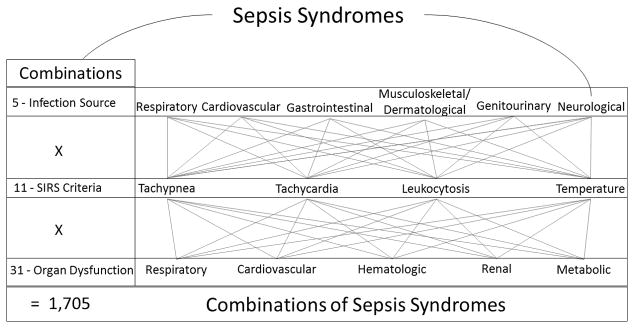
Licensing ourselves to hyperbole, sepsis may be one of the most diverse syndromes in the modern clinical vocabulary (1). For starters, it is a syndrome with etiologic agents not only across 3 taxonomic kingdoms of life (Bacteria, Protozoa and Fungi) but also outside of the taxonomy of life (Viruses). Multiplying the possible flavors of sepsis, many of these pathogens can infect a variety human tissues and each infective episode can in turn induce different combinations of systemic inflammatory responses and distant organ failures. Just how many flavors of sepsis can there be? As a simple thought experiment, let’s ignore the rainbow of etiologic agents, assume one source of infection per sepsis episode and narrow the field of organ dysfunctions to 5. To get the total number of sepsis combinations under these stipulations, we multiply the 5 infection sources by the 11 different combinations of SIRS criteria that meet a definition of sepsis by the 31 combinations of organ dysfunctions (Figure 1). This gives us 1,705 shades of sepsis, admittedly only the tip of the iceberg given that we have ignored the myriad of etiologic agents. Yet, like choosing which variation of beige to paint your dining room, the important question is whether there is any meaningful difference between these myriad tones of sepsis? The original article by paper by Shankar-Hari et al. adds to a body of evidence suggesting that there is a meaningful difference (2).
Figure 1. Conservative Estimate of the Number of Combinations of Clinical Criteria Comprising Sepsis, Assuming a Single Pathogen and Single Infected Source Organ.
SIRS = Systemic inflammatory response syndrome. Combinations calculated by Σ (n!/r!(n−r)!); where n is number of distinct items in a given set and r is one of the range of numbers of items selected from that set. For the SIRS example, n = 4 SIRS criteria and r = 2, 3 or 4 for the number of criteria we can select from this set to meet the definition of sepsis.
To summarize, the article is a cohort study of the 241,603 adults admitted with a diagnosis of infection to a general critical care unit in England from 2000–2012. Systemic inflammatory response and organ dysfunction criteria were extracted from physiologic data within the first 24 hours of critical care admission and hospital mortality was the primary outcome. The study uses data from the Case Mix Programme, a high quality, professionally and independently maintained database of nearly every patient of every general critical care unit in the United Kingdom (3). The analyses examine the unadjusted hospital mortality of sepsis from many different angles: source of infection, quantity and combination of SIRS criteria, and quantity and combination of organ dysfunctions. The results display a wide range of hospital mortality along every facet. For source of infection, hospital mortality was lowest for genitourinary infections (19.4%) and highest for cardiovascular infections (44.2%). For SIRS criteria, even within groupings of 2 and 3 total criteria met within the first 24 hours of critical care admission, different combinations were associated with wide within-group variation in unadjusted hospital mortality (from approximately 20%–40%, estimating from the graphs). Similarly, among different combinations of organ dysfunctions, there is a considerable range of unadjusted hospital mortality rates. From this data, the investigators create 4 risk categories of organ dysfunction combinations, grouped by the associated hospital mortality rates rather than the sum or physiologic relationships of organ dysfunctions. They demonstrate that within each risk category, hospital mortality has followed a similar downward trend from 2000–2012, an observation that increases confidence in the notion that overall sepsis mortality is improving due to changes in care rather than increases in diagnosis of less severe cases. Furthermore, the investigators use these risk categories to hypothesize an explanation as to why the hospital mortality from sepsis in England was on average 1.5 times higher than that reported in a well-known study over the same time period in Australia and New Zealand (4). Their analyses suggest that the sepsis case mix in England represents a higher risk case mix than that found in Australia and New Zealand. However, while they illustrate that overall mortality reported in Australia and New Zealand is similar to the mortality from risk category 2 sepsis in England, we do not see the risk categories of the Australia and New Zealand data, limiting any direct comparisons.
The study by Shankar-Hari et al. graphically depicts the wide variations in unadjusted hospital mortality of a wide variety sepsis syndromes defined by various combinations of infection source, SIRS criteria and organ dysfunctions. While this is an important and well-presented reminder of the significant variety under the umbrella of sepsis, many of the concepts are not new. The notion that increasing numbers of abnormal vital signs and organ dysfunctions was associated with increased mortality in sepsis was demonstrated in the early clinical studies of sepsis from the mid-1990s (5–7). Furthermore, the effects of different combinations and severities of acute physiology on the mortality of critically ill sepsis patients has been skillfully modeled since the original Sepsis-related Organ Failure Assessment (SOFA) score in 1996 (7). Given that the SOFA is advocated as a defining feature of sepsis among the critically ill in the Third International Consensus Guidelines and that Dr. Shankar-Hari was the lead investigator on the development of these new definitions, it is unclear why the SOFA score was not employed in the present work to explore the variations in mortality (1, 9). Instead we are presented with a new and unvalidated risk categorization based on combinations of organ dysfunctions that may be less useful in benchmarking and comparing sepsis mortality across nations and healthcare systems than a more well-known, widely-used and validated model, of which SOFA is only one of many. Therefore, while this manuscript clearly demonstrates the importance of case-mix adjustment and severity of illness adjustment in comparing sepsis outcomes, the Third Consensus Guidelines’ employment of the SOFA score may offer a more cohesive method for both defining sepsis and adjusting for case mix based on acute physiology.
As Shankar-Hari et al. allude to in their article’s title, the issue of utilizing a method for mortality risk adjustment specific to sepsis is of utmost importance when comparing sepsis outcomes across boundaries of institution, nation and time. In considering international comparisons, it is challenging to conceive of sepsis outcome benchmarks that could be globally generalizable given this inherently heterogeneous syndrome and the wide variety of regional variation in available resources, endemic pathogens, and prevalent comorbidities. Furthermore, this provides an important frame of reference for understanding the difficulty in finding effective medical interventions in sepsis and testing them in international, multicenter trials. Additionally, these issues of comparison and benchmarking are of importance even within a single country. This may currently be very salient to readers from the United States, as the recognition and treatment of sepsis has now become a core quality measure for assessing hospital performance in this country (10). Particularly in benchmarking, assessing and comparing the effects of the core measures on sepsis mortality across time and healthcare systems, the issue of whether or not there is a method for mortality risk adjustment specific to sepsis will become critically important. Despite these challenges, it is exciting to be witness to these changes in the field of sepsis that may be beginning to move from increasing global awareness to improving global quality of care.
Footnotes
Financial Disclosure: JAK, and this work, is supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1 TR000454, KL2 TR000455) and GSM is supported by the Food and Drug Administration (R01 FD003440), the National Institute for General Medical Sciences (R01 GM113228) and the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1 TR000454).
Copyright form disclosures: Dr. Martin received funding from Bard, Grifols (Medical Advisory Boards) and received support for article research from the National Institutes of Health (NIH). His institution received funding from the NIH, FDA and Baxter Healthcare. Dr. Kempker disclosed that he does not have any potential conflicts of interest.
Contributor Information
Jordan A. Kempker, Email: jkempke@emory.edu, Assistant Professor of Medicine, Division of Pulmonary, Allergy, Critical Care and Sleep Medicine, Emory University School of Medicine, 49 Jesse Hill Jr Drive, Atlanta, GA 30303, Phone: 404-616-9175.
Greg S. Martin, Email: greg.martin@emory.edu, Professor of Medicine and Associate Division Director, Division of Pulmonary, Allergy, Critical Care and Sleep Medicine, Emory University School of Medicine, 49 Jesse Hill Jr Drive, Atlanta, GA 30303, Phone: 404-616-0148.
References
- 1.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shankar-Hari M, Harrison DA, Rowan KM. Differences in impact of definitional elements on mortality precludes international comparisons of sepsis epidemiology - a cohort study illustrating the need for standardized reporting. Crit Care Med. 2016 doi: 10.1097/CCM.0000000000001876. in press. [DOI] [PubMed] [Google Scholar]
- 3.Intensive Care National Audit & Research Center. [Accessed March 31, 2016];Case Mix Programme Participation. ( https://www.icnarc.org/Our-Audit/Audits/Cmp/About/Participation)
- 4.Kaukonen KM, Bailey M, Suzuki S, et al. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA. 2014;311(13):1308–1316. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 5.Brun-Buisson C, Doyon F, Carlet J, et al. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. French ICU Group for Severe Sepsis. Jama. 1995;274(12):968–974. [PubMed] [Google Scholar]
- 6.Rangel-Frausto MS, Pittet D, Costigan M, et al. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. Jama. 1995;273(2):117–123. [PubMed] [Google Scholar]
- 7.Salvo I, de Cian W, Musicco M, et al. The Italian SEPSIS study: preliminary results on the incidence and evolution of SIRS, sepsis, severe sepsis and septic shock. Intensive care medicine. 1995;21(Suppl 2):S244–249. doi: 10.1007/BF01740762. [DOI] [PubMed] [Google Scholar]
- 8.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive care medicine. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 9.Shankar-Hari M, Phillips GS, Levy ML, et al. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):775–787. doi: 10.1001/jama.2016.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Joint Commision. [Accessed April 1, 2016];Specifications Manual for National Hospital Inpatient Quality Measures. ( http://www.jointcommission.org/specifications_manual_for_national_hospital_inpatient_quality_measures.aspx)