Abstract
Background
In the current antiretroviral (ART) era, the evolution of HIV guidelines and emergence of new antiretroviral agents might be expected to impact the times to ART initiation and HIV virologic suppression. We sought to determine if times to ART initiation and virologic suppression decreased and if disparities exist by age, race/ethnicity, and HIV risk.
Methods
We performed a retrospective cohort study of data from 12 sites of the HIV Research Network, a consortium of US clinics caring for HIV-infected patients. HIV-infected adults (≥18 years old) newly presenting for care between 2003 and 2013 were included in this study. Times to ART initiation and virologic suppression were defined as time from enrollment to ART initiation and HIV RNA <400 copies/ml, respectively. We conducted time to event analyses using competing risk regression in the HIVRN cohort from 2003-2012 in 2 year intervals, with follow-up through 2013.
Results
Among 15,272 participants, 76.9% were male, 48.4% black, and 10.9% were IDU with median age of 38 years (interquartile range: 29-46 years). The adjusted subdistribution hazards ratios (SHR) for ART initiation and virologic suppression each increased for years 2007-08 (SHR 1.23 ‘1.16-1.30’, and SHR 1.25 ‘1.17-1.34’, respectively), 2009-10 (1.55 ‘1.46-1.64’, and 1.54 ‘1.43-1.65’, respectively), and 2011-12 (1.94 ‘1.83-2.07’, and 1.73 ‘1.61-1.86’, respectively) compared to 2003-04. Blacks had a lower probability of ART initiation than whites and Hispanics.
Conclusions
Since 2007, times from enrollment to ART initiation and virologic suppression have decreased significantly compared to 2003-2004 but persisting disparities should be addressed.
Keywords: HIV, antiretroviral therapy, HIV suppression, public health, HIV clinical care
Background
Over the modern ART era, there have been marked advances in knowledge about the effectiveness of ART initiation on mortality and secondary transmission. From 2001 to 2007 the Department of Health and Human Services (DHHS) HIV treatment guidelines recommended initiation of ART at a CD4 count (CD4) of 200 cells/mm3. These guidelines additionally acknowledged that there may be benefit to initiating ART at higher CD4 counts, but data needed to support such a recommendation were lacking1. Based on data from the ART cohort collaboration (ART-CC), the SMART trial, and others, the DHHS revised the guidelines to initiate ART at a CD4 threshold of 350 cells/mm3 in December 2007.2 The DHHS HIV treatment guideline ART initiation CD4 threshold was increased to 500 cells/mm3 in December 20093 and extended to all HIV-infected individual in February 20134. Several studies have demonstrated improved outcomes in mortality and/or progression to AIDS when initiating ART at a CD4 threshold of 500 cells/mm3.5-8 A single study showed mortality benefit at CD4>500.6 Subsequently, in 2011 HPTN 0529 demonstrated decreased secondary transmission, an additional benefit of early ART.
A recent study from the North American Aids Cohort Collaboration on Research and Design (NA-ACCORD) demonstrated increases in the cumulative incidence of ART initiation and HIV virologic suppression among a large multistate population of persons living with HIV (PLWH) from 2001-2009. In addition, the NA-ACCORD study noted disparities in cumulative incidence of ART initiation by CD4, race/ethnicity, age, and HIV risk among individuals eligible for ART.10
Based on these results and changes in ART guidelines, we hypothesized that the time from initial presentation to HIV care to ART initiation and virologic suppression decreased over the course of the modern ART era and that these trends vary by CD4 at presentation, race/ethnicity, and age. Thus, we examined times to ART initiation and virologic suppression among ART-naïve subjects in a large multi-site clinical cohort during the years 2003-2013.
Methods
Participants
The HIV Research Network (HIVRN) is a consortium of primary and subspecialty medical care providers for HIV-infected patients. Participating sites abstract specified data elements from patients' medical records; abstracted data are assembled into a uniform database.11-14 The HIVRN currently consists of 18 sites that treat adult and pediatric patients; analyses included 13 sites that collect comprehensive utilization data on adults. Sites are located in the Northeastern (6), Midwestern (1), Southern (3), and Western U.S. (3). Ten sites have academic affiliations; 3 are non-academic, community-based. Analyses were limited to adult patients (≥18 years old) at these sites who were newly presenting to HIV care and enrolled at an HIVRN clinic site between January 1, 2003 and December 31, 2012. Data collection continued through December 31, 2013, with the exception of 1 site that left the Network after 2011.
Data Collection
Data including CD4 lymphocyte count, HIV-1 RNA, HIV acquisition risk factors, and demographics were collected prospectively, both electronically and by paper abstraction from medical records at each site. Abstracted data were sent in electronic format to a data-coordinating center after personal identifying information was removed. Electronic data received by the coordinating center were reviewed to ensure that each data element was correctly formatted and that all elements were captured. Data elements with incorrect formatting, unknown or incomplete information, or other inaccuracies were reviewed with the site and corrected. These data were combined across sites to achieve a uniformly constructed multi-site database. A variable identifying the site was included in the database. The study was approved by the Institutional Review Board of the Johns Hopkins School of Medicine as well as by each of the participating sites.
Definitions
Enrollment year was categorized into 2 year increments for analysis from 2003-04 to 2011-12 in order to increase sample in each period and decrease computational intensity. New presenters were defined as patients presenting with: (i) HIV RNA > 400 copies/ml, (ii) no antiretroviral therapy (ART) prior to enrollment, and (iii) no HIV clinic visits at the HIVRN site prior to enrollment month. Data abstracted from medical records included race/ethnicity (White, Black, Hispanic, and other/unknown), male/female, and HIV acquisition risk factors, coded as men who have sex with men (MSM), heterosexual transmission (HET), injection drug use (IDU), and other/unknown (OTH). In subjects with multiple HIV risk factors, a single category was designated based on the following order of priority: IDU, MSM, HET, OTH. Time to ART initiation was defined as the number of days from enrollment in an HIVRN clinic to ART initiation. Time to virologic suppression was defined as the number of days from enrollment in an HIVRN clinic to the first HIV RNA <400 copies/ml. CD4 count and HIV-1 RNA at enrollment were defined as the CD4 count and HIV RNA nearest to the enrollment date but not >6 months from enrollment. CD4 count at ART initiation was defined as the CD4 count nearest to ART initiation date but not greater than 30 days after ART initiation. CD4 count at enrollment was categorized as <200, 200-349, 350-500, and ≥500 cells/mm3. Subjects were censored at the end of follow-up, i.e., after 2013 except for one site that was censored after 2011.
Statistical Analyses
We examined both time from enrollment to ART initiation and time from enrollment to virologic suppression. The primary variable of interest was enrollment year. Studies indicate that risk of loss to follow-up (LTFU) varies by race/ethnicity, HIV risk factor, age, and initial CD4.15 Given that the study sample is drawn from individuals receiving clinical care, individuals who are LTFU prior to ART initiation are unlikely to initiate treatment or experience viral suppression, with the exception of those who enroll in another HIV clinic. Therefore this creates a competing risk situation, in which an event such as LTFU, or death, occurring before initiation of treatment prevents the occurrence of the events of interest (i.e., ART initiation and viral suppression). Standard time-to-event analyses can produce misleading results in the presence of competing risks, and special analytic techniques are required. Thus, time to ART initiation and time to virologic suppression were analyzed using both unadjusted and adjusted Fine and Gray 16 competing risk regression models. The covariates for the adjusted models included CD4 count at enrollment, age, gender, race/ethnicity, HIV risk, and clinic site. The competing risks were loss to follow-up (LTFU) and death. The results of the competing risk model should be interpreted as the subdistribution hazard (hazard), the hazard prior to the competing events, i.e., LTFU or death.17 A larger hazard implies a shorter time to an event. The hazard of virologic suppression includes all new presenters, not restricted to those receiving ART. We performed two LTFU analyses using the same competing risk model as the adjusted ART initiation and virologic suppression analyses. First we modeled the time to LTFU prior to ART initiation or death and second, the time to LTFU prior to virologic suppression or death.
Modification of the effect of enrollment year on times to ART initiation and virologic suppression was examined by enrollment CD4 count category, age, gender, and race. Table 3 shows comparisons of hazards with a reference category. Comparisons of hazards in non-referent categories were performed with linear combinations and described in the text. For each interaction, we assessed effects of enrollment year on the hazard within categories of the other variable. To interpret interactions, we estimated the linear year trend (slope) of the hazard within categories of the other variable. Cumulative incidence curves were generated to display the cumulative incidence of ART initiation and virologic suppression prior to the competing risks by enrollment year. A non-parametric cumulative incidence function was used to estimate cumulative incidence in a competing risk situation. Cuzick's test was used for non-parametric trends 18. Statistical analysis was performed using STATA 12.119 and R 3.0.120.
Table 3. Relative Hazard of Event by Enrollment CD4 Count*, Race/Ethnicity**, and Age†.
| CD4 <200 cells/mm3 | CD4 200-349 cells/mm3 | CD4 350-499 cells/mm3 | CD4 ≥500 cells/mm3 | Test of Category Effects†† | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| ART Initiation | |||||||||
| Enrollment Year | SHR (95% CI) | P value | SHR (95% CI) | P value | SHR (95% CI) | P value | SHR (95% CI) | P value | P value |
| 2003-04 | Ref | 0.59 (0.53 - 0.66) | <0.001 | 0.33 (0.29 - 0.37) | <0.001 | 0.25 (0.22 - 0.29) | <0.001 | <0.001 | |
| 2005-06 | 1.07 (0.98 - 1.17) | 0.146 | 0.68 (0.61 - 0.76) | <0.001 | 0.33 (0.30 - 0.38) | <0.001 | 0.22 (0.19 - 0.25) | <0.001 | <0.001 |
| 2007-08 | 1.15 (1.05 - 1.25) | 0.002 | 0.82 (0.74 - 0.90) | <0.001 | 0.43 (0.38 - 0.48) | <0.001 | 0.30 (0.27 - 0.34) | <0.001 | <0.001 |
| 2009-10 | 1.40 (1.28 - 1.53) | <0.001 | 1.02 (0.92 - 1.13) | 0.743 | 0.63 (0.57 - 0.70) | <0.001 | 0.35 (0.32 - 0.40) | <0.001 | <0.001 |
| 2011-12 | 1.63 (1.48 - 1.79) | <0.001 | 1.24 (1.12 - 1.38) | <0.001 | 0.82 (0.73 - 0.92) | 0.001 | 0.51 (0.46 - 0.57) | <0.001 | <0.001 |
| Test of year effects†† | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| ART initiation linear trend‡ | 1.06 (1.05 - 1.07) | <0.001 | 1.10 (1.08 - 1.12) | <0.001 | 1.13 (1.12 - 1.15) | <0.001 | 1.11 (1.09 - 1.12) | <0.001 | <0.001 |
| HIV Suppression | |||||||||
| Enrollment Year | SHR (95% CI) | P value | SHR (95% CI) | P value | SHR (95% CI) | P value | SHR (95% CI) | P value | P value |
| 2003-04 | Ref | 0.71 (0.62 - 0.81) | <0.001 | 0.52 (0.46 - 0.60) | <0.001 | 0.38 (0.33 - 0.44) | <0.001 | <0.001 | |
| 2005-06 | 1.01 (0.90 - 1.12) | 0.927 | 0.88 (0.77 - 1.00) | 0.049 | 0.48 (0.42 - 0.56) | <0.001 | 0.34 (0.30 - 0.40) | <0.001 | <0.001 |
| 2007-08 | 1.17 (1.05 - 1.30) | 0.005 | 1.04 (0.92 - 1.17) | 0.537 | 0.63 (0.55 - 0.72) | <0.001 | 0.48 (0.42 - 0.55) | <0.001 | <0.001 |
| 2009-10 | 1.35 (1.21 - 1.51) | <0.001 | 1.25 (1.10 - 1.41) | <0.001 | 0.91 (0.81 - 1.04) | 0.168 | 0.59 (0.52 - 0.67) | <0.001 | <0.001 |
| 2011-12 | 1.37 (1.22 - 1.54) | <0.001 | 1.27 (1.12 - 1.44) | <0.001 | 1.17 (1.03 - 1.33) | 0.020 | 0.79 (0.70 - 0.89) | <0.001 | <0.001 |
| Test of year effects†† | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| HIV suppression linear trend‡ | 1.05 (1.03 - 1.06) | <0.001 | 1.08 (1.06 - 1.09) | <0.001 | 1.12 (1.10 - 1.14) | <0.001 | 1.11 (1.09 - 1.13) | <0.001 | <0.001 |
| Black | White | Hispanic | Test of Category Effects†† | ||||||
|
|
|||||||||
| ART Initiation | |||||||||
| Enrollment Year | SHR (95% CI) | P value | SHR (95% CI) | P value | SHR (95% CI) | P value | P value | ||
| 2003-04 | Ref | 1.01 (0.91 - 1.13) | 0.801 | 1.04 (0.93 - 1.16) | 0.490 | 0.834 | |||
| 2005-06 | 1.04 (0.95 - 1.13) | 0.414 | 0.96 (0.86 - 1.07) | 0.446 | 1.28 (1.14 - 1.42) | <0.001 | <0.001 | ||
| 2007-08 | 1.18 (1.08 - 1.28) | <0.001 | 1.23 (1.11 - 1.36) | <0.001 | 1.45 (1.31 - 1.60) | <0.001 | <0.001 | ||
| 2009-10 | 1.46 (1.35 - 1.59) | <0.001 | 1.74 (1.56 - 1.93) | <0.001 | 1.66 (1.50 - 1.85) | <0.001 | <0.001 | ||
| 2011-12 | 1.86 (1.70 - 2.03) | <0.001 | 2.16 (1.95 - 2.40) | <0.001 | 2.05 (1.84 - 2.28) | <0.001 | 0.007 | ||
| Test of year effects†† | <0.001 | <0.001 | <0.001 | ||||||
| ART initiation linear trend‡ | 1.08 (1.07 - 1.09) | <0.001 | 1.11 (1.09 - 1.13) | <0.001 | 1.08 (1.07 - 1.10) | <0.001 | 0.002 | ||
| HIV Suppression | |||||||||
| Enrollment Year | SHR (95% CI) | P value | SHR (95% CI) | P value | SHR (95% CI) | P value | P value | ||
| 2003-04 | Ref | 1.00 (0.88 - 1.13) | 0.956 | 1.11 (0.97 - 1.26) | 0.128 | 0.417 | |||
| 2005-06 | 1.02 (0.92 - 1.14) | 0.663 | 0.95 (0.84 - 1.08) | 0.423 | 1.28 (1.12 - 1.46) | <0.001 | <0.001 | ||
| 2007-08 | 1.19 (1.07 - 1.31) | 0.001 | 1.26 (1.12 - 1.42) | <0.001 | 1.59 (1.41 - 1.79) | <0.001 | <0.001 | ||
| 2009-10 | 1.41 (1.28 - 1.56) | <0.001 | 1.60 (1.41 - 1.81) | <0.001 | 1.92 (1.70 - 2.18) | <0.001 | <0.001 | ||
| 2011-12 | 1.63 (1.47 - 1.81) | <0.001 | 1.76 (1.55 - 2.00) | <0.001 | 2.06 (1.82 - 2.33) | <0.001 | <0.001 | ||
| Test of year effects†† | <0.001 | <0.001 | <0.001 | ||||||
| HIV suppression linear trend‡ | 1.07 (1.06 - 1.08) | <0.001 | 1.09 (1.07 - 1.10) | <0.001 | 1.08 (1.07 - 1.10) | <0.001 | 0.001 | ||
| Age ≤30 | Age 31-40 | Age 41-50 | Age >50 | Test of Category Effects†† | |||||
|
| |||||||||
| ART Initiation | |||||||||
| Enrollment Year | SHR (95% CI) | P value | SHR (95% CI) | P value | SHR (95% CI) | P value | SHR (95% CI) | P value | P value |
| 2003-04 | Ref | 1.24 (1.11 - 1.40) | <0.001 | 1.28 (1.13 - 1.45) | <0.001 | 1.39 (1.19 - 1.62) | <0.001 | <0.001 | |
| 2005-06 | 1.07 (0.95 - 1.22) | 0.273 | 1.31 (1.16 - 1.48) | <0.001 | 1.32 (1.17 - 1.49) | <0.001 | 1.42 (1.21 - 1.67) | <0.001 | <0.001 |
| 2007-08 | 1.21 (1.07 - 1.36) | 0.002 | 1.58 (1.40 - 1.77) | <0.001 | 1.59 (1.41 - 1.79) | <0.001 | 1.59 (1.38 - 1.83) | <0.001 | <0.001 |
| 2009-10 | 1.62 (1.44 - 1.82) | <0.001 | 1.98 (1.76 - 2.23) | <0.001 | 1.94 (1.72 - 2.19) | <0.001 | 1.99 (1.73 - 2.30) | <0.001 | 0.003 |
| 2011-12 | 2.13 (1.89 - 2.40) | <0.001 | 2.32 (2.05 - 2.64) | <0.001 | 2.43 (2.15 - 2.75) | <0.001 | 2.57 (2.22 - 2.96) | <0.001 | 0.003 |
| Test of year effects†† | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| ART initiation linear trend‡ | 1.11 (1.09 - 1.12) | <0.001 | 1.09 (1.07 - 1.10) | <0.001 | 1.09 (1.07 - 1.10) | <0.001 | 1.08 (1.06 - 1.10) | <0.001 | <0.001 |
| HIV Suppression | |||||||||
| Enrollment Year | SHR (95% CI) | P value | SHR (95% CI) | P value | SHR (95% CI) | P value | SHR (95% CI) | P value | P value |
| 2003-04 | Ref | 1.13 (0.98 - 1.30) | 0.095 | 1.22 (1.05 - 1.41) | 0.010 | 1.77 (1.48 - 2.12) | <0.001 | <0.001 | |
| 2005-06 | 0.95 (0.81 - 1.11) | 0.493 | 1.21 (1.05 - 1.39) | 0.010 | 1.34 (1.16 - 1.54) | <0.001 | 1.65 (1.37 - 1.99) | <0.001 | <0.001 |
| 2007-08 | 1.28 (1.11 - 1.48) | 0.001 | 1.57 (1.36 - 1.81) | <0.001 | 1.63 (1.42 - 1.88) | <0.001 | 1.84 (1.56 - 2.17) | <0.001 | <0.001 |
| 2009-10 | 1.71 (1.48 - 1.97) | <0.001 | 2.04 (1.77 - 2.36) | <0.001 | 1.89 (1.64 - 2.18) | <0.001 | 2.25 (1.90 - 2.67) | <0.001 | 0.002 |
| 2011-12 | 2.15 (1.87 - 2.48) | <0.001 | 2.15 (1.86 - 2.49) | <0.001 | 2.26 (1.95 - 2.62) | <0.001 | 2.56 (2.17 - 3.03) | <0.001 | 0.014 |
| Test of year effects†† | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| HIV suppression linear trend‡ | 1.10 (1.08 - 1.12) | <0.001 | 1.08 (1.07 - 1.10) | <0.001 | 1.07 (1.05 - 1.09) | <0.001 | 1.05 (1.02 - 1.07) | <0.001 | <0.001 |
Statistically significant results (at a <0.05 level) are displayed in bold; adjusted for race/ethnicity, age, gender, HIV risk, and clinic site
Adjusted for CD4 count, age, gender, HIV risk, and clinic site
Adjusted for CD4 count, race/ethnicity, gender, HIV risk, and clinic site
Linear contrasts used to perform a joint test (χ2) of difference in effects within a given year or category
Enrollment year modeled as a continuous variable to determine a linear trend (i.e. slope) for time to ART initiation and virologic suppression
Results
Our study population consisted of 15,272 new presenters who enrolled in the HIVRN between 2003 and 2012. We excluded 466 subjects who were missing either a CD4 count or HIV-1 RNA at enrollment and 142 transgender and 5 unknown gender subjects. Subject characteristics stratified by enrollment year intervals are reported in Table 1. The median CD4 count at enrollment increased from 273.5 cells/mm3 in subjects enrolled from 2003-04 to 332 cells/mm3 in subjects enrolled from 2011-12 (test for trend p<0.001). The median CD4 count at ART initiation was 201 cells/mm3 in subjects enrolled from 2003-04 and increased to 285 cells/mm3 in subjects enrolled from 2011-12 (test for trend p<0.001).
Table 1. Basic Demographic and Clinical Characteristics of New Presenters, by Enrollment Year.
| No. (%) | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| 2003-04 | 2005-06 | 2007-08 | 2009-10 | 2011-12 | 2003-2012 | |
| Variable | N = 2920 | N = 2808 | N = 3274 | N = 3097 | N = 3173 | N=15,272 |
| Age(years) | 38.2 (9.7) | 38.3 (10.2) | 38.6 (11.0) | 37.7 (11.3) | 37.6 (11.5) | 38.1 (10.8) |
| Age Category† | ||||||
| ≤30 | 654 (22.4) | 695 (24.8) | 872 (26.6) | 959 (31.0) | 1056 (33.3) | 4236 (27.7) |
| 31-40 | 1114 (38.2) | 923 (32.9) | 948 (29.0) | 826 (26.7) | 820 (25.8) | 4631 (30.3) |
| 41-50 | 828 (28.4) | 877 (31.2) | 988 (30.2) | 887 (28.6) | 831 (26.2) | 4411 (28.9) |
| >50 | 324 (11.1) | 313 (11.1) | 466 (14.2) | 425 (13.7) | 466 (14.7) | 1994 (13.1) |
| Race/Ethnicity† | ||||||
| Black | 1327 (45.4) | 1347 (48.0) | 1623 (49.6) | 1604 (51.8) | 1496 (47.1) | 7397 (48.4) |
| White | 862 (29.5) | 774 (27.6) | 832 (25.4) | 741 (23.9) | 787 (24.8) | 3996 (26.2) |
| Hispanic | 660 (22.6) | 564 (20.1) | 696 (21.3) | 625 (20.2) | 765 (24.1) | 3310 (21.7) |
| Other | 71 (2.4) | 123 (4.4) | 123 (3.8) | 127 (4.1) | 125 (3.9) | 569 (3.7) |
| Gender† | ||||||
| Male | 2195 (75.2) | 2119 (75.5) | 2470 (75.4) | 2388 (77.1) | 2579 (81.3) | 11751(76.9) |
| Female | 725(24.8) | 689(24.5) | 804(24.6) | 709(22.9) | 975 (30.7) | 3521(23.1) |
| HIV Risk† | ||||||
| HET | 1078 (36.9) | 1093 (38.9) | 1236 (37.8) | 1165 (37.6) | 975 (30.7) | 5547 (36.3) |
| MSM | 1173 (40.2) | 1208 (43.0) | 1407 (43.0) | 1468 (47.4) | 1727 (54.4) | 6983 (45.7) |
| IDU | 440 (15.1) | 344 (12.3) | 409 (12.5) | 267 (8.6) | 199 (6.3) | 1659 (10.9) |
| Other | 229 (7.8) | 163 (5.8) | 222 (6.8) | 197 (6.4) | 272 (8.6) | 1083 (7.1) |
| CD4 Category (cells/mm3) † | ||||||
| ≥500 | 570 (19.5) | 568 (20.2) | 674 (20.6) | 696 (22.5) | 870 (27.4) | 3378 (22.1) |
| 350-499 | 561 (19.2) | 540 (19.2) | 643 (19.6) | 631 (20.4) | 633 (20.0) | 3008 (19.7) |
| 200-349 | 627 (21.5) | 607 (21.6) | 759 (23.2) | 715 (23.1) | 716 (22.6) | 3424 (22.4) |
| <200 | 1162 (39.8) | 1093 (38.9) | 1198 (36.6) | 1055 (34.1) | 954 (30.1) | 5462 (35.8) |
| Log10 HIV RNA (copies/ml), median(IQR) † | ||||||
| 4.6 | 4.7 | 4.5 | 4.4 | 4.5 | 4.5 | |
| (4.0 - 5.2) | (4.0 - 5.1) | (3.8 - 5.0) | (3.8 - 5.0) | (3.7 - 5.0) | (3.8-5.1) | |
| Survival Time (days), 50th Percentile (25th-75th) | ||||||
| AI | 109 | 131 | 75 | 40 | 15 | 54 |
| (9-1041) | (10-1001) | (6-672) | (0-389) | (0-183) | (1-626) | |
| VS | 763 | 702 | 508 | 303 | 235 | 458 |
| (155-1675) | (165-1583) | (131-1165) | (100-965) | (95-770) | (121-1296) | |
| Cumulative Incidence (%) | ||||||
| AI | 69.7 | 69.9 | 74.5 | 80.1 | 84.0 | |
| VS | 51.0 | 52.2 | 57.9 | 63.7 | 66.6 | |
AI – ART initiation, VS – HIV virologic suppression
Association of variable with biennial period is significant, p<0.001.
On average there were 3,054 new presenters per 2 year interval with a median survival time of 54 days prior to ART initiation, LTFU, death, or censoring. Of all subjects, 75.6% of subjects initiated ART and 58.6% achieved virologic suppression. Among those subjects who started ART, the median time to ART initiation was 19 days ‘95% confidence interval ‘CI’ 18-20 days’ and among those subjects who achieved suppression, the median time from enrollment to virologic suppression was 142 days ‘95% CI 139-147 days’(See supplemental Table 1 for median times to ART initiation and virologic suppression stratified by relevant categories). The cumulative incidence of ART initiation prior to a competing event rose from 69.7% in 2003-04 to 84.0% in 2011-12 (Table 1), and the cumulative incidence of virologic suppression prior to a competing event rose from 51.0% in 2003-04 to 66.6% in 2011-12 (Table 1).
ART initiation
The time to ART initiation decreased in subjects enrolled in 2011-12 compared to those enrolled in 2003-04 (unadjusted subdistribution hazard ratio (SHR) = 1.67 ‘(95% CI 1.58-1.77’; adjusted SHR = 1.94 ‘1.83-2.07’) (Table 2 and Figure 1). There was no difference in the adjusted time to ART initiation comparing years 2003-04 to 2005-06 (Table 2). Comparing hazards in adjacent intervals (not shown in Table 2), the adjusted time to ART initiation decreased for years 2005-06 to 2007-08 (SHR =(1.23/1.05)=1.17 ‘1.11-1.24’), 2007-08 to 2009-10 (SHR=(1.55/1.23)= 1.26 ‘1.20-1.33’), and 2009-10 to 2011-12 (SHR=(1.94/1.55)= 1.25 ‘1.19-1.33’). Patients older than 30 years had a shorter time to ART initiation than did patients 30 years or younger (Table 2). Hispanics had a shorter time to ART initiation than whites (Table 2) or blacks (SHR 1.14 ‘1.09-1.20’). IDU had a longer time to ART initiation than did HET (SHR=(0.84/0.94)= 0.89 ‘0.83 - 0.95’) or MSM (Table 2).
Table 2. Multivariable Model of Relative Hazard of Event.
| ART Initiation | HIV Suppression | |||
|---|---|---|---|---|
|
|
||||
| Variable | SHR | P value | SHR | P value |
| Enrollment Year | ||||
| 2003-04 | Ref | Ref | ||
| 2005-06 | 1.05 (0.99 - 1.11) | 0.135 | 1.03 (0.95 - 1.10) | 0.483 |
| 2007-08 | 1.23 (1.16 - 1.30) | <0.001 | 1.25 (1.17 - 1.34) | <0.001 |
| 2009-10 | 1.55 (1.46 - 1.64) | <0.001 | 1.54 (1.43 - 1.65) | <0.001 |
| 2011-12 | 1.94 (1.83 - 2.07) | <0.001 | 1.73 (1.61 - 1.86) | <0.001 |
| CD4 Count | ||||
| ≥500 | Ref | Ref | ||
| 350-499 | 1.52 (1.43 - 1.61) | <0.001 | 1.41 (1.32 - 1.51) | <0.001 |
| 200-349 | 2.64 (2.49 - 2.80) | <0.001 | 1.98 (1.86 - 2.12) | <0.001 |
| <200 | 3.85 (3.64 - 4.08) | <0.001 | 2.30 (2.16 - 2.44) | <0.001 |
| Age (years) | ||||
| ≤30 | Ref | Ref | ||
| 31-40 | 1.21 (1.15 - 1.27) | <0.001 | 1.12 (1.05 - 1.18) | <0.001 |
| 41-50 | 1.22 (1.16 - 1.29) | <0.001 | 1.16 (1.10 - 1.23) | <0.001 |
| >50 | 1.28 (1.20 - 1.36) | <0.001 | 1.39 (1.29 - 1.50) | <0.001 |
| Gender | ||||
| Female | Ref | Ref | ||
| Male | 0.99 (0.94 - 1.04) | 0.708 | 0.89 (0.84 - 0.95) | <0.001 |
| Race/Ethnicity | ||||
| White | Ref | Ref | ||
| Black | 0.94 (0.89 - 0.99) | 0.012 | 0.96 (0.90 - 1.02) | 0.157 |
| Hispanic | 1.07 (1.01 - 1.13) | 0.017 | 1.21 (1.14 - 1.29) | <0.001 |
| Other | 0.83 (0.74 - 0.92) | 0.001 | 0.90 (0.79 - 1.03) | 0.119 |
| HIV Risk | ||||
| MSM | Ref | Ref | ||
| HET | 0.94 (0.90 - 0.99) | 0.029 | 0.81 (0.76 - 0.86) | <0.001 |
| IDU | 0.84 (0.78 - 0.90) | <0.001 | 0.61 (0.57 - 0.67) | <0.001 |
| Other | 0.67 (0.61 - 0.73) | <0.001 | 0.46 (0.42 - 0.52) | <0.001 |
Statistically significant results (at a <0.05 level) are displayed in bold. SHR: Subdistribution Hazard Ratio
Figure 1. Cumulative Incidence of ART Initiation and Loss to Follow-up Stratified by Enrollment Year*.
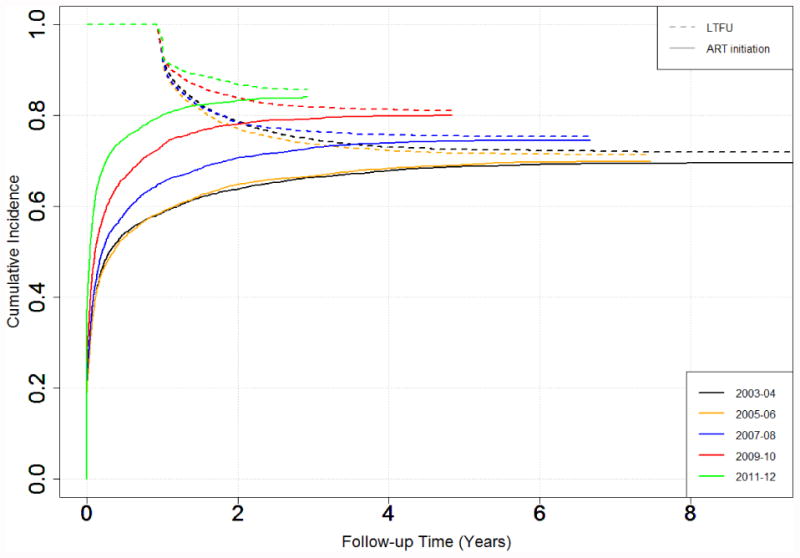
*LTFU curves are plotted as 1-cumulative incidence of LTFU prior to ART initiation
Comparing 2003-04 to 2011-12, the time to ART initiation decreased for all CD4, race/ethnicity, and age categories (Table 3 and Supplemental Figure 1). For most comparisons of successive time periods within categories of CD4, race/ethnicity, and age, the time to ART initiation decreased (i.e., higher hazard ratio). Tests for interactions showed that the decrease in the time to ART initiation by year varied by CD4 category (p<0.001) and race/ethnicity (p<0.001) but not by age. The magnitude of the linear time trend in the hazard was greatest among CD4 350-499 cells/mm3 (p<0.001 vs. CD4 200-349 cells/mm3), and among whites (p=0.005 vs. Hispanic). Within a given year, the time to ART initiation differed by CD4 category, race/ethnicity, and age (Table 3).
Virologic suppression
The time to HIV virologic suppression decreased in subjects enrolled in 2011-12 compared to those enrolled in 2003-04 (unadjusted SHR = 1.64 ‘95% CI 1.53-1.75’; adjusted SHR = 1.73 ‘1.61-1.86’) (Table 2 and Figure 2). The adjusted time tor virologic suppression did not decrease from 2003-04 to 2005-06 (1.03 ‘0.95-1.10’) (Table 2) but did decrease from 2005-06 to 2007-08 (SHR=(1.25/1.03)= 1.22 ‘1.14-1.31’), in 2007-08 to 2009-10 (SHR=(1.54/1.25)= 1.23 ‘1.15-1.31’), and in 2009-10 to 2011-12 (SHR=(1.73/1.54)= 1.13 ‘1.05-1.20’). Patients older than 30 years had a shorter time to virologic suppression than did patients 30 years or younger (Table 2). Hispanics had a shorter time to virologic suppression than whites (Table 2) or blacks (SHR=(1.21/0.96)= 1.27 ‘1.20-1.34’). IDU had a longer time to virologic suppression than did HET (SHR=(0.76 ‘0.70 - 0.83’) or MSM (Table 2).
Figure 2. Cumulative Incidence of HIV Suppression and Loss to Follow-up Stratified by Enrollment Year*.
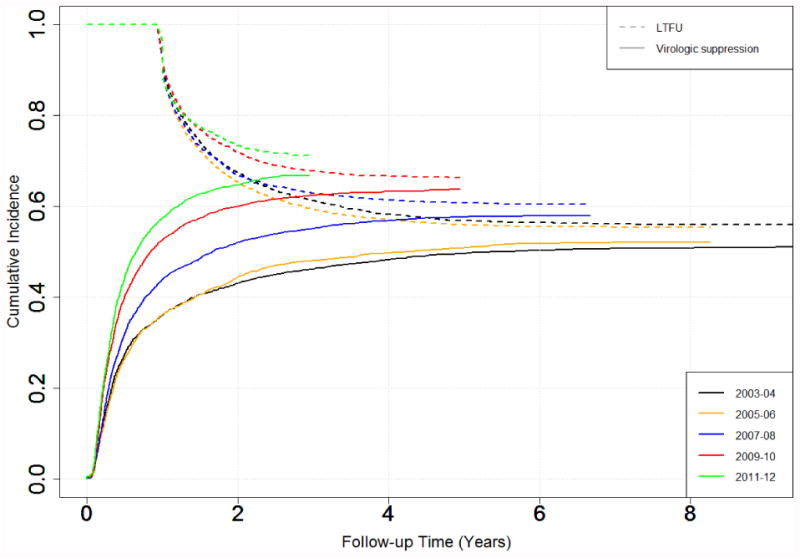
*LTFU curves are plotted as 1-cumulative incidence of LTFU prior to virologic suppression
The time to virologic suppression decreased from 2003-04 to 2011-12 for all CD4 categories, races/ethnicities, and ages (Table 3 and Supplemental Figure 1). Tests for interaction showed that the amount of decrease in the time to virologic suppression by year varied by CD4 categories (p<0.001), race/ethnicity (p<0.03), and age (0.002). Time to virologic suppression decreased, with minor exceptions, with each successive year for all CD4, age, and race/ethnicity categories (Table 3). The magnitude of the linear time trend in the hazard was greatest among CD4 350-499 cells/mm3 (p<0.001 vs. CD4 200-349 cells/mm3), age ≤ 30 (p<0.006 vs. age 31-40), and white (p=0.004 vs. black, p=0.715 vs. Hispanic). Within a specific time period, the time to virologic suppression differed across CD4, race/ethnicity, and age categories.
Loss to follow-up
In the adjusted analyses, the time to LTFU prior to ART initiation increased (SHR 0.39, ‘0.34-0.44’ from 2003-04 to 2011-12 and time to LTFU prior to virologic suppression increased (0.55 ‘0.50-0.60’ from 2003-04 to 2011-12 (Supplemental Table 2). The cumulative incidence at 2 years of follow-up time of LTFU prior to ART initiation decreased from 0.21 in 2003-04 to 0.13 in 2011-12 (Figure 1). Similarly the cumulative incidence at 2 years of follow-up time of LTFU prior to virologic suppression decreased from 0.33 in 2003-04 to 0.27 in 2011-12 (Figure 2). At 2 years of follow-up, the cumulative incidence of participants who had LTFU after ART initiation but before virologic suppression was 0.13 (0.27-0.13).
Discussion
In ART-naïve subjects in the HIVRN over the years 2003-2012, we demonstrate decreases in the times to ART initiation and virologic suppression in years 2007-08, 2009-10, and 2011-12 compared to the preceding years. These changes are consistent with changes in clinical practice and HIV treatment guidelines among HIV providers. Additionally, we note within-period disparities in the times to ART initiation and virologic suppression by race/ethnicity and age, and unfortunately, none of these disparities were eliminated over the study period.
The trends of decreasing times to ART initiation and virologic suppression are important because of their impact on both the health of PLWH and secondary HIV transmission. While we show relatively minor impacts on median number of days until ART initiation and virologic suppression, this translates to an increase in cumulative incidence of ART initiation and virologic suppression of 20.5% and 30.5%, respectively. The CDC reported 47,352 new HIV infections in 2013.21 The CDC estimates that 35% of HIV diagnosed patients are suppressed.22 Assuming a 30% increase in virologic suppression from 2003-04 to 2011-12, nearly 5,000 additional newly diagnosed PLWH achieved virologic suppression (i.e., 47352*0.35*0.30).
The changes in times to ART initiation and virologic suppression varied by enrollment CD4. This is consistent with the changes in the DHHS guidelines for ART initiation that were based on CD4 thresholds. 1-3 Interestingly, we observed decreases in the times to ART initiation and virologic suppression that preceded the publication of the DHHS HIV treatment guideline in December 2007 and in December 2009. Factors external to the published guidelines likely contributed to the observed changes. HIV clinicians may have learned about the latest HIV care practices from sources outside of official guidelines such as journals or HIV conferences, and as such, changes in clinical practice may precede the publication of official HIV treatment guidelines. This is in contrast to clinical care of other diseases, where clinical practice may not change prior to publication of guidelines or clinical alerts23.
Our study found evidence of racial/ethnic disparities in times to ART initiation and virologic suppression over the study period. Hispanics had shorter times to ART initiation and to virologic suppression than whites, while blacks had longer time to ART initiation than whites. Consistent with our findings, a study from the HIV Outpatient Study (HOPS) found non-Hispanic blacks had longer times to ART initiation and virologic suppression than whites.24 This association may be due to residual confounding with other factors such as poverty, access to HIV care, or other structural or psychosocial factors not examined in this study.10 Another multicenter study found that blacks had significantly lower adherence to ART than whites or Hispanics after adjusting for other potential confounders25. This study also found no difference in Hispanics and whites in ART adherence25; whereas several other studies found lower adherence among Hispanics than whites.26,27 We were unable to adjust specifically for adherence related confounders, which may explain our findings regarding shorter time to virologic suppression in Hispanics compared to whites.
Our finding of shorter times to ART initiation and virologic suppression with age greater than 30 years is consistent with previously published works.10,24,28 There are several possible explanations for these findings. Physicians may perceive young patients as high risk for non-adherence to ART and as a result, may delay ART initiation28. Physicians may also delay ART initiation in young patients due to concerns over the potential adverse events with long-term ART usage. Younger patients may also be more likely to decline treatment. This is of significant concern given the high risk of further HIV sexual transmission in this age group. Continued efforts to reduce age-related disparities are still needed.
We show that there have been marked improvements in LTFU over the study period. A recent study from the HOPS cohort found retention in care of about 85% and ART was the factor most associated with retention. It is plausible that the observed reduction in LTFU could be driven by the decreasing time to ART initiation. Nevertheless, maintaining engagement in care continues to be the single largest competing risk for ART initiation and virologic suppression and requires ongoing emphasis to drive continued improvements. We also show that 13% patients start ART but are LTFU prior to virologic suppression. This diagnosed and treated, but unsuppressed population should also be a priority for public health HIV prevention policy.
This study has several limitations. Changes in cost and availability of ART to individual patients occurred during the study period but were heterogeneous across the study population. We did not collect information on these changes, so this represents a potential source of residual confounding. Our study population was comprised of newly enrolled patients. It is possible that a small subset of patients previously receiving HIV care elsewhere were misclassified by our selection algorithm. Such selection bias should be consistent across time so should not impact our inference regarding changes in ART initiation and virologic suppression across time. Finally, the majority of our study population is from urban academic centers, where the practice patterns may not be representative of all HIV care sites in the United States.
In summary, we demonstrate decreasing times to ART initiation and to virologic suppression from 2003-04 to 2011-12. Furthermore, these decreases over this time period were greater among patients with enrollment CD4 ≥200 cells/mm3, compared to CD4 <200 cells/mm3 consistent with the changes in the DHHS HIV treatment guidelines of the time. Age and race disparities in times to ART initiation and virologic suppression are present. Addressing these persistent disparities will likely require targeted strategies focused on overcoming the drivers of disparities in these groups.
Supplementary Material
Acknowledgments
The authors would like to thank Cindy Voss for the management, cleaning, and quality assurance of the data used in this manuscript.
Role of the Sponsors: The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Funding/Support: This work was supported by the National Institutes of Health ‘5KL2-RR025006 to CFH, K23-AI084854 to SAB, K23-MH097647-01A1 to BRY, K23- AI084549 to ALA, R01-DA11602, K24-DA00432, R01-AA16893, R01-AG026250, U01 DA036935, P30 AI094189’; Agency for Healthcare Research and Quality ‘HHSA290201100007C’; the Health Resources and Services Administration ‘HHSH250201200008C’; and the Clinical Investigation and Biostatistics Core of the UC San Diego Center for AIDS Research ‘AI036214’.
Footnotes
Author Contribution: Dr. Haines had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Haines, Fleishman, Yehia, Gebo, Agwu
Acquisition of data: Moore, Gebo
Analysis and interpretation of data: Haines, Yehia, Lau, Fleishman, Berry, Gebo, Agwu
Drafting of the manuscript: Haines
Critical revision of the manuscript for important intellectual content: Haines, Fleishman, Yehia, Berry, Lau, Moore, Agwu, Gebo
Statistical analysis: Haines, Fleishman, Lau
Obtained Funding: Moore Gebo
Administrative, technical, or material support: Moore Gebo
Study supervision: Moore, Gebo
Participating Sites: Alameda County Medical Center, Oakland, California (Howard Edelstein, M.D.)
Children's Hospital of Philadelphia, Philadelphia, Pennsylvania (Richard Rutstein, M.D.)
Drexel University, Philadelphia, Pennsylvania (Jeffrey Jacobson, M.D., Sara Allen, C.R.N.P.)
Fenway Health, Boston, Massachusetts (Stephen Boswell, M.D.)
Johns Hopkins University, Baltimore, Maryland (Kelly Gebo, M.D., Richard Moore, M.D., Allison Agwu M.D.)
Montefiore Medical Group, Bronx, New York (Robert Beil, M.D.)
Montefiore Medical Center, Bronx, New York (Uriel Felsen, M.D.)
Oregon Health and Science University, Portland, Oregon (P. Todd Korthuis, M.D.)
Parkland Health and Hospital System, Dallas, Texas (Ank Nijhawan, M.D., Muhammad Akbar, M.D.)
St. Jude's Children's Research Hospital and University of Tennessee, Memphis,
Tennessee (Aditya Gaur, M.D.)
St. Luke's Roosevelt Hospital Center, New York, New York (Judith Aberg, M.D., Antonio Urbina, M.D.)
Tampa General Health Care, Tampa, Florida (Charurut Somboonwit, M.D.)
Trillium Health, Rochester, New York (William Valenti, MD., Roberto Corales, D.O.)
University of California, San Diego, California (W. Christopher Mathews, M.D.)
Sponsoring Agencies: Agency for Healthcare Research and Quality, Rockville, Maryland (Fred Hellinger, Ph.D., John Fleishman, Ph.D., Irene Fraser, Ph.D.)
Health Resources and Services Administration, Rockville, Maryland (Robert Mills, Ph.D., Faye Malitz, M.S.)
Data Coordinating Center: Johns Hopkins University (Richard Moore, M.D., Jeanne Keruly, C.R.N.P., Kelly Gebo, M.D., Cindy Voss, M.A., Nikki Balding, M.S.)
Publisher's Disclaimer: Disclaimer: The views expressed in this article are those of the authors. No official endorsement by the Department of Health and Human Services or the Agency for Healthcare Research and Quality is intended or should be inferred.
Conflict of Interest Disclosures: Dr. Gebo reported having served as a consultant, served on a scientific advisory board, and having received research funding from Tibotec. Dr. Berry has been a consultant for Bristol-Myers Squibb. No other disclosures were reported.
Contributor Information
Charles F. Haines, The Johns Hopkins Department of Medicine, Division of Infectious Diseases, Baltimore, MD
John A. Fleishman, Agency for Healthcare Research and Quality (AHRQ)
Baligh R. Yehia, University of Pennsylvania, Perelman School of Medicine, Philadelphia, PA
Bryan Lau, The Johns Hopkins University School of Public Health, Department of Epidemiology, Baltimore, MD
Stephen A. Berry, The Johns Hopkins Department of Medicine, Division of Infectious Diseases, Baltimore, MD
Allison L. Agwu, The Johns Hopkins Department of Pediatrics, Division of Infectious Diseases, Baltimore, MD
Richard D. Moore, The Johns Hopkins Department of Medicine, Division of Infectious Diseases, Baltimore, MD
Kelly A. Gebo, The Johns Hopkins Department of Medicine, Division of Infectious Diseases, Baltimore, MD
References
- 1.Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. HIV clinical trials. 2001 May-Jun;2(3):227–306. doi: 10.1310/RWG0-49RM-GQH4-5BB3. February 5, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Adolescents DPoAGfAa. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services; Dec 1, 2007. p. 2007. [Google Scholar]
- 3.Adolescents PoAGfAa. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2009 Dec 1;:2009. [Google Scholar]
- 4.Adolescents DPoAGfAa. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services; Feb 12, 2013. p. 2013. [Google Scholar]
- 5.Timing of HAART initiation and clinical outcomes in human immunodeficiency virus type 1 seroconverters. Archives of internal medicine. 2011 Sep 26;171(17):1560–1569. doi: 10.1001/archinternmed.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. The New England journal of medicine. 2009 Apr 30;360(18):1815–1826. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sterne JA, May M, Costagliola D, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009 Apr 18;373(9672):1352–1363. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cain LE, Logan R, Robins JM, et al. When to initiate combined antiretroviral therapy to reduce mortality and AIDS-defining illness in HIV-infected persons in developed countries: an observational study. Annals of internal medicine. 2011 Apr 19;154(8):509–515. doi: 10.1059/0003-4819-154-8-201104190-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. The New England journal of medicine. 2011 Aug 11;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanna DB, Buchacz K, Gebo KA, et al. Trends and disparities in antiretroviral therapy initiation and virologic suppression among newly treatment-eligible HIV-infected individuals in North America, 2001-2009. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013 Apr;56(8):1174–1182. doi: 10.1093/cid/cit003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleishman JA, Gebo KA, Reilly ED, et al. Hospital and outpatient health services utilization among HIV-infected adults in care 2000-2002. Medical care. 2005 Sep;43(9 Suppl):III40–52. doi: 10.1097/01.mlr.0000175621.65005.c6. [DOI] [PubMed] [Google Scholar]
- 12.Network HIVR. Hospital and outpatient health services utilization among HIV-infected patients in care in 1999. Journal of acquired immune deficiency syndromes. 2002 May 1;30(1):21–26. doi: 10.1097/00126334-200205010-00003. [DOI] [PubMed] [Google Scholar]
- 13.Gebo KA, Fleishman JA, Conviser R, et al. Racial and gender disparities in receipt of highly active antiretroviral therapy persist in a multistate sample of HIV patients in 2001. Journal of acquired immune deficiency syndromes. 2005 Jan 1;38(1):96–103. doi: 10.1097/00126334-200501010-00017. [DOI] [PubMed] [Google Scholar]
- 14.Gebo KA, Fleishman JA, Reilly ED, Moore RD, Network HIVR High rates of primary Mycobacterium avium complex and Pneumocystis jiroveci prophylaxis in the United States. Medical care. 2005 Sep;43(9 Suppl):III23–30. doi: 10.1097/01.mlr.0000175631.34438.1e. [DOI] [PubMed] [Google Scholar]
- 15.Fleishman JA, Yehia BR, Moore RD, Korthuis PT, Gebo KA. Establishment, retention, and loss to follow-up in outpatient HIV care. Journal of acquired immune deficiency syndromes. 2012 Jul 1;60(3):249–259. doi: 10.1097/QAI.0b013e318258c696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999 Jun;94(446):496–509. [Google Scholar]
- 17.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. American journal of epidemiology. 2009 Jul 15;170(2):244–256. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coviello V, Boggess M. Cumulative incidence estimation in the presence of competing risks. The Stata Journal. 2004;4(2):103–112. [Google Scholar]
- 19.Stata Statistical Software: Release 12 ‘computer program’. College Station, TX: StataCorp LP; 2011. [Google Scholar]
- 20.R: A Language and Environment for Statistical Computing ‘computer program’. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 21.Diagnoses of HIV Infection in the United States and Dependent Areas, 2013. Centers for Disease Control and Prevention; Feb, 2015. p. 2015. [Google Scholar]
- 22.Vital signs: HIV prevention through care and treatment--United States. MMWR Morbidity and mortality weekly report. 2011 Dec 2;60(47):1618–1623. [PubMed] [Google Scholar]
- 23.Gross CP, Steiner CA, Bass EB, Powe NR. Relation between prepublication release of clinical trial results and the practice of carotid endarterectomy. JAMA : the journal of the American Medical Association. 2000 Dec 13;284(22):2886–2893. doi: 10.1001/jama.284.22.2886. [DOI] [PubMed] [Google Scholar]
- 24.Novak RM, Hart RL, Chmiel JS, Brooks JT, Buchacz K. Disparities in Initiation of Combination Antiretroviral Treatment and in Virologic Suppression Among Patients in the HIV Outpatient Study, 2000-2013. Journal of acquired immune deficiency syndromes. 2015 Sep 1;70(1):23–32. doi: 10.1097/QAI.0000000000000652. [DOI] [PubMed] [Google Scholar]
- 25.Simoni JM, Huh D, Wilson IB, et al. Racial/Ethnic disparities in ART adherence in the United States: findings from the MACH14 study. Journal of acquired immune deficiency syndromes. 2012 Aug 15;60(5):466–472. doi: 10.1097/QAI.0b013e31825db0bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silverberg MJ, Leyden W, Quesenberry CP, Jr, Horberg MA. Race/ethnicity and risk of AIDS and death among HIV-infected patients with access to care. Journal of general internal medicine. 2009 Sep;24(9):1065–1072. doi: 10.1007/s11606-009-1049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh DL, Sarafian F, Silvestre A, et al. Evaluation of adherence and factors affecting adherence to combination antiretroviral therapy among White, Hispanic, and Black men in the MACS Cohort. Journal of acquired immune deficiency syndromes. 2009 Oct 1;52(2):290–293. doi: 10.1097/QAI.0b013e3181ab6d48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleishman JA, Yehia BR, Moore RD, Gebo KA, Agwu AL. Disparities in receipt of antiretroviral therapy among HIV-infected adults (2002-2008) Medical care. 2012 May;50(5):419–427. doi: 10.1097/MLR.0b013e31824e3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


