Abstract
Salmonellosis, caused by members of the genus Salmonella, is responsible for considerable global morbidity and mortality in both animals and humans. In this review, we will discuss the pathogenesis of Salmonella enterica serovar Typhi and Salmonella enterica serovar Typhimurium, focusing on human Salmonella infections. We will trace the path of Salmonella through the body, including host entry sites, tissues and organs affected, and mechanisms involved in both pathogenesis and stimulation of host immunity. Careful consideration of the natural progression of disease provides an important context in which attenuated live oral vaccines can be rationally designed and developed. With this in mind, we will describe a series of attenuated live oral vaccines that have been successfully tested in clinical trials and demonstrated to be both safe and highly immunogenic. The attenuation strategies summarized in this review offer important insights into further development of attenuated vaccines against other Salmonella for which live oral candidates are currently unavailable.
INTRODUCTION
Salmonellosis, caused by oral infection with members of the genus Salmonella, is responsible for considerable global morbidity and mortality, in both animals and humans (1, 2). The genus Salmonella contains two species, Salmonella enterica and Salmonella bongori, each of which contains multiple serotypes of genetically distinct organisms (3). There are currently over 2,500 serotypes (referred to as serovars) of Salmonella as defined by immunologic identification of somatic O and flagellar H antigens. In turn, S. enterica is divided into six subspecies (subsp.), of which only one, S. enterica subsp. enterica colonizes warm-blooded animals; the remaining subspecies are typically isolated from cold-blooded animals and the environment (3). It was recently reported that, worldwide, the highest morbidities from human infections resulting from food-borne diseases involved food contaminated with S. enterica (1, 2).
In this review, we will focus on S. enterica subsp. enterica, encompassing the majority of serovars identified thus far, and limit our discussion to vaccine candidates developed to prevent human disease that have been tested in human clinical trials. We will first describe the features of S. enterica infections in humans, including the host entry sites, tissues and organs affected, and mechanisms involved in pathogenesis; this information will provide a context for later description of vaccine candidates and the genetic strategies employed to rationally attenuate Salmonella. We will then describe general immune responses elicited by natural oral infection with Salmonella and discuss how these responses originate in relation to immunologically primed tissue. Finally, we will present clinical data derived from studies involving S. enterica serovars Typhi (the causative agent of typhoid fever) and Typhimurium (which typically causes gastroenteritis in humans). The pathogenic mechanisms explored here for two clinically relevant serovars, and genetic strategies employed to create attenuated but highly immunogenic live oral mucosal vaccines, can be applied to other Salmonella serovars for which vaccines are urgently needed (4).
PATHOBIOLOGY OF SALMONELLA INFECTIONS IN HUMANS
Overcoming the Gastric Acid Barrier
Infection with Salmonella is initiated following oral ingestion of contaminated food or water (see Fig. 1A). The minimum infectious dose required for establishing a productive infection depends on several key microbiological factors including the strain and serovar of Salmonella involved, as well as important host factors including the host mammalian species to be colonized and various gastrointestinal barriers to infection.
Figure 1.
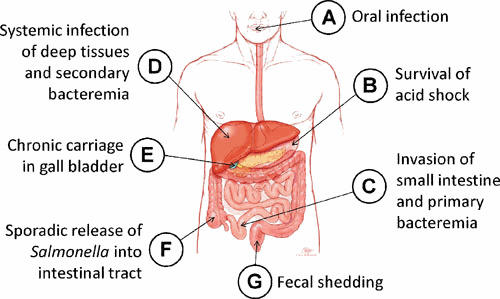
Pathobiology of human Salmonella infections. Infection with Salmonella is initiated following oral ingestion of contaminated food or water (A). Salmonella must then overcome potentially lethal levels of inorganic acid (H+) which produce pHs as low as 2 in the stomach of healthy adults (B). Salmonella organisms surviving the extreme acidic conditions of the stomach eventually drain into the small intestine, the portal for invasion into deeper tissues and development of systemic disease (C). Salmonella invade tissues of both villus epithelial tissue as well as lymphoid Peyer’s patches. Following transit of invading Salmonella out of the lumen and across the epithelial barrier of the small intestine, bacteria eventually gain transient access to the bloodstream to eventually colonize deep tissues including the liver (D), spleen, and bone marrow. It is at this stage that infection with S. Typhimurium is typically halted and does not progress to systemic disease in immunologically competent humans. However, S. Typhi can be released from deep tissues back into the bloodstream, triggering a more substantial secondary bacteremia which precedes the onset of classic typhoid fever. In rare cases, typhoid fever can progress to an asymptomatic chronic infection in which S. Typhi can migrate down the hepatic ducts of the liver and into the gallbladder (E), setting up a convalescent carrier state in which very high levels of organisms can be intermittently released back into the small intestine, passing through the large intestine (F) and being released in the feces (G).
In humans, one significant physiological barrier strongly influencing the infectious dose is gastrointestinal acidity. Salmonella must overcome potentially lethal levels of inorganic acid (H+) which produce a pH as low as 2 in the stomach of healthy adults (Fig. 1B) (5). In human challenge studies conducted in the early 1970s at the University of Maryland, ∼107 colony forming units (CFUs) was the minimum infectious dose required to establish infection in >50% of volunteers orally challenged with a fully virulent S. Typhi Quailes strain given with milk (6); however, in recent challenge studies conducted at the University of Oxford, using the identical Quailes strain administered to volunteers who first ingested bicarbonate solution to neutralize stomach acidity, the dose required to establish infection in >50% of volunteers was reduced by a factor of 4 logs to 103 CFUs (7).
Encountering low pH is believed to provide an important environmental signal to Salmonella for deploying a cascade of virulence factors necessary for host cell invasion (8). Salmonella is equipped with a variety of genetic strategies that contribute to their survival and growth. Two key proteins involved in reallocation of metabolic resources to survive acid stress (called the acid tolerance response [ATR]) are RpoS and OmpR. RpoS is an alternate sigma factor used by RNA polymerase to enhance cell survival under acidic conditions by switching RNA transcription and subsequent protein synthesis during exponential growth in nutrient-rich conditions to a much slower stationary phase growth in which a variety of acid resistance genes are induced (8). OmpR is also involved in this ATR, and is the effector component of a two-component environmental sensor, in which the sensor EnvZ activates OmpR upon exposure to both acidity and osmolarity (8). In addition to osmoregulation and acid tolerance, OmpR is also involved in the induction of intracellular survival factors required for survival of Salmonella within intracellular vacuoles, following invasion of permissive eukaryotic cells such as macrophages and intestinal epithelial cells (9).
The central role of RpoS in the survival of Salmonella has been exploited for the development of live attenuated vaccines against typhoid fever. Several candidate vaccines tested in clinical trials have been engineered from wild-type S. Typhi Ty2 strains in which the rpoS gene has been naturally inactivated (10). When attenuated vaccine candidates derived from Ty2 were compared with identically attenuated candidates derived from wild-type S. Typhi ISP1820 strains, in which rpoS was intact, these RpoS+ strains were unacceptably reactogenic (11). It has also been shown that the only licensed and well-tolerated live oral vaccine against typhoid fever, Ty21a, which was created by chemical mutagenesis of Ty2 resulting in multiple attenuating chromosomal lesions, is also deficient in synthesis of RpoS; consequently, this vaccine requires multiple doses to confer protection against disease and displays no acid tolerance response (12).
Invasion of the Small Intestine
Salmonella organisms that can survive the extreme acidic conditions of the stomach eventually drain into the small intestine, the portal for invasion into deeper tissues and development of systemic disease (Fig. 1C). It is at this stage of infection that differences in the serovar-specific progression of disease most clearly manifest themselves. S. Typhi possesses several essential clusters of pathogenicity genes, grouped into distinctly regulated chromosomal locations called Salmonella pathogenicity islands (SPIs), which enable invading S. Typhi organisms to reach deeper tissues of the human liver, spleen, and bone marrow, and bypass innate immunity clearance mechanisms (13, 14). In contrast, S. Typhimurium is not equipped with several of these essential intracellular survival mechanisms required for deep penetration of human tissues; therefore, infection of immunocompetent individuals with S. Typhimurium typically results only in local tissue invasion and a self-limiting gastroenteritis (13).
It is believed that Peyer’s patches are important sites involved in transepithelial migration for Salmonella across the luminal surface of the small intestine, based on murine experimental challenge studies with S. Typhimurium (15). While direct in vivo evidence supporting invasion of Peyer’s patches by S. Typhi in humans is lacking, several observations support this notion. Examination of intestinal mucosal biopsies from volunteers orally challenged with 109 CFUs of the Quailes strain showed granulomatous lesions throughout the small intestine including the duodenum, jejunum, and ileum, accompanied by infiltration of inflammatory cells, and coinciding with systemic clinical symptoms including fever and positive blood cultures (16). Peyer’s patches are present throughout the human small intestine, with densities increasing through the jejunum and the largest patches typically residing in the terminal ileum in both children and adults (17, 18), and Salmonella is believed to take advantage of this antigen-sampling compartment of the gastrointestinal tract to invade deeper tissues (19).
In addition to invading Peyer’s patches, Salmonella is also likely to invade villous epithelial tissue of the small intestine. Salmonella are equipped with a molecular arsenal of pathogenicity factors, some of which are common to both S. Typhimurium and S. Typhi, enabling rapid and efficient invasion of intestinal epithelial tissues. One critical environmental signal orchestrating a cascade of virulence factors that participate in the invasion process at precisely the right time is osmolarity. Villi of the small intestine possess a gradient of osmolarity that is highest at the luminal surface of villi (∼700 mOsm kg−1 H2O) and decreases to physiological levels (∼300 mOsm kg−1 H2O) in the lower crypts of the villus (20, 21). Incoming luminal Salmonella sense the high osmolarity of villus surfaces, which in turn signals induction of a pathogenicity island common to all S. enterica serovars capable of infecting mammals, called SPI-1. This locus encodes a type III secretion apparatus that injects effector proteins into target eukaryotic cells, resulting in ruffling of the outer membrane and engulfment of invading bacteria (22).
The transition from extracellular to intracellular pathogen induces an extensive reorganization of both bacterial metabolism and virulence factors. Upon transitioning across the epithelial barrier, the osmolarity of the surrounding tissue drops to physiological levels, providing a critical signal for Salmonella to downregulate SPI-1 while inducing synthesis of intracellular survival proteins injected into infected cells by a separate and distinct SPI-2 type III secretion system (23). At this stage of infection, crucial genetic differences between invading S. Typhimurium and S. Typhi begin to strongly influence the course and manifestation of disease (24). Although both serovars possess fully functional SPI-2 and ancillary effector virulence proteins, S. Typhi is also equipped with several additional genomic modifications, allowing it to avoid the host natural inflammatory response to invading organisms, which is characterized by a massive and rapid influx of neutrophils; this is not the case for S. Typhimurium, which causes acute gut inflammation.
S. Typhi has been characterized as an excellent example of “reductive genomic evolution” of a human pathogen (25). In contrast to S. Typhimurium, S. Typhi has evolved to become an exclusive human pathogen, incapable of establishing a productive natural infection in any other mammalian species, and relying on the host to provide multiple essential factors required for survival and growth. In adapting exclusively to infection of humans, S. Typhi has naturally accumulated a series of genetic disruptions and inactivations involving approximately 5% of its genome (26). Such inactivations include both loss of metabolic capacity and modifications to the bacterial outer membrane surface that reduce interaction and signaling via Toll-like Receptors (TLRs) expressed by innate immune cells. In contrast, recent data suggest that S. Typhimurium may actually benefit from initiation of an inflammatory response because incoming innate immune cells inadvertently generate a critical metabolite called tetrathionate from the oxidative burst; tetrathionate is used exclusively by S. Typhimurium as an alternate electron acceptor in anaerobic respiration, and therefore becomes available for enhanced growth prior to excretion from the colon (27, 28). Given that S. Typhi spends relatively little time in the intestine prior to invasion and systemic infection, it comes as no surprise that S. Typhi has lost the ability to utilize tetrathionate for anaerobic respiration (29).
Transepithelial migration and the accompanying drop in osmolarity induces yet another pathogenicity island, exclusive to S. Typhi, called SPI-7 (30, 31, 32), which encodes an outer polysaccharide capsule called Vi (26). The Vi capsule shields lipopolysaccharide (LPS) in the bacterial outer membrane from signaling an inflammatory sensor on the surface of phagocytic cells called Toll-like receptor 4 (TLR4) (33). Signaling of TLR4 induces the secretion of cytokines tumor necrosis factor alpha (TNF-α) and interleukin (IL)-6, which are responsible for recruitment of neutrophils and other inflammatory cells to the site of infection (13). To further reduce TLR4 interaction and signaling by invading organisms progressing to systemic tissues, S. Typhi has acquired an inactivating mutation in fepE, a gene controlling the length of the repeating O antigen comprising S. Typhi LPS. The result of losing fepE is that synthesis of extremely long LPS (>200 repeat units) is now blocked, and LPS is no longer able to protrude through the protective layer of the Vi capsule (34). Surface expression of the Vi capsule also interferes with neutrophil chemotaxis and phagocytic killing of invading organisms by blocking complement deposition onto the surface of S. Typhi; the Vi capsule is composed of a homopolymer of saccharides devoid of free hydroxyl groups required for deposition of the complement component C3b, which in turn activates the complement cascade through the alternative pathway, thereby generating the chemoattractant C5a which attracts neutrophils (35).
Remarkably, the SPI-7 locus also encodes a regulatory protein called TviA, which, while upregulating expression of Vi, also downregulates expression of flagellin (31), another powerful innate signaling protein that binds to and activates TLR5 in the basolateral membrane of intestinal epithelial cells (36, 37). Activation of TLR5 induces secretion of another potent cytokine, IL-8, which is also a powerful recruiter of neutrophils and other inflammatory cells to the site of invading pathogens. Importantly, flagellin is not expressed once S. Typhi has invaded macrophages, thereby reducing pyroptosis of infected cells and further recruitment of neutrophils (38). This strategy of reducing pyroptosis also prevents release of S. Typhi from its protected intracellular niche, thus facilitating systemic dissemination.
Transient Primary Bacteremia
Following transit of invading Salmonella out of the lumen and across the epithelial barrier of the small intestine, bacteria eventually gain access to the bloodstream for a brief period of time (39), facilitating a more sustained infection of the liver, spleen, and bone marrow (Fig. 1D). The passage of bacteria into the bloodstream involves intracellular persistence within human macrophages. The ability of Salmonella to survive and replicate within these cells, thereby facilitating systemic infection, clearly differentiates S. Typhi from S. Typhimurium. Compared with S. Typhimurium, S. Typhi has up to 100-fold higher survival rates in elutriated primary human macrophages from peripheral blood, with intracellular replication resulting in very little cell death (40); interestingly, the rpoS+ strain ISP1820 survives better in human macrophages than the rpoS– strain Ty2 (40), again suggesting a role for rpoS in host survival.
It has been reported that bacteremia can be detected by PCR in volunteers challenged with the S. Typhi Quailes strain within 12 hours after oral ingestion of organisms (41). Culture positive detection of S. Typhi is observed in the monocyte fraction of the peripheral blood cells recovered from naturally infected typhoid patients (42). S. Typhi is present at very low levels of ∼2 CFU/ml in blood from patients with uncomplicated enteric fever during the first week of illness. This level drops to ∼1 CFU/ml during the second and third weeks, and declines to ∼0.3 CFU/ml during the fourth week; blood-borne S. Typhi is not usually present as extracellular organisms, with approximately 63% of viable bacteria residing intracellularly in peripheral blood cells (43). Much higher levels of S. Typhi can be detected in the bone marrow of patients with confirmed uncomplicated typhoid fever, with ∼5 CFU/ml in bone marrow aspirate recovered in the first week of illness and increasing to ∼160 CFU/ml during the third week; again most bacteria are intracellular and therefore less susceptible to antibiotic treatment (44).
Establishment of the Carrier State and Shedding
Invading S. Typhi reaching the liver, spleen, and bone marrow can replicate within resident macrophages, and can then be released back into the bloodstream, triggering a more substantial secondary bacteremia that precedes the onset of classic typhoid fever (45, 46). However, 2 to 5% of typhoid cases (47, 48) eventually progress to an asymptomatic chronic infection in which S. Typhi can migrate through the hepatic ducts of the liver and into the gallbladder (Fig. 1E), setting up a convalescent carrier state in which very high levels of organisms can be intermittently released back into the small intestine, passing through the large intestine (Fig. 1F), and being shed in the feces at levels as high as 106 to 1010 viable organisms per gram of stool (Fig. 1G) (49). This intermittent shedding facilitates further spread of the disease to susceptible individuals through contaminated food or water. Chronic infection of the gallbladder is often accompanied by the presence of gallstones, upon which S. Typhi are able to establish robust colonization through the formation of biofilms. These biofilms are composed of polysaccharides including Vi and LPS but only minor amounts of flagellar proteins (50). S. Typhi residing in biofilms is recalcitrant to antibiotic treatment regardless of the inherent susceptibility of the pathogen. Further direct infection of gallbladder epithelium by free S. Typhi growing in the gallbladder lumen can take place via osmotic activation of the SPI-1 invasion locus, resulting in tissue damage and sloughing of epithelial cells down the bile duct and back into the duodenum of the small intestine (51, 52). Interestingly, although acute typhoid fever does not elicit appreciable antibody responses against Vi antigen, chronic carriers are able to mount a very high Vi-specific serum antibody response, which has been shown to be an excellent diagnostic marker for the carrier state (53, 54).
Late-Stage Complications and Death
Throughout the discussion of S. Typhi infections, we have described the pathobiology of acute typhoid fever, without the involvement or influence of life-threatening extraintestinal complications. However, while the vast majority of typhoid cases resolve without life-threatening sequelae, up to 10% of typhoid patients can develop serious complications, including death from gastrointestinal hemorrhages and peritonitis from intestinal perforation (39). The rate of intestinal perforations in patients with typhoid fever worldwide has been estimated to be 3% (55). The average case fatality rate for intestinal perforation was reported to be 15%, with rates as high as 40% depending on geographic location (56). Of note, intestinal perforations usually occur within 45 cm of the ileocecal valve (57), an area with the highest density of Peyer’s patches and an important portal of invasion for S. Typhi (17, 18).
STRATEGIES FOR ATTENUATION AND DESIGN OF HUMAN LIVE ORAL SALMONELLA VACCINES
Immune Responses to Natural Infection
Understanding the progression of human Salmonella infections and immune mechanisms that can control infection greatly facilitates the rational design of efficacious live attenuated vaccines against these organisms. Unlike many other enteric pathogens, infection with Salmonella does not typically induce a long-lasting protective immunity. Pathogen-specific antibodies, used as readouts of pathogen exposure and immunological priming, are important for clearance only while Salmonella is extracellular or within the lumen of the intestines. These antibodies block motility and facilitate complement-mediated lysis or phagocytic killing. Studies in typhoid patients from Bangladesh, where Salmonella is endemic, have shown elevated serum IgA and IgG antibodies against LPS, whole-cell extract, and membrane preparations, during infection (58), although membrane preparation-specific IgA antibodies were more prevalent than IgG. These responses are highest in adults, yet children also develop IgA antibodies specific for S. Typhi membrane antigens which decline during late convalescence (59).
Although it is evident that both innate and humoral immune responses are required for control of Salmonella infections, cellular immunity is believed to play a crucial role in the clearance of Salmonella residing intracellularly. However, the exact roles of individual cell types and mechanisms underlying protective immunity remain to be elucidated. Upon infection of phagocytic and professional antigen-presenting cells (i.e., macrophages and dendritic cells) Salmonella antigens are processed and presented for stimulation of CD4 and CD8 T cells, resulting in activation and differentiation of T helper (Th) 1, Th2, and Th17 cells (60, 61, 62), as well as T cytotoxic (Tc) (63, 64) and T regulatory (Treg) cells (65), which are believed to be essential for protection against disease (66). Natural infection also results in the induction of Th1 type CD4+ and CD8+ T cells that produce high levels of interferon gamma (IFN-γ) during acute and convalescent phases of infection (60). Peripheral blood cells isolated from typhoid patients and stimulated with bacterial antigens produced higher levels of other cytokines, including IFN-γ, macrophage inflammatory protein-1β, soluble CD40 ligand, TNF-β, IL-13, and IL-9 during convalescence, which are likely required for an effective immune response that leads to bacterial clearance (62). Recent studies have also shown that natural infection results in the upregulation of the gut-homing integrin α4β7 on T regulatory cells (65), which may play a role in downregulating proinflammatory CD4 and CD8 T-cell responses.
General Strategies for Live Oral Vaccine Design
As discussed above, Salmonella possess specialized virulence mechanisms that allow them to reach permissive niches within the human host, establishing reservoirs for subsequent replication. Therefore, genetically inactivating one or more of these essential virulence factors constitutes one highly successful approach for weakening fully virulent organisms and constructing attenuated live Salmonella vaccines intended for oral immunization of humans. A more subtle but equally effective strategy for attenuating pathogens targets metabolic pathways required to sustain infection in the host, but this approach must be accomplished in such a way that the resulting live vaccine remains metabolically fit enough to reach immune inductive sites and elicit biologically relevant protective immunity in the absence of overt disease. Overattenuation of a candidate vaccine, while resulting in a highly safe and nonreactogenic organism, will require the administration of multiple oral doses to achieve protective immunity, a requirement that complicates deployment of the vaccine into the field. These key concepts for designing live oral vaccine candidates are particularly well illustrated with a select group of attenuated candidate live oral vaccines that are summarized in Table 1 and individually examined in detail below.
Table 1.
Selected candidate live oral Salmonella vaccines against human disease tested in phase 1/phase 2 clinical trials
| Attenuation strategy | Vaccine strain | Serovar; strain; relevant genotype | Relevant phenotype | References |
|---|---|---|---|---|
| Chemical mutagenesis | Ty21a | Typhi; Ty2; ΔrpoS ΔgalE ΔgalK ΔilvD ΔvexD | Natural RpoS-dependent sensitivity to environmental stressors; defective synthesis of LPS; auxotrophic for isoleucine and valine; defective synthesis of Vi | 69 |
| Engineered deletions in rpoS | χ9639a | Typhi; Ty2; ΔrpoS | Natural RpoS-dependent sensitivity to environmental stressors | 90 |
| χ9640a | Typhi; Ty2; rpoS+ | Engineered RpoS-mediated resistance to environmental stressors | 90 | |
| χ9633a | Typhi; ISP1820; rpoS+ | Natural RpoS-mediated resistance to environmental stressors | 90 | |
| Engineered deletions in phoP/phoQ | Ty800 | Typhi; Ty2; ΔrpoS ΔphoP ΔphoQ | Natural RpoS-dependent sensitivity to environmental stressors; defective in pH and osmolarity induction of invasion virulence factors | 94 |
| Ty1033b | Typhi; Ty2; ΔrpoS ΔphoP ΔphoQ | Natural RpoS-dependent sensitivity to environmental stressors; defective in pH and osmolarity induction of invasion virulence factors | 96 | |
| LH1160b | Typhimurium; ATCC 14028; ΔphoP ΔphoQ | Defective in pH and osmolarity induction of invasion virulence factors | 95 | |
| Engineered deletions in ssaV | M01ZH09 | Typhi; Ty2; ΔrpoS ΔaroC ΔssaV | Natural RpoS-dependent sensitivity to environmental stressors; auxotrophic for aromatic amino acids; defective for proper secretion of SPI-2 effector proteins | 97, 99, 100,101, 102 |
| WT05 | Typhimurium; TML; ΔaroC ΔssaV | Auxotrophic for aromatic amino acids; defective for proper secretion of SPI-2 effector proteins | 97 | |
| Engineered deletions in aroC, aroD, and htrA | CVD 908 | Typhi; Ty2; ΔrpoS ΔaroC ΔaroD | Natural RpoS-dependent sensitivity to environmental stressors; auxotrophic for aromatic amino acids | 105 |
| CVD 908-htrA | Typhi; Ty2; ΔrpoS ΔaroC ΔaroD ΔhtrA | Natural RpoS-dependent sensitivity to environmental stressors; auxotrophic for aromatic amino acids; sensitive to environmental heat shock | 107 | |
| CVD 909 | Typhi; Ty2; ΔrpoS Ptac-tviA ΔaroC ΔaroD ΔhtrA | Natural RpoS-dependent sensitivity to environmental stressors; constitutive expression of TviA regulator and Vi antigen; auxotrophic for aromatic amino acids; sensitive to environmental heat shock | 77, 108, 110 |
These vaccines were engineered as live vector vaccines, carrying additional chromosomal mutations, as well as carrying multicopy plasmids expressing a pneumococcal foreign antigen. However, all candidate vaccines carried the same attenuating lesions and differed only with respect to parental strain used (Ty2 versus ISP1820) and the presence of rpoS.
These vaccines were engineered as live vector vaccines, carrying one additional chromosomal deletion in the purB gene, as well as carrying multicopy plasmids encoding both purB and the foreign antigen urease from H. pylori. However, both S. Typhi and S. Typhimurium candidate vaccines were isogenic for the attenuating lesions tested in clinical trials.
Chemical Mutagenesis and Ty21a
The only licensed vaccine against human infections caused by Salmonella is Ty21a, a typhoid fever vaccine derived from the parental wild-type S. Typhi strain Ty2 by chemical mutagenesis using N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) (67). MNNG is an alkylating agent that induces transition mutations (purine to purine or pyrimidine to pyrimidine base transitions) in replicating DNA, causing pleiotropic mutations to arise in multiple genes that can affect a wide variety of unrelated cell functions (68). Consequently, sequencing of the chromosome of Ty21a has revealed that this vaccine contains multiple mutations in a metabolic pathway controlling the incorporation of galactose into properly synthesized LPS (69). Ty21a requires growth in the presence of trace amounts (0.001%) of galactose to synthesize full-length LPS, but growth in the presence of higher concentrations (0.1%) leads to the intracellular accumulation of toxic intermediates that causes premature lysis of the vaccine strain (69). Ty21a contains additional metabolic mutations in amino acid synthesis pathways, leading to a requirement for isoleucine and valine (in addition to tryptophan and cysteine inherited from the parent strain Ty2) for growth under nutrient limiting conditions (69).
As a result of multiple mutations in critical metabolic pathways, Ty21a grows slower than the wild-type Ty2 in vitro (67), and cannot be recovered from the small intestine of orally vaccinated humans regardless of the number of organisms or vaccine doses administered (70). For this reason, successful oral immunization of humans requires a minimum of 3 doses to provide durable protection against challenge, with efficacy also depending on growth conditions and specific formulation of the vaccine. Ty21a grown in the absence of galactose results in a rough vaccine strain lacking O antigen that was poorly immunogenic and failed to protect volunteers challenged with 105 CFUs of the Quailes strain (70). In contrast, 3 oral doses of O-expressing Ty21a, administered orally every other day in enteric-coated capsules, conferred 62% protection in field trials over a 7-year period; 3 doses of a liquid formulation, reconstituted from buffered lyophilized sachets, elicited 78% protection over a 5-year period (71). These data support the hypothesis that O-antigen-specific immunity plays an important role in protection against typhoid fever. Interestingly, the recurrence rate for exposed individuals who recovered from a previous episode of typhoid fever ranged from 20% in an endemic region (72) to 23% in convalescent volunteers challenged with 105 CFUs of the Quailes strain (73), suggesting that natural infection does not necessarily elicit the robust protective immune effector mechanisms against reinfection as observed in vaccinees receiving multiple oral doses of Ty21a.
Both serum antibody titers and the frequency of circulating antibody-secreting cells (ASCs) have historically been the primary method to ascertain immunogenicity of orally delivered live Salmonella vaccines (74). The B cell responses induced by Ty21a include LPS-specific serum IgG and IgA antibodies (75) and mucosally primed ASCs bearing the α4β7 gut homing integrin (76). Among individuals who received the routine 4 doses of Ty21a in the United States, half of the recipients developed strong anti-LPS IgA B memory responses and 30 to 40% developed antiflagella IgG and IgA B memory responses (77). Ty21a-induced antibodies have been shown to bind to S. Typhi and enhance phagocytosis and bactericidal activity (75, 78).
Ty21a has also been shown to induce IFN-γ-producing CD4 and CD8 T terminal effector memory cells expressing α4β7 (79). Peripheral blood mononuclear cells from Ty21a-vaccinated volunteers stimulated with S. Typhi flagella produced cytokines required for clearance of intracellular pathogens and cell-mediated cytotoxicity including IFN-γ, TNF-α, IL-1β, IL-6, and IL-10 (80). In an early study, >90% of Ty21a-vaccinated volunteers developed LPS- and flagella-specific antibodies (78). Interestingly, when Salmonella-specific IgA antibodies were incubated together with CD4 T cells, antibody-dependent cellular cytotoxicity was observed against infected cells (81). Ty21a vaccination has also been show to elicit IFN-γ-secreting CD8 T cells that exhibited cytotoxic activity against infected cells (82, 83, 84). Further analysis showed that the vaccine-induced CD8 T cells were long-lived memory cells with the capacity to produce multiple cytokines including IFN-γ, TNF-α, IL-2, and IL-17 (64, 85). Taken together, these data suggest that, in contrast to natural infection, repeated vaccination with Ty21a appears more effective than wild-type organisms at presenting a variety of otherwise immunologically muted antigens such as LPS, flagella, and other outer membrane proteins to the immune system, thereby resulting in robust pathogen-specific humoral and cellular immunity.
Genetically Engineered Vaccines with Mutations in rpoS
In addition to mutations in genes controlling the synthesis of tryptophan and cysteine, the parent strain Ty2 used to create the vaccine Ty21a also contains a naturally occurring mutation inactivating rpoS (10, 86). The role of rpoS in adapting to environmental stresses, including its role in the acid tolerance response, was described previously in the pathobiology of Salmonella infections. However, rpoS also regulates the synthesis of Vi capsular polysaccharide (87, 88), which plays a critical role in the systemic survival of invading S. Typhi in natural disease. 99.5% of 2,222 S. Typhi blood-borne clinical isolates express the Vi polysaccharide (89), and human clinical trials clearly demonstrate that Vi+ wild-type S. Typhi challenge strains are more virulent than Vi– strains (6). However, in other studies, 36% (15/41) of S. Typhi clinical isolates were proven to carry defective rpoS alleles; interestingly, no inactivating mutations were identified in S. Typhimurium clinical isolates (86), suggesting different roles for rpoS in the physiology and resulting pathogenic potential of S. Typhimurium versus S. Typhi in humans. It has also been shown in vitro that RpoS– strains such as Ty2 express more Vi capsular antigen than wild-type RpoS+ strains under inducing osmolarity conditions (87, 88). The picture that emerges is that, under the high-osmolarity conditions of the intestinal lumen, rpoS is upregulated, which represses synthesis of Vi; this negative control is further strengthened by the osmotic repression of tviA, which is a positive activator of Vi synthesis. Since these conditions induce SPI-1 and the invasion of the small intestine, transepithelial migration results in a reduction of osmolarity to physiological levels, thereby repressing rpoS and activating tviA for induction of Vi synthesis (Fig. 2).
Figure 2.
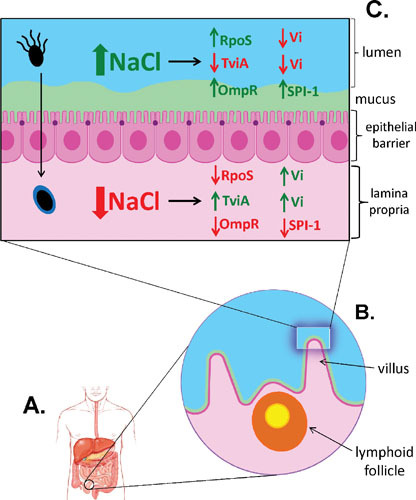
Induction of villus invasion by Salmonella. Invasion of Salmonella into deeper tissues of the human host occurs primarily in the small intestine (A) and is triggered by environmental signals including osmolarity. Villi of the small intestine possess a gradient of osmolarity, which is highest at the luminal surface of villi and decreases to physiological osmolarity in the lower crypts of the villus. Incoming luminal Salmonella sense the high osmolarity of villus surfaces that induce invasion (B). High osmolarity in the lumen upregulates rpoS, which in turn represses synthesis of Vi in S. Typhi to enhance invasion; this negative control is further strengthened by the osmotic repression of tviA, which is a positive activator of Vi synthesis (C). In addition, high osmolarity signals OmpR to upregulate Salmonella Pathogenicity Island 1 (SPI-1) to inject effector proteins into target eukaryotic cells, resulting in ruffling of the outer membrane and engulfment of invading bacteria. Transepithelial migration then reduces the osmolarity to physiological levels, repressing rpoS and activating tviA for induction of Vi synthesis. TviA is also a repressor of flagellar synthesis; therefore, S. Typhi is motile in the intestinal lumen when TviA is repressed (C, top left), but replaces flagella with the Vi capsule upon entry into host tissue (C, bottom left).
It is therefore plausible that although RpoS– strains are attenuated with respect to survival in environmentally stressful conditions such as intragastric acidity (controlling successful passage into the small intestine), vaccines derived from the RpoS– Ty2 parent might nonetheless be more immunogenic versus isogenic RpoS+ strains because of better intracellular survival within macrophages, allowing invading organisms to penetrate deeper into the host and reach immune inductive sites at levels high enough to elicit protective immunity. However, this hypothesis was recently shown to be incorrect in a phase 1 clinical trial in which volunteers were orally immunized with a single dose of 1010 CFUs of two isogenic vaccine strains derived from Ty2 (χ9639 and χ9640, Table 1) (61, 90), one carrying the original mutated rpoS allele and the other carrying a genetically engineered wild-type rpoS allele. Although not achieving statistical significance, a trend for higher serum IgA and IgA ASCs, specific for S. Typhi surface antigens, was observed for RpoS+ vaccines versus RpoS– strains (90). Interestingly, a third arm of this study was included in which volunteers were orally vaccinated with 1010 CFUs of an identically attenuated S. Typhi vaccine strain, derived from ISP1820 and designated χ9633, which carried the endogenous wild-type allele of RpoS. One subject from this group had a single positive blood culture 5 days following vaccination that spontaneously resolved without clinical intervention (90). These results seem to suggest an important role for RpoS in the early stages of vaccination with attenuated strains of S. Typhi, in which limited invasion of the host must be carefully balanced by additional chromosomal mutations in engineered vaccine strains to ensure both safety and immunogenicity. It is likely that the candidate vaccines included in this study may have proven more immunogenic if given in more than a single dose. Nonetheless, it was concluded from this trial that future vaccine development by this group would focus on RpoS+ Ty2 derivatives.
Engineered Vaccines with Deletions Blocking Systemic Infection: phoPQ
The licensed and experimental vaccines discussed thus far have relied on interference of essential metabolic pathways for construction of safe live oral vaccines. However, careful selection of virulence factors for inactivation can also yield safe vaccines that are highly immunogenic. PhoP/PhoQ is a pleiotropic two-component signal transduction system in which the environmental sensor PhoQ (activated either by low pH or low concentrations of extracellular Mg2+) triggers the transcriptional regulator PhoP to induce the synthesis of the SPI-2 locus controlling intracellular survival functions (91). In addition, PhoP/PhoQ regulates expression of other genes within unlinked pathogenicity islands such as SPI-11, in which the pagC gene is involved with intracellular survival of Salmonella within macrophages (92, 93). Therefore, inactivation of PhoP/PhoQ would be expected to interrupt the systemic phase of Salmonella infection. When this attenuating strategy was used as the sole means for construction of a single dose of live oral typhoid vaccine, again derived from the parent strain Ty2 and designated Ty800 (Table 1), the resulting candidate vaccine was shown in phase 1 clinical trials to be safe, well-tolerated, and immunogenic after single oral doses of up to 1010 CFUs (94). Humoral immunity was comparable to responses from a second cohort of the study receiving 4 doses of Ty21a, and robust dose-dependent S. Typhi LPS-specific IgA-secreting cell responses were detected in 10 of 11 subjects (94).
Given that the PhoP/PhoQ regulon is highly conserved between S. Typhi and S. Typhimurium, construction and initial clinical testing of Ty800 offered an intriguing opportunity to specifically investigate the immunogenicity in humans of two closely related serovars of Salmonella (i.e., S. Typhi versus S. Typhimurium) in which systemic dissemination of the organism was now blocked in both cases, and observed immunogenicity would therefore theoretically depend only on local immune induction sites. Two parallel phase 1 studies were performed in which S. Typhi Ty1033 and S. Typhimurium LH1160 candidate vaccine strains (Table 1), similarly deleted for PhoP/PhoQ, were administered to volunteers, with subjects vaccinated orally with S. Typhi Ty1033 receiving ≥1010 CFUs (95) and those vaccinated with S. Typhimurium LH1160 receiving an oral dose of 5 to 8 × 107 CFUs (96). As expected, no bacteremia was observed for either serovar, and both vaccines were highly immunogenic with no adverse reactions. However, despite the fact that subjects immunized with the attenuated S. Typhimurium vaccine received a 3 log unit lower dose of vaccine than those vaccinated with attenuated S. Typhi, 3 of 6 volunteers were durably colonized and excreted vaccine organisms for up to 10 days (96). Despite the absence of clinical symptoms, volunteers vaccinated with LH1160 were treated prophylactically with antibiotics to hasten complete elimination of this S. Typhimurium vaccine. In contrast, shedding of S. Typhi Ty1033 vaccine organisms was limited to no longer than 4 days in 9 of 9 volunteers, requiring no therapeutic intervention with antibiotics. Interestingly, volunteers more durably colonized with the S. Typhimurium vaccine mounted the most robust vaccine-specific humoral (anti-LPS serum IgA and IgG) and mucosal (anti-LPS ASCs) immune responses. Only 1 of 6 subjects immunized with S. Typhimurium LH1160 failed to mount significant mucosal or serological responses against any S. Typhimurium antigens, but this subject only excreted vaccine organisms for 2 days, suggesting that prolonged intestinal colonization can enhance immunogenicity (96). This surprising difference in fecal shedding of vaccine organisms seems to underscore the fact that S. Typhimurium is metabolically adapted to survival and growth within the human intestinal tract, whereas S. Typhi spends relatively little time in this environment because it quickly invades into deeper and more permissive tissues of the human host.
Engineered Vaccines with Deletions Blocking Systemic Infection: ssaV
A similar study design was pursued in a separate investigation, this time involving a direct comparison in a single phase 1 clinical trial of identically attenuated S. Typhi versus S. Typhimurium vaccines, in which a more narrowly focused deletion in ssaV targeted only the SPI-2 secretion apparatus to again interrupt systemic dissemination (Table 1). In this study, 18 volunteers were randomly assigned to two groups and orally immunized with a single escalating dose of either S. Typhi vaccine M01ZH09 or S. Typhimurium vaccine WT05, in doses ranging from 107 to 109 CFUs (97). Importantly, both vaccines also carried an additional metabolic deletion mutation in the aroC gene encoding chorismate synthase, rendering both strains auxotrophic for the biosynthesis of aromatic amino acids (98). As with the PhoP/PhoQ vaccines, no bacteremia was observed after vaccination with either serovar, and both vaccines were highly immunogenic with no adverse reactions. However, prolonged excretion of vaccine organisms for 12 to 23 days was again observed in 5 of 6 subjects receiving either 108 or 109 CFUs of the S. Typhimurium vaccine, regardless of the engineered auxotrophy for aromatic amino acids (97). Despite the limitation of these amino acids within the tissues of human hosts, enough of these nutrients are freely available within the lumen of the intestinal tract to support extended growth and excretion of S. Typhimurium vaccines. Therefore, this strategy for metabolic attenuation does not by itself ensure sufficient attenuation for S. Typhimurium vaccines.
However, the S. Typhi vaccine M01ZH09, which carries only these two deletion mutations in aroC and ssaV, has been shown to elicit excellent mucosal and humoral immunity. Next to Ty21a, M01ZH09 is the most extensively evaluated typhoid vaccine to date, having successfully completed four phase 1 clinical trials and two phase 2 clinical trials, involving a total of 356 orally immunized subjects from North American, Europe, and endemic Asian populations (97, 99, 100, 101, 102). The vaccine given orally as a single dose of up to 1010 CFUs is safe, causes no bacteremia, and engenders both mucosal and serum antibody responses comparable to those observed in individuals immunized with 3 doses of Ty21a, with aggregate S. Typhi LPS-specific seroconversions between 50 and 92% for M01ZH09 versus 50 to 64% for Ty21a and LPS-specific ASCs in 90 to 100% of vaccinees receiving M01ZH09 versus 63 to 96% for Ty21a (102).
Engineered Vaccines with Deletions Blocking Systemic Infection: htrA
Engineered auxotrophic dependence on supplementation with aromatic amino acids for growth was also exploited in another successful live oral typhoid vaccine that underwent three iterations of refinements, each tested in phase1 or phase 2 clinical trials to guide further development (Table 1). CVD 908 was first engineered from wild-type Ty2 to carry deletion mutations in aroC (encoding chorismate synthase) and aroD (encoding 3-dehydroxoquinate dehydrogenase), two independent nonreverting mutations in the essential aromatic amino acid biosynthesis pathway (11, 98). Following a single oral dose of CVD 908, the majority of vaccine recipients responded with LPS-specific serum IgG (83%) and all of them with LPS-specific IgA ASCs (103). In the presence of S. Typhi flagella and killed organisms, peripheral blood mononuclear cells from vaccinated individuals proliferated and produced IFN-γ and IL-6 indicative of a Th1 type/proinflammatory response (104). However, despite the presence of two distinct deletion mutations in aroC and aroD (plus the rpoS mutation from Ty2), vaccine organisms were still able to cross the intestinal epithelial barrier and were detected in the blood of vaccinees receiving oral doses as low as 5 × 107 CFUs (105). Therefore, it was deemed prudent to engineer additional chromosomal deletions to prevent this self-limiting bacteremia, even though no symptoms were documented in any of these volunteers and no therapeutic intervention was required.
The resulting candidate vaccine, CVD 908-htrA, contained a new deletion in htrA encoding a heat shock protease (105); previous data obtained in vitro with S. Typhimurium suggested that htrA enhanced survival within macrophages (106), and thus deletion of this gene might limit systemic spread of the vaccine. Indeed, phase 1 clinical trials of CVD 908-htrA conclusively demonstrated that the desired balance between reactogenicity and immunogenicity had been achieved. No vaccine organisms were detected in the blood, and limited shedding of vaccine organisms for less than 3 days was seen in volunteers orally vaccinated with up to 5 × 109 CFUs of freshly harvested organisms. In addition, excellent mucosal, humoral, and cellular immune responses were observed. One hundred percent (15/15) of volunteers vaccinated with 5 × 108 or 5 × 109 CFUs seroconverted to serum IgG against S. Typhi LPS and 73% (11/15) against flagella. IgA anti-LPS ASCs were detected in all vaccinees receiving 5 × 108 or 5 × 109 CFUs as well, and lymphoproliferative responses against flagella or inactivated whole-cell antigen were detected in 69% (9/13) and 77% (10/13) of subjects, respectively (105). Interestingly, when lyophilized vaccine was further tested in phase 2 clinical trials, mucosal ASC responses were maintained (94 to 100% LPS-specific ASCs and 50 to 82% flagella-specific ASCs) at doses of 4.5 × 108 CFUs (the highest dose given orally), but serum antibody responses declined slightly with only 49% of volunteers mounting anti-LPS IgG responses and 41% generating antiflagella responses; 63% of vaccinees had lymphoproliferative responses to flagella, and 44% responded to particulate inactivated whole cell (107).
CVD 908-htrA was then further genetically modified to constitutively express Vi polysaccharide (108). This novel approach was based on a hypothesis proposed by Levine et al., who observed that Ty21a live oral vaccine and a subunit parenteral vaccine composed of purified Vi polysaccharide both confer substantial protection against typhoid disease after multiple doses, despite the fact that Ty21a does not synthesize Vi polysaccharide and that the purified Vi vaccine is a monovalent vaccine lacking other surface antigens from S. Typhi (108). This suggested that immunity to typhoid disease may be manifested by at least two distinct immune mechanisms, one involving targeted antibody responses against Vi and the other involving more broad humoral and cell-mediated immunity against S. Typhi surface antigens other than Vi. Given that all genetically engineered live oral vaccines tested in clinical trials to date have elicited very poor serum immunity to Vi, Levine et al. proposed that perhaps a more broadly immunogenic vaccine could be developed, eliciting immunity against surface antigens including Vi, by further engineering constitutive expression of Vi in CVD 908-htrA.
To accomplish this, the powerful constitutive promoter Ptac was used to replace the highly regulated and osmotically controlled PtviA promoter controlling expression of the Vi operon viaB encoded within the SPI-7 pathogenicity island. It was then confirmed in vitro that excellent expression of Vi antigen in the resulting vaccine candidate CVD 909 (Table 1) was now independent of osmotic induction. Interestingly, it was also demonstrated that CVD 909 was less invasive for Henle 407 cells, a human embryonic intestinal epithelial cell line, than the parent CVD 908-htrA at low osmolarity (108); this observation agrees with previously published in vitro data in which induced high-level expression of Vi polysaccharide by osmotic induction of wild-type S. Typhi Ty2 significantly reduced invasion of intestinal epithelial cells (109).
CVD 909 proved to be safe and immunogenic in phase 1 clinical trials despite overexpression of the Vi polysaccharide virulence factor (77, 110). However, serum antibody responses against S. Typhi LPS and flagella were lower in comparison with the parent vaccine CVD 908-htrA. Only 2 of 6 (33%) volunteers orally vaccinated with a single dose of 2.5 × 109 freshly harvested vaccine organisms mounted anti-LPS IgG serum antibody responses (110) versus 8 of 8 vaccinated with a 10-fold lower dose of 5 × 108 CFUs of CVD 908-htrA (105); similarly, only 1 of 6 CVD 909 vaccinated subjects had antiflagellar serum IgG antibody responses versus 6 of 8 for CVD 908-htrA. This reduction in surface antigen-specific antibody responses is consistent with strong expression of the regulator TviA, which downregulates flagellar expression while upregulating synthesis of Vi capsule to mask surface LPS (31, 38). Curiously, despite overexpression of the capsular polysaccharide in CVD 909, Vi-specific serum immune responses were not observed (110).
In a subsequent clinical trial, volunteers primed with a single oral dose of 5 × 109 CFUs of CVD 909 and boosted intramuscularly with 25 μg of the licensed Vi polysaccharide vaccine Typhim Vi did not have Vi-specific IgM, IgG, or IgA antibodies significantly elevated above volunteers who received a placebo prime and boost with Typhim Vi (77). Sixty-four percent of the CVD909 recipients developed anti-LPS serum IgG and IgA while only 18 to 27% developed antiflagella serum antibodies (77). Importantly, these antibodies exhibited functional opsonophagocytic activity against wild-type S. Typhi (75). Over half (55%) of CVD909 primed individuals developed anti-Vi IgA B memory cells, compared with 12.5% in the placebo-primed group. This vaccine strain also resulted in the production of antiflagellar IgA B memory cells that remained for at least 1 year postvaccination (77). It remains puzzling that no Vi-specific antibody responses were observed in subjects primed with a Vi overexpressing live vaccine prior to boosting with a purified Vi subunit vaccine. The fact that robust Vi-specific antibody responses are observed in asymptomatic human carriers chronically colonized with S. Typhi underscores the fact that much still remains to be elucidated regarding induction of protective immunity against Salmonella and how to exploit this information in the rational design of efficacious live oral vaccines.
CONCLUSIONS AND FUTURE DIRECTIONS
Herein, we have summarized the pathogenesis of human Salmonella infections, contrasting S. Typhi and S. Typhimurium with regard to niches colonized and immune responses elicited by wild-type organisms. Understanding the natural progression of disease provides an important context in which attenuated live vaccines can be rationally designed and developed. With this in mind, we have reviewed a series of attenuated live vaccines that have been tested in clinical trials, and demonstrated to be both safe and highly immunogenic in the case of S. Typhi typhoid vaccines. However, we have also pointed out that correlates of protection against enteric fever have yet to be adequately defined. Therefore, at this point, immune responses elicited by candidate vaccines remain only suggestive of protective efficacy in the absence of challenge studies conducted with vaccinated volunteers. However, it is encouraging that a human typhoid challenge model has now been reestablished (7), offering the opportunity to compare the various attenuation strategies for currently available S. Typhi vaccine candidates, and their ability to induce immune responses that can protect against disease. Given the paucity of data relevant to mechanisms involved in disease clearance, it is entirely possible that some vaccines clinically proven to be safe and highly immunogenic will nonetheless fail to offer significant protection when administered orally as a single dose. Such future challenge studies may specifically inform the development of more efficacious vaccine schedules involving two or more doses, without having to reengineer further vaccine candidates, to elicit durable protective immunity.
Although there are now multiple examples of attenuated oral S. Typhi vaccines that have been clinically demonstrated to be safe and immunogenic, all S. Typhimurium vaccine candidates tested to date have displayed unacceptable safety profiles, with prolonged colonization of human volunteers leading to unacceptable shedding of viable vaccine organisms over several weeks (96, 97). It became evident from these important studies that strategies proven successful for attenuation of S. Typhi do not necessarily guarantee success when applied to S. Typhimurium. This is undoubtedly related, at least in part, to the differences in metabolic niches exploited by these two serovars, with S. Typhimurium typically thriving extracellularly in the gastrointestinal lumen, while S. Typhi proliferates intracellularly in deeper tissues of the host. However, the pathogenicity of S. Typhimurium has recently been changing, with more invasive and multidrug-resistant strains being increasingly isolated from the blood of malnourished children and immunocompromised adults living in sub-Saharan Africa (111, 112, 113). These newly emerging strains have developed an improved ability to replicate within human macrophages while downregulating production of flagella to reduce innate immune recognition (114). With this unsettling rise in unconventional invasive nontyphoidal Salmonella (iNTS) strains, it may therefore be appropriate to revisit available attenuated S. Typhimurium vaccines with the goal of improving the safety of existing vaccine candidates. We recognize, however, that, while development of improved second-generation S. Typhimurium live vaccines might prove to be safe and highly immunogenic in developed countries, they could nonetheless prove to be far less immunogenic in endemic regions of developing countries where malnourished children and immunocompromised adults suffering from coinfection with malaria parasites or HIV would be severely compromised for humoral and cell-mediated immunity (112). Notwithstanding this caveat, it would be intriguing to target the intestinal proliferation of S. Typhimurium to reduce or eliminate the unacceptable shedding of vaccine organisms. In theory, this might be accomplished by inactivating the ability to exploit tetrathionate as an alternate electron acceptor for anaerobic respiration, thereby eliminating the metabolic advantage of S. Typhimurium over competing flora to enhance clearance and reduce unacceptably high levels of shedding (27, 28). Another possible approach could involve introduction of proven attenuation strategies into an iNTS isolate of S. Typhimurium, with further deletion of a novel invasion gene called st313-td, recently reported to enhance systemic invasion in experimental animal models of infection (115). We conclude that the attenuation strategies we have summarized offer important insights into further development of attenuated S. Typhimurium vaccines, as well as for other serovars for which vaccines are currently unavailable.
ACKNOWLEDGMENTS
This work was supported by several grants from the National Institutes of Health, National Institute of Allergy and Infectious Disease; S.M.T. was supported by NIH, NIAID U19 AI109776-01 (Project 4); M.F.P. was supported by NIH, NIAID R01 AI089519; and J.E.G. was supported by NIH, NIAID R01 AI095309. The graphic elements for Figures 1 and 2 were provided by Timothy Phelps, Department of Art as Applied to Medicine, Johns Hopkins University.
Conflicts of interest: The authors declare no conflicts.
Contributor Information
James E. Galen, Center for Vaccine Development, Institute for Global Health, University of Maryland School of Medicine, Baltimore, MD 21201
Amanda D. Buskirk, Center for Vaccine Development, Institute for Global Health, University of Maryland School of Medicine, Baltimore MD 21201
Sharon M. Tennant, Center for Vaccine Development, Institute for Global Health, University of Maryland School of Medicine, Baltimore MD 21201
Marcela F. Pasetti, Center for Vaccine Development, Institute for Global Health, University of Maryland School of Medicine, Baltimore MD 21201
REFERENCES
- 1.Kirk MD, Pires SM, Black RE, Caipo M, Crump JA, Devleesschauwer B, Döpfer D, Fazil A, Fischer-Walker CL, Hald T, Hall AJ, Keddy KH, Lake RJ, Lanata CF, Torgerson PR, Havelaar AH, Angulo FJ. 2015. World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLoS Med 12:e1001921. 10.1371/journal.pmed.1001921. [PubMed] 10.1371/journal.pmed.1001921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirk MD, Pires SM, Black RE, Caipo M, Crump JA, Devleesschauwer B, Döpfer D, Fazil A, Fischer-Walker CL, Hald T, Hall AJ, Keddy KH, Lake RJ, Lanata CF, Torgerson PR, Havelaar AH, Angulo FJ. 2015. Correction: World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLoS Med 12:e1001940. 10.1371/journal.pmed.1001940 [PubMed] 10.1371/journal.pmed.1001940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grimont PAD, Weill F-X. 2007. Antigenic formulae of the Salmonella serovars. Salmonella WCCfRaRo. Institute Pasteur, Paris, France. [Google Scholar]
- 4.Tennant SM, Levine MM. 2015. Live attenuated vaccines for invasive Salmonella infections. Vaccine 33(Suppl 3):C36–C41. [PubMed] 10.1016/j.vaccine.2015.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verdu E, Viani F, Armstrong D, Fraser R, Siegrist HH, Pignatelli B, Idström JP, Cederberg C, Blum AL, Fried M. 1994. Effect of omeprazole on intragastric bacterial counts, nitrates, nitrites, and N-nitroso compounds. Gut 35:455–460. [PubMed] 10.1136/gut.35.4.455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hornick RB, Greisman SE, Woodward TE, DuPont HL, Dawkins AT, Snyder MJ. 1970. Typhoid fever: pathogenesis and immunologic control. N Engl J Med 283:686–691. [PubMed] 10.1056/NEJM197009242831306 [DOI] [PubMed] [Google Scholar]
- 7.Waddington CS, Darton TC, Jones C, Haworth K, Peters A, John T, Thompson BA, Kerridge SA, Kingsley RA, Zhou L, Holt KE, Yu LM, Lockhart S, Farrar JJ, Sztein MB, Dougan G, Angus B, Levine MM, Pollard AJ. 2014. An outpatient, ambulant-design, controlled human infection model using escalating doses of Salmonella Typhi challenge delivered in sodium bicarbonate solution. Clin Infect Dis 58:1230–1240. [PubMed] 10.1093/cid/ciu078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Audia JP, Webb CC, Foster JW. 2001. Breaking through the acid barrier: an orchestrated response to proton stress by enteric bacteria. Int J Med Microbiol 291:97–106. [PubMed] 10.1078/1438-4221-00106 [DOI] [PubMed] [Google Scholar]
- 9.Garmendia J, Beuzón CR, Ruiz-Albert J, Holden DW. 2003. The roles of SsrA-SsrB and OmpR-EnvZ in the regulation of genes encoding the Salmonella typhimurium SPI-2 type III secretion system. Microbiology 149:2385–2396. [PubMed] 10.1099/mic.0.26397-0 [DOI] [PubMed] [Google Scholar]
- 10.Robbe-Saule V, Norel F. 1999. The rpoS mutant allele of Salmonella typhi Ty2 is identical to that of the live typhoid vaccine Ty21a. FEMS Microbiol Lett 170:141–143. [PubMed] 10.1111/j.1574-6968.1999.tb13366.x [DOI] [PubMed] [Google Scholar]
- 11.Tacket CO, Hone DM, Curtiss R III, Kelly SM, Losonsky G, Guers L, Harris AM, Edelman R, Levine MM. 1992. Comparison of the safety and immunogenicity of delta aroC delta aroD and delta cya delta crp Salmonella typhi strains in adult volunteers. Infect Immun 60:536–541. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hone DM, Harris AM, Levine MM. 1994. Adaptive acid tolerance response by Salmonella typhi and candidate live oral typhoid vaccine strains. Vaccine 12:895–898. [PubMed] 10.1016/0264-410X(94)90031-0 [DOI] [PubMed] [Google Scholar]
- 13.Tsolis RM, Young GM, Solnick JV, Bäumler AJ. 2008. From bench to bedside: stealth of enteroinvasive pathogens. Nat Rev Microbiol 6:883–892. [PubMed] 10.1038/nrmicro2012 [DOI] [PubMed] [Google Scholar]
- 14.Keestra-Gounder AM, Tsolis RM, Bäumler AJ. 2015. Now you see me, now you don’t: the interaction of Salmonella with innate immune receptors. Nat Rev Microbiol 13:206–216. [PubMed] 10.1038/nrmicro3428 [DOI] [PubMed] [Google Scholar]
- 15.Jones BD, Ghori N, Falkow S. 1994. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer’s patches. J Exp Med 180:15–23. [PubMed] 10.1084/jem.180.1.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sprinz H, Gangarosa EJ, Williams M, Hornick RB, Woodward TE. 1966. Histopathology of the upper small intestines in typhoid fever. Biopsy study of experimental disease in man. Am J Dig Dis 11:615–624. [PubMed] 10.1007/BF02233509 [DOI] [PubMed] [Google Scholar]
- 17.Cornes JS. 1965. Number, size, and distribution of Peyer’s patches in the human small intestine: Part I The development of Peyer’s patches. Gut 6:225–229. [PubMed] 10.1136/gut.6.3.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cornes JS. 1965. Peyer’s patches in the human gut. Proc R Soc Med 58:716. [PubMed] 10.1177/003591576505800930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulz O, Pabst O. 2013. Antigen sampling in the small intestine. Trends Immunol 34:155–161. [PubMed] 10.1016/j.it.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 20.Hallbäck DA, Hultén L, Jodal M, Lindhagen J, Lundgren O. 1978. Evidence for the existence of a countercurrent exchanger in the small intestine in man. Gastroenterology 74:683–690. [PubMed] [PubMed] [Google Scholar]
- 21.Hallbäck DA, Jodal M, Mannischeff M, Lundgren O. 1991. Tissue osmolality in intestinal villi of four mammals in vivo and in vitro. Acta Physiol Scand 143:271–277. [PubMed] 10.1111/j.1748-1716.1991.tb09232.x [DOI] [PubMed] [Google Scholar]
- 22.Zhou D, Galán J. 2001. Salmonella entry into host cells: the work in concert of type III secreted effector proteins. Microbes Infect 3:1293–1298. [PubMed] 10.1016/S1286-4579(01)01489-7 [DOI] [PubMed] [Google Scholar]
- 23.Kuhle V, Hensel M. 2004. Cellular microbiology of intracellular Salmonella enterica: functions of the type III secretion system encoded by Salmonella pathogenicity island 2. Cell Mol Life Sci 61:2812–2826. [PubMed] 10.1007/s00018-004-4248-z [DOI] [PubMed] [Google Scholar]
- 24.Winter SE, Winter MG, Godinez I, Yang HJ, Rüssmann H, Andrews-Polymenis HL, Bäumler AJ. 2010. A rapid change in virulence gene expression during the transition from the intestinal lumen into tissue promotes systemic dissemination of Salmonella. PLoS Pathog 6:e1001060. 10.1371/journal.ppat.1001060. [PubMed] 10.1371/journal.ppat.1001060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dagan T, Blekhman R, Graur D. 2006. The “domino theory” of gene death: gradual and mass gene extinction events in three lineages of obligate symbiotic bacterial pathogens. Mol Biol Evol 23:310–316. [PubMed] 10.1093/molbev/msj036 [DOI] [PubMed] [Google Scholar]
- 26.Parkhill J, Dougan G, James KD, Thomson NR, Pickard D, Wain J, Churcher C, Mungall KL, Bentley SD, Holden MTG, Sebaihia M, Baker S, Basham D, Brooks K, Chillingworth T, Connerton P, Cronin A, Davis P, Davies RM, Dowd L, White N, Farrar J, Feltwell T, Hamlin N, Haque A, Hien TT, Holroyd S, Jagels K, Krogh A, Larsen TS, Leather S, Moule S, O’Gaora P, Parry C, Quail M, Rutherford K, Simmonds M, Skelton J, Stevens K, Whitehead S, Barrell BG. 2001. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature 413:848–852. [PubMed] 10.1038/35101607 [DOI] [PubMed] [Google Scholar]
- 27.Winter SE, Thiennimitr P, Winter MG, Butler BP, Huseby DL, Crawford RW, Russell JM, Bevins CL, Adams LG, Tsolis RM, Roth JR, Bäumler AJ. 2010. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature 467:426–429. [PubMed] 10.1038/nature09415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivera-Chávez F, Bäumler AJ. 2015. The pyromaniac inside you: Salmonella metabolism in the host gut. Annu Rev Microbiol 69:31–48. [PubMed] 10.1146/annurev-micro-091014-104108 [DOI] [PubMed] [Google Scholar]
- 29.Nuccio SP, Bäumler AJ. 2014. Comparative analysis of Salmonella genomes identifies a metabolic network for escalating growth in the inflamed gut. MBio 5:e00929-14. 10.1128/mBio.00929-14. [PubMed] 10.1128/mBio.00929-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seth-Smith HM. 2008. SPI-7: Salmonella’s Vi-encoding pathogenicity island. J Infect Dev Ctries 2:267–271. [PubMed] 10.3855/jidc.220 [DOI] [PubMed] [Google Scholar]
- 31.Winter SE, Winter MG, Thiennimitr P, Gerriets VA, Nuccio SP, Rüssmann H, Bäumler AJ. 2009. The TviA auxiliary protein renders the Salmonella enterica serotype Typhi RcsB regulon responsive to changes in osmolarity. Mol Microbiol 74:175–193. [PubMed] 10.1111/j.1365-2958.2009.06859.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tran QT, Gomez G, Khare S, Lawhon SD, Raffatellu M, Bäumler AJ, Ajithdoss D, Dhavala S, Adams LG. 2010. The Salmonella enterica serotype Typhi Vi capsular antigen is expressed after the bacterium enters the ileal mucosa. Infect Immun 78:527–535. [PubMed] 10.1128/IAI.00972-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson RP, Raffatellu M, Chessa D, Winter SE, Tükel C, Bäumler AJ. 2008. The Vi-capsule prevents Toll-like receptor 4 recognition of Salmonella. Cell Microbiol 10:876–890. [PubMed] 10.1111/j.1462-5822.2007.01090.x [DOI] [PubMed] [Google Scholar]
- 34.Crawford RW, Wangdi T, Spees AM, Xavier MN, Tsolis RM, Bäumler AJ. 2013. Loss of very-long O-antigen chains optimizes capsule-mediated immune evasion by Salmonella enterica serovar Typhi. MBio 4:e00232-13. 10.1128/mBio.00232-13. [PubMed] 10.1128/mBio.00232-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wangdi T, Lee CY, Spees AM, Yu C, Kingsbury DD, Winter SE, Hastey CJ, Wilson RP, Heinrich V, Bäumler AJ. 2014. The Vi capsular polysaccharide enables Salmonella enterica serovar typhi to evade microbe-guided neutrophil chemotaxis. PLoS Pathog 10:e1004306. 10.1371/journal.ppat.1004306. [PubMed] 10.1371/journal.ppat.1004306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. 2001. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol 167:1882–1885. [PubMed] 10.4049/jimmunol.167.4.1882 [DOI] [PubMed] [Google Scholar]
- 37.Abreu MT. 2010. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol 10:131–144. [PubMed] 10.1038/nri2707 [DOI] [PubMed] [Google Scholar]
- 38.Winter SE, Winter MG, Atluri V, Poon V, Romão EL, Tsolis RM, Bäumler AJ. 2015. The flagellar regulator TviA reduces pyroptosis by Salmonella enterica serovar Typhi. Infect Immun 83:1546–1555. [PubMed] 10.1128/IAI.02803-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.WHO. 2003. Background Document: The Diagnosis, Treatment, and Prevention of Typhoid Fever. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 40.Schwan WR, Huang XZ, Hu L, Kopecko DJ. 2000. Differential bacterial survival, replication, and apoptosis-inducing ability of Salmonella serovars within human and murine macrophages. Infect Immun 68:1005–1013. [PubMed] 10.1128/IAI.68.3.1005-1013.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Darton TC, Jones C, Waddington CS, Dougan G, Sztein M, Levine M, Angus B, Farrar J, Lockhart S, Crook D, Pollard AJ, Zhou L. 2012. Demonstration of primary and asymptomatic DNAaemia in participants challenged with Salmonella Typhi (Quailes strain) during the development of a human model of typhoid infection. Int J Infect Dis 16S:e215. 10.1016/j.ijid.2012.05.807. 10.1016/j.ijid.2012.05.807 [DOI] [Google Scholar]
- 42.Rubin FA, McWhirter PD, Burr D, Punjabi NH, Lane E, Kumala S, Sudarmono P, Pulungsih SP, Lesmana M, Tjaniadi P, Sukri N, Hoffman SL. 1990. Rapid diagnosis of typhoid fever through identification of Salmonella typhi within 18 hours of specimen acquisition by culture of the mononuclear cell-platelet fraction of blood. J Clin Microbiol 28:825–827. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wain J, Diep TS, Ho VA, Walsh AM, Nguyen TT, Parry CM, White NJ. 1998. Quantitation of bacteria in blood of typhoid fever patients and relationship between counts and clinical features, transmissibility, and antibiotic resistance. J Clin Microbiol 36:1683–1687. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wain J, Pham VB, Ha V, Nguyen NM, To SD, Walsh AL, Parry CM, Hasserjian RP, HoHo VA, Tran TH, Farrar J, White NJ, Day NP. 2001. Quantitation of bacteria in bone marrow from patients with typhoid fever: relationship between counts and clinical features. J Clin Microbiol 39:1571–1576. [PubMed] 10.1128/JCM.39.4.1571-1576.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Everest P, Wain J, Roberts M, Rook G, Dougan G. 2001. The molecular mechanisms of severe typhoid fever. Trends Microbiol 9:316–320. [PubMed] 10.1016/S0966-842X(01)02067-4 [DOI] [PubMed] [Google Scholar]
- 46.Dougan G, Baker S. 2014. Salmonella enterica serovar Typhi and the pathogenesis of typhoid fever. Annu Rev Microbiol 68:317–336. [PubMed] 10.1146/annurev-micro-091313-103739 [DOI] [PubMed] [Google Scholar]
- 47.Ramsey GH. 1934. What are the essentials of typhoid fever control today? Am J Public Health Nations Health 24:355–362. [PubMed] 10.2105/AJPH.24.4.355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson GW, Hamblen AD, Smith HM. 1936. Typhoid carriers -a study of their disease producing potentialities over a series of years as indicated by a study of cases. Am J Public Health Nations Health 26:396–405. [PubMed] 10.2105/AJPH.26.4.396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Merselis JG Jr, Kaye D, Connolly CS, Hook EW. 1964. Quantitative bacteriology of the typhoid carrier state. Am J Trop Med Hyg 13:425–429. [PubMed] [DOI] [PubMed] [Google Scholar]
- 50.Marshall JM, Flechtner AD, La Perle KM, Gunn JS. 2014. Visualization of extracellular matrix components within sectioned Salmonella biofilms on the surface of human gallstones. PLoS One 9:e89243. 10.1371/journal.pone.0089243. [PubMed] 10.1371/journal.pone.0089243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Knodler LA, Vallance BA, Celli J, Winfree S, Hansen B, Montero M, Steele-Mortimer O. 2010. Dissemination of invasive Salmonella via bacterial-induced extrusion of mucosal epithelia. Proc Natl Acad Sci USA 107:17733–17738. [PubMed] 10.1073/pnas.1006098107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gonzalez-Escobedo G, Marshall JM, Gunn JS. 2011. Chronic and acute infection of the gall bladder by Salmonella Typhi: understanding the carrier state. Nat Rev Microbiol 9:9–14. [PubMed] 10.1038/nrmicro2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Felix A. 1938. Detection of chronic typhoid carriers by agglutination tests. Lancet Infect Dis ii:738–741. 10.1016/s0140-6736(00)40980-3 [DOI] [Google Scholar]
- 54.Lanata CF, Levine MM, Ristori C, Black RE, Jimenez L, Salcedo M, Garcia J, Sotomayor V. 1983. Vi serology in detection of chronic Salmonella typhi carriers in an endemic area. Lancet 2:441–443. [PubMed] 10.1016/S0140-6736(83)90401-4 [DOI] [PubMed] [Google Scholar]
- 55.van Basten JP, Stockenbrügger R. 1994. Typhoid perforation. A review of the literature since 1960. Trop Geogr Med 46:336–339. [PubMed] [PubMed] [Google Scholar]
- 56.Mogasale V, Desai SN, Mogasale VV, Park JK, Ochiai RL, Wierzba TF. 2014. Case fatality rate and length of hospital stay among patients with typhoid intestinal perforation in developing countries: a systematic literature review. PLoS One 9:e93784. 10.1371/journal.pone.0093784. [PubMed] 10.1371/journal.pone.0093784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Archampong EQ. 1969. Operative treatment of typhoid perforation of the bowel. BMJ 3:273–276. [PubMed] 10.1136/bmj.3.5665.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sheikh A, Bhuiyan MS, Khanam F, Chowdhury F, Saha A, Ahmed D, Jamil KM, LaRocque RC, Harris JB, Ahmad MM, Charles R, Brooks WA, Calderwood SB, Cravioto A, Ryan ET, Qadri F. 2009. Salmonella enterica serovar Typhi-specific immunoglobulin A antibody responses in plasma and antibody in lymphocyte supernatant specimens in Bangladeshi patients with suspected typhoid fever. Clin Vaccine Immunol 16:1587–1594. [PubMed] 10.1128/CVI.00311-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khanam F, Sayeed MA, Choudhury FK, Sheikh A, Ahmed D, Goswami D, Hossain ML, Brooks A, Calderwood SB, Charles RC, Cravioto A, Ryan ET, Qadri F. 2015. Typhoid fever in young children in Bangladesh: clinical findings, antibiotic susceptibility pattern and immune responses. PLoS Negl Trop Dis 9:e0003619. 10.1371/journal.pntd.0003619. [PubMed] 10.1371/journal.pntd.0003619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sheikh A, Khanam F, Sayeed MA, Rahman T, Pacek M, Hu Y, Rollins A, Bhuiyan MS, Rollins S, Kalsy A, Arifuzzaman M, Leung DT, Sarracino DA, Krastins B, Charles RC, Larocque RC, Cravioto A, Calderwood SB, Brooks WA, Harris JB, Labaer J, Qadri F, Ryan ET. 2011. Interferon-γ and proliferation responses to Salmonella enterica Serotype Typhi proteins in patients with S. Typhi Bacteremia in Dhaka, Bangladesh. PLoS Negl Trop Dis 5:e1193. 10.1371/journal.pntd.0001193. 10.1371/journal.pntd.0001193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shi H, Santander J, Brenneman KE, Wanda SY, Wang S, Senechal P, Sun W, Roland KL, Curtiss R. 2010. Live recombinant Salmonella Typhi vaccines constructed to investigate the role of rpoS in eliciting immunity to a heterologous antigen. PLoS One 5:e11142. 10.1371/journal.pone.0011142. [PubMed] 10.1371/journal.pone.0011142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bhuiyan S, Sayeed A, Khanam F, Leung DT, Rahman Bhuiyan T, Sheikh A, Salma U, LaRocque RC, Harris JB, Pacek M, Calderwood SB, LaBaer J, Ryan ET, Qadri F, Charles RC. 2014. Cellular and cytokine responses to Salmonella enterica serotype Typhi proteins in patients with typhoid fever in Bangladesh. Am J Trop Med Hyg 90:1024–1030. [PubMed] 10.4269/ajtmh.13-0261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Wit J, Souwer Y, Jorritsma T, Klaasse Bos H, ten Brinke A, Neefjes J, van Ham SM. 2010. Antigen-specific B cells reactivate an effective cytotoxic T cell response against phagocytosed Salmonella through cross-presentation. PLoS One 5:e13016. 10.1371/journal.pone.0013016. [PubMed] 10.1371/journal.pone.0013016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McArthur MA, Sztein MB. 2012. Heterogeneity of multifunctional IL-17A producing S. Typhi-specific CD8+ T cells in volunteers following Ty21a typhoid immunization. PLoS One 7:e38408. 10.1371/journal.pone.0038408. [PubMed] 10.1371/journal.pone.0038408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McArthur MA, Fresnay S, Magder LS, Darton TC, Jones C, Waddington CS, Blohmke CJ, Dougan G, Angus B, Levine MM, Pollard AJ, Sztein MB. 2015. Activation of Salmonella Typhi-specific regulatory T cells in typhoid disease in a wild-type S. Typhi challenge model. PLoS Pathog 11:e1004914. 10.1371/journal.ppat.1004914. 10.1371/journal.ppat.1004914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ravindran R, McSorley SJ. 2005. Tracking the dynamics of T-cell activation in response to Salmonella infection. Immunology 114:450–458. [PubMed] 10.1111/j.1365-2567.2005.02140.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Germanier R, Füer E. 1975. Isolation and characterization of Gal E mutant Ty 21a of Salmonella typhi: a candidate strain for a live, oral typhoid vaccine. J Infect Dis 131:553–558. [PubMed] 10.1093/infdis/131.5.553 [DOI] [PubMed] [Google Scholar]
- 68.Schendel PF, Michaeli I. 1984. A model for the mechanism of alkylation mutagenesis. Mutat Res 125:1–14. [PubMed] 10.1016/0027-5107(84)90026-5 [DOI] [PubMed] [Google Scholar]
- 69.Kopecko DJ, Sieber H, Ures JA, Fürer A, Schlup J, Knof U, Collioud A, Xu D, Colburn K, Dietrich G. 2009. Genetic stability of vaccine strain Salmonella Typhi Ty21a over 25 years. Int J Med Microbiol 299:233–246. [PubMed] 10.1016/j.ijmm.2008.09.003 [DOI] [PubMed] [Google Scholar]
- 70.Gilman RH, Hornick RB, Woodard WE, DuPont HL, Snyder MJ, Levine MM, Libonati JP. 1977. Evaluation of a UDP-glucose-4-epimeraseless mutant of Salmonella typhi as a liver oral vaccine. J Infect Dis 136:717–723. [PubMed] 10.1093/infdis/136.6.717 [DOI] [PubMed] [Google Scholar]
- 71.Levine MM, Ferreccio C, Abrego P, Martin OS, Ortiz E, Cryz S. 1999. Duration of efficacy of Ty21a, attenuated Salmonella typhi live oral vaccine. Vaccine 17(Suppl 2):S22–S27. [PubMed] 10.1016/S0264-410X(99)00231-5 [DOI] [PubMed] [Google Scholar]
- 72.Marmion DE, Naylor GR, Stewart IO. 1953. Second attacks of typhoid fever. J Hyg (Lond) 51:260–267. [PubMed] 10.1017/S0022172400015680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dupont HL, Hornick RB, Snyder MJ, Dawkins AT, Heiner GG, Woodward TE. 1971. Studies of immunity in typhoid fever. Protection induced by killed oral antigens or by primary infection. Bull World Health Organ 44:667–672. [PubMed] [PMC free article] [PubMed] [Google Scholar]
- 74.Otczyk DC, Cripps AW. 2010. Mucosal immunization: a realistic alternative. Hum Vaccin 6:978–1006. [PubMed] 10.4161/hv.6.12.13142 [DOI] [PubMed] [Google Scholar]
- 75.Wahid R, Zafar SJ, McArthur MA, Pasetti MF, Levine MM, Sztein MB. 2014. Live oral Salmonella enterica serovar Typhi vaccines Ty21a and CVD 909 induce opsonophagocytic functional antibodies in humans that cross-react with S. Paratyphi A and S. Paratyphi B. Clin Vaccine Immunol 21:427–434. [PubMed] 10.1128/CVI.00786-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kantele A, Kantele JM, Savilahti E, Westerholm M, Arvilommi H, Lazarovits A, Butcher EC, Mäkelä PH. 1997. Homing potentials of circulating lymphocytes in humans depend on the site of activation: oral, but not parenteral, typhoid vaccination induces circulating antibody-secreting cells that all bear homing receptors directing them to the gut. J Immunol 158:574–579. [PubMed] [PubMed] [Google Scholar]
- 77.Wahid R, Pasetti MF, Maciel M Jr, Simon JK, Tacket CO, Levine MM, Sztein MB. 2011. Oral priming with Salmonella Typhi vaccine strain CVD 909 followed by parenteral boost with the S. Typhi Vi capsular polysaccharide vaccine induces CD27+IgD-S. Typhi-specific IgA and IgG B memory cells in humans. Clin Immunol 138:187–200. [PubMed] 10.1016/j.clim.2010.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tagliabue A, Villa L, De Magistris MT, Romano M, Silvestri S, Boraschi D, Nencioni L. 1986. IgA-driven T cell-mediated anti-bacterial immunity in man after live oral Ty 21a vaccine. J Immunol 137:1504–1510. [PubMed] [PubMed] [Google Scholar]
- 79.Wahid R, Salerno-Gonçalves R, Tacket CO, Levine MM, Sztein MB. 2008. Generation of specific effector and memory T cells with gut- and secondary lymphoid tissue-homing potential by oral attenuated CVD 909 typhoid vaccine in humans. Mucosal Immunol 1:389–398. [PubMed] 10.1038/mi.2008.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wyant TL, Tanner MK, Sztein MB. 1999. Salmonella typhi flagella are potent inducers of proinflammatory cytokine secretion by human monocytes. Infect Immun 67:3619–3624. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tagliabue A, Nencioni L, Caffarena A, Villa L, Boraschi D, Cazzola G, Cavalieri S. 1985. Cellular immunity against Salmonella typhi after live oral vaccine. Clin Exp Immunol 62:242–247. [PubMed] [PMC free article] [PubMed] [Google Scholar]
- 82.Lundin BS, Johansson C, Svennerholm AM. 2002. Oral immunization with a Salmonella enterica serovar typhi vaccine induces specific circulating mucosa-homing CD4(+) and CD8(+) T cells in humans. Infect Immun 70:5622–5627. [PubMed] 10.1128/IAI.70.10.5622-5627.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Salerno-Goncalves R, Pasetti MF, Sztein MB. 2002. Characterization of CD8(+) effector T cell responses in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J Immunol 169:2196–2203. [PubMed] 10.4049/jimmunol.169.4.2196 [DOI] [PubMed] [Google Scholar]
- 84.Salerno-Gonçalves R, Wahid R, Sztein MB. 2005. Immunization of volunteers with Salmonella enterica serovar Typhi strain Ty21a elicits the oligoclonal expansion of CD8+ T cells with predominant Vbeta repertoires. Infect Immun 73:3521–3530. [PubMed] 10.1128/IAI.73.6.3521-3530.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Salerno-Goncalves R, Wahid R, Sztein MB. 2010. Ex Vivo kinetics of early and long-term multifunctional human leukocyte antigen E-specific CD8+ cells in volunteers immunized with the Ty21a typhoid vaccine. Clin Vaccine Immunol 17:1305–1314. [PubMed] 10.1128/CVI.00234-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Robbe-Saule V, Algorta G, Rouilhac I, Norel F. 2003. Characterization of the RpoS status of clinical isolates of Salmonella enterica. Appl Environ Microbiol 69:4352–4358. [PubMed] 10.1128/AEM.69.8.4352-4358.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Santander J, Wanda SY, Nickerson CA, Curtiss R III. 2007. Role of RpoS in fine-tuning the synthesis of Vi capsular polysaccharide in Salmonella enterica serotype Typhi. Infect Immun 75:1382–1392. [PubMed] 10.1128/IAI.00888-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Santander J, Roland KL, Curtiss R III. 2008. Regulation of Vi capsular polysaccharide synthesis in Salmonella enterica serotype Typhi. J Infect Dev Ctries 2:412–420. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wain J, House D, Zafar A, Baker S, Nair S, Kidgell C, Bhutta Z, Dougan G, Hasan R. 2005. Vi antigen expression in Salmonella enterica serovar Typhi clinical isolates from Pakistan. J Clin Microbiol 43:1158–1165. [PubMed] 10.1128/JCM.43.3.1158-1165.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Frey SE, Lottenbach KR, Hill H, Blevins TP, Yu Y, Zhang Y, Brenneman KE, Kelly-Aehle SM, McDonald C, Jansen A, Curtiss R III. 2013. A Phase I, dose-escalation trial in adults of three recombinant attenuated Salmonella Typhi vaccine vectors producing Streptococcus pneumoniae surface protein antigen PspA. Vaccine 31:4874–4880. [PubMed] 10.1016/j.vaccine.2013.07.049 [DOI] [PubMed] [Google Scholar]
- 91.Fass E, Groisman EA. 2009. Control of Salmonella pathogenicity island-2 gene expression. Curr Opin Microbiol 12:199–204. [PubMed] 10.1016/j.mib.2009.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sabbagh SC, Forest CG, Lepage C, Leclerc JM, Daigle F. 2010. So similar, yet so different: uncovering distinctive features in the genomes of Salmonella enterica serovars Typhimurium and Typhi. FEMS Microbiol Lett 305:1–13. [PubMed] 10.1111/j.1574-6968.2010.01904.x [DOI] [PubMed] [Google Scholar]
- 93.Miller VL, Beer KB, Loomis WP, Olson JA, Miller SI. 1992. An unusual pagC:TnphoA mutation leads to an invasion- and virulence-defective phenotype in Salmonellae. Infect Immun 60:3763–3770. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hohmann EL, Oletta CA, Killeen KP, Miller SI. 1996. phoP/phoQ-deleted Salmonella typhi (Ty800) is a safe and immunogenic single-dose typhoid fever vaccine in volunteers. J Infect Dis 173:1408–1414. [PubMed] 10.1093/infdis/173.6.1408 [DOI] [PubMed] [Google Scholar]
- 95.DiPetrillo MD, Tibbetts T, Kleanthous H, Killeen KP, Hohmann EL. 1999. Safety and immunogenicity of phoP/phoQ-deleted Salmonella typhi expressing Helicobacter pylori urease in adult volunteers. Vaccine 18:449–459. 10.1016/S0264-410X(99)00246-7 [DOI] [PubMed] [Google Scholar]
- 96.Angelakopoulos H, Hohmann EL. 2000. Pilot study of phoP/phoQ-deleted Salmonella enterica serovar typhimurium expressing Helicobacter pylori urease in adult volunteers. Infect Immun 68:2135–2141. [PubMed] 10.1128/IAI.68.4.2135-2141.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hindle Z, Chatfield SN, Phillimore J, Bentley M, Johnson J, Cosgrove CA, Ghaem-Maghami M, Sexton A, Khan M, Brennan FR, Everest P, Wu T, Pickard D, Holden DW, Dougan G, Griffin GE, House D, Santangelo JD, Khan SA, Shea JE, Feldman RG, Lewis DJ. 2002. Characterization of Salmonella enterica derivatives harboring defined aroC and Salmonella pathogenicity island 2 type III secretion system (ssaV) mutations by immunization of healthy volunteers. Infect Immun 70:3457–3467. [PubMed] 10.1128/IAI.70.7.3457-3467.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pittard AJ. 1996. Biosynthesis of the aromatic amino acids, p 458–484. In Neidhardt FC, Curtiss Iii R, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE (ed), Escherichia coli and Salmonella: Cellular and Molecular Biology, vol 2. ASM Press, Washington, DC. [Google Scholar]
- 99.Kirkpatrick BD, Tenney KM, Larsson CJ, O’Neill JP, Ventrone C, Bentley M, Upton A, Hindle Z, Fidler C, Kutzko D, Holdridge R, Lapointe C, Hamlet S, Chatfield SN. 2005. The novel oral typhoid vaccine M01ZH09 is well tolerated and highly immunogenic in 2 vaccine presentations. J Infect Dis 192:360–366. [PubMed] 10.1086/431605 [DOI] [PubMed] [Google Scholar]
- 100.Kirkpatrick BD, McKenzie R, O’Neill JP, Larsson CJ, Bourgeois AL, Shimko J, Bentley M, Makin J, Chatfield S, Hindle Z, Fidler C, Robinson BE, Ventrone CH, Bansal N, Carpenter CM, Kutzko D, Hamlet S, LaPointe C, Taylor DN. 2006. Evaluation of Salmonella enterica serovar Typhi (Ty2 aroC-ssaV-) M01ZH09, with a defined mutation in the Salmonella pathogenicity island 2, as a live, oral typhoid vaccine in human volunteers. Vaccine 24:116–123. [PubMed] 10.1016/j.vaccine.2005.08.008 [DOI] [PubMed] [Google Scholar]
- 101.Tran TH, Nguyen TD, Nguyen TT, Ninh TT, Tran NB, Nguyen VM, Tran TT, Cao TT, Pham VM, Nguyen TC, Tran TD, Pham VT, To SD, Campbell JI, Stockwell E, Schultsz C, Simmons CP, Glover C, Lam W, Marques F, May JP, Upton A, Budhram R, Dougan G, Farrar J, Nguyen VV, Dolecek C. 2010. A randomised trial evaluating the safety and immunogenicity of the novel single oral dose typhoid vaccine M01ZH09 in healthy Vietnamese children. PLoS One 5:e11778. 10.1371/journal.pone.0011778. [PubMed] 10.1371/journal.pone.0011778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lyon CE, Sadigh KS, Carmolli MP, Harro C, Sheldon E, Lindow JC, Larsson CJ, Martinez T, Feller A, Ventrone CH, Sack DA, DeNearing B, Fingar A, Pierce K, Dill EA, Schwartz HI, Beardsworth EE, Kilonzo B, May JP, Lam W, Upton A, Budhram R, Kirkpatrick BD. 2010. In a randomized, double-blinded, placebo-controlled trial, the single oral dose typhoid vaccine, M01ZH09, is safe and immunogenic at doses up to 1.7 x 10(10) colony-forming units. Vaccine 28:3602–3608. [PubMed] 10.1016/j.vaccine.2010.02.017 [DOI] [PubMed] [Google Scholar]
- 103.Tacket CO, Hone DM, Losonsky GA, Guers L, Edelman R, Levine MM. 1992. Clinical acceptability and immunogenicity of CVD 908 Salmonella typhi vaccine strain. Vaccine 10:443–446. [PubMed] 10.1016/0264-410X(92)90392-W [DOI] [PubMed] [Google Scholar]
- 104.Sztein MB, Wasserman SS, Tacket CO, Edelman R, Hone D, Lindberg AA, Levine MM. 1994. Cytokine production patterns and lymphoproliferative responses in volunteers orally immunized with attenuated vaccine strains of Salmonella typhi. J Infect Dis 170:1508–1517. [PubMed] 10.1093/infdis/170.6.1508 [DOI] [PubMed] [Google Scholar]
- 105.Tacket CO, Sztein MB, Losonsky GA, Wasserman SS, Nataro JP, Edelman R, Pickard D, Dougan G, Chatfield SN, Levine MM. 1997. Safety of live oral Salmonella typhi vaccine strains with deletions in htrA and aroC aroD and immune response in humans. Infect Immun 65:452–456. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bäumler AJ, Kusters JG, Stojiljkovic I, Heffron F. 1994. Salmonella typhimurium loci involved in survival within macrophages. Infect Immun 62:1623–1630. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tacket CO, Sztein MB, Wasserman SS, Losonsky G, Kotloff KL, Wyant TL, Nataro JP, Edelman R, Perry J, Bedford P, Brown D, Chatfield S, Dougan G, Levine MM. 2000. Phase 2 clinical trial of attenuated Salmonella enterica serovar typhi oral live vector vaccine CVD 908-htrA in U.S. volunteers. Infect Immun 68:1196–1201. [PubMed] 10.1128/IAI.68.3.1196-1201.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang JY, Noriega FR, Galen JE, Barry E, Levine MM. 2000. Constitutive expression of the Vi polysaccharide capsular antigen in attenuated Salmonella enterica serovar typhi oral vaccine strain CVD 909. Infect Immun 68:4647–4652. [PubMed] 10.1128/IAI.68.8.4647-4652.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhao L, Ezak T, Li ZY, Kawamura Y, Hirose K, Watanabe H. 2001. Vi-Suppressed wild strain Salmonella typhi cultured in high osmolarity is hyperinvasive toward epithelial cells and destructive of Peyer’s patches. Microbiol Immunol 45:149–158. [PubMed] 10.1111/j.1348-0421.2001.tb01283.x [DOI] [PubMed] [Google Scholar]
- 110.Tacket CO, Pasetti MF, Sztein MB, Livio S, Levine MM. 2004. Immune responses to an oral typhoid vaccine strain that is modified to constitutively express Vi capsular polysaccharide. J Infect Dis 190:565–570. [PubMed] 10.1086/421469 [DOI] [PubMed] [Google Scholar]
- 111.Okoro CK, Kingsley RA, Connor TR, Harris SR, Parry CM, Al-Mashhadani MN, Kariuki S, Msefula CL, Gordon MA, de Pinna E, Wain J, Heyderman RS, Obaro S, Alonso PL, Mandomando I, MacLennan CA, Tapia MD, Levine MM, Tennant SM, Parkhill J, Dougan G. 2012. Intracontinental spread of human invasive Salmonella Typhimurium pathovariants in sub-Saharan Africa. Nat Genet 44:1215–1221. [PubMed] 10.1038/ng.2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ao TT, Feasey NA, Gordon MA, Keddy KH, Angulo FJ, Crump JA. 2015. Global burden of invasive nontyphoidal Salmonella disease, 2010. Emerg Infect Dis 21:941–949. [PubMed] 10.3201/eid2106.140999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kingsley RA, Msefula CL, Thomson NR, Kariuki S, Holt KE, Gordon MA, Harris D, Clarke L, Whitehead S, Sangal V, Marsh K, Achtman M, Molyneux ME, Cormican M, Parkhill J, MacLennan CA, Heyderman RS, Dougan G. 2009. Epidemic multiple drug resistant Salmonella Typhimurium causing invasive disease in sub-Saharan Africa have a distinct genotype. Genome Res 19:2279–2287. [PubMed] 10.1101/gr.091017.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ramachandran G, Perkins DJ, Schmidlein PJ, Tulapurkar ME, Tennant SM. 2015. Invasive Salmonella Typhimurium ST313 with naturally attenuated flagellin elicits reduced inflammation and replicates within macrophages. PLoS Negl Trop Dis 9:e3394. 10.1371/journal.pntd.0003394. [PubMed] 10.1371/journal.pntd.0003394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Herrero-Fresno A, Wallrodt I, Leekitcharoenphon P, Olsen JE, Aarestrup FM, Hendriksen RS. 2014. The role of the st313-td gene in virulence of Salmonella Typhimurium ST313. PLoS One 9:e84566. 10.1371/journal.pone.0084566. [PubMed] 10.1371/journal.pone.0084566 [DOI] [PMC free article] [PubMed] [Google Scholar]


