Abstract
Background
Cardiorespiratory fitness measured by treadmill testing has prognostic significance in determining mortality with cardiovascular and other chronic disease states. The accuracy of a recently developed method for estimating maximal oxygen uptake (VO2peak), the heart rate index (HRI), is dependent only on heart rate (HR) and was tested against oxygen uptake (VO2), either measured or predicted from conventional treadmill parameters (speed, incline, protocol time).
Methods
The HRI equation, METs = 6 x HRI– 5, where HRI = maximal HR/resting HR, provides a surrogate measure of VO2peak. Forty large scale treadmill studies were identified through a systematic search using MEDLINE, Google Scholar and Web of Science in which VO2peak was either measured (TM-VO2meas; n = 20) or predicted (TM-VO2pred; n = 20) based on treadmill parameters. All studies were required to have reported group mean data of both resting and maximal HRs for determination of HR index-derived oxygen uptake (HRI-VO2).
Results
The 20 studies with measured VO2 (TM-VO2meas), involved 11,477 participants (median 337) with a total of 105,044 participants (median 3,736) in the 20 studies with predicted VO2 (TM-VO2pred). A difference of only 0.4% was seen between mean (±SD) VO2peak for TM- VO2meas and HRI-VO2 (6.51±2.25 METs and 6.54±2.28, respectively; p = 0.84). In contrast, there was a highly significant 21.1% difference between mean (±SD) TM-VO2pred and HRI-VO2 (8.12±1.85 METs and 6.71±1.92, respectively; p<0.001).
Conclusion
Although mean TM-VO2meas and HRI-VO2 were almost identical, mean TM-VO2pred was more than 20% greater than mean HRI-VO2.
Introduction
When assessed as oxygen consumption (VO2), cardiorespiratory fitness (CRF) may be measured either using a treadmill with conventional gas analysis equipment (TM-VO2meas) or predicted from equations based on treadmill speed, incline or treadmill time (TM-VO2pred)[1]. The prognostic importance of CRF has been extensively investigated in recent meta-analyses confirming the strong inverse relationships between CRF and all-cause mortality in healthy individuals [2] and in patients with either coronary artery disease (CAD) or congestive heart failure (CHF) [3–6]. The prospective studies included in these reviews involve large numbers of subjects and have shown that a 1 MET (equal to 3.5 mL O2 ·kg-1·min-1) increment increase in CRF is associated with an approximate 10–20% reduction in all cause and cardiovascular mortality [2,7] with a similar effect being observed with CHF [6,8].
Logistics of large studies necessitate prediction of peak VO2 (VO2peak) as measurement of VO2 is costly and time consuming. Equations have been determined for the various treadmill protocols based on the variables of treadmill speed, incline or the test time for a particular protocol, a common reference being ACSM publications [1]. However, many factors may contribute to the error of TM-VO2pred. They include 1) treadmill handrail support [9–13], 2) failure to use population specific equations [14–18], 3) inappropriate testing protocol [19–21], 4) delayed oxygen kinetics [22–24], 5) reproducibility of cardiopulmonary parameters [25,26], 6) altered mechanical efficiency with treadmill walking [27] and 7) lack of treadmill calibration [28].
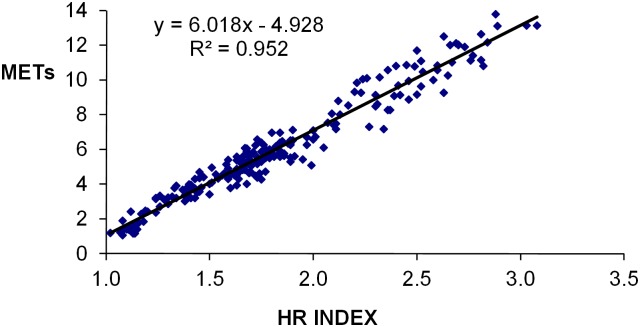
Cardiovascular pathology frequently screened for with treadmill testing includes both CAD and CHF. In using CRF as an outcome measure from a treadmill test, VO2peak is commonly expressed as METs with 1 MET being the VO2 at rest with current convention stating that it is equal to 3.5 mL O2 ·kg-1·min-1 [29]. Kaplan-Meier curves have been used extensively to document the link between CRF and long-term morbidity/mortality [30,31]. Although VO2 can be predicted from treadmill speed, incline or the test time for a particular protocol, currently the only way to ensure an accurate measurement of VO2 is direct measurement with gas analysis. Using only two simple measurements, rest HR and an activity HR (either sub-maximal or maximal), the recently published HR index (HRI = activity HR/rest HR), equation for predicting VO2 expressed as METs is associated with a high correlation between HRI and VO2, the equation being METs = 6 x HRI -5 [32]. The HRI equation was derived from group mean data from 60 studies in which an exercise test contained a resting HR (HRrest), and a VO2 measured at the activity HR (either submaximal or peak) and expressed in the form of mLO2 ·kg-1·min-1 or METs. The original data are shown as a regression plot in Fig 1. The utility of this equation is that it provides a simple independent surrogate method of estimating VO2 using only the rest and either the sub-maximal or maximal activity HR measurements. Though the HRI equation was developed from aggregate data, there has been no analysis to date that has established its predictive accuracy for assessment of VO2.
Fig 1. Linear regression plot of HR index equation.
An analysis from data (n = 220) derived from 60 studies with the HR index equation simplified to METs = 6 x HR index– 5.
The objective of this study was to compare aggregate HRI-derived VO2 (HRI-VO2) data against VO2peak from two different treadmill tests, either: 1) VO2 measured with conventional gas analysis equipment (TM-VO2meas) or 2) VO2 predicted from equations based on treadmill speed, incline or treadmill time (TM-VO2pred).
Methods
Study selection
Treadmill studies involving assessment of VO2peak, reporting either TM-VO2meas or TM-VO2pred, were identified through a systematic search conducted on at least a monthly basis from October 2011 till March 2013 using MEDLINE, Google Scholar and Web of Science. Search terms included (in various combinations) exercise testing, oxygen uptake, VO2, CRF, cardiovascular disease (CVD), CAD, CHF and physical activity. With publications having the prerequisite HR data extensive cross-referencing was undertaken to source other publications with eligible criteria [33].
Eligibility criteria for study inclusion are 1) >100 patients enrolled, 2) documented VO2peak (either measured or predicted) expressed as either mL O2 ·kg-1·min-1 or as METs, 3) measured maximal HR (HRmax) associated with VO2peak, and 4) measured HRrest. Where large scale studies included cycle ergometry in conjunction with treadmill testing, the study was excluded. In publications likely to have used a similar subject cohort based on 1. participating authors, 2. study location, 3. time period when the study was performed and 4. characteristics of the study population e.g. healthy, suspected or known CAD the most recent publication was chosen. From the HR data, a predicted MET value (VO2peak) was derived using the HRI equation (METs = 6 x HR index– 5, where HR index is HRmax/HRrest).
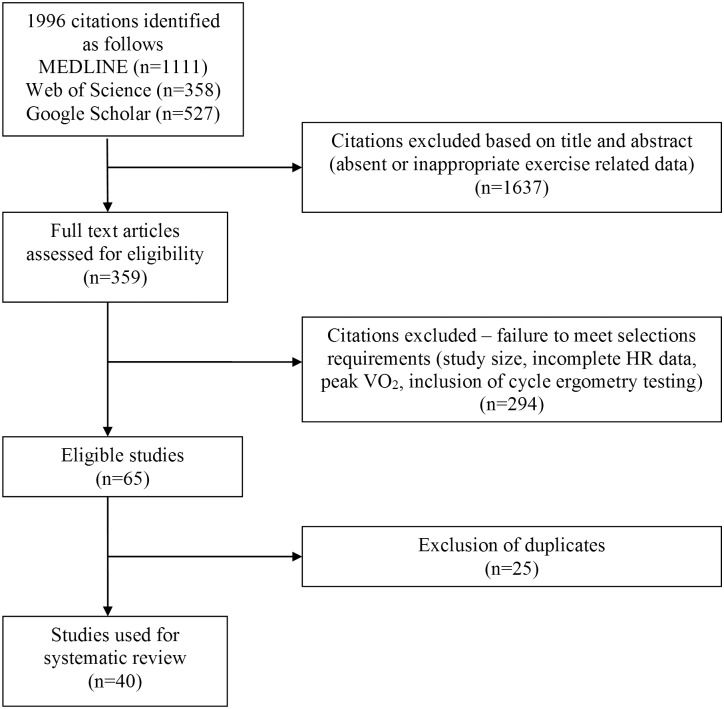
At the time of closure of data acquisition in March 2013 a total of 40 studies (TM-VO2meas; n = 20 studies, TM-VO2pred; n = 20 studies) had been identified with all but one being published since 1991. MEDLINE searching identified 19 of the 40 studies (TM-VO2meas; n = 11 studies, TM-VO2pred; n = 8 studies) used in this analysis with the remaining 21 studies being sourced through Web of Science, Google Scholar and cross referencing. The TM-VO2meas studies had a bias towards clinical outcomes related to CHF whereas the TM-VO2pred studies were frequently associated with long-term outcome (survival) in screening for CVD. Though multiple search strategies were used to obtain studies meeting selection criteria it is acknowledged that even with rigorous attention to search detail, suitable studies may have been missed.
Fig 2 details the study selection process at the completion of data acquisition in March 2013.
Fig 2. Study selection process used for data acquisition.
Statistical analysis
Categorical variables were expressed as numbers and percentages with continuous variables expressed as mean ± standard deviation. Student’s paired t-test was used to compare HRI-VO2 against both TM-VO2meas and TM-VO2pred. Results are expressed in two formats, namely 1) pooled data for each of TM-VO2meas and TM-VO2pred against HRI-VO2 expressed as group means and shown in the form of line of identity and Bland Altman plots [34] and 2) CRF data shown in tertiles for both TM-VO2meas and TM-VO2pred groups against HRI-VO2.
Results
Studies used in the analyses
There were 11,477 subjects in the 20 TM-VO2meas studies (range 110 to 4631, median 337) and, with each study mean VO2meas value representing a data point, there was a total of 45 data points. There was a considerably larger number of subjects at 105,044 (range 772 to 22,275, median 3,736) in the 20 TM-VO2pred studies and, with each study mean VO2pred value representing a data point, there were 57 data points. Age and gender distribution was similar for the TM-VO2meas (51.0 years and 64.9% males) and TM-VO2pred groups (52.9 years and 71.0% males).
The principal details of the 40 treadmill studies used in the analysis are outlined in Table 1. These include the test protocol, use of handrail support and the health status of participants. Of the 20 TM-VO2meas studies, 14 (70%) involved subjects with CHF and all 14 used protocols other than the standard Bruce protocol [35]. The design of these alternate protocols reduced the stage increment of VO2 usually to 2 METs or less with certain ramp protocols having increments of less than 1 MET per minute. In only two of the TM-VO2meas studies was hand rail support mentioned, being ‘not permitted’ in one study (Dressendorfer [36]) and ‘discouraged’ in the other (Oliveira [37]).
Table 1. Description of studies, patient diagnosis, and test protocol in which oxygen uptake was either measured or predicted using a prediction equation (Pred EQ).
| First Author | Year | n | Age (years) | Male% | Category | Test | Rail support | Pred EQ |
|---|---|---|---|---|---|---|---|---|
| Measured VO2 | ||||||||
| Bard | 2006 | 355 | 51 | 72 | CHF | ramp | ns | |
| Diller | 2006 | 727 | 33 | 52 | ACHD | MB | ns | |
| Dressendorfer | 1993 | 182 | 57 | 100 | CAD | MB | NP | |
| Elmariah | 2006 | 594 | 52 | 72 | CHF | ramp | ns | |
| Harrington | 1997 | 131 | 59 | 100 | CHF, H | MB | ns | |
| Ingle | 2007 | 394 | 65 | 74 | CHF | MB | ns | |
| Jorde | 2008 | 278 | 52 | 77 | CHF | Na | ns | |
| Kohrt | 1991 | 110 | 64 | 50 | H | B,O | ns | |
| Kubrychtova | 2009 | 712 | 56 | 72 | CHF | O | ns | |
| Lanier | 2012 | 320 | 52 | 75 | CHF | Na | ns | |
| McDonough | 1970 | 144 | 51 | 100 | H | B | ns | |
| Nes | 2012 | 4631 | 48 | 49 | H | ramp | ns | |
| Oliveira | 2009 | 948 | 57 | 100 | CPD, H | ramp | DIS | |
| Osada | 1998 | 154 | 52 | 75 | CHF | MB, MNa | ns | |
| Peterson | 2003 | 369 | 51 | 72 | CHF | O | ns | |
| Robbins | 1999 | 487 | 52 | 71 | CHF, H | Na | ns | |
| Schalcher | 2003 | 146 | 52 | 88 | CHF | ramp | ns | |
| Stolker | 2006 | 221 | 49 | 68 | CHF | O | ns | |
| Williams | 2001 | 219 | 56 | 76 | CHF | B, MB | ns | |
| Witte | 2006 | 355 | 66 | 68 | CHF, H | MB | ns | |
| Predicted VO2 | ||||||||
| Adabag | 2008 | 12555 | 46 | 100 | CAD° | B | ns | EQ-S |
| Aijaz | 2008 | 10897 | 54 | 75 | CVD, CVD° | B | ns | ns |
| Arruda-Olson | 2002 | 5798 | 62 | 57 | CAD, CAD? | B, MB, Na | ns | ns |
| Carnethon | 2003 | 4487 | 25 | 45 | H | MBa | ns | ns |
| Cheng | 2003 | 2333 | 49 | 100 | DM | MBa | ns | EQ-S |
| Elhendy | 2001 | 1618 | 55 | 35 | CAD? | B, MB, Na | ns | ns |
| Gulati | 2010 | 5437 | 52 | 0 | CAD° | B | LS | EQ-R |
| Kim | 2007 | 22275 | 51 | 59 | CVD° | B, MB, O | NP | EQ-R |
| Kokkinos | 2009 | 4631 | 61 | 100 | HT | B, ramp | DIS | EQ-R |
| Lai | 2004 | 5625 | 59 | 100 | CVD° | ramp, O | ns | EQ-R |
| Lauer | 1999 | 2953 | 58 | 64 | CVD°, CVD? | B, MB | NP | EQ-R |
| Lipinski | 2005 | 1914 | 52 | 100 | CAD, CHF, CAD° | ramp, O | ns | ns |
| Mahenthiran | 2005 | 1268 | 60 | 52 | CAD, CAD° | B | ns | ns |
| McAuley | 2007 | 6876 | 58 | 97 | CAD, CAD° | ramp | DIS | EQ-R |
| Mora | 2003 | 2985 | 47 | 0 | CAD° | B | ns | EQ-R |
| Morrow | 1993 | 2546 | 59 | 100 | CAD°, CAD, CHF | ramp, O | ns | EQ-R |
| Myers | 2002 | 6213 | 59 | 100 | CAD, CAD° | ramp | DIS | EQ-R |
| Negishi | 2013 | 914 | 56 | 56 | DM | B, MB | NP | EQ-R |
| Peteiro | 2010 | 2947 | 62 | 61 | CAD, CAD? | B, MB, Na | ns | ns |
| Shaw | 2011 | 772 | 63 | 0 | CAD? | B, MB | ns | ns |
References are available in the supplementary digital content. Category: ACHD, adult congenital heart disease; CAD, coronary artery disease (CAD°, absent; CAD?, suspected); CHF, congestive heart failure; CPD, cardiopulmonary disease; CVD, cardiovascular disease; DM, diabetes mellitus; H, healthy; HT, hypertension. Treadmill test: B, Bruce protocol; Ba, Balke protocol; Na, Naughton protocol; ramp, ramp protocol; M, modified protocol; O, other protocol; Rail support: ns, not stated; NP, not permitted; DIS, discouraged; LS, light support; Equation: EQ-S, stated equation; EQ-R, referenced equation; ns, not stated.
Typically, subjects with known or suspected CVD or with significant cardiovascular risk factors were involved in the TM-VO2pred studies (Table 1). A Bruce protocol, either as the standard or a modified protocol, was used in 13 (65%) of the 20 TM-VO2pred studies. With TM-VO2pred studies, the use of handrail support was defined in seven studies (35%) and not stated in the remaining 13 studies. Descriptors of handrail support used for these seven studies were ‘discouraged’ in 3 studies, ‘not permitted’ in 3 studies and ‘light hand rail support’ in 1 study. Predictive treadmill equations in TM-VO2pred studies were either given or referenced in only 12 (60%) of the 20 studies.
Characterization of study groups
A. Group means: oxygen consumption and heart rate
The mean TM-VO2pred reported in the 20 studies was 8.12 METS; the mean TM-VO2meas reported in the 20 studies was 6.51 METS, a difference of 1.61 Mets or 24.7% (Table 2). The mean HRrest with TM-VO2pred was 75.6 beats∙min-1 and with TM-VO2meas was 77.6 beats∙min-1; the mean HRmax for TM-VO2pred 146.3 beats∙min-1 and TM-VO2meas 147.1 beats∙min-1 (Table 2). However, the absolute differences in group means for HRrest and HRmax between TM-VO2pred and TM-VO2meas were small at 2.0 beats∙min-1 for HRrest and only 0.8 beat∙min-1 for HRmax (Table 2).
Table 2. Heart rate and oxygen consumption data for TM-VO2meas and TM-VO2pred.
Group mean (± 1SD) heart rate (HR) and oxygen consumption (VO2) data. HRrest, HRpeak, HRI-VO2 and VO2peak for TM-VO2meas and TM-VO2pred.
| Studies | Data points | HRrest beats∙min-1 | HRpeak beats∙min-1 | VO2peak METs | HRI-VO2 METs | |
|---|---|---|---|---|---|---|
| TM-VO2pred | 20 | 57 | 75.6 ± 5.3 | 146.3 ± 16.6 | 8.12 ± 1.85 | 6.71 ± 1.92 |
| TM-VO2meas | 20 | 45 | 77.6 ± 7.7 | 147.1 ± 18.8 | 6.51 ± 2.25 | 6.54 ± 2.28 |
Alternatively if VO2peak is determined by HRI-VO2 the difference between TM-VO2pred and TM-VO2meas is reduced to only 0.17 MET or 2.6% (TM-VO2pred 6.71 METs, TM-VO2meas 6.54 METs), a not unexpected result in view of the small differences in HRrest and HRmax between these two groups (Table 2).
B. Comparison of measured VO2 and predicted VO2 versus VO2 predicted by HRI
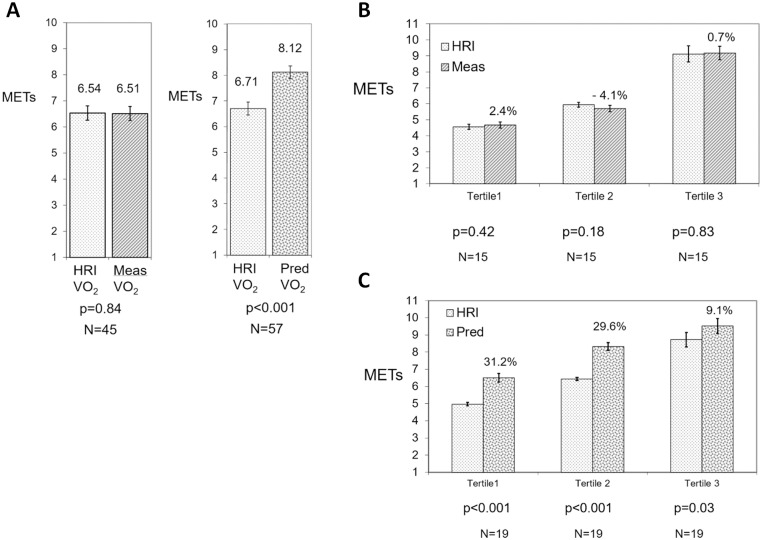
When using the HRI to calculate VO2peak, there was no significant difference (0.4%, p = 0.84) in the pooled VO2 data with mean (± SD) MET values of 6.51(±2.25) for TM-VO2meas and 6.54 (±2.28) for HRI-VO2 (Fig 3A). However, a highly significant difference (21.1%, p<0.001) was seen between TM-VO2pred and HRI-VO2 with respective values of 8.12 (±1.85) METs and 6.71 (±1.92) METs (Fig 3A).
Fig 3. Comparison of pooled data from 20 studies for TM-VO2meas and TM-VO2pred against HRI-VO2.
A. Comparison of group mean data for 20 TM-VO2meas and TM-VO2pred studies against HRI-VO2 (mean ± SE), B. Comparison of cardiorespiratory fitness tertiles from 20 studies for TM-VO2meas against HRI-VO2 (mean ±SE). Percentage difference between TM-VO2meas and HRI-VO2 shown within figure and C. Comparison of cardiorespiratory fitness tertiles from 20 studies for TM-VO2pred against HRI-VO2 (mean ±SE). Percentage difference between TM-VO2pred and HRI-VO2 shown within figure.
Even when expressed in tertiles based on HRI-VO2, there were no significant differences between TM-VO2meas and HRI-VO2 by VO2 tertile; tertile 1, 2.4% (p = 0.42), tertile 2, -4.1% (p = 0.18) and tertile 3, 0.7% (p = 0.83) (Fig 3B). By comparison, each tertile for the TM-VO2pred groups showed a significant difference from HRI-VO2; tertile 1, 31.2% (p<0.001), tertile 2, 29.6% (p<0.001) and tertile 3, 9.1% (p = 0.03) (Fig 3C).
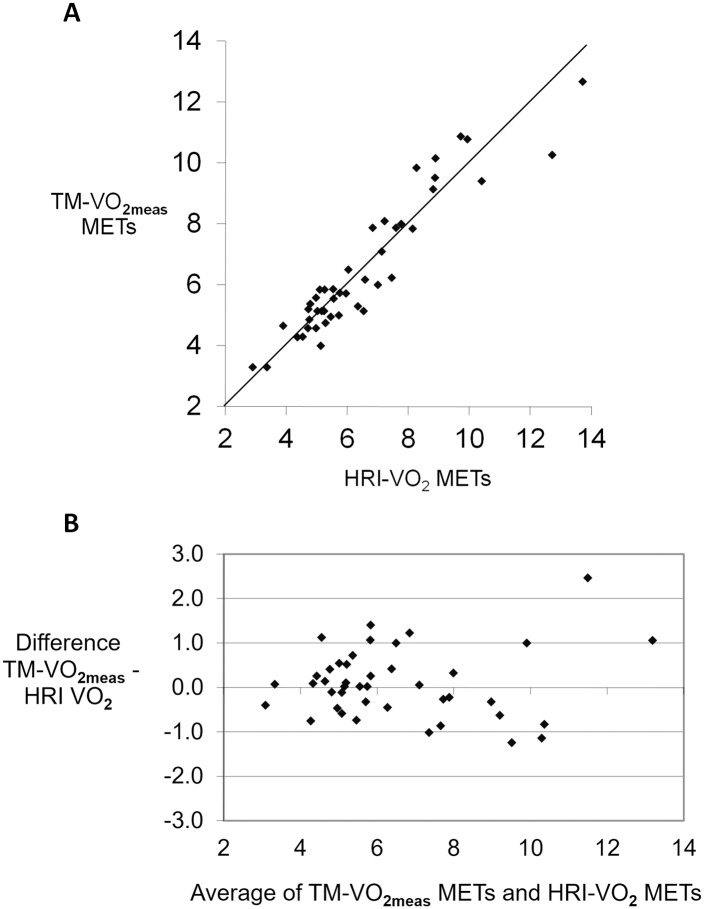
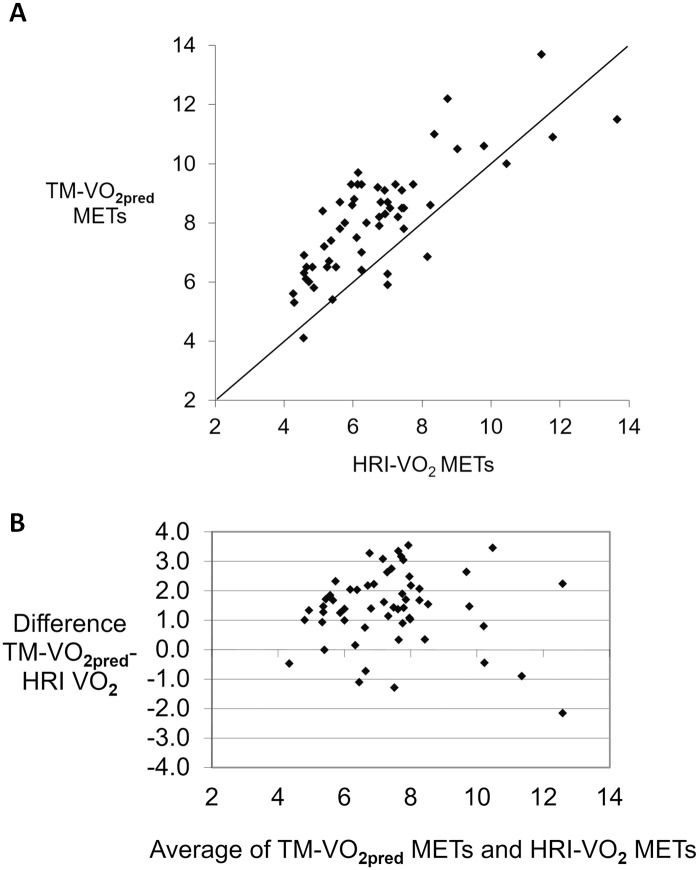
The plot of TM-VO2meas against HRI-VO2 shows a uniform distribution around the line of identity with the Bland Altman plot suggesting that there is no bias between these two separate methods of determining VO2peak (Fig 4A and 4B). However, a similar line of identity plot for TM-VO2pred against HRI-VO2 indicates a strong bias with the Bland Altman plot indicating a systematic error in support of over-prediction of TM-VO2pred (Fig 5A and 5B).
Fig 4. Line of identity and Bland Altman plot for TM-VO2meas.
A. Line of identity for TM-VO2meas and B. against Bland Altman plot for TM-VO2meas against HRI-VO2.
Fig 5. Line of identity and Bland Altman plot for TM-VO2pred.
A. Line of identity for TM-VO2pred and B. against Bland Altman plot for TM-VO2pred against HRI-VO2.
Discussion
It is crucial to have high quality CRF data for use in epidemiological studies as management strategies involving both pharmacological and lifestyle intervention rely on this accuracy. The utility of the HRI equation [32] as a surrogate measure of VO2 expressed in METs is confirmed in this study when assessed against VO2peak for both TM-VO2meas measured with conventional gas analysis equipment and for TM-VO2pred predicted from equations based on treadmill speed, incline or treadmill time. A close agreement between HRI-VO2 and TM-VO2meas was observed in the 20 TM-VO2meas studies with only a 0.4% difference (p = 0.84) between group means. By comparison, a highly significant 21.1% (p<0.001) over-prediction of VO2peak was observed when comparing HRI-VO2 against TM-VO2pred in the 20 TM-VO2pred studies. The magnitude of the potential error using TM-VO2pred challenges the current methods of treadmill prediction of CRF which appear to lead to overestimation of CRF and potentially to false prognostic classification.
If the magnitude of the disparity between HRI-VO2 and TM-VO2pred as shown in this study is, for example, applied to the outcome data of CRF as expressed in METs in the meta-analysis by Kodama [2], there is a strong likelihood of a false classification based on the over-prediction of CRF. For example, in treadmill studies investigating the effect of handrail support, a practice that lengthens treadmill time, VO2peak is over-predicted by 20% to 30% [9–13,17] which would lead to a potentially false prognostic classification of CRF. To correct for the consistently observed over-prediction of VO2peak of around 20% resulting from the use of handrail support, Foster has developed simple modifications of the ACSM equations for use when handrail support is observed during treadmill testing [17]. None of the 20 TM-VO2pred studies used in this analysis referenced use of the Foster or similar equations to correct for observed handrail support. This prediction error could potentially apply to other published studies that express results in the form of survival tables and Kaplan-Meier curves. The measurement of CRF is not only limited to CVD. CRF also defines long-term risk in both healthy subjects and other common medical conditions, such as stroke [38], dementia [39] and diabetes mellitus [40]. In the TM-VO2pred group of studies, the smallest difference (9.1%) between HRI-VO2 and TM-VO2pred was observed in the highest CRF tertile. Presumably, the fittest subjects find less difficulty with treadmill walking and so have less need for handrail support. Conversely, the least fit, i.e., the lowest tertile, are most likely to utilize handrail support, even when instructed otherwise, and, in the present study, they demonstrated a 31.2% difference between HRI-VO2 and TM-VO2pred. Results from the HUNT 3 Fitness Study also noted the greatest overestimation of VO2peak in the least fit subjects [18].
Collectively the 20 TM-VO2pred studies used in this analysis involve a tenfold greater number of subjects when compared with the 20 TM-VO2meas studies, whether considering the total number of subjects (105,044 TM-VO2pred versus 11,477 TM-VO2meas) or the median number (3,736 TM-VO2pred versus 337 TM-VO2meas). This observation indicates an inherent bias in using predicted VO2 studies for epidemiological purposes. In recognizing the need for high quality population CRF data, the Fitness Registry and the Importance of Exercise: A National Database (FRIEND) was established in 2014 [41]. A recent publication from this group has provided age-related reference standards of CRF from 7783 tests in which VO2max was determined by gas analysis, the authors highlighting the shortcomings of using TM-VO2pred largely because of over-prediction of VO2max associated with hand rail support [42]. Their statement together with the observations in the present review suggest that, for the continued use of TM-VO2pred data, a reappraisal of current methods used for prediction of VO2peak warrants consideration.
One important question arising from this analysis is the value of using maximal HRI to predict VO2peak from HR derived values (rest and peak) as opposed to treadmill parameters (speed, incline or treadmill time). When calculating maximal HRI, two independent predictors of future CVD risk, namely an estimated VO2peak [2,43] and HRrest [44] are incorporated within the HRI. The maximal HRI is based on two measured values of HR and, when used as an index, there is minimal predictive error especially when compared to VO2pred using equations based on speed, incline or treadmill time. As a 1.0 MET increment corresponds to a HRI increment of 0.167, Kaplan-Meier curves ranging from <5 to >10 METs have a corresponding HRI range from <1.67 to >2.50 (e.g., 5 METs = Rest [HRI = 1] + 4 METs [HRI = 4 x 0.167] = 1.67). In considering a range of activity from rest (1.0 MET) to the maximum aerobic performance of an elite athlete (e.g. 19 METs), the corresponding range of HRI would be from 1 to 4. The simplicity of calculating HRI together with the range of index used for clinical evaluation suggests that it could provide a useful addition to the assessment of CRF. To illustrate this, a range of 5, 10 and 15 MET levels have corresponding HRIs of 1.67, 2.5 and 3.33.
Study Limitations
This review has used the simple concept of HRI as a surrogate measure of VO2. The equation was established from aggregate data acquired from 60 studies. In applying the HR index to this analysis, we have compared aggregate data from TM-VO2pred and TM-VO2meas against HRI-VO2 with no intention of indicating the individual predictive accuracy of the equation. Ideally the use of individual, as opposed to aggregate data would have been preferable but it was beyond the capability of this analysis.
Conclusions
The usefulness of CRF is well established for assessing CV risk with treadmill testing providing a simple and convenient method of assessing CRF. The aggregate analysis used in this study shows a close relationship, i.e., a non-significant 0.4% difference, between HRI-VO2 and TM-VO2meas but a large and highly significant 21.1% difference between HRI-VO2 and TM-VO2pred.This overestimation of TM-VO2pred, and so CRF, challenges the validity of predicting VO2 peak from equations based on treadmill speed, incline or protocol time when attempting to document a link between CRF and long-term morbidity/mortality.
Supporting Information
File listing the 40 treadmill studies used for analysis.
(RTF)
Acknowledgments
Neither author has any conflict of interest nor was any financial assistance provided for this study. The authors thank the staff of the Gold Coast Hospital Library for their invaluable help in sourcing reference materials and assistance offered in developing search strategies provided by Ms Sarah Thorning, senior librarian, Gold Coast University Hospital Library and Professor Paul Glasziou, Department of Evidence Based Medicine, Faculty of Health Sciences and Medicine, Bond University, Queensland Australia.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.ACSM (2000) ACSM's Guidelines for Exercise Testing and Prescription. 6th edition Philadelphia, PA: Lippincott Williams & Wilkins. [Google Scholar]
- 2.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, et al. (2009) Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women. JAMA 301: 2024–2035. 10.1001/jama.2009.681 [DOI] [PubMed] [Google Scholar]
- 3.Echouffo-Tcheugui JB, Butler J, Yancy CW, Fonarow GC (2015) Association of physical activity or fitness with incident heart failure: A systematic review and meta-analysis. Circ Heart Fail 8: 853–861. 10.1161/CIRCHEARTFAILURE.115.002070 [DOI] [PubMed] [Google Scholar]
- 4.Keteyian SJ, Brawner CA, Savage PD, Ehrman JK, Schairer J, Divine G, et al. (2008) Peak aerobic capacity predicts prognosis in patients with coronary heart disease. Am Heart J 156: 292–300. 10.1016/j.ahj.2008.03.017 [DOI] [PubMed] [Google Scholar]
- 5.McNeer JF, Margolis JR, Lee KL, Kisslo JA, Peter RH, Kong Y, et al. (1978) The role of the exercise test in the evaluation of patients for ischemic heart disease. Circulation 57: 64–70. [DOI] [PubMed] [Google Scholar]
- 6.Berry JD, Pandey A, Gao A, Leonard D, Farzaneh-Far R, Ayers C, et al. (2013) Physical fitness and risk for heart failure and coronary artery disease. Circ Heart Fail 6: 627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gander JC, Sui X, Hébert JR, Hazlett LJ, Cai B, Lavie CJ, et al. (2015) Association of cardiorespiratory fitness with coronary heart disease in asymptomatic men. Mayo Clin Proc 90: 1372–1379. 10.1016/j.mayocp.2015.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan H, Kunutsor S, Rauramaa R, Savonen K, Kalogeropoulos AP, Georgiopoulou VV, et al. (2014) Cardiorespiratory fitness and risk of heart failure: a population‐based follow‐up study. Eur J Heart Fail 16: 180–188. 10.1111/ejhf.37 [DOI] [PubMed] [Google Scholar]
- 9.Pinkstaff S, Peberdy MA, Kontos MC, Fabiato A, Finucane S, Arena R (2011) Overestimation of aerobic capacity with the bruce treadmill protocol in patients being assessed for suspected myocardial ischemia. J Cardiopulm Rehabil Prev 31: 254–260. 10.1097/HCR.0b013e318211e3ed [DOI] [PubMed] [Google Scholar]
- 10.Berling JM, Foster C, Gibson M, Doberstein S, Porcari JP (2006) The effect of handrail support on oxygen uptake during steady state treadmill exercise. J Cardiopulm Rehabil Prev 26: 250. [DOI] [PubMed] [Google Scholar]
- 11.Zeimetz G, McNeil J, Hall J, Moss R (1985) Quantifiable changes in oxygen uptake, heart rate, and time to target heart rate when hand support is allowed during treadmill exercise. J Cardiopulm Rehabil 5: 525–530. [Google Scholar]
- 12.Haskell WL, Savin W, Oldridge N, DeBusk R (1982) Factors influencing estimated oxygen uptake during exercise testing soon after myocardial infarction. Am J Cardiol 50: 299–304. [DOI] [PubMed] [Google Scholar]
- 13.Manfre MJ, Yu GH, Varmá AA, Mallis GI, Kearney K, Karageorgis MA (1994) The effect of limited handrail support on total treadmill time and the prediction of VO2 max. Clin Cardiol 17: 445–450. [DOI] [PubMed] [Google Scholar]
- 14.Foster C, Hare J, Taylor M, Goldstein T, Anholm J, Pollock M (1984) Prediction of oxygen uptake during exercise testing in cardiac patients and healthy volunteers. J Cardiac Rehabil 4: 537–542. [Google Scholar]
- 15.Foster C, Jackson AS, Pollock ML, Taylor MM, Hare J, Sennett SM, et al. (1984) Generalized equations for predicting functional capacity from treadmill performance. Am Heart J 107: 1229–1234. [DOI] [PubMed] [Google Scholar]
- 16.McConnell TR, Foster C, Conlin NC, Thompson NN (1991) Prediction of functional capacity during treadmill testing: effect of handrail support. J Cardiopulm Rehabil Prev 11: 255–260. [Google Scholar]
- 17.Foster C, Crowe A, Daines E, Dumit M, Green MA, Lettau S, et al. (1996) Predicting functional capacity during treadmill testing independent of exercise protocol. Med Sci Sports Exerc 28: 752–756. [DOI] [PubMed] [Google Scholar]
- 18.Loe H, Nes BM, Wisløff U (2016) Predicting VO 2peak from Submaximal-and Peak Exercise Models: The HUNT 3 Fitness Study, Norway. PLoS ONE 11: e0144873 10.1371/journal.pone.0144873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchfuhrer MJ, Hansen JE, Robinson TE, Sue DY, Wasserman K, Whipp BJ (1983) Optimizing the exercise protocol for cardiopulmonary assessment. J Appl Physiol 55: 1558–1564. [DOI] [PubMed] [Google Scholar]
- 20.Myers J, Buchanan N, Walsh D, Kraemer M, McAuley P, Hamilton-Wessler M, et al. (1991) Comparison of the ramp versus standard exercise protocols. J Am Coll Cardiol 17: 1334–1342. [DOI] [PubMed] [Google Scholar]
- 21.Opasich C, Myers J (2001) Working Group Report. Recommendations for exercise testing in chronic heart failure patients. Eur Heart J 22: 37–45. [DOI] [PubMed] [Google Scholar]
- 22.Roberts JM, Sullivan M, Froelicher VF, Genter F, Myers J (1984) Predicting oxygen uptake from treadmill testing in normal subjects and coronary artery disease patients. Am Heart J 108: 1454–1460. [DOI] [PubMed] [Google Scholar]
- 23.Alexander NB, Dengel DR, Olson RJ, Krajewski KM (2003) Oxygen-uptake (VO2) kinetics and functional mobility performance in impaired older adults. J Gerontol A Biol Sci Med Sci 58: M734–M739. [DOI] [PubMed] [Google Scholar]
- 24.Mezzani A, Agostoni P, Cohen-Solal A, Corra U, Jegier A, Kouidi E, et al. (2009) Standards for the use of cardiopulmonary exercise testing for the functional evaluation of cardiac patients: a report from the Exercise Physiology Section of the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil 16: 249–267. 10.1097/HJR.0b013e32832914c8 [DOI] [PubMed] [Google Scholar]
- 25.Sullivan M, Genter F, Savvides M, Roberts M, Myers J, Froelicher V (1984) The reproducibility of hemodynamic, electrocardiographic, and gas exchange data during treadmill exercise in patients with stable angina pectoris. Chest 86: 375–382. [DOI] [PubMed] [Google Scholar]
- 26.Elborn J, Stanford C, Nicholls D (1990) Reproducibility of cardiopulmonary parameters during exercise in patients with chronic cardiac failure. The need for a preliminary test. Eur Heart J 11: 75–81. [DOI] [PubMed] [Google Scholar]
- 27.Levy WC, Maichel BA, Steele NP, Leclerc KM, Stratton JR (2004) Biomechanical efficiency is decreased in heart failure during low-level steady state and maximal ramp exercise. Eur J Heart Fail 6: 917–926. [DOI] [PubMed] [Google Scholar]
- 28.Hiatt WR, Cox L, Greenwalt M, Griffin A, Schechter C (2005) Quality of the assessment of primary and secondary endpoints in claudication and critical leg ischemia trials. Vasc Med 10: 207–213. [DOI] [PubMed] [Google Scholar]
- 29.Gagge AP, Burton AC, Bazett HC (1941) A practical system of units for the description of the heat exchange of man with his environment. Science 94: 428–430. [DOI] [PubMed] [Google Scholar]
- 30.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE (2002) Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 346: 793–801. [DOI] [PubMed] [Google Scholar]
- 31.Kubrychtova V, Olson TP, Bailey KR, Thapa P, Allison TG, Johnson BD (2009) Heart rate recovery and prognosis in heart failure patients. Eur J Appl Physiol 105: 37–45. 10.1007/s00421-008-0870-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wicks JR, Oldridge NB, Nielsen LK, Vickers CE (2011) HR index-a simple method for the prediction of oxygen uptake. Med Sci Sports Exerc 43: 2005–2012. 10.1249/MSS.0b013e318217276e [DOI] [PubMed] [Google Scholar]
- 33.Robinson KA, Dunn AG, Tsafnat G, Glasziou P (2014) Citation networks of related trials are often disconnected: implications for bidirectional citation searches. J Clin Epidemiol 67: 793–799. 10.1016/j.jclinepi.2013.11.015 [DOI] [PubMed] [Google Scholar]
- 34.Bland JM, Altman D (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 327: 307–310. [PubMed] [Google Scholar]
- 35.Bruce RA, Kusumi F, Hosmer D (1973) Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J 85: 546–562. [DOI] [PubMed] [Google Scholar]
- 36.Dressendorfer R, Franklin B, Gordon S, Timmis G (1993) Resting oxygen uptake in coronary artery disease. Influence of chronic beta-blockade. Chest 104: 1269–1272. [DOI] [PubMed] [Google Scholar]
- 37.Oliveira RB, Myers J, Araujo CGS, Abella J, Mandic S, Froelicher V (2009) Maximal exercise oxygen pulse as a predictor of mortality among male veterans referred for exercise testing. Eur J Cardiovasc Prev Rehabil 16: 358–364. 10.1097/HJR.0b013e3283292fe8 [DOI] [PubMed] [Google Scholar]
- 38.Do Lee C, Folsom AR, Blair SN (2003) Physical activity and stroke risk a meta-analysis. Stroke 34: 2475–2481. 10.1161/01.STR.0000091843.02517.9D [DOI] [PubMed] [Google Scholar]
- 39.DeFina LF, Willis BL, Radford NB, Gao A, Leonard D, Haskell WL, et al. (2013) The association between midlife cardiorespiratory fitness levels and later-life dementia: a cohort study. Ann Intern Med 158: 162–168. 10.7326/0003-4819-158-3-201302050-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helmrich SP, Ragland DR, Leung RW, Paffenbarger RS Jr (1991) Physical activity and reduced occurrence of non-insulin-dependent diabetes mellitus. N Engl J Med 325: 147–152. 10.1056/NEJM199107183250302 [DOI] [PubMed] [Google Scholar]
- 41.Kaminsky LA, Arena R, Beckie TM, Brubaker PH, Church TS, Forman DE, et al. (2013) The importance of cardiorespiratory fitness in the United States: the need for a national registry a policy statement from the American Heart Association. Circulation 127: 652–662. 10.1161/CIR.0b013e31827ee100 [DOI] [PubMed] [Google Scholar]
- 42.Kaminsky LA, Arena R, Myers J (2015) Reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing: Data from the Fitness Registry and the Importance of Exercise National Database. Mayo Clin Proc 90: 1515–1523. 10.1016/j.mayocp.2015.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blair SN, Kohl HW 3rd, Paffenbarger RS Jr., Clark DG, Cooper KH, Gibbons LW (1989) Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 262: 2395–2401. [DOI] [PubMed] [Google Scholar]
- 44.Fox K, Borer JS, Camm AJ, Danchin N, Ferrari R, Sendon JLL, et al. (2007) Resting heart rate in cardiovascular disease. J Am Coll Cardiol 50: 823–830. 10.1016/j.jacc.2007.04.079 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File listing the 40 treadmill studies used for analysis.
(RTF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.