Abstract
Oral cavity cancer (OC) has steadily decreased in the United States (US) since 1973 whereas oropharyngeal cancer (OP) has increased. We analyzed OC and OP cases from the Oklahoma Central Cancer Registry and Surveillance, Epidemiology, and End Results program comparing those diagnosed from 1997–1999 to those diagnosed from 2010–2012. We compared the incidence of OC and OP cases between Oklahoma and the US and by demographic factors. We observed an increase in OP cases, but no change in OC cases in both the US and in Oklahoma, and observed some differences between Oklahoma and the US by race, gender, and age group. A possible explanation for the increasing incidence of OP cancers may be the increasing prevalence of HPV. This study highlighted the differences in temporal trends of OC and OP cancers and the importance of changing risk factors for these cancers.
INTRODUCTION
While uncommon, head and neck cancers are associated with several modifiable risk factors, including tobacco, alcohol, and infection with a high risk human papilloma virus (HPV) strain.1 Cancers arising from the paranasal sinuses, nasal cavity, salivary glands, oral cavity, oropharynx, nasopharynx, hypopharynx, and larynx are collectively referred to as head and neck cancers.2,3 Etiology, risk factors, incidence, treatment, and mortality vary for each site. Squamous cell carcinoma (SCC) is the most common cancer of the head and neck,1 but the incidence has decreased over the past 30 years.4 Unlike oral cavity (OC) and other head and neck cancer sites, the incidence of oropharyngeal (OP) SCC, however, has increased.4,5 Traditionally, most patients diagnosed with head and neck SCC were older and 90% of those diagnosed with oral cancer had a history of tobacco and/or alcohol use,4,6 but the characteristics of the head and neck cancer patient have changed over time.4 This change has been attributed to changes in social behaviors and the increase in prevalence of the HPV.7
Prior to the investigation of HPV, studies reported primarily tobacco and alcohol usage as a risk factors for both OC and OP cancers. While not completely understood, it is speculated that alcohol becomes a solvent for the carcinogenic cigarette smoke and allows for better absorption through the mucosal layer.3,8 Tobacco has long been a known risk factor for OC and OP cancers,1 with a strong dose-response effect.8 Current cigarette smokers compared to never-smokers were 8.53 (95% CI: 3.38, 21.55) times more likely to develop oro-/hypopharyngeal cancer, a cancer involving the oropharynx and/or the hypopharynx.3 Additionally, former smokers were at an increased risk of oro-/hypopharyngeal compared to never-smokers.3 Studies have also reported a positive multiplicative interaction when cigarette use and alcohol use were combined.1,3,9 Heavy drinkers that smoke ≥20 cigarettes per day were 8.28 (95% CI: 3.98, 17.22) times more likely to develop head and neck cancer than never-smoker/non-drinkers.3
Regarding alcohol as an individual risk factor, the International Agency for Research on Cancer (IARC) has identified ethanol alcohol as a human carcinogen.8 In a study by Maasland et al, those with heavy alcohol consumption (≥30 g per day) compared to those with no alcohol consumption were 6.39 (95% CI 3.13, 13.03) times more likely to develop oral cavity cancer and 3.52 (95% CI 1.69, 7.36) times more likely to develop oro-/hypopharyngeal cancer.3 The odds of OP cancer increases with both increased dose and increased duration of alcohol consumption.10
HPV is another known risk factor for OP cancers, particularly for SCC, according to IARC.8,7,11 While HPV is not currently listed as a risk factor for OC cancer by IARC, HPV has been implicated in the development of OC cancer.7 Cancer is induced by inactivating the tumor suppressor gene TP53, which aids in the up-regulation of squamous cell carcinoma.10 The high-risk strains most notably associated with cervical cancers are HPV-16 and HPV-18.11 Oral HPV infection is assumed to be a sexually transmitted infection.12 In a study by Tezel et al, 70% of the base of tongue tumors, which are included in OP cancers, were HPV-16 positive, while none were positive for HPV-18.13 HPV-16 is most strongly associated with head and neck squamous cell carcinoma, which includes OP and OC cases (Adjusted Odds Ration [AOR]=18.3, 95% CI: 6.8, 49.0)10 Furthermore, Nasman et al observed that 74% of tonsillar or base of tongue SCC contained HPV DNA, with the most common strain being HPV-16 (84%). Oral cavity cancers were not evaluated in this study.12 In contrast, a review of 60 studies reported only 35.6% of OP cancers were HPV positive and 23.5% of oral cavity cancers were HPV positive. Of those that were HPV positive, 87% of OP cancers and 68% of OC cancers were HPV-16 positive.9 While HPV-16 is the most common, high risk strains 31 and 33 have also been linked to OP SCC.14
Studies have indicated a reduced association between OP cancers and smoking and alcohol when HPV is present in patients. Non-smokers are 15 times more likely to present with HPV positive tumors than smokers.7 In a separate study, among the HPV-16 negative, the odds of oropharyngeal cancer was highest among those reporting greater than 50 packs years of smoking (AOR = 4.9, 95% CI: 2.1 to 11.3), those reporting 40–49 pack years (AOR = 3.2, 95% CI: 1.1 to 9.0), and those reporting 20–39 pack years (AOR = 2.6, 95% CI: 1.3 to 5.1) compared to never smokers.10 Similar results were observed with alcohol use.7 HPV-16 positive tumors in those with low tobacco exposure were biologically different and responded better to therapy than HPV-16 negative tumors of heavy drinkers and smokers.1 Additionally, Mehta, Yu, and Schantz observed that survival for less differentiated tumors increased by 57% compared to only 15.5% increase in survival for well-differentiated OC and OP tumors.7
Comparison of previous studies has been difficult due to lack of a standardized case definition.15 Each cancer site has different risk factors and how they are grouped together will dictate the trends observed. The purpose of this study was to evaluate the distribution of OC and OP cancers in Oklahoma and the US by grouping cancers based on risk factors instead of anatomical locations.15
METHODS
To examine incidence of OC and OP cancers in Oklahoma, we analyzed data from the Oklahoma Central Cancer Registry (OCCR). The OCCR is a part of the National Program of Central Registries (NPCR) of the Centers for Disease Control and Prevention (CDC). The registry has catalogued all cancers in Oklahoma residents since January 1, 1997. We included those with OP and OC cancers using SEER site recodes based on ICD-O-3/WHO 2008 diagnosed from 1997–2012 (Table 1).16 We excluded lymphomas, mesotheliomas, and Kaposi sarcoma from all sites. To avoid misclassification, lip not otherwise specified (NOS), pharynx NOS, tongue NOS, palate NOS, and mouth NOS were excluded. Analyses of Oklahoma data were conducted in SAS (Cary, NC, version 9.4).
Table 1.
Oropharyngeal and Oral Cavity Cancer Site Recodes.16
| Oropharyngeal Cancer Site Recodes | Oral Cavity Cancer Site Recodes | ||
|---|---|---|---|
| Site | Recode | Site | Recode |
| Base of tongue | C01.9 | Mucosa of upper lip | C00.3 |
| Lingual tonsils | C02.4 | Mucosa of lower lip | C00.4 |
| Overlapping lesions of the tongue | C02.8 | Mucosa of lip NOS | C00.5 |
| Soft palate not otherwise specified (NOS) | C05.1 | Overlapping lesion of the lip | C00.8 |
| Uvula | C05.2 | Dorsal surface of tongue | C02.0 |
| Overlapping lesion of the palate | C05.8 | Border of tongue | C02.1 |
| Tonsillar fossa | C09.0 | Ventral surface of tongue | C02.2 |
| Tonsillar pillar | C09.1 | Anterior two-thirds tongue | C02.3 |
| Overlapping lesion of the tonsils | C09.8 | Overlapping lesion of tongue | C02.8 |
| Tonsils NOS | C09.9 | Upper gum | C03.0 |
| Lateral walls of the oropharynx | C10.2 | Lower gum | C03.1 |
| Posterior walls of the oropharynx | C10.3 | Gum NOS | C03.9 |
| Overlapping lesion of the oropharynx | C10.8 | Anterior floor of mouth | C04.0 |
| Oropharynx NOS | C10.9 | Lateral floor of mouth | C04.1 |
| Waldeyer’s ring | C14.2 | Overlapping lesion of floor of mouth | C04.8 |
| Floor of mouth NOS | C04.9 | ||
| Hard palate | C05.0 | ||
| Overlapping lesion of palate | C05.8 | ||
| Cheek mucosa | C06.0 | ||
| Retromolar area | C06.2 | ||
| Overlapping lesion other | C06.8 | ||
| Overlapping lesion of lips and oral cavity | C14.8 | ||
To obtain OC and OP incidence rates for the US, we used SEER*Stat software (Surveillance Research Program, National Cancer Institute, version 8.2.1). The Surveillance, Epidemiology, and End Results (SEER) Program, of the National Cancer Institute, has cancer data available from 1973 to 2012 and collects cancer data from cancer registries across the US designed to be representative of the US.17
To evaluate changes in incidence over time, we compared data from 1997–1999 and 2010–2012 in Oklahoma to the US by age group, race, and gender. Age was grouped into five-year age groups, with those 85 and older combined. Comparisons by age were limited to those 35 and older due to limited cases among younger age groups (<2% of OC cancers and <1% of OP cancers). In the Oklahoma data, we used the primary race variable to classify race and linked the data with Indian Health Service (IHS) records to reduce misclassification among the American Indian/Alaska Native population.
This study was approved by the Institutional Review Boards of the University of Oklahoma Health Sciences Center and the Oklahoma State Department of Health.
RESULTS
Overall
As observed in Figure 1, the age-adjusted incidence rate (AAIR) of OC cancer appears to have decreased from 3.7 (95% CI: 3.4, 4.1) in 1973 to 3.2 (95% CI: 3.0, 3.4) in 2012 in the US, whereas the AAIR of OP cancer has increased from 3.0 (95% CI: 2.7, 3.3) in 1973 to 4.4 (4.2, 4.7) in 2012. When comparing OC and OP cancer in Oklahoma, the AAIR of OP cancer appears to have increased over time, whereas the AAIR of OC cancer appears stable, resulting in a widening gap between OC and OP cancer incidence over time (Figure 2). There was no change in the OC cancer incidence rate in Oklahoma, but we observed an increase in the age-adjusted OP cancer incidence from 3.2 (95% CI: 2.6, 3.8) per 100,000 in 1997 to 5.1 (95% CI: 4.4, 5.8) per 100,000 in 2012.
Figure 1. Age-adjusted incidence rates of oral cavity (OC) and oropharyngeal (OP) cancers in the US from 1973 to 2012.
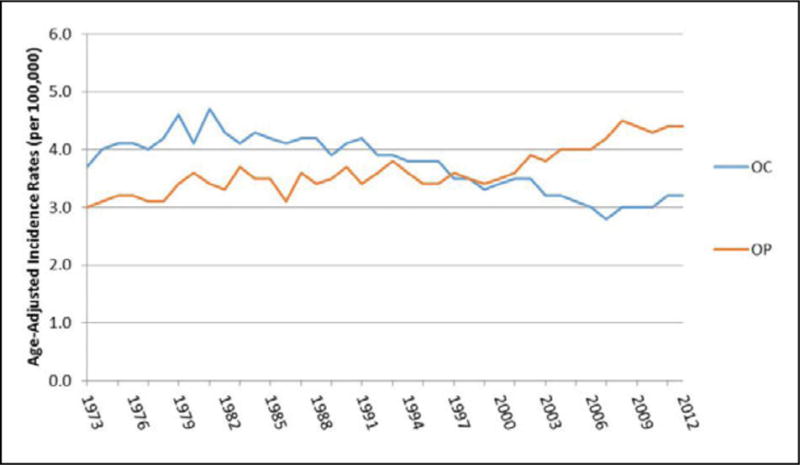
Source: Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2014 Sub (1973–2012) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2013
Figure 2.
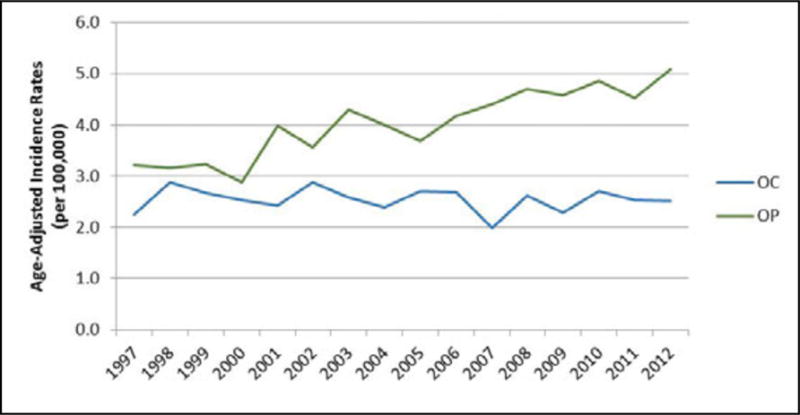
Age-adjusted incidence rates of oral cavity (OC) cancer and oropharyngeal (OP) cancer in Oklahoma from 1997–2012.
Oral Cavity Cancer
From 1997–1999 to 2010–2012 in the US, the AAIR of OC cancer decreased for Caucasians from 3.6 to 3.1 per 100,000 and African Americans from 3.4 to 2.3 per 100,000, but remained stable among American Indian/Alaska Natives and Asian/Pacific Islanders (Table 2). The AAIR for OC cancer in Oklahoma remained stable for Caucasians, African Americans, and American Indian/Alaska Natives during this time frame. We were unable to compare the AAIR for Asian/Pacific Islanders in Oklahoma as no cases occurred from 1997–1999. Regarding differences by gender, the AAIR of OC cancer in the US decreased among males from 4.5 to 3.6 per 100,000 and among females from 2.6 to 2.3 per 100,000. In Oklahoma, the AAIRs for OC cancer remained stable in both males and females. The AAIR of OC cancer in the US significantly decreased in the 65–69 age group from 13.0 to 9.8 per 100,000, but remained stable in Oklahoma.
Table 2.
Age-adjusted incidence rates of oral cavity cancer in the US and Oklahoma in 1997–1999 and 2010–2012.
| OK | US | |||
|---|---|---|---|---|
| 1997–1999 | 2010–2012 | 1997–1999 | 2010–2012 | |
| Characteristic | Age-Adjusted Incidence Rate per 100,000 (95% CI) | Age-Adjusted Incidence Rate per 100,000 (95% CI) | Age-Adjusted Incidence Rate per 100,000 (95% CI) | Age-Adjusted Incidence Rate per 100,000 (95% CI) |
| Race | ||||
| White | 2.6 (2.3, 2.9) | 2.6 (2.3, 2.9) | 3.6 (3.5, 4.0) | 3.1 (3.0, 3.3) |
| African American | 2.4 (1.1, 3.8) | 2.8 (1.6, 4.1) | 3.4 (3.1, 3.8) | 2.3 (2.0, 2.6) |
| American Indian/Alaska Native | 3.2 (1.7, 4.8) | 2.4 (1.4, 3.4) | 1.9 (1.1, 3.1) | 1.8 (1.2, 2.5) |
| Asian/Pacific Islander | N/Aa | 2.4 (0.0, 5.4) | 2.2 (1.9, 2.5) | 2.0 (1.8, 2.2) |
| Gender | ||||
| Male | 3.3 (2.8, 3.9) | 3.3 (2.8, 3.7) | 4.5 (4.3, 4.7) | 3.6 (3.5, 3.8) |
| Female | 2.0 (1.6, 2.3) | 1.9 (1.6, 2.2) | 2.6 (2.5, 2.7) | 2.3 (2.2, 2.4) |
| Age | ||||
| 35–39 | 0.6 (0.1, 1.2) | 1.0 (0.3, 1.8) | 0.9 (0.7, 1.1) | 0.9 (0.7, 1.1) |
| 40–44 | 2.3 (1.2, 3.4) | 1.4 (0.5, 2.3) | 1.7 (1.4, 1.9) | 1.4 (1.1, 1.6) |
| 45–49 | 3.2 (1.9, 4.5) | 2.5 (1.4, 3.7) | 3.1 (2.8, 3.6) | 2.7 (2.3, 3.0) |
| 50–54 | 5.2 (3.4, 7.0) | 4.1 (2.7, 5.6) | 5.4 (4.8, 6.0) | 4.7 (4.3, 5.2) |
| 55–59 | 5.5 (3.5, 7.6) | 7.4 (5.4, 9.4) | 7.8 (7.0 8.6) | 6.9 (6.3, 7.5) |
| 60–64 | 6.2 (3.8, 8.6) | 5.2 (3.4, 7.0) | 10.1 (9.1, 11.1) | 7.5 (6.9, 8.2) |
| 65–69 | 8.2 (5.4, 11.1) | 8.5 (5.9, 11.0) | 13.0 (11.8, 14.2) | 9.8 (8.9, 10.7) |
| 70–74 | 8.2 (5.2, 11.3) | 11.2 (7.8, 14.6) | 13.7 (12.4, 15) | 11.3 (10.2, 12.5) |
| 75–80 | 10.1 (6.4, 13.9) | 11.8 (7.8, 15.8) | 16.1 (14.6, 17.7) | 13.9 (12.5, 15.4) |
| 80–84 | 14.1 (8.7, 19.6) | 11.0 (6.5, 15.5) | 16.8 (15.0, 18.8) | 13.9 (12.4, 15.6) |
| 85+ | 11.2 (6.2, 16.3) | 10.4 (5.9, 15.0) | 15.9 (13.9, 18) | 15.3 (13.8, 17.1) |
No cases observed
Oropharyngeal Cancer
From 1997–1999 to 2010–2012, OP cancer AAIR in the US increased in Caucasians from 3.4 to 4.7 per 100,000 (Table 3). The AAIR decreased in African Americans from 5.2 to 3.8 per 100,000, but remained stable in American Indian/Alaska Natives and Asian/Pacific Islanders. Similar to the US, the AAIR of OP cancer in Oklahoma increased among Caucasians from 3.2 to 5.0 per 100,000, while the AAIR remained stable among African Americans and Asian/Pacific Islanders. Although not statistically significant, the AAIR for the American Indian/Alaska Native population in Oklahoma increased from 2.7 to 5.2 per 100,000 during the time frame. The incidence rates of OP cancers in the US remained relatively stable in females, but increased in males from 5.4 to 7.0 per 100,000. Similarly, the incidence of OP cancer in Oklahoma in males increased from 5.1 to 8.2 per 100,000, but remained stable among females. Regarding differences by age group, the AAIR of OP cancer in the US increased in most age groups, but only significantly among those between 50 and 69 years of age, which was similar to rates observed in Oklahoma.
Table 3.
Age-adjusted incidence rates of oropharyngeal cancer in the US and Oklahoma in 1997–1999 and 2010–2012.
| OK | US | |||
|---|---|---|---|---|
| 1997–1999 | 2010–2012 | 1997–1999 | 2010–2012 | |
| Characteristic | Age-Adjusted Incidence Rate per 100,000 (95% CI) | Age-Adjusted Incidence Rate per 100,000 (95% CI) | Age-Adjusted Incidence Rate per 100,000 (95% CI) | Age-Adjusted Incidence Rate per 100,000 (95% CI) |
| Race | ||||
| White | 3.2 (2.8, 3.5) | 5.0 (4.6, 5.5) | 3.4 (3.3, 3.6) | 4.7 (4.5, 4.8) |
| African American | 4.5 (2.8, 6.3) | 3.3 (1.9, 4.6) | 5.2 (4.8, 5.7) | 3.8 (3.5, 4.2) |
| American Indian/Alaska Native | 2.7 (1.4, 3.9) | 5.2 (3.7, 6.7) | 2.3 (1.4, 3.5) | 2.7 (2.0, 3.6) |
| Asian/Pacific Islander | 1.9 (0.0, 4.4) | 0.4 (0.0, 1.2) | 2.0 (1.7, 2.3) | 1.7 (1.5, 2.0) |
| Gender | ||||
| Male | 5.1 (4.4, 5.7) | 8.2 (7.5, 9.0) | 5.4 (5.2, 5.6) | 7.0 (6.8, 7.2) |
| Female | 1.6 (1.3, 1.9) | 1.7 (1.4, 2.0) | 1.8 (1.7, 1.9) | 1.7 (1.6, 1.8) |
| Age | ||||
| 35–39 | 0.9 (0.2, 1.5) | 0.9 (0.2, 1.6) | 0.9 (0.7, 1.1) | 0.6 (0.5, 0.8) |
| 40–44 | 2.7 (1.6, 3.9) | 3.4 (2.1, 4.8) | 2.0 (1.7, 2.3) | 2.1 (1.8, 2.4) |
| 45–49 | 6.1 (4.3, 8.0) | 8.0 (6.0, 10.0) | 4.6 (4.1, 5.1) | 5.1 (4.7, 5.6) |
| 50–54 | 7.7 (5.5, 9.9) | 12.3 (9.9, 14.7) | 7.3 (6.6, 8.0) | 9.3 (8.7, 10.0) |
| 55–59 | 9.2 (6.5, 11.9) | 15.0 (12.1, 17.8) | 9.4 (8.5, 10.3) | 12.9 (12.1, 13.7) |
| 60–64 | 8.8 (6.0, 11.7) | 16.6 (13.4, 19.7) | 10.8 (9.8, 11.9) | 16.5 (15.5, 17.5) |
| 65–69 | 10.8 (7.5, 14.1) | 21.2 (17.1, 25.2) | 12.2 (11.0, 13.4) | 15.9 (14.8, 17.0) |
| 70–74 | 12.4 (8.6, 16.1) | 11.5 (8.1, 14.9) | 14.8 (13.5, 16.2) | 14.9 (13.6, 16.2) |
| 75–80 | 7.2 (4.1, 10.4) | 12.8 (8.7, 17.0) | 11.6 (10.3, 12.9) | 13.4 (12.1, 14.9) |
| 80–84 | 8.7 (4.4, 13.0) | 12.4 (7.7, 17.2) | 10.4 (9.0, 12.0) | 11.6 (10.2, 13.1) |
| 85+ | 4.1 (1.1, 7.2) | 4.2 (1.3, 7.1) | 8.0 (6.6, 9.6) | 8.0 (6.9, 9.2) |
DISCUSSION
This study examined trends in the incidence of OC and OP cancers in both Oklahoma and the US. The longitudinal data confirm that the incidence of head and neck cancers vary by site in both Oklahoma and the US. Specifically, we observed that the incidence of OC declined over time while OP cancers increased in the US. OP cancers have also increased in Oklahoma while there has been no change in the incidence of OC cancers. In general, the trends in Oklahoma by time, race, sex, and age are similar to those in the US.
It is likely OC and OP cancers now consist of two separate etiologic pathways: those made up of cancers associated with alcohol and tobacco exposures and those more strongly associated with exposure to high risk HPV infection.18 While head and neck cancers due to alcohol and tobacco use have steadily decreased in the US since 1973, OP cancers have increased, likely due to the prevalence of HPV.2 Our study observed a decline in OC incidence in the US, which may suggest a decrease in alcohol and tobacco usage at the national level, but this needs further investigation. While not forgetting the classic risk factors, health care providers must consider a new risk factor, HPV, and those at risk of HPV infection.
Changing social behaviors and prevalence of HPV have changed the demographics of the OC and OP cancer patient. HPV positive head and neck cancer patients are younger than the head and neck cancer patients of the past.4 Today a higher proportion of the head and neck cancer patients are younger white14 males that have limited or no alcohol/tobacco exposures2 as opposed to previous years where African Americans had a higher risk of these cancers.6 Recent head and neck cancer patients tend to have a history of increased sexual partners11 and specifically an increase in oral sex partners.4,9
This study had several strengths, one of which was that we were able to use data from population-based registries in Oklahoma and the US, which reduced the chance of selection bias. We were also able to select specific cancer sites to classify cases as having OC or OP cancer according to risk factors rather than anatomical sites due to the level of detail available from both sources of data. Furthermore, we used the same classifications to compare Oklahoma and US data based on the SEER registry.
However, there were also several limitations in this study. Both OC and OP are rare diseases, resulting in the need to combine multiple years for comparison. Furthermore, the rarity of these diseases lead to imprecision in the AAIR estimates so some true differences may be masked. We observed a nonsignificant increase in OP cancer among American Indians/Alaska Natives in Oklahoma, with little difference in the US data. SEER is designed to be representative of the US population.19 However, the American Indian/Alaska Native population in SEER primarily originates from the New Mexico and Alaska SEER registries, which are not necessarily representative of all American Indians/Alaska Natives in the US.20 Using IHS-linked data to estimate the AAIR of both OC and OP cancer in the US would provide a better comparison to the Oklahoma data. The study required the ability to identify specific sites of OP and OC cancers, which was not available using the data available in the CDC’s Wide-ranging Online Data for Epidemiologic Research (WONDER) database.21
In summary, while the AAIR of both OP and OC cancers is similar in the US and Oklahoma, the incidence of OP cancers has increased, which may be related to increasing prevalence of HPV. This study highlighted the differences in trends of OC and OP cancers over time and the importance of changing risk factors for these cancers, primarily OP cancers. The relationship between OC and OP cancers becomes increasingly complex in individuals who smoke, drink and have HPV.1 More studies are needed to understand the interaction between an established HPV infection and modifiable risk factors such as smoking and drinking. In addition future studies evaluating the differences in smoking, alcohol, and HPV status among OC and OP cancer cases by gender, race, and age are recommended to better understand the importance of these modifiable risk factors.
Acknowledgments
FUNDING
JC and AJ were partially supported by grants NU58DP005513 from the Centers for Disease Control and Prevention. The content is solely the responsibility of the authors and does not necessarily represent the official views of the CDC. JC was partially supported by grant AIAMP120011 from the Office of Minority Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the OMH.
References
- 1.Huber MA, Tantiwongkosi B. Oral and oropharyngeal cancer. Med Clin North Am. 2014;98(6):1299–1321. doi: 10.1016/j.mcna.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Howard JD, Chung CH. Biology of human papillomavirus-related oropharyngeal cancer. Semin Radiat Oncol. 2012;22(3):187–193. doi: 10.1016/j.semradonc.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maasland DH, van den Brandt PA, Kremer B, Goldbohm RA, Schouten LJ. Alcohol consumption, cigarette smoking and the risk of subtypes of head-neck cancer: results from the Netherlands Cohort Study. BMC Cancer. 2014;14:187. doi: 10.1186/1471-2407-14-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deschler DG, Richmon JD, Khariwala SS, Ferris RL, Wang MB. The “new” head and neck cancer patient-young, nonsmoker, nondrinker, and HPV positive: evaluation. Otolaryngol Head Neck Surg. 2014;151(3):375–380. doi: 10.1177/0194599814538605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrews E, Seaman WT, Webster-Cyriaque J. Oropharyngeal carcinoma in non-smokers and non-drinkers: a role for HPV. Oral Oncol. 2009;45(6):486–491. doi: 10.1016/j.oraloncology.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Brown LM, Check DP, Devesa SS. Oropharyngeal cancer incidence trends: diminishing racial disparities. Cancer Causes Control. 2011;22(5):753–763. doi: 10.1007/s10552-011-9748-1. [DOI] [PubMed] [Google Scholar]
- 7.Mehta V, Yu GP, Schantz SP. Population-based analysis of oral and oropharyngeal carcinoma: changing trends of histopathologic differentiation, survival and patient demographics. Laryngoscope. 2010;120(11):2203–2212. doi: 10.1002/lary.21129. [DOI] [PubMed] [Google Scholar]
- 8.Lambert R, Sauvaget C, de Camargo Cancela M, Sankaranarayanan R. Epidemiology of cancer from the oral cavity and oropharynx. Eur J Gastroenterol Hepatol. 2011;23(8):633–641. doi: 10.1097/MEG.0b013e3283484795. [DOI] [PubMed] [Google Scholar]
- 9.Ryerson AB, Peters ES, Coughlin SS, et al. Burden of potentially human papillomavirus-associated cancers of the oropharynx and oral cavity in the US, 1998–2003. Cancer. 2008;113(10 Suppl):2901–2909. doi: 10.1002/cncr.23745. [DOI] [PubMed] [Google Scholar]
- 10.Gillison ML, D’Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. Journal of the National Cancer Institute. 2008;100(6):407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 11.Chaturvedi AK. Beyond cervical cancer: burden of other HPV-related cancers among men and women. J Adolesc Health. 2010;46(4 Suppl):S20–26. doi: 10.1016/j.jadohealth.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Nasman A, Nordfors C, Holzhauser S, et al. Incidence of human papillomavirus positive tonsillar and base of tongue carcinoma: a stabilisation of an epidemic of viral induced carcinoma? European journal of cancer (Oxford, England: 1990) 2015;51(1):55–61. doi: 10.1016/j.ejca.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 13.Tezal M, Sullivan Nasca M, Stoler DL, et al. Chronic periodontitis-human papillomavirus synergy in base of tongue cancers. Archives of otolaryngology–head & neck surgery. 2009;135(4):391–396. doi: 10.1001/archoto.2009.6. [DOI] [PubMed] [Google Scholar]
- 14.Wang XI, Thomas J, Zhang S. Changing trends in human papillomavirus-associated head and neck squamous cell carcinoma. Annals of diagnostic pathology. 2012;16(1):7–12. doi: 10.1016/j.anndiagpath.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Radoi L, Luce D. A review of risk factors for oral cavity cancer: the importance of a standardized case definition. Community dentistry and oral epidemiology. 2013;41(2):97–109. e178–191. doi: 10.1111/j.1600-0528.2012.00710.x. [DOI] [PubMed] [Google Scholar]
- 16.Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute Site Recode. 2015 http://seer.cancer.gov/siterecode/2015.
- 17.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 13 Regs Research Data, Nov 2014 Sub (1992–2012) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2013 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2015, based on the November 2014 submission. 2015.
- 18.Mettner J. A cancer gone viral. Minnesota medicine. 2011;94(11):22–25. [PubMed] [Google Scholar]
- 19.Lazaar N, Aucouturier J, Ratel S, Rance M, Meyer M, Duche P. Effect of physical activity intervention on body composition in young children: influence of body mass index status and gender. Acta Paediatr. 2007;96(9):1315–1320. doi: 10.1111/j.1651-2227.2007.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lohman T, Thompson J, Going S, et al. Indices of changes in adiposity in American Indian children. Prev Med. 2003;37(6 Pt 2):S91–96. doi: 10.1016/j.ypmed.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Lohman TG, Caballero B, Himes JH, et al. Body composition assessment in American Indian children. Am J Clin Nutr. 1999;69(4 Suppl):764S–766S. doi: 10.1093/ajcn/69.4.764S. [DOI] [PubMed] [Google Scholar]


