Abstract
Recent studies have shown an apparent increase in thyroid cancer in the United States. Whether is due to an actual increase or increased screening is disputed. We analyzed thyroid cancer incidence and mortality across age and racial groups in Oklahoma (using data from the Oklahoma Central Cancer Registry) against Surveillance, Epidemiology, and End Results (SEER) program national data – using SEER*Stat software for mortality. In the US and Oklahoma, females had a higher AAIR compared to males, but it was lower in Oklahoma than in the US (Females: US 15.5 per 100,000, OK 10.9 per 100,000; Males: US 5.4 per 100,000, OK 3.8 per 100,000). Overall, five-year relative survival was lower, yet still high, for Oklahoma than in the US (92.1% v. 97.1%). Survival by stage was lower in Oklahoma compared to the United States for localized (97.8% v. 99.8%), regional (92.0% v. 97.0%), and distant (36.6% v. 55.3%) stage cancers.
INTRODUCTION
Recent data have shown an apparent increase in the incidence of thyroid cancer in the United States.1 The annual percent change (for both men and women) from 1980 – 1997 was a 2.4% increase per year, but from 1997 to 2009, this increased to 6.6% per year.1 There is disagreement on the exact cause of this increase – particularly whether it is an accurate reflection on thyroid cancer cases actually increasing, or rather due to increased screening.2 Literature reviews suggests controversy, with evidence that could be interpreted to support either idea. Studies have shown that increased detection of the preclinical manifestations of thyroid cancer are a key contribution factor of the increased incidence rate.2 However, others have reported evidence of a true increase in incidence, that cannot be fully explained simply by an increase in diagnosis – and thus suggesting that the increase could be due to lifestyle and environmental factors, as well.3
The carcinomas of the thyroid are demarcated and named for the primary cell type within the thyroid that is affected.4 Typically, they are all referred to as thyroid cancer. Thyroid cancer is a relatively rare cancer, but it is the most common cancer of the endocrine system.5 The four subtypes of this cancer are papillary carcinoma, follicular carcinoma, medullary carcinoma, and anaplastic carcinoma.6 Papillary carcinoma, which comprises approximately 80 – 85% of all thyroid cancers, has the highest prognosis, with ten-year survival being more than 95%.6 Although frequently diagnosed at early stages, papillary carcinoma can spread to the cervical lymph nodes, and is highly associated with exposure to ionizing radiation.6 Follicular carcinoma comprises ten percent of thyroid cancer cases, and is one of the few types among all cancer types to spread through hematogenous routes instead of lymph pathways. Medullary carcinoma (comprising five percent of thyroid cancer cases) is associated with hereditary mutations of the RET oncogene. Lastly, anaplastic (or undifferentiated) carcinomas – which have the poorest prognosis – comprise less than five percent of all thyroid cancer cases. Generally, anaplastic carcinomas afflict the elderly far more than those of younger ages. These carcinomas are typically fatal within a few years.6
The American Cancer Society suggests routine screening as part of a periodic physical exam and health counseling to include thyroid cancer screening for men and women 20 years and older.10 The USPSTF is currently reviewing and updating their thyroid cancer screening recommendations.11 However, in 1996, their published recommendations for asymptomatic thyroid cancer screening using palpation or ultrasound was a grade D, meaning they recommend against the use of these screenings. Asymptomatic screening for those with childhood head and neck irradiation was a grade C, meaning it is suggested that these screenings are selectively offered based on patient preference and provider judgment.11
The purpose of this study was to analyze thyroid cancer incidence and mortality across age and racial groups in Oklahoma against Surveillance, Epidemiology, and End Results (SEER) program data.
METHODS
To determine incidence rates of thyroid cancer in Oklahoma, we used existing data from the Oklahoma Central Cancer Registry (OCCR), which includes data on all cancer cases diagnosed in Oklahoma residents from 1997–2012. The OCCR is a participant in the National Program of Cancer Registries and follows all guidelines of the North American Association of Central Cancer Registries. To obtain data on incidence of thyroid cancer in the US, we used the Surveillance, Epidemiology, and End Results (SEER) program data from 1997–2012. We restricted this analysis to those diagnosed with thyroid cancer using the SEER site recodes for thyroid cancer, which included International Classification of Diseases of Oncology, Third Edition/World Health Organization (ICDO-3/WHO) 2008 site codes of C739, excluding histology codes of 9050-9055, 9140, 9590-9992 (lymphomas, Kaposi sarcoma, and mesothelioma).12 We used SAS v. 9.4 (Cary, NC) to calculate age-adjusted incidence rates (AAIR) per 100,000 by the direct method using the 2000 standard population.13 To calculate AAIR for the US, we used SEER*Stat software (v. 8.2.1) using the SEER 13 region data, which included more detailed information on race and Hispanic ethnicity.14 We compared AAIR and 95% confidence intervals over time from 1997–2012, by demographic groups of sex, and race/ethnicity.14 We classified race/ethnicity as white non-Hispanic (NH), African American NH, American Indian/Alaska Native (AI/AN) NH, Asian/Pacific Islander NH, and Hispanic. To reduce misclassification of the AI/AN population, we linked the cancer records with the Indian Health Service (IHS) records. For this study, AI/ANs who either reported their primary race as AI/AN or who linked with an IHS record regardless of the primary race selected, were reclassified as AI/AN. Age-specific incidence rates were calculated in five-year age groups, combining those under 20 years of age and 75 years and older for stability and confidentiality reasons. We also evaluated stage at diagnosis using the SEER Summary Stage 2000 for those diagnosed between 1998 and 2003 and the derived SEER Summary Stage 2000 for those diagnosed between 2004 and 2012 for Oklahoma and the Summary Stage 2000 for the SEER data from 1998–2012. We excluded cancers reported on a death certificate only or at autopsy for analysis of staging.
To evaluate survival from thyroid cancer, we used data from the OCCR and SEER using SEER*Stat software. We calculated five-year relative survival by age, sex, race, stage, and year of diagnosis for Oklahoma and SEER data using the expected survival life tables provided by SEER.16 By comparing the observed and expected survival in the population, relative survival allows for the estimation of excess deaths due to cancer in the population without the need for underlying cause of death.15 Expected survival was only available for the racial groups of white, African American, and other (AI/NA and Asians/Pacific Islanders). Hispanic ethnicity was also not available. We grouped year of diagnosis into 1997–2000, 2001–2003, and 2004–2008 for stability purposes. We used the same SEER Summary Stage classification as in the incidence estimates. The OCCR conducts routine follow-up on all cancer cases and links with the Oklahoma Mortality Data, the Social Security Death Index, and the National Death Index to determine mortality. Follow-up was conducted through November 1, 2014, thus we limited our analysis of survival from 1997–2008 to allow for more complete five-year survival for all cases of thyroid cancer.
This study was approved by the Institutional Review Boards of the University of Oklahoma Health Sciences Center and the Oklahoma State Department of Health.
RESULTS
Incidence
Thyroid cancer is the 8th leading cause of cancer in the United States with an estimated 62,450 new cases in 2015 accounting for 3.8% of cancers.1 In 2015, there will be an estimated 1,950 deaths due to thyroid cancer which accounts for 0.3% of all cancer mortality.1 In the US, the age-adjusted incidence rate of thyroid cancer from 1997–2012 was 10.5 per 100,000 (95% CI: 10.4, 10.6 per 100,000) compared to 7.4 per 100,000 in Oklahoma (95% CI: 7.2, 7.6) (Table 1). In both the US and in Oklahoma, females had a higher AAIR compared to males, but the estimate was lower in Oklahoma than in the US (Females: US 15.5 per 100,000, OK 10.9 per 100,000; Males: US 5.4 per 100,000, OK 3.8 per 100,000). When evaluating race/ethnicity, the AAIR was higher in the US for all race/ethnicity groups except AI/AN NH, which were similar at 8.2 per 100,000 in the US (95% CI: 7.5, 9.0) and 8.7 in Oklahoma (95% CI: 7.8, 9.6). Among Asian/Pacific Islander NHs, the incidence in the US was 10.9 per 100,000 (95% CI: 9.1, 9.5) compared to 3.5 per 100,000 (95% CI: 1.9, 5.1) in Oklahoma, though the Oklahoma estimate was imprecise. Age-specific incidence increased with increasing age until 75 years, when it decreased to 14.0 per 100,000 in the US and 11.7 per 100,000 in Oklahoma. Again, the age-specific incidence was higher in the US and Oklahoma for all age groups. Regarding stage, 66.5% were diagnosed at local stage, 18.6% at regional stage, and 4.9% at distant stage (10.1% unknown) in Oklahoma, compared to the US with 64.0% at local stage, 25.7% at regional stage, and 4.7% at distant stage (2.0% unknown). In our analysis by year of diagnosis, the AAIR increased in both the US and Oklahoma, though remained lower in Oklahoma than the US throughout the period (Figure 1). In Oklahoma, the AAIR increased from 3.9 per 100,000 in 1997 to 12.3 per 100,000 in 2012. In the US, the AAIR increased from 6.7 per 100,000 in 1997 to 14.2 per 100,000 in 2012.
Table 1.
Oklahoma and United States incidence (per 100,000) of thyroid cancer by gender, age, and race (1997 – 2012)21
| Characteristic | Oklahoma Incidence % (95% CI) | United States Incidence % (95% CI) |
|---|---|---|
| Overall | 7.4 (7.2, 7.6) | 10.5 (10.4, 10.6) |
| Sex | ||
| Male | 3.8 (3.6, 4.1) | 5.4 (5.3, 5.4) |
| Female | 10.9 (10.5, 11.3) | 15.5 (15.4, 15.6) |
| Race/Ethnicity | ||
| White NH | 7.6 (7.3, 7.9) | 11.5 (11.4, 11.6) |
| African American NH | 4.9 (4.2, 5.6) | 6.6 (6.4, 6.8) |
| American Indian/Alaska Native NH | 8.7 (7.8, 9.6) | 8.2 (7.5, 9.0) |
| Asian or Pacific Islander NH | 3.5 (1.9, 5.1) | 10.9 (10.7, 11.2) |
| Hispanic | 7.2 (5.9, 8.5) | 9.3 (9.1, 9.5) |
| Age at Diagnosis | ||
| 0–19 years | 0.4 (0.3, 0.5) | 0.7 (0.7, 0.7) |
| 20–24 years | 3.8 (3.2, 4.4) | 4.9 (4.7, 5.1) |
| 25–29 years | 6.3 (5.5, 7.1) | 8.4 (8.1, 8.7) |
| 30–34 years | 8.5 (7.5, 9.4) | 11.3 (11.0, 11.6) |
| 35–39 years | 9.6 (8.6, 10.6) | 13.9 (13.6, 14.3) |
| 40–44 years | 9.8 (8.9, 10.8) | 15.4 (15.0, 15.7) |
| 45–49 years | 11.6 (10.6, 12.7) | 16.9 (16.6, 17.3) |
| 50–54 years | 12.7 (11.6, 13.9) | 18.8 (18.3, 19.2) |
| 55–59 years | 12.8 (11.5, 14.0) | 19.2 (18.7, 19.6) |
| 60–64 years | 13.1 (11.8, 14.5) | 19.4 (18.9, 19.9) |
| 65–69 years | 15.4 (13.7, 17.0) | 20.8 (20.2, 21.5) |
| 70–74 years | 14.3 (12.6, 16.0) | 19.0 (18.3, 19.6) |
| 75+ years | 11.7 (10.6, 12.8) | 14.0 (13.6, 14.4) |
Figure 1.
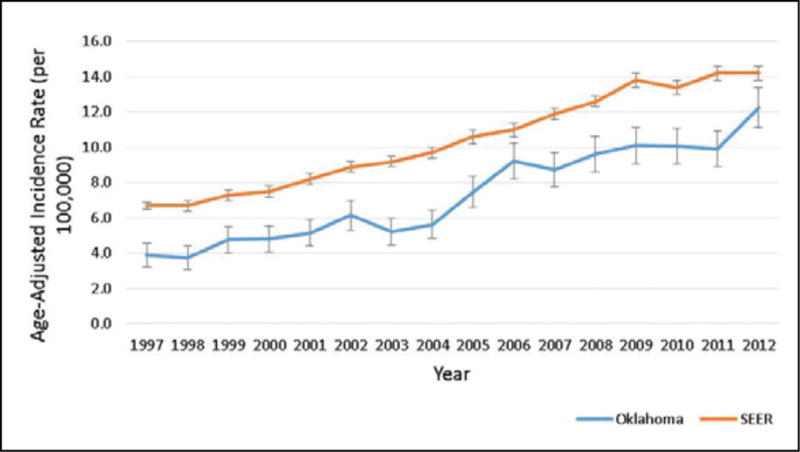
Age-adjusted incidence rates per 100,000 and 95% confidence intervals by year for the US and Oklahoma, 1997–2012.
Survival
Overall, five-year relative survival was lower, yet still high, for Oklahoma than in the US (92.1% v. 97.1%) (Table 2). In general, survival was higher for females than males, but was lower for both males (84.2% v. 94.4%) and females (94.7% v. 98.0%) in Oklahoma compared to the US, respectively. Regarding race, survival was highest in the unknown and white population in the US, followed by African Americans and those in the Other category. In Oklahoma, there were no significant differences by race; however, African Americans had a lower estimate compared to other racial groups. When comparing Oklahoma and the US, white (92.2% v. 97.4%) and African Americans (85.9% v. 95.3%) in Oklahoma had lower survival than those in the US, respectively, but the confidence intervals overlapped for Other and unknown racial groups. While survival generally decreased with increasing age, based on confidence intervals, Oklahomans from ages 40–44 years, 50–54 years, 55–59 years, and 75 years and older at cancer diagnosis had poorer five-year relative survival compared to these same age groups in SEER, but the confidence intervals overlapped for the remaining age groups. Over time, survival increased slightly in the US from 95.9% in 1997–2000 to 97.6% in 2004–2008. While there was no significant increase observed in Oklahoma, the survival estimates increased from 90.4% in 1997–2000 to 93.3% in 2004–2008. When comparing Oklahoma and the US, survival was lower, yet still above 90%, for each category of year of diagnosis in Oklahoma. Survival by stage was also lower in Oklahoma for localized (97.8% v. 99.8%), regional (92.0% v. 97.0%), and distant (36.6% v. 55.3%) stage cancers, but followed similar trends.
Table 2.
Five-year relative survival for thyroid cancer in Oklahoma and the US by selected characteristics, 1997–2008.16
| Characteristic | Oklahoma 5-Year Relative Survival % (95% CI) | SEER 5-Year Relative Survival % (95% CI) |
|---|---|---|
| Overall | 92.1 (90.8, 93.5) | 97.1 (96.9, 97.3) |
| Sex | ||
| Male | 84.2 (80.7, 87.6) | 94.4 (93.8, 94.9) |
| Female | 94.7 (93.4, 96.1) | 98.0 (97.8, 98.2) |
| Race/Ethnicity | ||
| White | 92.2 (90.8, 93.7) | 97.4 (97.2, 97.6) |
| African American | 85.9 (77.7, 94.1) | 95.3 (94.2, 96.1) |
| Othera | 93.5 (90.2, 96.9) | 95.9 (95.2, 96.4) |
| Unknown | 96.2 (86.0, 100.0) | 99.8 (94.8, 100.0) |
| Age at Diagnosis | ||
| 0–19 years | 97.9 (93.4, 100.0) | 98.7 (97.9, 99.2) |
| 20–24 years | 99.3 (97.5, 100.0) | 99.5 (99.1, 99.8) |
| 25–29 years | 99.2 (97.5, 100.0) | 99.7 (99.3, 99.9) |
| 30–34 years | 99.5 (98.1, 100.0) | 99.7 (99.4, 99.8) |
| 35–39 years | 98.9 (97.2, 100.0) | 99.5 (99.2, 99.7) |
| 40–44 years | 95.9 (93.2, 98.6) | 99.4 (99.1, 99.6) |
| 45–49 years | 97.2 (94.8, 99.6) | 98.9 (98.5, 99.2) |
| 50–54 years | 93.9 (90.7, 97.2) | 98.3 (97.8, 98.7) |
| 55–59 years | 90.5 (86.0, 94.9) | 96.8 (96.2, 97.4) |
| 60–64 years | 89.1 (83.7, 94.5) | 95.4 (94.4, 96.2) |
| 65–69 years | 90.8 (85.3, 96.4) | 94.7 (93.5, 95.8) |
| 70–74 years | 82.9 (74.9, 90.8) | 89.8 (87.9, 91.5) |
| 75+ years | 69.2 (60.1, 78.3) | 81.9 (79.9, 83.8) |
| Year of Diagnosis | ||
| 1997–2000 | 90.4 (87.4, 93.5) | 95.9 (95.4, 96.4) |
| 2001–2003 | 90.6 (87.7, 93.6) | 96.9 (96.4, 97.2) |
| 2004–2008 | 93.3 (91.6, 95.0) | 97.6 (97.4, 97.9) |
| Stage | ||
| Localized | 97.8 (96.6, 99.1) | 99.8 (99.7, 99.9) |
| Regional | 92.0 (88.9, 95.1) | 97.0 (96.6, 97.4) |
| Distant | 36.6 (27.7, 45.6) | 55.3 (53.4, 57.1) |
| Unknown/unstaged | 84.5 (79.5, 89.5) | 88.3 (86.3, 90.1) |
DISCUSSION
Thyroid cancer is not a particularly common cause of cancer, even with the recent apparent increases in incidence. Historically thyroid cancer survivability has been very high – among the highest of all types of cancer, with the five-year relative rate having been consistently above ninety percent.1 Currently, that rate is almost ninety-eight percent in the US.1 Though the incidence continues to rise, there does not appear to be a concurrent effect on survival, as evidence by our data. The relationship between this admittedly minute uptick and the recent increases in incidence have not yet been conclusively demonstrated. The high overall relative survival suggests that the cases being detected are either not clinically important or are being effectively managed with the treatment options currently at hand.
Women in general, across all races, have a higher incidence of thyroid cancer than men.1 In the United States, whites and Asians/Pacific Islanders have a higher incidence rate of thyroid cancer, with African Americans having a particularly low incidence rate.1 However, this may not be reflective of actual incidence rates, and may be more reflective of increased detection (medical surveillance bias), in turn stemming from increased access to healthcare – which itself is correlated with increased wealth.17
The clinical stage at which thyroid cancer is first detected can have quite a pronounced effect on survivability. As is the case with most all cancer scenarios, an earlier diagnosis yields higher rates of survival. Metastasis to regional lymph nodes, which can make the cancer more likely to spread to other parts of the body,6 does not decrease survivability considerably. However, metastasis to more distant sites does dramatically reduce survivability (from nearly one hundred percent to almost half at fifty-four percent).1
All the characteristic-specific rates in Oklahoma are lower than the corresponding rates in the United States. This can be because of a truly lower incidence across all groups, or from a lower rate of diagnosis across all groups – or even a combination of both. As mentioned previously, there is conflicting evidence and marked controversy about the recent increase in thyroid cancer incidence.2,3 That same uncertainty can be present in Oklahoma as well. This disparity presents an intriguing avenue for future study. Furthermore, future studies should consider evaluating specific subtypes of thyroid cancer by race/ethnicity to understand whether disparities in incidence and survival exist. Oklahoma’s survivability figures were less than those of the United States in virtually every characteristic. This may be due to Oklahoma’s relative poverty compared to the United States as a whole.18 As established above, socioeconomic status (SES) is correlated with relative increases in diagnosis and incidence of thyroid cancer, but SES is also correlated with increased use of medical care.17,19 Poverty may be associated with a relative decreases in diagnosis and incidence of thyroid cancer.20 Following diagnosis, the various treatment options for thyroid cancer all require significant medical care by specialists.21–23 Since specialist medical care is disproportionately available to and used by the affluent,17 an economically disadvantaged population will have less access to such care. This will have a negative effect on survivability.21–23 Thus, a state like Oklahoma, with generally lower SES, can be expected to have both lower incidence rates and lower survivability for thyroid cancer.
Although thyroid cancer is not an extensive cause of cancer mortality, the increasing incidence rate makes it of great interest. The exact cause of increase has not yet been explained and is probably mutlifactorial. Prevention guidelines have not been put forth as evidence-based strategies have not yet been published. Further research is needed to identify the factors contributing to the increasing incidence of thyroid cancer, both in the United States and Oklahoma. Further, the deviation from national data for thyroid cancer incidence across racial groups is also a possible avenue for further study. The reasons for Oklahoma’s lower incidence rates and survivability are also as yet inconclusive, and certainly merit further study. This information will assist in establishing solid prevention recommendations which may decrease the incidence of thyroid cancers in the future. Additionally, it can guide the recommendations for diagnosis treatment so as not to over treat subclinical cancers.
Acknowledgments
FUNDING
JC and AJ were partially supported by grants NU58DP005513 from the Centers for Disease Control and Prevention. The content is solely the responsibility of the authors and does not necessarily represent the official views of the CDC. JC was partially supported by grant AIAMP120011 from the Office of Minority Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the OMH.
Footnotes
DISCLOSURES
The authors have no financial disclosures.
References
- 1.Thyroid cancer facts and figures. National Cancer Institute Surveillance, Epidemiology, and End Results Program Web Site. http://seer.cancer.gov/statfacts/html/thyro.html. Accessed September 20, 2015.
- 2.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;285:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 3.Enewold, Zhu K, Ron E, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiology Biomarkers & Prevention. 2009;18:784–791. doi: 10.1158/1055-9965.EPI-08-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thyroid cancer treatment. National Cancer Institute at the National Institutes of Health Web site. http://www.cancer.gov/types/thyroid/patient/thyroid-treatment-pdq. Accessed 20 July 2015.
- 5.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the US, 1985–1995. Cancer. 83(12):2638–2648. doi: 10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 6.Sattar HA. Fundamentals of Pathology. Chicago: Pathoma.com; 2011. [Google Scholar]
- 7.Thyroid cancer. American Cancer Society Web Site. http://www.cancer.org/acs/groups/cid/documents/webcontent/003144-pdf. Accessed Jun 10, 2015.
- 8.NCCN clinical practice guidelines in oncology: thyroid carcinoma Version 1.2105. National Comprehensive Cancer Network Web Site. doi: 10.6004/jnccn.2022.0040. http://www.nccn.org/professionals/physician_gls/PDF/thyroid.pdf. Accessed June 10, 2015. [DOI] [PubMed]
- 9.Thyroid cancer. American Thyroid Association. http://www.thyroid.org/cancer-of-the-thyroid-gland/. Accessed Jun 10, 2015.
- 10.Screening guidelines. American Cancer Society Web Site. http://www.cancer.org/healthy/findcancerearly/cancerscreeningguidelines/chronological-history-of-acs-recommendations. Accessed Jun 20, 2015.
- 11.Thyroid cancer: screening. US Preventive Service Task Force Web Site. http://www.uspreventiveservicestaskforce.org/BrowseRec/Search?s=thyroid+cancer. Accessed Jun 10, 2015.
- 12.Site Recode ICD-O-3/WHO 2008 Definition. National Cancer Institute Surveillance, Epidemiology, and End Results Program Web Site. http://seer.cancer.gov/siterecode/icdo3_dwhoheme/index.html. Accessed October 3, 2015.
- 13.Standard Populations. National Cancer Institute Surveillance, Epidemiology, and End Results Program Web Site. http://seer.cancer.gov/stdpopulations/stdpop.19ages.html. Accessed October 3, 2015.
- 14.Surveillance, Epidemiology, and End Results (SEER) Program SEER*Stat Database: Incidence - SEER 13 Regs Research Data, Nov 2014 Sub (1992–2012) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2013 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2015, based on the November 2014 submission. http://www.seer.cancer.gov. Accessed October 3, 2015
- 15.Mariotto AB, Noone AM, Howlader N, Cho H, Keel GE, Garshell J, et al. Cancer survival: an overview of measures, uses, and interpretation. J Natl Cancer Inst Monogr. 2014;2014(49):145–186. doi: 10.1093/jncimonographs/lgu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howlader N, Noone AM, Krapcho M, et al., editors. SEER cancer statistics review, 1975–2012. National Cancer Institute Surveillance Epidemiology, and End Results Program Web Site. http://seer.cancer.gov/csr/1975_2012/, Updated April 2015. Accessed September 29, 2015.
- 17.Van Doorslaer E, Masseria C, Koolman X. Inequalities in access to medical care by income in developed countries. Canadian Med Assoc J. 2006;174:177–183. doi: 10.1503/cmaj.050584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Median Household Income by Race: Oklahoma. US Census Web Site. http://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=ACS_10_SF4_B19013&prodType=table. Accessed September 25, 2015.
- 19.Ahn HS, Hyun JK, Welch HG. Korea’s thyroid-cancer “epidemic”-screening and overdiagnosis. N Engl J Med. 2014;371:1765–1767. doi: 10.1056/NEJMp1409841. [DOI] [PubMed] [Google Scholar]
- 20.Morris LGT, Sikora AG, Tosteson TD, Davies L. The Increasing Incidence of Thyroid Cancer: The Influence of Access to Care. Thyroid. 2013;23:885–891. doi: 10.1089/thy.2013.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bilimoria KY, Bentrem DJ, Ko CY, et al. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg. 2007;246(3):375–81. doi: 10.1097/SLA.0b013e31814697d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hay ID, Grant CS, Bergstralh EJ, Thompson GB, Van Heerden JA, Goellner JR. Unilateral total lobectomy: is it sufficient surgical treatment for patients with AMES low-risk papillary thyroid carcinoma? Surgery. 1998;124:958–64. [PubMed] [Google Scholar]
- 23.Biondi B, Filetti S, Schlumberger M. Thyroid-hormone therapy and thyroid cancer: a reassessment. Nat Clin Pract Endocrinol Metab. 2005;1:32–40. doi: 10.1038/ncpendmet0020. [DOI] [PubMed] [Google Scholar]


