Abstract
To test whether a modified version of prolonged exposure (mPE) can effectively treat posttraumatic stress disorder (PTSD) in individuals with co-occurring PTSD and substance dependence, an efficacy trial was conducted in which substance dependent treatment-seekers with PTSD (N = 126, male = 54.0%, White = 79.4%) were randomly assigned to mPE, mPE + trauma-focused motivational enhancement session (mPE+MET-PTSD), or a health information-based control condition (HLS). All participants were multiply traumatized; the median number of reported traumas that satisfied DSM-IV Criterion A for PTSD was 8. Treatment consisted of 9–12 60 min. individual therapy sessions plus substance abuse treatment-as-usual. Participants were assessed at baseline, end-of-treatment, and at 3- and 6-months posttreatment. Both the mPE and mPE+MET-PTSD conditions achieved significantly better PTSD outcome than the control condition. The mPE+MET-PTSD and mPE conditions did not differ from one another on PTSD symptoms at end of treatment, 3-, or 6-month follow-up. Substance use outcomes did not differ between groups with all groups achieving 85.7–97.9% days abstinent at follow-up. In regard to clinically significant improvement in trauma symptoms, 75.8 % of the mPE participants, 60.0% of the mPE+MET-PTSD participants, and 44.4% of the HLS participants experienced clinically significant improvement at the end-of-treatment. Results indicate mPE, with or without an MET-PTSD session, can effectively treat PTSD in patients with co-occurring PTSD and substance dependence. In addition, mPE session lengths may better suit standard clinical practice and are associated with medium effect sizes.
Keywords: Posttraumatic Stress Disorder, Alcoholism, Drug Abuse, Exposure Therapy, Comorbidity
Traumatic life events, such as physical or sexual assaults, are unusually common among substance users (e.g., Brown et al., 2014; Goldstein et al., 2016). Distressing recollections of these traumatic experiences, accompanied by avoidance of trauma reminders and increased arousal, are characteristic of posttraumatic stress disorder (PTSD), which occurs in approximately 15–42% of individuals with a substance use disorder (SUD; e.g., Driessen et al., 2008; Ouimette, Goodwin, & Brown, 2006; Reynolds, Hinchliffe, Asamoah, & Kouimtsidis, 2011). Unfortunately, PTSD predicts unfavorable SUD treatment outcomes and substance users with PTSD tend to relapse faster following standard addictions treatment (e.g., Read, Brown & Kahler, 2004). Also, they demonstrate poorer social functioning, greater psychiatric comorbidity and higher healthcare costs than substance users without trauma histories (e.g., Ouimette et al., 2006; Read et al., 2004; Tate, Norman, McQuaid & Brown, 2007).
Importantly, symptoms of PTSD and SUD appear to covary with one another (e.g., Back, Brady, Sonne, & Verduin, 2006; Read et al., 2004) and there is some evidence that patients would prefer receiving concurrent PTSD and SUD treatment (e.g., Back, Brady, Jaanimägi, & Jackson, 2006). Fortunately, research examining the efficacy of treating both PTSD and SUD symptoms at the same time has been emerging in recent years. For example, research on the efficacy of prolonged exposure (e.g., Foa, Hembree, & Rothbaum, 2007) concurrent with substance use treatment is emerging. Prolonged exposure is a cognitive-behavioral therapy considered a first line treatment for PTSD (Foa, Keane, Friedman, & Cohen, 2008). It is designed to disrupt the cycle of anxiety and avoidance that characterizes PTSD via exposure techniques; helping clients gradually and systematically face their painful memories and current, real-life trauma reminders. Although prolonged exposure treatment studies typically exclude participants with a SUD, a recent meta-analysis of 13 RCTs found that prolonged exposure produced large decreases in PTSD symptoms, with the average exposure-treated patient faring better than 86% of control patients at post-treatment (Powers, Halpern, Ferenschak, Gillihan, & Foa, 2010).
Despite its demonstrated effectiveness, only a few randomized controlled trials (RCT) have examined the efficacy of using exposure-based PTSD treatment within substance using samples (Mills et al., 2012; Sannibale et al., 2013; Foa et al., 2013). In two of these studies (Mills et al., and Sannibale et al.), greater PTSD symptom reduction was observed for the group receiving an exposure-based PTSD treatment protocol as part of the combined PTSD/SUD intervention. However, across the three studies results for substance use outcomes were more variable. One study showed small gains in both the exposure and control condition (Mills et al.), a second study showed greater gains in the control condition (Sannibale et al.), and a third study, a combined pharmacotherapy/psychotherapy RCT, showed similar gains for both treatment conditions at the end of treatment, but less of an increase in alcohol use during the follow-up period for the group receiving prolonged exposure plus naltrexone (Foa et al.). Importantly, the type of control condition also varied and neither Mills et al. nor Foa et al. employed a matched control group to control for time and other nonspecific therapeutic factors (e.g., greater attention, alliance, positive regard, time spent in PTSD treatment). Thus, it remains unclear whether significant reductions in PTSD symptoms are due to the exposure therapy content or due to other nonspecific factors.
While valuable advances have been made toward the treatment of PTSD and SUD, additional research examining the efficacy of prolonged exposure in SUD populations is essential. For example, refinement of prolonged exposure protocols to maximize treatment completion, convenience, and ease of implementation is necessary. Some previous RCTs combine treatment of PTSD and SUD within one protocol, under one care provider (e.g., Mills et al., 2012; Sannibale et al., 2013). Although this effectively consolidates care and reduces the required number of therapists, it increases the level of expertise required of each clinician. As securing the resources, training, and expertise required to implement protocols requiring extensive training may be challenging for some practitioners and agencies, examination of alternative models is needed.
The primary aim of the present work is to test the efficacy of a modified version of prolonged exposure (mPE) as an augmentation to a 6-week traditional, 12-Step residential SUD treatment program. Because treatment dropout can be high when prolonged exposure is used with substance abusers with PTSD (van Dam, Vedel, Ehring, & Emmelkamp, 2012), a second aim was to test whether a 90-min trauma-focused motivational enhancement session administered prior to mPE (mPE+MET-PTSD) would increase study treatment completion.
In light of the primary aim, we hypothesized that relative to individuals receiving the control condition, individuals receiving mPE or mPE+MET-PTSD would: 1) show significantly greater decreases in PTSD symptoms at follow-up; 2) show significantly greater decreases in depressive symptoms at follow-up; 3) evidence better SUD outcomes at follow-up; 4) demonstrate higher rates of clinically significant improvement on trauma symptoms and substance use outcomes; and 5) report less craving. In light of the second aim, we hypothesized that study treatment completion would be greater for those individuals who received a trauma-focused motivational enhancement session prior to beginning mPE.
Method
Participants
Participants (N = 126) were recruited from an unlocked 6-week community residential SUD treatment facility. Potential participants were approached within the first week of their treatment. Recruitment occurred between 2008 and 2011. Inclusion criteria were 1) Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev.; DSM-IV-TR; American Psychiatric Association, 2000) diagnosis of both PTSD (stemming from any trauma except combat) and alcohol dependence (AD), 2) one heavy drinking day in the past 60 days, as defined by consumption of 4 standard drinks for women and 5 standard drinks for men, and 3) age between 18–64. Exclusion criteria were 1) the presence of an acute psychotic disorder, 2) bipolar disorder with an active manic episode (but not simply the presence of bipolar disorder), 3) imminent risk for suicide, 4) prescription of craving reducing medications (e.g., naltrexone) or medications to reduce alcohol use (e.g., disulfiram), 5) current self-reported use, or urine drug screen indicating use, of a benzodiazapine, 6) judged to have a medical condition that might limit cooperation or compromise the integrity of the data (e.g., organic brain syndrome, dementia, head injury, neuropathy, etc.), and 7) illiteracy in English.
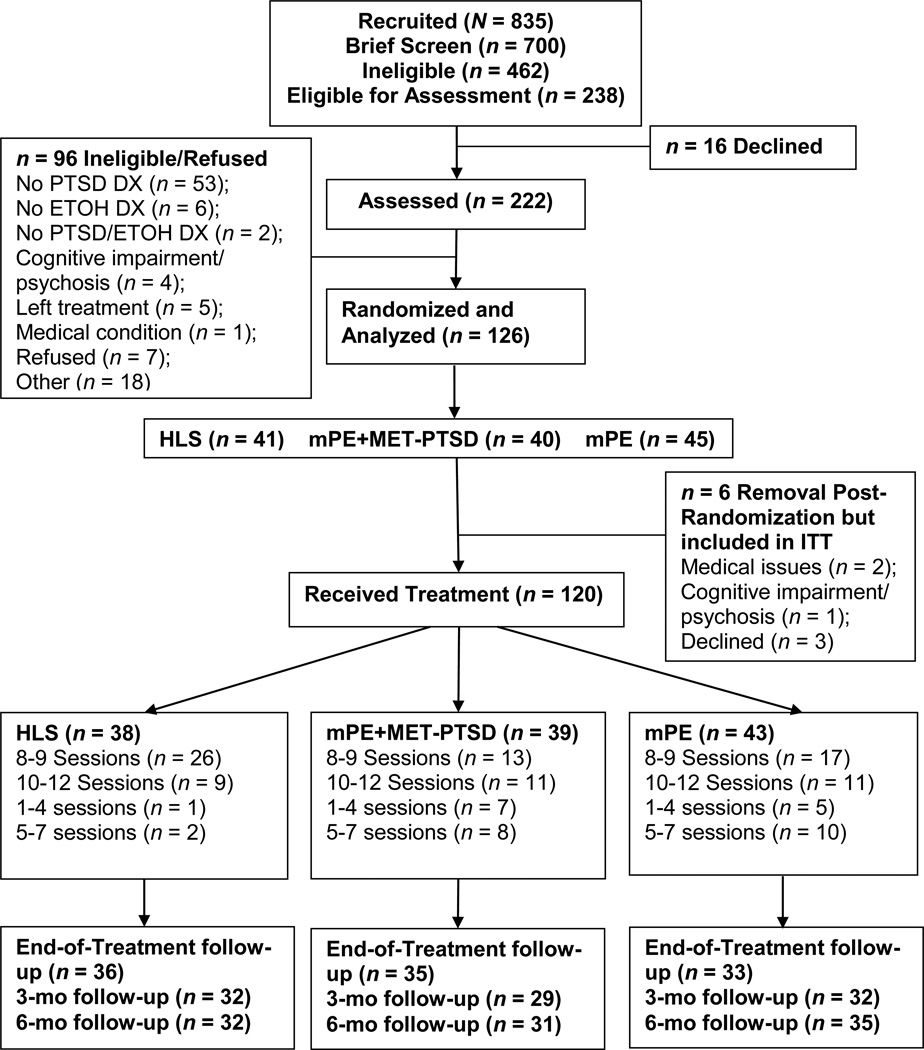
Figure 1 summarizes participants’ flow from recruitment to 6-month follow-up. Study eligibility was determined through a two-step process: a brief screening followed by an assessment. A total of 835 individuals were approached and 700 completed a brief screening. Of those 700, 238 passed the brief screening and were eligible for an assessment. Two hundred twenty-two individuals were consented and assessed. The intent-to-treat sample consisted of 126 participants and these participants were randomized to a treatment condition resulting in a response rate of 94.7% (i.e., proportion of eligible participants who agreed to participate). Forty-five were assigned to mPE, 40 to mPE+MET-PTSD, and 41 to the health information control condition (HLS). One hundred twenty participants received treatment with 43 receiving mPE, 39 receiving mPE+MET-PTSD, and 38 receiving HLS.
Figure 1.
CONSORT figure depicting participant flow from recruitment through 6-month follow-up. mPE = modified prolonged exposure; mPE+MET-PTSD = modified prolonged exposure plus trauma-focused motivational enhancement therapy; HLS = healthy lifestyles sessions.
The study protocol for this randomized controlled efficacy trial was approved by the [redacted] Institutional Review Board (IRB). All participants provided written informed consent prior to enrollment in the study and were compensated by check for the baseline, end-of-treatment, and 3- and 6-month follow-up assessments. Participants were not compensated for therapy sessions.
Measures
Diagnostic measures administered at baseline
The National Women's Study (NWS) PTSD Module (Resnick, 1996) was used to assess participants’ trauma history and establish DSM-IV PTSD Criterion A (APA, 2000). The NWS PTSD Module is a structured interview and has been used to assess PTSD Criterion A in numerous studies involving both men and women (e.g., Coffey et al., 2006).The Clinician Administered PTSD Scale (CAPS; Blake et al., 1995), a well-established and psychometrically sound structured clinical interview designed to assess the 17 symptoms of PTSD, was used as the diagnostic tool for current PTSD according to DSM-IV (i.e., symptoms satisfying Criterion B-F present in the past month). Both frequency and severity ratings range from 0 to 4 and a symptom was considered to be present when it had a frequency rating of at least 1 and a severity rating of at least 2 (Weathers, Keane, & Davidson, 2001). A total severity score is calculated by summing the frequency and severity ratings of the 17 symptoms of PTSD (possible range = 0–136). The Computerized Diagnostic Interview Schedule (C-DIS IV;Robins et al., 2000) is a computerized version of the Diagnostic Interview Schedule, a fully structured, psychometrically sound, diagnostic interview for Axis I psychiatric disorders in DSM-IV. The current study used the C-DIS to establish substance use disorder diagnostic status (past 12 months).The Mini-International Neuropsychiatric Interview (M.I.N.I.;Sheehan et al., 1998)is a widely used structured, psychometrically sound, diagnostic interview. The M.I.N.I was used to establish current DSM-IV Axis I psychiatric diagnostic status for mood disorders and all anxiety disorders except PTSD.
Self-report questionnaires
Unless otherwise noted, self-report questionnaires were administered at baseline, end-of-treatment, 3-, 6-month follow-up assessments. The Impact of Event Scale-Revised (IES-R; Weiss & Marmar, 1997) was used to measure trauma symptoms. The IES-R consists of 22 items rated on a 0–4 scale. It has demonstrated good internal consistency in a PTSD-SUD sample (Rash, Coffey, Baschnagel, Drobes, & Saladin, 2008). In the current study, Cronbach’s alpha was 0.90. The Beck Depression Inventory-II (BDI-II; Beck et al., 1996) is a widely used, psychometrically sound measure of depressive symptoms and consists of 21 items. In the current study, Cronbach’s alpha for the BDI-II was 0.91. The Alcohol Craving Questionnaire-Now (ACQ-Now; Singleton et al., 1994) is a 47-item questionnaire designed to measure self-reported craving symptoms in the present moment. The ACQ-Now has strong psychometric properties (Connolly, Coffey, Baschnagel, Drobes, & Saladin, 2009). In the current study, Cronbach’s alpha was 0.87. The Alcohol Dependence Scale (ADS; Skinner & Horn, 1984) is a 25-item questionnaire and was administered to participants to measure alcohol related symptoms. Numerous studies have demonstrated the ADS to have strong psychometric properties (e.g., Drake, McHugo, & Biesanz, 1995). In the current study, Cronbach’s alpha was 0.89. The ADS was only administered at the baseline assessment.
Substance use and abstinence compliance
The Time Line Follow-Back (TLFB; Sobell & Sobell, 1992) is a commonly used calendar method to gather retrospective information on substance use for a specified time. The primary alcohol and drug use outcome measures used in the current study were percent days abstinent (PDA), which represents the percentage of days not in a controlled environment in which the participant did not use alcohol or drugs. As secondary measures to validate the TLFB, urine drug screens were administered to test for the presence of metabolites of illicit drugs (iCup, Instant Technologies, Inc., Norfolk, VA) and recent alcohol use was assessed with a breathalyzer (Alco-sensor IV, Intoximeters, Inc., St. Louis, MO). Participants were not informed of UDS or breathalyzer test results during the assessment so as to not bias the TLFB interview.
Treatment conditions
Regardless of treatment assignment, all participants were provided two study therapy sessions per week over the course of 5 to 8 weeks. Therapy was provided by Masters and PhD level clinicians who were not affiliated with the residential treatment program. Specifically, therapy was provided by 5 PhD level clinicians (i.e., psychologists) and 3 Masters level clinicians over the course of the four-year intervention period of the project.
Modified prolonged exposure (mPE)
Prolonged exposure is a well-described psychotherapy that utilizes imaginal and in vivo exposure techniques to reduce the symptoms of PTSD (see Foa et al., 2007). In addition to imaginal and in vivo exposure techniques, patients are provided psychoeducation about PTSD, a rationale for mPE, and are taught breathing retraining as a method to manage PTSD-associated arousal. Imaginal exposures were audio taped and participants were instructed to listen to the tapes daily. Consistent with Foa et al. (2005), 9–12 sessions of mPE were provided to participants in the mPE and mPE+MET-PTSD conditions. Nine sessions of mPE were offered initially and, if PTSD symptom severity did no missing t decrease by at least 70%, an additional 3 sessions of mPE were offered.
Although the mPE intervention was largely informed by conventional prolonged exposure (Foa et al., 2007), several adaptations were made to conventional prolonged exposure. Traditionally, prolonged exposure sessions are 90 min but since 90 min psychotherapy sessions may prove to be a barrier when attempting to implement prolonged exposure in SUD treatment facilities, the current study utilized 60 min sessions (Nacasch et al., 2015; van Minnen, & Foa, 2006). Additionally, although participants were explicitly informed that mPE would not focus on SUD symptoms (which were addressed through TAU), the mPE protocol did contain added psychoeducation about the relationship between PTSD and SUD symptoms and weekly check-ins about substance use. Given there is evidence that substance craving in patients with PTSD-SUD can be elicited by trauma-related negative affect (e.g., Coffey et al. 2006), a few minutes of diaphragmatic breathing was completed at the end of each imaginal exposure session to return distress to baseline levels.
Trauma-focused motivational enhancement therapy for PTSD
Approximately one half of the participants receiving mPE were provided a 90 min trauma-focused motivational enhancement therapy for PTSD session prior to beginning mPE (i.e., mPE+MET-PTSD condition). The other half of the mPE participants (i.e., mPE condition) and all of the HLS participants were provided a 60 min relaxation session prior to the first scheduled treatment session. The MET-PTSD session consisted of four main phases: opening statements; eliciting/developing discrepancy; presenting feedback; and future directions and was designed to increase motivation to address PTSD issues during substance use treatment.
Healthy Lifestyles Sessions (HLS)
HLS is a structured 9–12 session intervention that provides education about a variety of health-related topics and is similar to the control condition used in (Stasiewicz et al., 2013). HLS was designed to involve a similar amount of therapist contact as mPE. Unlike mPE, in which a 70% symptom reduction in trauma symptoms at session 8 could initiate a termination session at session 9 (otherwise treatment continued until session 12), a participant’s HLS treatment completion was yoked to another participant’s successful mPE treatment completion (not mPE+MET-PTSD) to equalize treatment dose between the treatment conditions. Therefore, if an mPE participant successfully completed treatment in 9 sessions, the maximum number of HLS sessions offered to the HLS participant would be 9. Likewise, 12 would be offered if the mPE participant completed 12 sessions. Topics covered included an introduction to treatment; sleep hygiene; progressive muscle’s relaxation; starting/maintaining an exercise program; personal role identification; healthy eating and nutrition (two sessions); diabetes (prevention or diabetes treatment adherence, depending on diabetes status); monitoring goals and values; cancer (a focus on breast cancer for women and colon cancer for men); HIV (reducing HIV risk or adhering to HIV treatment, depending on HIV status); and a final review session. Sessions included the provision of information, discussing participants’ understanding of information, and answering questions about the information provided.
Substance abuse treatment as usual
All participants received standard TAU for substance abuse within a 6-week community residential treatment facility. TAU consisted of daily group therapy for approximately 3 hours each day, daily recreation therapy, AA and NA meetings, individual drug counseling sessions, and completion of drug counseling homework. TAU was provided by drug and alcohol counselors unaffiliated with the current study.
Procedure
Brief pre-screening
Following a brief description of the study, individuals expressing interest in the trial were administered the DSM-IV version of the PTSD Checklist (PCL; Weathers, Litz, Herman, Huska, & Keane, 1993) to assess the likelihood for meeting diagnostic criteria for PTSD. The PCL has strong psychometric properties (e.g., Ruggiero, Del Ben, Scotti & Rabalais, 2003) and consists of 17 items rated on a 1–5 scale. To screen for problematic drinking, the Alcohol Use Disorder Identification Test (AUDIT; Saunders, Aasland, Babor, de la Fuente, & Grant, 1993) was used. The AUDIT has strong psychometric properties (Saunders et al., 1993) and consists of 10 items rated on a 0–4 scale. Individuals whose score on the PCL equaled or exceed 44, a score suggesting significant PTSD symptoms, and an AUDIT score equaling or exceeding 8, a score suggesting problematic drinking, provided informed consent and were scheduled for a comprehensive assessment. To satisfy pre-screening criteria, all potential participants affirmed that he or she had experienced “emotional, physical, or sexual trauma at some time in [their lives], either recently or in the past”, scored 44 or higher on the PCL, and scored 8 or higher on the AUDIT. Participants who passed the pre-screening described above were scheduled for an in-person clinical assessment to provide informed consent and determine study eligibility. Once eligibility was determined, participants were randomized to treatment condition.
Randomization
Eligible participants were randomly assigned, using urn randomization (Wei, 1978), to a treatment condition: mPE, mPE+MET-PTSD, or HLS using the computer program developed for Project MATCH. Variables in the urn included sex, alcohol abuse severity (ADS), PTSD symptom severity (IES-R), and motivation to address trauma issues (University of Rhode Island Change Assessment-Trauma; Hunt, Linkovich-Kyle, Coffey, Stasiewicz, & Schumacher, 2006).
Assessments
Participants completed in-person assessments at pre-treatment and completed either in-person or telephone assessments at end-of-treatment, and 3- and 6-months post-treatment. In-person assessment was encouraged at 3- and 6-month assessment points but was not possible for some participants. Prior to administering the CAPS, all research staff received training in the administration of the CAPS by the first author. Upon gaining proficiency under supervision, the research staff member was allowed to interview study participants. The CAPS were audiotaped and the first 5 audiotapes were reviewed by senior research staff members. Research staff conducting the assessments were blind to treatment condition. In the present study, the level of diagnostic agreement for a random sample of CAPS (approximately 20% of all interviews) was high (kappa = .94).
Training and fidelity monitoring
All treatment sessions were administered by advanced psychology graduate students, psychology postdoctoral fellows, or licensed psychologists. Therapists were trained in mPE by the first author and both HLS and MET-PTSD by the second author. To reduce the possible influence of therapist effects, therapists administered all three treatment conditions. Therapist training included having the therapist read the treatment manual, listen to recorded exemplary sessions, attend separate didactic series on the treatment approaches, and submit practice therapy audiotapes to the trainers. Weekly group supervision was provided and audiotapes were monitored throughout the study to reduce therapist drift. All therapists were provided with, and followed, mPE, MET-PTSD, and HLS treatment manuals. A senior clinician independent of treatment delivery listened to randomly selected audiotaped sessions for fidelity to the treatment manual and for competence in providing the treatments.
mPE adherence and competence
One hundred mPE and mPE+MET-PTSD audiotaped therapy sessions were rated for mPE treatment fidelity (15% of mPE and mPE+MET-PTSD sessions) and these therapy sessions contained a total of 1,276 essential elements. mPE essential elements included therapist behaviors such as presenting an overview of treatment, providing a rationale for exposure therapy, providing breathing retraining instruction, assigning homework, reviewing homework, conducting imaginal exposure, etc. A total of 51 (4%) elements were not provided by therapists during the therapy session. Therapy session audiotapes were also rated for the inclusion of therapy elements not contained in the treatment manual. Three percent of sessions contained therapeutic techniques not included in the manual (e.g., a mindfulness technique, a passing suggestion to try progressive muscle relaxation, etc.). Competence providing the essential and unique elements (e.g., describing imaginal exposure and providing rationale) and the essential but not unique elements (e.g., assigning homework, establishing good rapport, etc.) were rated separately using a 5-point scale ranging from poor to excellent. When providing essential and unique elements, 77% of the samples were rated excellent, 95% were rated good or excellent, and none were rated below satisfactory. When providing the essential but not unique elements, 87% of the samples were rated excellent, 95% were rated good or excellent, and none were rated below satisfactory. The mPE adherence and competence measure was created for the current study.
HLS adherence and competence
Forty-five, randomly selected, audiotaped HLS therapy sessions (15% of HLS sessions) were rated for treatment fidelity and these therapy sessions contained a total of 564 essential elements. A total of 3 (0.5%) elements were not provided by therapists during the therapy session. Therapy session audiotapes were also rated for the inclusion of therapy elements not contained in the HLS treatment manual. One session contained a therapeutic technique not included in the manual (i.e., did not minimize discussion of substance use or trauma symptoms when brought up the by the participant). Competence providing each element of HLS (e.g., discuss healthy lifestyle choices in daily life) was rated using a 7-point scale ranging from poor to excellent. When providing HLS treatment elements, 94% of the elements were rated excellent and none were rated below average. Adherence and competence ratings for HLS were modeled after those used in Stasiewicz et al. (2013).
Data Analytic Plan Demographic information and symptom severity
Baseline measures for the treatment groups were compared with one-way analysis of variance (ANOVA) for continuous variables and the chi-square test for categorical variables. Diagnostic agreement on the CAPS was assessed with kappa. Study treatment completer status was assessed with the chi-square test for categorical variables. For all analyses, alpha was set at 0.05.
PTSD and SUD outcomes (Hypotheses 1 – 5)
Intent-to-treat analysis (ITT) analyses were conducted using multilevel mixed-effects models (MLMM) with the xtmixed feature in Stata 14. The xtmixed feature uses maximum likelihood to account for missing data (Allison, 2012; Graham, 2009). The analytic strategy was modeled on Foa et al. (2005). To examine treatment impact, substance use variables (i.e., PDA for alcohol and drugs) were examined at baseline and 3-month, while maintenance of these changes were examined at 3- and 6-month follow-up. To examine treatment impact, self-report measures (i.e., IES-R, BDI-II, and ACQ-Now) were examined at baseline and end-of-treatment. To examine stability of change, self-report measures were examined at end-of-treatment, 3-, and 6-month follow-up. Statistically significant analyses were submitted to pairwise comparisons. Study participants were free to terminate study-related therapy but continue to participate in the end of treatment, 3-, and 6-month follow-up assessments as part of the ITT analyses. Clinically significant improvement was calculated based on recommendations by Jacobson and Truax (1991). Data from three studies of PTSD-SUD participants using the IES-R and TLFB were pooled to establish more stable norms by which to judge clinical improvement. Clinically significant improvement was defined as scores at least two standard deviations lower than the pooled mean.
Motivational enhancement session and treatment retention (Hypothesis 6)
MLMM was utilized to examine the number of treatment sessions attended between mPE participants receiving either the MET-PTSD session or the relaxation session. Differences were not predicted a priori on measures of PTSD, depression, and substance outcomes.
Effect sizes and power analysis
The between group effect sizes are reported as Cohen’s d (Cohen, 1988). The G*Power statistical software version 3.1 (Faul, Erdfelder, Lang, & Buchner, 2007) was used to conduct power analysis on outcome variables (i.e., IES-R total score). Assuming a type I error protection level of 0.05 and a longitudinal design with four measurement points, a total sample of 51 has 0.90 power to detect an effect size of f = 0.25 (a medium effect size on trauma symptoms). Assuming a type I error protection level of 0.05 and a longitudinal design with three measurement points, a total sample of 93 has 0.90 power to detect an effect size of f = 0.2 on percent days abstinent from alcohol and drugs. The final sample size was 126 due to a better than expected recruitment rate. Given the actual effect sizes calculated from the current data, final sample had greater than 0.99 power to detect a significant Treatment X Time interaction on trauma symptoms, 0.19 power on percent days abstinent from alcohol, and 0.70 power on percent days abstinent from drugs.
Results
Pretreatment differences
To assess for possible pretreatment differences on key variables at baseline, continuous summary variables for the treatment conditions (mPE+MET-PTSD vs. mPE vs. HLS) were submitted to one-way ANOVAs. The three treatment groups did not differ at baseline on age, F(2, 123) = .361, p = .70, ADS total score, F(2, 123) = 1.69, p = .19, IES-R total score, F(2, 123) = 1.93, p = .15, CAPS total severity score, F(2, 123) = 1.00, p = .37, baseline percent days abstinent from alcohol, F(2, 123) = .43, p = .65, or baseline percent days abstinent from drugs, F(2, 123) = 2.05, p = .13. Categorical variables were submitted to separate chi-square tests. Treatment groups did not differ at baseline on sex, χ2 (2, N=126) = .72, p = .70, or self-identified race, χ2 (6, N=126) = 4.77, p = .57. The mean CAPS total score for all participants was 79.0 (SD = 18.1). Therefore, the CAPS total score fell between the severe and extreme level of PTSD symptoms at baseline (Weathers et al., 2001). See Table 1 for demographic information.
Table 1.
Baseline characteristics by groupa. Number of participants and percentage of group are reported unless otherwise noted.
| Variable | mPE (n = 45) |
mPE+MET- PTSD (n = 40) |
HLS (n = 41) |
|---|---|---|---|
| Age, mean (95% CI), yr | 34.7 (31.6–37.8) | 34.4 (31.0–37.8) | 32.9 (29.5–36.2) |
| Sex | |||
| Male | 26 (57.8) | 22 (55.0) | 20 (48.8) |
| Female | 19 (42.2) | 18 (45.0) | 21 (51.2) |
| Race | |||
| White | 35 (77.8) | 31 (77.5) | 34 (82.9) |
| Black/African American | 10 (22.2) | 7 (17.5) | 7 (17.1) |
| Other | ---- | 2 (5.0) | ---- |
| Education | |||
| ≤ 12th | 4 (8.9) | 6 (15.0) | 5 (12.2) |
| H.S. diploma | 12 (26.7) | 9 (22.5) | 12 (29.3) |
| Post H.S. | 23 (51.1) | 20 (50.0) | 18 (43.9) |
| ≥ 4 yr degree | 6 (13.3) | 5 (12.5) | 6 (14.6) |
| URICA-T | 10.6 (10.1–11.1) | 10.3 (9.8–10.8) | 10.3 (9.9–10.7) |
| CAPS Total Score, mean (95% CI) | 78.5 (72.7–84.3) | 81.5 (76.8–86.3) | 75.9 (69.4–81.9) |
| Trauma History | |||
| Sexual assaultb | 26 (57.8) | 22 (55.0) | 25 (61.0) |
| Attacked with weapon | 29 (64.4) | 24 (60.0) | 27 (65.9) |
| Attacked without weapon | 22 (48.9) | 24 (60.0) | 25 (61.0) |
| Accident | 21 (52.5) | 28 (62.2) | 27 (65.9) |
| Childhood physical abuse | 18 (40.0) | 18 (45.0) | 16 (39.0) |
| Natural disaster | 19 (42.2) | 16 (40.0) | 9 (22.0) |
| Total Criterion A events, median (interquartile range) |
9.0 (6.5) | 9.0 (9.8) | 8.0 (4.0) |
| Current Psychiatric and Substance Use Diagnoses | |||
| Current alcohol dependence | 45 (100) | 40 (100) | 41 (100) |
| Any current drug dependence | 45 (100) | 40 (100) | 39 (95.1) |
| Major depressive disorder | 36 (80.0) | 35 (87.5) | 30 (73.2) |
| Additional anxiety disorder(s) | 33 (73.3) | 32 (80.0) | 22 (53.7) |
Abbreviations: mPE = modified prolonged exposure; mPE+MET-PTSD = modified prolonged exposure plus trauma-focused motivational enhancement therapy; HLS = Healthy Lifestyle Sessions; URICA-T = University of Rhode Island Change Assessment-Trauma Readiness to Change Score; CAPS = Clinician Administered PTSD Scale.
Values are expressed as numbers (percentages) unless otherwise noted.
Any sexual assault occurring in either adulthood or childhood.
Study treatment completion and assessment completion rates
Study treatment completion was defined as attending 8 or more therapy sessions (Foa et al., 2005). Of those randomized to treatment, 28 (62.2%) completed mPE, 24 (60.0%) completed mPE+MET-PTSD, and 35 (87.8%) completed HLS. Overall, study treatment retention was 69% (n = 87). Baseline demographic variables were not associated with treatment completion. Study treatment completers did not differ from non-completers on age, F(1, 125) = .30, p = .59, sex, χ2 (1, N=126) = .35, p = .56, race, χ2 (2, N=126) = .98, p = .81, lifetime number of PTSD Criterion A events experienced, F(1, 125) = .02, p = .89, or baseline PTSD symptom severity, F(1, 125) = .58, p = .45.
Consistent with the ITT approach, all randomized participants were eligible for end-of-treatment, 3-, and 6-month follow-up assessments regardless of whether they completed their study treatment. End-of-treatment, 3-, and 6-month follow-up assessments were completed on 33 (73.3%), 32 (71.1%), and 35 (77.8%) participants, respectively, in the mPE condition. End-of-treatment, 3-, and 6-month follow-up assessments were completed on 35 (87.5%), 29 (72.5%), and 31 (77.5%) participants, respectively, in the mPE+MET-PTSD condition. End-of-treatment, 3-, and 6-month follow-up assessments were completed on 36 (87.8%), 32 (78.0%), and 32 (78.0%) participants, respectively, in the HLS condition.
PTSD, Depression, and SUD outcomes (Hypotheses 1 – 5)
Missing data
Overall, 16.47% of total data points were missing. At baseline, no data points were missing. However, 17.46%, 26.19 %, and 22.22 % of data points were missing at end-of-treatment, 3-month follow-up, and 6-month follow-up, respectively. MLMM in Stata 14 uses maximum likelihood to account for missing data (Allison, 2012; Graham, 2009). Results from sensitivity analyses suggest the results from the ITT analyses presented below are robust (reported in Supplementary Material).
Hypothesis 1: PTSD outcomes
MLMM was used to assess the impact of treatment condition on the IES-R scores at pre-treatment and end-of-treatment. A significant Treatment Condition X Time interaction was revealed for PTSD symptoms, χ2 (2, N=126) = 7.25, p = .03. All three conditions demonstrated reductions on the IES-R, χ2 (1, N=126) = 316.29, p = .0001, and the three treatment conditions differed significantly from one another, χ2 (2, N=126) = 6.30, p = .04. Specifically, the mPE (p = .008) and mPE+MET-PTSD (p = .04) conditions evidenced significantly greater reductions at end-of-treatment compared to the control condition. The reduction in IES-R scores in the mPE+MET-PTSD condition did not differ from the score reduction in the mPE condition at the end of treatment (p = .55).
To test if these score reductions were maintained at follow-up, MLMM was applied to IES-R scores at end of treatment, 3-, and 6-month follow-up. Neither the Treatment Condition X Time interaction, χ2 (4, N=126) = .32, p = .99, nor the main effect for time, χ2 (2, N=126) = 1.06, p = .59, were statistically significant for IES-R scores. However, the main effect for treatment condition was statistically significant, χ2 (2, N=126) = 8.93, p = .01. The mPE (p = .02) and mPE+MET-PTSD (p = .03) conditions demonstrated significantly lower trauma symptoms at 3-month follow-up compared to the control condition but the treatment conditions did not differ from one another (p = .84). At 6-month follow-up, the mPE condition evidenced lower trauma symptoms compared to the HLS condition (p < .05) but did not differ from the mPE+MET-PTSD condition (p = .67). The mPE+MET-PTSD condition versus HLS condition comparison did not reach statistical significance (p = .13). The means and tests of simple effects for IES-R scores are reported in Table 2. Effect sizes are reported in Table 3.
Table 2.
Means and 95% CI of posttraumatic stress disorder and substance use outcomes for intent to treat sample (N=126).
| mPE n = 45; mPE+MET-PTSD n = 40; HLS n = 41 | ||||
|---|---|---|---|---|
| Baseline | End-of- treatment |
3-month | 6-month | |
| IES-R | ||||
| mPE | 48.56 (43.43–53.68) |
16.20a (10.48–21.92) |
14.11a (8.33–19.90) |
16.45a (10.85–22.06) |
| mPE+MET- PTSD |
54.95 (49.51–60.39) |
20.49a (14.77–26.20) |
19.10a (13.01–25.18) |
20.48 (14.53–26.42) |
| HLS | 51.02 (45.65–56.40) |
27.40b (21.80–33.01) |
26.00b (20.15–31.85) |
26.50b (20.52–32.50) |
| BDI-II | ||||
| mPE | 29.49 (26.48–32.49) |
7.08a (3.49–10.66) |
10.21a (5.90–14.51) |
6.60a (2.16–11.19) |
| mPE+MET- PTSD |
32.40 (29.21–35.59) |
10.78 (7.07–14.50) |
14.34 (9.82–18.87) |
13.39b (8.49–18.30 |
| HLS | 29.80 (26.61–32.90) |
13.33b (9.89–16.77) |
16.84b (12.94–20.73) |
13.59b (8.97–18.22) |
| Alcohol PDA | ||||
| PE | 46.13 (39.04–52.61) |
------ | 97.32 (90.76–103.87) |
94.49 (87.94–101.05) |
| mPE+MET- PTSD |
48.70 (41.82–55.57) |
------ | 92.46 (85.66–99.25) |
85.73 (78.94–92.52) |
| HLS | 52.23 (45.53–59.03) |
------ | 97.08 (90.28–103.88) |
93.58 (86.78–100.37) |
| Drug PDA | ||||
| mPE | 53.44 (46.87–60.01) |
------ | 97.52 (90.87–104.16) |
96.94 (90.87–104.16) |
| mPE+MET- PTSD |
45.47 (38.50–52.43) |
------ | 93.37 (86.49–100.26) |
91.97 (85.08–98.85) |
| HLS | 59.59 (52.71–66.48) |
------ | 97.94 (91.06–104.83) |
91.70 (84.82–98.59) |
| ACQ | ||||
| mPE | 143.65 (128.05–159.25) |
95.85 (78.58–113.12) |
103.15 (83.32–122.97) |
96.40 (75.89–116.91) |
| mPE+MET- PTSD |
172.31 (155.93–188.69) |
114.23 (96.08–132.38) |
104.12 (83.66–124.57) |
95.63 (72.95–118.31) |
| HLS | 145.45 (128.85–162.1) |
117.13 (100.00–134.26) |
109.34 (90.62–128.05) |
108.35 (87.26–129.44) |
Note: mPE = modified prolonged exposure; mPE+MET-PTSD = modified prolonged exposure plus trauma-focused motivation enhancement therapy; HLS = Healthy Lifestyle Sessions; IES-R = Impact of Event Scale-Revised; BDI-II = Beck Depression Inventory-II; PDA = percent days abstinent, ACQ = Alcohol Craving Questionnaire-Now.
Significant differences (≤ .05) between groups at each assessment point are denoted by unmatched letters (i.e., a and b).
Table 3.
Between group effect size (Cohen's d) for the modified prolonged exposure (mPE) and modified prolonged exposure plus trauma-focused motivation enhancement therapy (mPE+MET-PTSD) compared to the healthy lifestyles (HLS) group.
| End-of-treatment | 3-month | 6-month | ||
|---|---|---|---|---|
| IES-R | mPE | 0.62 | 0.65 | 0.55 |
| mPE+MET-PTSD | 0.36 | 0.36 | 0.31 | |
| BDI-II | mPE | 0.26 | 0.48 | 0.46 |
| mPE+MET-PTSD | 0.22 | 0.18 | 0.006 | |
| ACQ | mPE | 0.37 | 0.10 | 0.17 |
| mPE+MET-PTSD | 0.05 | 0.08 | 0.18 | |
| PDA alcohol | mPE | --- | 0.01 | 0.04 |
| mPE+MET-PTSD | --- | 0.21 | 0.36 | |
| PDA drug | mPE | --- | 0.02 | 0.21 |
| mPE+MET-PTSD | --- | 0.24 | 0.02 |
Note: IES-R = Impact of Event Scale-Revised; BDI-II = Beck Depression Inventory-II; ACQ = Alcohol Craving Questionnaire-Now; PDA = percent days abstinent.
Hypothesis 2: Depression outcomes
MLMM was used to assess the impact of treatment condition on the BDI-II scores at pre-treatment and end-of-treatment. A marginally significant Treatment Condition X Time interaction was revealed for depressive symptoms, χ2 (2, N=126) = 5.16, p = .08. All three conditions demonstrated reductions on the BDI-II, χ2 (1, N=126) = 268. 91, p = .0001, however, the mPE condition evidenced significantly greater reductions at end-of-treatment compared to the control condition. The reduction in BDI-II scores in the mPE+MET-PTSD condition did not differ from the score reduction in either the mPE or HLS conditions at end of treatment.
To test if these score reductions were maintained at follow-up, MLMM was applied to BDI-II scores at end of treatment, 3-, and 6-month follow-up. The Treatment Condition X Time interaction for depressive symptoms was not statistically significant, χ2 (4, N=126) = 1.03, p = .91. However, the main effect for treatment condition was statistically significant, χ2 (2, N=126) = 11.57, p = .003, as was the main effect for time, χ2 (2, N=126) = 6.93, p = .03. Means and tests of simple effects are reported in Table 2. Effect sizes are reported in Table 3.
Hypothesis 3: Substance use outcomes
To test the effect of PTSD treatment on SUD outcomes, 90-day PDA from alcohol and drugs were assessed at baseline, 3- and 6-month follow-up. Given that participants were in a residential treatment facility (i.e., a controlled environment), end-of-treatment SUD outcomes were not included in the models. MLMM was used to assess the impact of treatment condition on alcohol PDA at pre-treatment and 3-month follow-up. A non-significant Treatment Condition X Time interaction was revealed for alcohol PDA, χ2 (2, N=126) = 1.51, p = .47. All three treatment conditions demonstrated reductions in alcohol PDA, χ2 (1, N=126) = 211.54, p = .0001, but the mPE, mPE+MET-PTSD, and HLS conditions did not differ from another at 3-month follow-up.
To test if these reductions in drinking were maintained at follow-up, MLMM was applied to alcohol PDA at 3- and 6-month follow-up. The Treatment Condition X Time interaction for alcohol PDA was not statistically significant, χ2 (2, N=126) = 1.11, p = .58. In addition, the main effect for time was not statistically significant, χ2 (1, N=126) = 3.33, p = .07, nor was the main effect for treatment condition, χ2 (2, N=126) = 5.46, p = .07.
In regard to drug use, a non-significant Treatment Condition X Time interaction was revealed for drug PDA at pre-treatment and 3-month follow-up, χ2 (2, N=126) = .92, p = .63. All three treatment conditions demonstrated reductions in drug PDA, χ2 (1, N=126) = 154.57, p = .0001, but the mPE, mPE+MET-PTSD, and HLS conditions did not differ from another at the 3-month follow-up.
To test if these reductions in drug use were maintained at follow-up, MLMM was applied to drug PDA at 3- and 6-month follow-up. The Treatment Condition X Time interaction for drug PDA was not statistically significant, χ2 (2, N=126) = 2.20, p = .33, nor was the main effect for time, χ2 (1, N=126) = 3.56, p = .06, or treatment condition, χ2 (2, N=126) = 3.74, p = .15. The means and tests of simple effects for alcohol and drug PDA are reported in Table 2.
In an effort to validate the primary dependent substance use measures (i.e., TLFB), biological measures were collected. At the 3-month follow-up assessment, urine drug screens on participants completing an in-person interview identified 2 out of 19 (10.5%) participants who tested positive for at least one illicit substance in the mPE+MET-PTSD condition, 1 out of 21 (4.8%) in the mPE condition, and 3 out of 24 (12.5%) in the HLS condition. No participant provided an expired air sample indicating recent alcohol consumption at the 3-month follow-up assessment. Every participant who tested positive for an illicit substance at 3-month follow-up also reported drug use on the TLFB interview. At the 6-month follow-up assessment, urine drug screens identified 1 out of 16 (6.3%) participants who tested positive for at least one illicit substance in the mPE+MET-PTSD condition, 2 out of 19 (10.5%) in the mPE condition, and 2 out of 16 (12.5%) in the HLS condition. All of the participants who tested positive for an illicit substance at 6-month follow-up also reported drug use on the TLFB interview. One participant in the mPE+MET-PTSD condition and one participant in the HLS condition provided expired air sample at the beginning of the 6-month follow-up assessment indicating recent alcohol use (BAL = .030 and .012, respectively).
Hypothesis 4: Clinically Significant Improvement
Clinically significant improvement was calculated following the recommendations by Jacobson and Truax (1991). Specifically, data from three studies of SUD participants with PTSD in which the IES-R and TLFB were administered were pooled to establish more stable norms by which to judge clinical improvement. Data from the current study (N = 126), [reference redacted] (N = 43), and [reference redacted] (N = 65) were included. (Only data from the substance dependent-PTSD subset of participants were drawn from [reference redacted]). All three data sets used similar inclusion/exclusion criteria and the samples were drawn from geographically and racially diverse cities in the US. The mean baseline IES-R total score of this aggregate sample was 50.43 and the standard deviation was 15.10. Applying the conservative two standard deviations recommendation of Jacobson and Truax, a cutoff score of 20.23 was established to describe clinically significant improvement on the IES-R. Applying this cutoff to the data in the current study revealed 75.8% of the participants in the mPE group, 60.0% of the participants in the mPE+MET-PTSD, and 44.4% of the participants in the HLS condition experienced clinically significant improvement in trauma symptoms at the end-of-treatment and these differences in percentage of clinical improvement were statistically significant, χ2 (2, N=104) = 7.02, p = .03. The HLS, χ2 (1, N=71) = 1.72, p = .19, and mPE groups, χ2 (1, N=68) = 1.93, p = .17, did not differ from the mPE+MET-PTSD group. However, the mPE group, χ2 (1, N=69) = 7.00, p = .008, experienced significantly more clinical improvement compared to the HLS group. The mean PDA-alcohol and PDA-drugs of this aggregate sample was 48.92 and 53.38, respectively, and the standard deviation was 30.40 and 34.88, respectively. Applying the two standard deviations recommendation resulted in a cutoff score of 100.00 PDA for alcohol and drugs. Applying this cutoff to the PDA-alcohol and PDA-drug data in the current study revealed 78.1% and 84.4% of the participants in the mPE group, 58.6% and 65.5% of the participants in the mPE+MET-PTSD, and 71.9% and 71.9% of the participants in the HLS condition, respectively, experienced clinically significant improvement in drinking and illicit drug use at the 3 month follow-up. Clinically significant improvement in drinking, χ2 (2, N= 93) = 2.84, p = .24, and illicit drug use, χ2 (2, N= 93) = 2.96, p = .23, did not differ between the three treatment conditions.
Hypothesis 5: Alcohol craving
To test the effect of PTSD treatment on alcohol craving, the ACQ-Now was administered at baseline, end-of-treatment, 3- and 6-month follow-up. MLMM was used to assess the impact of treatment condition on the ACQ-Now scores at pre-treatment and end-of-treatment. A significant Treatment Condition X Time interaction was revealed for alcohol craving, χ2 (2, N=126) = 8.24, p = .02. All three conditions demonstrated reductions on the ACQ-Now, χ2 (1, N=126) = 115.37, p = .0001, and the test of treatment condition approached significance, χ2 (2, N=126) = 4.87, p = .09. However, pairwise comparisons did not reveal significant differences in craving reductions between any of the treatment conditions.
To test if these score reductions were maintained at follow-up, MLMM was applied to ACQ-Now scores at end of treatment, 3-, and 6-month follow-up. The Treatment Condition X Time interaction for alcohol craving was not statistically significant, χ2 (4, N=126) = 1.02, p = .91, nor was the main effect for time, χ2 (2, N=126) = 4.51, p = .11. However, the main effect for treatment condition was statistically significant, χ2 (2, N=126) = 6.89, p = .03. Pairwise comparisons did not reveal significant differences in craving between any of the treatment conditions. The means, including tests of simple effects, are reported in Table 2 for ACQ-Now scores.
Hypothesis 6: Motivational enhancement session and treatment completion
There was no difference in the number of treatment sessions attended for those who received the motivational enhancement session vs. the relaxation session prior to initiating mPE. Based on prior work (i.e., Foa et al., 2005), completion of 8 or more therapy sessions (mPE, mPE+MET-PE, or HLS) was considered a complete dose of treatment. Since study participants were free to terminate study-related therapy but continue to receive TAU at the recruitment site, the term study treatment completer only refers to completion of study treatments and not completion of TAU. In addition, participants could drop out of study treatments and complete all follow-up assessments as part of the ITT analyses. Using the 8 session criterion, 69.8% of the overall sample was classified as study treatment completers. Of the participants receiving mPE+MET-PTSD, mPE, and HLS, 60.0%, 62.2%, and 87.8%, respectively, completed study treatments. The completion rate in the HLS condition was significantly higher than the completion rates in the mPE+MET-PTSD and mPE conditions, χ2 (2, N= 126) = 9.36, p = .009. Examining the standardized residuals, the significant difference in completion rate between the HLS condition and mPE+MET-PTSD and mPE conditions was largely driven by the unexpectedly low noncompletion rate (n = 5) in the HLS condition. The median number of study treatment sessions completed in the mPE+MET-PTSD, mPE, and HLS conditions was 9.0. Completion of TAU did not differ as a function of treatment condition, χ2 (2, N= 126) = .81, p = .67.Completion rates of TAU was as follows: mPE = 38 (84.4%), mPE+MET-PTSD = 31 (77.5%), and HLS = 32 (78.0%).
Completers versus non-completers for primary outcomes
While mPE and mPE+MET-PTSD treatment completers realized significantly larger PTSD treatment gains, as measured by the IES-R, compared to non-completers, F(1, 68) = 26.63, p = .001, mPE and mPE+MET-PTSD non-completers reported substantial decreases in PTSD symptoms. For example, mPE and mPE+MET-PTSD non-completers evidenced a 38.0% reduction in trauma symptoms at end of treatment. In contrast, HLS non-completers evidenced a 0.8% increase in trauma symptoms at the end of treatment.
Effect sizes
Between group effect sizes are reported as Cohen’s d and reflect differences between the mPE group and HLS group and the mPE+MET-PTSD group and HLS group (Table 3). Given that participants were treated at a residential treatment facility and did not have access to alcohol and illicit drugs, the TLFB was not administered at the end-of-treatment assessment.
Discussion
The purpose of the current study was to examine whether adding modified prolonged exposure (mPE) to traditional 12-Step substance use treatment improves mental health outcomes more than a health-focused control treatment matched for therapist time (HLS) in participants with PTSD-SUD. In addition, one half of the participants receiving mPE received a 90 min trauma-focused motivational enhancement session prior to beginning mPE (mPE+MET-PTSD) in an effort to improve study treatment retention. Results from this study add to the growing body of research finding that PTSD can be treated safely and effectively with exposure therapy during the early phases of substance abuse treatment. Further, results suggest that mPE, both with and without MET-PTSD, produces significantly greater improvements in symptoms of PTSD compared to the HLS condition and this result is consistent with our first hypothesis. Our second hypothesis was partially supported in that one of the exposure therapy conditions, mPE, improved depressive symptoms significantly more than the control condition while the mPE+MET-PTSD condition did not. In contrast, our third hypothesis, that individuals receiving mPE or mPE+MET-PTSD would enjoy better substance use outcomes, was not supported. Substance use outcomes in all conditions were quite positive, with participants in all three treatment conditions maintaining abstinence for over 85% of days during 6-month follow-up. However, contrary to our hypothesis, proportion of days abstinent did not differ statistically between participants receiving mPE or mPE+MET-PTSD compared to those receiving HLS. This may be the result of a ceiling effect due to the high percent days abstinent from both drugs and alcohol. Our fourth hypothesis, that individuals receiving mPE, both with and without METPTSD, would achieve greater clinically significant improvement in trauma symptoms and substance use outcomes compared to participants receiving HLS, was partially supported with the mPE group achieving greater clinically significant improvement in trauma symptoms compared to HLS while the mPE+MET-PTSD group did not differ from the mPE or HLS groups. Clinically significant improvement in substance use outcomes did not differ between treatment groups. Our fifth hypothesis also was partially supported, in that craving declined over the course of treatment and these improvements were maintained at follow-up. However, the decline in craving did not differ between the three treatment groups. Finally, our hypothesis that a trauma-focused motivational enhancement therapy session would improve mPE retention was not supported; retention in the mPE condition did not differ from retention in the mPE+MET-PTSD condition.
Results from the current study compare favorably to other studies testing prolonged exposure for the treatment of PTSD. Using conventional PE to treat PTSD without SUD, the ITT sample in Foa et al. (2005) enjoyed a 47% reduction in PTSD symptoms. Examining reductions in PTSD symptoms from exposure-based studies in PTSD-SUD samples, Sannibale et al. (2013) reported a 37% reduction in trauma symptoms at posttreatment while Foa et al. (2013) reported 60% and 52% reductions at the end of treatment in the PE plus naltrexone and PE plus placebo conditions, respectively. Mills et al. (2012) reported a 42% reduction in PTSD symptoms at 9-month assessment (participants took up to 9 months to complete exposure therapy). In the current study, participants in the mPE and mPE+MET-PTSD conditions realized a 65% reduction in PTSD symptoms.
Overall, substance use outcomes were excellent with all participants achieving an average of over 85% days abstinent from both illicit drugs and alcohol at 3- and 6-month follow-up. Although the current study was not designed as a noninferiority trial, the similarity in substance use outcomes between the HLS and PE conditions is striking and suggests that mPE did not negatively impact substance use outcomes.
In the current study, study treatment completion was significantly lower in the mPE (60.0%) and mPE+MET-PTSD (62.2%) conditions compared to the HLS condition (87.8%). Given that treatment studies of co-occurring PTSD and SUD established different definitions of treatment completion, the mean or median number of sessions completed may be a better context in which to compare participation in exposure-based interventions across studies. In an early study, Brady et al. (2001) reported participants completed an average of 8.18 sessions (in vivo exposure began at session 6, imaginal exposure began at session 7). Mills et al. (2013) reported the median number of COPE sessions completed was 5 (in vivo exposure began at session 5, imaginal exposure began at session 6). The range of exposure therapy treatment sessions reported in Sannibale et al. (2013) was 0–16 with participants completing an average of 11 sessions (in vivo and imaginal exposure began at session 5 and 6). Using conventional prolonged exposure, Foa et al. (2013) reported completing an average of approximately 6 prolonged exposure sessions, where imaginal exposure begins at session 3. In comparison, participants in the current study receiving mPE completed 9 therapy sessions (in vivo exposure began at session 3, imaginal exposure began at session 4). Study treatment dropout in the two mPE conditions did not appear to be associated with any particular treatment component, such as the initiation of imaginal exposure and, instead, reflected a relatively steady attrition across the course of treatment, with predictable increases in completion rate at session 9 and session 12 (the two predefined treatment completion points). Despite the lower completion rate, the ITT analyses that included all participants randomized to treatment (participants received anywhere from 0–12 therapy sessions), illustrate the substantial impact of mPE on PTSD symptoms.
Importantly, the current study builds on prior research by demonstrating that mPE, provided within a standard 60 min therapy hour and consisting of approximately 30 min of in-session imaginal exposure and between session in vivo exposure, produced substantial treatment gains in participants with PTSD-SUD. Prolonged exposure was modified in the hope that therapy provided within a standard therapy hour might better fit standard clinical practice and, therefore, facilitate implementation in SUD treatment settings. To accommodate briefer session times, information that is typically presented in session 1 and 2 of conventional prolonged exposure (e.g., psychoeducation about PTSD, providing a rationale for treatment, breathing retraining, etc.) was presented in sessions 1–3. Imaginal exposure in conventional prolonged exposure begins at session 3, whereas in mPE, imaginal exposure began at session 4. Data from the current study suggest that this briefer form of prolonged exposure produces PTSD treatment outcomes comparable to those associated with conventional PE (e.g., Foa et al., 2005) and may be better suited to usual clinical practice.
In addition to demonstrating the effectiveness and safety of mPE, another goal was to demonstrate that PTSD treatment and substance abuse treatment, provided by different therapists, could effectively treat PTSD and SUD. This form of integrated treatment has the advantage of not requiring one therapist to be knowledgeable about both the treatment of substance abuse and the use of prolonged exposure to treat PTSD. In the current study, integration of treatment was realized by maintaining strong lines of communication between treatment providers. Certainly, communication lines found in residential treatment centers, such as multidisciplinary treatment team meetings, can be created in outpatient settings but other communication approaches might be difficult to duplicate. For example, in a residential treatment facility an addition therapist can be recruited to help problem solve homework compliance or attendance issues and this may be difficult to replicate an outpatient setting.
Although treatment gains were much more pronounced in the mPE conditions compared to the HLS condition, it is important to note that substantial treatment gains were realized in the HLS condition. This is not surprising given the literature reporting PTSD and SUD symptom improvement during standard SUD treatments in both longitudinal assessment studies (Coffey et al., 2007) and RCTs (e.g., Mills et al., 2012; Sannibale et al, 2013). Improvements in both PTSD and SUD outcomes are typical in TAU control conditions in RCTs involving participants with co-occurring PTSD and SUD (see Torchalla et al., 2012; van Dam et al., 2012). Importantly, the current study compares an exposure-based treatment for PTSD to a control condition in a PTSD-SUD sample. By providing a control condition, the impact of PTSD treatment, above and beyond both assessment reactivity (e.g., repeated assessment of PTSD, SUD) and nonspecific psychotherapy factors (e.g., time spent with a therapist, warmth, genuineness, positive regard), can be assessed. However, it should be noted it is possible that components of HLS (e.g., relaxation, sleep hygiene) were beneficial but, without an assessment-only condition, this potential benefit cannot be evaluated in the current study.
The current study was conducted in a 6-week residential substance abuse treatment facility so it is reasonable to question whether findings would generalize to an outpatient setting. Certainly, there are clear advantages to providing treatment within a residential treatment program, such as, the elimination of missed appointments due to either childcare coverage or transportation difficulties. In addition, providing treatment within a residential treatment facility avoided treatment scheduling problems with complicated work schedules. In addition, PTSD treatment provided within a residential treatment facility may reduce the risk of elevated substance craving and substance use relapse secondary to elevations in trauma-related negative affect. However, the manner in which exposure therapy was provided in the current study may reduce the risk of elevated craving or substance use relapse in response to elevations in trauma-related negative affect thereby increasing the possible generalization to outpatient treatment settings. At the end of each imaginal exposure session, negative affect was reduced to baseline levels through the use of diaphragmatic breathing, an affect management tool taught to all participants receiving mPE. This portion of each session was included in the recorded imaginal exposure sessions so participants engaged in diaphragmatic breathing at the end of each imaginal exposure homework; a practice that may reduce risk of elevated substance craving or relapse an outpatient settings.
Another advantage of both providing treatment within a residential treatment facility and maintaining separate substance abuse and PTSD therapists is that if a participant’s motivation to receive a study treatment waivered, it was not uncommon for a substance abuse counselor to encourage a participant to remain in the study treatment. Unfortunately, this encouragement was not systematically documented within the study so this point must remain anecdotal. It is likely that this encouragement could occur in an outpatient substance abuse treatment setting and increase retention but that issue will need to be evaluated in future studies. In addition, if motivation to receive mPE waivered, the effort required to attend treatment was relatively low (e.g., participants were almost always at the treatment facility) and this minimal effort may have obfuscated MET treatment effects. However, it is not known whether MET-PTSD may prove to be beneficial in outpatient treatment settings where barriers to attending treatment are greater.
The study had at least three limitations. First, the follow-up period was relatively brief (i.e., 6-months). Ideally, a longer follow-up period (e.g., 1 to 2 years) should be utilized when studying a chronic, relapsing condition such as substance abuse. This limitation is mitigated somewhat by the fact that time to first use following substance abuse treatment most often occurs in the first six months following treatment (e.g., Lowman, Allen, Stout, & The Relapse Research Group, 1996). Another limitation of this study is that biological verification of substance abstinence (e.g., UDS) was not performed for all follow-up assessments. Although treatment was provided near a moderately sized city, our recruitment site treated many patients living a considerable distance from the treatment site, typically in rural counties. Given that a sizable portion of our sample were unable to participate in an in-person assessment, we chose to utilize telephone-based assessments. Lastly, while a possible limitation of treatment approaches that provides PTSD and substance abuse treatment within one protocol (e.g., COPE) is that they require substantial clinical expertise in both PTSD and substance abuse treatment, the currently tested model (i.e., PTSD and a substance abuse treatment provided separately) requires multiple providers and treatment sessions, which may be a limitation.
Future directions
Treatment completion continues to be problematic in studies examining prolonged exposure administered to individuals with co-occurring PTSD and substance use disorder (e.g., Foa et al., 2013; Mills et al., 2012; Sannibale et al., 2013). The current study attempted to address this concern by providing a 90 min trauma-focused motivational enhancement session prior to beginning mPE but this brief intervention failed to improve study treatment completion. Although there is concern that the relatively low study treatment completion rate may indicate exposure therapy is not well tolerated in patients with co-occurring PTSD-SUD, it may be that discontinuation is due to PTSD symptom improvement. For example, mPE and mPE+MET-PTSD treatment non-completers enjoyed a 38% reduction in trauma symptoms, which is in sharp contrast to the 0.8% increase realized in the HLS non-completers. Future studies should examine the possibility that the less than optimal completion rate of prolonged exposure in substance abusing patients with PTSD may, in part, be attributed to perceived improvements in PTSD symptoms among some patients.
Supplementary Material
Acknowledgments
This research was supported, in part, by National Institute on Alcohol Abuse and Alcoholism grant R01AA016816 (PI: Coffey). The authors wish to thank M. Trost Friedler, Jackie Lampley, and the staff and patients of Harbor House Recovery Center for their cooperation on this study.
Contributor Information
Scott F. Coffey, Department of Psychiatry and Human Behavior, The University of Mississippi Medical Center
Julie A. Schumacher, Department of Psychiatry and Human Behavior, The University of Mississippi Medical Center
Elizabeth Nosen, Department of Psychiatry and Human Behavior, The University of Mississippi Medical Center.
Andrew K. Littlefield, Department of Psychology, Texas Tech University
Amber M. Henslee, Department of Psychology, Missouri University of Science and Technology
Amy Lappen, Rossier School of Education, University of Southern California.
Paul R. Stasiewicz, Research Institute on Addictions, University at Buffalo
References
- Allison PD. Handling missing data by maximum likelihood. Orlando, Florida: Keynote presentation at the SAS Global Forum; 2012. Apr, Retrieved from http://www.statisticalhorizons.com/wp-content/uploads/MissingDataByML.pdf. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Association; 2000. (text revision) [Google Scholar]
- Back SE, Brady KT, Jaanimägi U, Jackson JL. Cocaine dependence and PTSD: A pilot study of symptom interplay and treatment preferences. Addictive behaviors. 2006;31:351–354. doi: 10.1016/j.addbeh.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Back SE, Brady KT, Sonne SC, Verduin ML. Symptom improvement in co-occurring PTSD and alcohol dependence. Journal of Nervous and Mental Disorders. 2006;194:690–696. doi: 10.1097/01.nmd.0000235794.12794.8a. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. Journal of Traumatic Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Brady KT, Back S, Coffey SF. Substance abuse and posttraumatic stress disorder. Current Directions in Psychological Science. 2004;13:206–209. [Google Scholar]
- Brown RC, Berenz EC, Aggen SH, Gardner CO, Knudsen GP, Reichborn-Kjennerud T, Amstadter AB. Trauma exposure and Axis I psychopathology: A cotwin control analysis in Norwegian young adults. Psychological Trauma: Theory, Research, Practice, and Policy. 2014;6:652–660. doi: 10.1037/a0034326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SF, Schumacher JA, Brady KT, Dansky Cotton B. Changes in PTSD symptomatology during acute and protracted alcohol and cocaine abstinence. Drug and Alcohol Dependence. 2007;87:241–248. doi: 10.1016/j.drugalcdep.2006.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SF, Stasiewicz PR, Hughes P, Brimo ML. Trauma-focused imaginal exposure with comorbid PTSD- alcohol dependent individuals: Revealing mechanisms of alcohol craving in a cue reactivity paradigm. Psychology of Addictive Behaviors. 2006;20:425–435. doi: 10.1037/0893-164X.20.4.425. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- Connolly K, Coffey SF, Baschnagel JS, Drobes DJ, Saladin ME. Evaluation of the Alcohol Craving Questionnaire-Now factor structures: Application of a cue reactivity paradigm. Drug and Alcohol Dependence. 2009;103:84–91. doi: 10.1016/j.drugalcdep.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake RE, McHugo GJ, Biesanz JC. The test–retest reliability of standardized instruments among homeless persons with substance use disorders. Journal of Studies on Alcohol. 1995;56:161–167. doi: 10.15288/jsa.1995.56.161. doi: http://dx.doi.org/10.15288/jsa.1995.56.161. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Foa E, Keane T, Friedman M, Cohen J, editors. Effective treatments for PTSD: Practice guidelines from the International Society for Traumatic Stress Studies. 2nd. New York: Guilford Press; 2008. [Google Scholar]
- Foa E, Hembree E, Cahill S, Rauch S, Riggs D, Feeny N, Yadin E. Randomized trial of prolonged exposure for posttraumatic stress disorder with and without cognitive restructuring: Outcome at academic and community clinics. Journal of Consulting and Clinical Psychology. 2005:953–964. doi: 10.1037/0022-006X.73.5.953. [DOI] [PubMed] [Google Scholar]
- Foa E, Hembree E, Rothbaum BO. Prolonged exposure therapy for PTSD: Emotional processing of traumatic experiences therapist guide. New York: Oxford University Press; 2007. [Google Scholar]
- Foa EB, Yusko DA, McLean CP, Suvak MK, Bux DA, Oslin D, Volpicelli J. Concurrent naltrexone and prolonged exposure therapy for patients with comorbid alcohol dependence and PTSD: A randomized clinical trial. JAMA. 2013;310:488–495. doi: 10.1001/jama.2013.8268. [DOI] [PubMed] [Google Scholar]
- Goldstein RB, Smith SM, Chou SP, Saha TD, Jung J, Zhang H, Pickering RP, Ruan WJ, Huang B, Grant BF. The epidemiology of DSM-5 posttraumatic stress disorder in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. Social Psychiatry and Psychiatric Epidemiology. 2016 doi: 10.1007/s00127-016-1208-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JW. Missing data analysis: Making it work in the real world. Annual Review of Psychology. 2009;60:549–576. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- Hunt Y, Linkovich-Kyle T, Coffey SF, Stasiewicz PR, Schumacher JA. University of Rhode Island Change Assessment-Trauma: Preliminary psychometric properties in an alcohol dependent-PTSD sample. Journal of Traumatic Stress. 2006;19:915–921. doi: 10.1002/jts.20161. [DOI] [PubMed] [Google Scholar]
- Jacobson NS, Truax P. Clinical significance: A statistical approach to denning meaningful change in psychotherapy research. Journal of Consulting and Clinical Psychology. 1991;59:12–19. doi: 10.1037//0022-006x.59.1.12. doi.org/10.1037/0022-006X.59.1.12. [DOI] [PubMed] [Google Scholar]
- Lowman C, Allen J, Stout RL The Relapse Research Group. Replication and extension of Marlatt's taxonomy of relapse precipitants: Overview of procedures and results. Addiction. 1996;91(Supplement):S51–S71. [PubMed] [Google Scholar]
- Mills KL, Teesson M, Back SE, Brady KT, Baker AL, Hopwood S, Ewer PL. Integrated exposure-based therapy for co-occurring posttraumatic stress disorder and substance dependence: A randomized controlled trial. JAMA. 2012;308:690–699. doi: 10.1001/jama.2012.9071. [DOI] [PubMed] [Google Scholar]
- Nacasch N, Huppert JD, Su Y-J, Kivity Y, Dinshtein Y, Yeh R, Foa EB. Are 60-minute prolonged exposure sessions with 20-minute imaginal exposure to traumatic memories sufficient to successfully treat PTSD? A randomized noninferiority clinical trial. Behavior Therapy. 2015 doi: 10.1016/j.beth.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Ouimette P, Goodman E, Brown PJ. Health and well being of substance use disorder patients with and without posttraumatic stress disorder. Addictive Behaviors. 2006;31:1415–1423. doi: 10.1016/j.addbeh.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Powers MB, Halpern JM, Ferenschak MP, Gillihan SJ, Foa EB. A meta-analytic review of prolonged exposure for posttraumatic stress disorder. Clinical Psychology Review. 2010;30:635–641. doi: 10.1016/j.cpr.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Rash CJ, Coffey SF, Baschnagel JS, Drobes DJ, Saladin ME. Psychometric properties of the IES-R in a sample of traumatized substance users. Addictive Behaviors. 2008;33:1039–1047. doi: 10.1016/j.addbeh.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read JP, Brown PJ, Kahler CW. Substance use and posttraumatic stress disorder: Symptom interplay and effects on outcome. Addictive Behaviors. 2004;29:1665–1672. doi: 10.1016/j.addbeh.2004.02.061. [DOI] [PubMed] [Google Scholar]
- Resnick H. Psychometric review of National Women's Study (NWS) Event History-PTSD Module. In: Stamm BH, editor. Measurement of stress, trauma, and adaptation. Lutherville, MD: Sidran Press; 1996. pp. 214–217. [Google Scholar]
- Robins LN, Cottler LB, Bucholz KK, Compton WM, North CS, Rourke KM. Diagnostic Interview Schedule for the DSM-IV (DIS-IV) St. Louis, MO: Washington University; 2000. [Google Scholar]
- Ruggiero KJ, Del Ben K, Scotti JR, Rabalais AE. Psychometric properties of the PTSD Checklist-Clinician Version. Journal of Traumatic Stress. 2003;16:495–502. doi: 10.1023/A:1025714729117. [DOI] [PubMed] [Google Scholar]
- Sannibale C, Teesson M, Creamer M, Sitharthan T, Bryant RA, Sutherland K, Peek-O’Leary M. Randomized controlled trial of cognitive behaviour therapy for comorbid post-traumatic stress disorder and alcohol use disorders. Addiction. 2013;108:1397–1410. doi: 10.1111/add.12167. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Screening Test (AUDIT). WHO collaborative project on early detection of persons with harmful alcohol consumption. II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Schnurr PP, Friedman MJ, Engel CC, Foa EB, Shea MT, Chow BK, Bernardy B. Cognitive behavioral therapy for posttraumatic stress disorder in women: A randomized controlled trial. JAMA. 2007;297:820–830. doi: 10.1001/jama.297.8.820. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier V, Sheehan KH, Amorim P, Janavs J, Weiller E, Dunbar G. The Mini-International Neuropsychiatric Interview (MINI): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59(suppl 20):22–33. [PubMed] [Google Scholar]
- Singleton EG, Tiffany ST, Henningfield JE. Development and validation of a new questionnaire to assess craving for alcohol; Proceedings of the 56th Annual Meeting, The College on Problems of Drug Dependence.1994. [Google Scholar]
- Skinner HA, Horn JL. Alcohol Dependence Scale: Users guide. Toronto: Addiction Research Foundation; 1984. [Google Scholar]
- Sobell LC, Sobell MB. Timeline Followback user's guide: A calendar method for assessing alcohol and drug use. Toronto, Ontario: Addiction Research Foundation; 1996. [Google Scholar]
- Stasiewicz PR, Bradizza CM, Schlauch RC, Coffey SF, Gulliver SB, Gudleski G, Bole CW. Affect Regulation Training (ART) for alcohol use disorders: Development of a novel intervention for negative affect drinkers. Journal of Substance Abuse Treatment. 2013;45:1159–1166. doi: 10.1016/j.jsat.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate SR, Norman SB, McQuaid JR, Brown SA. Health problems of substance-dependent veterans with and those without trauma history. Journal of Substance Abuse Treatment. 2007;33:25–32. doi: 10.1016/j.jsat.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Torchalla I, Nosen L, Rostam H, Allen P. Integrated treatment programs for individuals with concurrent substance use disorders and trauma experiences: A systematic review and meta-analysis. Journal of Substance Abuse Treatment. 2012;42:65–77. doi: 10.1016/j.jsat.2011.09.001. [DOI] [PubMed] [Google Scholar]
- van Dam D, Vedel E, Ehring T, Emmelkamp PM. Psychological treatments for concurrent posttraumatic stress disorder and substance use disorder: A systematic review. Clinical Psychology Review. 2012;32:202–214. doi: 10.1016/j.cpr.2012.01.004. [DOI] [PubMed] [Google Scholar]
- van Minnen A, Foa EB. The effect of imaginal exposure length on outcome of treatment for PTSD. Journal of Traumatic Stress. 2006;19:427–438. doi: 10.1002/jts.20146. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JRT. Clinician-Administered PTSD Scale: A review of the first ten years of research. Depression and Anxiety. 2001;13:132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Weathers F, Litz B, Herman D, Huska J, Keane T. The PTSD Checklist (PCL): Reliability, validity, and diagnostic utility; Paper presented at the Annual Convention of the International Society for Traumatic Stress Studies; San Antonio, TX. 1993. Oct, [Google Scholar]
- Wei LJ. An application of an urn model to the design of sequential controlled trials. Journal of American Statistics Association. 1978;73:559–563. [Google Scholar]
- Weiss DS, Marmar CR. The Impact of Event Scale-Revised. In: Wilson JP, Keane TM, editors. Assessing psychological trauma and PTSD. New York: Guilford Press; 1997. pp. 399–411. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.