Summary
Introduction
Recent acquisitions of the complex mechanisms of osseointegration between implants and host bone have gained attention, accordingly to the methods of evaluation of these interactions. DEXA analysis is considered an useful tool to assess such phenomena, in order to analyse in a quantitative manner the local metabolic activity of the bone, and to evaluate over the time the integration between host bone and prosthetic components. The purpose of the present study is to report about a preliminary experience in the analysis of osseointegration processes of patients undergoing a primary Total Hip Arthroplasty (THA) or a revision Total Knee Arthroplasty (rTKA).
Materials and methods
Thirty patients undergoing THA and nineteen undergoing rTKA were included in this study. In fifteen cases of THA a standard cementless stem was used; in the other fifteen a short cementless stem was chosen. In all cases a cementless cup was implanted. In all patients undergoing rTKA, all implants had pressfit femoral and tibial diaphyseal stems; only the femoral component and the tibial plateau were cemented. DEXA evaluation was performed preoperatively, and at 3, 6, 12, and 24 months postoperatively for rTKA, and at 6 and 12 months for THA.
Results
DEXA in THA showed a significant decrease at the femoral ROIs 1 and 7, and an increase in ROI 4. In rTKA a reduction of femoral BMD in R1, R7, and R4 was found, with maximum values of −13.6% in R1 and −11.89% in R7 at 24 months and a value of −2.55% in R4 at 12 months. On the tibial side, an increase in BMD R4 (with values of 2.18% still at 24 months), and a reduction in R7 (progressively lesser over the time) and in R1 (progressively higher) were found.
Conclusions
After a joint replacement a full adhesion of the prosthetic surface to the host bone should be achieved through a local biological process named osseointegration. In some cases this process may not fully realize, so the secondary stability of the implant may fail. DXA is a valuable tool to follow over time the bone remodelling at the bone-prosthesis counterface in THA and in rTKA, in order to early detect any alterations of such phenomenon.
Keywords: total knee arthroplasty, total hip arthroplasty, revision, bone remodelling, DXA
Introduction
Total Hip Arthroplasty (THA) and Total Knee Arthroplasty (TKA) represent the most successful procedures in orthopaedic surgery for the treatment of primary and secondary osteoarthritis in terms of pain relief, functional recovery, and improvement of quality of life (1–6). Given the increase of the mean age of general population and the following arising number of joint replacements in the next decades, it is also expected an increase of failures of such implants (7, 8). Historically, failures of THAs and TKAs have been related to three main causes: aseptic loosening, infection, and instability. These conditions usually affect the long-term survival of the implants (9). In the last years, atypical patterns of presentation of failures of orthopaedic implants have been described and defined “painful prostheses”. In these clinical settings, major causes of failure may be excluded and often the only available clue is the persistent pain. This specific type of pain is usually induced by a wide spectrum of underlining mechanisms: hypersensitivity to metals or cement, local manifestations of an extraarticular process, poor integration of the prosthetic surface to the host bone (10–13). Such cases may be treated by a revision arthroplasty.
New materials, modern designs, and meticulous and less invasive surgical techniques have contributed to a longer survivorship of the implants (2, 5, 14–16). Among all the mentioned improvements, the recent acquisition of the complex mechanisms of osseointegration between implants and host bone have gained attention, accordingly to the methods of evaluation of these peculiar interactions (17–21). One of those methods is the Dual-Energy X-rays Absorptiobiometry (DXA), an accurate tool for the assessment of bone mineral density (BMD) and its minor changes (22–26). The analysis of the periprosthetic bone by DXA has two main purposes: on one hand, it is useful to verify in a quantitative manner the local metabolic activity of the bone; on the other hand, to evaluate the integration between host bone and prosthetic components over the time.
The purpose of the present study is to report about a preliminary experience in the analysis of osseointegration processes of THAs and revision TKAs (rTKA) in a cohort of patients undergoing a joint replacement or a revision respectively, and to compare the results with similar studies in literature.
Materials and methods
Thirty consecutive patients undergoing THA between 2011 and 2012 and nineteen consecutive patients undergoing rTKA between 2011 and 2013 were selected at the Authors’ Institution for a DXA study before and after surgery at specific intervals. The Institutional Review Board approved the study and follow-up, respecting the principles of the Declaration of Helsinki, and after an adequate informed consent of patients. Inclusion criteria were: adult patients candidate to THA and rTKA; no recent use of corticosteroids (<1 year before surgery) and collaborative patients able to express an informed consent. Exclusion criteria were: periprosthetic fractures, use of corticosteroids less than 1 year with respect to the index operation, patients affected by comorbidities treated by corticosteroids or biologic agents (as Rheumatoid Arthritis, Haemophilic Arthropathy) and subjects operated for proximal femoral fractures.
In fifteen cases of THA a PPF® cementless stem (Biomet, Warsaw, IN) was used; in the other fifteen a GTS® cementless stem (Biomet, Warsaw, IN) was chosen. In all cases a Regenerex® cementless cup (Biomet, Warsaw, IN) was implanted. The use of a stem or another was made according to the age of patients at the time of surgery: subjects <70 years of age were selected for a smaller stem (GTS), while patients >71 years of age received a standard stem (PPF). The overall mean age was 60,3 (range: 50–78), and the mean Body Mass Index (BMI) was 29,4 (range: 28,5–38,6). In all patients undergoing a rTKA the Legion® knee system (Smith & Nephew, Memphis, TN) was used, characterized by cementless femoral and tibial stems. The mean age was 71,3 (range: 49–83), and the mean Body Mass Index (BMI) was 30,3 (range: 20,3–40,4).
The preoperative evaluation was performed by dedicated scores: Knee Society rating Score (KSS) and Harris Hip Score (HHS) (27, 28). The radiographic study was performed following the criteria of DeLee and Charnley and Knee Society Roentgengraphic Evaluation System for THA and rTKA respectively (29, 30). A standard orthopaedic evaluation (Range of Motion, radiographic alignment) was conducted in all patients.
At the time of inclusion in the study a DXA was performed for the evaluation of the lumbar and periprosthetic bone (Figures 1, 2). A follow-up by DXA was made at 6 and 12 months for THAs, and 3, 6, 12 and 24 months for rTKAs. In all cases the Hologic® Scanner QDR 4500/DELPHI (HOLOGIC Inc, Zaventem, Belgium) was used. For the evaluation of THAs, the periprosthetic bone was studied by the analysis of the 7 Region of Interests (ROI) around the stem according to Gruen (31) (Figures 3, 4). In case of rTKAs 8 ROI were evaluated (32–34) (Figures 5, 6).
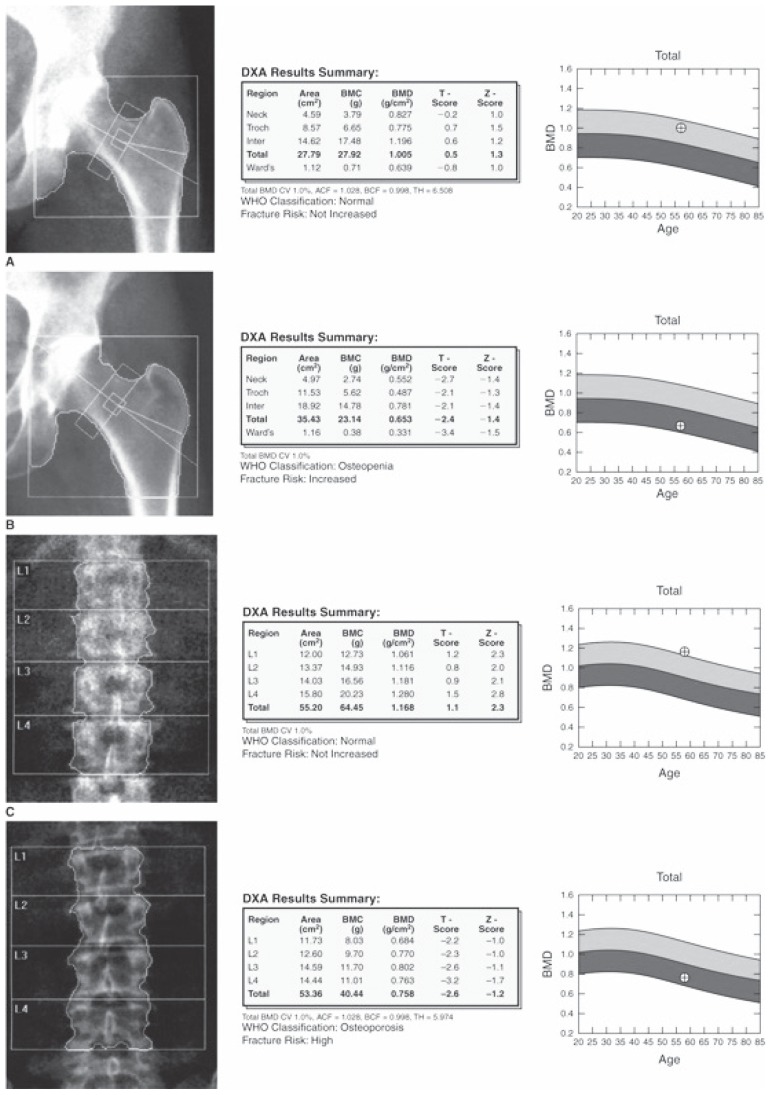
Figure 1.
Example of a DXA scan: hip and lumbar spine are divided in several zones. Values of BMD are expressed in g/cm2.
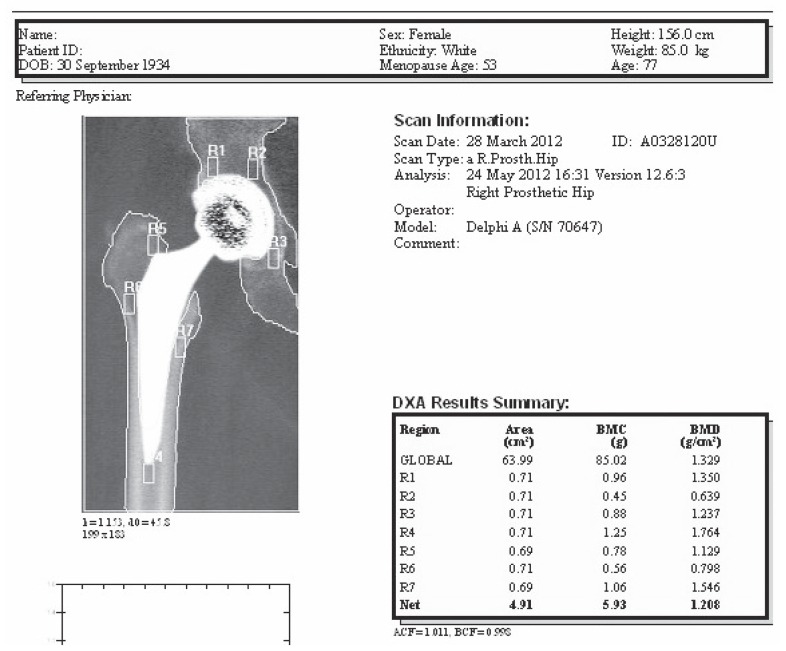
Figure 2.
Example of a prosthetic hip DXA scan: 7 zones are defined. Values of BMD are expressed in g/cm2.
Figure 3.
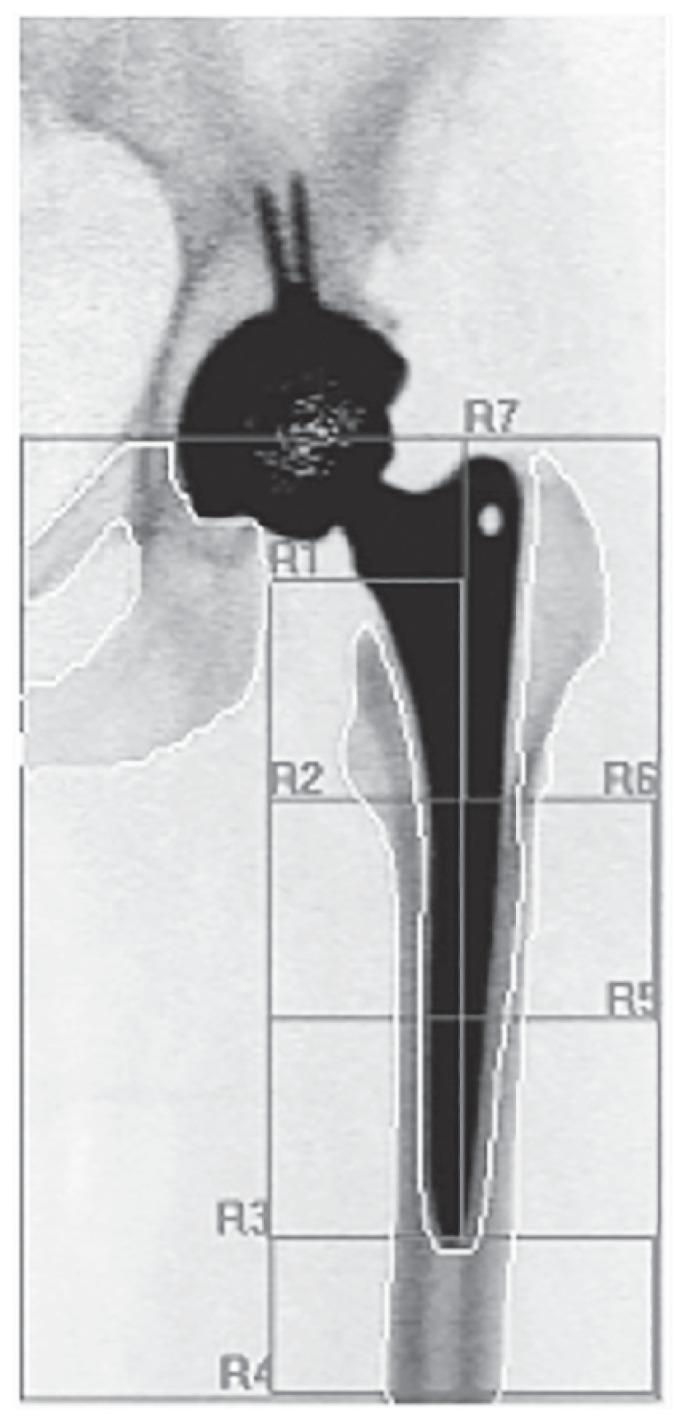
Red areas around a standard stem represent the 7 Gruen zones, in order to analyse BMD variations. R1 and R7 represent the proximal areas, while R4 is related to the tip of the stem.
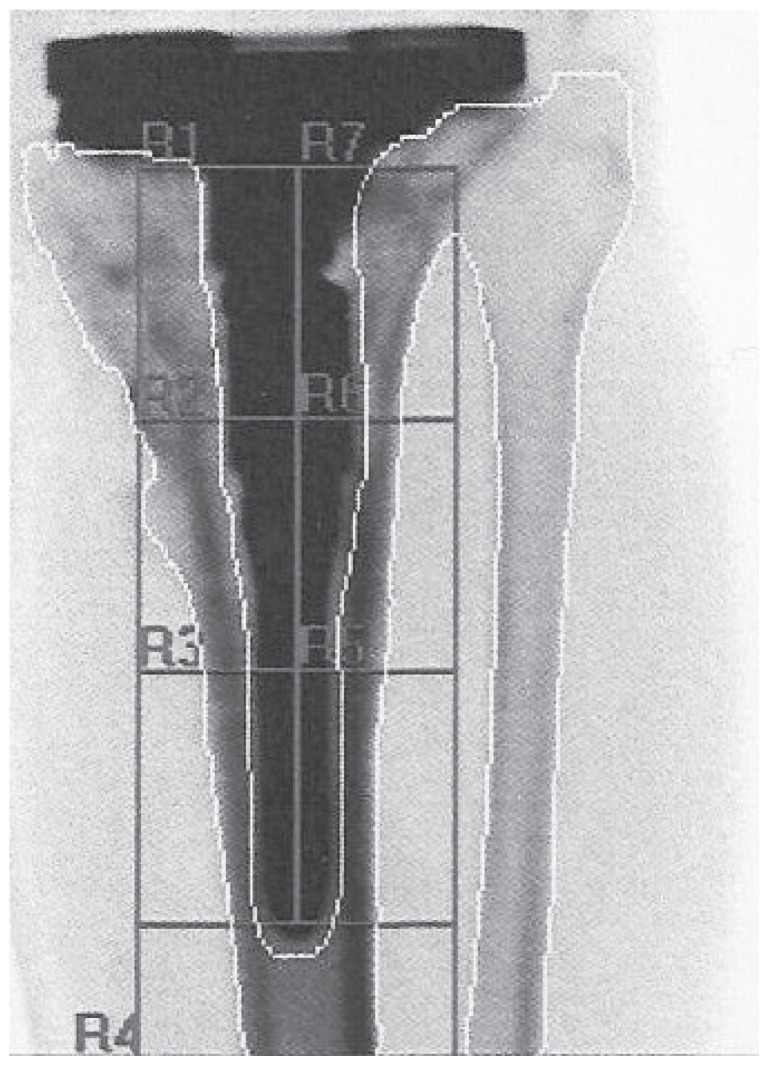
Figure 4.
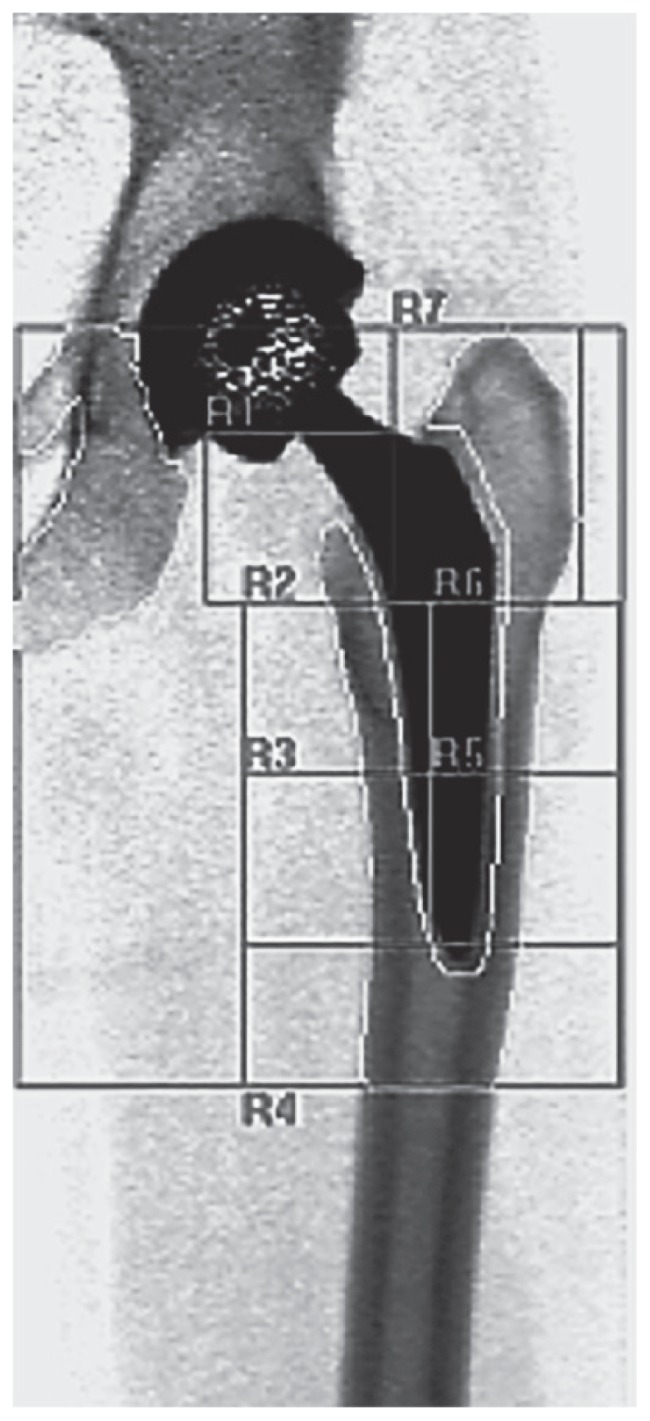
Red areas around a short stem represent the 7 Gruen zones. BMD ROIs are the same of Figure 3.
Figure 5.
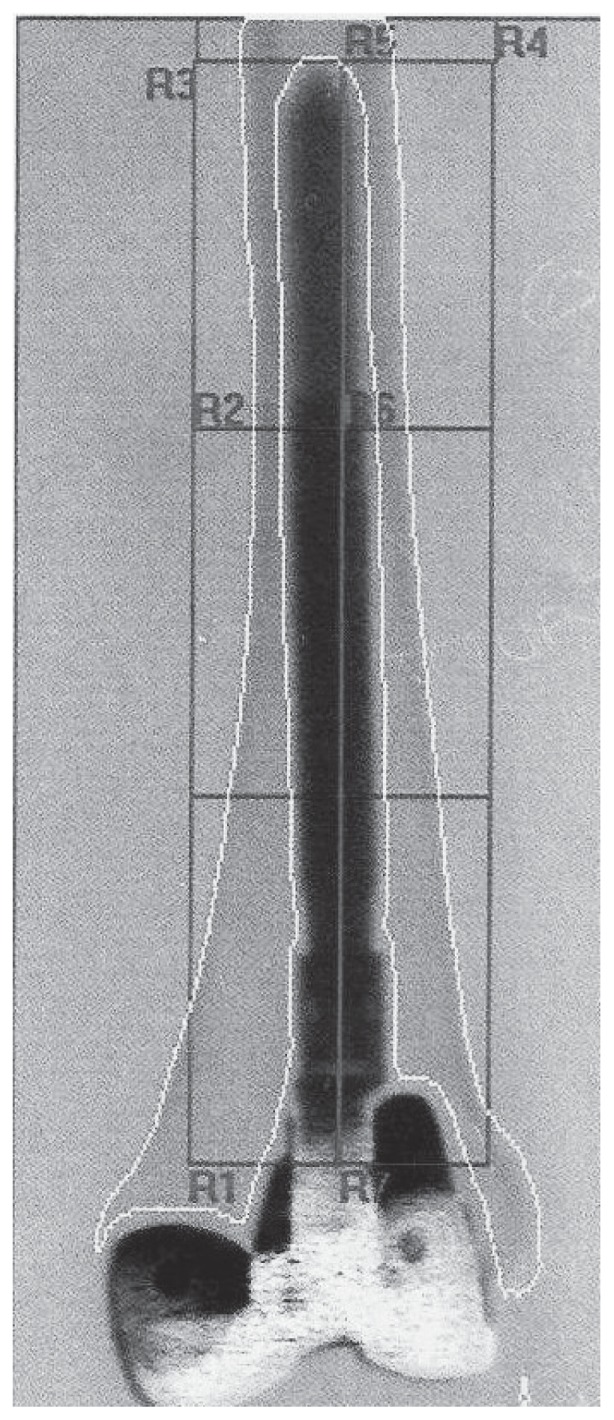
Red areas around a rTKA femoral stem represent the 7 Gruen zones. BMD ROIs follow to the previous scheme.
Figure 6.
Red areas around a rTKA tibial stem represent the 7 Gruen zones. BMD ROIs are the same of Figure 5.
Results
All patients completed the minimum follow-up of 12 months for the group of THA and 24 months for the group of rTKA. No intraoperative or postoperative complications and no failures were reported at the latest follow-up in both groups. The mean HHS showed an increase from a mean score of 42.1 to 63.7. Similarly, the mean KSS increased from a mean preoperative value of 41.3 to 73.4. Non progressive and <1mm radiolucencies were found in 2 THAs in zone 2 of DeLee and Charnley, without any complaint of patients and no indications to revision. No radiolucencies were founded at the radiologic follow-up of all rTKAs. All patients referred satisfaction for the procedure, showed an increase of ROM after surgery and a good level of functional ability.
At 12 months DXA showed a decreased BMD in ROIs 1 and 7 in all patients after THA for both stems, with lower values for GTS. On the contrary, an increased BMD was found in ROI 4 in all THAs, with higher values for PPF stems. The other ROIs remained substantially unchanged. The overall BMD similarly showed a decrease in ROIs 1 and 7, and an increase in ROI 4 (Table 1).
Table 1.
Total Hip Arthroplasty: BMD variations at 6 and 12 months (values are expressed in %).
| AREAS | 6 mo | 12 mo | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Short stem | Standard stem | Average of both stems | Short stem | Standard stem | Average of both stems | |
| R1 | −0,5 | −4,0 | −2,25 | −1,3 | −4,2 | −2,75 |
| R2 | 0,9 | −1,5 | −0,30 | 0,1 | −1,0 | −0,45 |
| R3 | 1,0 | 2,8 | 1,90 | 0,005 | 0,2 | 0,10 |
| R4 | 1,6 | 2,2 | 1,90 | 0,9 | 4,1 | 2,50 |
| R5 | 1,2 | 3,2 | 2,20 | 0,01 | 0,1 | 0,06 |
| R6 | −0,1 | −1,8 | −0,95 | 0,2 | −1,3 | −0,55 |
| R7 | −0,9 | −3,8 | −2,35 | −1,6 | −4,3 | −2,95 |
At 24 months in the group of patients treated by rTKAs, BMD showed a clear reduction in ROI 1, 4, and 7 of femur. In the tibial side BMD increased significantly in ROI 4, particularly at 3 months and decreased in ROI 1 and 7 (Table 2).
Table 2.
Revision Total Knee Arthroplasty: BMD variations in Tibia and Femur at 3, 6, 12 and 24 months (values are expressed in %).
| AREAS | 3 mo | 6 mo | 12 mo | 24 mo |
|---|---|---|---|---|
|
| ||||
| Tibial Stem | ||||
| GLOBAL | −1,62 | −1,93 | −2,42 | −5,80 |
| R1 | −15,05 | −11,63 | −11,49 | −14,15 |
| R2 | 2,48 | 0,13 | −3,80 | −6,82 |
| R3 | 9,67 | 4,86 | 5,18 | 2,33 |
| R4 | 10,74 | 7,03 | 6,91 | 2,18 |
| R5 | 0,63 | −5,73 | 7,69 | −6,11 |
| R6 | 2,07 | −11,97 | −3,20 | −12,71 |
| R7 | −4,24 | −17,36 | −16,06 | −12,95 |
|
| ||||
| Femoral Stem | ||||
|
| ||||
| GLOBAL | −1,58 | −2,92 | −4,80 | −6,14 |
| R1 | −2,96 | −7,73 | −9,45 | −13,60 |
| R2 | 0,17 | −2,35 | −6,55 | −6,66 |
| R3 | 3,22 | 1,57 | −1,55 | 2,78 |
| R4 | −1,12 | −1,25 | −2,55 | −1,45 |
| R5 | −4,10 | −0,77 | −5,87 | −6,61 |
| R6 | −3,18 | −8,10 | −3,52 | −3,37 |
| R7 | 4,14 | −5,73 | −7,88 | −11,89 |
Discussion
After a joint replacement a local biological process named “osseointegration” usually occurs, theoretically leading to a full adhesion of prosthetic surface to the host bone. This phenomenon is related to several factors: periprosthetic bone remodelling, positioning of the components, and properties of implants surface. If the implant is correctly positioned, a wide distribution of forces occurs at the bone-prosthesis counterface: the integration is oriented to be adequate and long lasting. Empty areas may however be present at the bone-prosthesis counterface. In these gaps, an ischemia followed by a bone resorption immediately occurs. In cases of primary stability of prosthetic components, circulating stem cells are recalled and specialised in osteoblastic elements with a consequent bone formation and increase of the local BMD to achieve a “secondary stability” of the implant. If the implant is not correctly positioned, or in case of host bone alterations, or in case of a poorly adequate bioactive surface (often for the first generation hip and knee implants), the mentioned process may not realize. Gaps may be filled by fibroblastic elements producing a fibrous tissue absolutely not appropriate for a long stability of the components. No bone formation but even resorption may occur with the local BMD decreasing, and configuring the so called “stress shielding” (31, 35–37). This may result in a painful implant or even in an early loosening of the components (19, 20, 30, 38–42).
Over the decades, many attempts to quantify or to early reveal any alterations of this process have been proposed, with variable results (35, 37). The radiographic assessment of the host bone-prosthetic components counterface after surgery is completely inadequate and tardive, as demonstrated by several studies confirming that a bone loss from 30 to 50% may actually occur before a radiologic evidence (43, 44).
One of the most useful tool is the DXA, and many studies have confirmed that it represents the most simple and efficient way to analyse the periprosthetic bone remodelling. Dedicated softwares for the densitometric evaluation allow a close analysis of specific areas in order to detect any BMD changes in all zones (45–50).
By using DXA at specific intervals over the time it is possible to detect any increase or decrease in the BMD, and to early understand where bone formation or resorption occurs (17, 31, 32). In the present study primary hip and revision knee implants were selected for the presence of stems, particularly interesting to be studied by DXA. Primary knee arthroplasty was not considered given the absence of stems in osteoarthritic patients. Our preliminary results confirm what reported in literature: in specific zones of femur in THAs there is a postoperative reduction of BMD (ROI 1 and 7), as an increase in ROI 4 (47, 51).
Moreover, a short stem (GTS) induces a higher periprosthetic bone remodelling with respect to a standard stem (PPF), preserving also the bone stock. The choice to not consider the acetabular cup depends on the not confirmed precision and accuracy of BMD measurements to identify the bone remodelling around the cup; the difference in patient posture affects the result: Mogens B. Laursen showed on one hand that the time elapsed since surgery does not inflict on reproducibility but on the other hand that scanning of the periacetabular bone can be performed as AP scans, with acceptable precision as long as pelvic tilt of more than 10 degrees is avoided. However he supports the assumption that load is beneficial to bone remodelling. While in the laboratory study small bony defects were found, no differences in bone remodelling in the clinical study related to the use of HA coating of the cups were showed (52).
Regarding rTKAs, our data suggest that in the distal femur and proximal tibia a bone resorption is consistent, while no substantial modification is revealed at the tip of femoral stems (ROI 4). This is significantly different from what reported in a previous study, in which an increase in the femur at the tip of stems has been found (34). By using a high press-fit stem for the tibial component in rTKA we have observed a bone apposition at 3 and 6 months, and a lesser resorption at 24 months compared to the low press-fit implantation (53).
Bone remodelling activity in THA and in rTKA is a process that seems to intensively develop during the first year after surgery, then reducing in the second year (32, 34, 47, 54–57).
Such observations, associated to other reported in literature have aroused a great interest in the proposal of bone metabolic therapies to improve the quality of osseointegration, particularly in the first months after an arthroplasty (12, 57, 58–60). However, further studies and randomized trials have to be conducted to ascertain any positive effect in such attempt.
Conclusions
A primary mechanical stability of a prosthetic implant should be intraoperatively achieved followed by a full osseointegration (secondary stability). However, in a small percentage of cases this mechanism may not completely realize leading to several clinical settings as painful prostheses, aseptic loosening, and failure of the implant. With respect to the past decades, an early detection of stress-shielding or loosening of the implants by a radiologic evaluation and DXA is possible. We consider DXA evaluation an ideal tool to assess early alterations at the bone-component counterface in primary THA and rTKA, in order to adopt strategies to limit any mechanical failure of the implant.
References
- 1.Innocenti M, Matassi F, Carulli C, Nistri L, Civinini R. Oxidized zirconium femoral component for TKA: a follow-up note of a previous report at a minimum of 10 years. Knee. 2014;21:858–61. doi: 10.1016/j.knee.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Buechel FF. Long-term follow-up after mobile-bearing total knee replacement. Clin Orthop Relat Res. 2002;404:40–50. doi: 10.1097/00003086-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Meding JB1, Ritter MA, Keating EM, Berend ME. Twenty-year Followup of an Uncemented Stem in Primary THA. Clin Orthop Relat Res. 2015 Feb;473(2):543–8. doi: 10.1007/s11999-014-3763-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Innocenti M, Civinini R, Carulli C, Villano M, Linari S, Morfini M. A modular total knee arthroplasty in haemophilic arthropathy. Knee. 2007;14:264–8. doi: 10.1016/j.knee.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Carulli C, Matassi F, Nistri L, Civinini R, Innocenti M. Long-term survival of a flat-on-flat total condylar knee arthroplasty fixed with a hybrid cementing technique for tibial components. J Long Term Eff Med Implants. 2012;22:305–12. doi: 10.1615/jlongtermeffmedimplants.2013007289. [DOI] [PubMed] [Google Scholar]
- 6.Solarino G, Piazzolla A, Notarnicola A, Moretti L, Tafuri S, De Giorgi S, Moretti B. Long-term results of 32-mm alumina-on-alumina THA for avascular necrosis of the femoral head. J Orthopaed Traumatol. 2012;13:21–27. doi: 10.1007/s10195-011-0174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Innocenti M, Civinini R, Carulli C, Matassi F. Proximal femural fractures: epidemiology. Clinical Case Bone Min Metab. 2009;6:117–119. [PMC free article] [PubMed] [Google Scholar]
- 8.Carulli C, Matassi F, Civinini R, Villano M, Innocenti M. Surgical prosthetic treatment. Clinical Case Bone Min Metab. 2010;7:32–38. [PMC free article] [PubMed] [Google Scholar]
- 9.Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7–13. doi: 10.1097/00003086-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Carulli C, Villano M, Bucciarelli G, Martini C, Innocenti M. Painful knee arthroplasty: definition and overview. Clin Cases Miner Bone Metab. 2011;8:23–5. [PMC free article] [PubMed] [Google Scholar]
- 11.Villano M, Carulli C, Puccini S, Soderi S, Innocenti M. Painful knee prosthesis: surgical approach. Clin Cases Miner Bone Metab. 2011;8:26–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Carulli C, Civinini R, Matassi F, Villano M, Innocenti M. The use of drugs antiosteoporotic in Total Knee Arthroplasty. Aging Clin Exp Res. 2011;2(Suppl 1):38–39. [PubMed] [Google Scholar]
- 13.Piscitelli P, Iolascon G, Innocenti M, Civinini R, Rubinacci A, Muratore M, D’Arienzo M, Leali PT, Carossino AM, Brandi ML. Painful prosthesis: approaching the patient with persistent pain following total hip and knee arthroplasty. Clin Cases Miner Bone Metab. 2013;10:97–110. [PMC free article] [PubMed] [Google Scholar]
- 14.Butler JB, Lansky D, Duwellius PJ. Prospective evaluation of total hip arthroplasty with a cementless, anatomically designed, porous-coated femoral implant: mean 11-year follow-up. J Arthroplasty. 2005;20:709–16. doi: 10.1016/j.arth.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Innocenti M, Carulli C, Matassi F, Carossino AM, Brandi ML, Civinini R. Total knee arthroplasty in patients with hypersensitivity to metals. Int Orthop. 2014;38:329–33. doi: 10.1007/s00264-013-2229-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matassi F, Botti A, Sirleo L, Carulli C, Innocenti M. Porous metal for orthopedics implants. Clin Cases Miner Bone Metab. 2013;10:111–5. [PMC free article] [PubMed] [Google Scholar]
- 17.Kiratli BJ, Heiner JP, McBeath AA, Wilson MA. Determination of bone mineral density by dual x-ray absorptiometry in patients with uncemented total hip arthroplasty. J Orthop Res. 1992;10:836–44. doi: 10.1002/jor.1100100613. [DOI] [PubMed] [Google Scholar]
- 18.Cohen B, Rushton N. Accuracy of DEXA measurement of bone mineral density after total hip arthroplasty. J Bone Joint Surg Br. 1995;77:479–83. [PubMed] [Google Scholar]
- 19.Engh CA, Bobyn JD. The influence of stem size and extent of porous coating on femoral bone resorption after primary cementless hip arthroplasty. Clin Orthop. 1988;231:7–28. [PubMed] [Google Scholar]
- 20.Mintzer CM, Robertson DD, Rackemann S, Ewald FC, Scott RD, Spector M. Bone loss in the distal anterior femur after TKA. Clinical Orthopaedics. 1990;260:135–43. [PubMed] [Google Scholar]
- 21.Okano T, Hagino H, Otsuka T, Teshima R, Yamamoto K, Hirano Y, Nakamura K. Measurement of periprosthetic bone mineral density by dual energy X-ray absorptiometry is useful for estimating fixation between the bone and the prosthesis in an early stage. J Arthroplasty. 2002;17:49–55. doi: 10.1054/arth.2002.28729. [DOI] [PubMed] [Google Scholar]
- 22.Windisch C, Windisch B, Kolb W, Kolb K, Grutzner P, Roth A. Osteo-densitometry measurements of periprosthetic bone using dual energy X-ray absorptiometry following total knee arthroplasty. Arch Orthop Trauma Surg. 2012;132:1595–601. doi: 10.1007/s00402-012-1601-9. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez-Vaquero D1, Garcia-Sandoval MA, Fernandez-Carreira JM, Suarez-Vazquez A, Perez-Hernandez D. Measurement of bone mineral density is possible with standard radiographs: a study involving total knee replacement. Acta Orthop. 2005;76:791–5. doi: 10.1080/17453670510045381. [DOI] [PubMed] [Google Scholar]
- 24.Soininvaara T1, Kroger H, Jurvelin JS, Miettinen H, Suomalainen O, Alhava E. Measurement of bone density around total knee arthroplasty using fan-beam dual energy X-ray absorptiometry. Calcif Tissue Int. 2000;67:267–72. doi: 10.1007/s002230001111. [DOI] [PubMed] [Google Scholar]
- 25.Karbowski A1, Schwitalle M, Eckardt A, Heine J. Periprosthetic bone remodelling after total knee arthroplasty: early assessment by dual energy X-ray absorptiometry. Arch Orthop Trauma Surg. 1999;119:324–6. doi: 10.1007/s004020050419. [DOI] [PubMed] [Google Scholar]
- 26.Parchi PD, Cervi V, Piolanti N, Ciapini G, Andreani L, Castellini I, Poggetti A, Lisanti M. Densitometric evaluation of periprosthetic bone remodeling. Clin Cases Miner Bone Metab. 2014;11:226–31. [PMC free article] [PubMed] [Google Scholar]
- 27.Ewald FC. The knee society total knee arthroplasty roentgen graphic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9–12. [PubMed] [Google Scholar]
- 28.Harris VM. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–55. [PubMed] [Google Scholar]
- 29.DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976;121:20–32. [PubMed] [Google Scholar]
- 30.Sundfeldt M, Carlsson LV, Johansson CB, et al. Aseptic loosening, not only a question of wear: a review of different theories. Acta Orthop. 2006;77:177–97. doi: 10.1080/17453670610045902. [DOI] [PubMed] [Google Scholar]
- 31.Gruen TA, McNeice GM, Amstutz HC. Modes of failure of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop. 1979;141:17–27. [PubMed] [Google Scholar]
- 32.Giorgini M, Matassi F, Cozzi Lepri A, Nistri L, Carulli C, Civinini R, Innocenti M. Analysis of bone remodeling in the revision of periprosthetic knee prosthesis with stems by diaphyseal densitometric evaluation. J Orthopaed Traumatol. 2014;15(Suppl 1):S88–S89. [Google Scholar]
- 33.Van Lenthe GH, Willems MMM, Verdonschot N, de Waal Malefijt MC, Huiskes R. Stemmed femoral knee prostheses. Acta Orthop Scand. 2002;73:630–637. doi: 10.1080/000164702321039589. [DOI] [PubMed] [Google Scholar]
- 34.Jensen CL, Petersen MM, Schroder HM, Lund B. Bone mineral density changes of the proximal tibia after revision total knee arthroplasty. A randomised study with the use of porous tantalum metaphyseal cones. Int Orthop. 2012;36:1857–1863. doi: 10.1007/s00264-012-1601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hinderling T, Ruegsegger P, Anliker M, Dietschi C. Computed tomography reconstruction from hollow projections. An application to in vivo evaluation of artificial hip joints. J Comput Assist Tomogr. 1979;3:52–57. [PubMed] [Google Scholar]
- 36.Rawlinson JJ, Peters LE, Campbell DA, Windsor R, Wright T, Bartel D. Cancellous bone strains indicate efficacy of stem augumentation in constrained condylar knees. Clin Orthop Relate Res. 2005;400:107–116. doi: 10.1097/01.blo.0000187340.10003.68. [DOI] [PubMed] [Google Scholar]
- 37.Health and Public Policy Committee, American College of Physicians. Radiologic methods to evaluate bone-mineral content. Ann Intern Med. 1984;100:908–910. [PubMed] [Google Scholar]
- 38.Pritchett JW. Following hip replacement femoral bone loss. Clin Orthop Relat Res. 1995;314:156–161. [PubMed] [Google Scholar]
- 39.Cristofolini L, Affatato S, Erani P, Leardini W, Tigani D, Viceconti M. Long-term implant-bone fixation of the femoral component in total knee replacement. Proc Inst Mech Eng H J Eng Med. 2008;222:319–331. doi: 10.1243/09544119JEIM328. [DOI] [PubMed] [Google Scholar]
- 40.Aspenberg P, Herbertsson P. Periprosthetic bone resorption. Particles versus movement. J Bone Joint Surg Br. 1996;78:641–646. [PubMed] [Google Scholar]
- 41.Bauer TW. Particles and periimplant bone resorption. Clin Orthop Relat Res. 2002;405:138–143. doi: 10.1097/00003086-200212000-00016. [DOI] [PubMed] [Google Scholar]
- 42.Stern SH, Wills RD, Gilbert JL. The effect of tibial stem design on component micromotion in knee arthroplasty. Clin Orthop Relat Res. 1997;345:44–52. [PubMed] [Google Scholar]
- 43.Ardran GM. Bone destruction not demonstrable by radiography. Br J Radiol. 1951;24:107–109. doi: 10.1259/0007-1285-24-278-107. [DOI] [PubMed] [Google Scholar]
- 44.Lachmann E. Osteoporosis: the potentialities and limitations of its roentgenologic diagnosis. Am J Roentgenol. 1955;74:712–715. [Google Scholar]
- 45.Sartoris DJ, Resnick D. Dual-energy radiographic absorptiometry for bone densitometry. Current status and perspective. Am J Roentgenol. 1989;152:241–246. doi: 10.2214/ajr.152.2.241. [DOI] [PubMed] [Google Scholar]
- 46.Wahner HW, Dunn WI, Brown ML, Morin RL, Riggs BL. Comparison of dual energy X-ray absorptiometry and dual photon absorptiometry for bone mineral measurements of the lumbar spine. Mayo Clin Proc. 1978;62:1075–1084. doi: 10.1016/s0025-6196(12)65502-5. [DOI] [PubMed] [Google Scholar]
- 47.Boden HS, Skoldenberg OG, Salemyr MO, Lundberg HJ, Adolphson PY. Continuous bone loss around a tapered uncemented femoral stem: a long-term evaluation with DEXA. Acta Orthop. 2006;77:877–885. doi: 10.1080/17453670610013169. [DOI] [PubMed] [Google Scholar]
- 48.Skoldenberg OG, Boden HS, Salemyr MO, Ahl TE, Adolphson PY. Periprosthetic proximal bone loss after uncemented hip arthroplasty is related to stem size: DXA measurements in 138 patients followed for 2–7 years. Acta Orthop. 2006;77:386–392. doi: 10.1080/17453670610046307. [DOI] [PubMed] [Google Scholar]
- 49.Venesmaa PK, Kroger HP, Jurvelin JS, Miettinen HJ, Suomalainen OT, Alhava EM. Periprosthetic bone loss after cemented total hip arthroplasty: a prospective 5-year dual energy radiographic absorptiometry study of 15 patients. Acta Orthop Scand. 2003;74:31–36. doi: 10.1080/00016470310013617. [DOI] [PubMed] [Google Scholar]
- 50.Wixson RL, Stulberg SD, Van Flandern GJ, Puri L. Maintenance of proximal bone mass with an uncemented femoral stem analysis with dual-energy x-ray absorptiometry. J Arthroplasty. 1997;12:365–372. doi: 10.1016/s0883-5403(97)90191-1. [DOI] [PubMed] [Google Scholar]
- 51.Dan D, Germann D, Burki H, Hausner P, Kappeler U, Meyer RP, Klaghofer R, Stoll T. Bone loss after total hip arthroplasty. Rheumatol Int. 2006;26:792–8. doi: 10.1007/s00296-005-0077-0. [DOI] [PubMed] [Google Scholar]
- 52.Laursen Mogens Berg. PhD thesis. Faculty of Health Sciences, University of Aarhus Northern Orthopaedic Division Aalborg Hospital, Aarhus University Hospital; 2005. DEXA-scanning in description of bone remodeling and osteolysis aroundcementless acetabular cups. [Google Scholar]
- 53.Marshall AD, Mokris JG, Reitman RD, Dandar A, Mauerhan DR. Cementless titanium tapered-wedge femoral stem: 10 - to 15-year followup. J Arthroplasty. 2004;19:546–52. doi: 10.1016/j.arth.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 54.Kroger H, Venesmaa P, Jurvelin J, et al. Bone density at the proximal femur after total hip arthroplasty. Clin Orthop. 1998;352:66–74. [PubMed] [Google Scholar]
- 55.Shanbhag AS, Hasselman CT, Rubash HE. The John Charnley Award. Inhibition of wear debris mediated osteolysis in a canine total hip arthroplasty model. Clin Orthop Relat Res. 1997;344:33–43. [PubMed] [Google Scholar]
- 56.Seki T, Omori G, Koga Y, et al. Is bone density in the distal femur affected by use of cement and by femoral component design in total knee arthroplasty? J Orthop Sci. 1999;4:180–186. doi: 10.1007/s007760050091. [DOI] [PubMed] [Google Scholar]
- 57.Cavalli L, Brandi ML. Periprosthetic bone loss: diagnostic and therapeutic approaches. F1000Res. 2014 Jun 17;2:266. doi: 10.12688/f1000research.2-266.v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Knusten R, Ebramzadeh E, Longjohn DB, Sangiorgio SN. Systematic Analysis of Bisphosphonate Intervention on Periprosthetic BMD as a Function of Stem Design. J Arthroplasty. 2014;29:1292–97. doi: 10.1016/j.arth.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 59.Tapaninen TS, Venesmaa PK, Jurvelin JS, Miettinen HJ, Kroger HP. Alendronate reduces periprosthetic bone loss after uncemented primary total hip arthroplasty - a 5-year follow-up of 16 patients. Scand J Surg. 2010;99:32–7. doi: 10.1177/145749691009900108. [DOI] [PubMed] [Google Scholar]
- 60.Carulli C, Matassi F, Civinini R, Innocenti M. Tissue engineering applications in the management of bone loss. Clin Cases Miner Bone Metab. 2013;10:22–5. doi: 10.11138/ccmbm/2013.10.1.022. [DOI] [PMC free article] [PubMed] [Google Scholar]