Abstract
The pathogenetic heterogeneity of pulmonary fibrosis yields both challenges and opportunities for therapy. Its complexity implicates a variety of cellular processes, signaling pathways, and genetics as drivers of disease. TGF-β stimulation is one avenue, and is central to pro-fibrotic protein expression, leading to decreased pulmonary function. Here we report our recent findings, introducing the E3 ligase Fibrosis Inducing E3 Ligase 1 (FIEL1) as an important regulator of TGF-β signaling through the selective degradation of PIAS4. FIEL1 exacerbates bleomycin-induced murine pulmonary fibrosis, while its silencing attenuates the fibrotic phenotype. Further, we developed a small molecule inhibitor of FIEL1 (BC-1485) that inhibits the degradation of PIAS4, and ameliorates fibrosis in murine models. New understanding of this pathway illustrates the many targeting opportunities among the complexity of pulmonary fibrosis in the continuing search for therapy.
Idiopathic pulmonary fibrosis (IPF) is the most common interstitial lung disease with no cure and a grim median survival time of 3 years. This disease afflicts over 50 people per 100,000; and disproportionately affects the elderly: up to 0.2% of those over 75 years of age (1). Excessive fibrous deposition in the alveolar space results in pulmonary failure. Interestingly, there is profound heterogeneity in disease course and severity among patients, necessitating research into the molecular mechanisms of pathogenicity. Despite years of research, the sub-cellular molecular etiology of pulmonary fibrosis remains unclear. Several groups have sought to clarify the pathogenesis of pulmonary fibrosis.
Both genetic and environmental insults show association with the development of IPF. Research from the Armanios lab has implicated telomere distress as a causal agent in a subset of cases, as part of generational genetic anticipation (2). Additionally, research has shown several genetic hotspots, such as the MUC5B promoter, associate with a worsened phenotype (3). Extra-cellular matrix proteins such as integrins and matricellular proteins have been associated with fibrosis (4, 5). Most saliently important is the cytokine Transforming Growth Factor β (TGF-β), which is well accepted as a driver of cellular fibrosis (5, 6). TGF-β is sequestered in the extracellular matrix of cells in a latent form prior to cleavage and activation. Reed and colleagues demonstrated the importance of integrin-mediated latent-TGF-β activation in the context of liver and pulmonary fibrosis, as well as the potential for therapeutic targeting (7). Active TGF-β is a ligand for the TGF-β receptor, which initiates the SMAD signaling cascade (8). TGF-β signaling transduces through SMAD proteins, which dimerize prior to nuclear translocation and regulation of gene expression (9). Gao and colleagues demonstrated Ubiquitin E3 ligases directly regulate SMAD signal transduction, and Imoto and colleagues observed direct SUMOylation of SMADs leading to signaling suppression (10–12).
Until recently, IPF had few therapeutic options and was an orphan drug disease. Researchers halted triple treatment of immuno-suppressants and N-acetylcysteine due to concerns of increased mortality among study patients (13). However, in 2014, the FDA approved two therapeutics for IPF treatment. Several groups developed Nintedanib as a small-molecule treatment for idiopathic pulmonary fibrosis, culminating in the INPULSIS trials and FDA approval (14). Nintedanib inhibits multiple tyrosine kinases, such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and platelet-derived growth factor (PDGF). This therapy shows efficacy in critical metrics of pulmonary function. Concurrently, several research groups developed Pirfenidone, culminating in the ASCEND Study trial (15). The discreet targets of Pirfenidone still remain unclear, yet it shows efficacy in delaying disease progression (16). Due to the heterogeneity of this disease, multiple targets in the fibrotic pathway lay open for potential intervention (17).
We investigated additional E3 ligase-mediated regulation of the SMAD signaling pathway in the context of pulmonary fibrosis. Through candidate screening we characterized a novel HECT E3 ligase, Fibrosis-Inducing E3 Ligase 1 (FIEL1), which regulates and ubiquitinates PIAS4 (18). The selective degradation of the PIAS4 isoform has downstream effects on SMAD signaling, as the depletion of FIEL1 encourages the repression of SMAD translocation and fibrotic gene expression. We observed that PIAS4-FIEL1 binding requires a unique “double-locking” mechanism, necessitating mutual E3 and substrate phosphorylation by different kinases. Lentiviral silencing of FIEL1 ameliorated fibrotic phenotype, progression, and mortality in an experimental bleomycin murine fibrosis model. Further, we designed a small-molecule inhibitor of FIEL1 termed BC-1485, which protects PIAS4 from ubiquitin-mediated degradation. BC-1485 intervention to murine bleomycin-induced fibrosis resulted in a time and dose-dependent rescue of fibrotic phenotype, progression, and mortality. In addition to the in vitro and in vivo characterization of FIEL1 and PIAS4, we observed differential protein expression of both proteins in parenchymal tissue samples between IPF patients and controls, suggesting the clinical importance of this pathway. This study offers a novel pathway for pre-clinical targeting of the proteolytic pathway in the context of pulmonary fibrosis. Continuing research will further elucidate the molecular mechanisms at play, and the therapeutic potential of this inhibition.
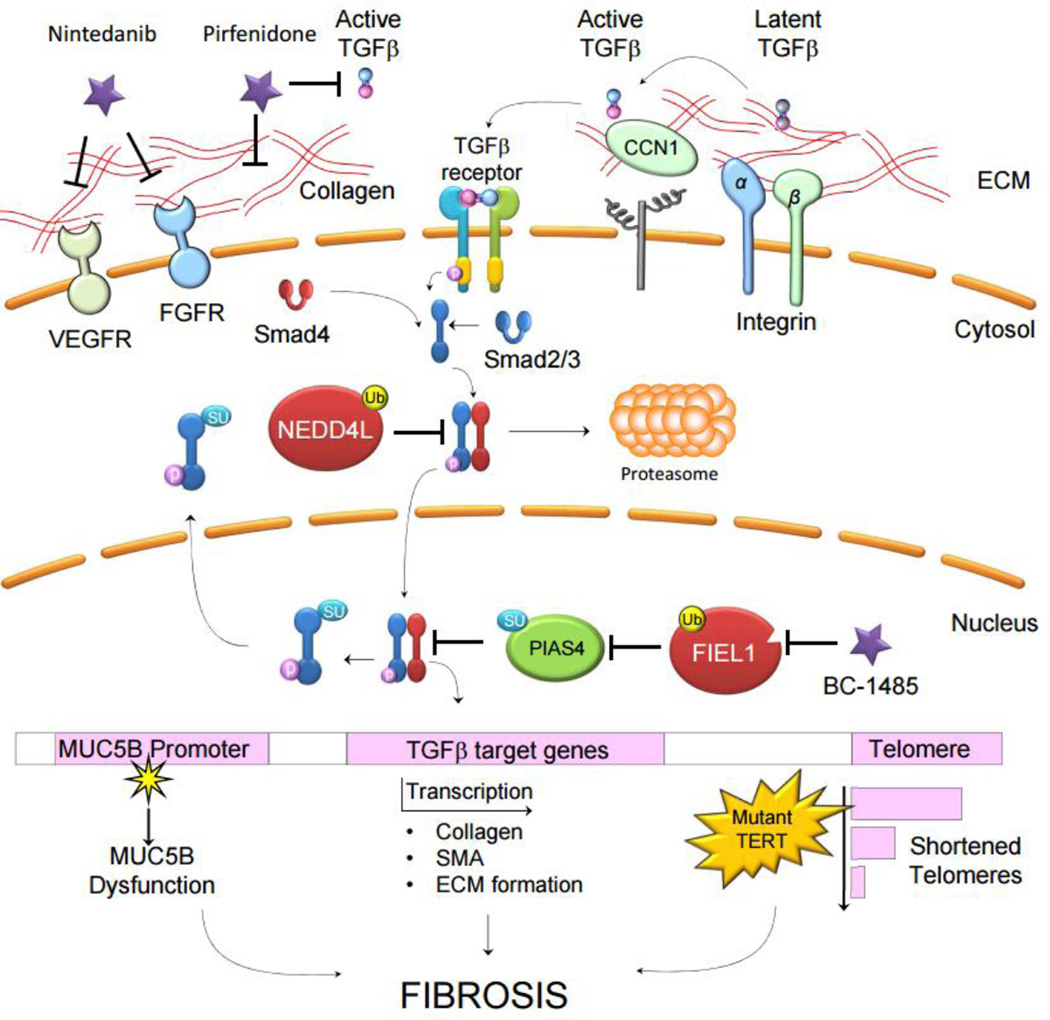
Figure 1.
Membrane proteins such as integrins and secreted proteins in the extracellular matrix (ECM) such as CCN1 interact and facilitate the conversion of latent, ECM-sequestered TGFβ to its active form. Active TGFβ is ligand to the TGFβ receptor on epithelia, fibroblasts, and immune cells. Receptor stimulation causes a signaling cascade via SMAD proteins, leading to the nuclear translocation of a SMAD complex and the transcriptional activation of pro-fibrotic genes. Ubiquitin-E3 ligases, such as NEDD4L, regulate SMAD signaling through proteasome-mediated degradation. Additionally, SUMO-E3 ligases, such as PIAS4, regulate SMAD signaling by forcing its nuclear export. Direct regulators of SMAD signaling are themselves regulated, as FIEL1 catalyzes the degradation of PIAS4. In addition to transcriptional regulation of pro-fibrotic genes and gene products such as collagens, smooth muscle action and fibronectin, genetic abnormalities associate with fibrosis. Mutation within the MUC5B promoter and shortened telomeres caused by telomerase mutations both associate with worsened fibrosis. The current therapeutics Nintedanib and Pirfenidone target growth receptors, ECM formation, and TGFβ. Novel compound BC-1485 targets downstream profibrotic protein FIEL1, which may serve as a new therapeutic approach for the future IPF therapy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wolters PJ, Collard HR, Jones KD. Pathogenesis of idiopathic pulmonary fibrosis. Annual review of pathology. 2014;9:157–179. doi: 10.1146/annurev-pathol-012513-104706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armanios M. Telomerase and idiopathic pulmonary fibrosis. Mutation research. 2012;730:52–58. doi: 10.1016/j.mrfmmm.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peljto AL, et al. Association between the MUC5B promoter polymorphism and survival in patients with idiopathic pulmonary fibrosis. Jama. 2013;309:2232–2239. doi: 10.1001/jama.2013.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurundkar AR, et al. The matricellular protein CCN1 enhances TGF-beta1/SMAD3-dependent profibrotic signaling in fibroblasts and contributes to fibrogenic responses to lung injury. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2016 doi: 10.1096/fj.201500173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheppard D. Epithelial-mesenchymal interactions in fibrosis and repair. Transforming growth factor-beta activation by epithelial cells and fibroblasts. Annals of the American Thoracic Society. 2015;12(Suppl 1):S21–S23. doi: 10.1513/AnnalsATS.201406-245MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luzina IG, Todd NW, Sundararajan S, Atamas SP. The cytokines of pulmonary fibrosis: Much learned, much more to learn. Cytokine. 2015;74:88–100. doi: 10.1016/j.cyto.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Reed NI, et al. The alphavbeta1 integrin plays a critical in vivo role in tissue fibrosis. Science translational medicine. 2015;7:288ra279. doi: 10.1126/scitranslmed.aaa5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 9.Macias MJ, Martin-Malpartida P, Massague J. Structural determinants of Smad function in TGF-beta signaling. Trends in biochemical sciences. 2015;40:296–308. doi: 10.1016/j.tibs.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao S, et al. Ubiquitin ligase Nedd4L targets activated Smad2/3 to limit TGF-beta signaling. Molecular cell. 2009;36:457–468. doi: 10.1016/j.molcel.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imoto S, et al. Sumoylation of Smad3 stimulates its nuclear export during PIASy-mediated suppression of TGF-beta signaling. Biochemical and biophysical research communications. 2008;370:359–365. doi: 10.1016/j.bbrc.2008.03.116. [DOI] [PubMed] [Google Scholar]
- 12.Imoto S, et al. Regulation of transforming growth factor-beta signaling by protein inhibitor of activated STAT, PIASy through Smad3. The Journal of biological chemistry. 2003;278:34253–34258. doi: 10.1074/jbc.M304961200. [DOI] [PubMed] [Google Scholar]
- 13.T. I. P. F. C. R. Network, Prednisone, Azathioprine, and N-Acetylcysteine for Pulmonary Fibrosis. New England Journal of Medicine. 2012;366:1968–1977. doi: 10.1056/NEJMoa1113354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richeldi L, et al. Efficacy and Safety of Nintedanib in Idiopathic Pulmonary Fibrosis. New England Journal of Medicine. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 15.King TE, et al. A Phase 3 Trial of Pirfenidone in Patients with Idiopathic Pulmonary Fibrosis. New England Journal of Medicine. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 16.Selvaggio AS, Noble PW. Pirfenidone Initiates a New Era in the Treatment of Idiopathic Pulmonary Fibrosis. Annual review of medicine. 2016;67:487–495. doi: 10.1146/annurev-med-120214-013614. [DOI] [PubMed] [Google Scholar]
- 17.Rangarajan S, Locy ML, Luckhardt TR, Thannickal VJ. Targeted Therapy for Idiopathic Pulmonary Fibrosis: Where To Now? Drugs. 2016;76:291–300. doi: 10.1007/s40265-015-0523-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lear T, et al. Ubiquitin E3 ligase FIEL1 regulates fibrotic lung injury through SUMO-E3 ligase PIAS4. The Journal of Experimental Medicine. 2016;213:1029–1046. doi: 10.1084/jem.20151229. [DOI] [PMC free article] [PubMed] [Google Scholar]