Abstract
Climate change has affected the seasonal phenology of a variety of taxa, including that of migratory birds and their critical food resources. However, whether climate-induced changes in breeding phenology affect individual fitness, and how these changes might, therefore, influence selection on breeding date remain unresolved. Here, we use a 36-year dataset from a long-term, individual-based study of House Wrens (Troglodytes aedon) to test whether the timing of avian breeding seasons is associated with annual changes in temperature, which have increased to a small but significant extent locally since the onset of the study in 1980. Increasing temperature was associated with an advancement of breeding date in the population, as the onset of breeding within years was closely associated with daily spring temperatures. Warmer springs were also associated with a reduced incubation period, but reduced incubation periods were associated with a prolonged duration of nestling provisioning. Nest productivity, in terms of fledgling production, was not associated with temperature, but wetter springs reduced fledging success. Most years were characterized by selection for earlier breeding, but cool and wet years resulted in stabilizing selection on breeding date. Our results indicate that climate change and increasing spring temperatures can affect suites of life-history traits, including selection on breeding date. Increasing temperatures may favor earlier breeding, but the extent to which the phenology of populations might advance may be constrained by reductions in fitness associated with early breeding during cool, wet years. Variability in climatic conditions will, therefore, shape the extent to which seasonal organisms can respond to changes in their environment.
Keywords: climate change, house wren, laying date, life history, long-term study
Introduction
Seasonal environments constrain reproduction in many organisms to times of the year at which resources for breeding are optimal. In birds, for example, breeding activity is generally matched to the availability of food for provisioning young and sustaining high workloads associated with providing parental care. Moreover, the close temporal association between migration, breeding, and molt imposes a trade-off in energy that must be partitioned among these processes, and the timing of breeding should be optimized to minimize the extent to which energy is invested in any of these stages simultaneously, such as reproduction and molt (Marshall 1961, Murton and Westwood 1977). Phenotypic plasticity in physiological processes may permit adjustments in the timing of these costly activities (Charmantier et al. 2008, Carey 2009, Dawson 2008, Vedder et al. 2013), but transitions between stages of the annual cycle require anatomical and physiological changes (e.g., gonadal recrudescence, fattening) that must be coordinated in advance by predictable environmental cues. Predictable variation in photoperiod is the primary cue used by many birds to prepare for transitions between stages of the annual cycle (Dawson 2008). However, birds also use a range of supplemental cues, including temperature and food availability, to adjust the timing of activities flexibly within seasons (reviewed in Dawson 2008), but the relevance of these cues varies among populations because of differences in the consistency of the environment (Wingfield 1993, Carey 2009).
Accumulating evidence has revealed that the onset of avian breeding seasons varies in response to local temperatures (McCleery and Perrins 1998, Dunn and Winkler 1999, Both et al. 2004, Dunn 2004, Visser et al. 2009, Williams et al. 2015), and the existence of genetic and phenotypic correlations between various life-history traits suggests that suites of these traits likely change in concert with each other as a response to long-term climate change (Winkler et al. 2002, Sheldon et al. 2003, Both and Visser 2005). For example, populations of the Pied Flycatcher (Ficedula hypoleuca) have recently exhibited an advance in clutch-initiation dates (Both and Visser 2001, Sanz 2003, Both et al. 2004), an increase in clutch sizes (Winkel and Hudde 1997, Both et al. 2004), and a reduction in the incubation period (Both and Visser 2005). However, the phenological changes observed may not be sufficient to match the change in peak food availability for nestlings (Both and Visser 2001; Visser et al. 2004, 2006). Indeed, although climate change is known to have affected the seasonal phenology in a variety of taxa, the consequences of these changes are poorly understood. Understanding how selection acts on phenological responses to environmental cues, such as changing temperatures, are critical to assessing how populations might be affected by climate change. Here, we assess changes in climatic conditions that have occurred as part of a long-term study of House Wrens (Troglodytes aedon), and how these changes affect life-history traits, individual fitness, and the shape and strength of selection acting on breeding date.
Selection for early breeding is strong in the House Wren, a short-distance migratory bird. House Wrens are common throughout much of the Americas from southern to northern temperate latitudes. In a population in central Illinois, USA, they return from the wintering grounds in April, typically initiating the nesting cycle at the end of April and early May, and breed through August (Fig. 3 in Johnson 2014). Previous work has indicated that birds that are able to fledge a brood of young early within a given breeding season are more likely to produce a second or third brood than those producing their first brood of the year at a later date (Bowers et al. 2012, Hodges et al. 2015). Because House Wrens are short-lived, with most adults breeding in only one or two years, selection for producing multiple broods of high-quality young within seasons is likely high (Johnson 2014, Bowers et al. 2015). Moreover, competition for limiting nesting sites for breeding is intense, and males experience strong intrasexual competition to arrive early and secure high-quality sites with which to attract females (e.g., Muller et al. 1997, Johnson et al. 2002). Finally, offspring produced earlier within breeding seasons are consistently more likely to survive and recruit as adults in the breeding population in subsequent years than are offspring produced later within breeding seasons (Bowers et al. 2013, 2014), a result that is not attributable to differences in natal dispersal between offspring produced early vs. late within seasons (Drilling and Thompson 1988). Thus, these birds are under strong selection for early arrival to the breeding grounds and to breed early. However, low temperatures early in the breeding season can directly affect parent or offspring survival through increased energetic demands imposed both to parents and their offspring (Stevenson and Bryant 2000; Sanz et al. 2003). Moreover, inclement weather and storms can reduce the success of nests that are initiated too early within breeding seasons (e.g., Wingfield et al. 1983), and breeding too early may also impose negative effects indirectly by creating a phenological mismatch between prey availability and the demands of rearing altricial young (e.g., Thomas et al. 2001, Both et al. 2009, 2010, Jones and Cresswell 2010, Reed et al. 2013, Dunn and Møller 2014). Therefore, the timing of reproduction is subject to opposing selective forces that act, in one direction, to advance the timing of breeding, and, in the other, to delay it.
Fig 3.
Monthly temperatures spanning the duration of the breeding season (April-August). Each line represents a reaction norm for a given year; the 36 years of study are plotted in four groups for visualization only.
In this study, we use a long-term dataset collected over 36 breeding seasons from an individual-based study to test whether climatic variation predicts breeding date and components of the avian breeding cycle, including the duration of the incubation and nestling-provisioning stages and fledging success. We predicted that average daily temperature following spring migration and at the onset of the breeding season would be negatively associated with clutch-initiation dates (i.e., warmer springs characterized by earlier breeding) and with the duration of incubation periods. We also monitored the recruitment of offspring from nests, and we expected that broods produced earlier within breeding seasons would produce more recruits than those produced later. However, we also predicted that the effect of breeding date on offspring recruitment would differ among years, such that selection for earlier breeding would increase the risk of a phenological mismatch for the earliest breeders in cooler years. Thus, we predicted a hump-shaped relationship between breeding date and recruitment within cooler years.
Methods
Study population
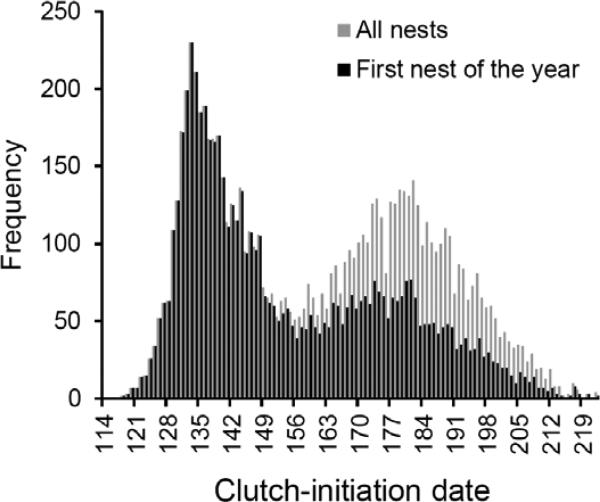
Northern House Wrens are secondary-cavity-nesting songbirds and readily accept nestboxes (Johnson 2014). We studied a population breeding in secondary deciduous forest (dominated by Quercus, Carya, and Acer spp.) in central Illinois, USA (40.665°N, 88.89°W) from 1980-2015. The study area was established in 1980 with 327 nestboxes. An additional 148 boxes were added in 1981, and 110 more in 1982, totaling 585 nestboxes from 1982-2003 (Fig. 1 in Eckerle and Thompson 2006). In 2004 the study area was expanded by an additional 115 boxes, bringing the total to 700 (Fig. 1 in DeMory et al. 2010). Nestboxes are spaced 30 m apart along north-south-oriented transects that are 60 m apart. The density of nestboxes has remained constant at 5.4 boxes/ha during the study, as has the size of nestboxes (Lambrechts et al. 2010 provide further details on nestboxes). Nestboxes were mounted on tree trunks in 1980, and since 1981 all have been mounted on 1.5-m metal poles to which axle grease was regularly applied to discourage terrestrial predators. Beginning in 2004, 48.3-cm-diameter predator baffles were mounted directly beneath the boxes until all were mounted atop baffles by the end of the 2010 field season, and the application of grease as a deterrent had ceased by 2010. Despite efforts to reduce nest depredation, rates of nest failure vary among years from ca. 31%-52% of all nests, even in the presence of predator baffles (Bowers et al. 2016). Approximately 95% of the nests in any given year are produced in the nestboxes (Drilling and Thompson 1988; data for nests produced in natural tree cavities are not included here), and the sexes do not show pronounced differences in return rate as recruits (average return rate ± SE: females = 4.1% ± 0.6%, males = 5.2% ± 0.6%; Bowers et al. 2014), or in natal dispersal distances in the population (median distances ± SD: females = 674 ± 469 m, males = 608 ± 467 m; Drilling and Thompson 1988). Birds in the study population winter along the northern coast of the Gulf of Mexico. Johnson and Wise (2000) provide details on the overwintering locations and migratory routes of northern House Wrens. The breeding season extends from late April to August, and 50-70% of the females in the study population produce a second clutch after fledging a brood of young earlier that season (Johnson 2014), but breeding success generally declines over the course of the season (Drilling and Thompson 1991). For early-season nests (Fig. 1), females in the current study produced an average clutch size of 6.8 eggs, and, for late-season clutches, 5.7 eggs. For nests that fledged at least one young, early-season nests produced an average of 5.7 fledglings and late-season nests an average of 4.7 fledglings.
Fig 1.
Frequency of clutch-initiation dates. Approximately half of the nests initiated in the second peak were produced by females new to the study area.
General field procedures
Each year, nestboxes were checked at least twice weekly from the start of the breeding season through August to determine clutch-initiation date and clutch size. Females lay one egg per day; thus, when clutches were newly discovered and contained more than one egg, we estimated the date of clutch initiation assuming the female had laid one egg per day. Clutch sizes were recorded only when the same number of eggs was present on two nest visits and there was evidence of incubation (eggs warm to the touch); only the female incubates and broods. After egg laying was complete and females commenced full incubation, we captured, measured, and banded females with a numbered, aluminum U. S. Geological Survey leg band. Capturing females too early during the nesting cycle (i.e., during egg production and early incubation) dramatically increases rates of nest abandonment; thus, the capture of adults at nests was typically delayed until half-way through the incubation period, at which time females can be handled with a low risk of abandonment. We captured females and males either by capturing them inside their nestbox or using mist nets outside the nestbox. Males received three additional colored leg bands arranged in a unique combination so they could be identified visually without being recaptured. We then visited nests daily when hatching was expected to determine the day of the year on which hatching within a nest began. We visited nests periodically during the nestling stage to monitor their progress, and nestlings were weighed and banded 11-13 days posthatching. We subsequently visited nests daily to determine fledging dates (13-17 days posthatching).
Climate data
We obtained daily temperature (the average of the minimum and maximum temperature on a given day) and rainfall data from the National Climatic Data Center (National Oceanographic and Atmospheric Association) for the weather station at Chenoa, McLean County, IL (40.733°N, 88.717°W). This station is ca. 16 km east-northeast of the study area, with a similar altitude, and was the closest weather station with nearly complete coverage during the study period. Measures at this station are unlikely to have been affected by heat-island effects associated with urbanization during the study, because the population size of Chenoa was less than 2000 and did not change substantially between 1980 (N = 1847) and 2010 (N = 1785) (United States Census Bureau 1982, 2012). The second-closest weather station to our study site that would have provided useable data was ca. 38 km north of the study site (Minonk, IL), and had larger gaps in coverage. Data obtained from the two stations were strongly correlated for both temperature (r12458 = 0.975, P < 0.001) and rainfall (r12329 = 0.543, P < 0.001).
Data analysis
All analyses were performed in SAS (version 9.4), all tests are two-tailed (α = 0.05), and we centered and standardized variables following Schielzeth (2010). Nests in which experimental manipulations altered clutch or brood size were excluded. Because we could not capture females early during the nesting cycle, some nests failed at early stages before we could identify the female producing the clutch. Thus, to limit the risk of pseudoreplication in our analysis of clutch size and breeding date, we excluded those nests at which the identity of the female producing the clutch was unknown, but analyzing the larger set of data (total N = 11,412 nests with known clutch-initiation dates, 8,185 of which were produced by females of known identity) that includes failed nests of unidentified females produces qualitatively similar results (data not shown). To assess changes in spring temperature, and its effect on the onset of breeding, we used daily temperature and rainfall averaged across the month of April, as conditions at this time reflect conditions that the birds experience upon their arrival to the study area while preparing to produce their first clutch of the season. The first egg of the breeding season at our site is generally laid on ca. the 5th of May. Thus, we used 01-30 April to reflect spring temperatures because this window covers an approximately 2-week period prior to and after the birds’ arrival before the onset of egg laying. Using temperature and rainfall from other windows of time during this period produces qualitatively similar results (data not shown).
We first used linear mixed models (LMMs; PROC GLIMMIX in SAS) that included year as a random effect to analyze variation in the daily temperature and rainfall during the month of April. We then used LMMs that included year and maternal identity as random effects to analyze variation in clutch-initiation dates and clutch size. We analyzed the date of clutch initiation for all nests that were produced by females of known identity (Fig. 1), as the effect of spring temperature on clutch-initiation date was identical across the full length of the breeding season. For clutch size, we analyzed effects on the number of eggs per clutch within the first peak of activity (Fig. 1) to assess effects on females’ first clutches (clutch sizes later within years may be reduced because of investment in early-season nests), and we also analyzed all nests to observe patterns in clutch size for the whole population. The trough between the two peaks in Figure 1 occurred on day 156 (05 June in non-leap years); thus, to analyze effects on early-season clutch sizes, we included a female's first clutch of the season, provided that it was produced before this date. We analyzed the length of the incubation and nestling periods as the time elapsed from clutch completion to hatching, and from hatching to fledging, using a Cox regression (survival analysis; PROC PHREG) with broods that failed prior to fledging as censored values. As the time until hatching and fledging represent time to an event occurring, we analyzed these data using survival analysis, in which failed broods included as censored values contribute to the analysis until the point at which they failed. For our analysis of incubation duration, we assessed effects of temperature and rainfall on the day clutches were completed, and we analyzed the time until fledging in relation to temperature and rainfall on the day hatching began within a nest. We included clutch size, clutch-initiation date, and year as covariates in the two analyses, and we also included the incubation duration of a given nest in our analysis of the time until fledging to test for a correlation between the duration of incubation and the length of the nestling stage. We accounted for non-independence of nests within the same year and nests that were produced by the same female in these survival analyses, similar to the use of random effects in a LMM, following Allison (2010). We also analyzed the length of each breeding season, quantified as the interval between the laying of the first egg of a year until the laying of the last egg of the last clutch of that year, as increases in spring temperatures and an advance in breeding dates may increase the length of the season overall. We analyzed this as a LMM with year, average April temperature, and average April precipitation per day as fixed effects. We then analyzed hatching success based on the number of unhatched eggs in relation to the total number of eggs laid in events/trials syntax using a generalized linear mixed model (GLMM; PROC GLIMMIX) with year as a random effect and a binomial distribution. We analyzed fledging success with a similar approach, but analyzed the total number of young fledged by a female across the breeding season (i.e., the sum of their first and second broods) instead of including each brood separately, using a GLMM with a Poisson distribution. Finally, we analyzed the number of recruits from all broods from years for which data were available (1981-1983, 1985-1992, 1994, 1998, 2001-2014) using a GLMM with a Poisson distribution and year as a random effect. We used breeding date as both linear and quadratic terms in this analysis to obtain non-linear selection gradients for each year as the coefficient of the quadratic term (sensu Lande and Arnold 1983, Brodie et al. 1995, see also Charmantier et al. 2008, Visser et al. 2015).
Results
Climate in central Illinois
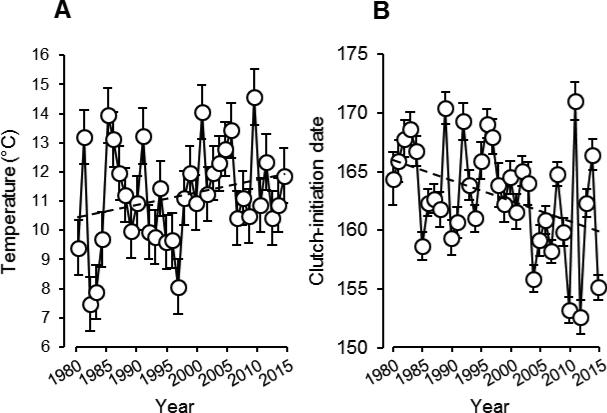
From 1980-2015, the average daily temperature during the month of April increased significantly (LMM with year as a random effect: estimate ± SE = 0.039 ± 0.014, F1, 1073 = 7.43, P = 0.007; Figs. 2A, 3). There was no change in daily precipitation during this time (estimate ± SE = 0.022 ± 0.026, F1, 1077 = 0.68, P = 0.408), and average daily temperature and rainfall were not associated with each other (estimate ± SE = 0.090 ± 0.055, F1, 1074 = 2.69, P = 0.101).
Fig 2.
Inter-annual variation in spring temperature (A) and clutch-initiation date (B). Plotted are least-squares means ± SE for each year.
Breeding date and clutch size
In response to the increases in spring temperatures, the average date of clutch initiation has advanced significantly since 1980 (Figs. 2, 3; Table 1), and this has been accompanied by an increase in the temporal synchrony of clutch initiation early within breeding seasons (Fig. 3). April temperatures did not predict the number of eggs per clutch for early-season nests, but the sizes of these clutches have declined since the onset of the study, and April temperatures were negatively correlated with the number of eggs per clutch when all nests were analyzed (Table 2). Thus, the decline in clutch size for early season nests appears to be attributable, at least in part, to increasing temperature. Neither clutch-initiation dates nor clutch sizes varied significantly with daily rainfall in April (Tables 1, 2).
Table 1.
Effects on clutch-initiation date.
| Estimate ± SE | F | df | P | |
|---|---|---|---|---|
| April temperature | –0.109 ± 0.030 | 12.76 | 1, 33.1 | 0.001 |
| April rainfall | 0.052 ± 0.031 | 2.76 | 1, 33.3 | 0.106 |
| Year | –0.073 ± 0.030 | 5.92 | 1, 29.8 | 0.021 |
| Intercept | –0.003 ± 0.030 |
Table 2.
Effects on clutch size.
| Early-season nests |
All nests |
|||||||
|---|---|---|---|---|---|---|---|---|
| Estimate ± SE | F | df | P | Estimate ± SE | F | df | P | |
| April temperature | –0.002 ± 0.019 | 0.02 | 1, 30.1 | 0.896 | –0.054 ± 0.024 | 5.14 | 1, 32.7 | 0.030 |
| April rainfall | –0.019 ± 0.019 | 0.93 | 1, 33.2 | 0.343 | 0.008 ± 0.024 | 0.1 | 1, 32.7 | 0.756 |
| Clutch-initiation date | –0.396 ± 0.035 | 125.14 | 1, 3399 | < 0.001 | –0.584 ± 0.008 | 6006.24 | 1, 6486 | < 0.001 |
| Year | –0.049 ± 0.018 | 7.22 | 1, 27.4 | 0.012 | –0.018 ± 0.024 | 0.57 | 1, 30.7 | 0.454 |
| Intercept | 0.169 ± 0.039 | 0.035 ± 0.023 | ||||||
Length of the incubation and nestling periods, and duration of the laying season
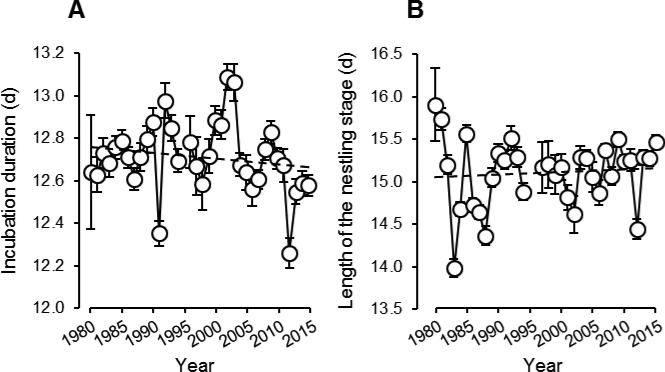
As expected, the length of the incubation period varied negatively with average daily temperature on the day of clutch completion, while controlling for variation in clutch-initiation date and clutch size (Table 3A). Parameter estimates produced by the Cox regression implemented in PROC PHREG represent the probability of ‘failure’ (i.e., hatching or fledging) at any point in time. Thus, a positive parameter estimate reflects a negative correlation between temperature and incubation duration, and vice versa. Consistent with the negative relationship between temperature and incubation duration, and with the increase in average daily temperature since 1980, there was also a trend for incubation duration to decline from 1980 to 2015 (Fig. 4A, Table 3A). The effect of year on incubation duration is statistically significant if temperature is omitted from the model (estimate ± SE = 0.023 ± 0.011, = 4.92, P = 0.027; Fig. 4A), suggesting that the decline in incubation duration over time is primarily attributable to changes in temperature. Incubation duration also declined over the course of the breeding season, and with increasing clutch size (Table 3A). The length of the nestling period was also negatively correlated with daily temperatures during the nestling stage (Table 3B), indicating that warmer conditions were associated with earlier fledging. The length of the nestling period was also strongly negatively correlated with the duration of incubation (Table 3B), and increased from 1980 to 2015 (Fig. 4B, Table 3B).
TABLE 3.
Effects on the duration of incubation and nestling stages. Temperature and precipitation represent values observed on the day of clutch completion and hatching for individual nests in analyses of incubation duration and length of the nestling stage, respectively. Positive parameter estimates (not standardized) reflect a negative relationship between the independent variable and the duration of the incubation and nestling periods.
| Estimate ± SE | χ 2 | df | P | |
|---|---|---|---|---|
| A. Incubation duration | ||||
| Temperature | 0.216 ± 0.012 | 340.79 | 1 | < 0.001 |
| Precipitation | –0.003 ± 0.010 | 0.10 | 1 | 0.750 |
| Clutch-initiation date | 0.366 ± 0.017 | 456.99 | 1 | < 0.001 |
| Clutch size | 0.381 ± 0.019 | 402.90 | 1 | < 0.001 |
| Year | 0.018 ± 0.011 | 2.57 | 1 | 0.109 |
| B. Length of the nestling stage | ||||
| Temperature | 0.060 ± 0.013 | 22.74 | 1 | < 0.001 |
| Precipitation | –0.002 ± 0.012 | 0.03 | 1 | 0.869 |
| Clutch-initiation date | –0.003 ± 0.019 | 0.02 | 1 | 0.882 |
| Clutch size | 0.007 ± 0.022 | 0.10 | 1 | 0.757 |
| Incubation duration | 0.151 ± 0.023 | 43.68 | 1 | < 0.001 |
| Year | –0.082 ± 0.011 | 51.79 | 1 | < 0.001 |
Fig 4.
Length of the incubation (A) and nestling (B) periods of the reproductive cycle in relation to year (least-squares means ± SE).
The length of the laying season varied among years (overall mean ± SD = 92.4 ± 6.2 d), and spring temperatures had a strong effect on the length of the season (estimate ± SE = 2.36 ± 0.92, F1, 32 = 6.55, P = 0.015), but season length was not associated with spring rainfall (estimate ± SE = 0.74 ± 0.91, F1, 32 = 0.67, P = 0.420), nor has it changed in a discernible direction since the onset of the study in 1980 (effect of year: estimate ± SE = −1.25 ± 0.96, F1, 32 = 1.71, P = 0.200).
Hatching and fledging success
Hatching success was unaffected by the variables considered here, and did not change significantly from 1980-2015 (Table 4A). However, fledging success was negatively correlated with daily rainfall in spring (Table 4B). The number of fledglings also declined over the course of the breeding season, while controlling for variation in clutch size (Table 4B).
TABLE 4.
Effects on hatching and fledging success. Hatching success is analyzed at the level of the nest, whereas fledging success represents the total number of offspring fledged across all broods within a year for individual females.
| Estimate ± SE | F | df | P | |
|---|---|---|---|---|
| A. Number of unhatched eggs | ||||
| April temperature | –0.055 ± 0.056 | 0.97 | 1, 21.46 | 0.336 |
| April rainfall | 0.001 ± 0.051 | 0.00 | 1, 19.51 | 0.993 |
| Year | –0.032 ± 0.058 | 0.31 | 1, 16.87 | 0.587 |
| Clutch-initiation date | –0.025 ± 0.028 | 0.84 | 1, 4844 | 0.360 |
| Intercept | –2.839 ± 0.049 | |||
| B. Fledging success | ||||
| April temperature | 0.003 ± 0.025 | 0.01 | 1, 30.76 | 0.919 |
| April rainfall | –0.052 ± 0.025 | 4.24 | 1, 31.02 | 0.048 |
| Year | 0.013 ± 0.025 | 0.26 | 1, 28.96 | 0.614 |
| Clutch-initiation date | –0.173 ± 0.009 | 386.98 | 1, 6057 | < 0.001 |
| Total eggs produced | 0.239 ± 0.006 | 1419.60 | 1, 6057 | < 0.001 |
| Intercept | 1.420 ± 0.024 |
Offspring recruitment and selection for breeding date
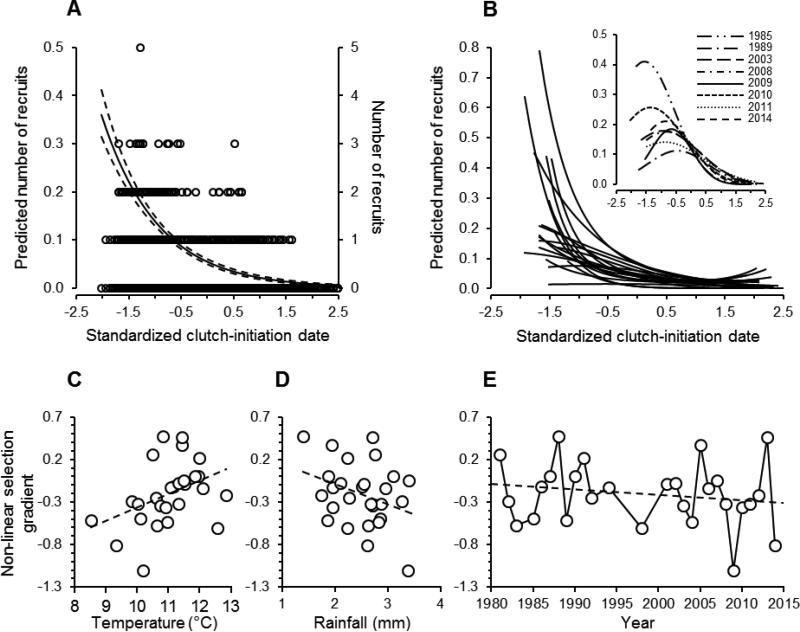
Overall, there was a consistent decline over the course of the breeding season in the number of recruits produced by a given brood (effect of clutch-initiation date: linear term estimate ± SE = – 0.902 ± 0.146, F1, 10346 = 261.34, P < 0.001; quadratic term estimate ± SE = –0.055 ± 0.057, F1, 10346 = 0.93, P = 0.336; Fig. 5A). However, the strength and direction of this effect varied among years (Fig. 5B). In some years, there was a sharp decline in the number of recruits produced with clutch-initiation date, while in other years there was a hump-shaped relationship between the number of recruits and clutch-initiation date (Fig. 5B). We obtained linear and non-linear selection differentials for each year, predicting that those years in which the earliest of broods suffered a reduction in the number of recruits would more likely be colder and wetter than average. This was indeed the case, as stabilizing selection for breeding date was strongest in colder years (r25 = 0.402, P = 0.038; Fig. 5C) and also in wetter years, although this latter effect was marginally non-significant (r25 = −0.334, P = 0.089; Fig. 5D). Moreover, these cooler years were associated with an overall reduction in recruitment relative to warmer years overall (effect of spring temperature: estimate ± SE = 0.357 ± 0.138, F1, 23.73 = 6.72, P = 0.016). Recruitment was unrelated to rainfall (estimate ± SE = −0.105 ± 0.145, F1, 22.57 = 0.52, P = 0.479).
Fig 5.
Selection on breeding date. (A) Number of recruits produced in relation to clutch-initiation date. Curves (left vertical axis) represent the predicted number of recruits per nest from a generalized linear mixed model ± 95% CI, and individual data are plotted against the right vertical axis (N = 10,383 nests). (B) Number of recruits predicted by a generalized linear mixed model (as in A) in relation to breeding date for each year (each curve depicts a single year). In most years, the earliest breeders produced the most recruits, but there was a cost to breeding too early in others (inset). (C-E) Non-linear selection gradient for the relationship between recruitment and clutch-initiation date in relation to annual variation in (C) average daily temperatures, (D) rainfall, and (E) year. Most years were characterized by selection for earlier breeding overall, but cool and wet years were characterized by stabilizing selection on breeding date.
Discussion
Average daily temperature during the early stages of the breeding season has increased to a small but significant extent since 1980, and changes in temperature strongly predict breeding date in the population. The advance of laying date since 1980 in relation to spring temperatures has also been accompanied by an increase in the temporal synchrony with which clutches are initiated at the onset of breeding seasons. Clutch sizes early within breeding seasons have also declined, on average, since the onset of the study in 1980, a decline that appears to be attributable, in part, to increasing temperature. Although spring rainfall has not changed in a discernible direction since the onset of the study, changes in rainfall associated with global climate change may not be without costs. While rainfall is often an important predictor of breeding date, particularly for tropical birds (e.g., Styrsky and Brawn 2011; Oppel et al. 2013), its effects on nest success are complex, and have been shown to be both positive (Rottenberry and Wiens 1991) and negative (Rodríguez and Bustamante 2003), perhaps by reducing the ability of parents to forage for themselves or their nestlings. In the current study, wetter springs were associated with a reduction in the total number of offspring fledged within years and also tended to reduce recruitment from early-season nests.
In addition to advancing the timing of breeding, warmer springs were also associated with a reduction in the duration of the incubation period. This could have been brought about in a number of ways. For example, warmer temperatures may have directly increased the rate of embryonic development (e.g., Olson et al. 2006, Hepp et al. 2006, Nilsson et al. 2008), but may also have affected maternal incubation behavior, including the constancy with which heat was applied to eggs (Bryan and Bryant 1999, Ardia et al. 2009). Warmer temperatures were also associated with early onset of incubation relative to clutch completion in Tree Swallows (Tachycineta bicolor; Ardia et al. 2006), and such a process may have contributed to the reduction in the time between clutch completion and hatching in the current study. Regardless of the mechanism relating ambient temperature to incubation duration, the decline in incubation period with increasing temperature and year was associated with an increase in the length of the nestling period, indicating that changing spring temperatures can affect a suite of life-history traits in addition to the timing of breeding.
Although a number of studies have documented temperature effects on the timing of breeding, only a small handful have investigated consequences for incubation duration. Pied Flycatchers seem to modulate incubation duration in warmer years to match hatching with peak food availability (Both and Visser 2005). In a Dutch population of Great Tits (Parus major), spring temperatures have increased, peak food availability for nestlings has advanced, and the strength of selection for earlier breeding has intensified, but the tits’ egg-laying dates in early spring have not advanced in parallel with these conditions (Visser et al. 1998). Intriguingly, the interval between laying and hatching has decreased over time (Visser et al. 1998). In contrast, laying date has advanced in response to warmer spring temperatures in a British population of Great Tits (McCleery and Perrins 1998), and hatching in this population remains closely linked to peak food availability (Cresswell and McCleery 2003). In the British population, the incubation period has increased since 1960 (Cresswell and McCleery 2003). Thus, the length of the incubation period in both the Dutch and British tit populations has been altered, but in opposite ways, in response to changes in spring temperatures and peak food availability.
In our study population, an increase in spring temperatures was associated with a reduction in the duration of incubation, and also a reduction in the length of the nestling stage. The effect of temperature on the duration of the nestling stage is consistent with a potential increase in food availability and growth rates of ectothermic young with increased temperature. However, a negative correlation between the duration of the incubation and nestling stages, and the declined incubation since 1980, has led to a concomitant increase in the length of the nestling stage over this time. These changes may reflect a change in life history, whereby rapid embryonic development and earlier hatching necessitates a prolonged period of posthatching parental care before nestlings are able to fledge (see also Bowers et al. 2013). The reduction in incubation duration may not be without costs, particularly if it results in an increase in the duration of posthatching parental care. Moreover, experimentally increased incubation temperatures were recently shown to induce persistent effects on offspring survival and adult body mass (Nord and Nilsson 2016). Thus, increasing temperatures per se not only imposes immediate consequences for the duration of incubation and the nestling period (e.g., costs of reproduction on parents’ reproductive value, increased risk of predation or parasitism to parents and their offspring), but can also carry long-term effects on offspring phenotype and fitness after they have left the nest.
The production of fledglings and recruits into local breeding populations has long been known to vary with date in a number of species, generally resulting in strong selection for earlier breeding within seasons (e.g., van Noordwijk et al. 1995, Visser et al. 2015, reviewed in Williams 2012). We also observed a pronounced decline in the number of recruits over the course of the breeding season, a decline that is not attributable to seasonal variation in natal dispersal (Drilling and Thompson 1988). However, the strength of this selection varied widely among years, with certain years, particularly cooler and wetter ones, resulting in stabilizing selection on breeding date and, in the case of cooler years, reduced recruitment overall. Although temperature is expected to continue to increase in future years, inter-annual variability in environmental conditions among years may constrain the degree to which breeding birds can respond, plastically or adaptively, to a changing climate.
In conclusion, temperature in central Illinois has increased since 1980, and this slight but significant increase is associated with an advancement of breeding date overall in the population. Warmer temperatures were associated with a reduction in the duration of the incubation and nestling stages, but a negative correlation between these stages overall has led to a decline in the duration of the incubation period and a concomitant increase in the duration of the nestling period since the onset of the study in 1980. Wetter springs were associated with a reduction in the number of fledglings produced. Whereas spring temperatures did not affect fledging success, temperature was positively associated with the number of recruits produced by a given brood, and cooler springs were associated with stabilizing selection on breeding date. Our results indicate that climate change and increasing spring temperatures can affect a suite of interrelated life-history traits, including the shape and strength of selection acting on breeding date. Although climate predictions indicate a general increase in temperature, variability in future climatic conditions will shape the accuracy with which seasonal organisms can respond to changes in their environment.
Acknowledgments
Data were collected over the 36 breeding seasons by field and laboratory workers who participated in Wren Crew, without whom the study would not have been possible. Robert Werkman, Lyonel Leonard, James Williamson, and James Dunham helped with nestbox design and construction over the study period. We are grateful to David Davis; Mr. and Mrs. Dean Sears; the Davis, Sears, and Butler families; and the ParkLands Foundation (Merwin Nature Preserve) for use of their properties. We also thank two anonymous reviewers for helpful comments that improved the manuscript. Funding was provided at various times by the School of Biological Sciences, College of Arts and Sciences, Honors Program, and Graduate School of Illinois State University; the Beta Lambda Chapter of the Phi Sigma Biological Honor Society; the Sigma Xi Society; the Whitehall Foundation; Research Internships in Science and Engineering (RISE) from the Deutscher Akademischer Austauschdienst; the National Science Foundation (DEB 7707246, DEB 8104687, INT 8410955, BSR 8615296, INT 9123572, IBN 0316580, IOS 0718140); and the National Institutes of Health (R15HD076308-01).
Literature Cited
- Allison PD. Survival analysis using SAS: a practical guide. Second edition SAS Institute, Inc.; Cary, North Carolina, USA: 2010. [Google Scholar]
- Ardia DR, Cooper CB, Dhondt AA. Warm temperatures lead to early onset of incubation, shorter incubation periods and greater hatching asynchrony in tree swallows Tachycineta bicolor at the extremes of their range. Journal of Avian Biology. 2006;37:137–142. [Google Scholar]
- Ardia DR, Pérez JH, Chad EK, Voss MA, Clotfelter ED. Temperature and life history: experimental heating leads female tree swallows to modulate egg temperature and incubation behaviour. Journal of Animal Ecology. 2009;78:4–13. doi: 10.1111/j.1365-2656.2008.01453.x. [DOI] [PubMed] [Google Scholar]
- Both C, Visser ME. Adjustment to climate change is constrained by arrival date in a long-distance migrant bird. Nature. 2001;411:296–298. doi: 10.1038/35077063. [DOI] [PubMed] [Google Scholar]
- Both C, et al. Large-scale geographical variation confirms that climate change causes birds to lay earlier. Proceedings of the Royal Society of London B. 2004;271:1657–1662. doi: 10.1098/rspb.2004.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both C, Visser ME. The effect of climate change on the correlation between avian life-history traits. Global Change Biology. 2005;11:1606–1613. [Google Scholar]
- Both C, van Asch M, Bijlsma RG, van den Burg AB, Visser ME. Climate change and unequal phenological changes across four trophic levels: constraints or adaptations? Journal of Animal Ecology. 2009;78:73–83. doi: 10.1111/j.1365-2656.2008.01458.x. [DOI] [PubMed] [Google Scholar]
- Both C, Van Turnhout CAM, Bijlsma RG, Siepel H, Van Strien AJ, Foppen RPB. Avian population consequences of climate change are most severe for long-distance migrants in seasonal habitats. Proceedings of the Royal Society B. 2010;277:1259–1266. doi: 10.1098/rspb.2009.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Sakaluk SK, Thompson CF. Experimentally increased egg production constrains future reproduction of female house wrens. Animal Behaviour. 2012;83:495–500. [Google Scholar]
- Bowers EK, Sakaluk SK, Thompson CF. Sibling cooperation influences the age of nest-leaving in an altricial bird. American Naturalist. 2013;181:775–786. doi: 10.1086/670244. [DOI] [PubMed] [Google Scholar]
- Bowers EK, Hodges CJ, Forsman AM, Vogel LA, Masters BS, Johnson BGP, Johnson LS, Thompson CF, Sakaluk SK. Neonatal body condition, immune responsiveness, and hematocrit predict longevity in a wild bird population. Ecology. 2014;95:3027–3034. doi: 10.1890/14-0418.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Bowden RM, Sakaluk SK, Thompson CF. Immune activation generates corticosterone-mediated terminal reproductive investment in a wild bird. American Naturalist. 2015;185:769–783. doi: 10.1086/681017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Sakaluk SK, Thompson CF. No effect of blood sampling or phytohaemagglutinin injection on postfledging survival in a wild songbird. Ecology and Evolution. 2016 doi: 10.1002/ece3.2112. doi:10.1002/ece3.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie ED, III, Moore AJ, Janzen FJ. Visualizing and quantifying natural selection. Trends Ecology and Evolution. 1995;10:313–318. doi: 10.1016/s0169-5347(00)89117-x. [DOI] [PubMed] [Google Scholar]
- Bryan SM, Bryant DM. Heating nest-boxes reveals an energetic constraint on incubation behaviour in great tits, Parus major. Proceedings of the Royal Society of London B. 1999;266:157–162. [Google Scholar]
- Carey C. The impacts of climate change on the annual cycles of birds. Philosophical Transactions of the Royal Society B. 2009;364:3321–3330. doi: 10.1098/rstb.2009.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charmantier A, McCleery RH, Cole LR, Perrins C, Kruuk LEB, Sheldon BC. Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science. 2008;320:800–803. doi: 10.1126/science.1157174. [DOI] [PubMed] [Google Scholar]
- Cresswell W, McCleery R. How great tits maintain synchronization of their hatch date with food supply in response to long-term variability in temperature. Journal of Animal Ecology. 2003;72:356–366. [Google Scholar]
- Dawson A. Control of the annual cycle in birds: endocrine constraints and plasticity in response to ecological variability. Philosophical Transactions of the Royal Society B. 2008;363:1621–1633. doi: 10.1098/rstb.2007.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMory ML, Thompson CF, Sakaluk SK. Male quality influences male provisioning in house wrens independent of attractiveness. Behavioral Ecology. 2010;21:1156–1164. [Google Scholar]
- Drilling NE, Thompson CF. Natal and breeding dispersal in House Wrens (Troglodytes aedon). Auk. 1988;105:480–491. [Google Scholar]
- Drilling NE, Thompson CF. Mate switching in multibrooded House Wrens. Auk. 1991;108:60–70. [Google Scholar]
- Dunn P. Breeding dates and reproductive performance. Advances in Ecological Research. 2004;35:69–87. [Google Scholar]
- Dunn PO, Møller AP. Changes in breeding phenology and population size of birds. Journal of Animal Ecology. 2014;83:729–739. doi: 10.1111/1365-2656.12162. [DOI] [PubMed] [Google Scholar]
- Dunn PO, Winker DW. Climate change has affected the breeding date of tree swallows throughout North America. Proceedings of the Royal Society of London B. 1999;266:2487–2490. doi: 10.1098/rspb.1999.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckerle KP, Thompson CF. Mate choice in house wrens: nest cavities trump male characteristics. Behaviour. 2006;143:253–271. [Google Scholar]
- Hepp GR, Kennamer RA, Johnson MH. Maternal effects in Wood Ducks: incubation temperature influences incubation period and neonate phenotype. Functional Ecology. 2006;20:307–314. [Google Scholar]
- Hodges CJ, Bowers EK, Thompson CF, Sakaluk SK. Cascading costs of reproduction in female house wrens induced to lay larger clutches. Journal of Evolutionary Biology. 2015;28:1383–1393. doi: 10.1111/jeb.12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LS. House Wren (Troglodytes aedon), 2nd edition. Number 380. In: Poole A, editor. The Birds of North America Online. Cornell Lab of Ornithology and American Ornithologists’ Union; Ithaca, New York, USA: 2014. [Google Scholar]
- Johnson LS, Wise J. Wintering grounds of North American house wrens as revealed by band recoveries. Journal of Field Ornithology. 2000;71:501–505. [Google Scholar]
- Johnson LS, Hicks BG, Masters BS. Increased cuckoldry as a cost of breeding late for male house wrens (Troglodytes aedon). Behavioral Ecology. 2002;13:670–675. [Google Scholar]
- Jones T, Cresswell W. The phenology mismatch hypothesis: are declines of migrant birds linked to uneven global climate change? Journal of Animal Ecology. 2010;79:98–108. doi: 10.1111/j.1365-2656.2009.01610.x. [DOI] [PubMed] [Google Scholar]
- Lambrechts MM, et al. The design of artificial nestboxes for the study of secondary hole-nesting birds: a review of methodological inconsistencies and potential biases. Acta Ornithologica. 2010;45:1–26. [Google Scholar]
- Lande R, Arnold SJ. The measurement of selection on correlated characters. Evolution. 1983;37:1210–1226. doi: 10.1111/j.1558-5646.1983.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Marshall AJ. Breeding seasons and migration. In: Marshall AJ, editor. Biology and comparative physiology of birds, v. II. Academic Press; New York, USA: 1961. pp. 307–339. [Google Scholar]
- McCleery RH, Perrins CM. temperature and egg-laying trends. Nature. 1998;391:30–31. [Google Scholar]
- Muller KL, Stamps JA, Krishnan VV, Willits NH. The effects of conspecific attraction and habitat quality on habitat selection in territorial birds (Troglodytes aedon). American Naturalist. 1997;150:650–661. doi: 10.1086/286087. [DOI] [PubMed] [Google Scholar]
- Murton RK, Westwood NJ. Avian breeding cycles. Oxford University Press; Oxford, UK: 1977. [Google Scholar]
- Nilsson JF, Stjernman M, Nilsson J-Å. Experimental reduction of incubation temperature affects both nestling and adult blue tits Cyanistes caeruleus. Journal of Avian Biology. 2008;39:553–559. [Google Scholar]
- Nord A, Nilsson J-Å. Long-term consequences of high incubation temperature in a wild bird population. Biology Letters. 2016;12:20160087. doi: 10.1098/rsbl.2016.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson CR, Vleck CM, Vleck D. Plasticity of embryonic growth rate and metabolism in the face of periodic cooling in House Wrens. Journal of Ornithology. 2006;147:222–223. [Google Scholar]
- Oppel S, Hilton GM, Allcorn R, Fenton C, Matthews AJ, Gibbons DW. The effects of rainfall on different components of seasonal fecundity in a tropical forest passerine. Ibis. 2013;155:464–475. [Google Scholar]
- Reed TE, Jenouvrier S, Visser ME. Phenological mismatch strongly affects individual fitness but not population demography in a woodland passerine. Journal of Animal Ecology. 2013;82:131–144. doi: 10.1111/j.1365-2656.2012.02020.x. [DOI] [PubMed] [Google Scholar]
- Rodríguez C, Bustamante J. The effect of weather on lesser kestrel breeding success: can climate change explain historical population declines? Journal of Animal Ecology. 2003;72:793–810. [Google Scholar]
- Rottenberry JT, Wiens JA. Weather and reproductive variation in shrubsteppe sparrows: a hierarchical analysis. Ecology. 1991;72:1325–1335. [Google Scholar]
- Sanz JJ. Large-scale effect of climate change on breeding parameters of pied flycatchers in Western Europe. Ecography. 2003;26:45–50. [Google Scholar]
- Sanz JJ, Potti J, Moreno J, Merino S, Frías O. Climate change and fitness components of a migratory bird breeding in the Mediterranean region. Global Change Biology. 2003;9:461–472. [Google Scholar]
- Schielzeth H. Simple means to improve the interpretability of regression coefficients. Methods in Ecology and Evolution. 2010;1:103–113. [Google Scholar]
- Sheldon BC, Kruuk LEB, Merilä J. Natural selection and the inheritance of breeding time and clutch size in the collared flycatcher. Evolution. 2003;57:406–420. doi: 10.1111/j.0014-3820.2003.tb00274.x. [DOI] [PubMed] [Google Scholar]
- Stevenson IR, Bryant DM. Avian phenology: climate change and constraints on breeding. Nature. 2000;406:366–367. doi: 10.1038/35019151. [DOI] [PubMed] [Google Scholar]
- Styrsky JN, Brawn JD. Annual fecundity of a Neotropical bird during years of high and low rainfall. Condor. 2011;113:194–199. [Google Scholar]
- Thomas DW, Blondel J, Perret P, Lambrechts MM, Speakman JR. Energetic and fitness costs of mismatching resource supply and demand in seasonally breeding birds. Science. 2001;291:2598–2600. doi: 10.1126/science.1057487. [DOI] [PubMed] [Google Scholar]
- United States Census Bureau . 1980 Census of Population, volume 1, Characteristics of the Population, PC80-1-A15, Illinois. Washington, DC, USA: 1982. [Google Scholar]
- United States Census Bureau . 2010 Census of Population and Housing, Summary Population and Housing Characteristics, CPH-1-15, Illinois. Washington, DC, USA: 2012. [Google Scholar]
- van Noordwijk AJ, McCleery RH, Perrins CM. Selection for the timing of great tit breeding in relation to caterpillar growth and temperature. Journal of Animal Ecology. 1995;64:451–458. [Google Scholar]
- Vedder O, Bouwhuis S, Sheldon BC. Quantitative assessment of the importance of phenotypic plasticity in adaptation to climate change in wild bird populations. PLoS Biology. 2013;11:e1001605. doi: 10.1371/journal.pbio.1001605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser ME, Both C, Lambrechts MM. Global climate change leads to mistimed avian reproduction. Advances in Ecological Research. 2004;35:89–110. [Google Scholar]
- Visser ME, Holleman LJM, Gienapp P. Shifts in caterpillar biomass phenology due to climate change and its impact on the breeding biology of an insectivorous bird. Oecologia. 2006;147:164–172. doi: 10.1007/s00442-005-0299-6. [DOI] [PubMed] [Google Scholar]
- Visser ME, Holleman LJM, Caro SP. Temperature has a causal effect on avian timing of reproduction. Proceedings of the Royal Society B. 2009;276:2323–2331. doi: 10.1098/rspb.2009.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser ME, van Noordwijk AJ, Tinbergen JM, Lessells CM. Warmer springs lead to mistimed reproduction in great tits (Parus major). Proceedings of the Royal Society of London B. 1998;265:1867–1870. [Google Scholar]
- Visser ME, Gienapp P, Husby A, Morrisey M, de la Hera I, Pulido F, Both C. Effects of spring temperatures on the strength of selection on timing of reproduction in a long-distance migratory bird. PLoS Biology. 2015;13:e1002120. doi: 10.1371/journal.pbio.1002120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TD. Physiological adaptations for breeding in birds. Princeton University Press; Princeton, New Jersey, USA: 2012. [Google Scholar]
- Williams TD, Bourgeon S, Cornell A, Ferguson L, Fowler M, Fronstin RB, Love OP. Mid-winter temperatures, not spring temperatures, predict breeding phenology in the European starling Sturnus vulgaris. Royal Society Open Science. 2015;2:140301. doi: 10.1098/rsos.140301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield JC. Control of testicular cycles in the song sparrow, Melospiza melodia melodia-interaction of photoperiod and an endogenous program. General and Comparative Endocrinology. 1993;92:388–401. doi: 10.1006/gcen.1993.1176. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Moore MC, Farner DS. Endocrine responses to inclement weather in naturally breeding populations of white-crowned sparrows (Zonotrichia leucophrys pugetensis). Auk. 1983;100:56–62. [Google Scholar]
- Winkel W, Hudde H. Long-term trends in reproductive traits of tits (Parus major, P. caeruleus) and Pied Flycatchers Ficedula hypoleuca. Journal of Avian Biology. 1997;28:187–190. [Google Scholar]
- Winkler DW, Dunn PO, McCulloch MC. Predicting the effects of climate change on avian life-history traits. Proceedings of the National Academy of Sciences USA. 2002;99:13595–13599. doi: 10.1073/pnas.212251999. [DOI] [PMC free article] [PubMed] [Google Scholar]