Summary
Hereditary multiple exostoses (HME) is an inherited genetic condition characterized by the presence of multiple exostoses (osteochondromas). MHE is a relatively rare autosomal dominant disorder, mainly caused by loss of function mutations in two genes: exostosin-1 (EXT1) and exostosin-2 (EXT2). These genes are linked to heparan sulfate (HS) synthesis, but the specific molecular mechanism leading to the disruption of the cartilage structure and the consequent exostoses formation is still not resolved. The aim of this paper is to encounter the main aspects of HME reviewing the literature, in order to improve clinical features and evolution, and the metabolic-pathogenetic mechanisms underlying.
Although MHE may be asymptomatic, a wide spectrum of clinical manifestations is found in paediatric patients with this disorder. Pain is experienced by the majority of patients, even restricted motion of the joint is often encountered. Sometimes exostoses can interfere with normal development of the growth plate, giving rise to limb deformities, low stature and scoliosis. Other many neurovascular and associated disorders can lead to surgery. The most feared complication is the malignant transformation of an existing osteochondroma into a secondary peripheral chondrosarcoma, during adulthood. The therapeutic approach to HME is substantially surgical, whereas the medical one is still at an experimental level.
In conclusion, HME is a complex disease where the paediatrician, the geneticist and the orthopaedic surgeon play an interchangeable role in diagnosis, research and therapy. We are waiting for new studies able to explain better the role of HS in signal transduction, because it plays a role in other bone and cartilage diseases (in particular malignant degeneration) as well as in skeletal embryology.
Keywords: Hereditary Multiple Exostoses, osteochondromas, EXT1, EXT2, HS synthesis
Introduction
Hereditary multiple exostoses (HME), also known as familial ostechondromatosis or diaphyseal aclasis, is an inherited genetic condition characterized by the presence of multiple exostoses (osteochondromas) (1).
Osteochondroma is a benign tumour relatively frequent, defined by the WHO as a cartilage-capped bony outgrowth, which is broad-based (sessile) or stemmed (pedunculated) and is made up of cortex and a marrow cavity both continuous with the host bone. The pedunculated osteochondromas are always directed away from the growth plate and the joint. In broad-based osteochondromas it can be difficult to differentiate the tumour from the normal underlying bone. The most common location for osteochondromas is at the lateral side of the most active growth plate of a long bone. Osteochondromas develop and increase in number and size during childhood until closure of the growth plates.
In MHE, the osteochondromas are often highly irregular and can become extremely large. The most common clinical problems include pain and functional impairment. Additionally, growth deformities of bones and short stature are present in a considerable number of patients. In MHE, malignant degeneration of osteochondroma is a rare but important complication, especially in adult patients. The aim of this paper is to encounter the main aspects of HME reviewing the literature, in order to improve clinical features and evolution, and the metabolic-pathogenetic mechanisms underlying.
Epidemiology
MHE is relatively rare with an estimated prevalence in Caucasians of 1 per 50,000 individuals (2), as an incidence in the western population of 1,5% (3). However these values are probably underestimated, because individuals without complaints or asymptomatic lesions are often not diagnosed as HME.
In approximately 50% of individuals, multiple osteochondromas are initially diagnosed before 3,5 years of age. In more than 80%, it is diagnosed before the end of the first decade (2). In previous studies, there seemed to be a higher prevalence of MHE in males (4), while more recent studies have found no evidence of sexual predominance (5, 6).
Etiology and pathophysiology
MHE is a monogenetic, autosomal dominant disorder, mainly caused by loss of function mutations in two genes: exostosin-1 (EXT1) and exostosin-2 (EXT2). The first gene was located on chromosome 8 (locus 8q24.1) and was discovered by Cook in 1993. Later, EXT2 gene was identified on chromosome 11 (locus 11p11–13) (7). These mutations have been detected in 70–94% of human patients, with a higher frequency in EXT1 (8). The existence of a third MHE locus EXT3 has been discussed for many years, and also located on chromosome 19, but it has so far not been identified and its linkage to MHE has been questioned. Recently, somatic mosaic mutations in EXT1 or EXT2 have been proposed to cause MHE in patients previously tested negative for mutations in any of the two genes (9). According to the Multiple Osteochondroma Mutation Database, 429 mutations in EXT1 and 223 in EXT2 have been described (10). Most of these mutations (~77%) are sporadic, but at the same time very rare in population: in fact, only 30% of the affected individuals are spontaneous genetic mutations, whereas residual 70% have a family history. Ethnic concentration of these mutations has led to the speculation that their increased frequency is probably due to a common ancestor and founder effect rather than mutational sensitivity of the loci (11). We can observe it, for example, in the Chinese population, where EXT2 mutations have been reported to occur more frequently than EXT1 mutations, whereas it is demonstrated that EXT1 mutations are nearly twice as common as EXT2 mutations (12).
Due to the dominant inheritance of the disease, two models, haploinsufficiency and loss-of-heterozygosity (LOH), have been discussed as the molecular cause of HME. The first view expressed was that the “gene dosage” model might not be correct. This model hypothesizes that proper cartilage development and long bone growth would require normal levels of heparan sulfate (HS) synthesis and when levels dropped below a certain “threshold” due to EXT haploinsufficiency, an environment would be generated that is conducive to osteochondroma formation. Alternatively, LOH model has been proposed such that MHE affected individuals inheriting a single dysfunctional copy of either EXT1 or EXT2 would undergo a second somatic mutation within a normal EXT allele, resulting in localized osteochondroma formation. Jones confirmed that in 2010 with a genetically engineered mouse model: clonal inactivation of EXT1 in postnatal chondrocyte by a doxycicline inducible allele led to postnatal tumours developing at the side of the growth plate (13). Another recent study showed second hits in 63% of analysed osteochondromas and revealed a mixture of HS positive and negative cells in the cartilage caps. These results raise the possibility of undetected or masked second hits in negatively tested samples and support LOH as a molecular cause of HME (14). The researchers concluded that EXT genes functioned as tumour suppressor genes (15).
EXT1 and EXT2 are very similar genes: that suggests a similar biological work. The protein sequence (both of 80kD) showed structural similarities. But their functions remained unknown until two independent studies in 1998 that linked EXT1 and EXT2 to HS synthesis (16, 17): in particular these proteins result transmembrane glycoproteins with glycosyltransferase activity (Box 1). By researching homologous sequences others three EXT-like (EXTL) genes have been identified in mammals: EXTL1, EXTL2 and EXTL3, but they have not been linked to HME (18). Since the three EXTL-proteins share amino acid sequence homology with EXT1 and EXT2, it is expected that these proteins also might be involved in HS synthesis (Table 1).
BOX 1. Background.
| Ossification mechanism |
| The vertebrate skeleton develops by two mechanisms: intramembranous ossification and endochondral ossification. The first one gives origin to flat bones of the skull, parts of the craniofacial skeleton and clavicles, through a direct mechanism. Endochondral ossification generates the rest of the craniofacial bones and the axial and appendicular skeleton and is initiated with the formation of a cartilaginous template, subsequently replaced by bone. |
| Endochondral Ossification |
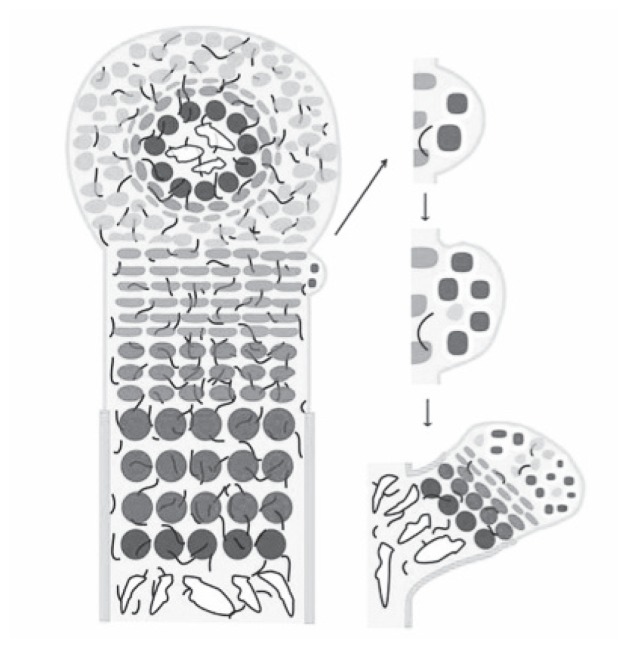
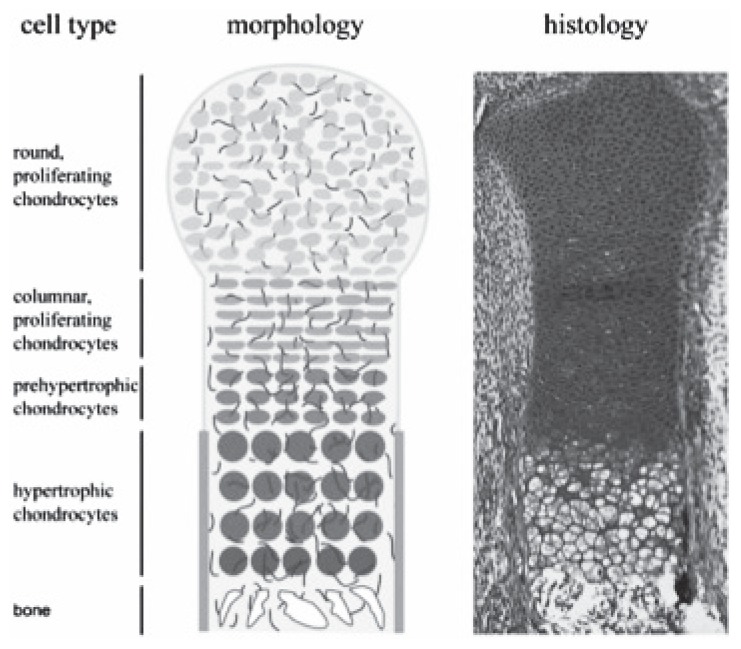
| During embryonic development, mesenchymal progenitor cells condensate at the site of the future bone and then differentiate into two cell types: in the centre they differentiate into chondrocytes, whereas the outer cells develop into a fibroblastic cell layer, the perichondrium, which surrounds the cartilage core (Figure 1). The cartilage elements grow by proliferation of chondrocytes, which align in columns. In the centre of the cartilage anlagen cells exit the cell cycle and differentiate into prehypertrophic and hypertrophic chondrocytes that finally undergo apoptosis. In parallel, cells in the flanking perichondrium differentiate into osteoblasts, forming the periosteum and the bone collar. The hypertrophic chondrocytes secrete blood vessel attracting factors leading to the vascularization. Accompanying osteoblasts and osteoclasts enter the hypertrophic region and replace the cartilage remnants with trabecular bone and bone marrow. Postnatally, these cells maintain their embryonic organization and form the growth plate, which enables the longitudinal growth of bones. |
| Growth factors role |
| Every step of endochondral ossification is tightly regulated by the concerted action of various growth factors, signalling molecules and cytokines. One of the key factors is the secreted growth factor Indian hedgehog (IHH). IHH acts together with Parathyroid Hormone related Peptide (PTHrP) in a negative feedback mechanism. IHH is expressed in prehypertrophic chondrocytes and signals to the proliferating chondrocytes and the flanking perichondrium, where it increases the proliferation rate and the differentiation of osteoblasts, respectively. Moreover, IHH induces the expression of PTHrP in periarticular chondrocytes. PTHrP signals back to the proliferating chondrocytes, keeping them in a proliferating state. With increasing distance from the producing cells, PTHrP levels decrease. Once they decline below a certain threshold, hypertrophic differentiation is initiated and the differentiating chondrocytes start to express IHH. Further signalling molecules controlling chondrocyte proliferation and differentiation include bone morphogenetic proteins (BMP), fibroblast growth factors (FGF), WNT proteins and many others. BMP signalling is required to form the cartilage anlagen. At later stages of chondrogenesis, BMP induces chondrocyte proliferation and up-regulate IHH expression, while FGF inhibits these processes. In addition, BMP signalling induces hypertrophic differentiation and FGF signals accelerate the turnover of hypertrophic cells. High WNT signalling is required for the condensation of mesenchymal cells and differentiation of the osteoblasts. A decrease in WNT signalling allows the differentiation of chondrocytes. Although the genetic interactions of these and other signalling molecules in regulating endochondral bone formation have been intensively studied, mechanisms that regulate their extracellular distribution are less well understood. A recent study demonstrates that HS plays a crucial role in these signalling pathways. |
| HS biosynthesis |
| The assembly of the HS chains takes place in the Golgi apparatus using nucleotide sugars imported from the cytoplasm. Heteromeric complexes of the glycosyltransferases exostosin-1 and exostosin-2 catalyse the elongation of the HS chains. Simultaneously, the HS chain is modified by several sulfotransferases and one epimerase. |
| HS carrying proteoglycans |
| HS carrying proteoglycans are main components of the extracellular matrix. They consist of carrier proteins and one or more HS glycosaminoglycan chains (linear polysaccharides). Depending on the subcellular localization of the HS carrying proteoglycans, three subfamilies can be distinguished: membrane spanning (syndecans and betaglycans), phosphatidylinositol-linked (glypicans), and proteoglycans secreted into the extracellular space (perlecan and agrin). |
| HS role |
| HS binds to extracellular proteins through interactions between the highly sulphated, negatively charged sugar motifs and clusters of positively charged amino acids on the protein surface. Tissue specific patterns of differently modified HS chains provide a highly complex, extra-cellular control system regulating signalling events in tissue specific ways. During recent years, many lines of evidence have identified HS as important regulators controlling the activity and signalling range of many secreted factors in the vertebrate organism, in particular in endochondral ossification. |
Table 1.
Exostosin human genes.
| Gene | Chromosome number | Amino acid |
|---|---|---|
| EXT 1 | 8 | 746 |
| EXT 2 | 11 | 718 |
| EXT-L 1 | 1 | 676 |
| EXT-L 2 | 1 | 330 |
| EXT-L 3 | 8 | 919 |
In HME, EXT genes may have mutations, chain elongations and non-sense coding, all leading to structural changes in EXT glycosyltransferases. Structural changes in these proteins lead to disturbed interactions and lower enzymatic activity, with a consequent lower production of HS. So a disturbance in the HS synthesis is the cause of the formation of exostoses. Moreover it has been proved that the severity of the disease is inversely correlated to HS level (19). Also the penetrance (~95%) tends to be complete when the fall in HS is greater (5). The reduction of HS in cell surface-associated proteoglycans and matrix-associated proteoglycans, impacts a number of signalizing proteins critical to skeletal development, such as IHH, PTHrP, FGF, BMP and WNT: distribution, range of activity, stability and action on target cells can be influenced (20).
Besides studying the role of HS in osteochondroma formation, several groups have started to use different mouse models with engineered EXT genes to investigate the function of HS as a regulator of endochondral ossification. Stickens et al. proposed in 2000 that exostoses are caused by a disturbed coordination of chondrocyte maturation and perichondrial bone formation (21). The mutation leading to a complete loss of expression of EXT1/EXT2 genes disturbs the crosstalk via IHH and PTHrP between chondrocytes and perichondrial osteogenic cells. This local defect of regulation leads to a hiatus in the forming of the bony collar. Even the tumour seems to start from early differentiate chondrocytes (and not from an osteoblasts) perichondrial cells play a crucial role because never in HME can be seen enchondromas or other benign tumours located within the bone or bone marrow (Figure 2). A novel theory based on previous findings was proposed. EXT mutations cause abnormal HS chains and this leads to disturbed binding of IHH. Loss of binding leads to abnormal diffusion and an excess of free IHH which leads to disturbance of terminal differentiation of chondrocytes at the growth plate, as well as disturbed perichondrial bone formation. With changes in diffusion area of IHH there is a loss of polar organisation on the growth plate. Combined with a defect in the bony collar, this leads to a pathological process. Chondrocytes proliferate further and migrate through the defect, which leads to abnormal growth and abnormal bone formation. The process exacerbates and there is uncontrolled growth of bone and cartilage in an angle opposed to normal growth (22).
Figure 2.
Model of ostochondroma formation (69).
In 2005 however another new model by Stickens et al. was introduced. There were suggestions that besides the IHH-PTHrP pathway, the major defects in HME would be located in the FGF and BMP pathway. In this model disturbed HS synthesis leads to lower FGF and disturbed differentiation of cartilage (23).
Collectively, these studies suggest that localized and/or tissue specific disruptions in HS-mediated signalling may be important for contributing to the various aspects of the MHE pathology. On the other hand, growth factors alteration may also represent a secondary consequence of osteochondroma formation. Although progress has been made, the specific molecular mechanism leading to the disruption of the cartilage structure and the consequent exostoses formation after HS loss is still not resolved.
Clinical presentation
Although MHE may be asymptomatic, a wide spectrum of clinical manifestations is found in patients with this disorder. As seen above, the severity of phenotype may vary because of genetic factors such as the EXT1 mutation and the incomplete penetrance; but recent studies found a significant correlation between male sex and more severe clinical presentations, especially for patients over 18 years old (24). Possible explanations may relate to physeal closure at an older age in male (prolonging the effects of the EXT gene), hormonal differences between genders, or X-linked genes that may interact and accentuate the effect of the EXT1 and EXT2 genotypes.
Real symptoms will only manifest during growth and specifically during childhood. Most patients present with an average of 6 exostoses, but anatomical distribution and burden can vary largely (25). The most commonly involved bones include the distal femur (90%), proximal tibia (84%), fibula (76%), and humerus (72%) (26). Osteochondromas are often first discovered on the ribs and the proximal tibia, where they can be clearly visible and palpable. They are rarely located in carpal and tarsal bones, and never in the facial bones because they develop by intramembranous ossification.
Recently, a clinical classification system for MHE has been developed (27). In this classification system, MHE is divided into three classes according to the number of bone segments affected and the presence of skeletal deformities and/or functional limitations. This classification has been validated through a Switching Neural Network approach and provides an efficient method to characterize MHE (Table 2).
Table 2.
Clinical classification of MHE.
| I No deformities and no functional limitations | |
| II Deformities and no functional limitations | |
| III Deformities and functional limitations |
Pain is experienced by the majority of patients and often leads to the surgery (28). It may be caused by compression of tendons and muscles, with possible consequent chronic irritation (impingement, entrapment) and ruptures. Sometimes, bursitis over the cartilage cup may develop and, as the result of a trauma, peduncolated exostoses can fracture.
Even restricted motion of the joint is often encountered. For example, forearm rotation may be limited because of osteochondromas extending into the interosseous interval between radio and ulna; or in the knee, maximum flexion may be avoided by an osteochondroma in the popliteal region.
Sometimes exostoses can interfere with normal development of the growth plate, giving rise to limb deformities. Osteochondromas found in the forearm, can lead to unbalanced shortening of the ulna, with consequent higher curved radius. That often impairs function with a progressive limitation pronation. Shortening of the ulna, also can cause subluxation or dislocation of the radial head. These deformities are described as Madelung-type deformities (29) and are classified by Masada into three groups based on morphological aspects (30).
Hand involvement is often described, with an increase seen around the metacarpo-phalangeal joint. The most affected bones were the index and small finger metacarpals. Brachydactyly, angular deformity (more often seen on the ulnar side) or pseudo-mallet fingers represent the result (31).
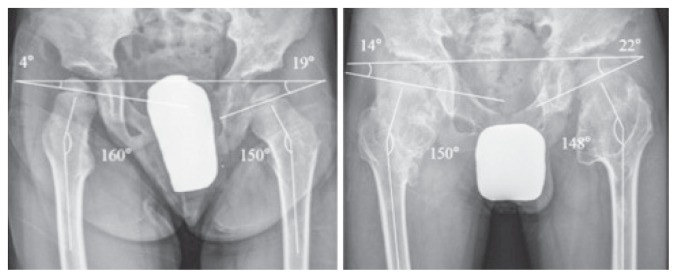
The principal developmental pattern of the hip in patients with HME is coxa valga, due to exostoses near the minor trochanter (32) (Figure 3). More rarely, we can observe acetabular exostoses and deformations at the medial femur and consequent ischio-femoral impingement or acetabular dysplasia that may require major surgery such as juvenile arthroplasty (33).
Figure 3.
Clinical evolution of a coxa valga in a young HME (32).
There is a high rate of knee deformity in patients with HME, with nearly a third of patients developing genu valgum (34). It is most often found in the proximal tibial metaphysis, although angulation of the femur might also be present. This could even lead to an oblique joint line orientation that may predispose to the development of degenerative joint disease at relatively early age. Furthermore, progression of the angulation might lead to lateral patella subluxation and patello-femoral complaints.
Even ankle joint is typically characterized by a valgus angulation. A complex growth disorder is carried out with relative shortening of the fibula, an oblique course of the distal tibial epiphysis and a medial subluxation of the talus (35).
Last but not the least, the exostoses are also thought to influence longitudinal limb growth, with a quarter of patients having a limb length discrepancy that often require surgical interventions.
Polidistrectual sequelae are often described and may have non-orthopaedic implications.
Neurovascular disorder
Impingement, entrapment or irritation of neural structure can occur when osteochondromas are located in particular regions such as axilla, proximal fibula or where the noble structures are located near bone surfaces. The result is a neuropathy with pain, paresthesias or functional impairment, more frequently seen in superficial peroneal nerve. In the vertebral column, osteochondromas can occur in up to 68% of the patients. The most are located in the posterior part of the vertebrae, but in 27% of patients MRI can reveal the extension into the spinal canal. Although rare, a symptomatic compression of the medulla can occur with serious neurologic consequences (36). Therefore, spinal osteochondromas require careful management, thorough follow-up and a MRI screening for patients in the growing years in order to prevent serious disability (37).
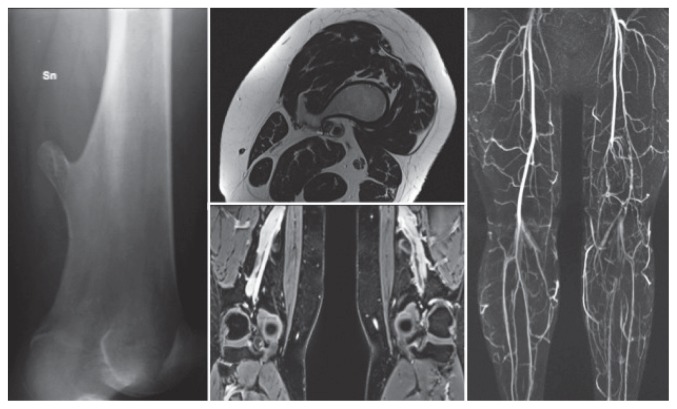
More rarely vascular compressions are seen, especially at the lower limb. Popliteal artery is most often involved, with pseudo aneurysm formation or thrombosis, claudication (Figure 4) and ischaemia (38). As case report, is described in literature an acute coronary syndrome due to dynamic extrinsic coronary artery compression by a rib exostoses (39).
Figure 4.
A case of a 35-year woman with a vascular claudication, due to a chronic obstruction of the popliteal artery.
Low stature
HME can lead to disproportionate short stature. This is not only due to the involvement of long bones growth plates, but new findings support a systemic influence of the gene defect on growth rate. For example, it is observed in adolescents – particularly boys – an earlier or faster closure of the growth plates (40). Moreover, growth hormone (GH) deficiency has rarely been found in HME patients, but no pathogenetic connection has been identified and GH replacement therapy has not been well described in this disease (41).
Scoliosis
Scoliosis associated with MHE has been described in literature, without data about prevalence and severity. A recent study confirmed scoliosis as a common feature of MHE and disease severity is a predictor of moderate scoliosis (>20°) (42).
Erosion of bone
In the forearm and lower leg, growth of an osteochondroma can give rise to erosion of the adjacent bone. In the forearm this might lead to limited rotation and pain. In the lower leg, erosion of the distal fibula is most often seen and caused by an osteochondroma arising from the lateral side of the distal aspect of the tibia. Ankle pain, a palpable mass and plastic deformation of the eroded bone can be the reason for surgical intervention.
Spontaneous hemotorax
Costal exostoses are mostly asymptomatic, but can rarely cause intra-thoracic complications, including haemothorax, pneumothorax as well as injury to the pleura, lung, diaphragm or pericardium. The association between spontaneous haemothorax and rib exostoses may be because of rupture of markedly dilated pleural vessels due to prolonged friction between the exostoses and the pleura (43).
Obstetric problems
Complications can occur during delivery due to the aberrant morphology of the pelvis or the presence of osteochondromas on the interior pelvis. Wicklund et al. (5) reported in a paper that 2/3 of women with MHE presented delivery by caesarean section, with 29% of these procedures being secondary to pelvic osteochondromas (5). Women with MHE were at least twice as likely as the general population to have a caesarean section.
Psychosocial problems
MHE is not only limited to physical complaints and inconvenience, but has a severe impact on daily life (12). More than half children in school encounter serious problems, especially with physical education, writing, and computer-related tasks. Half of the adult patients with paid employment had experienced problems during their occupation, whereas 28% had to stop their activities and 21% needed adjustment to the workplace. Patients with MHE were substantially incapacitated in their ability to perform sports. Moreover limb deformities and short stature are often considered important cosmetic disfiguring.
For all these reasons, HME can have great psychological repercussions on affected people.
Associate disorders
HS is located not only in growth plate and doesn’t plays a role only in chondrogenesis and endochondal ossification, but it is widespread in many others districts and takes part to others several biological processes. For example, HS plays a crucial role in endothelial function. Mooij et al. investigated the effect of HS chain length in relation to endothelial function and nitric oxide availability. They demonstrated that HS elongation genes EXT1 and EXT2 are involved in maintaining endothelial homeostasis, presumably via increased nitric oxide bioavailability. So endothelial HS might be interesting therapeutic targets for endothelial dysfunction and subsequent cardiovascular disease in humans (44).
Another recent study by Mooij et al. supports the concept that, although minor, HS-proteoglycans do contribute to human hepatic triglyceride-rich-lipoproteins clearance. So EXT1 heterozygosity causes a modest effect on postprandial lipid clearance (45).
Interestingly, HS plays a role in pancreas development and beta – cell function; genetic variations in EXT2 are associated with an increased risk for type 2 diabetes mellitus. It is hypothesized that loss of function of EXT1 or EXT2 in subjects with HME can affects pancreatic insulin secretion capacity and development (46).
With only four cases of coexistence of HME and ankylosing spondylitis so far, it seems that either association of HME and ankylosing spondylitis could be a rare entity or a coincidence (47). The increase in the number of reported patients who have a coexistence of these two diseases might suggest that this association is stronger than a coincidence. The role of genetic history, gene expression and a pre-existing inflammatory process or tissue destruction should be under consideration. To obtain clear and conclusive information and to reach a better understanding of this association, further studies are suggested.
Malignant degeneration
The most feared complication of HME is malignant transformation of an existing osteochondroma into a secondary peripheral chondrosarcoma, during adulthood. There is no evidence that chondrosarcomas might occur more frequently in relation with sex, severity of the disease, EXT1 or EXT2 mutations (6–24). Also, there is no consensus regarding the lifelong risk of malignant transformation: it varies from less than 1% in recent papers, up to 25% in the older series. In 2015, a wide multicentre survey by Czajka et al. reported a proportion of 2,7% (48). In the same study – and in according with previous findings – the districts more frequently involved are the pelvis, the scapula, the proximal part of the femur and the humerus. Malignant transformation in MHE seems to be diagnosed at a younger age than chondrosarcomas in non-MHE patients, most often in the first half of the fourth decade (2–49).
An interesting study published by Musso at al. in 2015 can clarify the genetic basis of malignant transformation: they observed that osteochondromas and their associated secondary peripheral chondrosarcomas have different initiating cells (50). In particular they reported the analysis of the mutational status of the EXT2 gene in tumour samples derived from a patient affected by HME, documenting the somatic loss of the germline mutation in a giant chondrosarcoma and in a rapidly growing osteochondroma. In the classic two-hit model of hereditary tumorigensis, the second somatic event is usually responsible for the loss of the wild-type allele, whereas in the case reported by Musso et al., they observed the loss of the mutated allele and the retention of the wild-type one. Such a condition could be referred to as an “isoallelic two-hit model” to highlight that the same allele is affected twice, as opposed to the classic “alloallelic two-hit hypothesis” that predicts the different alleles are affected by the two hits. Interestingly, a similar observation was reported in 1997 for the EXT1 gene (51).
These tumours develop in the cartilage cap of an existing osteochondroma and symptoms of such change include growth after skeletal maturity. The patient can experience pain, neuropraxia due to compression of nerves and pressure related symptoms at other nearby organs. Malignant transformation causes a surface irregularity and unorganised chalk deposits with light areas in the middle of the tumour as well as in the cartilage cap. Sometimes calcifications are seen in the surrounding organs. However, definitive diagnosis requires a biopsy.
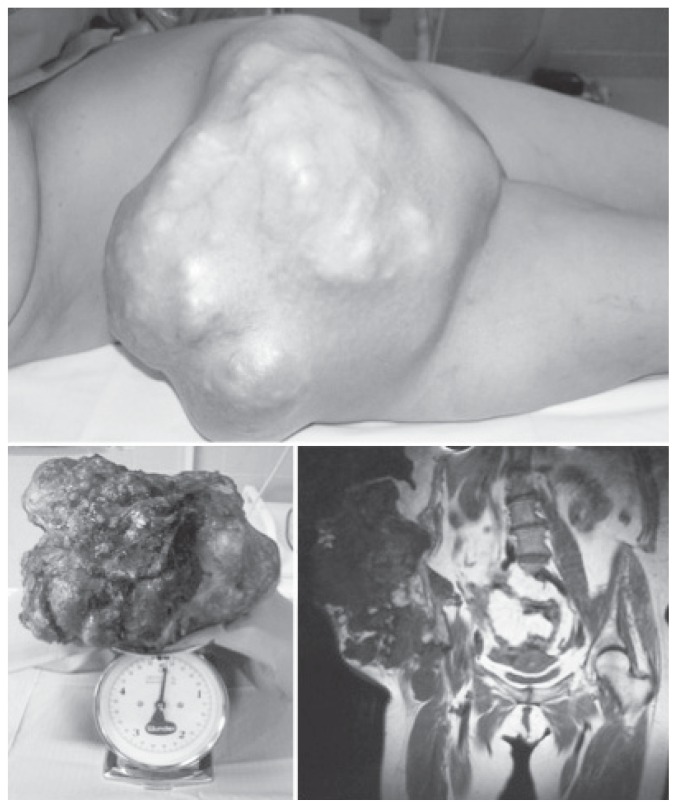
Patients with HME seem to develop low-grade chondrosarcomas. These tumours grow slowly, and rarely metastasise haematologically to other organs. 10% of secondary chondrosarcomas are classified as dedifferentiated chondrosarcomas (Figure 5), a very aggressive type of tumour consisting of two components: a low grade, well-differentiated chondrosarcoma, and a high-grade (non-cartilage) sarcoma (52). They have high metastatic risk and a bad prognosis. EXT gene mutations have been found in these tumours, and defects in TGF-β and FGF are described.
Figure 5.
A case of a dedifferentiated chondrosarcoma.
In the literature we can found a few paper that report other type of degeneration linked to HME. A recent review found 14 cases showing transformation of osteochondromas into osteosarcomas and 1 case of degeneration in Ewing sarcoma (53). In this paper it is underlined that Ewing sarcoma has never been reported on a previous bony lesion such as an osteochondroma. No genetic or molecular deepening was reported.
HME is also associated with intraosseous chondroid neoplasms, potentially resulting in central chondrosarcoma. This interesting new is reported by Gound et al. in 2015: in their cohort of patients with HME, they identified 7 cases of histologically confirmed low-grade central chondrosarcoma, giving a prevalence of 3,6%. Second this finding, central chondrosarcoma appears to have a similar lifetime incidence as the peripheral one. Once again, the pathogenetic mechanisms underlying are unknown (54).
EXT gene mutations are described in lung-, liver-, breast-, prostate-, brain-, skin- and haematologic cancer. For example, is described a case-report of atypical teratoid/rhabdoid tumour (an aggressive embryonic brain tumour, predominantly found in children) affecting an HME young man (55).
Diagnosis
Since most of the patients are asymptomatic at birth, early diagnosis can only made by genetic screening, such as with registered affected families. During childhood, when symptoms become evident, diagnosis result simple.
About the imaging, conventional radiographs are able to focus osteochondromas in the appendicular skeleton, in the most cases. Computerized tomography can be considered for regions that are difficult to visualize (thorax, spine, pelvis).
Magnetic resonance imaging (MRI) allows accurate measurement of cartilage cap thickness and the effect of the lesion on surrounding soft tissue structures. It also clearly demonstrates continuity of the cortex and medulla from the osteochondroma to the parent bone. The high water content of the cartilaginous cap ensures high signal intensity on T2 weighted images, allowing it to be easily identified in relation to surrounding soft tissues. The thickness of the cartilage cap must be measured from the osseous surface to the outermost edge of the cartilage cap at the thickest portion. Peterson claimed that a thickness of more than one centimetre should raise suspicion (56), whereas many others Authors set the limit to more than 2 centimetres. In particular, Kok et al. in 2013 asserted such measurements have been shown to have sensitivity and specificity greater than 95% for the detection of secondary chondrosarcomas if a 2 cm threshold is used (57).
For detection of malignant transformation, also positron emission tomography can be used, but increased metabolic radionucleotide uptake may be secondary to benign osteochondroma activity. Even bone scintigraphy has a low specificity, but can be useful such as alternative. However, the use of these techniques is therefore controversial, especially given the dose of radiation needed.
It seems that the risk of sarcomatous change among patients with HME is of the same magnitude as for the breast or cervix cancer. For this reason, Sonne-Holm et al. pointed out that a screening programme is needed for patients after the termination of bone growth (58). They proposed the following screening programme: a thorough clinical examination along with a full body MRI should be offered to adult HME patients (> 16 years of age or when growth pates are closed) every second year in order to map all of the patient’s exostoses. If MRI is not possible due to technical, logistic or patient related reasons, a bone scintigraphy is recommended instead.
Differential diagnosis
Actually, no problems of differential diagnosis are reported, but certain disease can have some affinities with HME.
Hardly exostoses may be confused with enchondromas. They are benign, generally asymptomatic, intra-osseous tumours of hyaline cartilage that most commonly affect medullary canal of the phalanges of the hand. Rarely, they arise on the surface of the bone. Enchondromas are the second most common benign cartilaginous tumour after osteochondromas. Ollier disease is characterized by multiple enchondromas, whereas Maffucci syndrome is characterized by multiple enchondromas and soft tissue haemangioma. These pathologies fall into a group of disease named enchondromatosis (59). A recent genetic analysis suggests that somatic mosaic mutations of IDH1 and IDH2 genes cause these disorders (60). So the etiology is different from HME, but the pathogenesis may be similar: the development of enchondromas in these disorders is thought to be due to derangement of cartilaginous growth, resulting in migration of cartilaginous rests from the epiphyseal plate into the metaphyseal regions. Moreover, malignant degeneration in chondrosarcoma can be observed in both these disease.
Metachondromatosis is another disease, taking part to the encondromatosis family. It occurs with multiple enchondromas and exostoses, configuring a clinical appearance most close to HME (61). Recently, it has been reported that loss of function mutations of the SHP2 gene (which encodes the SHP2 protein tyrosine phosphatase) are associated with metachondromatosis. At molecular levels, we observed an abundant expression of IHH and FGF2, as it is done in HME (62).
Another condition where exostoses can be found is Langer-Giedion syndrome, a very rare congenital disease caused by deletion of chromosome 8. Clinical manifestations include (over the multiple exostoses) short stature, intellectual disability and typical facial dysmorphism.
All patients show a de novo mutation, spanning the TRPS I and EXT1 genes, resulting in a phenotype that is a combination of two independent: tricho-rhino-phalangeal syndrome type I, and HME, respectively (63).
Unlikely achondroplasia can be mistake with HME, but presents analogies in clinical features and pathogenesis. Achondroplasia is a human bone genetic disorder of the growth plate and represents the most common form of dwarfism. Achondroplasia is caused by autosomal dominant mutations of the transmembrane receptor FGF-receptor 3, an important regulator of linear bone growth. Patients have no exostoses, but present characteristic short stature with disproportionate shortening of the limb and metaphyseal irregularities (64).
Prognosis
HME is a chronic disease that requires a careful follow-up to avoid many possible complications.
In children, periodic screening every 6–12 months is expected because several growth disturbances can be early treated by relatively simple procedures, and also it is necessary to monitor critical districts such as the spine. Roach et al. recommend a full columna MRI every second year in children with HME in order to identify intraspinal exostoses and potential intramedullary affection, to prevent neurological damage (37).
In adults, regular clinical examination is recommended for early detection and adequate treatment of malignant transformation; this should be done every 12–24 months depending on the locations at major risk (pelvis, scapula, proximal femur). However, the prognosis of secondary chondrosarcomas is good, since these tumours rarely metastasise: the 5-year survival is estimated to be 90% (3).
Treatment
The therapeutic approach to HME is substantially surgical, whereas the medical one is still at an experimental level.
Must be underlined that treatment of exostoses may be conservative if there are no clinical problems to avoid eventual surgical complications; also, spontaneous regression of the lesions has been documented in single cases during childhood and adolescence (65). Surgery may be considered when symptoms appear: individuals affected by HME often undergo multiple interventions (sometimes greater than 20) to remove symptomatic osteochondromas or address deformities (66). Excision is a relatively easy procedure with low morbidity that consists in a resection at the base of the exostoses. The largest lesions may require reconstructive techniques such as internal fixation and allografting. Local recurrence may occur for incomplete excision (to minimise the risk, is important to remove all the cartilage cap and the overlying perichondrium) or the development of a new lesion at the same location especially in very young children with open physis. To correct deformities, misalignment and limb length inequality more complex procedures are often necessary, such as osteotomies, bone lengthening and epiphysiodesis (in children).
Even in case of malignant degeneration in chondrosarcoma surgery represents the first choice: surgical resection alone is usually adequate because these tend to be low-grade lesions. A role for adjuvant radiotherapy and chemotherapy has not been proved in secondary chondrosarcomas, and their use remains controversial. Also the few cases of chondorsarcoma dedifferentiated need surgery: they tend to be even more resistant to radiotherapy and chemotherapy, because of poor vascularisation, low pH and high interstitial pressure.
However, HME is first of all a genetic disease. Today, research in this field is very active and recent discovers about physio-pathology can individuate useful therapeutic target. In particular, IHH signalisation defect are found in HME and promising treatment with blocking agents are currently under investigation. IHH blocking agents, such as triparanol, can be used in a neo-adjuvant fashion, to reduce tumour load before surgery and are currently used in several studies on brain and skin cancer (67).
A recent study by Huegel et al. highlights one additional possible pathway in pathophysiology of HME: the role of heparanase (68). This protein is easily detectable in growth plates of unaffected people, only in hypertrophic zone, and has the ability to stimulate chondrogenesis, BMP signalling, cell migration and cell proliferation. Through testing on mouse models, the Authors experimented a potent heparanase inhibitor, which strongly inhibited chondrogenesis. These properties make heparanase a conceivable therapeutic target for the future, in HME.
Conclusions
HME is a complex disease where the paediatrician, the geneticist and the orthopaedic surgeon play an interchangeable role in diagnosis, research and therapy.
Despite several findings in recent years, HME still holds many secrets. But, the complex and fascinating genetic mechanisms that lead to the interactions of cells and molecules at the growth plate remain under investigation. The importance of these researches lies in the fact that they offer an insight on other – more important – diseases. They give us a better understanding of the role of HS in signal transduction, and of the physiology of other bone and cartilage diseases as well as of skeletal embryology.
Now we look at the EXT-genes as tumour-suppressor genes with a major role in angiogenesis and malignant degeneration. The hope is that new discovers on HS pathways may one day lead to the development of an effective cancer therapy. For example, the finding that regulation defects of IHH are present in the pathogenesis of the whole spectrum of benign and malignant cartilage tumours can become a major therapeutic target for treating these tumours.
Figure 1.
Organization of the embryonic growth plate (69).
References
- 1.Campanacci M. Bone and soft tissue tumors. Bologna: Springer-Verlag Wien GmbH; 1990. [Google Scholar]
- 2.Schmale GA, Conrad EU, III, Raskind WH. The natural history of hereditary multiple exostoses. J Bone Joint Surg Am. 1994;76A:986e92. doi: 10.2106/00004623-199407000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Ryckx A, Somers JF, Allaert L. Hereditary multiple exostosis. Acta Orthop Belg. 2013;79(6):597–607. [PubMed] [Google Scholar]
- 4.Shapiro F, Simon S, Glimcher HJ. Hereditary multiple exostoses. Anthropometric, roentgenographic, and clinical aspects. J Bone Joint Surg Am. 1979;61A:815e24. [PubMed] [Google Scholar]
- 5.Wicklund CL, Pauli RM, Johnston D, Hecht JT. Natural history study of hereditary multiple exostoses. Am J Med Genet. 1995;55:43e6. doi: 10.1002/ajmg.1320550113. [DOI] [PubMed] [Google Scholar]
- 6.Porter DE, Lonie L, Fraser M, et al. Severity of disease and risk of malignant change in hereditary multiple exostoses. A genotypee phenotype study. J Bone Joint Surg Br. 2004;86B:1041e6. doi: 10.1302/0301-620x.86b7.14815. [DOI] [PubMed] [Google Scholar]
- 7.Stickens D, Clines G, Burbee D, et al. The EXT2 multiple hereditary exostoses gene defines a family of putative tumor suppressor genes. Nat Genet. 1996;14:25–32. doi: 10.1038/ng0996-25. [DOI] [PubMed] [Google Scholar]
- 8.Jennes I, Pedrini E, Zuntini M, et al. Multiple osteochondromas: mutation up- date and description of the multiple osteochondromas mutation database (MOdb) Hum Mutat. 2009;30(12):1620–1627. doi: 10.1002/humu.21123. [DOI] [PubMed] [Google Scholar]
- 9.Szuhai K, Jennes I, de Jong D, et al. Tiling resolution array-CGH shows that somatic mosaic deletion of the EXT gene is causative in EXT gene mutation negative multiple osteochondromas patients. Hum Mutat. 2011;32(2):E2036–E2049. doi: 10.1002/humu.21423. [DOI] [PubMed] [Google Scholar]
- 10.Goud AL, Lange J, de Scholtes VAB, et al. Pain, physical and social functioning and quality of life in individuals with hereditary multiple exostoses in the Netherlands. A national cohort study. J Bone Joint Surg Am. 2012;94A:1013e20. doi: 10.2106/JBJS.K.00406. [DOI] [PubMed] [Google Scholar]
- 11.Malini K, Gudi Narayan S, Kutty AVM, et al. Mutational Analysis of Exostosin 1 and 2 Genes in Multiple Osteochondroma. Indian J Pediatr. 2015;82(7):649–650. doi: 10.1007/s12098-014-1675-1. [DOI] [PubMed] [Google Scholar]
- 12.Kang Qing-lin, Xu Jia, Zhang Zeng, et al. Mutation Screening for the EXT1 and EXT2 Genes in Chinese Patients with Multiple Osteochondromas. Arch Med Res. 2013;44(7):542–8. doi: 10.1016/j.arcmed.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Jones KB, Piombo V, Searby C, et al. A mouse model of osteochondromagenesis from clonal inactivation of Ext1 in chondrocytes. Proc Natl Acad Sci USA. 2010;107(5):2054–2059. doi: 10.1073/pnas.0910875107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reijnders CM, Waaijer CJ, Hamilton A, et al. No haploinsufficiency but loss of heterozygosity for EXT in multiple osteochondromas. Am J Pathol. 2010;177(4):1946–1957. doi: 10.2353/ajpath.2010.100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hecht JT, Hogue D, Strong LC, et al. Hereditary multiple exostosis and chondrosarcoma: linkage to chromosome 2 and loss of heterozygosity for EXT-linked markers on chromosomes 2 and 8. Am J Hum Genet. 1995;56:1125–1131. [PMC free article] [PubMed] [Google Scholar]
- 16.Lind T, Tufaro F, McCormick C, et al. The putative tumor suppressors EXT1 and EXT2 are glycosyltransferases required for the biosynthesis of heparan sulfate. J Biol Chem. 1998;273:26265–26268. doi: 10.1074/jbc.273.41.26265. [DOI] [PubMed] [Google Scholar]
- 17.McCormick C, Leduc Y, Martindale D, et al. The putative tumour suppressor EXT1 alters the expression of cell-surface heparan sulfate. Nat Genet. 1998;19:158–161. doi: 10.1038/514. [DOI] [PubMed] [Google Scholar]
- 18.Busse-Wicher M, Wicher KB, Kusche-Gullberg M. The exostosin family: proteins with many functions. Matrix Biol. 2014;35:25–33. doi: 10.1016/j.matbio.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Zak BM, Schuksz M, Koyama E, et al. Compound heterozygous loss of Ext1 and Ext2 is sufficient for formation of multiple exostoses in mouse ribs and long bones. Bone. 2011;48(5):979–987. doi: 10.1016/j.bone.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones KB, Pacifici M, Hilton MJ. Multiple hereditary exostoses (MHE): elucidating the pathogenesis of a rare skeletal disorder through interdisciplinary research. Connect Tissue Res. 2014;55(2):80–8. doi: 10.3109/03008207.2013.867957. [DOI] [PubMed] [Google Scholar]
- 21.Stickens D, Brown D, Evans GA. EXT genes are differentially expressed in bone and cartilage during mouse embryogenesis. Dev Dyn. 2000;218:452–464. doi: 10.1002/1097-0177(200007)218:3<452::AID-DVDY1000>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 22.Benoist-Lasselin C, de Margerie E, Gibbs L, et al. Defective chondrocyte proliferation and differentiation in osteochondromas of MHE patients. Bone. 2006;39:17–26. doi: 10.1016/j.bone.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Stickens D, Zak BM, Rougier N, et al. Mice deficient in Ext2 lack heparan sulfate and develop exostoses. Development. 2005;132:5055–5068. doi: 10.1242/dev.02088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pedrini E, Jennes I, Tremosini M, et al. Genotype-phenotype correlation study in 529 patients with multiple hereditary exostoses: identification of “protective” and “risk” factors. J Bone Joint Surg Am. 2011;2011(93):2294e302. doi: 10.2106/JBJS.J.00949. [DOI] [PubMed] [Google Scholar]
- 25.Clement ND, Porter DE. Hereditary multiple exostoses: anatomical distribution and burden of exostoses is dependent upon genotype and gender. Scott Med J. 2014;59(1):35–44. doi: 10.1177/0036933013518150. [DOI] [PubMed] [Google Scholar]
- 26.Hennekam RC. Hereditary multiple exostoses. J Med Genet. 1991;28:262e266. doi: 10.1136/jmg.28.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mordenti M, Ferrari E, Pedrini E, et al. Validation of a new multiple osteochondromas classification through Switching Neural Networks. Am J Med Genet. 2013;161A:556–560. doi: 10.1002/ajmg.a.35819. [DOI] [PubMed] [Google Scholar]
- 28.Darilek S, Wicklund C, Novy D, et al. Hereditary multiple exostosis and pain. J Pediatr Orthop. 2005;2005(25):369e76. doi: 10.1097/01.bpo.0000150813.18673.ad. [DOI] [PubMed] [Google Scholar]
- 29.Ali S, Kaplan S, Kaufman T, et al. Madelung deformity and Madelung-type deformities: a review of the clinical and radiological characteristics. Pediatr Radiol. 2015;45:1856–1863. doi: 10.1007/s00247-015-3390-0. [DOI] [PubMed] [Google Scholar]
- 30.Masada K, Tsuyuguchi Y, Kawai H, et al. Operations for forearm deformity caused by multiple osteochondromas. J Bone Joint Surg Br. 1989;71B:24e9. doi: 10.1302/0301-620X.71B1.2914999. [DOI] [PubMed] [Google Scholar]
- 31.Woodside CJ, Ganey T, Gaston RG. Multiple osteochondroma of the hand: initial and long-term follow-up study. HAND. 2015;10:616–620. doi: 10.1007/s11552-015-9775-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang YZ, Park KW, Oh CS, et al. Developmental pattern of the hip in patients with hereditary multiple exostoses. BMC Musculoskelet Disord. 2015;16:54. doi: 10.1186/s12891-015-0514-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaishya R, Swami S, Vijay V, et al. Bilateral total hip arthroplasty in a young man with hereditary multiple exostoses. BMJ Case Rep. 2015 doi: 10.1136/bcr-2014-207853.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clement ND, Porter DE. Can deformity of the knee and longitudinal growth of the leg be predicted in patients with hereditary multiple exostoses? A cross-sectional study. The Knee. 2014;21:299–303. doi: 10.1016/j.knee.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 35.Noonan KJ, Feinberg JR, Levenda A, et al. Natural history of multiple hereditary osteochondromatosis of the lower extremity and ankle. J Pediatr Orthop. 2002;22:120–4. [PubMed] [Google Scholar]
- 36.Sciubba DM, Macki M, Bydon M, et al. Long-term outcomes in primary spinal osteochondroma: a multicenter study of 27 patients. J Neuro-surg Spine. 2015;22(6):582–8. doi: 10.3171/2014.10.SPINE14501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roach JW, Klatt JWB, Faulkner ND. Involvement of the spine in patients with multiple hereditary exostoses. J Bone Joint Surg Am. 2009;91:1942–8. doi: 10.2106/JBJS.H.00762. [DOI] [PubMed] [Google Scholar]
- 38.Nasr B, Albert B, David CH, et al. Exostoses and vascular complications in the lower limbs: two case reports and review of the literature. Ann Vasc Surg. 2015;29(6):1315.e7–1315.e14. doi: 10.1016/j.avsg.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 39.Rodrigues JC, Mathias HC, Lyen SM, et al. A Novel Cause of Acute Coronary Syndrome Due to Dynamic Extrinsic Coronary Artery Compression by a Rib Exostosis: Multimodality Imaging Diagnosis. Can J Cardiol. 2015;31(10):1303.e9–1303.e11. doi: 10.1016/j.cjca.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 40.Staal HM, Goud AL, van der Woude HJ, et al. Skeletal maturity of children with multiple osteochondromas: is diminished stature due to a systemic influence? J Child Orthop. 2015;9(5):397–402. doi: 10.1007/s11832-015-0680-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bozzola M, Gertosio C, Gnoli M, et al. Hereditary multiple exostoses and solitary osteochondroma associated with growth hormone deficiency: to treat or not to treat? Ital J Pediatr. 2015;41:53. doi: 10.1186/s13052-015-0162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsumoto Y, Matsumoto K, Harimaya K, et al. Scoliosis in patients with multiple hereditary exostoses. Eur Spine J. 2015;24(7):1568–73. doi: 10.1007/s00586-015-3883-4. [DOI] [PubMed] [Google Scholar]
- 43.Ravindran R, Jordan S, Bush A. An extra piece of grey. Thorax. 2015;70(7):705–6. doi: 10.1136/thoraxjnl-2015-207061. [DOI] [PubMed] [Google Scholar]
- 44.Mooij HL, Cabrales P, Bernelot Moens SJ, et al. Loss of function in heparan sulfate elongation genes EXT1 and EXT 2 results in improved nitric oxide bioavailability and endothelial function. J Am Heart Assoc. 2014;3(6):e001274. doi: 10.1161/JAHA.114.001274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mooij HL, Bernelot Moens SJ, Gordts PL, et al. Ext1 heterozygosity causes a modest effect on postprandial lipid clearance in humans. J Lipid Res. 2015;56(3):665–73. doi: 10.1194/jlr.M053504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bernelot Moens SJ, Mooij HL, Hassing HC, et al. Carriers of loss-of-function mutations in EXT display impaired pancreatic beta-cell reserve due to smaller pancreas volume. PLoS One. 2014;9(12):e115662. doi: 10.1371/journal.pone.0115662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rostamian A, Mazoochy H, Movassaghi S. Coexistence of ankylosing spondylitis and hereditary multiple exostoses: coincidence or association. Iran J Radiol. 2014;11(1):e4242. doi: 10.5812/iranjradiol.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Czajka CM, DiCaprio MR. What is the Proportion of Patients With Multiple Hereditary Exostoses Who Undergo Malignant Degeneration? Clin Orthop Relat Res. 2015;473(7):2355–61. doi: 10.1007/s11999-015-4134-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Altay M, Yildiz Y, Erekul S, et al. Secondary chondrosarcoma in cartilage bone tumors: report of 32 patients. J Orthop Sci. 2007;12:415–23. doi: 10.1007/s00776-007-1152-z. [DOI] [PubMed] [Google Scholar]
- 50.Musso N, Caronia FP, Castorina S, et al. Somatic loss of an EXT2 gene mutation during malignant progression in a patient with hereditary multiple osteochondromas. Cancer Genet. 2015;208(3):62–7. doi: 10.1016/j.cancergen.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Hecht JT, Hogue D, Wang Y, et al. Hereditary multiple exostoses: mutational studies of familial EXT1 cases and EXT-associated malignancies. Am J Hum Genet. 1997;60:80–86. [PMC free article] [PubMed] [Google Scholar]
- 52.Rozeman LB, de Bruijn IH, Bacchini P, et al. Dedifferentiated peripheral chondrosarcomas: regulation of EXT-downstream molecules and differentiation related genes. Mod Path. 2009;22:1489–1498. doi: 10.1038/modpathol.2009.120. [DOI] [PubMed] [Google Scholar]
- 53.Marrero Barrera PA, Marrero Ortiz PV. Ewing sarcoma superimposed on a previous osteochondroma in multiple osteochondromatosis. Orthopedics. 2014;37(4):e403–6. doi: 10.3928/01477447-20140401-65. [DOI] [PubMed] [Google Scholar]
- 54.Goud AL, Wuyts W, Bessems J, et al. Intraosseous atypical chondroid tumor or chondrosarcoma grade 1 in patients with multiple osteochondromas. J Bone Joint Surg Am. 2015;97(1):24–31. doi: 10.2106/JBJS.N.00121. [DOI] [PubMed] [Google Scholar]
- 55.Wu Q, Xiao BO, Li L, et al. Atypical teratoid/rhabdoid tumor with hereditary multiple exostoses in an 18-year-old male: A case report. Oncol Lett. 2015;10(3):1561–1564. doi: 10.3892/ol.2015.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peterson HA. Multiple hereditary osteochondromata. Section Ped Orthoped. 1989;239:222–30. [PubMed] [Google Scholar]
- 57.Kok HK, Fitzgerald L, Campbell N, et al. Multimodality imaging features of hereditary multiple exostoses. Br J Radiol. 2013;86:20130398. doi: 10.1259/bjr.20130398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sonne-Holm E, Wong C, Sonne-Holm S. Multiple cartilaginous exostoses and development of chondrosarcomas – a systematic review. Dan Med J. 2014;61(9):A4895. [PubMed] [Google Scholar]
- 59.Northrup BE, Slat DF, Loomans RU, et al. The myriad of diseases that present with polyostotic bone lesions. Curr Probl Diagn Radiol. 2014;43(4):186–204. doi: 10.1067/j.cpradiol.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 60.Kumar A, Jain VK, Bharadwaj M, et al. Ollier Disease: Pathogenesis, Diagnosis, and Management. Orthopedics. 2015;38(6):e497–506. doi: 10.3928/01477447-20150603-58. [DOI] [PubMed] [Google Scholar]
- 61.Fisher TJ, Williams N, Morris L, et al. Metachondromatosis: more than just multiple osteochondromas. J Child Orthop. 2013;7(6):455–64. doi: 10.1007/s11832-013-0526-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim HK, Feng GS, Chen D, et al. Targeted disruption of Shp2 in chondrocytes leads to metachondromatosis with multiple cartilaginous protrusions. J Bone Miner Res. 2014;29(3):761–9. doi: 10.1002/jbmr.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cappuccio G, Genesio R, Ronga V, et al. Complex chromosomal rearrangements causing Langer-Giedion syndrome atypical phenotype: genotype-phenotype correlation and literature review. Am J Med Genet A. 2014;164A(3):753–9. doi: 10.1002/ajmg.a.36326. [DOI] [PubMed] [Google Scholar]
- 64.Faruqi T, Dhawan N, Bahl J, et al. Molecular, phenotypic aspects and therapeutic horizons of rare genetic bone disorders. Biomed Res Int. 2014;2014:670842. doi: 10.1155/2014/670842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Passanise AM, Mehlman CT, Wall EJ, et al. Radiographic evidence of regression of a solitary osteochondroma: a report of 4 cases and a literature review. J Pediatr Orthop. 2011;31:312–6. doi: 10.1097/BPO.0b013e31820fc676. [DOI] [PubMed] [Google Scholar]
- 66.Jager M, Westhoff B, Portier S, et al. Clinical outcome and genotype in patients with hereditary multiple exostoses. Clinical outcome and genotype in patients with hereditary multiple exostoses. J Orthop Res. 2007;25:1541–51. doi: 10.1002/jor.20479. [DOI] [PubMed] [Google Scholar]
- 67.Karibe T, Fukui H, Sekikawa A, et al. EXTL 3 promoter methylation down-regulates EXTL 3 and heparin sulphate expression in mucinous colorectal cancers. J Pathol. 2008;216:32–42. doi: 10.1002/path.2377. [DOI] [PubMed] [Google Scholar]
- 68.Huegel J, Enomoto-Iwamoto M, Sgariglia F, et al. Heparanase Stimulates Chondrogenesis and Is Up-Regulated in Human Ectopic Cartilage. A Mechanism Possibly Involved in Hereditary Multiple Exostoses. Am J Pathol. 2015;185:1676–1685. doi: 10.1016/j.ajpath.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jochmann K, Bachvarova V, Vortkamp A. Reprint of: Heparan sulfate as a regulator of endochondral ossification and osteochondroma development. Matrix Biol. 2014;35:239–47. doi: 10.1016/j.matbio.2014.04.001. [DOI] [PubMed] [Google Scholar]