Abstract
The utility of positron emission tomography (PET) with 18F-fluorodeoxyglucose (FDG) in prostate cancer depends on the phase of the disease along the natural history of this prevalent malignancy in men. Incidental high FDG uptake in the prostate gland, while rare, should prompt further investigation with at least a measurement of serum prostate specific antigen (PSA) level. While in general FDG uptake level may significantly overlap among normal, benign, and malignant tissues, aggressive primary tumors with Gleason score greater than 7 tend to display high FDG uptake. PET with FDG may be useful in staging of those patients with aggressive primary tumors and can localize the site of disease in a small fraction of men with biochemical failure and negative conventional imaging studies. FDG PET may be quite useful in treatment response assessment and prognostication of patients with castrate-resistant metastatic prostate cancer.
Keywords: PE, prostate, cancer, FDG
Positron emission tomography (PET) with 18F-fluorodeoxyglucose (FDG) has revolutionized the imaging evaluation of a wide variety of disorders and, in particular, cancer. Almost all PET scans are now perfumed in combination with computed tomography (CT). PET-CT with FDG is now a common procedure for guided biopsy, diagnosis, initial staging, therapy response assessment, radiation treatment planning, restaging, and prognostication for many cancers (1). The Society of Nuclear Medicine and Molecular Imaging (SNMMI) celebrated the 40th anniversary of the first human images with FDG during its annual meeting in San Diego, California, in June 2016. Recently hybrid PET and magnetic resonance imaging (MRI) imaging systems have also become commercially available. While the exact clinical indications, clinic workflow, and reimbursement issues will need to be worked out, PET-MRI will likely play an important role in those conditions where the excellent soft tissue contrast and multiparametric capability of MRI will be advantageous. These clinical settings may include neoplasms of the brain, head and neck, liver, female pelvis, prostate, and those in the pediatric and young adult patients (2, 3).
While the utility of FDG PET-CT in many cancers is relatively well established, its role in prostate cancer has been somewhat misunderstood. There appears to be a general misconception that FDG PET-CT is not useful in prostate cancer. This was probably due to few initial studies that reported less than favorable results compared to those from other cancers (4–6). However, further experience demonstrated that FDG PET-CT might indeed be useful in specific phases of the disease along the timeline of the natural history of prostate cancer. This article reviews briefly the literature on the utility and limitations of FDG PET-CT in prostate cancer.
Detection of Primary Prostate Tumor
In order to localize a potential primary tumor, one needs to know what the range of physiologic or benign FDG uptake level may be in the prostate gland. The normal prostate gland generally demonstrates relatively low and homogeneous FDG uptake. One study attempted to quantify the uptake level in the presumed normal prostate gland of 145 men with no documented signs or symptoms of prostate gland disease, normal serum prostate specific antigen (PSA) level, and no visible prostatic calcifications on CT or prostatic urethral urine activity on PET (7). The mean and maximum standardized uptake values (SUVmax) for the prostate gland were 1.3±0.4 (range 0.1–2.7) and 1.6±0.4 (range 1.1–3.7). While this study showed overall low grade FDG uptake in the “normal” prostate gland, but it was recognized that small pockets of cancer or foci of benign prostate hyperplasia (BPH) might have existed. Overall the level of FDG accumulation can overlap in normal prostate, BPH and prostate cancer tissues, which often coexist altogether in a heterogeneous pattern (8, 9).
Occasionally, incidental high FDG uptake may be seen in the prostate gland of patients who undergo FDG PET-CT for a condition unrelated to known prostate pathology. In a study of 6,128 men who had undergone FDG PET scans, the incidental prostatic FDG uptake was noted in 1.3% of patients (10). No significant correlation was found between the SUVmax and serum PSA levels. Another investigation reported that focal incidental prostate uptake with SUVmax greater than 6 should be further evaluated with multiparametric magnetic resonance imaging (mpMRI) (11). A systematic review and meta-analysis of 47,935 patients reported a pooled prevalence of 1.8% for incidental high FDG uptake in the prostate gland (12). The pooled risk of malignancy with biopsy verification was 62% (95% Confidence Interval [CI]: 54–71%). It has been suggested that incidental prostate uptake on FDG PET scans should not be ignored and at least a serum PSA measurement should be considered (13, 14).
Minamimoto et al assessed the utility of FDG PET/CT for detecting primary prostate cancer in 50 men with elevated serum PSA levels who underwent confirmatory prostate biopsy (15). The sensitivity and specificity were 51.9 % and 75.7 % for the entire prostate gland, 73 % and 64 % for the peripheral zone, and 22.7 % and 85.9 % for the central zone, respectively. A relatively low sensitivity of 37% has been reported in larger cohorts (16). However, higher FDG uptake levels in lesions that turned out to represent cancer on biopsy were associated with more aggressive cancers (Gleason score >7) (17).
Initial Staging of Prostate Cancer
There is a paucity of data on the utility of FDG PET-CT in the initial staging of primary prostate cancer since this modality is not generally advocated in the imaging evaluation of men suspected of harboring prostate cancer. Liu reported on a retrospective study of 9 patients (mean serum PSA level of 291±363 ng/ml with range of 6.1–980 ng/ml) who underwent FDG PET-CT at the time of initial staging of known primary prostate cancer (18). Standard of reference for the PET observations was by biopsy, regional diagnostic CT and/or whole-body bone scintigraphy. The sensitivity of FDG PET-CT in identifying untreated primary lesions was only 33%. However, FDG PET-CT detected metastatic disease in lymph nodes and/or bone in six of the nine (67%) patients. The authors suggested that while FDG PET-CT may not in general be useful for detection of primary cancer but in certain subgroup of high serum PSA level, it may be useful for initial staging. The challenge for detection of primary tumors may be related to the remarkable heterogeneity of the lesion that can be intermixed with normal and benign tissues, the small size of some tumors, the possible interference from the high activity level in the nearby urinary bladder, and the underlying biology of prostate cancer which in general may display low rate of glycolysis and prefer other pathways such fatty acid metabolism for growth (19–21). It is interesting, however, to note that In the early analysis of the National Oncologic PET Registry (NOPR) data in the US involving 2,042 scans for initial staging of prostate cancer, FDG PET-CT had an impact on clinical management in 32% (95% confidence interval (CI): 30.0–34.1%) of the patients (nopr). This suggests that in certain subgroups of patients with known aggressive prostate cancer (Gleason score > 7), FDG PET-CT may be useful in initial staging with diagnostic information that may impact clinical management (23). However, the exclusive knowledge that FDG PET-CT may provide in this particular clinical setting in comparison to conventional imaging remains to be investigated.
Localization of Disease in Biochemical Recurrence
Biochemical recurrence (aka. PSA relapse, biochemical failure) is defined differently depending upon the initial treatment for primary prostate cancer. In prostatectomized patients, biochemical recurrence is declared when there is an initial serum PSA of 0.2 ng/mL or higher with a second confirmatory PSA rise (24). In patients who had undergone external beam radiation therapy, this clinical condition is asserted when there is a serum PSA rise by 2 ng/mL or more above the nadir PSA level (25). Typically these patients undergo imaging (contrast-enhanced abdomen and pelvic CT, whole body bone scintigraphy, and often pelvis MRI) to localize disease sites whether in the treated prostate bed (local recurrence) or at distant sites (metastatic disease) (Fig. 1). Note that if conventional imaging detects the suspicious sites of disease that may explain the PSA rise after definitive primary treatment, then there would be no need for additional nonstandard imaging. However, in many cases, especially in those patients with low PSA levels, conventional imaging may be unrevealing. It is in this group of patients that there has been major recent work on deciphering the potential utility of PET with a number of non-FDG radiotracers (e.g. 11C-acetate, 11C-choline, 18F-fluorocholine, 18F-anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid, or tracers targeting prostate specific membrane antigen or gastrin-releasing peptide receptor)(26).
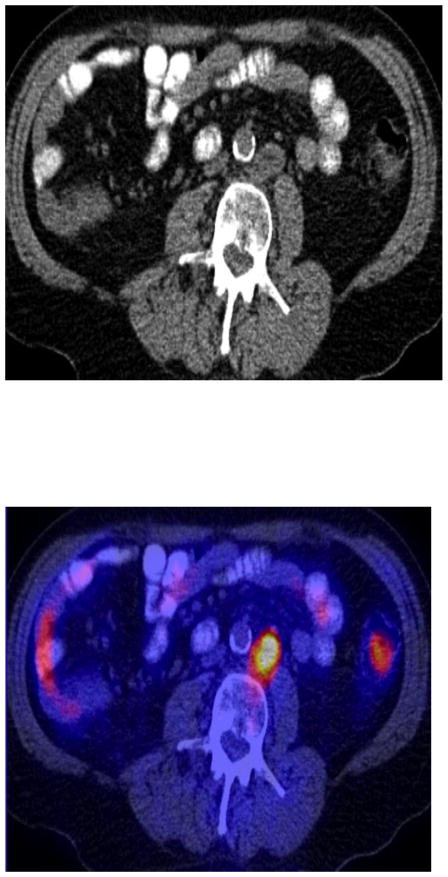
Fig. 1.
A man with biochemical failure (serum PSA of 22.5 ng/ml) after radical prostatectomy. Axial abdomen CT (A), and fused FDG PET-CT image (B) demonstrate an enlarged (short axis = 2.3 cm) hypermetabolic (SUVmax = 8) left paraaortic metastatic lymph node.
FDG PET is advantageous over the FDA approved 111In-capromab pendetide (Prostascint) scintigraphy in this clinical setting (29). In a recent investigation of 28 men with biochemical failure after definitive primary therapy (82.1% radical prostatectomy, 17.9% external beam radiation therapy), the sensitivity and specificity of FDG PET-CT were 61.6% and 75%, respectively (28). In one retrospective study of 91 men with biochemical recurrence following prostatectomy and validation of PET findings by biopsy or clinical and imaging follow-up, mean serum PSA levels were higher in the FDG PET-positive patients than in the FDG PET-negative patients (9.5±2.2 ng/ml vs. 2.1±3.3 ng/ml) with an overall PET detection rate of 31% (29). However, the reported detection rate was probably overestimated since some patients had disease already evident on conventional imaging. A prospective investigation of FDG PET-CT and 18F-NaF PET-CT in detection of occult metastases in 37 men with PSA relapse (range, 0.5–40.2 ng/mL) and negative standard imaging studies demonstrated a detection rate of only 8.1% for FDG PET-CT (30). Overall, the current evidence suggests that FDG PET may be useful in detecting disease in only a relatively small proportion of all patients with biochemical failure.
Therapy Response Assessment
A major utility of FDG PET in oncology has been to assess objectively the response to a variety of treatments at various periods in the clinical management of the disease such as at neoadjuvant, adjuvant, primary or salvage settings. Overall there is little data available for FDG PET-CT in the imaging evaluation of response to treatment in patients with metastatic prostate cancer. FDG uptake in metastatic lesions tends to decrease with androgen deprivation therapy or chemotherapy, but there may be mixed changes in individual lesions, the overall changes may be discordant with changes in the level of serum PSA or circulating tumor cells, and more importantly depend on the response criteria that is employed (e.g. Response Evaluation Criteria In Solid Tumors - RECIST 1.0 and RECIST 1.1, European Organization for Research and Treatment of Cancer - EORTC, or PET Response Criteria in Solid Tumors – PERCIST 1.0)(31, 32) (Fig. 2). A major issue in assessing therapy response in metastatic prostate cancer is that lesions in the bone (the most common site for metastases from prostate cancer) are considered non-target lesions for the structurally-based response criteria such as RECIST, and therefore metabolically-based criteria such as PERCIST may best serve such task. Since castrate-resistant metastatic prostate cancer is FDG avid and there are a growing number of novel drug regimens for therapy (e.g. enzalutamide, abiratierone, cabazitaxel, 223Ra dichloride), it seems prudent that FDG PET-CT may deserve further investigations in this clinical space.
Fig. 2.
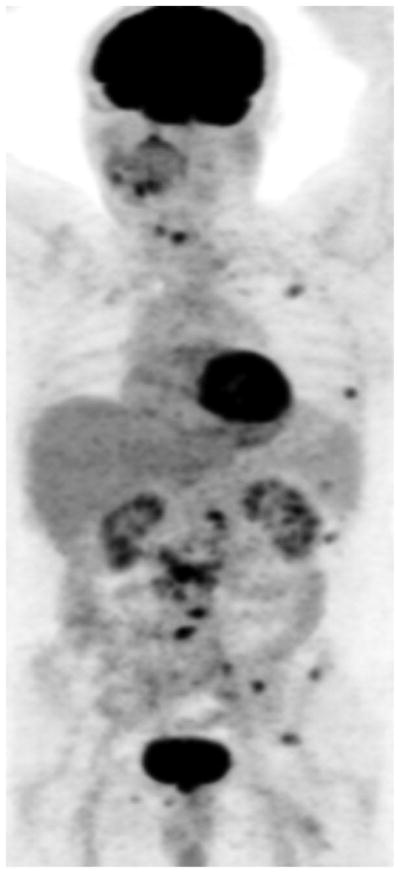
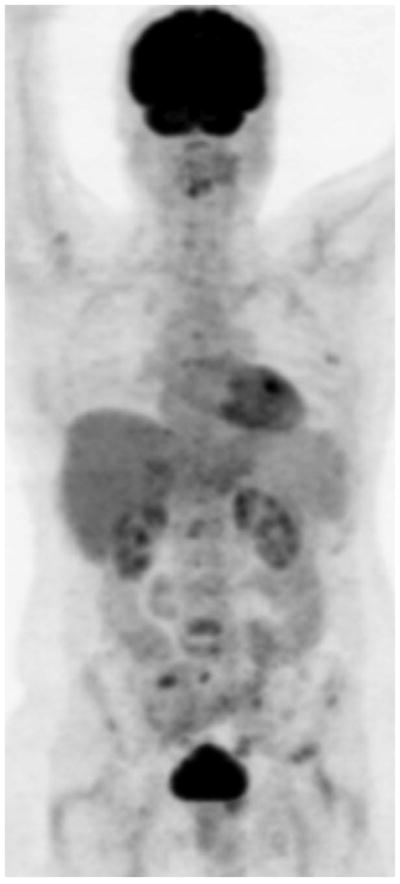
Maximum intensity projection FDG PET images show baseline castrate-sensitive state with hypermetabolic metastatic lesions (A), which responded favorably to androgen deprivation therapy with decline in metabolic activity of metastatic lesions (B).
Prognosis Assessment
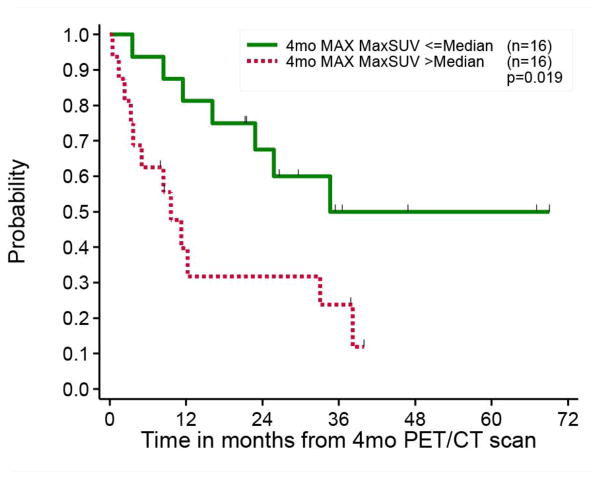
The ability to assess outcome in patients with cancer using non-invasive imaging is powerful and of major utility in clinical decision-making and individual patient management. Relevant outcome measures in prostate cancer may include, but are not limited to, time to biochemical recurrence (time to PSA progression), time to first metastasis, time to symptomatic progression, time to initiation of cytotoxic chemotherapy, time to radiographic progression, time to castrate resistance state, progression-free survival (PFS), metastasis-free survival, disease-specific survival, and overall survival (33). One study of 42 men with primary prostate cancer showed that patients with higher primary tumor FDG uptake had significantly poorer prognosis compared to those patients with tumors that showed lower FDG uptake (34). In another investigation of 43 men with metastatic castrate-resistant prostate, the FDG uptake in most active lesion was positively correlated with overall survival (35). A prospective study of 87 men with metastatic castrate-resistant prostate cancer showed, in a multivariate analysis controlling for confounding factors, that sum of the SUVmax of up to 25 metabolically active lesions (lymph nodes, bone, and soft tissue metastases) was statistically significant with a hazard ratio of 1.01 (95% CI 1.001–1.020; P=0.053) in predicting overall survival (36). The moving hazards of death in relation to sum of SUVmax (chance of death per person per month), showed a marked increase in chance of death for sum of SUVmax greater than 20. In another retrospective investigation, the association of CT patterns and glycolytic activity of prostate cancer bone metastases to overall survival was investigated in 38 patients (37). The number of lesions on CT or FDG PET, but not the intensity of FDG uptake, was associated with overall survival. These studies together suggested that the number of lesions and the highest intensity of FDG uptake might be independent prognostic variables for overall survival in this clinical setting (38). Finally, FDG PET-CT may be useful in predicting the transition from the castrate-sensitive state to the castrate-resistant state, which may potentially have important clinical management ramifications (39, 40) (Fig. 3).
Fig. 3.
Prediction of time to hormonal treatment failure with FDG PET-CT performed 4 months after the start of androgen deprivation therapy in 32 men with castrate-sensitive metastatic prostate cancer. The Kaplan-Meir survival curves show statistically significant difference in time to hormonal treatment failure when patients were dichotomized based on the median value of the most metabolically active lesion on scans (MAX = highest SUVmax of all active lesions).
Conclusion
FDG PET-CT may be useful in diagnosis and staging of aggressive primary prostate tumors (Gleason score >7) and as such incidental findings of high FDG uptake in the prostate gland should be further investigated. FDG PET-CT may also be useful in the detection of metastatic disease in a small fraction of men with biochemical failure with scan sensitivity that increases with increasing serum PSA level, in the assessment of extent of metabolically active castrate resistant metastatic disease, in monitoring response to androgen deprivation therapy and other treatments, and in prognostication.
Acknowledgments
H. Jadvar was supported in part by the National Institutes of Health grants R01-CA111613, R21-CA142426, R21-EB017568, and P30-CA014089.
Footnotes
Financial Disclosure: No conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alavi A, Lakhani P, Mavi A, et al. PET: a revolution in medical imaging. Radiol Clin North Am. 2004;42:983–1001. doi: 10.1016/j.rcl.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 2.Rosenkrantz AB, Friedman K, Chandarana H, et al. Current status of PET-MRI in oncologic imaging. Am J Rontgenol AJR. 2016;206:162–172. doi: 10.2214/AJR.15.14968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schafer JF, Gatidis S, Schmidt H, et al. Simultaneous whole-body PET/MR imaging in comparison to PET/CT in pediatric oncology: initial results. Radiology. 2014;273:221–231. doi: 10.1148/radiol.14131732. [DOI] [PubMed] [Google Scholar]
- 4.Hofer C, Laubenbacher C, Block T, et al. Fluorine-18-fluorodeoxyglucose positron emission tomography is useless for detection of local recurrence after radical prostatectomy. Eur Urol. 1999;36:31–5. doi: 10.1159/000019923. [DOI] [PubMed] [Google Scholar]
- 5.Liu IJ, Zafar MB, Lai YH, et al. Fluorodeoxyglucose positron emission tomography studies in diagnosis and staging of clinically organ-confined prostate cancer. Urology. 2001;57(1):108–11. doi: 10.1016/s0090-4295(00)00896-7. [DOI] [PubMed] [Google Scholar]
- 6.Salminen E, Hogg A, Binns D, et al. Investigations with FDG PET scanning in prostate cancer show limited value for clinical practice. Acta Oncol. 2002;41:425–9. doi: 10.1080/028418602320405005. [DOI] [PubMed] [Google Scholar]
- 7.Jadvar H, Ye W, Groshen S, et al. [F-18]-fluorodeoxyglucose PET-CT of the normal prostate gland. Ann Nucl Med. 2008;22:787–93. doi: 10.1007/s12149-008-0177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jadvar H. Molecular imaging of prostate cancer with [F-18]-fluorodeoxyglucose PET. Nat Rev Urol. 2009;6:317–23. doi: 10.1038/nrurol.2009.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jadvar H. Imaging evaluation of prostate cancer with 18F-flurodeoxyglucose PET/CT: utility and limitations. Eur J Nucl Med Mol Imaging. 2013;40(Suppl 1):5–10. doi: 10.1007/s00259-013-2361-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahin E, Elboga U, Kalender E, et al. Clinical significance of incidental FDG uptake in the prostate gland detected by PET/CT. Int J Clin Exp Med. 2015;8:10577–85. [PMC free article] [PubMed] [Google Scholar]
- 11.Brown AM, Lindenberg ML, Sankineni S, et al. Does focal incidental 18F-FDG uptake in the prostate gland have significance? Abdom Imaging. 2015;40:3222–9. doi: 10.1007/s00261-015-0520-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertagna F, Sadeghi R, Giovanella L, et al. Incidental uptake of 18F-fluorodeoxyglucose in the prostate gland. Systematic review and meta-analysis on prevalence and risk of malignancy. Nuklearmedizin. 2014;53:249–258. doi: 10.3413/Nukmed-0668-14-05. [DOI] [PubMed] [Google Scholar]
- 13.Kang PM, Seo WI, Lee SS, et al. Incidental abnormal FDG uptake in the prostate on 18-fluoro-2-deoxyglucose positron emission tomography-computed tomography. Asian Pac J Cancer Prev. 2014;15:8699–703. doi: 10.7314/apjcp.2014.15.20.8699. [DOI] [PubMed] [Google Scholar]
- 14.Seino H, Ono S, Miura H, et al. Incidental prostate 18F-FDG uptake without calcification indicates possibility of prostate cancer. Oncol Rep. 2014;31:1517–22. doi: 10.3892/or.2014.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minamimoto R, Uemura H, Sano F, et al. The potential of FDG PET/CT for detecting prostate cancer in patients with an elevated serum PSA level. Ann Nucl Med. 2011;25:21–7. doi: 10.1007/s12149-010-0424-4. [DOI] [PubMed] [Google Scholar]
- 16.Minamimoto R, Senda M, Jinnouchi S, et al. The current status of an FDG-PET cancer screening program in Japan based on a 4-year (2006–2009) nationwide survey. Ann Nucl Med. 2013;27:46–57. doi: 10.1007/s12149-012-0660-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang I, Chong A, Jung SI, et al. Is further evaluation needed for incidental focal uptake in the prostate in 18-fluoro-2-deoxyglucose positron emission tomography-computed tomography images? Ann Nucl Med. 2013;27(2):140–5. doi: 10.1007/s12149-012-0663-7. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y. Diagnostic role of fluorodeoxyglucose positron emission tomography-computed tomography in prostate cancer. Oncol Lett. 2014;7:2013–18. doi: 10.3892/ol.2014.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Candler JD, Williams ED, Slavin JL, Best JD, Rogers S. Expression and localization of GLUT1 and GLUT12 in prostate carcinoma. Cancer. 2003;97:2035–2042. doi: 10.1002/cncr.11293. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Zuckier LS, Ghesani NV. Dominant uptake of fatty acid over glucose by prostate cells: a potential new diagnostic and therapeutic approach. Anticancer Res. 2010;30:369–374. [PubMed] [Google Scholar]
- 21.Jadvar H. Prostate cancer: PET with 18F-FDG, 18F- or 11C-acetate, and 18F- or 11C-choline. J Nucl Med. 2011;52(1):81–9. doi: 10.2967/jnumed.110.077941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hillner BE, Siegel BA, Shields AF, et al. Relationship between cancer type and impact of PET and PET/CT on intended management: findings of the National Oncologic PET Registry. J Nucl Med. 2008;49:1928–35. doi: 10.2967/jnumed.108.056713. [DOI] [PubMed] [Google Scholar]
- 23.Oyama N, Akino H, Suzuki Y, et al. The increased accumulation of [18F]fluorodeoxyglucose in untreated prostate cancer. Jpn J Clin Oncol. 1999;29:623–9. doi: 10.1093/jjco/29.12.623. [DOI] [PubMed] [Google Scholar]
- 24.Cookson MS, Aus G, Burnett AL, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association prostate guidelines for localized prostate cancer update panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol. 2007;177:540–5. doi: 10.1016/j.juro.2006.10.097. [DOI] [PubMed] [Google Scholar]
- 25.Roach M, III, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix consensus conference. Int J Radiat Oncol Biol Phys. 2006;65:965–74. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 26.Jadvar H. Molecular imaging of prostate cancer: PET radiotracers. Am J Roentgenol AJR. 2012;199(2):278–91. doi: 10.2214/AJR.12.8816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schöder H, Herrmann K, Gönen M, et al. 2-[18F]fluoro-2-deoxyglucose positron emission tomography for detection of disease in patients with prostate specific antigen relapse after radical prostatectomy. Clin Cancer Res. 2005;11:4761–9. doi: 10.1158/1078-0432.CCR-05-0249. [DOI] [PubMed] [Google Scholar]
- 28.Seltzer MA, Barbaric Z, Belldegrun A, et al. Comparison of helical computerized tomography, positron emission tomography and monoclonal antibody scans for evaluation of lymph node metastases in patients with prostate specific antigen relapse after treatment for localized prostate cancer. J Urol. 1999;162:1322–8. [PubMed] [Google Scholar]
- 29.Jadvar H, Desai B, Ji L, et al. Prospective evaluation of 18F-NaF and 18F-FDG PET/CT in detection of occult metastatic disease in biochemical recurrence of prostate cancer. Clin Nucl Med. 2012;37:637–643. doi: 10.1097/RLU.0b013e318252d829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozturk H, Karapolat I. 18F- fluorodeoxyglucose PET/CT for detection of disease in patients with prostate-specific antigen relapse following radical treatment of a local-stage prostate cancer. Oncol Lett. 2016;11:316–22. doi: 10.3892/ol.2015.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oyama N, Akino H, Suzuki Y, et al. FDG PET for evaluating the change of glucose metabolism in prostate cancer after androgen ablation. Nucl Med Commun. 2001;22:963–9. doi: 10.1097/00006231-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Jadvar H, Desai B, Quinn D, et al. Treatment response assessment of metastatic prostate cancer with FDG PET/CT. J Nucl Med. 2011;52(Suppl 1):1908. [Google Scholar]
- 33.Jadvar H. Prognostic utility of PET in prostate cancer. PET Clin. 2015;10:255–63. doi: 10.1016/j.cpet.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oyama N, Akino H, Suzuki Y, et al. Prognostic value of 2- deoxy-2-[F-18]fluoro-D-glucose positron emission tomography imaging for patients with prostate cancer. Mol Imaging Biol. 2002;4:99–104. doi: 10.1016/s1095-0397(01)00065-6. [DOI] [PubMed] [Google Scholar]
- 35.Meirelles GS, Schoder H, Ravizzini GC, et al. Prognostic value of baseline [18F]fluorodeoxyglucose positron emission tomography and 99mTc-MDP bone scan in progressing metastatic prostate cancer. Clin Cancer Res. 2010;16:6093–6096. doi: 10.1158/1078-0432.CCR-10-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jadvar H, Desai B, Ji L, et al. Baseline 18F-FDG PET/CT parameters as imaging biomarkers of overall survival in castrate-resistant metastatic prostate cancer. J Nucl Med. 2013;54:1195–1201. doi: 10.2967/jnumed.112.114116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vargas HA, Wassberg C, Fox JJ, et al. Bone metastases in castration-resistant prostate cancer: associations between morphologic CT patterns, glycolytic activity, and androgen receptor expression on PET and overall survival. Radiology. 2014;271:220–9. doi: 10.1148/radiol.13130625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jadvar H, Groshen SG, Quinn DI. Association of overall survival with glycolytic activity of castrate-resistant prostate cancer metastases. Radiology. 2015;274:624–625. doi: 10.1148/radiol.14141593. [DOI] [PubMed] [Google Scholar]
- 39.Jadvar H, Desai B, Ji L, et al. Prediction of time to hormonal treatment failure or death in men with castrate-sensitive metastatic prostate cancer. RSNA 98th Scientific Assembly & Ann Meeting; Chicago, IL. 2012. [Google Scholar]
- 40.Jadvar H, Desai B, Ji L, et al. Prognostic implication of changes on FDG PET/CT following early hormonal treatment in castrate-sensitive metastatic prostate cancer. Proc SNM 59th Ann Meeting; Vancouver, BC, Canada. 2013. [Google Scholar]