Abstract
Objective
Depression and anxiety are considered risk factors for cardiovascular disease (CVD); however, the explanatory mechanisms are still to be characterized. One proposed pathophysiological pathway is dysregulation of the autonomic nervous system, including heightened sympathetic nervous system (SNS) activity. This study examined the relationship between symptoms of depression, anxiety and SNS activity in individuals with untreated high blood pressure.
Methods
140 participants with untreated high blood pressure (55% White, 38.5% Female, mean age ± SD: 45.5±8.55 years) collected urine over a 24-hour period on 3 separate occasions. Urine samples were assayed for mean 24-hour epinephrine (EPI24) and norepinephrine (NE24) excretion. Depressive symptoms were assessed using the Beck Depression Inventory, with anxiety symptoms assessed by the Spielberger State-Trait Anxiety Inventory.
Results
Depression and anxiety scores were inter-correlated (r = .76, p < .001). EPI24 was positively correlated with anxiety (r = .20, p = .02) but not depression (r = .02, p = .77), while NE24 was not correlated with anxiety (r = .10, p = .21) or with depression (r = .07, p = .39). Regression models, accounting for gender, Age, BMI, race, mean systolic ambulatory blood pressure, tobacco use, alcohol use, physical activity, and sleep efficacy confirmed that anxiety was associated with EPI24 excretion (p = .023), and that depressive symptoms were not (p = .54).
Conclusions
Anxiety was associated with heightened sympathoadrenal activity, suggesting a biological pathway through which anxiety could increase CVD risk. Anxiety and depression may confer increased CVD risk via different mechanisms.
Keywords: depression, anxiety, urinary catecholamines, epinephrine, norepinephrine
Introduction
Cardiovascular disease (CVD) is the most common cause of morbidity and mortality in the United States (1). In addition to traditional CVD risk factors, including smoking, obesity and physical inactivity, accumulating evidence now exists indicating that depression is a risk factor for the development and exacerbation of CVD (2, 3), and other acute coronary syndromes (ACS) (4). Anxiety also is associated with increased risk of mortality in CVD patients (2, 1–7) as well as in otherwise healthy populations (8). There is considerable overlap between symptoms of anxiety and depression (9), with anxiety disorders often co-occurring with depression (10). Importantly, the presence of both elevated depressive symptoms and elevated anxiety further heightens the risk of mortality in patients with CVD (2), as well as in healthy populations (11).
The mechanisms responsible for the association between depression, anxiety and CVD risk have not been fully elucidated, but evidence suggests that the dysregulation of the autonomic nervous system (ANS) may be one plausible physiological pathway (12). For example, depressed CAD patients have an elevated incidence of ventricular tachycardia in contrast to their non-depressed counterparts (13). Increased SNS activation is detrimental to cardiovascular health, as evidenced by triggering cardiac arrhythmias and sudden cardiac death (14, 15), and may also lead to increased risk of morbidity and mortality through promotion of vascular injury and inhibition of normal healing (16). Depressed psychiatric patients have been shown to exhibit increased levels of both plasma (17) and urinary norepinephrine (NE) (18, 19), and depressed CAD patients exhibit elevated norepinephrine excretion (20). However, other studies have found no evidence that depressive symptoms and diagnoses are linked to hyperactivity of the SNS (21–23).
Anxiety disorders may be even more common than depression in the US population with estimates of a lifetime prevalence of approximately 29% (24). Moreover, anxiety is highly prevalent in cardiac populations, with estimates ranging from 25% to 44% (25). A recent meta-analysis revealed that, like depression, anxiety is also a risk factor for CHD (6), with heightened anxiety in the 3 month period following MI being predictive of future cardiac events and mortality (7). Anxiety has been linked to reduced parasympathetic nervous system control of heart rate in several different study samples, including patients with anxiety disorders (26), CHD (27), as well as healthy volunteers (28). Elevated SNS activity also may contribute to increased CVD risk associated with anxiety symptoms and diagnoses (19, 21–32). However, regarding epinephrine specifically, there is evidence that its chronic administration improves glucose tolerance, lowers blood pressure and increases muscle growth (33).
The studies that have investigated the relationships between urinary catecholamines and depression and anxiety have done so in either depressed populations, or populations with existing documented CVD. The current study aims to further this area of research by investigating these relationships in a population that are at risk for CVD development. Further, given the considerable overlap between the symptoms and frequent co-morbid presence of anxiety and depression (9, 10), and associated heightening of CVD-related mortality (2, 11), this study also will examine the relationship between urinary catecholamines and both anxiety and depressive symptoms.
The objective of the present study was to examine the relationship between symptoms of depression and anxiety and 24-hour urinary catecholamine excretion in a secondary analysis of a study sample of men and women with untreated high blood pressure. We hypothesized that elevated symptoms of depression assessed using the Beck Depression inventory (BDI; (34)), and elevated symptoms of anxiety assessed by the Spielberger State–Trait Anxiety Inventory (STAI; (35)) would be associated with heightened 24-hour urinary NE and EPI excretion.
Methods
Participants
The study sample consisted of 140 participants who were recruited as part of a larger study examining blood pressure dipping, full details of which can be found elsewhere (see (36, 37)). Data collection was completed from 2001–2009. Participants were recruited from the general population within a 30-mile radius of Duke University Medical Center (DUMC) by posting of study flyers in regional family medicine clinics, as well as through newspaper advertisements. Inclusion criteria were clinic systolic BP (SBP) 130–159mmHg and/or diastolic BP (DBP) 85–99mmHg (which includes the JNC 7 criteria for Stage 1 hypertension and the upper half of the range defined for prehypertension). The full exclusion criteria can be found elsewhere (36, 37) but, included BMI greater than 35kg/m2, alcohol or drug abuse or use of antihypertensive medication within 12 months, oral contraceptive use, pregnancy, use of hormone replacement therapy, diabetes mellitus, current use of cardiovascular medications and cardiovascular conditions including heart failure, pacemaker, atrial fibrillation, myocardial infarction, percutaneous coronary intervention or coronary artery bypass graft surgery within 6 months of enrollment; or inability to provide informed consent. Women who reported being amenorrheic for at least 12 months prior to the commencement of the study, or surgical menopause, were classed as post-menopausal. The study protocol was approved by Duke University Health System (DUHS) Institutional Review Board. All eligible individuals provided written informed consent prior to participation in the study.
Assessment of Depression and Anxiety
Depressive symptoms were assessed using the BDI (34), which is a self-report measure consisting of 21 items, with response options for each item reflecting varying degrees of depressive symptoms. Numerical values for each depressive symptom are summed for a total depressive symptoms score. The BDI is related to clinical ratings of depression (38), with a BDI score > 10 previously shown to detect psychiatric depression with moderate sensitivity and high specificity (39). The BDI has been shown to be a reliable, valid, and sensitive measure of depressive symptoms (34, 38). In cardiac populations, depressive symptoms assessed using the BDI have been related to adverse clinical outcomes (41–43). The Spielberger State-Trait Anxiety Inventory (STAI) is a 40-item inventory that is used to assess state and trait anxiety symptoms. The state anxiety components assess how responders feel in their current state (35) while trait anxiety assesses more generalized anxiety (35), and was the focus of the current study. The BDI reports high internal consistency, as demonstrated by Cronbach alpha scores of α=.91 (44). Likewise, the STAI also reports high internal consistency scores of 0.86 to 0.95 (45).
24-hour Urinary Catecholamines
Participants were asked to collect all urine over a 24-hour period on 3 separate days, one week apart, during which ambulatory BP monitoring was also undertaken (data reported previously, see (36, 37)). No preservatives were added to the urine collection containers; although some investigators and commercial labs utilize preservatives, a systematic evaluation of their effects on urinary catecholamine measures indicate they were unnecessary in the context of our study’s urine collection protocol (46). During the 24-hour collection process, the urine was maintained at 1–7 degrees C. All urine samples were kept cold by storage in a portable cooler and refrigerator during each 24-hour sampling period. Once the participants had returned to our laboratory in the morning after 24 hour urine collection, the collected sample was immediately aliquoted and frozen at −80 degrees C. Each sample collected on each of the collection days were then used to calculate an overall mean 24-hour EPI and NE excretion, subsequently referred to as EPI24 and NE24 for EPI and NE respectively. Urinary concentrations of NE and EPI were determined by high-pressure liquid chromatography (HPLC) with electrochemical detection. Urine creatinine was determined using the Jaffe method as modified by Slot, with kits supplied by Sigma Chemical Company (St. Louis, MO). The co-efficient of variation and within-person reproducibility for all biological assays was less than 10%. The detection range was 5 pg/ml for EPI and 25 pg/ml for NE. Catecholamine levels were expressed as urine concentration (ng/mL) per urine concentration of creatinine (mg/mL), yielding norepinephrine and epinephrine values of ng/mg (adjusted for creatinine) for each sample. Using creatinine-adjusted EPI24 and NE24 indices of catecholamine excretion account for individual differences in body size (47). However, it should be noted that creatinine adjustment is not without limitations (48).
Data Analysis
Repeated measures ANOVAs and correlational analyses were undertaken to examine the variation in catecholamine excretion across our sampling time points to ascertain if it would be suitable to create one mean catecholamine excretion value from our three time points of assessment. Repeated measures ANOVAs reveal no time effects for EPI (F (2, 136) = 0.31, p = 0.74) or NE (F (2, 136) = 1.19, p = 0.31) which indicate that there were no differences in catecholamine excretion over the 3 sampling time points. Pearson correlational analyses revealed consistency between the catecholamine values across each of our time points. For both epinephrine and norepinephine excretion, significant correlations were observed across all time points (p < .001), with correlation coefficients ranging from r=0.41–0.72. These observation support our use of a single averaged value, defined by the mean across the three 24-hour assessment periods, to represent a robust index of individual difference in catecholamine excretion. Bivariate correlations were used to evaluate the relationship between depressive symptoms, anxiety and 24-hour urinary catecholamine excretion (EPI24 and NE24). Multivariate regression analyses were used to examine the association between depressive symptoms, anxiety and urinary catecholamines in models which accounted for other participant characteristics related to catecholamine excretion, such as Gender, Age, BMI, Race, mean systolic ambulatory blood pressure, tobacco use, alcohol use, physical activity and sleep efficacy. Examination of the combined effects of depressive symptoms and anxiety on catecholamine excretion was undertaken by examining the interaction of depressive and anxiety symptom, and adding this into the regression models which also included other participant characteristics related to catecholamine excretion. All statistical analyses were performed using SAS software (SAS, Cary, NC), with statistical significance set at p < .05.
Results
Demographic and Psychometric Characteristics
The sample was comprised of 63 African Americans (22.1% Female) and 77 Caucasians (16.4% Female) participants, aged between 40 and 60 years (mean age ± SD: 45.5±8.55 years). Eighteen participants were smokers. Across all participants, BDI scores ranged from 0 to 33 with a mean score of 5.7 (SD = 6.4) and trait anxiety scores ranged from 21 to 67 with a mean score of 34.7 (SD = 9.0). Positive associations were evident between all of the psychological measures assessed. Depressive symptoms were associated with trait anxiety (r (134) = .76, p < .001). Additional sample characteristics are summarized in Table 1.
Table 1.
Descriptive measures of study sample (N = 140)
| Variable | Mean ± SD or % | Correlation with Depression Symptoms | p | Correlation with Anxiety Symptoms | p |
|---|---|---|---|---|---|
| Age (years) | 45.49 ± 8.55 | −0.20 | 0.02 | −0.12 | 0.16 |
| Gender (% Female) | 38.5 | −0.02 | 0.86 | 0.05 | 0.53 |
| Race (% White) | 55 | −0.15 | 0.08 | −0.00 | 0.99 |
| BMI (kg/m2) | 28.43 ± 3.85 | 0.12 | 0.18 | 0.02 | 0.78 |
| Clinic SBP (mmHg) | 136.8 ± 10.67 | 0.03 | 0.76 | −0.01 | 0.91 |
| Clinic DBP (mmHg) | 83.78 ± 8.09 | 0.07 | 0.45 | 0.03 | 0.76 |
| Creatinine 24h excretion (mg/mL) | 1.46 ± 0.72 | 0.02 | 0.84 | −0.11 | 0.19 |
| EPI24 (ng/mg) | 2.87 ± 1.78 | 0.03 | 0.77 | 0.20 | 0.02 |
| NE24 (ng/mg) | 20.83 ± 10.03 | 0.07 | 0.40 | 0.11 | 0.21 |
Note: BMI = Body Mass Index; SBP = Systolic Blood Pressure; DBP = Diastolic Blood Pressure; EPI24 = 24 hour epinephrine excretion; NE24 = 24 hour norepinephrine excretion
Correlations between depressive symptoms, anxiety symptoms and 24 hour catecholamine excretion
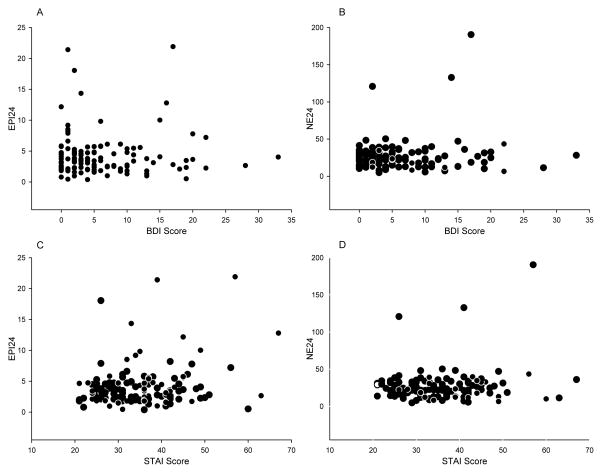
24 hour epinephrine excretion (EPI24) was positively correlated with anxiety scores (r (133) = .20, p = .022) but not with depressive symptoms (r (133) = .02, p = .77). 24 hour norepinephrine excretion (NE24) was not correlated with anxiety scores (r (133) = .10, p = .21) or with depressive symptoms (r (133) = .07, p = .39). These relationships are illustrated in Figure 1.
Figure 1.
Scattergrams showing the relationships between EPI24 and BDI Scores (A), NE24 and BDI Scores (B), EPI24 and STAI Scores (C) and NE24 and STAI Scores (D). EPI24 = 24-hour urinary epinephrine excretion; BDI = Beck Depression Inventory; NE24 = 24-hour urinary norepinephrine excretion; STAI = Spielberger State-Trait Anxiety Inventory.
Multivariate Linear Regression Models
Multivariate linear regression models were utilized to account for potential confounding factors that may influence the relationships between depressive symptoms and anxiety and urinary catecholamine excretion. Gender, Age, Race, BMI, mean ambulatory systolic blood pressure, alcohol use, tobacco use, physical activity and sleep efficacy were included into each model alongside depressive symptoms and anxiety when analyzing the relationships with norepinephrine (Table 2) and epinephrine (Table 3) excretion.
Table 2.
Multivariate Regression Analysis of Depression and Anxiety symptoms predicting 24 hour norepinephrine excretion
| B | p | Adjusted R2 | Increase in Adjusted R2 | Model F | Model p | |
|---|---|---|---|---|---|---|
| Model 1 | 0.06 | 1.52 | 0.15 | |||
| Gender | 0.01 | 0.90 | ||||
| Age | 0.28 | 0.026 | ||||
| Race | −0.12 | 0.38 | ||||
| BMI | −0.02 | 0.84 | ||||
| Mean | 0.19 | 0.12 | ||||
| Ambulatory SBP | ||||||
| Alcohol Use | −0.12 | 0.29 | ||||
| Tobacco Use | 0.12 | 0.15 | ||||
| Physical Activity | −0.07 | 0.55 | ||||
| Sleep Efficacy | 0.19 | 0.24 | ||||
| BDI Score | 0.12 | 0.31 | ||||
|
| ||||||
| Model 2 | 0.07 | 0.01 | 1.55 | 0.14 | ||
| Gender | 0.00 | 0.99 | ||||
| Age | 0.27 | 0.028 | ||||
| Race | −0.15 | 0.29 | ||||
| BMI | −0.02 | 0.86 | ||||
| Mean | 0.20 | 0.11 | ||||
| Ambulatory SBP | ||||||
| Alcohol Use | −0.13 | 0.27 | ||||
| Tobacco Use | 0.11 | 0.47 | ||||
| Physical Activity | −0.05 | 0.64 | ||||
| Sleep Efficacy | 0.20 | 0.21 | ||||
| STAI Score | 0.13 | 0.26 | ||||
Table 3.
Multivariate Regression Analysis of Depression and Anxiety predicting 24 hour epinephrine excretion
| B | p | Adjusted R2 | Increase in Adjusted R2 | Model F | Model p | |
|---|---|---|---|---|---|---|
| Model 1 | −0.00 | 0.95 | 0.49 | |||
| Gender | −0.06 | 0.66 | ||||
| Age | 0.12 | 0.34 | ||||
| Race | −0.12 | 0.38 | ||||
| BMI | −0.23 | 0.073 | ||||
| Mean | −0.05 | 0.67 | ||||
| Ambulatory SBP | ||||||
| Alcohol Use | 0.10 | 0.41 | ||||
| Tobacco Use | 0.06 | 0.69 | ||||
| Physical Activity | −0.14 | 0.24 | ||||
| Sleep Efficacy | 0.05 | 0.78 | ||||
| BDI Score | 0.08 | 0.54 | ||||
|
| ||||||
| Model 2 | 0.07 | 0.07 | 1.53 | 0.15 | ||
| Gender | −0.09 | 0.45 | ||||
| Age | 0.13 | 0.30 | ||||
| Race | −0.18 | 0.19 | ||||
| BMI | −0.23 | 0.058 | ||||
| Mean | −0.00 | 0.96 | ||||
| Ambulatory SBP | ||||||
| Alcohol Use | 0.09 | 0.46 | ||||
| Tobacco Use | 0.04 | 0.78 | ||||
| Physical Activity | −0.12 | 0.30 | ||||
| Sleep Efficacy | 0.12 | 0.46 | ||||
| STAI Score | 0.28 | 0.023 | ||||
NE24: Table 2 illustrates the relationships between norepinephrine excretion and depression and anxiety symptoms. Model 1 examined the relationships between norepinephrine excretion and BDI Score. Age was related to 24 hour norepinephrine excretion (NE24) (t = 2.27, b = 0.28, p = .026), such that older individuals produced higher NE24 excretion. BDI Score did not significantly account for norepinephrine excretion. Model 2, which examined the relationship between anxiety and norepinephrine, also did not show that anxiety significantly accounted for norepinephrine excretion. Finally, addition of a depressive symptoms by anxiety symptoms interaction term did not improve the model and was unrelated to 24 hour norepinephrine excretion (NE24) (p = .77). Likewise, addition of both BDI and anxiety scores alongside Model 1 factors did not reveal relationships with 24 hour norepinephrine excretion (NE24).
EPI24: Table 3 illustrates the relationships between norepinephrine excretion and depressive and anxiety symptoms. Model 1 reveals evidence of a trend between BMI and 24 hour epinephrine excretion (EPI24) (t = −1.82, b = −0.23, p = .073), such that higher BMI was associated with lower EPI24 excretion. No other Model 1 factor was related to epinephrine excretion, including BDI Score. Model 2 which examined the relationships between epinephrine and anxiety. Both BMI (t = −1.93, b = −0.23, p =.058) and anxiety were related to epinephrine excretion (t = 2.33, b = 0.28, p =.023). Addition of a Depression by Anxiety symptoms interaction term did not improve the model and was unrelated to 24 hour epinephrine excretion (EPI24) (p = .42) excretion. Addition of both BDI and anxiety scores revealed that 24 hour epinephrine excretion (EPI24) was related to both anxiety (t = 3.13, b = 0.61, p = .003) and depressive symptoms (t = −2.12, b = −0.41, p = .040).
Finally, all analyses were completed using unadjusted catecholamine concentrations and 24-hour total output. These analyses did not alter the pattern of the reported findings, with the key finding that 24 hour epinephrine excretion was related to anxiety remaining unaltered. Additionally, analysis with raw catecholamine concentration and 24 hour total output revealed that norepinephrine was still related to advancing age (raw concentration: t = 2.76, b = 0.30, p = .008; 24-hour volume: t = 2.55, b = 0.28, p = .013).
Discussion
In this study sample of men and women with untreated high blood pressure, anxiety symptoms were related to 24-hour epinephrine excretion. Advancing age and BMI were also associated with increased catecholamine excretion, but controlling for them did not diminish the effects related to anxiety. Importantly, age was still related to catecholamine excretion when raw catecholamine concentration and 24-hour total output was examined. Depressive symptoms were not directly related to catecholamine excretion.
Very few studies have examined the relationships between urinary catecholamines and anxiety, and the current study adds to this under-examined area of the literature by demonstrating associations between epinephrine and anxiety symptoms. This is in contrast to previous studies that have either revealed associations between anxiety symptoms and norepinephrine (19) or found no associations at all (49). Despite no longer being classified as an anxiety disorder, there is evidence that PTSD is associated with catecholamine excretion. Male veterans with PTSD had higher levels of 24-h urinary epinephrine and norepinephrine in contrast to male veterans without PTSD symptoms (50), with similar associations seen in females who have abuse-related PTSD in contrast to women without PTSD (51). 24-hour urine epinephrine excretion is a marker of sympathetic activity (as well as possible hyper-activation of the SNS), and the associations evident in the current study might provide a potential mechanism through which anxiety be a risk factor for CVD. However, it is important to note that our regression models reveal that the relationship between EPI24 and anxiety symptoms explains only 4% of variance, and as such the effect of anxiety symptoms on epinephrine excretion should be considered modest. Previous studies have demonstrated that anxiety is linked to accelerated atherosclerosis and CVD development (52, 53), with suggestions that this might be attributed to physical inactivity, chronic inflammation, hypertension, cardiac autonomic abnormalities and metabolic syndrome (51–57). However, the associations seen in the current study suggest that epinephrine and sympathetic activation might be an additional mechanism for consideration, however further work is needed to confirm this proposed mechanism.
Our study did not show evidence of a relationship between depressive symptoms and 24-hour urinary catecholamine excretion. However the majority of studies showing links between norepinephrine and depression have done so in samples where clinical levels of depression were observed (17, 18, 20, 58). Indirect evidence of a relationship between 24-hour urinary norepinephrine excretion and depression comes from a study of the alleles of the serotonin transporter gene (5-HTTLPR) (59). To our knowledge, only two studies have examined depressive symptoms and urinary catecholamines in a ‘non-depressed’ population, with equivocal results (19, 49). Splitting our sample into individuals with ‘high’ and ‘low’ depressive symptoms yielded no associations between depression and catecholamines. However, it is important to note that our sample was not recruited with a focus on studying clinical depression, and included participants displaying a relatively restricted range of depressive symptoms. Given that HPA axis hyperactivity is more likely to be present in more severely depressed patients than in mildly depressed patients or non-depressed controls (60), it is possible that if a broader range of depressive symptoms were observed in our study sample, including individuals with major depressive disorder, stronger and statistically significant relationships between norepinephrine and depressive symptoms might have been evident.
It is unclear why anxiety symptoms were related to urinary epinephrine but not urinary norepinephrine. The observation is consistent with the view that epinephrine is a “stress” hormone released by the adrenal medulla, whereas norepinephrine is released predominantly from sympathetic nerve terminals, where its active reuptake occurs, with only spillover from this source ultimately becoming present in urine (61). Epinephrine also acts more widely on beta-adrenergic receptors, and some types of anxiety can be managed effectively with beta-adrenergic antagonists. Interestingly, our study revealed that anxiety symptoms remained positively associated with EPI24 in the presence of depressive symptoms, while depressive symptoms became inversely associated with EPI24 in the presence of anxiety symptoms. Therefore it appears to indicate that the unique variance of the STAI is important, and may be reflective of the different biological mechanisms which are responsive for each psychological construct.
Alternatively, methodological considerations may explain these differences. Given that the concentration of plasma NE reflects sympathoadrenal activity, elevated NE would suggest elevated sympathoadrenal activation. However, if the antecubital vein is used as the site for the venous blood draw, plasma drawn from this site may capture local sympathetic forearm activity, which might not mirror cardiac or total body sympathetic activity levels (62). Further, elevated NE might result due to diminished NE clearance, sympathetic hyperactivity, or possibly both of these events (63). These confounding factors might explain the differences in the studies which have seen associations between depressive symptoms and catecholamine excretion (64).
Of tangential interest was the finding that increased epinephrine excretion was associated with lower BMI; such that thinner participants excreted a greater epinephrine volume. Similar observations have been seen in a study of middle aged participants with various components of metabolic syndrome (65). As epinephrine is stimulated by low glucose levels, it is possible that epinephrine might be elevated in individuals with lowered BMI who do not tend toward obesity related hyperglycemia. However, further confirmation in a larger sample is needed.
Limitations
Our sample was diverse in terms of both gender and race, but the generalizability may be affected because the study sample was selected for having elevated clinic blood pressure. Elevated BP and Hypertension are important risk factors for CVD development and is associated with hyper-activation of the SNS (66), and anxiety can produce symptoms and behavioral responses that elicit increases in blood pressure (67). Several studies have reported an association between hypertension and anxiety (61–71), while others have not (71–75). However, given that our sample consisted only of adults with untreated high blood pressure, this may have restricted the range of SNS activation and result in low statistical power. With this perspective in mind, the “trends” observed for an association between NE24 and both anxiety and depressive symptoms may be especially noteworthy. Although it is possible that our observations may reflect the associations of anxiety and depressive symptoms with catecholamine excretion in the context of high blood pressure, it is of note that our regression models accounted for the potentially confounding effects of blood pressure. However, while this statistical adjustment for elevated blood pressure helps to minimize the issue of external validity, it does so only to a limited extent. Another limitation may be sample size and inadequate statistical power to detect all potentially meaningful associations as statistically significant, which might explain the trends which are evident in our results, particularly regarding the relationships to norepinephrine. The study was cross sectional in nature, which does not allow us to make cause-effect inferences. Future work, and possible utilization of a prospective design, should aim to examine this. Given the methodological considerations with assessing urinary and plasma catecholamines, future studies may also which to assess both urinary and plasma catecholamines and their associations with each other, as well as with depressive and anxiety symptoms.
In conclusion, symptoms of anxiety, but not depressive symptoms, were associated with heightened urinary epinephrine excretion. These findings may indicate that the CVD risk associated with depression and anxiety may manifest through different biological pathways.
Acknowledgments
Source of Funding: This study was supported by Grant HL072390 awarded to Dr. Sherwood by the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, and grant M01-RR-30 from the General Clinical Research Center program, National Center for Research Resources, National Institutes of Health.
Acronyms
- CVD
Cardiovascular Disease
- ACS
Acute Coronary Syndrome
- MI
Myocardial Infarction
- CHD
Coronary Heart Disease
- ANS
Autonomic Nervous System
- CAD
Coronary Artery Disease
- NE
Norepinephrine
- NE24
24 hour urinary norepinephrine excretion
- EPI
Epinephrine
- EPI24
24 hour urinary epinephrine excretion
- SNS
Sympathetic Nervous System
- BDI
Beck Depression Inventory
- STAI
Spielberger State-Trait Anxiety Inventory
- SBP
Systolic Blood Pressure
- DBP
Diastolic Blood Pressure
- BMI
Body Mass Index
- HPLC
high-pressure liquid chromatography
- MHPG
3-Methoxy-4-hydroxyphenylglycol
- CES-D
Center for Epidemiologic Studies Depression Scale
- 5 HTTLPR
serotonin-transporter-linked polymorphic region
- HPA
Hypothalamic Pituitary Adrenal Axis
- PTSD
Post Traumatic Stress Disorder
Footnotes
Conflict of Interest: The authors declare there are no conflicts of interest
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watkins LL, Koch GG, Sherwood A, Blumenthal JA, Davidson JR, O’Connor C, Sketch MH. Association of anxiety and depression with all-cause mortality in individuals with coronary heart disease. Journal of the American Heart Association. 2013;2:e000068. doi: 10.1161/JAHA.112.000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakamura S, Kato K, Yoshida A, Fukuma N, Okumura Y, Ito H, Mizuno K. Prognostic value of depression, anxiety, and anger in hospitalized cardiovascular disease patients for predicting adverse cardiac outcomes. Am J Cardiol. 2013;111:1431–6. doi: 10.1016/j.amjcard.2013.01.293. [DOI] [PubMed] [Google Scholar]
- 4.Lichtman JH, Froelicher ES, Blumenthal JA, Carney RM, Doering LV, Frasure-Smith N, Freedland KE, Jaffe AS, Leifheit-Limson EC, Sheps DS, Vaccarino V, Wulsin L. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the american heart association. Circulation. 2014;129:1351–69. doi: 10.1161/CIR.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 5.Tully PJ, Baune BT. Comorbid anxiety disorders alter the association between cardiovascular diseases and depression: the German National Health Interview and Examination Survey. Social psychiatry and psychiatric epidemiology. 2014;49:681–91. doi: 10.1007/s00127-013-0784-x. [DOI] [PubMed] [Google Scholar]
- 6.Roest AM, Martens EJ, de Jonge P, Denollet J. Anxiety and Risk of Incident Coronary Heart Disease: A Meta-Analysis. Journal of the American College of Cardiology. 2010;56:31–46. doi: 10.1016/j.jacc.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 7.Roest AM, Martens EJ, Denollet J, De Jonge P. Prognostic association of anxiety post myocardial infarction with mortality and new cardiac events: A meta-analysis. Psychosomatic Medicine. 2010;72:561–9. doi: 10.1097/PSY.0b013e3181dbff97. [DOI] [PubMed] [Google Scholar]
- 8.Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychol Bull. 2005;131:261–300. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- 9.Munk PS, Isaksen K, Bronnick K, Kurz MW, Butt N, Larsen AI. Symptoms of anxiety and depression after percutaneous coronary intervention are associated with decreased heart rate variability, impaired endothelial function and increased inflammation. Int J Cardiol. 2012;158:171–6. doi: 10.1016/j.ijcard.2012.04.085. [DOI] [PubMed] [Google Scholar]
- 10.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder - Results from the National Comorbidity Survey Replication (NCS-R) Jama-Journal of the American Medical Association. 2003;289:3091–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 11.Garfield LD, Scherrer JF, Hauptman PJ, Freedland KE, Chrusciel T, Balasubramanian S, Carney RM, Newcomer JW, Owen R, Bucholz KK, Lustman PJ. Association of Anxiety Disorders and Depression With Incident Heart Failure. Psychosom Med. 2014;76:121–36. doi: 10.1097/PSY.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joynt KE, Whellan DJ, O’Connor CM. Depression and cardiovascular disease: mechanisms of interaction. Biological Psychiatry. 2003;54:241–61. doi: 10.1016/s0006-3223(03)00568-7. [DOI] [PubMed] [Google Scholar]
- 13.Carney RM, Freedland KE, Rich MW, Smith LJ, Jaffe AS. Ventricular tachycardia and psychiatric depression in patients with coronary artery disease. The American Journal of Medicine. 1993;95:21–8. doi: 10.1016/0002-9343(93)90228-h. [DOI] [PubMed] [Google Scholar]
- 14.Verrier RL, Dickerson LW, Nearing BD. Behavioral states and sudden cardiac death. PACE - Pacing and Clinical Electrophysiology. 1992;15:1381–93. [PubMed] [Google Scholar]
- 15.Podrid PJ, Fuchs T, Candinas R. Role of the sympathetic nervous system in the genesis of ventricular arrhythmia. Circulation. 1990;82:I101–13. [PubMed] [Google Scholar]
- 16.Anfossi G, Trovati M. Role of catecholamines in platelet function: Pathophysiological and clinical significance. European Journal of Clinical Investigation. 1996;26:351–70. doi: 10.1046/j.1365-2362.1996.150293.x. [DOI] [PubMed] [Google Scholar]
- 17.Roy A, Pickar D, Douillet P, Karoum F, Linnoila M. Urinary monoamines and monoamine metabolites in subtypes of unipolar depressive disorder and normal controls. Psychological Medicine. 1986;16:541–6. doi: 10.1017/s0033291700010308. [DOI] [PubMed] [Google Scholar]
- 18.Grossman F, Potter WZ. Catecholamines in depression: A cumulative study of urinary norepinephrine and its major metabolites in unipolar and bipolar depressed patients versus healthy volunteers at the NIMH. Psychiatry Research. 1999;87:21–7. doi: 10.1016/s0165-1781(99)00055-4. [DOI] [PubMed] [Google Scholar]
- 19.Hughes JW, Watkins L, Blumenthal JA, Kuhn C, Sherwood A. Depression and anxiety symptoms are related to increased 24-hour urinary norepinephrine excretion among healthy middle-aged women. Journal of Psychosomatic Research. 2004;57:351–8. doi: 10.1016/j.jpsychores.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 20.Otte C, Neylan TC, Pipkin SS, Browner WS, Whooley MA. Depressive symptoms and 24-hour urinary norepinephrine excretion levels in patients with coronary disease: findings from the Heart and Soul Study. Am J Psychiatry. 2005;162:2131–45. doi: 10.1176/appi.ajp.162.11.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Light KC, Kothandapani RV, Allen MT. Enhanced cardiovascular and catecholamine responses in women with depressive symptoms. International Journal of Psychophysiology. 1998;28:151–66. doi: 10.1016/s0167-8760(97)00093-7. [DOI] [PubMed] [Google Scholar]
- 22.Roy A, Pickar D, Linnoila M, Potter WZ. Plasma norepinephrine level in affective disorders. Relationship to melancholia. Archives of General Psychiatry. 1985;42:1181–5. doi: 10.1001/archpsyc.1985.01790350055010. [DOI] [PubMed] [Google Scholar]
- 23.Carney RM, Freedland KE, Veith RC, Cryer PE, Skala JA, Lynch T, Jaffe AS. Major depression, heart rate, and plasma norepinephrine in patients with coronary heart disease. Biological Psychiatry. 1999;45:451–63. doi: 10.1016/s0006-3223(98)00049-3. [DOI] [PubMed] [Google Scholar]
- 24.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:591–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 25.Meyer T, Buss U, Herrmann-Lingen C. Role of cardiac disease severity in the predictive value of anxiety for all-cause mortality. Psychosom Med. 2010;72:1–15. doi: 10.1097/PSY.0b013e3181c64fc0. [DOI] [PubMed] [Google Scholar]
- 26.Friedman BH, Thayer JF. Autonomic balance revisited: panic anxiety and heart rate variability. J Psychosom Res. 1998;44:131–51. doi: 10.1016/s0022-3999(97)00202-x. [DOI] [PubMed] [Google Scholar]
- 27.Watkins LL, Blumenthal JA, Carney RM. Association of anxiety with reduced baroreflex cardiac control in patients after acute myocardial infarction. Am Heart J. 2002;143:461–6. doi: 10.1067/mhj.2002.120404. [DOI] [PubMed] [Google Scholar]
- 28.Watkins LL, Grossman P, Krishnan R, Sherwood A. Anxiety and vagal control of heart rate. Psychosom Med. 1998;60:491–502. doi: 10.1097/00006842-199807000-00018. [DOI] [PubMed] [Google Scholar]
- 29.Alvarenga ME, Richards JC, Lambert G, Esler MD. Psychophysiological mechanisms in panic disorder: a correlative analysis of noradrenaline spillover, neuronal noradrenaline reuptake, power spectral analysis of heart rate variability, and psychological variables. Psychosom Med. 2006;68:1–16. doi: 10.1097/01.psy.0000195872.00987.db. [DOI] [PubMed] [Google Scholar]
- 30.Nesse RM, Cameron OG, Curtis GC, McCann DS, Huber-Smith MJ. Adrenergic function in patients with panic anxiety. Archives of General Psychiatry. 1984;41:771–6. doi: 10.1001/archpsyc.1984.01790190045005. [DOI] [PubMed] [Google Scholar]
- 31.Dimsdale JE. What does heart disease have to do with anxiety? J Am Coll Cardiol. 2010;56:41–8. doi: 10.1016/j.jacc.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 32.Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev. 2010;90:511–57. doi: 10.1152/physrev.00007.2009. [DOI] [PubMed] [Google Scholar]
- 33.Ziegler MG, Elayan H, Milic M, Sun P, Gharaibeh M. Epinephrine and the metabolic syndrome. Curr Hypertens Rep. 2012;14:1–7. doi: 10.1007/s11906-011-0243-6. [DOI] [PubMed] [Google Scholar]
- 34.Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. Modern problems of pharmacopsychiatry. 1974;7:151–69. doi: 10.1159/000395074. [DOI] [PubMed] [Google Scholar]
- 35.Spielberger CDGR, Luchene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologist Press; 1970. [Google Scholar]
- 36.Hinderliter AL, Routledge FS, Blumenthal JA, Koch G, Hussey MA, Wohlgemuth WK, Sherwood A. Reproducibility of blood pressure dipping: relation to day-to-day variability in sleep quality. Journal of the American Society of Hypertension : JASH. 2013;7:431–9. doi: 10.1016/j.jash.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sherwood A, Routledge FS, Wohlgemuth WK, Hinderliter AL, Kuhn CM, Blumenthal JA. Blood Pressure Dipping: Ethnicity, Sleep Quality, and Sympathetic Nervous System Activity. American Journal of Hypertension. 2011;24:981–8. doi: 10.1038/ajh.2011.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8:71–100. [Google Scholar]
- 39.Carney RM, Rich MW, Tevelde A, Saini J, Clark K, Jaffe AS. Major depressive disorder in coronary artery disease. The American Journal of Cardiology. 1987;60:1271–5. doi: 10.1016/0002-9149(87)90607-2. [DOI] [PubMed] [Google Scholar]
- 40.Carney RM, Blumenthal JA, Freedland KE, Youngblood M, Veith RC, Burg MM, Cornell C, Saab PG, Kaufmann PG, Czajkowski SM, Jaffe AS. Depression and late mortality after myocardial infarction in the Enhancing Recovery in Coronary Heart Disease (ENRICHD) study. Psychosom Med. 2004;66:461–74. doi: 10.1097/01.psy.0000133362.75075.a6. [DOI] [PubMed] [Google Scholar]
- 41.Lesperance F, Frasure-Smith N, Talajic M, Bourassa MG. Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation. 2002;105:1041–53. doi: 10.1161/hc0902.104707. [DOI] [PubMed] [Google Scholar]
- 42.Wassertheil-Smoller S, Applegate WB, Berge K, Chang CJ, Davis BR, Grimm R, Jr, Kostis J, Pressel S, Schron E. Change in depression as a precursor of cardiovascular events. SHEP Cooperative Research Group (Systoloc Hypertension in the elderly) Arch Intern Med. 1996;156:551–61. [PubMed] [Google Scholar]
- 43.Sherwood A, Blumenthal JA, Trivedi R, Johnson KS, O’Connor CM, Adams KF, Jr, Dupree CS, Waugh RA, Bensimhon DR, Gaulden L, Christenson RH, Koch GG, Hinderliter AL. Relationship of depression to death or hospitalization in patients with heart failure. Arch Intern Med. 2007;167:361–73. doi: 10.1001/archinte.167.4.367. [DOI] [PubMed] [Google Scholar]
- 44.Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories-IA and-II in Psychiatric Outpatients. Journal of personality assessment. 1996;67:581–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 45.Spielberger CDJG, Russell S, Crane RS. Assessment of Anger: The State-Trait Anger Scale. In: Butcher JNS, CD, editors. Advances in Personality Assessment. Routledge; 1983. [Google Scholar]
- 46.Boomsma F, Alberts G, van Eijk L, Man in ‘t Veld AJ, Schalekamp MA. Optimal collection and storage conditions for catecholamine measurements in human plasma and urine. Clin Chem. 1993;39:2501–8. [PubMed] [Google Scholar]
- 47.White IR, Brunner EJ, Barron JL. A comparison of overnight and 24 hour collection to measure urinary catecholamines. Journal of clinical epidemiology. 1995;48:261–7. doi: 10.1016/0895-4356(94)00127-c. [DOI] [PubMed] [Google Scholar]
- 48.Masi CM, Rickett EM, Hawkley LC, Cacioppo JT. Gender and ethnic differences in urinary stress hormones: the population-based Chicago Health, Aging, and Social Relations Study. Journal of Applied Physiology. 2004;97:941–7. doi: 10.1152/japplphysiol.002562004. [DOI] [PubMed] [Google Scholar]
- 49.Castro-Diehl C, Roux AVD, Seeman T, Shea S, Shrager S, Tadros S. Associations of socioeconomic and psychosocial factors with urinary measures of cortisol and catecholamines in the Multi-Ethnic Study of Atherosclerosis (MESA) Psychoneuroendocrinology. 2014;41:131–41. doi: 10.1016/j.psyneuen.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yehuda R, Southwick S, Giller EL, Ma X, Mason JW. Urinary catecholamine excretion and severity of PTSD symptoms in Vietnam combat veterans. Journal of Nervous and Mental Disease. 1992;180:321–5. doi: 10.1097/00005053-199205000-00006. [DOI] [PubMed] [Google Scholar]
- 51.Lemieux AM, Coe CL. Abuse-related posttraumatic stress disorder: Evidence for chronic neuroendocrine activation in women. Psychosomatic Medicine. 1995;57:101–15. doi: 10.1097/00006842-199503000-00002. [DOI] [PubMed] [Google Scholar]
- 52.Paterniti S, Zureik M, Ducimetière P, Touboul PJ, Fève JM, Alpérovitch A. Sustained anxiety and 4-year progression of carotid atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2001;21:131–41. doi: 10.1161/01.atv.21.1.136. [DOI] [PubMed] [Google Scholar]
- 53.Seldenrijk A, Vogelzangs N, van Hout HP, van Marwijk HW, Diamant M, Penninx BW. Depressive and anxiety disorders and risk of subclinical atherosclerosis Findings from the Netherlands Study of Depression and Anxiety (NESDA) J Psychosom Res. 2010;69:201–10. doi: 10.1016/j.jpsychores.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 54.Piccirillo G, Elvira S, Bucca C, Viola E, Cacciafesta M, Marigliano V. Abnormal passive head-up tilt test in subjects with symptoms of anxiety power spectral analysis study of heart rate and blood pressure. International Journal of Cardiology. 1997;60:121–31. doi: 10.1016/s0167-5273(97)00088-0. [DOI] [PubMed] [Google Scholar]
- 55.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2191–217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- 56.Shih M, Hootman JM, Kruger J, Helmick CG. Physical Activity in Men and Women with Arthritis: National Health Interview Survey, 2002. American Journal of Preventive Medicine. 2006;30:381–93. doi: 10.1016/j.amepre.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 57.Engum A. The role of depression and anxiety in onset of diabetes in a large population-based study. Journal of Psychosomatic Research. 2007;62:31–8. doi: 10.1016/j.jpsychores.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 58.Hamer M, Tanaka G, Okamura H, Tsuda A, Steptoe A. The effects of depressive symptoms on cardiovascular and catecholamine responses to the induction of depressive mood. Biological Psychology. 2007;74:21–5. doi: 10.1016/j.biopsycho.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 59.Otte C, McCaffery J, Ali S, Whooley MA. Association of a serotonin transporter polymorphism (5-HTTLPR) with depression, perceived stress, and norepinephrine in patients with coronary disease: the Heart and Soul Study. Am J Psychiatry. 2007;164:1371–84. doi: 10.1176/appi.ajp.2007.06101617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends in Neurosciences. 2008;31:461–8. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 61.Grouzmann E, Lamine F. Determination of catecholamines in plasma and urine. Best practice & research Clinical endocrinology & metabolism. 2013;27:711–23. doi: 10.1016/j.beem.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 62.Veith RC, Best JD, Halter JB. Dose-dependent suppression of norepinephrine appearance rate in plasma by clonidine in man. The Journal of clinical endocrinology and metabolism. 1984;59:151–5. doi: 10.1210/jcem-59-1-151. [DOI] [PubMed] [Google Scholar]
- 63.Esler M, Jennings G, Korner P, Willett I, Dudley F, Hasking G, Anderson W, Lambert G. Assessment of human sympathetic nervous system activity from measurements of norepinephrine turnover. Hypertension. 1988;11:1–20. doi: 10.1161/01.hyp.11.1.3. [DOI] [PubMed] [Google Scholar]
- 64.Carney RM, Freedland KE, Veith RC. Depression, the autonomic nervous system, and coronary heart disease. Psychosom Med. 2005;67(Suppl 1):S21–33. doi: 10.1097/01.psy.0000162254.61556.d5. [DOI] [PubMed] [Google Scholar]
- 65.Lee ZS, Critchley JA, Tomlinson B, Young RP, Thomas GN, Cockram CS, Chan TY, Chan JC. Urinary epinephrine and norepinephrine interrelations with obesity, insulin, and the metabolic syndrome in Hong Kong Chinese. Metabolism. 2001;50:131–43. doi: 10.1053/meta.2001.19502. [DOI] [PubMed] [Google Scholar]
- 66.Palatini P. Sympathetic overactivity in hypertension: A risk factor for cardiovascular disease. Current Science Inc. 2001;3:S3–S9. doi: 10.1007/s11906-001-0065-z. [DOI] [PubMed] [Google Scholar]
- 67.Player MS, Peterson LE. Anxiety disorders, hypertension, and cardiovascular risk: a review. International journal of psychiatry in medicine. 2011;41:361–77. doi: 10.2190/PM.41.4.f. [DOI] [PubMed] [Google Scholar]
- 68.Cheung BM, Au T, Chan S, Lam C, Lau S, Lee R, Lee S, Lo W, Sin E, Tang M, Tsang H. The relationship between hypertension and anxiety or depression in Hong Kong Chinese. Experimental and clinical cardiology. 2005;10:21–4. [PMC free article] [PubMed] [Google Scholar]
- 69.Carroll D, Phillips AC, Gale CR, Batty GD. Generalized anxiety and major depressive disorders, their comorbidity and hypertension in middle-aged men. Psychosom Med. 2010;72:11–9. doi: 10.1097/PSY.0b013e3181c4fca1. [DOI] [PubMed] [Google Scholar]
- 70.Johannessen L, Strudsholm U, Foldager L, Munk-Jorgensen P. Increased risk of hypertension in patients with bipolar disorder and patients with anxiety compared to background population and patients with schizophrenia. Journal of affective disorders. 2006;95:11–7. doi: 10.1016/j.jad.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 71.Wei TM, Wang L. Anxiety symptoms in patients with hypertension: a community-based study. International journal of psychiatry in medicine. 2006;36:311–22. doi: 10.2190/5LX9-D3BH-FUA3-PQF0. [DOI] [PubMed] [Google Scholar]
- 72.Hildrum B, Mykletun A, Holmen J, Dahl AA. Effect of anxiety and depression on blood pressure: 11-year longitudinal population study. The British journal of psychiatry : the journal of mental science. 2008;193:101–13. doi: 10.1192/bjp.bp.107.045013. [DOI] [PubMed] [Google Scholar]
- 73.Hildrum B, Mykletun A, Stordal E, Bjelland I, Dahl AA, Holmen J. Association of low blood pressure with anxiety and depression: the Nord-Trondelag Health Study. J Epidemiol Community Health. 2007;61:51–8. doi: 10.1136/jech.2005.044966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Friedman R, Schwartz JE, Schnall PL, Landsbergis PA, Pieper C, Gerin W, Pickering TG. Psychological variables in hypertension: relationship to casual or ambulatory blood pressure in men. Psychosom Med. 2001;63:11–31. doi: 10.1097/00006842-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 75.Yan LL, Liu K, Matthews KA, Daviglus ML, Ferguson TF, Kiefe CI. Psychosocial factors and risk of hypertension: the Coronary Artery Risk Development in Young Adults (CARDIA) study. JAMA : the journal of the American Medical Association. 2003;290:2131–48. doi: 10.1001/jama.290.16.2138. [DOI] [PubMed] [Google Scholar]