Abstract
Objective
To determine the effect of diets low in saturated fatty acids (SFA), high in monounsaturated fatty acids (MUFA) or polyunsaturated fatty acids (PUFA) on body composition in participants at risk for MetS.
Methods;
The present study is a randomized, crossover, controlled feeding study. Participants (n = 101, ages 49.5 ± 1.2, BMI 29.4 ± 0.4 kg/m2) were randomized to five isocaloric diets contained treatment oils: Canola, CanolaOleic, CanolaDHA, Corn/Safflower and Flax/Safflower. Each diet period was 4-week followed by a 2–4 week washout period.
Results
Canola (3.1 kg, p=0.026) and CanolaOleic oil diets (3.1 kg, p=0.03) reduced android fat mass compared with the Flax/Saff oil diet (3.2 kg), particularly in males. The decrease in abdominal fat mass was correlated with the reduction in blood pressure after the Canola (SBP r = 0.26, p=0.062; DBP r=0.38, p=0.0049) and CanolaOleic oil diets (SBP r = 0.39 p=0.004; DBP r=0.45, p=0.0006). The decrease in abdominal fat mass also was associated with a reduction in TG levels after the CanolaOleic oil diet (r = 0.42, p=0.002).
Conclusion
Diets high in MUFA (compared with PUFA) reduced central obesity with an accompanying improvement in MetS risk factors. Our findings demonstrate that diets high in MUFA may be beneficial for treating and perhaps preventing MetS.
Keywords: Obesity, Fat Mass Loss, MUFA, Diet, Metabolic Sydrome
Introduction
Abdominal obesity is an important criterion for metabolic syndrome (MetS) along with glucose intolerance, dyslipidemia, and hypertension (1). MetS increases the risk of cardiovascular disease (CVD) and type 2 diabetes mellitus (2). Almost 40% of US adults have MetS based on the International Diabetes Federation definition (IDF) (3). Weight loss is the primary treatment for MetS (4). Evidence is emerging that demonstrates beneficial effects of dietary monounsaturated fatty acids (MUFA) in regulating body weight and cardiometabolic risk factors (5). The PREDIMED (Prevención con Dieta Mediterránea) study has shown that long-term consumption of a high MUFA diet provided by extra virgin olive oil or mixed nuts reduced central obesity (6). In addition, studies have shown that MUFA-enriched diets (21–23% of energy) reduce abdominal fat and central obesity (7, 8). A short-term study (3 weeks) by Kien et al. (9) reported that a diet rich in oleic acid versus palmitic acid was associated with a decreased android adiposity in healthy men and women. In the OmniHeart Trial, a diet with 21% of energy from MUFA (and 10% from PUFA) lowered blood pressure and coronary heart disease risk compared with the higher carbohydrate diet (5). Others have shown that high MUFA (20–23% of energy) diets improved the lipid/lipoprotein profile (10, 11), as well as insulin sensitivity and/or glycemic control (10, 12). Our understanding of the role that MUFA play in cardiometabolic disease risk reduction is in the early stages, and further research is needed to clarify the specific effects that MUFA have on central obesity, which is causally related to other MetS criteria.
The present study was conducted to evaluate the effects of five vegetable oil blends varying in MUFA and PUFA on body composition changes and cardiometabolic risk factors in individuals with or at risk for MetS. Our hypothesis was that high MUFA diets would beneficially affect central obesity in individuals with or at risk for MetS and also reduce other cardiometabolic risk factors.
Methods
Participants
We have published detailed information about the study design, participant characteristics, and diet design (13). Briefly, 130 participants were studied at 3 research centers: University of Manitoba (Canada), Laval University (Canada) and the Pennsylvania State University (United States). Inclusion criteria were men and women 20 to 65 years of age, BMI between 22 to 40 kg/m2 with central obesity (waist circumference: men, ≥ 94 cm; women, ≥ 80 cm) plus at least one other MetS criteria. Criteria included raised fasting blood glucose (≥5.6 mmol/L), decreased HDL cholesterol (men ≤ 1.0 mmol/L, women ≤ 1.3 mmol/L), increased triglycerides ( 1.7 mmol/L) and elevated blood pressure (SBP ≥ 130 mmHg or DBP ≥ 85 mmHg). Exclusion criteria were thyroid disease, diabetes mellitus, kidney disease, liver disease; current smokers; or consuming more than 2 alcoholic drinks per week. Individuals taking any medications for dyslipidemia, hypercholesterolemia or inflammation were not eligible to participate in the study. Recruitment criteria were based on the IDF criteria for MetS (14). Participants were recruited via flyers, local newspapers, radio advertisements, and campus mail. The study was approved by the Ethics Committee of University Manitoba, Laval University and Pennsylvania State University and carried out in accordance with the Helsinki Declaration.
Study protocol
A randomized, cross-over, 5 diet period, controlled feeding study was conducted. Each treatment period lasted 4 wks and was separated by a 2–4 week break during which time participants followed their habitual diet. After meeting eligibility criteria, participants were randomly assigned to a sequence of 5 experimental diets. The study coordinators and participants were blinded to the treatments as were all laboratory staff.
Dietary intervention
Test diets were created using Food Processor SQL software, version 10.8 (ESHA Research, Salem, OR). Participants were fed an isocaloric diet (calculated by Harris-Benedict equation) and advised to follow their routine physical activity practices. Baseline body weight was used to calculate basal metabolic rate (BMR). For men: BMR = 66 + [13.7 x weight (kg)] +[ 5 x height (cm)] − [6.8 x age (years)], for women: BMR = 655 + [9.6 x weight (kg)] + [1.8 x height (cm)] − [4.7 x age (years)]. Weight was measured daily (Monday through Friday). During the feeding periods, participants were provided with all of their food, which was prepared in the metabolic kitchen at each research center.
The macronutrient profiles of the study diets was based on a typical American diet with 50% of energy from carbohydrate, 35% of energy from fat (18% from treatment oils) and 15% of energy from protein. Five treatment oils: Canola oil (conventional canola oil), CanolaOleic (high-oleic acid canola oil), CanolaDHA (high-oleic acid canola oil with DHA), Corn/Saff (Corn/Safflower oil), and Flax/Saff (Flax/Safflower oil) were studied and incorporated into smoothies that participants consumed twice daily. The smoothie contained the treatment oil, frozen unsweetened strawberries, orange sherbet and non-fat milk. The quantity of oil was calculated based on participant energy needs. For a 3000 kcal diet, 60 g of treatment oil per day was required to provide 18% of total energy. Each smoothie contained 100 g orange sherbet, 100 g non-fat milk, 100 g frozen unsweetened strawberries and 30 g oil. Compliance was verified by assessing the total plasma fatty acid profile at the end of each intervention period, as described previously (15). In brief, fasted blood samples were collected. Fatty acid methyl esters were analyzed using an Agilent 6890 N gas chromatograph equipped with a flame ionization detector (Agilent Technologies, Ontario, Canada). The quantity of each fatty acid was calculated by using the corresponding peak area divided by the total area of the fatty acids assayed.
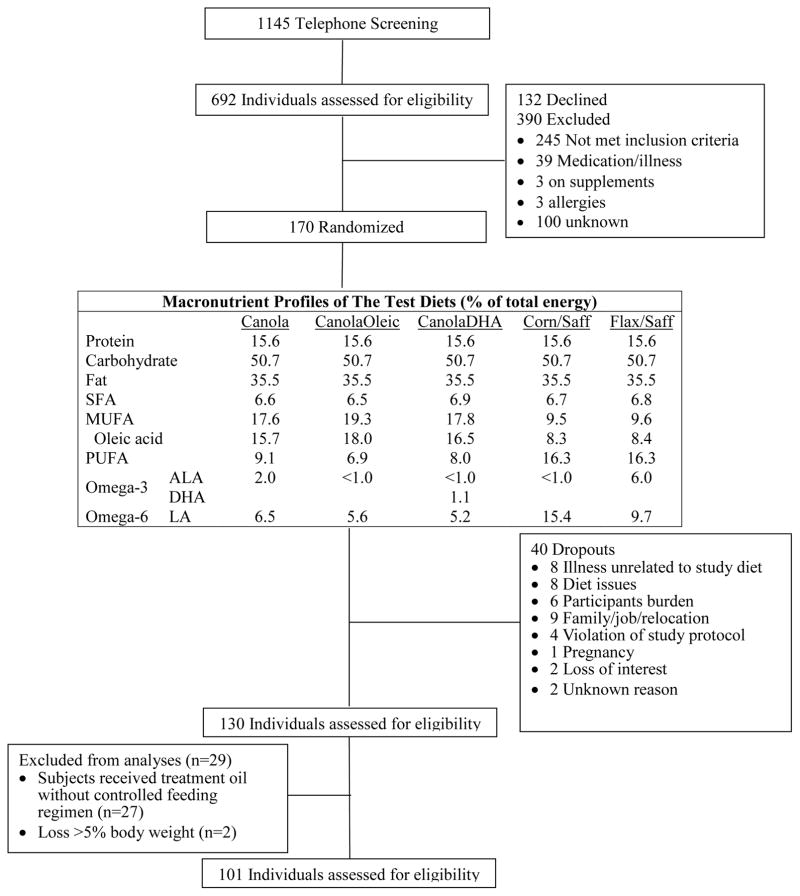
The macronutrient profiles of the study diets are presented in Figure 1. The fatty acid profiles of treatment oils are presented in Table S1. A subgroup of participants (n=27) from the University of Manitoba site prepared their own meals in a communal kitchen under supervision due to cultural habits, and consumed smoothies with the treatment oils that were pre-made and delivered from the University of Manitoba. Since they did not receive a controlled feeding regimen, these participants were excluded from the analysis (15). Two participants who lost more than 5% of body weight due to illness unrelated to the study were also excluded from the analysis (Figure 1).
Figure 1.
Participant screening, enrollment and the macronutrient profiles of the study diets in the COMIT study
Main Outcome and Measures
Dual-Energy X-Ray Absorptiometry (DXA) measurements
Body composition was assessed by DXA according to the manufacturer’s recommendations (Lunar Prodigy Advance, Madison, WI, USA; QDR-4500W; Hologic Corp, Waltham, MA). Total and regional body composition were determined with Prodigy Encore 2005 software (version 9.30.044) and APEX System software (version 4.0). Criteria used to identify the anatomical region of interest were identical across all sites. A subset of study population from two sites (n=54) had baseline body composition assessment (measured at the beginning of the study). All sites performed DXA scans at the end of each diet period (n=101).
Statistics
Variables were tested for normality and reported as the mean ± SE. Statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, NC, US) mixed model (PROC MIXED) procedure. Models included the following factors as fixed effects: diet, period, age, gender, diet by period, diet by gender interaction with participants and center as random effects. No diet carryover effect was observed. A Tukey adjustment was used for multiple comparisons. Within the 54 participants who had baseline body composition assessment, changes from baseline were calculated by subtracting the day 1 measurements from the day 29 measurements. For end-of-diet measurements, the sample size of n=101 offered at least 90% of power to detect a mean of 3% differences in android fat mass between treatments with α=0.05. For changes from baseline in response to treatment diets, the sample size of n= 54 had 80% of power to detect a mean of 3% of android fat mass change between treatment with α=0.05. The Benjamini–Hochberg procedure was used to control for the false discovery rate. With the Benjamini-Hochberg critical value for a false discovery rate of 10%, the corrected level of significance was 0.03. Pearson correlation was used to calculate the correlation coefficient.
Results
Participant characteristics
Participant screening and enrollment in the study are shown in Figure 1. The study was conducted between July 2010 and April 2012. One hundred and one participants completed all 5 controlled feeding diet periods and were included in the analysis. The mean energy intake for all participants was the same between treatments (Table S1). Participants’ baseline anthropometric and metabolic characteristics are summarized in Table 1. The majority of participants were Caucasian (95%). All participants had at least one MetS criterion in addition to central obesity, which characterized them as a population with or “at risk” for MetS (14). Specifically, 39 participants had elevated blood pressure, 44 had elevated triglycerides, 49 had low HDL-C, and 34 had elevated fasted glucose. In addition to increased waist circumference, 50 participants had one additional criterion for MetS, 33 participants had two additional criteria, 16 had three additional criteria, and 2 had all five criteria for MetS. Fifty-one participants met the criteria for MetS.
Table 1.
Subject metabolic characteristics at screening (n=101)
| Characteristic | All participants (n=101) | Males (50) | Females (51) |
|---|---|---|---|
|
Anthropometric measurements
| |||
| Age | 49.5±1.2 | 46.6±1.9 | 52.2±1.5 |
| Body mass (kg) | 85.8±1.5 | 95.8±1.9* | 75.9±2.1* |
| Height (m) | 1.7±0.01 | 1.7±0.01 | 1.6±0.01 |
| Body mass index (kg/m2) | 29.4±0.4 | 30.2±0.6 | 28.6±0.6 |
| Waist circumference | 101.9±1.1 | 107.0±1.2* | 96.8±1.4* |
|
| |||
|
Metabolic syndrome risk factors
| |||
| Glucose (mmol/L) | 5.2±0.1 | 5.2±0.1 | 5.3±0.2 |
| HDL-cholesterol (mmol/L) | 1.3±0.03 | 1.1±0.04 | 1.4±0.1 |
| Triglycerides (mmol/L) | 1.8±0.09 | 2.0±0.2 | 1.7±0.1 |
| Blood pressure SBP/DPB(mmHg) | 122/78 | 123/78 | 118/78 |
|
| |||
|
Number of Metabolic syndrome risk factors per participant
| |||
| 1 factor | 57 | 25 | 32 |
| 2 factors | 24 | 17 | 7 |
| 3 factors | 19 | 7 | 12 |
| 4 factors | 1 | 1 | |
Values are expressed as means ± SEM
Values were significantly different between genders p<0.0001
Adherence
Plasma fatty acid profiles at the end of each diet period confirmed diet adherence (Table S2). Between-diet comparisons revealed that total MUFA concentration was the highest in participants on the CanolaOleic oil diet (18.5 ± 0.3, p<0.0001), followed by the Canola oil diet (17.9 ± 0.2, p<0.0001) versus the other diets. The Corn/Saff oil diet resulted in the highest total n-6 PUFA (38.7 ± 0.3, p<0.0001), followed by the Flax/Saff oil diet (35.9 ± 0.3, p=0.0043). The Flax/Saff oil diet resulted in the highest ALA (1.7 ± 0.04, p<0.0001). The CanolaDHA oil diet resulted in the highest total n-3 PUFA plasma concentration (9.6 ± 0.1), mainly contributed by DHA (7.1 ± 0.08) and EPA (1.6±0.06).
Outcomes
Correlations between android fat mass and cardiometabolic risk factors (n=54)
Baseline correlations between android fat mass and cardiometabolic risk factors (n=54) are presented in Table 2. Android fat mass was positively correlated with CRP (r=0.28, p=0.04), TG levels (r=0.27, p =0.04), SBP (r=0.32, p=0.02) and DBP (r=0.32, p=0.019). Changes in android fat mass from baseline after the Canola and CanolaOleic oil diets were positively associated with decreases in SBP (Canola, r=0.26, p=0.06; CanolaOleic, r=0.39, p=0.004 and DBP (Canola, r=0.38, p=0.005; CanolaOleic, r=0.45, p=0.0006). Changes in android fat mass were positively correlated with decreases in plasma TG levels after consumption of the CanolaOleic oil (r=0.42, p=0.002) and the Flax/Saff oil (r=0.41, p=0.002) diets. Thus, as android fat mass decreased, there was a corresponding decrease in both SBP and DBP in the Canola and CanolaOleic oil diets, and a decrease in TG with the CanolaOleic oil diet.
Table 2.
Correlations between android fat mass and cardiovascular risk factors at baseline and after test diets (n=54)
| Baseline | Canola | CanolaOleic | CanolaDHA | Corn/Saff | Flax/Saff | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Android fat mass | Changes of android fat mass from baseline | |||||||||||
|
|
||||||||||||
| r | p | r | p | r | p | r | p | r | p | r | p | |
| TG | 0.27 | 0.045 | 0.07 | NS | 0.42 | 0.0017 | 0.09 | NS | 0.26 | NS | 0.41 | 0.002 |
| CRP | 0.28 | 0.0043 | 0.0012 | NS | 0.013 | NS | 0.11 | NS | 0.11 | NS | 0.19 | NS |
| SBP | 0.31 | 0.023 | 0.26 | 0.06 | 0.39 | 0.004 | 0.28 | 0.044 | 0.19 | NS | 0.37 | 0.0052 |
| DBP | 0.32 | 0.019 | 0.38 | 0.0049 | 0.45 | 0.0006 | 0.30 | 0.028 | 0.21 | NS | 0.35 | 0.010 |
NS: non-significant
Between-diet comparisons following the end-of-diet period body weight and body composition (n=101)
Body composition results are presented in Table 3. Participants had lower body weights at the end of the Canola (p=0.007) and CanolaOleic oil diets (p=0.02) compared with the Flax/Saff oil diet. There was a strong trend for the Canola oil diet to decrease trunk fat mass compared with the Flax/Saff oil diet (p=0.05). There was a diet effect on android fat mass (p = 0.01). Participants had a lower android fat mass in response to the two high MUFA diets, CanolaOleic oil diet (3.09 ± 0.1 kg, p=0.03), and the Canola oil diet (3.1 ± 0.1 kg, p=0.03) compared with the Flax/Saff oil diet (3.2 ± 0.1 kg). The decrease in android fat mass was affected by gender (p=0.03 for diet by gender interaction). Specifically, males had a lower android fat mass (3.3 ± 0.1 kg) as well as the android-to-gynoid fat mass ratio (0.71 ± 0.02) in response to the CanolaOleic oil diet compared with the Flax/Saff oil diet (3.5 ± 0.1 kg, 0.73 ± 0.03, p=0.003, p=0.0067, respectively). In contrast, there were no differences in females across the five treatment diets.
Table 3.
Body weight, body composition after each test diets (All participants n=101, males n=50, females n=51)
| Body composition measurements (kg) | Canola | CanolaOleic | CanolaDHA | Corn/Saff | Flax/Saff | p-value | |
|---|---|---|---|---|---|---|---|
| Body mass | All | 86.6±1.5a | 86.8±1.5a | 86.9±1.5ab | 87.0±1.5ab | 87.3±1.56b | 0.0073 |
| Male | 95.3±1.2ab | 95.0±1.9a | 95.4±1.9ab | 95.5±1.9ab | 96.0±1.9b | 0.01ǂ | |
| Female | 76.8±1.6 | 77.3±1.6 | 77.3±1.6 | 77.2±1.6 | 77.4±1.6 | NS | |
|
| |||||||
| Total fat mass | All | 31.2±0.8 | 31.2±0.8 | 31.3±0.8 | 31.4±0.8 | 31.6±0.8 | 0.083 |
| Male | 30.7±1.1 | 30.4±1.1 | 30.6±1.1 | 30.5±1.1 | 31.1±1.2 | NS | |
| Female | 31.7±1.2 | 32.1±1.2 | 32.0±1.2 | 32.3±1.2 | 32.0±1.2 | NS | |
|
| |||||||
| Total lean mass | All | 51.3±1.2 | 51.3±1.2 | 51.4±1.2 | 51.4±1.2 | 51.4±1.2 | NS |
| Male | 60.7±1.2 | 60.8±1.2 | 61.0±1.2 | 61.0±1.2 | 60.9±1.2 | NS | |
| Female | 42.1±0.7 | 42.1±0.7 | 42.1±0.7 | 42.0±0.6 | 42.1±0.7 | NS | |
|
| |||||||
| Trunk fat mass | All | 16.7±0.51 | 16.7±0.5 | 16.9±0.5 | 17.0±0.5 | 17.0±0.51 | 0.013 |
| Male | 17.9±0.7ab | 17.6±0.6a | 17.9±0.7ab | 17.9±0.7ab | 18.2±0.7b | 0.04ǂ | |
| Female | 15.6±0.7a | 15.8±0.7ab | 15.9±0.7ab | 16.1±0.7b | 15.8±0.7ab | 0.04ǂ | |
|
| |||||||
| Trunk lean mass | All | 24.2±0.6 | 24.2±0.6 | 24.3±0.6 | 24.3±0.6 | 24.3±0.6 | NS |
| Male | 28.2±0.7 | 28.3±0.7 | 28.4±0.7 | 28.5±0.7 | 28.4±0.7 | NS | |
| Female | 20.2±0.4 | 20.2±0.4 | 20.3±0.4 | 20.2±0.4 | 20.2±0.4 | NS | |
|
| |||||||
| Android fat mass | All | 3.1±0.1a | 3.09±0.1a | 3.2±0.1ab | 3.1±0.1ab | 3.2±0.1b | 0.010ǂ |
| Male | 3.4±0.1ab | 3.3±0.1a | 3.4±0.1ab | 3.4±0.1ab | 3.5±0.1b | 0.0027ǂ | |
| Female | 2.9±0.1 | 2.9±0.1 | 2.9±0.1 | 2.9±0.1 | 2.9±0.1 | NS | |
|
| |||||||
| Android lean mass | All | 3.6±0.1 | 3.6±0.1 | 3.7±0.1 | 3.6±0.1 | 3.7±0.1 | NS |
| Male | 4.3±0.1 | 4.3±0.1 | 4.3±0.1 | 4.3±0.1 | 4.3±0.1 | NS | |
| Female | 2.9±0.1 | 3.0±0.1 | 3.1±0.1 | 3.0±0.1 | 3.0±0.1 | NS | |
|
| |||||||
| Gynoid fat mass | All | 5.3±0.2 | 5.3±0.2 | 5.3±0.2 | 5.3±0.2 | 5.3±0.2 | NS |
| Male | 4.9±0.2 | 4.9±0.2 | 4.9±0.2 | 4.8±0.2 | 4.9±0.2 | NS | |
| Female | 5.7±0.2 | 5.7±0.2 | 5.8±0.2 | 5.7±0.2 | 5.7±0.2 | NS | |
|
| |||||||
| Gynoid lean mass | All | 7.7±0.2 | 7.7±0.2 | 7.7±0.2 | 7.7±0.2 | 7.7±0.2 | NS |
| Male | 9.0±0.2 | 0.9±0.2 | 9.1±0.2 | 9.1±0.2 | 9.1±0.2 | NS | |
| Female | 6.4±0.1 | 6.4±0.1 | 6.3±0.1 | 6.4±0.1 | 6.3±0.1 | NS | |
|
| |||||||
| A/G¶ | All | 0.61±0.02 | 0.60±0.02 | 0.61±0.02 | 0.62±0.02 | 0.62±0.02 | 0.05 |
| Male | 0.72±0.02ab | 0.71±0.02a | 0.72±0.02ab | 0.72±0.03ab | 0.73±0.03b | 0.0067ǂ | |
| Female | 0.51±0.02 | 0.51±0.02 | 0.51±0.02 | 0.51±0.02 | 0.51±0.02 | NS | |
Values are expressed as means ± SEM. Values in a row with different superscript letters indicate significant differences between diets p<0.05.
Diet by gender effect;
Canola vs. Flax/Saff (p=0.0518); NS: non-significant;
Android-to-gynoid fat mass ratio
Between-diet comparisons for changes in android fat mass from baseline (n=54)
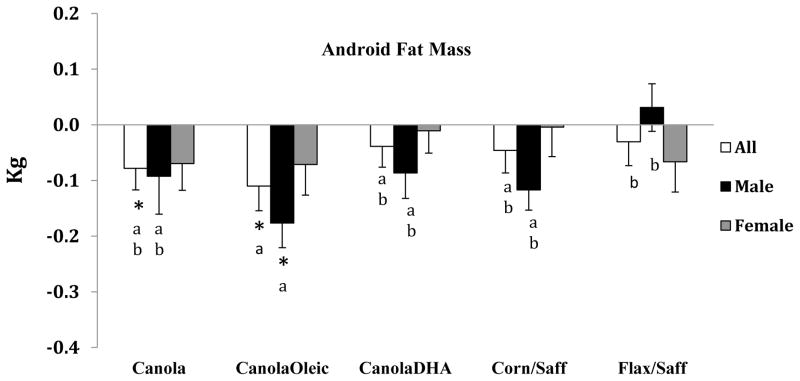
Android fat mass was decreased from baseline on the Canola oil (p=0.04) and CanolaOleic oil diets (p=0.007); the reduction in response to the CanolaOleic oil diet was greater than that for the Flax/Saff oil diet (p=0.02) Figure 2. No between diet differences were observed among the Canola, CanolaDHA and Corn/Saff oil diets in android fat mass. A gender difference was observed in android fat mass in response to the experimental diets (p=0.02). Male participants experienced a significant android fat mass loss (p=0.02) from baseline after the CanolaOleic oil diet compared with the Flax/Saff oil diet.
Figure 2.
Android fat mass changes in response to five experimental diets
□ All
■ Male
 Female
Female
*Values were significantly different from baseline p<0.05
Values with different superscript letters indicate significant differences between diets p<0.05
Discussion
Our results add to the emerging evidence that dietary MUFA decrease central obesity and improve cardiometabolic health compared with PUFA, specifically, with ALA (18). After just 28 days, the two MUFA rich diets, CanolaOleic oil and Canola oil diets reduced android fat mass (about 3%) compared to a n-6 PUFA-enriched Flax/Saff oil diet (which was high in ALA). The changes in android fat mass were accompanied by a reduction in the android to gynoid fat mass ratio after the CanolaOleic oil diet in males. The reduction was due to a decrease in central body fat mass, rather than a redistribution of adipose tissue to the lower body, as indicated by no change in the gynoid fat mass. The CanolaOleic oil diet provided the most MUFA (19.3% of energy) compared with the Canola and CanolaDHA oil diets (17.6% and 17.8% of energy, respectively). In the Canola and CanolaOleic diets that decreased android fat mass, plasma oleic acid concentration was highest (14.9% and 15.6%, respectively) compared with the other oil diets. These findings add to the evidence base that dietary MUFA beneficially affects central adiposity in an isocaloric setting. In support of our findings, Gillingham et al. (7) reported a tendency for a reduction (p=0.055) in the android to gynoid fat mass ratio after consumption of a high oleic canola oil diet that provided 21% MUFA from canola oil for 28 days in hypercholesterolemic individuals. Results from the PREDIMED study corroborate our findings in which diets high in MUFA have a beneficial effect on reducing central obesity and CVD risk. The PREDIMED study reported a decrease in waist circumference, which is a surrogate marker for central obesity, and demonstrated cardiovascular benefits in response to long term consumption of Mediterranean diets supplemented with extra virgin olive oil or mixed nuts (6, 16, 17).
The mechanisms for the reduction in body weight and android fat mass may include a greater oxidation rate and/or increased energy expenditure in response to the consumption of a high MUFA diet. Previous studies have shown that diets high in MUFA affect fat balance, body mass and possibly energy expenditure (18, 19, 20). Paniagua et al. (8) reported that a MUFA-rich diet compared to a carbohydrate-rich diet resulted in greater fat oxidation rates and a decrease in the abdominal fat-to-leg fat ratio in insulin resistant participants. In addition, Schmidt et al. (21) reported that the fractional oleic acid oxidation rate was significantly greater (21%) than for palmitic acid in healthy men (n=10) after consuming meals that provided 16.5% energy from oleate and 16.3% from palmitate during an 8 hour postprandial period. Kien et al. (19) demonstrated that consumption (for 28 days) of a high oleic acid diet (1.7% palmitic acid and 31.4% oleic acid) increased the fatty acid oxidation rate in women compared to the control diet (8.4% palmitic acid and 13.1% oleic acid). In the same study, a high oleic acid diet increased daily energy expenditure compared to a high palmitic acid diet (16.8% palmitic acid, 16.4% oleic acid) in men (19). Collectively, the results from other studies are suggestive of increased oxidation and energy expenditure mechanisms that may explain the decrease in body weight and android fat mass in response to the two high oleic acid diets evaluated in the present study. In contrast, a recent study by Kien et al. (22) reported that a lower rate of total fatty acid oxidation was observed when palmitic acid (PA: 16.0% of energy; OA: 16.2% of energy) was replaced with oleic acid (OA: 28.8% of energy) in eighteen healthy participants. The authors suggested that the discrepancy with their previous finding about fatty acid oxidation could due to the methods of food delivery and the high serum concentration of estradiol, which may have masked the effects of MUFA on fatty acid oxidation.
Related to this, MUFA have been shown to activate PPAR-delta and thereby increase fatty acid oxidative capacity (23, 24), a finding that provides a plausible molecular mechanism for the body weight and android fat mass decreases we report herein for the diets highest in MUFA. Moreover, a derivative of oleic acid, oleoylethanolamide (OEA), has been shown to induce lipolysis through the activation of PPAR-alpha, which provides a possible mechanism of action for the favorable body composition changes in participants on the high MUFA diets (25, 26, 27). In addition, a higher MUFA diet has been associated with lower inflammatory activity that may regulate adipose tissue mass (28). Recent evidence has shown that inhibition of inflammasome-mediated caspase-1 activity and reduction of obesity-associated inflammation (i.e., IL-1β) attenuated an increase in body weight despite a similar daily food intake in animal models (29, 30). High MUFA consumption may also contribute to this particular pathway in regulating body weight and fat mass.
Numerous studies have shown that android fat mass is positively correlated with BP, TG and CRP levels (31, 32, 33). In our study, the reduction in android fat mass was positively correlated with decreases in cardiometabolic risk factors including triglycerides, SBP and DBP after the Canola, CanolaOleic, CanolaDHA, and Flax/Saff oil diets, but not the Corn/Saff oil diet. Previous evidence has shown that dietary MUFA decrease SBP (5). A recent cross-sectional study reported that MUFA intake (ranging from 8.1% kcal to 12.2% kcal), especially oleic acid from vegetable oil sources, may contribute to the prevention of high blood pressure (34). Research has shown that weight loss and a reduction in android adiposity decrease MetS risk (35). The positive correlation between the reduction in android fat mass and the decrease in cardiometabolic risk factors demonstrates unique effects of dietary MUFA on MetS criteria that could be due to a decrease in android adiposity. Fatty acids have depot-specific effects on lipid accumulation (36) in that MUFA preferentially accumulate in subcutaneous adipose tissue in contrast to SFA that preferentially accumulate in visceral adipose tissue (36, 37, 38). Consumption of a high oleic acid diet may therefore contribute to preferential fat deposition in subcutaneous adipose tissue and thereby decrease visceral adiposity and accompanying adverse metabolic effects.
A strength of the present study is the controlled diet design, the trial achieved high rates of dietary adherence as evidenced by plasma fatty acid concentrations, the delivery of the treatment oils, all of which were incorporated into smoothies for easy and accurate administration of the specified dose of oil. Second, the cross-over study design minimized the influence of genetic polymorphisms that contribute to variations in diet responsiveness and consequently inter-individual differences in results among study participants (39). Third, results presented herein are applicable to a large proportion of the US population with almost 40% of adults having MetS.
Limitations of the study include the 4-week duration of each diet period. Further studies are needed to evaluate the specific effects of a high MUFA diet longer term on body weight and android fat mass changes in free-living individuals on self-selected diets. As is always the case, it not possible to definitively conclude that increasing MUFA versus decreasing PUFA accounts for the observed effects. Nonetheless, increasing MUFA is both at the expense of decreasing PUFA (specifically ALA) and decreasing SFA. One recent study has shown erythrocyte linoleic acid is associated with decreased trunk fat mass in men and women (40). In conjunction with our finding, this suggests that varying PUFA (LA vs. ALA) may have a different effect on android fat mass. It will be important to conduct additional studies that confirm increasing MUFA is the mechanistically important event. In addition, while the baseline body composition is a reasonable covariate for assessing the effects of the experimental diets, comparisons with the baseline diet are limited because it was always ingested first and nutrient composition was only estimated based on the habitual intake of a Western population. Nonetheless, it is highly improbable that the SFA content of the habitual diets of our participants was as low as it was in the experimental diets, nor was MUFA as high.
The present study has important clinical implications. Our results add to the emerging evidence base that diets high in MUFA have beneficial effects on central obesity and cardiometabolic risk factors.
Conclusion
In summary, short-term consumption of diets high in MUFA provided by Canola oil and CanolaOleic oil was associated with a reduced android fat mass in participants with or at risk for MetS. These changes were associated with favorable shifts in cardiometabolic risk factors. Importantly, our findings provide evidence for a beneficial effect of dietary MUFA in lowering cardiometabolic risk that we suggest is mediated by a decrease in android fat mass.
Supplementary Material
Study Importance Questions.
What is already known about this subject?
Results from previous observational and prospective studies suggest that diets rich in MUFA have beneficial effects on body composition, especially in people with obesity.
What does your study add?
We found that diets high in MUFA and low in SFA (and ALA) reduce central obesity with an accompanying improvement in MetS risk factors, which may be of benefit for the treatment of and perhaps prevention of MetS.
Acknowledgments
We express our gratitude to the participants, the clinical coordinators and nurses for making this study possible.
Sources of support: The present study was supported by Agriculture and Agri Food Canada, Canola Council of Canada, Dow Agrosciences and Flax Council of Canada. The project described was supported by the National Center for Research Resources, Grant UL1 RR033184, and is now at the National Center for Advancing Translational Sciences, Grant UL1 TR000127. This project was also supported by NIH Grant 2T32DK007703. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations/acronyms used
- Canola
conventional canola oil
- CanolaOleic
high oleic canola oil
- CanolaDHA
DHA-enriched high oleic canola oil
- CornSaff
a blend of corn oil and safflower oil
- FlaxSaff
a blend of flax oil and safflower oil
- TC
total cholesterol
- HDL-C
HDL-cholesterol
- TG
triglycerides
- CRP
C-reactive protein
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
Footnotes
Written permission has been obtained from all persons named in the acknowledgment. This trial was registered at www.clinicaltrials.gov (NCT01351012).
Author contributions
PMK-E, SGW, JAF, BL, DJAJ and PJHJ: study concept design
PMK-E, SGW, JAF, BL, DJAJ, PJHJ, PWC, PC, XL, CEM and SP: data collection, carried out the study XL and CEM: statistical analysis XL and PMK-E: analysis and interpretation of the data, preparation of the manuscript PMK-E, SGW, PJHJ, BL, DJAJ, PWC, BL, PWC, PC: review manuscript All authors read and approved the final manuscript. The funders were not involved in the design, conduct, management, data collection and analysis, or preparation and review of the manuscript.
Conflict of Interest
PJHJ received grants from Advanced Foods and Materials Network, Danone, Enzymotec, Unilever, the Canadian Institutes of Health Research (CIHR) and Canada Research Chair Endowment of the Federal Government of Canada. PJHJ also serves as President of Nutritional Fundamentals for Health Inc.
BL and PC received research grants from CIHR, Agrifood and Agriculture Canada, the Dairy Farmers of Canada, Dairy Australia. BL received research funding from the Danone Institute and Atrium Innovations and honoraria from Unilever, Danone. BL is Chair of the Expert Scientific Advisory Council of Dairy Farmers of Canada and is Chair of Nutrition,supported by endowments from Provigo/Loblaws, Pfizer and Royal Bank of Canada.
DJAJ serves on the Scientific Advisory Board of Unilever, Sanitarium Company, California Strawberry Commission, Loblaw Supermarket, Herbal Life International, Nutritional Fundamental for Health, Pacific Health Laboratories, Metagenics, Bayer Consumer Care, Orafti, Dean Foods, Kellogg’s, Quaker Oats, Procter & Gamble, Coca-Cola, NuVal Griffin Hospital, Abbott, Pulse Canada, Saskatchewan Pulse Growers, and Canola Council of Canada. He has received honoraria from the Almond Board of California, International Tree Nut Council Nutrition Research and Education Foundation, Barilla, Unilever Canada, Solae, Oldways, Kellogg’s, Quaker Oats, Procter & Gamble, Coca-Cola, NuVal Griffin Hospital, Abbott, Canola Council of Canada, Dean Foods, California Strawberry Commission, Haine Celestial, and Alpro Foundation. He serves on the speakers panel for the Almond Board of California; has received research grants from Loblaw Brands Ltd, Unilever, Barilla, Almond Board of California, Solae, Haine Celestial, Sanitarium Company, Orafti, International Tree Nut Council, and the Peanut Institute; and travel support from the Almond Board of California, Unilever, Alpro Foundation, International Tree Nut Council, Canadian Institutes for Health Research, Canada Foundation for Innovation, and the Ontario Research Fund. He receives salary support as a Canada Research Chair from the federal government of Canada and his wife is a director of Glycemic Index Laboratories, Toronto, Ontario, Canada.
SGW has received research funding and consulting and travel fees from the Canola Council of Canada and Flax Canada 2013.
PMK-E serves on the California Walnut Commission Scientific Advisory Council, McDonald’s Global Advisory Council, and the Avocado Nutrition Science Advisory Other authors did not report any conflicts of financial interest.
References
- 1.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2010;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 2.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among us adults: Findings from the third national health and nutrition examination survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES. Prevalence of the Metabolic Syndrome Defined by the International Diabetes Federation Among Adults in the U.S. Diabetes Care. 2005;28:2745–2749. doi: 10.2337/diacare.28.11.2745. [DOI] [PubMed] [Google Scholar]
- 4.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 5.Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ERr, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: Results of the omniheart randomized trial. JAMA. 2005;294:2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 6.Damasceno NR, Sala-Vila A, Cofan M, Perez-Heras AM, Fito M, Ruiz-Gutierrez V, et al. Mediterranean diet supplemented with nuts reduces waist circumference and shifts lipoprotein subfractions to a less atherogenic pattern in subjects at high cardiovascular risk. Atherosclerosis. 2013;230:347–353. doi: 10.1016/j.atherosclerosis.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 7.Gillingham LG, Robinson KS, Jones PJ. Effect of high-oleic canola and flaxseed oils on energy expenditure and body composition in hypercholesterolemic subjects. Metabolism. 2012;61:1598–1605. doi: 10.1016/j.metabol.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 8.Paniagua JA, Gallego de la Sacristana A, Romero I, Vidal-Puig A, Latre JM, Sanchez E, et al. Monounsaturated fat-rich diet prevents central body fat distribution and decreases postprandial adiponectin expression induced by a carbohydrate-rich diet in insulin-resistant subjects. Diabetes Care. 2007;30:1717–1723. doi: 10.2337/dc06-2220. [DOI] [PubMed] [Google Scholar]
- 9.Kien CL, Bunn JY, Poynter ME, Stevens R, Bain J, Ikayeva O, et al. A lipidomics analysis of the relationship between dietary fatty acid composition and insulin sensitivity in young adults. Diabetes. 2013;62:1054–1063. doi: 10.2337/db12-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paniagua JA, de la Sacristana AG, Sanchez E, Romero I, Vidal-Puig A, Berral FJ, et al. A MUFA-rich diet improves posprandial glucose, lipid and GLP-1 responses in insulin-resistant subjects. J Am Coll Nutr. 2007;26:434–444. doi: 10.1080/07315724.2007.10719633. [DOI] [PubMed] [Google Scholar]
- 11.Gillingham LG, Gustafson JA, Han SY, Jassal DS, Jones PJ. High-oleic rapeseed (canola) and flaxseed oils modulate serum lipids and inflammatory biomarkers in hypercholesterolaemic subjects. Br J Nutr. 2011;105:417–427. doi: 10.1017/S0007114510003697. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins DJ, Kendall CW, Vuksan V, Faulkner D, Augustin LS, Mitchell S, et al. Effect of lowering the glycemic load with canola oil on glycemic control and cardiovascular risk factors: a randomized controlled trial. Diabetes Care. 2014;37:1806–1814. doi: 10.2337/dc13-2990. [DOI] [PubMed] [Google Scholar]
- 13.Jones PJ, Senanayake VK, Pu S, Jenkins DJ, Connelly PW, Lamarche B, et al. DHA-enriched high-oleic acid canola oil improves lipid profile and lowers predicted cardiovascular disease risk in the canola oil multicenter randomized controlled trial. Am J Clin Nutr. 2014;100:88–97. doi: 10.3945/ajcn.113.081133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimmet P, Alberti KG, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The metabolic syndrome in children and adolescents - an IDF consensus report. Pediatr Diabetes. 2007;8:299–306. doi: 10.1111/j.1399-5448.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 15.Senanayake VK, Pu S, Jenkins DA, Lamarche B, Kris-Etherton PM, West SG, et al. Plasma fatty acid changes following consumption of dietary oils containing n-3, n-6, and n-9 fatty acids at different proportions: preliminary findings of the Canola Oil Multicenter Intervention Trial (COMIT) Trials. 2014;15:136–148. doi: 10.1186/1745-6215-15-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salas-Salvadó J, Fernández-Ballart J, Ros E, et al. Effect of a mediterranean diet supplemented with nuts on metabolic syndrome status: One-year results of the predimed randomized trial. Arch Intern Med. 2008;168:2449–2458. doi: 10.1001/archinte.168.22.2449. [DOI] [PubMed] [Google Scholar]
- 17.Babio N, Toledo E, Estruch R, Ros E, Martínez-González MA, Castañer O, et al. Mediterranean diets and metabolic syndrome status in the PREDIMED randomized trial. CMAJ. 2014;186:E649–657. doi: 10.1503/cmaj.140764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piers LS, Walker KZ, Stoney RM, Soares MJ, O'Dea K. Substitution of saturated with monounsaturated fat in a 4-week diet affects body weight and composition of overweight and obese men. Br J Nutr. 2003;90:717–727. doi: 10.1079/bjn2003948. [DOI] [PubMed] [Google Scholar]
- 19.Kien CL, Bunn JY. Gender alters the effects of palmitate and oleate on fat oxidation and energy expenditure. Obesity (Silver Spring) 2008;16:29–33. doi: 10.1038/oby.2007.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones PJ, Jew S, AbuMweis S. The effect of dietary oleic, linoleic, and linolenic acids on fat oxidation and energy expenditure in healthy men. Metabolism. 2008;57:1198–1203. doi: 10.1016/j.metabol.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt DE, Allred JB, Kien CL. Fractional oxidation of chylomicron-derived oleate is greater than that of palmitate in healthy adults fed frequent small meals. J Lipid Res. 1999;40:2322–2332. [PubMed] [Google Scholar]
- 22.Kien CL, Bunn JY, Stevens R, Bain J, Ikayeva O, Crain K, et al. Dietary intake of palmitate and oleate has broad impact on systemic and tissue lipid profiles in humans. Am J Clin Nutr. 2014;99:436–445. doi: 10.3945/ajcn.113.070557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravnskjaer K, Frigerio F, Boergesen M, Nielsen T, Maechler P, Mandrup S. PPARdelta is a fatty acid sensor that enhances mitochondrial oxidation in insulin-secreting cells and protects against fatty acid-induced dysfunction. J Lipid Res. 2010;51:1370–1379. doi: 10.1194/jlr.M001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bojic LA, Huff MW. Peroxisome proliferator-activated receptor delta: a multifaceted metabolic player. Curr Opin Lipidol. 2013;24:171–177. doi: 10.1097/MOL.0b013e32835cc949. [DOI] [PubMed] [Google Scholar]
- 25.Yang Y, Chen M, Georgeson KE, Harmon CM. Mechanism of oleoylethanolamide on fatty acid uptake in small intestine after food intake and body weight reduction. Am J Physiol Regul Integr Comp Physiol. 2007;292:R235–241. doi: 10.1152/ajpregu.00270.2006. [DOI] [PubMed] [Google Scholar]
- 26.Yang ZH, Miyahara H, Iwasaki Y, Takeo J, Katayama M. Dietary supplementation with long-chain monounsaturated fatty acids attenuates obesity-related metabolic dysfunction and increases expression of PPAR gamma in adipose tissue in type 2 diabetic KK-Ay mice. Nutr Metab (Lond) 2013;10:16–26. doi: 10.1186/1743-7075-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodriguez De Fonseca F, et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425:90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- 28.Kien CL, Bunn JY, Fukagawa NK, Anathy V, Matthews DE, Crain KI, et al. Lipidomic evidence that lowering the typical dietary palmitate to oleate ratio in humans decreases the leukocyte production of proinflammatory cytokines and muscle expression of redox-sensitive genes. J Nutr Biochem. 2015;26:1599–1606. doi: 10.1016/j.jnutbio.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stienstra R, Joosten LA, Koenen T, van Tits B, van Diepen JA, van den Berg SA, et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 2010;12:593–605. doi: 10.1016/j.cmet.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tack CJ, Stienstra R, Joosten LA, Netea MG. Inflammation links excess fat to insulin resistance: the role of the interleukin-1 family. Immunol Rev. 2012;249:239–252. doi: 10.1111/j.1600-065X.2012.01145.x. [DOI] [PubMed] [Google Scholar]
- 31.Despres JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–1049. doi: 10.1161/ATVBAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- 32.Despres JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126:1301–1313. doi: 10.1161/CIRCULATIONAHA.111.067264. [DOI] [PubMed] [Google Scholar]
- 33.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 34.Intermap Research Group K. Relationship of dietary monounsaturated fatty acids to blood pressure: the International Study of Macro/Micronutrients and Blood Pressure. J Hypertens. 2013;31:1144–1150. doi: 10.1097/HJH.0b013e3283604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ibarrola-Jurado N, Bullo M, Guasch-Ferre M, Ros E, Martinez-Gonzalez MA, Corella D, et al. Cross-sectional assessment of nut consumption and obesity, metabolic syndrome and other cardiometabolic risk factors: the PREDIMED study. PLoS One. 2013;8:e57367. doi: 10.1371/journal.pone.0057367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sabin MA, Crowne EC, Stewart CE, Hunt LP, Turner SJ, Welsh GI, et al. Depot-specific effects of fatty acids on lipid accumulation in children's adipocytes. Biochem Biophys Res Commun. 2007;361:356–361. doi: 10.1016/j.bbrc.2007.06.184. [DOI] [PubMed] [Google Scholar]
- 37.Garaulet M, Perez-Llamas F, Perez-Ayala M, Martinez P, de Medina FS, Tebar FJ, et al. Site-specific differences in the fatty acid composition of abdominal adipose tissue in an obese population from a Mediterranean area: relation with dietary fatty acids, plasma lipid profile, serum insulin, and central obesity. Am J Clin Nutr. 2001;74:585–591. doi: 10.1093/ajcn/74.5.585. [DOI] [PubMed] [Google Scholar]
- 38.Bergouignan A, Momken I, Schoeller DA, Simon C, Blanc S. Metabolic fate of saturated and monounsaturated dietary fats: the Mediterranean diet revisited from epidemiological evidence to cellular mechanisms. Prog Lipid Res. 2009;48:128–147. doi: 10.1016/j.plipres.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 39.AbuMweis SS. Optimizing clinical trial design for assessing the efficacy of functional foods. Nutr Rev. 2010;68:485–499. doi: 10.1111/j.1753-4887.2010.00308.x. [DOI] [PubMed] [Google Scholar]
- 40.Belury MA, Cole RM, Bailey BE, Ke JY, Andridge RR, Kiecolt-Glaser JK. Erythrocyte linoleic acid, but not oleic acid, is associated with improvements in body composition in men and women. Mol Nutr Food Res. 2016 doi: 10.1002/mnfr.201500744. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.