Multidrug-resistant tuberculosis (MDR-TB) treatment is complicated by pill burden and drug toxicities, and is associated with high mortality in resource-limited settings. Tremendous financial and scientific resources are directed toward the investigation of new drugs for MDR-TB,1,2 but efforts to optimize and shorten treatment have been significantly hindered by a poor understanding of the individual contribution of each drug to a multidrug regimen, the exposure-response relationship for each drug, and how best to monitor treatment response. In addition, given that MDR-TB treatment is increasingly provided in community settings where directly observed therapy may be implemented on a case-by-case basis,3 better tools are needed to assess adherence to improve individual outcomes and reduce transmission.
We developed a multi-analyte quantitative assay for measuring second-line anti-tuberculosis drugs (levofloxacin [LFX], moxifloxacin [MFX], linezolid [LZD], and pyrazinamide [PZA]) in small hair samples using a liquid chromatography-tandem mass spectrometry (LC-MS/MS) system (Agilent LC 1260-AB Sciex API 5500, Agilent Technologies, Santa Clara, CA, USA). Sample preparation consisted of hair pulverization using an Omni Bead Ruptor homogenizer (OMNI International, NW Kennesaw, GA, USA), extraction with methanol, evaporation, and reconstitution to 20% (v/v) methanol with 1% formic acid. After injection of the sample extract (10 μl) into the LC-MS/MS, the analytes were separated by gradient elution on a Phenomenex Synergi Polar RP column (2.1 × 100 mm, 2.5 μm particle size, Phenomenex, Torrance, CA, USA) using water with 1% formic acid as mobile phase A (MPA) and methanol with 0.4% formic acid as mobile phase B (MPB). The gradient used for analyte separation consisted of 20% MPB at 0–0.5 min, gradient to 100% MPB from 0.5 to 4 min, 100% MPB at 4–5 min, and 20% MPB at 5.1–10 min. Mass spectrometric detection of each drug was achieved with electrospray ionization (ESI) in positive polarity, and mass scanning was performed via multiple reaction monitoring (MRM). Each analyte was monitored using the peak area ratio between two transitions and its retention time. Quantitation of each drug was performed by isotope dilution using deuterium- or 13C-labeled standard.
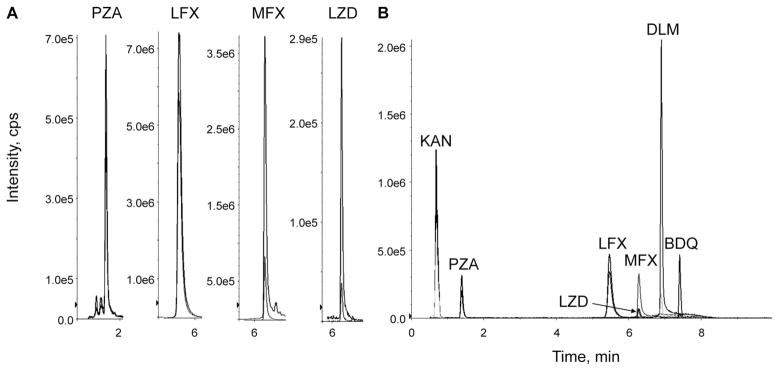
Using the LC-MS/MS method we developed, we extracted and quantified LFX (15.1 ng/mg), MFX (16.9 ng/mg), LZD (7.2 and 12.8 ng/mg), and PZA (15.1 ng/mg) from hair specimens in two patients on directly observed MDR-TB treatment (Figure, Panel A). Each participant provided written informed consent, and ethical approval was obtained from the University of California, San Francisco Human Research Protection Program.
Figure.
Chromatograms for drugs extracted from patient hair samples (Panel A) and MDR-TB drug panel (Panel B) showing multiple transitions monitored for each drug. PZA = pyrazinamide; LFX = levofloxacin; MFX = moxifloxacin; LZD = linezolid; KAN = kanamycin; DLM = delanamid; BDQ = bedaquiline; cps = counts per second; MDR-TB = multidrug-resistant tuberculosis.
Using a 6-point calibration curve, each assay showed high sensitivity (LLQ 0.1–1 ng/mg) and a wide linear dynamic range (0.1–40 ng/mg) using 10–25 strands of hair (~2 mg). The within-run precision (5 intra-subject iterations) of our method was within 15% coefficient of variation (CV%) (PZA 13.2%; LZD 6.6–12.9%; LFX 7.8%; MFX 6.5%). We further incorporated bedaquiline (BDQ), delamanid (DLM), and kanamycin A (KAN) into this multi-analyte panel (Figure, Panel B), and patient hair extraction protocols for these drugs are in progress.
We developed assays to accurately and non-invasively determine long-term exposure to key second-line anti-tuberculosis drugs in hair within a single panel. Although various groups have published multi-TB drug panels in plasma,4–6 to our knowledge this is the first report demonstrating a similar panel for MDR-TB drugs in hair. Further analytic validation of these hair assays and testing their utility in clinical settings is underway.
Acknowledgments
Funding for this work was provided by National Institutes of Health/National Institute of Infectious Diseases Grants R01 AI123024 (principal investigators [PI] Metcalfe, Gandhi) and R01 AI098472 (PI Gandhi).
Conflicts of interest: none declared.
References
- 1.Zumla AI, Gillespie SH, Hoelscher M, et al. New antituberculosis drugs, regimens, and adjunct therapies: needs, advances, and future prospects. Lancet Infect Dis. 2014;14:327–340. doi: 10.1016/S1473-3099(13)70328-1. [DOI] [PubMed] [Google Scholar]
- 2.Research Excellence to Stop TB Resistance (RESIST-TB) [Accessed January 2015];DR-TB Clinical Trial Progress Report. http://www.resisttb.org/?page_id=1602.
- 3.Metcalfe JZ, O’Donnell MR, Bangsberg DR. Moving beyond directly observed therapy for tuberculosis. PLoS Med. 2015;12(9):e1001877. doi: 10.1371/journal.pmed.1001877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim HJ, Seo KA, Kim HM, et al. Simple and accurate quantitative analysis of 20 anti-tuberculosis drugs in human plasma using liquid chromatography-electrospray ionization-tandem mass spectrometry. J Pharm Biomed Anal. 2015;102:9–16. doi: 10.1016/j.jpba.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 5.Song SH, Jun SH, Park KU, et al. Simultaneous determination of first-line anti-tuberculosis drugs and their major metabolic ratios by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21:1331–1338. doi: 10.1002/rcm.2961. [DOI] [PubMed] [Google Scholar]
- 6.Han M, Jun SH, Lee JH, Park KU, Song J, Song SH. Method for simultaneous analysis of nine second-line anti-tuberculosis drugs using UPLC-MS/MS. J Antimicrob Chemother. 2013;68:2066–2073. doi: 10.1093/jac/dkt154. [DOI] [PubMed] [Google Scholar]