Abstract
Objectives: This study sought to assess the value of differing pre-operative measures in prediction of post-operative non-surgical site infection (NSSI) and length of hospital stay following hip fracture surgery. Methods: All patients admitted during a one year period with a hip fracture to our department were included in the study (n=207). Primary outcome measures were ten independent risk factors correlated to the development of non-surgical site infection following surgery for hip fracture. Secondary outcome measures were duration of hospital stay and inpatient mortality. Results: The patients who had severe cognitive impairment had a 71.0% risk of developing non-surgical site infection. Patients who had multiple medical co-morbidities also had increased risk of developing non-surgical site infection at 59.1%. Patients who developed NSSI on average stayed in hospital 13.1 days longer than patients who did not (31.6 vs. 18.5, p < .001). Conclusions: This study demonstrates the importance of reducing post-operative infection in hip fracture patients in view of reducing morbidity, mortality and cost. These patients can be stratified by risk factors and interventions can be employed in view of reducing inpatient post-operative infection rates in this cohort.
Keywords: dementia, hip fracture, cognitive impairment, co-morbidity, risk factors, AMTS
Introduction
Hip fractures are common, accounting for 70,000 hospital admissions per year in the United Kingdom, a figure set to double as the population ages (National Institute for Health and Care Excellence [NICE], 2009). Hip fractures also represent a significant cause of morbidity and mortality for patients, as well as a large financial burden on health care provision with the estimated cost for inpatient treatment alone is £2 billion per year (NICE, 2009). A large proportion of the morbidity, mortality, and cost associated with hip fractures can be attributed to post-operative complications, of which infection is a major cause (Shah, Wainess, & Karunakar, 2005). Patients most at risk of a hip fractures tend to be older, less mobile, and to have other co-morbidities, all of which are risk factors for the development of an infection in any hospital inpatient. Infections can arise from a number of sources, including the lower respiratory tract (LRTI), urinary tract (UTI), and skin. Infections that arise from outside the surgical operative field are termed non-surgical site infections (NSSI).
Despite the important implications of developing an infection in the post-operative period following a hip fracture, there are currently no quantitative methods for pre-operatively predicting the risk of post-operative infection. The Nottingham Hip Fracture Score (NHFS) is a method of predicting mortality at both 30 days and 1 year (Moppett et al., 2012). This score incorporates a host of factors, including age, hemoglobin concentration, mental state, and medical co-morbidities. To measure the latter two, the abbreviated mental test score (AMTS) and the American Society of Anesthesiologist (ASA) scoring systems can be used. The AMTS is a well-validated measure of cognitive function (Hoskinson, 1972; Jackson, Naqvi, & Sheehan, 2013). The ASA is a grading system used to categorize the severity of a patient’s co-morbidities prior to undergoing a general anesthetic (Keats, 1978). Independent of the NHFS, both scoring systems have already been shown to have a role in predicting mortality following hip fracture (Bombaci et al., 2012; Chia, Gualano, Seevanayagam, & Weinberg, 2013).
This study builds on the work of Bombaci and Chia on identifying and reducing the rate of NSSI following surgical management of hip fractures (Bombaci et al., 2012; Chia et al., 2013). Using a variety of pre-operative and intra-operative measures, including the NHFS, we look at the predictive power of highlighting “the at risk population” within our cohort. We then hypothesize targeted interventions to reduce NSSI to those most at risk. We investigate the outcome of these infections, using the duration of stay and mortality observed in these cases.
Patients and Method
The study population included men and women of any age, admitted in a 1-year period, who received any surgery for hip fracture in our hospital. Data were retrieved from a prospectively constructed database. Patients were pre-operatively assessed for the seven factors that comprise the NHFS. These were admission hemoglobin concentration, age, gender, AMTS, ASA grade, presence of malignancy, and place of residence. The NHFS was then calculated and recorded.
The AMTS score stratified patients into one of three categories: severe (AMTS < 7), moderate (AMTS 7-9), and no cognitive impairment (AMTS 10). These groupings were based on previous studies using the same, or similar approaches (Jackson et al., 2013). ASA score stratified patients into one of four categories: those patients with no systemic disease (ASA 1), mild systemic disease (ASA 2), severe systemic disease (ASA 3), and disease that is constantly a threat to life (ASA 4). Hemoglobin concentration was divided into <10 g/dL and >10 g/dL on admission and age into three groups (<65 years, 65-75 years, and >75 years). These groupings not only correlate with previous work but also the NHFS.
Patients were screened for urinary tract infection (UTI) or LRTI on hospital admission and any resultant infection recorded. During admission, the development of any post-operative infection was recorded. NSSI was determined retrospectively from a combination of medical notes, nursing notes, discharge summaries, and investigation results. NSSI was only diagnosed if the patients met the criteria laid out in Figure 1, which were based on the Loeb criteria for diagnosing infection in nursing home patients (Loeb, 2003). The data were blinded and checked by two separate investigators. Any disputes were resolved by a third assessor. Patients who on admission had either an UTI or an LRTI were not deemed to have developed an NSSI unless the post-operative infection was deemed to be different from the original infection. Time from admission to theater was measured and correlated with post-operative NSSI.
Figure 1.

A table highlighting how NSSI was diagnosed.
Note. NSSI = non surgical-site infection.
Duration of hospital stay was recorded from operation to time of discharge from hospital. This enabled the operation to act as a baseline starting point for all patients. During their hospital stay, all patients received as similar care as possible with regular orthogeriatrician and medical review. Patients who died during their admission were excluded from calculations about duration of stay. Duration of time from admission to theater and catheter insertion post or intra-operatively was recorded.
The primary outcome measure was development of NSSI as a hospital inpatient. Secondary outcome measures were mortality as an inpatient and duration of inpatient stay.
Statistical Analysis
The chi-square test for trend was performed to assess significance between NSSI within the patient sub-groups. This was an appropriate test because these groups are both ordered categorical variables.
The values for length of stay were deemed not to be normally distributed by the Shapiro–Wilk test for normality. Therefore, the non-parametric Mann–Whitney U test was performed to elucidate any significant differences in length of hospital stay for those patients who developed a post-operative NSSI compared with those who did not. A one-way ANOVA was performed to compare the length of hospital stay across different sub-groups. Tukey’s post hoc analysis was then used to compare values between groups. For all statistical analyses, a p value of <.05 was deemed significant.
Results
Study Population Profile
Two hundred seven patients underwent surgery for hip fracture during the 1-year study period. All had a complete set of data recorded comprising age, gender, admission AMTS, ASA grade, presence of malignancy, place of residence, admission hemoglobin concentration, presence of catheter, fracture type, and infection recorded on admission.
Development of Infection, Length of Stay, and Mortality
Overall, 40.6% (84 / 207) patients developed a post-operative NSSI following surgery for hip fracture. The distribution of infection type is shown in Figure 2. Twenty patients (9.6%) had an infection on admission, either confirmed UTI or LRTI. Of these patients, five (25.0%) acquired further post-operative NSSIs during their admission. These were deemed to be different infections from the primary admission infection due either the type of infection or the causative organism of the infections identified.
Figure 2.
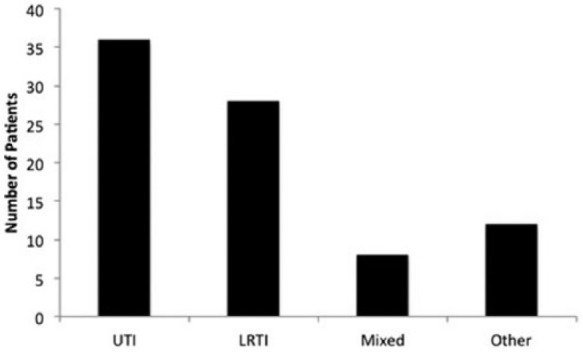
A graph showing the number of patients who developed each type of NSSI following surgery.
Note. UTI = urinary tract infection.
The average length of stay following hip fracture surgery was 23.9 days (SD = 18.1 days). Patients who did not develop an NSSI stayed in hospital for an average of 18.5 days (SD = 13.4 days), which was significantly shorter when compared with patients who developed an NSSI (31.6 days, SD = 24.5 days, p < .001).
Overall, 7.2% (15 / 207) of patients died during their hospital stay. Of these, 14 had infective causes (12 / 14 from LRTI; 2 / 14 from urosepsis), and one had cardiac arrest as the primary causes of death.
NHFS
Patients were all scored using the NHFS. The patients were divided into two groups, those scoring greater than 4 (high risk) and those scoring less than or equal to 4 (low risk). Of all, 41.1% (85 / 207) were classified as low-risk and 58.9% (122 / 207) were classified as high-risk patients. This scoring system achieved significance when assessing NSSI using the chi-square test.
AMTS
Thirty percent (62 / 207) patients had an AMTS of less than 7 and were deemed to have severe cognitive impairment; 25.6% (53 / 207) of patients were in the moderate cognitive impairment group; and most patients, 41.6% (86 / 207), had no cognitive impairment. Seventy-one percent (44 / 64) of patients with severe cognitive impairment developed an NSSI. This was more than double the proportion of patients with moderate impairment who developed a post-operative infection (34.0%, 18 / 53). In patients with no cognitive impairment, only 25.6% (22 / 86) developed a new NSSI following their operation. Chi-square test for trend showed a significant trend for a reduction in the probability of developing of post-operative infection with increasing pre-operative cognitive function (p < .001), summarized in Table 1.
Table 1.
The Number of Patients (and Percentage of Patients) Who Did and Who Did Not Develop NSSI With the χ2 p Value Given for Each Subgroup.
| Number of patients (% of each group) |
|||
|---|---|---|---|
| Inpatient NSSI | No inpatient NSSI | p value | |
| NHFS | |||
| ≤4 | 18 (21.2%) | 67 (78.8%) | <.001* |
| >4 | 66 (54.1%) | 56 (45.9%) | .002* |
| AMTS | |||
| <7 | 44 (71.0%) | 18 (29.0%) | <.001* |
| 7-9 | 18 (34.0%) | 35 (66.0%) | .327 |
| 10 | 22 (25.6%) | 64 (74.4%) | .005* |
| ASA | |||
| 1 | 0 (0.0%) | 10 (100.0%) | .009* |
| 2 | 14 (25.9%) | 40 (74.1%) | .028* |
| 3 | 54 (46.6%) | 62 (53.4%) | .190 |
| 4 | 16 (59.3%) | 11 (40.7%) | .048* |
| Age | |||
| <65 years | 3 (18.8%) | 13 (81.3%) | .075 |
| 65-75 years | 39 (36.5%) | 68 (63.6%) | .384 |
| >75 years | 42 (50.0%) | 42 (50.0%) | .079 |
| Hemoglobin | |||
| <10 g/dL | 6 (50.0%) | 6 (50.0%) | .506 |
| >10 g/dL | 78 (40.0%) | 117 (60%) | .869 |
| Residence | |||
| Nursing home | 24 (54.5%) | 20 (45.5%) | .059 |
| Own home | 60 (36.8%) | 103 (63.2%) | .327 |
| Gender | |||
| Male | 26 (37.1%) | 44 (62.9%) | .558 |
| Female | 58 (42.3%) | 79 (57.7%) | .676 |
| Fracture type | |||
| Intra-capsular (displaced) | 36 (40.9%) | 52 (59.1%) | .950 |
| Intra-capsular (undisplaced) | 3 (21.4%) | 11 (78.6%) | .144 |
| Intertrochanteric | 38 (41.3%) | 54 (58.7%) | .887 |
| Sub-trochanteric | 7 (53.8%) | 6 (46.2%) | .330 |
| Time to theater | |||
| <36 hr | 63 (39.4%) | 97 (60.6%) | .756 |
| >36 hr | 21 (44.7%) | 26 (55.3%) | .567 |
| Catheterization | |||
| Catheter | 60 (51.7%) | 56 (48.3%) | .015* |
| No catheter | 24 (26.4%) | 67 (73.6%) | .006* |
Note. NSSI = non surgical-site infection; NHFS = Nottingham Hip Fracture Score; AMTS = abbreviated mental test score; ASA = American Society of Anesthesiologist.
= p<0.05.
ASA
Most patients were classified as ASA 3 (116 / 207, 56.0%), followed by ASA 2 (54 / 207, 26.1%). Fewest patients were classified as ASA 1 (10 / 207, 4.8%) and ASA 4 (27 / 207, 13.4%). There was a significant trend for increasing risk of infection with increased pre-operative co-morbidities, as measured using the ASA grade (p < .05, test for trend). Patients with a high ASA grade of four had an absolute risk of developing an NSSI of 59.1%. None of those in the ASA 1 group developed an infection, a trend which was consistent across intermediate ASA groups (ASA 3: 46.6%; ASA 2: 25.9%). These results are summarized in Table 1. Of the ASA 4 group, 25.0% (4 / 16) of those who developed an infection and 14.8% (4 / 27) of the whole group died secondary to the LRTI that they developed during their hospital stay.
Age, Admission Hemoglobin, Gender, Place of Residence, and Malignancy
Eighty-four out of 207 (40.6%) patients were above 75 years old on admission, with the commonest age group being between 65 and 75 years at 51.7% (107 / 207) of patients. Only 16 (7.7%) patients were below 65 years of age. The majority of patients had a hemoglobin concentration of greater than 10 g/dL on admission (195 / 207, 94.2%). Forty-four out of 207 (21.3%) patients were admitted from a nursing home or residential home compared with 163 / 207 (78.7%) who were admitted from their own homes. Male patients comprised 33.8% (70 / 207) compared with 66.2% (137 / 207) patients who were female. Malignancy was not included in the calculations because of such small numbers in our cohort, 1.0% (2 / 194). Age, admission hemoglobin concentration, gender, or place of residence did not achieve significance as predictive measures of post-operative NSSI when tested using the chi-square test (Table 1).
Type of Fracture and Time to Theater
The most common fracture types were intertrochanteric (92 / 207, 44.4%) and displaced intra-capsular fractures (88 / 207, 42.5%); 6.8% (14 / 207) of fractures were undisplaced intra-capsular fractures; and 6.3% (13 / 207) of fractures were sub-trochanteric fractures. Most patients, 160 / 207 (77.3%), had their surgery within 36 hr of admission. Both of these measures did not achieve significance as predictive measures of post-operative NSSI when using the chi-square test (Table 1).
Catheterization
Post-operatively, 56.0% of patients (116 / 207) were catheterized, compared with 44.0% of patients (91 / 207) who were not. Of the catheterized patients, 51.7% (60 / 207) developed a post-operative NSSI (p < .05, χ2 test). Out of the 36 patients who developed an UTI, 86.1% had a catheter (p < .01, χ2 test); however, 27.2% (31 / 116) of patients who were catheterized developed an UTI, which did not achieve significance.
Discussion
This study has demonstrated that there is a correlation between certain risk factors and development of NSSI following hip fractures. It has also shown that the development of such an infection can significantly increase the length of inpatient stay and contribute to inpatient mortality.
Development of NSSI
Overall, 40.6% of patients in this study developed an NSSI, which is similar to infection rates from other hospitals (Poh & Lingaraj, 2013). The NHFS was used to find risk factors that may have increased the risk of NSSI post-operatively. Among the severely cognitively impaired patients, 71.0% developed an NSSI, the most common of which UTIs and LRTIs. One potential explanation for this increased risk concerns post-operative mobility and physiotherapy. Chest physiotherapy is an effective technique for decreasing the risk of LRTI post-operatively (Castro, Calil, Freitas, Oliveira, & Porto, 2013). The incidence of UTIs can be reduced by good personal hygiene (Wang et al., 2013). The ability to comply with both of these measures may be impaired in patients with poor cognitive function. In addition, patients with some level of confusion have a higher risk of incontinence (Prosser, 1997), and their likelihood of being managed with catheterization introduces a further risk factor for developing UTIs (Isikgoz Tasbakan et al., 2013), which was demonstrated by 86.1% of patients who had an UTI also had a catheter in situ.
Increasing ASA grade was shown to significantly increase the risk of post-operative infection with 59.1% of ASA 4 patients developing an NSSI post-operatively. This substantiates previous studies showing ASA to be a predictor of post-operative morbidity and mortality in hip fractures (Parker, Beatty, Giuffre, Scholes, & Coolican, 2011; Poh & Lingaraj, 2013). The reason for this relationship is likely multifactorial. Co-morbidities can act as independent risk factors for acquiring an infection, for example, chronic obstructive lung disease and the development of LRTI (Celli, 2010; Miravitlles & Anzueto, 2013). Other common co-morbidities linked with an increased risk of infection include diabetes mellitus, heart failure, and chronic kidney disease. The linear correlation between ASA and the acquisition of a post-operative NSSI suggests an association, rather than causation, of NSSI with increasing ASA (i.e., increasing co-morbidities).
One explanation for this is that, the presence of co-morbidities will often cause an individual to have a much lower baseline exercise tolerance (ET) compared with those who are systemically well. Good post-operative mobility resulting from an adequate ET has been established as a predictor of a better prognosis following recovery for hip surgery (Wall, Hossain, Beard, Murray, & Andrew, 2013).
Other factors that were recorded were not able to predict whether patients would go on to develop post-operative NSSI. Age range, hemoglobin concentration on admission, place of residence, and gender were all unable to predict the occurrence of post-operative NSSI. Despite this, the NHFS, when grouped into high risk (score greater than 4) and low risk (score less than 4) was still able to predict the occurrence of NSSI. A score of greater than 4 gave a 54.1% risk of infection and a score of less than 4 only a 21.2% risk of NSSI. This, therefore, gives a robust way of both prediction of mortality at 30 days and 1 year in addition to prediction of NSSI in this cohort.
Non-patient factors were also investigated. Fracture type, divided into intra-capsular (displaced and undisplaced) and extra-capsular (intertrochanteric and sub-trochanteric), did not have any bearing on development of NSSI following surgery. Time to surgery was also not deemed a risk for developing NSSI either.
Length of Stay
The development of NSSI resulted in an increased length of stay, by an average of 13.1 days (from 18.5 days to 31.6 days). Furthermore, the severe cognitive impairment group demonstrated the longest time discrepancy between those who contracted infection and those who did not (14.1 days). This suggests that severe cognitive impairment is a predictor of slower recovery from infection. In addition, those who had an AMTS of <7 without an infection stayed an average of 8 days longer than those who had an AMTS of 10 without an infection (22.1 vs. 14.1 days). This suggests that AMTS is a predictor of length of hospital stay, even in the absence of infection.
This prolonged stay could be attributed to lack of “self-motivated mobilization” between physiotherapy episodes. Alternatively, it could reflect increased caution of health care professionals. It is reasonable to suggest that discharge may be delayed in a patient who has an AMTS of <10. The presence of confusion creates a degree of uncertainty, which is best resolved by performing investigations. The request and interpretation of such investigations could account for the additional days spent in hospital. It is also important to note that patients with a reduced AMTS often require a far greater package of care to be put in place, whether this be increased home care or the finding of residential or nursing home placement all adds to discharge delay.
Mortality
Following hip fracture surgery, 15 (7.2%) patients died as an inpatient. Of these, the majority (14 / 15) had infection as the main cause of death. The majority of these were from LRTIs rather than UTIs. ASA grade was able to predict mortality from these infections with an NSSI mortality rate at 25.0% of patients in the ASA 4 category.
Strengths and Limitations
The strengths of this study include strict data collection, blinding those who were performing data collection, and ensuring that it was double checked and any discrepancies were resolved by a third party who could make a final decision. Due to the design, the majority of data were collected prospectively and it was the presence of NSSI that was collected retrospectively. Some limitations should be noted. The AMTS and ASA grade, although widely used, have their limitations. There may be an element of assessor bias inherent in scoring someone’s cognitive function, and the result may be influenced by various non-cognitive factors, such as hearing deficits, confusion, or the time available to the assessor. This may explain the relatively similar infection rates for patients with both normal and moderate cognitive impairment, as the narrower AMTS cutoff values in these two groups result in proportionally more overlap and so patients labeled moderately cognitively impaired or not impaired may have in fact been formed from a similar population (Hoskinson, 1972).
The ASA score’s potential for introducing noise into the results of this study is primarily a result of its subjectivity. There are criteria for assigning ASA scores, but they are loose and there is the potential for very similar patients, whose true risk of developing an NSSI might be comparable, to be given different ASA scores by different anesthetists (Mak, Campbell, & Irwin, 2002). ASA grade is a measure of patient co-morbidity, and although it does not classify exact co-morbidities into each category, it does take into account the patient’s current disease status and current co-morbidities that are inherently linked to operative risk. An alternative scoring system that could have been used is the Charlson age-adjusted score that gives a score for co-morbidities and their severity for prediction for mortality at 1 year and 10 years, originally designed for use in breast cancer patients (Charlson, Pompei, Ales, & MacKenzie, 1987). As with ASA, this scoring system has limitations. It was deemed that in this study, ASA was a more appropriate measurement of patient co-morbidity, and this is supported by our results where there is an increasing NSSI rate corresponding to both increasing ASA as well increasing severity of cognitive impairment.
Another limitation of the study is not to consider the impact of the immune systems on NSSI. Studies have found that the elderly are susceptible to immune system malfunction and that this is further compound by fracture trauma (Vester et al., 2014), suggesting increased susceptibility to post-operative NSSI. Although not explicitly explored in this study, given the lack of significance of age alone on developing an NSSI, but a significant association between increasing ASA grade and NSSI suggests that the impact of immunity is multifactorial and compounded by medical co-morbidities in addition to age and trauma. As previously discussed, given the great advantages of using the ASA scoring system, namely, widespread use and simplicity of application, it brings its own limitations. ASA grading fails to identify individual patient co-morbidities. How these individual co-morbidities affect hospital admission length, mortality, and more directly immunity and susceptibility to an NSSI is not further explored in this article.
Proposed Interventions
The results of this study have indicated a role for ASA and AMTS in predicting post-operative infection rates, and consequentially, length of hospital stay. With the cost of a hospital bed estimated to be approximately £200 per day in our institution (although there is a wide variation in these cost between trusts), an intervention which aimed to reduce the risk of patient infection would be invaluable.
There are two key areas that interventions could target: the pre-operative period and the post-operative period preceding the onset of infection. As it would be negligent to delay an operation for a patient in pain, we would recommend that the optimum time for intervention would be in the days immediately following the operation. Current evidence suggests that there is a role for staff education, prompt removal of catheters, daily cleansing of the urethral meatus, and a closed urinary drainage system for the prevention of UTIs (Wang et al., 2013). Hemoglobin levels that trigger transfusion are commonly a value of less than 70 g/L to 90 g/L dependant on co-morbidities (Joint United Kingdom [UK] Blood Transfusion and Tissue Transplantation Services Professional Advisory Committee [JPAC], 2014). However, increasing this trigger for transfusion to a higher number such as 100 g/L may have the benefit of reducing cardiovascular complications and post-operative fatigue resulting in an improvement of rehabilitation. Finally, reports have proposed that sitting a patient upright for 2 hr following a meal can help to reduce the risk of contracting an LRTI (Meguro et al., 1992).
It is well studied that early ambulation with physiotherapy can help ameliorate the incidence of delirium and post-operative pneumonia (Kamel, Iqbal, Mogallapu, Maas, & Hoffmann, 2003). Poor compliance and limited uptake of physiotherapy is often seen in the patients with impaired cognition and multiple medical co-morbidities, that is, the very patient group of patients we have highlighted are the most susceptible to NSSI. Working closer with physiotherapy teams to help identify those patients at risk may help improve this.
Summary
By combining the current findings with previous evidence, we propose that future research should use pre-operative cognitive and physical health screening tools to determine the risk of post-operative infection. Further investigation is needed to establish post-operative interventions that are especially effective in preventing infections, particularly for those populations at high risk of developing infection and more prone to slower recovery.
Footnotes
Authors’ Note: The work should be attributed to Department of Orthopaedics, Milton Keynes University Hospital, Milton Keynes, UK.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- Bombaci H., Erdoğan Ö., Çetinkaya F., Kuyumcu M., Kaya E., Bombaci E. (2012). Preoperative indicators affecting postoperative mortality in elderly patients with hip fractures. Acta Orthopaedica et Traumatologica Turcica, 46, 425-429. [DOI] [PubMed] [Google Scholar]
- Castro A. A. M., Calil S. R., Freitas S. A., Oliveira A. B., Porto E. F. (2013). Chest physiotherapy effectiveness to reduce hospitalization and mechanical ventilation length of stay, pulmonary infection rate and mortality in ICU patients. Respiratory Medicine, 107, 68-74. [DOI] [PubMed] [Google Scholar]
- Celli B. R. (2010). Predictors of mortality in COPD. Respiratory Medicine, 104, 773-779. [DOI] [PubMed] [Google Scholar]
- Charlson M. E., Pompei P., Ales K. L., MacKenzie C. R. (1987). A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases, 40, 373-383. [DOI] [PubMed] [Google Scholar]
- Chia P. H., Gualano L., Seevanayagam S., Weinberg L. (2013). Outcomes following fractured neck of femur in an Australian metropolitan teaching hospital. Bone & Joint Research, 2, 162-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskinson H. M. (1972). Evaluation of a mental test score for assessment of mental impairment in the elderly. Age and Ageing, 1, 233-238. [DOI] [PubMed] [Google Scholar]
- Isikgoz Tasbakan M., Durusoy R., Pullukcu H., Sipahi O. R., Ulusoy S. (2013). Hospital-acquired urinary tract infection point prevalence in Turkey: Differences in risk factors among patient groups. Annals of Clinical Microbiology and Antimicrobials, 12, Article 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson T. A., Naqvi S. H., Sheehan B. (2013). Screening for dementia in general hospital inpatients: A systematic review and meta-analysis of available instruments. Age and Ageing, 42, 689-695. [DOI] [PubMed] [Google Scholar]
- Joint United Kingdom Blood Transfusion and Tissue Transplantation Services Professional Advisory Committee. (2014). 7.2 Transfusion in critically ill patients. In Transfusion handbook (5th ed., pp. 71-87). UK: TSO publishing. [Google Scholar]
- Kamel H. K., Iqbal M. A., Mogallapu R., Maas D., Hoffmann R. G. (2003). Time to ambulation after hip fracture surgery: Relation to hospitalization outcomes. The Journals of Gerontology, Series A: Biological Sciences & Medical Sciences, 58, 1042-1045. [DOI] [PubMed] [Google Scholar]
- Keats A. S. (1978). The ASA classification of physical status—A recapitulation. The Journal of the American Society of Anesthesiologists, 49, 233-235. [DOI] [PubMed] [Google Scholar]
- Loeb M. (2003). Pneumonia in older persons. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 37, 1335-1339. [DOI] [PubMed] [Google Scholar]
- Mak P. H. K., Campbell R. C. H., Irwin M. G. (2002). The ASA physical status classification: Inter-observer consistency. Anaesthesia and Intensive Care, 30, 633-640. [DOI] [PubMed] [Google Scholar]
- Meguro K., Yamagauchi S., Doi C., Nakamura T., Sekizawa K., Sasaki H. (1992). Prevention of respiratory infections in elderly bed-bound nursing home patients. The Tohoku Journal of Experimental Medicine, 167, 135-142. [DOI] [PubMed] [Google Scholar]
- Miravitlles M., Anzueto A. (2013). Antibiotics for acute and chronic respiratory infection in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine, 188, 1052-1057. [DOI] [PubMed] [Google Scholar]
- Moppett I. K., Parker M., Griffiths R., Bowers T., White S. M., Moran C. G. (2012). Nottingham Hip Fracture Score: Longitudinal and multi-assessment. British Journal of Anaesthesia, 109, 546-550. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence. (2009). The management of hip fractures in adults. NICE guideline CG124. [PubMed]
- Parker D. A., Beatty K. T., Giuffre B., Scholes C. J., Coolican M. R. (2011). Articular cartilage changes in patients with osteoarthritis after osteotomy. The American Journal of Sports Medicine, 39, 1039-1045. [DOI] [PubMed] [Google Scholar]
- Poh K. S., Lingaraj K. (2013). Complications and their risk factors following hip fracture surgery. Journal of Orthopaedic Surgery (Hong Kong), 21, 154-157. [DOI] [PubMed] [Google Scholar]
- Prosser S. (1997). Case-finding incontinence in the over-75s. British Journal of General Practice, 47, 498-500. [PMC free article] [PubMed] [Google Scholar]
- Shah S. N., Wainess R. M., Karunakar M. A. (2005). Hemiarthroplasty for femoral neck fracture in the elderly: Surgeon and hospital volume-related outcomes. Journal of Arthroplasty, 20, 503-508. [DOI] [PubMed] [Google Scholar]
- Vester H., Huber-Lang M. S., Kida Q., Scola A., van Griensven M., Gebhard F., . . . Perl M. (2014). The immune response after fracture trauma is different in old compared to young patients. Immunity & Ageing, 11(1), Article 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall P. D. H., Hossain M., Beard D. J., Murray D. W., Andrew J. G. (2013). The effect of locomotion on the outcome following total hip arthroplasty. Hip International: The Journal of Clinical and Experimental Research on Hip Pathology and Therapy, 23, 193-198. [DOI] [PubMed] [Google Scholar]
- Wang F., Xing T., Li J., He Y., Bai M., Wang N. (2013). Survey on hospital-acquired urinary tract infection in neurological intensive care unit. Acta Pathologica, Microbiologica, et Immunologica Scandinavica, 121, 197-201. [DOI] [PubMed] [Google Scholar]


