related to prior findings showing that truncated P148 and its recombinant analogues from pig (rP147) and mouse (rM166) form random protein assemblies (Fang et al. 2011; Kwak et al. 2011; Wiedemann-Bidlack et al. 2011). In contrast, full-length P173, rP172, and rM179 (from mouse) undergo a stepwise hierarchical self-assembly process from monomers to nanospheres (Fang et al. 2011; Fang et al. 2013) to more highly ordered chain-like structures (Aichmayer et al. 2005; Aichmayer et al. 2010; Wiedemann-Bidlack et al. 2011).
On the basis of these collective findings, we further propose that growing and persisting higher levels of predominant truncated native amelogenins (e.g., P148 and M167) containing a single phosphorylated group play a key role in amelogenesis by preventing uncontrolled mineralization during the secretory stage where the mineral volume is only 10% to 20% (Fukae 2002; Robinson et al. 1988), while full-length phosphorylated amelogenins (e.g., P173 and M180) stabilize ACP precursors and guide their alignment and transformation to parallel arrays of needle-like enamel crystals, upon degradation by MMP20. Hence, as in the development of other mineralized tissues, inhibitors of mineralization play multiple roles in regulating enamel formation, by stabilizing precursor mineral phases, maintaining appropriate levels of supersaturation, preventing aberrant mineral formation, and controlling crystal morphology (Margolis et al. 2014). Consistent with these capacities, organized arrays of stable parallel-oriented ACP mineral particles have been observed in developing mouse incisor enamels, prior to their transformation to enamel crystallites (Beniash et al. 2009).
In the present study, the onset of HA formation was brought about by the partial proteolysis of P173 by MMP20. P173 concentration was reduced by approximately 67% to 0.33 mg/mL (Fig. 3E). As already noted, a similarly low concentration of P173 (0.4 mg/mL) was also found to guide oriented HA growth in vitro (Kwak et al. 2014). However, in the present study where P173 is being processed by added MMP20, a significant amount of P148 (~0.37 mg/mL) was generated along with lesser amounts of smaller cleavage products (Fig. 3A). Nevertheless, we observe similar behavior with respect to the regulation of oriented HA growth, when like concentrations of P173 are used or generated in situ. Upon closer examination of these findings, however, there is a distinct difference with respect to mineral formation that may be attributed to the combined presence of P173 and its cleavage products. In the present study, with a generated mixture of P173 and P148 (Fig. 3E), stabilized ACP was also observed throughout the reaction period, while ACP was not found in the previous study (Kwak et al. 2014) using P173 without added P148. Effects of small amounts of other degradation products generated in situ in the present study upon addition of MMP20, in addition to P148, cannot be ruled out, however, including a role in promoting mineralization.
In conclusion, we have provided new in vitro evidence that supports the hypothesis that ACP transformation to ordered arrays of enamel crystals is regulated in part by MMP20-induced proteolysis of full-length native amelogenin during initial stages of enamel formation. In addition, predominant amelogenin degradation products, like P148, may primarily serve to prevent uncontrolled aberrant mineralization during the secretory stage of amelogenesis.
Author Contributions
S.Y. Kwak, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; Y. Yamakoshi and J.P. Simmer, contributed to data acquisition, drafted the manuscript; H.C. Margolis, contributed to conception, design, data analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This study was supported by National Institutes of Dental and Craniofacial Research (NIDCR) grant R01-DE023091 (HCM).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Aichmayer B, Margolis HC, Sigel R, Yamakoshi Y, Simmer JP, Fratzl P. 2005. The onset of amelogenin nanosphere aggregation studied by small-angle X-ray scattering and dynamic light scattering. J Struct Biol. 151(3):239–249. [DOI] [PubMed] [Google Scholar]
- Aichmayer B, Wiedemann-Bidlack FB, Gilow C, Simmer JP, Yamakoshi Y, Emmerling F, Margolis HC, Fratzl P. 2010. Amelogenin nanoparticles in suspension: deviations from spherical shape and pH-dependent aggregation. Biomacromolecules. 11(2):369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoba T, Moreno EC. 1987. The enamel fluid in the early secretory stage of porcine amelogenesis: chemical composition and saturation with respect to enamel mineral. Calcif Tissue Int. 41(2):86–94. [DOI] [PubMed] [Google Scholar]
- Bartlett JD, Ryu OH, Xue J, Simmer JP, Margolis HC. 1998. Enamelysin mRNA displays a developmentally defined pattern of expression and encodes a protein which degrades amelogenin. Connect Tissue Res. 39(1–3):101–109; discussion 141–149. [DOI] [PubMed] [Google Scholar]
- Bartlett JD, Simmer JP, Xue J, Margolis HC, Moreno EC. 1996. Molecular cloning and mRNA tissue distribution of a novel matrix metalloproteinase isolated from porcine enamel organ. Gene. 183(1–2):123–128. [DOI] [PubMed] [Google Scholar]
- Bègue-Kirn C, Krebsbach PH, Bartlett JD, Butler WT. 1998. Dentin sialoprotein, dentin phosphoprotein, enamelysin and ameloblastin: tooth-specific molecules that are distinctively expressed during murine dental differentiation. Eur J Oral Sci. 106(5):963–970. [DOI] [PubMed] [Google Scholar]
- Beniash E, Metzler RA, Lam RS, Gilbert PU. 2009. Transient amorphous calcium phosphate in forming enamel. J Struct Biol. 166(2):133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beniash E, Simmer JP, Margolis HC. 2005. The effect of recombinant mouse amelogenins on the formation and organization of hydroxyapatite crystals in vitro. J Struct Biol. 149(2):182–190. [DOI] [PubMed] [Google Scholar]
- Caterina JJ, Skobe Z, Shi J, Ding Y, Simmer JP, Birkedal-Hansen H, Bartlett JD. 2002. Enamelysin (matrix metalloproteinase 20)–deficient mice display an amelogenesis imperfecta phenotype. J Biol Chem. 277(51):49598–49604. [DOI] [PubMed] [Google Scholar]
- Fang PA, Conway JF, Margolis HC, Simmer JP, Beniash E. 2011. Hierarchical self-assembly of amelogenin and the regulation of biomineralization at the nanoscale. Proc Natl Acad Sci USA. 108(34):14097–14102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang PA, Margolis HC, Conway JF, Simmer JP, Beniash E. 2013. CryoTEM study of effects of phosphorylation on the hierarchical assembly of porcine amelogenin and its regulation of mineralization in vitro. J Struct Biol. 183(2):250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukae M. 2002. Amelogenesis: three-dimensional structure of amelogenin micelles and their degradation by a cascade system. Tsurumi, Japan: International Symposium of Oral Science, Tsurumi University School of Dental Medicine. [Google Scholar]
- Gibson CW, Yuan ZA, Hall B, Longenecker G, Chen E, Thyagarajan T, Sreenath T, Wright JT, Decker S, Piddington R, et al. 2001. Amelogenin-deficient mice display an amelogenesis imperfecta phenotype. J Biol Chem. 276(34):31871–31875. [DOI] [PubMed] [Google Scholar]
- He X, Li W, Habelitz S. 2008. The cooperative self-assembly of 25 and 23 kDa amelogenins. J Struct Biol. 164(3):314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JC, Chun YH, Al Hazzazzi T, Simmer JP. 2007. Enamel formation and amelogenesis imperfecta. Cells Tissues Organs. 186(1):78–85. [DOI] [PubMed] [Google Scholar]
- Khan F, Liu H, Reyes A, Witkowska HE, Martinez-Avila O, Zhu L, Li W, Habelitz S. 2012. The proteolytic processing of amelogenin by enamel matrix metalloproteinase (MMP-20) is controlled by mineral ions. Biochim Biophys Acta. 1830(3):2600–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak SY, Green S, Wiedemann-Bidlack FB, Beniash E, Yamakoshi Y, Simmer JP, Margolis HC. 2011. Regulation of calcium phosphate formation by amelogenins under physiological conditions. Eur J Oral Sci. 119(Suppl 1):103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak SY, Kim S, Yamakoshi Y, Simmer JP, Beniash E, Margolis HC. 2014. Regulation of calcium phosphate formation by native amelogenins in vitro. Connect Tissue Res. 55(Suppl 1):21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak SY, Wiedemann-Bidlack FB, Beniash E, Yamakoshi Y, Simmer JP, Litman A, Margolis HC. 2009. Role of 20-kDa amelogenin (P148) phosphorylation in calcium phosphate formation in vitro. J Biol Chem. 284(28):18972–18979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Norcy E, Kwak SY, Wiedemann-Bidlack FB, Beniash E, Yamakoshi Y, Simmer JP, Margolis HC. 2011a. Leucine-rich amelogenin peptides regulate mineralization in vitro. J Dent Res. 90(9):1091–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Norcy E, Kwak SY, Wiedemann-Bidlack FB, Beniash E, Yamakoshi Y, Simmer JP, Margolis HC. 2011b. Potential role of the amelogenin N-terminus in the regulation of calcium phosphate formation in vitro. Cells Tissues Organs. 194(2–4):188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis HC, Kwak SY, Yamazaki H. 2014. Role of mineralization inhibitors in the regulation of hard tissue biomineralization: relevance to initial enamel formation and maturation. Front Physiol. 5:339 eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano T, Kakegawa A, Yamakoshi Y, Tsuchiya S, Hu JC, Gomi K, Arai T, Bartlett JD, Simmer JP. 2009. Mmp-20 and Klk4 cleavage site preferences for amelogenin sequences. J Dent Res. 88(9):823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson C, Kirkham J, Hallsworth AS. 1988. Volume distribution and concentration of protein, mineral and water in developing bovine enamel. Arch Oral Biol. 33(3):159–162. [DOI] [PubMed] [Google Scholar]
- Smith CE. 1998. Cellular and chemical events during enamel maturation. Crit Rev Oral Biol Med. 9(2):128–161. [DOI] [PubMed] [Google Scholar]
- Smith CE, Hu Y, Richardson AS, Bartlett JD, Hu JC, Simmer JP. 2011. Relationships between protein and mineral during enamel development in normal and genetically altered mice. Eur J Oral Sci. 119(Suppl 1):125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Carpiaux W, Fan D, Fan Y, Lakshminarayanan R, Moradian-Oldak J. 2010. Apatite reduces amelogenin proteolysis by MMP-20 and KLK4 in vitro. J Dent Res. 89(4):344–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Fan D, Fan Y, Du C, Moradian-Oldak J. 2008. Enamel proteases reduce amelogenin-apatite binding. J Dent Res. 87(12):1133–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T, Tanabe T, Fukae M, Shimizu M, Yamada M, Miake K, Kobayashi S. 1991. Immunochemical and immunohistochemical studies, using antisera against porcine 25 kDa amelogenin, 89 kDa enamelin and the 13–17 kDa nonamelogenins, on immature enamel of the pig and rat. Histochemistry. 96(2):129–138. [DOI] [PubMed] [Google Scholar]
- Uskokovic V, Kim MK, Li W, Habelitz S. 2008. Enzymatic processing of amelogenin during continuous crystallization of apatite. J Mater Res. 23(12):3184–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uskokovic V, Khan F, Liu H, Witkowska HE, Zhu L, Li W, Habelitz S. 2011. Hydrolysis of amelogenin by matrix metalloprotease-20 accelerates mineralization in vitro. Arch Oral Biol. 56(12):1548–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen HB, Moradian-Oldak J, Leung W, Bringas P, Jr, Fincham AG. 1999. Microstructures of an amelogenin gel matrix. J Struct Biol. 126(1):42–51. [DOI] [PubMed] [Google Scholar]
- Wiedemann-Bidlack FB, Beniash E, Yamakoshi Y, Simmer JP, Margolis HC. 2007. pH triggered self-assembly of native and recombinant amelogenins under physiological pH and temperature in vitro. J Struct Biol. 160(1):57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann-Bidlack FB, Kwak SY, Beniash E, Yamakoshi Y, Simmer JP, Margolis HC. 2011. Effects of phosphorylation on the self-assembly of native full-length porcine amelogenin and its regulation of calcium phosphate formation in vitro. J Struct Biol. 173(2):250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakoshi Y, Simmer JP, Bartlett JD, Karakida T, Oida S. 2013. MMP20 and KLK4 activation and inactivation interactions in vitro. Arch Oral Biol. 58(11):1569–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakoshi Y, Tanabe T, Fukae M, Shimizu M. 1994. Porcine amelogenins. Calcif Tissue Int. 54(1):69–75. [DOI] [PubMed] [Google Scholar]
- Yang X, Sun Z, Ma R, Fan D, Moradian-Oldak J. 2011. Amelogenin “nanorods” formation during proteolysis by Mmp-20. J Struct Biol. 176(2):220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.