Abstract
Aim
This study evaluates the effect of dapagliflozin, a SGLT2 inhibitor, on fluid/electrolyte balance and its effect on urea transporter-A1 (UT-A1), aquaporin-2 (AQP2) and Na-K-2Cl cotransporter (NKCC2) protein abundance in diabetic rats.
Methods
Diabetes mellitus was induced by injection of streptozotocin into the tail vein. Serum Na+, K+, Cl− concentration, urine Na+, K+, Cl− excretion, blood glucose, urine glucose excretion, urine volume, urine osmolality and urine urea excretion were analyzed after the administration of dapagliflozin. UT-A1, AQP2 and NKCC2 proteins were detected by western blot.
Results
Dapagliflozin treatment decreased blood glucose by 38% at day 7 and 47% at day 14 and increased the urinary glucose excretion rate compared with the untreated diabetic animals. Increased 24-h urine volume, decreased urine osmolality and hyponatremia, hypokalemia and hypochloremia observed in diabetic rats were attenuated by dapagliflozin treatment. Western analysis showed that UT-A1, AQP2 and NKCC2 proteins are up-regulated in DM rats over control rats; dapagliflozin treatment results in a further increase in IM tip UT-A1 protein abundance by 42% at day 7 and 46% at day 14, but it did not affect the DM-induced up-regulation of AQP2 and NKCC2 proteins.
Conclusion
Dapagliflozin treatment augmented the compensatory changes in medullary transport proteins in DM. These changes will tend to conserve solute and water even with persistent glycosuria. Therefore, diabetic rats treated with dapagliflozin have a mild osmotic diuresis compared to non-diabetic animals, but this does not result in an electrolyte disorder or significant volume depletion.
Keywords: diabetic mellitus, kidney, sodium glucose co-transporter 2, urine concentration
Introduction
Diabetes mellitus is a major health problem affecting people worldwide. Several lines of evidence suggest that there is increased tubular Na+-glucose reabsorption in uncontrolled diabetes, due to the increase in filtered glucose load and the increased expression of sodium glucose co-transporter 1 (SGLT1), SGLT2 and glucose transporter isotype 2 (GLUT2) transporters in proximal tubule cells[1]. So there is a growing interest in SGLT inhibitors to manage diabetic patients by inhibiting SGLTs in the kidney[2, 3]. Phlorizin, a nonspecific SGLT inhibitor, was suggested to be effective in controlling blood glucose and have a beneficial role in treating diabetic nephropathy in diabetic animal[4, 5]. However, oral phlorizin does meet therapeutic requirements as it blocks gastrointestinal absorption of glucose and thus produces osmotic diarrhea. SGLT2 reabsorbs 90% of filtered glucose load in the proximal tubule, therefore, specific SGLT2 inhibitors, such as dapagliflozin or canagliflozin, were produced to lower blood glucose in diabetic patients. The effectiveness of SGLT2 inhibitors in decreasing blood glucose in diabetic patients has been proven in phase I to III clinical trials[6, 7]. Some animal experiments also suggested that SGLT2 inhibitors were effective in preventing diabetic vascular complications including diabetic nephropathy[8, 9]. However, the increased urinary glucose excretion associated with SGLT2 inhibition would result in an osmotic diuresis that could be associated with a reduction in intravascular volume and electrolyte disorder. Thus, one point of concern in the adoption of SGLT2 inhibitor therapy is the possibility of osmotic diuresis and its effect on fluid/electrolyte balance in patients with T2DM. In clinical trials of dapagliflozin in patients with T2DM, although the number of events of hypovolemia was elevated in the dapagliflozin group versus the placebo group, the overall rates of volume-related events were low, and serum electrolytes were mostly unchanged[10]. This suggests the hypothesis that the degree of polyuria is mild due to compensatory changes that occur in the kidney during urine concentration to maintain fluid/electrolyte balance, despite persistent glycosuria resulting from dapagliflozin treatment.
The renal medulla is the primary site of urine concentration. There are three major transport proteins in the renal medulla that work in combination to produce an osmotic gradient that is necessary to produce concentrated urine: the UT-A1 urea transporter, the aquaporin-2 (AQP2) water channel, and the Na-K-2Cl cotransporter NKCC2. In the outer medulla, NaCl is the main constituent of the osmotic gradient and NKCC2, located in the medullary thick ascending limb, is chiefly responsible for the absorption of NaCl. The urea transporters UT-A1 and UT-A3 are present in the inner medulla and are responsible for the reabsorption of urea to increase the hypertonicity of the interstitium. AQP2, located in the apical plasma membrane of the inner medulla, is responsible for absorption of water. Our previous study found that the abundance of the three major medullary transport proteins involved in the urinary concentrating mechanism increased in uncontrolled diabetes mellitus to prevent a progressive decline in urinary concentrating ability despite the continuing osmotic diuresis [11]. However, it is not clear whether hyperglycemia or glycosuria play a key role in the DM-induced increases of the medullary transporter protein abundances. SGLT2 inhibitors decrease blood glucose by increasing urinary glucose excretion, resulting in the disassociation between blood glucose and urinary glucose level. Therefore, how SGLT2 inhibitor treatment may affect medullary transport protein expression is not clear. The present study evaluated the effects of an SGLT2 inhibitor, dapagliflozin, on plasma glucose, urinary glucose excretion, and parameters reflective of fluid/electrolyte balance, as well as on the expression of three major transport proteins UT-A1, AQP2, and NKCC2.
Methods
Animals
All animal protocols were approved by the Emory University Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA), weighing 150–200 g, received free access to water and standard rat chow (Teklad diet # 5001) containing 23% protein. Rats were made diabetic by injection of streptozotocin (STZ; 60 mg/kg) into the tail vein. Hyperglycemia was verified 24–48 h after injection using a Lifescan Ultra II glucometer. Dapagliflozin (1 mg/kg/day; Selleck Chemicals, Houston, TX) was started 2 days after STZ injection. Dapagliflozin was dissolved in 1% hydroxypropyl methylcellulose. The dose per animal was delivered in 2 ml of this solution by gavage for 7 days or 14 days.
Sample preparation
One day before sacrifice, a 24-h urine collection was obtained to measure urinary volume, osmolality and urinary urea excretion. Rats were killed by decapitation and blood was collected and assayed for glucose, blood urea, Na+, K+ and Cl− levels. Kidneys were removed and dissected into outer medulla (OM), base of the inner medulla (IM), and tip of the IM. Tissues were placed into ice-cold isolation buffer (10 mM triethanolamine, 250 mM sucrose, pH 7.6, 1 μg/ml leupeptin, 40 μg/ml PMSF) and homogenized with glass homogenizers. SDS was added to a final concentration of 1%, and the samples were sheared with a 25-gauge needle. Homogenates were centrifuged at 8,000xg for 15 min, and the protein in the supernatant fractions was measured by a modified Lowry method (DC Protein Assay Kit; Bio-Rad, Hercules, CA).
Western blot analysis
Proteins were size separated by SDS-PAGE by using 10 or 12.5% gels and then electroblotted to polyvinylidenedifluoride membranes (Imobilon, Millipore, Bedford, MA). Blots were blocked with 5% nonfat dry milk in Tris-buffered saline (TBS; 20 mM Tris·HCl, 0.5 M NaCl, pH 7.5) at room temperature for 1 h and then incubated with primary antibody overnight at 4°C. The primary antibodies were to the following proteins: UT-A1[12], NKCC2 [13, 14] and AQP2 [15, 16]. Blots were washed three times in TBS with 0.5% Tween-20 (TBS/Tween) and then incubated with Alexa Fluor 680-linked anti-rabbit IgG (Molecular Probes, Eugene, OR). Blots were washed three times with TBS/Tween, and then the bound secondary antibody was visualized using infrared detection with the Licor Odyssey protein analysis system. Blots were stained with Ponceau S for total protein and scanned. Image J (NIH) was used to quantitate lane protein density. We normalized each protein of interest to the loading control [17]. The amounts of protein loaded per well are as follows: 20μg for UT-A1, 30μg for AQP2 and 30μg for NKCC2.
Statistics
All data are presented as mean percent of control ± S.E. To test more than two groups, we used an ANOVA, followed by Fisher’s least significant difference (protected t-test) to determine statistical differences. The criterion for statistical significance is P<0.05.
Results
Animal parameters
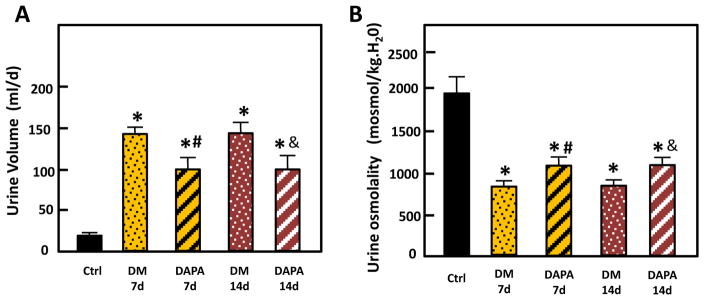
The weight gain of control rats was significantly greater than the weight gain of untreated diabetic rats and dapagliflozin-treated rats (7-day control: 87±11g vs untreated diabetic: 42±22g vs dapagliflozin treatment: 38±14g). Blood and urinary parameters are provided in Table 1. Blood glucose was significantly increased in diabetic over control rats, and dapagliflozin treatment decreased blood glucose significantly both at day 7 and day 14 when compared with untreated diabetic rats. The difference in 24h urinary glucose excretion amount between dapagliflozin treated and untreated diabetic groups was not statistically significant, but dapagliflozin treatment resulted in an increased urinary glucose excretion rate expressed as 24h urinary glucose (g)/blood glucose (blood glucose concentration × blood volume[18]) compared with the rate in untreated diabetic rats. Both untreated diabetic rats and dapagliflozin treatment rats showed polyuria and decreased urine osmolality compared with control rats, but when comparing with untreated diabetic rats, dapagliflozin treatment decreased the 24h-urine volume by 29% at day 7 and 30% at day 14, and increased urine osmolality by 29% at day 7 and 23% at day 14 (Fig. 1). Untreated, diabetic rats showed hyponatremia, hypochloremia and hypokalemia at both day 7 and day 14. With dapagliflozin treatment, blood Na+ and Cl− levels were not changed at either time point compared with control rats. The blood K+ level decreased at day 7 but increased to about the level of control rats at day 14. Comparing with control rats, all diabetic rats showed increased urinary excretion of Na+, K+ and Cl−. Dapagliflozin treatment appeared to decrease urinary excretion of these electrolytes when compared to untreated diabetic rats; however, these changes did not reach statistical significance. Diabetes produced a significant increase in urine urea excretion comparing with the control group, but dapagliflozin treatment decreased urinary urea excretion when compared to untreated diabetic rats.
Table 1.
In vivo blood and urinary parameters
| Control-7d1 | DM-7d2 | DAPA-7d3 | DM-14d4 | DAPA-14d5 | |
|---|---|---|---|---|---|
| Blood glucose concentration (mg/dl) | 177±10 | 469±19* | 291±33*# | 498±17* | 262±30*& |
| Urine glucose concentration (mg/dl) | 108±8 | 8055±766* | 8387±491* | 9740±174* | 8954±638* |
| Urine glucose excretion amount (g/d/kg body wt) | 0.06±0.01 | 38.5±2.8* | 31.3±3.3* | 46.7±3.9* | 39.3±1.9* |
| Urine glucose excretion rate (mg/min) | 0.01±0.002 | 7.3±1.0* | 6.0±0.7* | 8.9±1* | 7.0±0.6* |
| 24h urine glucose (g)/blood glucose content (g) 6 | 0.6±0.1 | 122.9±24.6* | 201.5±23.9*# | 148.8±15.5* | 239.0±29.6*& |
| Body weight (g) | 315.6±9.0 | 268.7±6.7 * | 263.9±12.5 * | 281.5±10.9* | 282.6±5.3* |
| 24h urine volume (ml) | 17.6±3.4 | 142.1±13.8* | 100.9±15.5*# | 143.5±11.6 * | 99.8±13.4 *& |
| Urine osmolality (mosmol/kg H20) | 1943±216 | 851±30* | 1094±92*# | 913±47* | 1122±47*& |
| Serum Na+ (mmol/L) | 138.9±0.5 | 133.2±0.3* | 138.8±2.1# | 130.6±1.7* | 138.7±1.0& |
| Serum Cl−(mmol/L) | 103.7±0.5 | 98.2±0.6* | 104.4±1.9# | 95.4±1.3* | 104.4±1.1& |
| Serum K+ (mmol/L) | 6.5±0.3 | 5.4±0.2* | 5.6±0.2* | 5.4±0.1* | 6.1±0.2& |
| Urine Na+ excretion amount (mmol/d/kg body wt) | 51.6±3.8 | 144.2±12.2* | 115.7±14.5* | 132.0±13.4* | 117.7±9.3* |
| Urine K+ excretion amount (mmol/d/kg body wt) | 103.6±7.5 | 319.3±39.2* | 263.4±27.4* | 305.8±24.9* | 216.1±18.9* |
| Urine Cl− excretion amount (mmol/d/kg body wt) | 208.0±31.0 | 288.1±44.7* | 264.8±49.2* | 268.6±16.9* | 259.4±27.3* |
| Blood urea (mg/L) | 5.4±0.9 | 5.4±0.5 | 5.0±1.0 | 4.7±1.0 | 5.2±0.4 |
| Urine urea excretion amount (mg/d/kg body wt) | 15.3±1.1 | 73.9±6.8* | 56.1±4.5*# | 59.2±5.0* | 43.4±4.5*& |
|
| |||||
| N | 5 | 10 | 10 | 10 | 10 |
Control-7d: control rat values were measured at day 7;
DM-7d: untreated diabetic rat values were measured at day 7;
DAPA-7d: dapagliflozin treated diabetic rat values were measured at day 7;
DM-14d: untreated diabetic rat values were measured at day 14;
DAPA-14d: dapagliflozin treated diabetic rat values were measured at day 14.
P<0.05, statistical significance relative to control values, determined by unpaired Student’s t-test.
P<0.05, statistical significance between DM-7d and DAPA-7d groups as determined by t-test.
P<0.05, statistical significance between DM-14d and DAPA-14d groups as determined by t-test.
Blood glucose content= blood glucose mg/dl×blood volume (dl); rat blood volume= 0.06×BW+0.77
Figure 1.
24-h urine volume (A) and urine osmolality (B) from control, untreated diabetic and dapagliflozin treated diabetic rats. Ctrl: control rats; DM 7d: untreated diabetic rats at 7 days after STZ injection; DAPA 7d: diabetic rats treated with DAPA for 7d; DM 14d: untreated diabetic rats at 14 days; DAPA 14d: diabetic rats treated with DAPA for 14d. Bar graphs: results of 2 combined experiments. The experimental conditions were performed two times with five animals per experimental group in each cohort. In total, 10 animals per experimental group were analyzed. *=P < 0.05 vs control; # =P < 0.05 vs DM-7d; &=P < 0.05 vs DM-14d.
Effects on concentrating mechanism transport proteins
Administration of dapagliflozin further increased the compensatory up-regulation of UT-A1 normally observed with DM
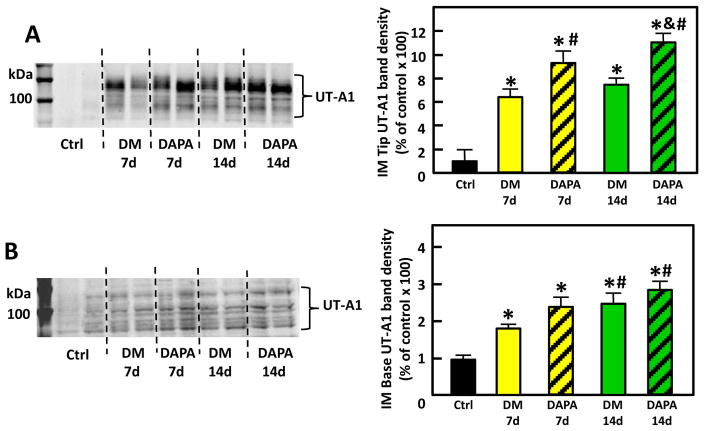
UT-A1 is expressed in both IM tip and base and has two glycoprotein forms of molecular weights 117 and 97-kDa. The total protein abundance of UT-A1 was significantly increased in the IM tip of untreated DM rats at both day 7 and day 14. UT-A1 increased to 646% of control at day 7 and 734% of control at day 14 (p<0.05), consistent with previous findings[11]. Dapagliflozin treatment further increased UT-A1 expression by 42% and 46% at day 7 and day 14 in IM tip, respectively (p<0.05). In the IM base, UT-A1 protein abundance increased to 164% of control at day 7 and 295% of control at day 14 after STZ induction of DM. Dapagliflozin treatment also appeared to further increase IM base UT-A1 expression by17% and 9% at day 7 and day 14; however, these changes did not reach statistical significance (Fig. 2).
Figure 2.
Representative Western blot of IM tip (A) and IM base (B) from control, untreated (Ctrl) diabetic (DM) and dapagliflozin-treated diabetic (DAPA) rats probed for UT-A1. The blot is from a single gel. Dashed lines were added only to aid in identification of sample groups. Ctrl: control rats; DM 7d: untreated diabetic rats at 7 days after STZ injection; DAPA 7d: diabetic rats treated with DAPA for 7d; DM 14d: untreated diabetic rats for 14 days; DAPA 14d: diabetic rats treated with DAPA for 14 days. Bar graphs: densitometry results of 2 combined experiments presented as percent of control. The experimental conditions were performed two times with five animals per experimental group in each cohort. In total, 10 animals per experimental group were analyzed.*=P < 0.05 vs control; # =P < 0.05 vs DM-7d; &=P < 0.05 vs DM-14d.
Dapagliflozin did not alter the DM-induced up-regulation of AQP2 and NKCC2
AQP2
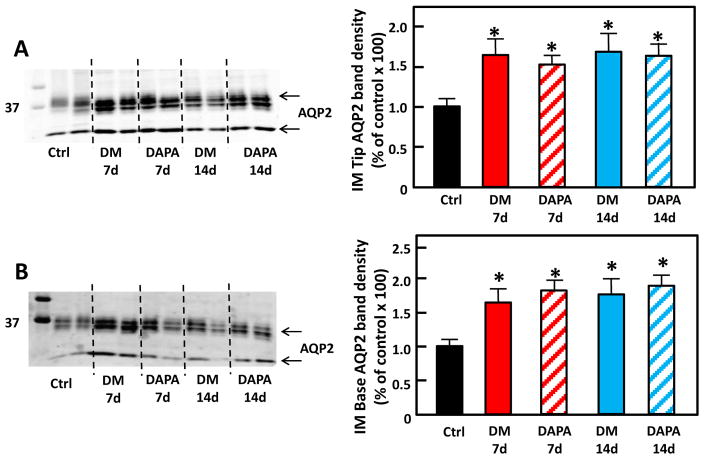
AQP2, the water channel, exists as glycosylated (35–50 kDa) and unglycosylated (29 kDa) forms. The total protein abundance of AQP2 significantly increased in both the IM tip and base (Fig. 3) in untreated DM, consistent with previous findings[11]. Dapagliflozin treatment did not interrupt the DM-induced up-regulation of AQP2 protein abundance in either the IM tip or base when compared with untreated diabetic rats.
Figure 3.
Representative Western blot of IM tip (A) and IM base (B) from control, untreated diabetic and dapagliflozin treated diabetic rats probed for AQP2. The blot is from a single gel. Dashed lines were added only to aid in identification of sample groups. Ctrl: control rats; DM 7d: untreated diabetic rats at 7 days after STZ injection; DAPA 7d: diabetic rats treated with DAPA for 7d; DM 14d: untreated diabetic rats for 14 days; DAPA 14d: diabetic rats treated with DAPA for 14 days. Bar graphs: densitometry results of 2 combined experiments presented as percent of control. The experimental conditions were performed two times with five animals per experimental group in each cohort. In total, 10 animals per experimental group were analyzed.*=P < 0.05 vs control.
NKCC2
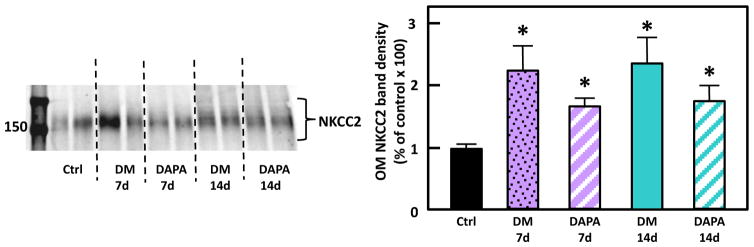
NKCC2 protein abundance increased (226%) in the untreated DM rats compared with control rats, consistent with previous findings[11]. Although there appeared to be a trend toward decrease of NKCC2 with dapagliflozin treatment of the DM rats, the decrease in NKCC2 in the OM of treated rats did not reach statistical significance (p= 0.19 at 7d and 0.21 at 14 d) (Fig. 4).
Figure 4.
Representative Western blot of OM from control, untreated diabetic and dapagliflozin treated diabetic rats probed for NKCC2. The blot is from a single gel. Dashed lines were added only to aid in identification of sample groups. Ctrl: control rats; DM 7d: untreated diabetic rats at 7 days after STZ injection; DAPA 7d: diabetic rats treated with DAPA for 7d; DM 14d: untreated diabetic rats for 14 days; DAPA 14d: diabetic rats treated with DAPA for 14 days. Bar graphs: densitometry results of 2 combined experiments presented as percent of control. The experimental conditions were performed two times with five animals per experimental group in each cohort. In total, 10 animals per experimental group were analyzed.*=P < 0.05 vs control.
Discussion
The goal of this study was to determine how SGLT2 inhibition affects fluid/electrolyte balance in DM with compromised urine concentrating mechanism. Using STZ-induced diabetic rats, we found that: 1) Dapagliflozin treatment decreased blood glucose and increased the urinary glucose excretion rate in diabetic rats; 2) Dapagliflozin treatment alleviated the polyuria observed in untreated diabetes; 3) Untreated DM is associated with hyponatremia, hypochloremia and hypokalemia, and dapagliflozin treatment showed a tendency to reverse these electrolyte disorders; and 4) UT-A1, AQP2 and NKCC2 proteins were upregulated in DM rats, and dapagliflozin treatment further increased UT-A1 protein abundance but did not affect the DM-induced up-regulation of AQP2 and NKCC2 proteins.
Consistent with previous studies [19, 20], dapagliflozin treatment decreased blood glucose level in this study, and the ability of dapagliflozin to improve glycemic control was achieved by inducing urinary glucose excretion. The glucose molecule can be freely filtered by glomeruli and more than 99.5% of the glucose filtered in mammalian kidneys is absorbed by the renal tubules. Detailed data have indicated that SGLT2 plays a major role in renal glucose reabsorption, which led many pharmaceutical companies to develop SGLT2 inhibitors as a way to improve glycemic control in patients with diabetes[21–23]. In this study, urinary glucose excretion as high as 40g/d/kg body wt was observed in dapagliflozin treated diabetic rats. The difference of 24h urinary glucose amount was not statistically significant in dapagliflozin treated and untreated diabetic rats. Increased urinary glucose excretion would result in an osmotic diuresis. Polyuria and decreased urinary osmolality were observed in both dapagliflozin treated and untreated diabetic rats over control rats. However, the urinary volume was decreased and the urinary osmolality was increased in dapagliflozin treated diabetic rats when comparing with untreated diabetic rats. Taken together, these data suggest that the dapagliflozin-induced increases in urinary glucose excretion lead to improve glycemic control and a milder osmotic diuresis than that in untreated diabetic animals.
In dapagliflozin-treated rats, the blood glucose level is lower than in uncontrolled diabetic rats. As a result, the total glucose amount filtered by glomeruli in dapagliflozin-treated rats is lower. We speculate that this is the reason why our results did not show increased glucose excretion in dapagliflozin-treated rats when compared with uncontrolled diabetic rats. However, the urine glucose concentration is similar between two diabetic groups, with or without dapagliflozin treatment. This result suggests dapagliflozin promotes urinary glucose excretion in diabetic rats. Because increased solute excretion, such as Na+, Cl− and K+, is believed to be attributable (at least in part) to osmotic diuresis in DM, we investigated whether dapagliflozin treatment leads to electrolyte disorder in diabetic rats. In this study, uncontrolled diabetic rats showed hyponatremia, hypochloremia and hypokalemia, but dapagliflozin treatment corrects these electrolyte disorders. This is consistent with the result of human clinical trials where serum electrolytes were mostly unchanged in patients receiving dapagliflozin treatment[24]. Increased urinary excretion of Na+, K+ and Cl− was observed in all diabetic rats when compared with control rats; dapagliflozin treatment appeared to decrease urinary excretion of these electrolytes when compared with untreated diabetic group; however, these changes did not reach statistical significance. These results suggest the correction of electrolyte disorder by dapagliflozin in diabetic rats may not rely solely on decreasing electrolyte excretion in urine.
Next, we examined the effect of dapagliflozin on the abundance of the main transporter proteins involved in urine concentration. Our previous study suggested that DM induced the up-regulation of UT-A1, AQP2 and NKCC2 to prevent excessive water and solute loss. The present study confirmed the earlier finding that UT-A1, AQP2 and NKCC2 proteins were upregulated in uncontrolled DM rats. Dapagliflozin treatment resulted in a further increase in UT-A1 protein abundance and did not affect the DM-induced up-regulation of AQP2 and NKCC2 proteins. These changes will tend to conserve solute and water despite the persistent glycosuria existing during dapagliflozin treatment. These changes also suggest the increases in abundance of urinary concentrating associated transport proteins result from persistent glycosuria but not hyperglycemia in diabetes.
The further increase of protein expression of UT-A1 in IM may explain, in part at least, how dapagliflozin partially alleviates pronounced polyuria even in the face of the same urinary glucose concentrations that are present in the untreated diabetic rats. However, the mechanism by which dapagliflozin treatment further increases IM UT-A1 protein expression is not clear. To our knowledge, in situations where the urea content of the urinary solute is low, UT-A1 protein abundance increases[25]. This increase in UT-A1 may be an effort to restore the hyperosmolality of the medullary interstitium to promote urine concentrating ability. This study showed that urea excretion in dapagliflozin treated diabetic rats is significantly lower than in untreated diabetic rats. Therefore, it was suggested that a reduction of urine urea excretion could be responsible for this further increase of UT-A1 protein expression in dapagliflozin treated diabetic rats. The reason why dapagliflozin treatment decreases urea excretion in diabetic rats is also not clear. A small number of studies have shown that SGLT2 inhibition induced SGLT1 up-regulation and activation[26, 27]. Some data indicate that SGLT1 may be an alternative channel for urea uptake[28]. It is possible that urea reabsorption by SGLT1 increases when SGLT2 is inhibited, which leads to the decrease of urea excretion, but this hypothesis needs to be further studied. In this study, there was no comparison to diabetic rats treated with insulin, so it is not known whether the decrease of blood glucose upon dapagliflozin treatment might contribute to UT-A1 up-regulation in diabetic rat IM. However, our previous study showed that absence of nitric oxide (NO) production in kidney reduced UT-A1 protein abundance in DM[29]. Hyperglycemia is believed to play a key role in reducing NO production in kidney[30]. Therefore, it is also possible that dapagliflozin alleviates the reduction of NO production in kidney by decreasing blood glucose, then up-regulating UT-A1 protein expression.
Our previous study showed that diabetic animals do not cope with osmotic diuresis effectively in the absence of UT-A1 and UT-A3 despite the fact that AQP2 levels still increased. This suggests that the two transport systems are dependent on each other for effective compensation for polyuria[31]. The present study showed that dapagliflozin treatment further increased UT-A1 protein abundance above levels in untreated diabetic animals, but did not result in a further up-regulation on AQP2 expression. These results suggest that dapagliflozin is specifically effecting urea transport rather than having a generalized effect on all urine concentrating transporter abundances.
In conclusion, diabetic rats treated with dapagliflozin have a mild osmotic diuresis compared to non-diabetic animals, but this does not result in an electrolyte disorder. Dapagliflozin treatment did not interrupt the DM-induced increases in either AQP2 or NKCC2 protein expression. Moreover, dapagliflozin treatment further increased UT-A1 protein abundance above the levels in uncontrolled DM rats. Taken together, the results of this study suggest that dapagliflozin treatment is unlikely to exacerbate any risk of worsening hypovolemia in diabetes despite the ongoing glycosuria.
Acknowledgments
This work was supported by NIH grants DK41707 and DK89828
Footnotes
Disclosures: No conflicts of interest are declared by the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vestri S, Okamoto M, De Freitas H, Dos Santos RA, Nunes M, Morimatsu M, et al. Changes in sodium or glucose filtration rate modulate expression of glucose transporters in renal proximal tubular cells of rat. The Journal of Membrane Biology. 2001;182(2):105–12. doi: 10.1007/s00232-001-0036-y. [DOI] [PubMed] [Google Scholar]
- 2.Chao EC, Henry RR. SGLT2 inhibition—a novel strategy for diabetes treatment. Nature Reviews Drug Discovery. 2010;9(7):551–9. doi: 10.1038/nrd3180. [DOI] [PubMed] [Google Scholar]
- 3.Mudaliar S, Polidori D, Zambrowicz B, Henry RR. Sodium–Glucose Cotransporter Inhibitors: Effects on Renal and Intestinal Glucose Transport From Bench to Bedside. Diabetes care. 2015;38(12):2344–53. doi: 10.2337/dc15-0642. [DOI] [PubMed] [Google Scholar]
- 4.Pei F, Li B-y, Zhang Z, Yu F, Li X-l, Cai Q, et al. Beneficial effects of phlorizin on diabetic nephropathy in diabetic db/db mice. Journal of diabetes and its complications. 2014;28(5):596–603. doi: 10.1016/j.jdiacomp.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Najafian M, Jahromi MZ, Nowroznejhad MJ, Khajeaian P, Kargar MM, Sadeghi M, et al. Phloridzin reduces blood glucose levels and improves lipids metabolism in streptozotocin-induced diabetic rats. Molecular Biology Reports. 2012;39(5):5299–306. doi: 10.1007/s11033-011-1328-7. [DOI] [PubMed] [Google Scholar]
- 6.Chen M, Xie C-G, Gao H, Zheng H, Chen Q, Fang J-Q. Comparative effectiveness of sodium-glucose co-transporter 2 inhibitors for controlling hyperglycaemia in patients with type 2 diabetes: protocol for a systematic review and network meta-analysis. BMJ open. 2016;6(1):e010252. doi: 10.1136/bmjopen-2015-010252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forst T, Guthrie R, Goldenberg R, Yee J, Vijapurkar U, Meininger G, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes, Obesity and Metabolism. 2014;16(5):467–77. doi: 10.1111/dom.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takakura S, Toyoshi T, Hayashizaki Y, Takasu T. Effect of ipragliflozin, an SGLT2 inhibitor, on progression of diabetic microvascular complications in spontaneously diabetic Torii fatty rats. Life Sciences. 2016;147:125–31. doi: 10.1016/j.lfs.2016.01.042. [DOI] [PubMed] [Google Scholar]
- 9.Gembardt F, Bartaun C, Jarzebska N, Mayoux E, Todorov VT, Hohenstein B, et al. The SGLT2 inhibitor empagliflozin ameliorates early features of diabetic nephropathy in BTBR ob/ob type 2 diabetic mice with and without hypertension. American Journal of Physiology-Renal Physiology. 2014;307(3):F317–F25. doi: 10.1152/ajprenal.00145.2014. [DOI] [PubMed] [Google Scholar]
- 10.Cefalu WT, Leiter LA, de Bruin TW, Gause-Nilsson I, Sugg J, Parikh SJ. Dapagliflozin’s Effects on Glycemia and Cardiovascular Risk Factors in High-Risk Patients With Type 2 Diabetes: A 24-Week, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study With a 28-Week Extension. Diabetes care. 2015;38(7):1218–27. doi: 10.2337/dc14-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim D-U, Sands JM, Klein JD. Changes in renal medullary transport proteins during uncontrolled diabetes mellitus in rats. American Journal of Physiology-Renal Physiology. 2003;285(2):F303–F9. doi: 10.1152/ajprenal.00438.2002. [DOI] [PubMed] [Google Scholar]
- 12.Klein JD, Price SR, Bailey JL, Jacobs JD, Sands JM. Glucocorticoids mediate a decrease in the AVP-regulated urea transporter in diabetic rat inner medulla. AmJPhysiol. 1997;273(6):F949–F53. doi: 10.1152/ajprenal.1997.273.6.F949. [DOI] [PubMed] [Google Scholar]
- 13.Kim GH, Ecelbarger CA, Mitchell C, Packer RK, Wade JB, Knepper MA. Vasopressin increases Na-K-2Cl cotransporter expression in thick ascending limb of Henle’s loop. AmJPhysiol. 1999;276(1):F96–F103. doi: 10.1152/ajprenal.1999.276.1.F96. [DOI] [PubMed] [Google Scholar]
- 14.Ecelbarger CA, Sands JM, Doran JJ, Cacini W, Kishore BK. Expression of salt and urea transporters in rat kidney during cisplatin-induced polyuria. Kidney International. 2001;60(6):2274–82. doi: 10.1046/j.1523-1755.2001.00048.x. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen S, DiGiovanni SR, Christensen EI, Knepper MA, Harris HW. Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc Natl Acad Sci USA. 1993;90:11663–7. doi: 10.1073/pnas.90.24.11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kishore BK, Krane CM, Di Iulio D, Menon AG, Cacini W. Expression of renal aquaporins 1, 2, and 3 in a rat model of cisplatin-induced polyuria. Kidney International. 2000;58(2):701–11. doi: 10.1046/j.1523-1755.2000.00216.x. [DOI] [PubMed] [Google Scholar]
- 17.Ren H, Yang B, Molina PA, Sands JM, Klein JD. NSAIDs alter phosphorylated forms of AQP2 in the inner medullary tip. PloS One. 2015;10(10):e0141714. doi: 10.1371/journal.pone.0141714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee HB, Blaufox MD. Blood volume in the rat. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 1985;26(1):72–6. [PubMed] [Google Scholar]
- 19.Han S, Hagan DL, Taylor JR, Xin L, Meng W, Biller SA, et al. Dapagliflozin, a selective SGLT2 inhibitor, improves glucose homeostasis in normal and diabetic rats. Diabetes. 2008;57(6):1723–9. doi: 10.2337/db07-1472. [DOI] [PubMed] [Google Scholar]
- 20.Thomson SC, Rieg T, Miracle C, Mansoury H, Whaley J, Vallon V, et al. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. American journal of physiology Regulatory, Integrative and Comparative Physiology. 2012;302(1):R75–83. doi: 10.1152/ajpregu.00357.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vallon V, Platt KA, Cunard R, Schroth J, Whaley J, Thomson SC, et al. SGLT2 mediates glucose reabsorption in the early proximal tubule. Journal of the American Society of Nephrology: JASN. 2011;22(1):104–12. doi: 10.1681/ASN.2010030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cherney DZ, Perkins BA, Soleymanlou N, Xiao F, Zimpelmann J, Woerle HJ, et al. Sodium glucose cotransport-2 inhibition and intrarenal RAS activity in people with type 1 diabetes. Kidney International. 2014;86(5):1057–8. doi: 10.1038/ki.2014.246. [DOI] [PubMed] [Google Scholar]
- 23.Poulsen SB, Fenton RA, Rieg T. Sodium-glucose cotransport. Current opinion in Nephrology and Hypertension. 2015;24(5):463–9. doi: 10.1097/MNH.0000000000000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paisley AJ, Yadav R, Younis N, Rao-Balakrishna P, Soran H. Dapagliflozin: a review on efficacy, clinical effectiveness and safety. Expert opinion on investigational drugs. 2013;22(1):131–40. doi: 10.1517/13543784.2013.740009. [DOI] [PubMed] [Google Scholar]
- 25.Kim D, Klein JD, Racine S, Murrell BP, Sands JM. Urea may regulate urea transporter protein abundance during osmotic diuresis. American Journal of Physiology-Renal Physiology. 2005;288(1):F188–F97. doi: 10.1152/ajprenal.00200.2004. [DOI] [PubMed] [Google Scholar]
- 26.Rieg T, Masuda T, Gerasimova M, Mayoux E, Platt K, Powell DR, et al. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. American Journal of Physiology- Renal Physiol. 2014;306(2):F188–93. doi: 10.1152/ajprenal.00518.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinstein AM. A mathematical model of the rat nephron: glucose transport. American Journal of Physiology-Renal Physiology. 2015;308(10):F1098–118. doi: 10.1152/ajprenal.00505.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leung DW, Loo DD, Hirayama BA, Zeuthen T, Wright EM. Urea transport by cotransporters. The Journal of Physiology. 2000;528(Pt 2):251–7. doi: 10.1111/j.1469-7793.2000.00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cipriani P, Kim SL, Klein JD, Sim JH, von Bergen TN, Blount MA. The role of nitric oxide in the dysregulation of the urine concentration mechanism in diabetes mellitus. Front Physiol. 2012;3:176. doi: 10.3389/fphys.2012.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang JS, Chuang LY, Guh JY, Huang YJ. Effects of nitric oxide and antioxidants on advanced glycation end products-induced hypertrophic growth in human renal tubular cells. Toxicological sciences: an official journal of the Society of Toxicology. 2009;111(1):109–19. doi: 10.1093/toxsci/kfp134. [DOI] [PubMed] [Google Scholar]
- 31.Ilori TO, Blount MA, Martin CF, Sands JM, Klein JD. Urine concentration in the diabetic mouse requires both urea and water transporters. American Journal of Physiology-Renal Physiology. 2013;304(1):F103–F11. doi: 10.1152/ajprenal.00385.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]