Abstract
Heterogeneity of psychosis presents significant challenges for classification. Between two and 12 symptom dimensions have been proposed, and consensus is lacking. The present study sought to identify uniquely informative models by comparing the validity of these alternatives. An epidemiologic cohort of 628 first-admission inpatients with psychosis was interviewed 6 times over two decades and completed an electrophysiological assessment of error processing at year 20. We first analyzed a comprehensive set of 49 symptoms rated by interviewers at baseline, progressively extracting from one to 12 factors. Next, we compared the ability of resulting factor solutions to (a) account for concurrent neural dysfunction and (b) predict 20-year role, social, residential, and global functioning, and life satisfaction. A four-factor model showed incremental validity with all outcomes, and more complex models did not improve explanatory power. The four dimensions—reality distortion, disorganization, inexpressivity, and apathy/asociality—were replicable in 5 follow-ups, internally consistent, stable across assessments, and showed strong discriminant validity. These results reaffirm the value of separating disorganization and reality distortion, are consistent with recent findings distinguishing inexpressivity and apathy/asociality, and suggest that these four dimensions are fundamental to understanding neural abnormalities and long-term outcomes in psychosis.
Keywords: Nosology, Classification, Thought Disorder, Factor Analysis, Event-Related Potentials
Psychiatric nosology has made substantial advances since 1970; nevertheless, critics continue to call attention to several significant shortcomings of traditional taxonomies (Cuthbert & Insel, 2010; Markon, 2013; Watson & Clark, 2006; Widiger & Trull, 2007). First, these taxonomies define mental disorders as categories, although evidence available to date suggests that psychopathology exists on a continuum with normality (Carragher et al., 2014; Haslam, Holland, & Kuppens, 2012; Markon & Krueger, 2005; Walton, Ormel, & Krueger, 2011; Widiger & Samuel, 2005; Wright et al., 2013). Accumulating data indicate that this also is true for psychotic disorders, as psychotic experiences are relatively common in the general population and appear to reflect a continuum of liability to psychosis (Johns & Van Os, 2001; Linscott & Van Os, 2013; Nelson, Seal, Pantelis, & Phillips, 2013; Zavos et al., 2014). Imposition of a categorical nomenclature on naturally dimensional phenomena is problematic as it leads to substantial loss of information and to diagnostic instability (MacCallum, Zhang, & Preacher, 2003; Markon, Chmielewski, & Miller, 2011; Morey et al., 2012). In fact, psychotic disorders show high levels of diagnostic instability over time (Bromet et al., 2011).
Second, many diagnoses are heterogeneous and encompass multiple distinct phenomena (Clark, Watson, & Reynolds, 1995; Hasler, Drevets, Manji, & Charney, 2004; Zimmerman et al., 2015). Phenotypic heterogeneity is very pronounced within psychotic disorders (Barch et al., 2013; Kirkpatrick, Buchanan, Ross, & Carpenter, 2001; Kirkpatrick & Galderisi, 2008; Strauss, Carpenter, & Bartko, 1974). Indeed, two people diagnosed with schizophrenia may have no symptoms in common, and the symptoms are only loosely related (Peralta, Moreno-Izco, Calvo-Barrena, & Cuesta, 2013). This heterogeneity hinders research on etiology and treatment of psychotic disorders and also limits the value of diagnosis in clinical settings (Heinrichs, 2004; Jablensky, 2006). Traditional taxonomies attempt to deal with this problem by specifying subtypes. However, most of these subtypes have been derived rationally rather than from structural research and do not produce homogenous subgroups (Watson, 2003a). In particular, schizophrenia subtypes were found to have low temporal stability and minimal prognostic value, and were removed from the DSM-5 (APA, 2013).
Third, co-occurrence of mental disorder, or comorbidity, is very common across all forms of psychopathology (Grant et al., 2004; Kessler, Chiu, Demler, & Walters, 2005; Teesson, Slade, & Mills, 2009). Comorbidity complicates research design and clinical decision-making. It often is unknown whether a given finding characterizes the target disorder or is driven entirely by a comorbid condition, and what treatment is appropriate for a patient suffering from multiple conditions (Westen, Novotny, & Thompson-Brenner, 2004; Zimmerman et al., in press).
On the other hand, comorbidity offers a window into the natural organization of psychopathology that can guide refinement of the classification system. Recent advances in comorbidity research revealed evidence of six major dimensions of mental illness: internalizing, externalizing, thought disorder, antagonism, detachment, and somatoform spectra (e.g., Achenbach & Rescorla, 2001; Forbush & Watson, 2013; Kotov, Ruggero et al., 2011; Krueger & Markon, 2006; Lahey et al., 2008; Roysamb et al., 2011; Wright & Simms, 2015). Internalizing is defined primarily by depressive, anxiety, trauma-related, and eating disorders; externalizing by substance-related and disruptive disorders; thought disorder by schizotypal, schizoid, and paranoid personality disorders (PDs) as well as schizophrenia spectrum, bipolar disorder with psychosis, and depressive disorder with psychosis; antagonism by antisocial, nacrissistic, and histrionic PDs; detachment by avoidant and schizoid PDs; somatoform by somatic symptom disorders. These spectra form the foundation of an emerging quantative classification system, the Hierarchical Taxonomy Of Psychopathology (HiTOP; Kotov et al., submitted; http://medicine.stonybrookmedicine.edu/HITOP). It provides a dimensional description of psychopathology, incorporates comorbidity into the model by classifying co-occuring conditions together, and addresses heterogeneity by identifying sets of narrow homogenous dimensions within each spectrum. Preliminary models of homogeneous dimensions have been proposed for each spectrum, but the lower-order structure of the thought disorder spectrum is contentious.
Some theories conceptualized psychosis as a single dimension (Craddock & Owen, 2010; Crow, 1990; Tamminga et al., 2013). Other influential models described two dimensions of psychosis symptoms: positive (hallucinations, delusions, and disorganized) and negative (Andreasen & Olsen, 1982; Crow, 1980). Further research subdivided positive symptoms into reality distortion and disorganization, resulting in a 3-factor model (Andreasen et al., 1995; Grube, Bilder & Goldman, 1998; Liddle, 1987; Smith, Mar, & Turoff, 1998). Recent studies divided negative symptoms into diminished expression and avolition/apathy (Kring et al., 2013; Strauss et al., 2012, 2013), resulting in four dimensions overall. DSM-5’s dimensional assessment of psychosis (APA, 2013) includes five dimensions: it combines negative symptoms into one dimension, but considers hallucinations and delusions separately, and also treats disorganized speech and disorganized behavior as separate dimensions. A number of studies subdivided symptoms even further, identifying up to 12 distinct dimensions (Blanchard & Cohen, 2006; Emsley, Rabinowitz, Torreman, & The RIS-INT-35 Early Psychosis Global Working Group, 2003; Peralta & Cuesta, 2001; Peralta et al., 2013).
Of note, a growing number of structural studies have utilized heterogeneous samples (e.g., first episode/admission cohorts) with various psychotic disorders (e.g., Peralta et al., 2013; Russo et al., 2014). This is consistent with the cross-diagnostic approach of the HiTOP and other new-generation dimensional models of psychosis (Barch et al., 2013; Kotov et al., submitted). Also, structural research has relied primarily on factor analysis, as this method was designed to examine associations among variables and group related variables together helping to identify coherent, homogeneous constructs (Fabrigar et al., 1999).
Importantly, the numerous aforementioned classification schemes can be conceptualized as different levels of a single symptom hierarchy (e.g., the 3-factor model is a more fine-grained version of the 2-factor model). There is not necessarily a single “right” level in this structure, rather the choice may depend on the question. For example, the 2-factor model may be particularly informative for identifying abnormalities in neural circuitry, whereas treatment decisions may require the more refined 5-factor description. A factor analytic technique designed for this particular situation—bass-ackwards method—can evaluate multiple levels of the hierarchy and their interrelations in a single study (Farmer et al., 2013; Goldberg, 2006; Kim & Eaton, in press; Markon, Krueger, & Watson, 2005). In fact, prior research has begun to explicate the hierarchy of psychosis symptoms in this fashion (Peralta & Cuesta, 2001; Peralta et al., 2013). However, very few studies looked at the longitudinal stability of these structures (Russo et al., 2014), and none directly compared the utility of different levels for clinical and scientific purposes.
Indeed, a major motivation behind the HiTOP and other modern dimensional systems is to enhance diagnostic validity, providing more tractable targets for etiology and treatment research as well as improving clinical decision-making and prognostication (Barch et al., 2013; Kotov et al., submitted). The most fundamental diagnostic validators may be (a) long-term outcome, (b) disorder pathophysiology (e.g., abnormalities in neural circuitry), and (c) genetic and environmental risk factors (Robins & Guze, 1970). In other words, the optimal classification scheme should maximize the ability to predict outcomes and to explain pathophysiologic abnormalities and risk factors. In principle, the optimal classification may differ by validator, a possibility that presents certain pragmatic challenges but deserves consideration. A natural next question is whether validators can guide the choice of classification scheme among the alternatives. Indeed, there already are examples of this approach. For instance, the meta-structure project recommended inclusion of five disorder clusters in the DSM-5 based on the similarity of associations between disorders and validators observed among conditions considered for a cluster (Andrews et al., 2009). Numerous studies examined associations among various symptom dimensions and validators, although none compared alternative sets of dimensions to select the most informative one.
In particular, follow-up studies found that symptom dimensions predict global outcome—total burden of symptoms and functional impairments—up to 10 years later (Milev, Ho, Arndt, & Andreasen, 2005; White et al., 2009; Whitty et al., 2008) and also specific functional outcomes, such as occupational functioning, social functioning, and quality of life (Ho, Nopoulos, Flaum, Arndt, & Andreasen, 1998; Siegel et al., 2006; Ventura et al., 2015). Negative symptoms severity was the most consistent predictor that emerged in these analyses. However, each study considered only one model of psychosis symptoms—typically either 2- or 3-factor—and it is uncertain what set of symptoms is needed for optimal prediction of outcome.
With regard to pathophysiology, psychotic disorders have been linked to a wide range of physiological and neural abnormalities (Carpenter et al., 2009; O’Donnell, Salisbury, Niznikiewicz, Brenner, & Vohs, 2011; Pearlson, 2015). Here we focus on neural indices of error processing—the error-related negativity (ERN) and the error positivity (Pe)—because impaired error processing was identified by the Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia (CNTRICS; Carter, Minzenberg, West, & Macdonald, 2011) initiative as one of key deficits in psychotic disorders. Also, the Research Domain Criteria (RDoC; Cuthbert & Insel, 2010; www.nimh.nih.gov/research-priorities/rdoc/index.shtml) project highlighted ERN in measurement of multiple dimensions, such as performance monitoring. The ERN occurs within 100ms following error commission on speeded response tasks (Falkenstein, Hohnsbein, Hoormann, & Blanke, 1991) and is thought to reflect automatic, pre-conscious error detection, whereas the Pe occurs 200–400ms following errors and is thought to reflect conscious error awareness (Overbeek, Nieuwenhuis, & Ridderinkhof, 2005). It is well-established that the ERN is substantially blunted in psychotic disorders, and recent studies also observed a blunted Pe in these conditions (Foti, Kotov, Bromet, & Hajcak, 2012; Kansal, Patriciu, & Kiang, 2014; Mathalon et al., 2002; Perez et al., 2012). Moreover, these neural markers may be associated with specific psychotic symptoms (Foti et al., 2012; Mathalon et al., 2002), suggesting that ERN and Pe may be useful as validators for psychosis dimensions.
Present study
The aim of the present study is to illustrate the use of validators in selecting an optimal level of a dimensional hierarchical taxonomy. We propose an approach that first describes the hierarchy comprehensively (the bass-ackwards method) and then evaluates the incremental validity of these levels to select the most informative ones. Another aim of this study was to facilitate application of selected models, and thus we constructed scales based on the most informative factor structures and evaluated reliability and validity of these measures. First, we analyzed a relatively comprehensive set of 49 symptoms rated around the time of first hospitalization with psychosis. We then considered several validators: neural markers (ERN, Pe) and 20-year outcomes, including a global index that encompasses both symptoms and functioning, and some of its facets (primary role, social, residential, and life satisfaction). Finally, we constructed scales to measure the identified symptom dimensions, and considered their reliability, convergent/discriminant validity, long-term stability, and predictive validity. We did not intend to comprehensively test all relevant validators and establish a specific set (or sets) of psychosis dimensions as superior to others—indeed this would require a long series of studies—but to begin the process of comparing alternative nosologic models systematically.
Methods
Participants
Data for this study came from the Suffolk County Mental Health Project, an epidemiologic study of first-admission patients with psychosis (Bromet et al., 1992, 2011; Kotov et al., 2013). Participants were recruited from the 12 psychiatric inpatient units of Suffolk County, NY, between 1989 and 1995. Inclusion criteria were first admission either current or within six months, clinical evidence of psychosis, ages 15–60, IQ >70, proficiency with English, resident of Suffolk County, and no apparent general medical etiology. The study was approved annually by the institutional review boards of Stony Brook University and the participating hospitals. Treating physicians determined capacity to provide consent. Written consent was obtained from adult participants and from parents of patients <18 years old (patients themselves provided verbal assent).
We initially interviewed 675 participants (72% of referrals); 628 of them met the eligibility criteria and constitute the baseline sample. At baseline, the sample was 30 years old on average (standard deviation [SD] = 9.7), 58% male, and 75% non-Hispanic Caucasian. Best-estimate diagnoses at baseline indicated that 29% had schizophrenia-spectrum disorder, 21% bipolar disorder with psychosis, 17% major depressive disorder with psychosis, 7% substance-induced psychosis, and 26% other psychoses.
Face-to-face follow-ups were conducted at 6-month (469 participants), 24-month (421 participants), 48-month (354 participants), 10-year (349 participants), and 20-year (249 participants) time points; analyses of symptom structure were based on these data. We conducted phone interviews with participants who were unable to travel to the lab to obtain outcome information. Thus, another 66 participants completed phone interviews at Year 20, resulting in 315 participants with 20-year outcomes. Finally, of the 249 participants interviewed in person at Year 20, EEG data was collected from 217. Forty-seven had unusable EEG data (26 due to poor task performance of <70% correct, 5 due to making no errors on the task, 3 for having only a single clean error trial, 11 for having poor quality EEG data, and 2 for being statistical outliers), resulting in 170 participants with analyzable neural data. We employed a pairwise deletion approach to management of missing data, so that analyses of long-term outcomes were all based on 315 participant and EEG analyses were based on 170 participants. The 315 participants with 20-year outcomes did not differ from the 313 who did not participate on baseline symptom dimensions, except for a slightly higher level of reality distortion in the former group (Cohen’s d = .17). The 170 participants with analyzable EEG data did not differ from the other 458 patients on baseline dimensions.
Measures
Interviews were conducted by master’s level mental health professionals. Medical records and interviews with significant others were also obtained at every assessment. This detailed information was used to complete two rating scales: the Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1983) and the Scale for the Assessment of Positive Symptoms (SAPS; Andreasen, 1984), which are among the most commonly used measures of psychotic symptoms. The SANS is composed of 19 items organized into five domains and a global rating for each domain; the item “inattention during mental status test” had a high level of missing data and was excluded from the analyses. The SAPS consists of 31 items tapping four symptom domains as well as the global ratings. SANS and SAPS items are rated on a six-point scale (0 = none, 5 = severe) to reflect the past four weeks. Consistent with prior studies, we used individual ratings but not the global items (Kotov, Guey, Bromet, & Schwartz, 2010; Peralta & Cuesta, 2001; Stuart, Pantelis, Klimidis, & Minas, 1999). These 49 specific rating provide wide coverage of psychotic symptoms (Foussias & Remington, 2010). The inter-rater reliability of these ratings was very high (mean intraclass correlation = .72; Brown et al., 2000).
We examined five outcome measures to target different aspects of functioning at Year 20. First, overall outcome was operationalized with Global Assessment of Functioning (GAF) rated for the best month of the year before the 20-year interview and considered both symptoms and functioning. It has the advantage of a broad summary index. Next, we examined specific outcomes within this general domain, including life satisfaction which was assessed with an item from the Quality of Life Scale (QLS; Heinrichs, Hanlon, & Carpenter, 1984). The QLS is a semi-structured interview with multiple probes providing information for each interviewer rating. The interviewer summarized results of this interview on a rating of satisfaction ranging from 1 (very poor) to 5 (very good). Also, role functioning was assessed with the QLS level of accomplishment item, where the interviewer rated participants’ performance in their primary role during the past month from 0 (attempted no rule functioning) to 6 (very good functioning). Furthermore, social functioning was a composite of three interpersonal relations items from the QLS: social activity, sociosexual relations, and relationships with friends. The composite score ranged from 1 (worst functioning) to 17 (best) and showed acceptable internal reliability (α = 0.79). Finally, residential independence was rated by interviewers at each assessment as independent (living in participant’s own household) vs. not independent (living with relatives or in institutional settings). We categorized the pattern from Month 6 (to allow recovery from the initial hospitalization) to Year 20 as (0) never independent, (1) independent at some points of the follow-up, and (2) independent during the entire follow-up. The inter-rater reliability of outcome ratings was very high, with intraclass correlations ranging from .87 to .95 based on joint interview of 30 participants.
Error-related brain activity was assessed using an arrow flankers task (Eriksen & Eriksen, 1974). A row of five arrowheads was presented on each trial, with half of the trials being compatible (< < < < < or > > > > >) and half incompatible (< < > < < or > > < > >). The arrows were presented for 200 ms, followed by an inter-trial interval of 2300–2800 ms. Participants were instructed to press the mouse button corresponding to the direction of the center arrow and to respond both quickly and accurately. The task consisted of 11 blocks of 300 trials. At the end of each block, participants received feedback emphasizing either speed or accuracy to keep performance between approximately 75–90% correct.
The EEG was sampled at 1024 Hz using an elastic cap and the ActiveTwo BioSemi System (BioSemi, Amsterdam, Netherlands). Electrodes were measured with respect to a common mode sense active electrode, and recordings were taken from 34 scalp electrodes based on the 10/20 system, as well as two mastoid electrodes. The electro-oculogram was recorded from four facial electrodes. Offline analysis was performed using Brain Vision Analyzer software (Brain Products, Munich, Germany). Data were re-referenced to the mastoid average and filtered from .1–30 Hz. The EEG was segmented for each trial, spanning −400 to 800 ms relative to the button press, and corrected for blinks and eye movements (Gratton, Coles, & Donchin, 1983). Channels were rejected for artifacts in each trial using a semi-automated procedure. Response-locked ERP averages were created for correct and incorrect responses, with a baseline of −400 to −200 ms. Difference waves (error minus correct) were created to isolate error-related brain activity (Luck, 2005). The ERN was scored as the mean activity from 0–100 ms at Cz, and the Pe as the mean from 200–400 ms at Pz (for additional details, also see Foti, Kotov, Bromet, & Hajcak, 2012).
Follow-up analyses controlled for three key covariates that may distort associations between psychosis symptoms and outcomes: symptoms of depression and mania contemporaneous with predictors (psychosis symptoms), and antipsychotic medication use (Yes/No) contemporaneous with 20-year outcomes. Depression was assessed with the total score of the Hamilton Depression Rating Scale (Hamilton, 1960). Mania was operationalized with the excitement rating of the Brief Psychiatric Rating Scale (Overall & Gorham, 1962). Data on antipsychotic medications was obtained at Year 20 by interviewing participants and examining bottles with their medications.
Descriptive characteristics of validators (neural correlates and outcomes) are given in Table 1. They indicate that a wide range of performance was observed on each measure at Year 20. GAF showed high correlations with social and role functioning, as expected from this summary measure. Social and role functioning also correlated substantially (r = .65), but all other correlations among the validators were modest, indicating that they are clearly distinct from each other.
Table 1.
Correlations among validators at Year 20
| Mean | SD | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|---|
| 1. GAF | 49.03 | 17.88 | ||||||
| 2. Social functioning | 10.21 | 4.53 | .70 | |||||
| 3. Role functioning | 3.42 | 1.60 | .83 | .65 | ||||
| 4. Residential independence | 0.94 | 0.74 | .28 | .20 | .24 | |||
| 5. Life satisfaction | 3.73 | 1.07 | .39 | .42 | .43 | .06 | ||
| 6. ERN | −1.02 | 4.70 | −.14 | −.08 | −.09 | −.03 | .08 | |
| 7. Pe | 4.83 | 5.12 | .20 | .19 | .19 | −.02 | .07 | .16 |
Note: |r| > .15 are significant at p < .05. GAF = Global Assessment of functioning; ERN = error-related negativity; Pe = error positivity.
Data Analysis
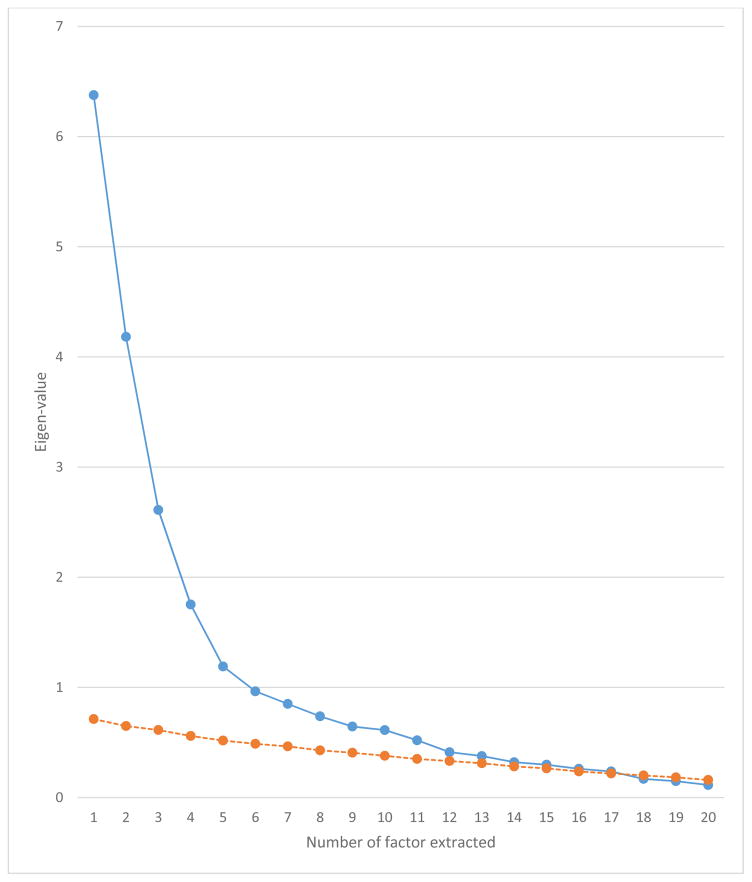
We investigated structure of psychosis symptoms by first performing a series of exploratory factor analyses of 49 specific items from the SANS and SAPS. To determine the maximum number of factors to extract, we performed parallel analyses (Fabrigar, Wegener, MacCallum, & Strahan, 1999). Extraction was stopped when eigen-value from common factors analysis of symptom data fell within 95% confidence interval of eigenvalue from randomly-generated data. We also considered interpretability of factor solutions, as parallel analysis has a tendency to overfactor. Next, we explicated the hierarchy up to the stopping point by using bass-ackwards method (Goldberg, 2006): we progressively extracted and rotated—with promax—an increasing number of factors starting with 1-factor solution and correlated adjacent levels of the hierarchy to describe transitions between them.
To test incremental validity of these models over each other, we constructed hierarchical regression models with a validator as the dependent variable and factors at each level of the hierarchy entering as a block (i.e., number of blocks equaled number of levels). Addition of predictors is known to increase R2 of the model by chance due to a greater number of predictors in the analysis; thus we reported adjusted R2 that corrects for this inflation and also tested R2 change between blocks for significance. Year 20 outcomes and neural markers served as dependent variables.
To test replicability of factor structures between waves, we calculated comparability coefficients, which provide a stringent test of factor similarity (Everett & Entrekin, 1980; Finn, 1986; Harman, 1976). Specifically, we derived regression-based factor scores for each solution at each time point, applied baseline regression weights to follow-up data, and at each follow-up correlated scores obtained with baseline weights and follow-up weights—correlations between corresponding scores are comparability coefficients. Next, we constructed symptom scales based on primary loadings of items in baseline factor analysis, then evaluated psychometric characteristics of these scales in follow-up data. Finally, we considered stability (convergent) correlations for these scales between baseline and each follow-up, and compared them to discriminant correlations between one scale at baseline and another scale at the follow-up. The pattern where discriminant correlations are lower than the two corresponding stability correlations would support convergent and discriminant validity of these scales.
Analyses were performed in SPSS Statistics version 23 and Mplus version 7 (to obtain fit indices).
Results
Hierarchical factor structure
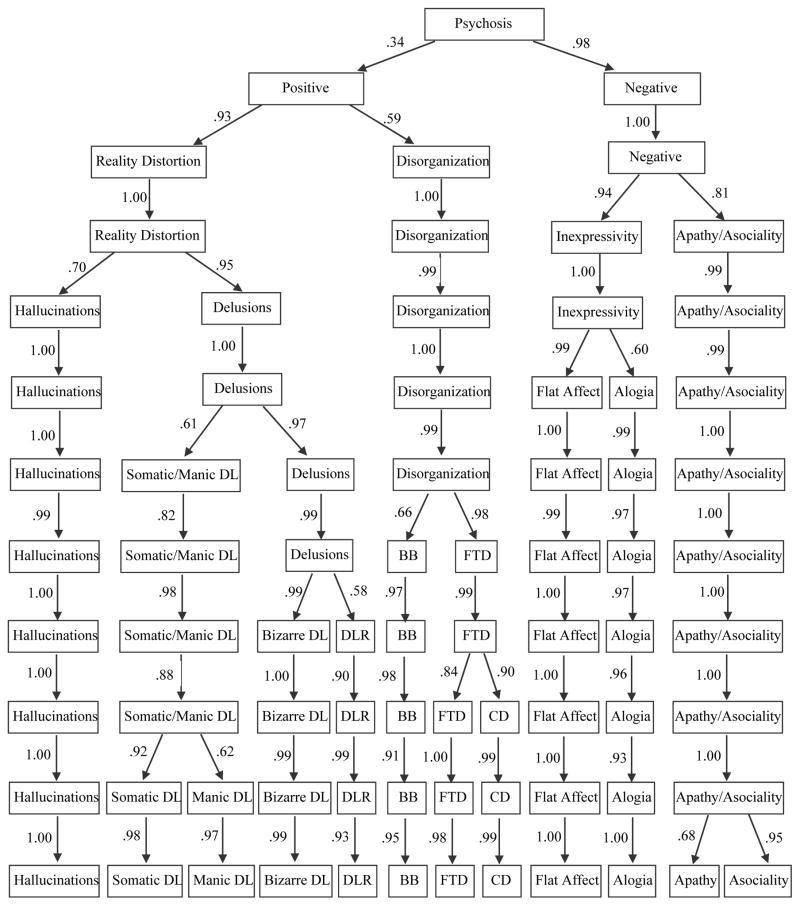
To fully explicate factor structures, we performed exploratory factor analyses of 49 SAPS and SANS ratings at baseline using bass-ackwards method (Goldberg, 2006). Correlations among the ratings are given in eTable 1. Parallel analyses suggested that up to 17 factors could be extracted (Figure 1). However, solutions 13 through 17 produced factors defined by a single primary loading and were not considered further. All models from a single factor to 12 factors were interpretable and are reported in Figure 2 with paths showing connections between levels. The 1-factor structure reflected the general burden of psychotic symptoms. The 2-factor revealed the expected positive and negative distinction. In the 3-factor structure, positive symptoms bifurcated into reality distortion and disorganized dimensions. In the 4-factor structure, negative symptoms bifurcated into inexpressivity and apathy/asociality. In the 5-factor structure, reality distortion split into hallucinations and delusions. Subsequent levels continued to narrow the content of factors. Of note, all transitions between levels resulted in bifurcation of a single factor rather than reorganization of content from multiple dimensions. This is not uncommon, as several studies that used bass-ackwards method did not observe reorganization (Kim & Eaton, in press; Markon, Krueger, & Watson, 2005), whereas several others found some reorganization (Farmer et al., 2013; Goldberg, 2006).
Figure 1.
Eigen-values for the first 20 factors in common factors analysis of baseline symptoms (blue curve) and of random values (orange curve). Orange curve is top end of the 95% confidence interval around the random-order eigen-value.
Figure 2.
Results of exploratory factor analyses. DL is delusions, DLR is delusions of reference, BB is bizarre behavior, FTD is formal thought disorder, and CD is communication difficulties. Paths are correlations between adjacent levels.
Most informative levels of the hierarchy
A common approach to selection of structural models is to compare fit indices. This produced inconsistent results, as the 12-factor solution showed the best AIC, whereas the 7-factor solution was the best-fitting model on the BIC (eTable 2). These indices reflect the ability of factor structures to explain associations among symptoms accurately and parsimoniously, and do not necessarily maximize validity of these factors. In contrast, our goal was to identify the most informative levels of the hierarchical structure, so we used 20-year outcomes as criteria and sought to predict them from symptom dimensions that emerged in the various factor analyses. In the interest of parsimony, we tested the incremental predictive power of each level of the hierarchy over and above all simpler levels. We used this stringent test, rather than comparing levels in pairs, to ensure that a significant result for level N was not due to a relative weakness of level N − 1 (e.g., when level N outperforms level N − 1 but not level N − 2), and reflects new information not captured by more general levels. For completeness, we also reported correlations between individual symptom ratings and outcomes (eTable 3).
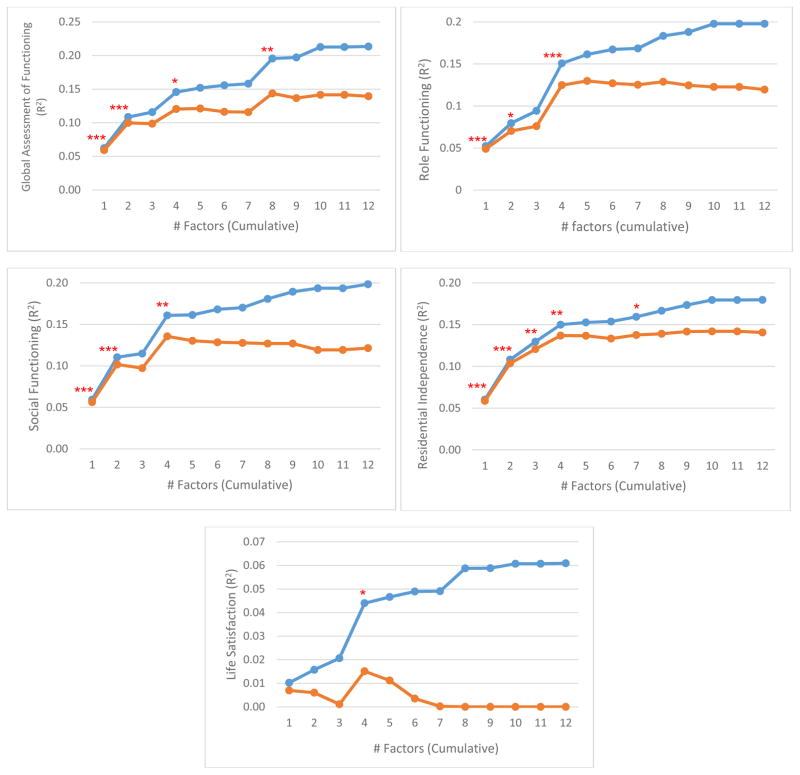
Prediction of global outcome (20-year GAF) revealed that the 1-factor solution was a significant predictor, and 2-, 4-, and 8-factor models showed significant incremental predictive power (Figure 3). Adjusted R2 supported this, as it increased through the 4-factor model (reaching .12), then remained steady until another increase at 8-factors (.14) and was flat thereafter. Evidently, observed increases in raw R2 outside these levels were due to chance (i.e., adding more predictors to the model). Prediction of 20-year role functioning followed a similar pattern: 1-, 2-, and 4-factor (but not 8-factor) models were significant, and adjusted R2 increased substantially through the 4-factor level (reaching .14) but remained steady after. The pattern of results for 20-year social functioning mirrored that of role functioning. For life satisfaction at year 20, only the 4-factor model was significant, which also was the peak of adjusted R2 (.02). Prediction of residential independence across the 20 years was significant for 1-, 2-, 3-, 4-, and 7-factor models. However, adjusted R2 increased very little after the 4-factor level, when it reached .14. Overall, the 4-factor solution was the only model to show incremental validity in every analysis.
Figure 3.
Prediction of 20-year outcomes from baseline symptom dimensions. Blue curves show cumulative predictive power (R2) for a given factor structure plus all simpler structures. Orange curves show the same but present adjusted R2, which controls for increase in fit due to chance, resulting from the greater number of predictors in the model. Asterisks indicate significant ΔR2 for that structure vs. all simpler structures combined (*p < .05, **p < .01, ***p < .001).
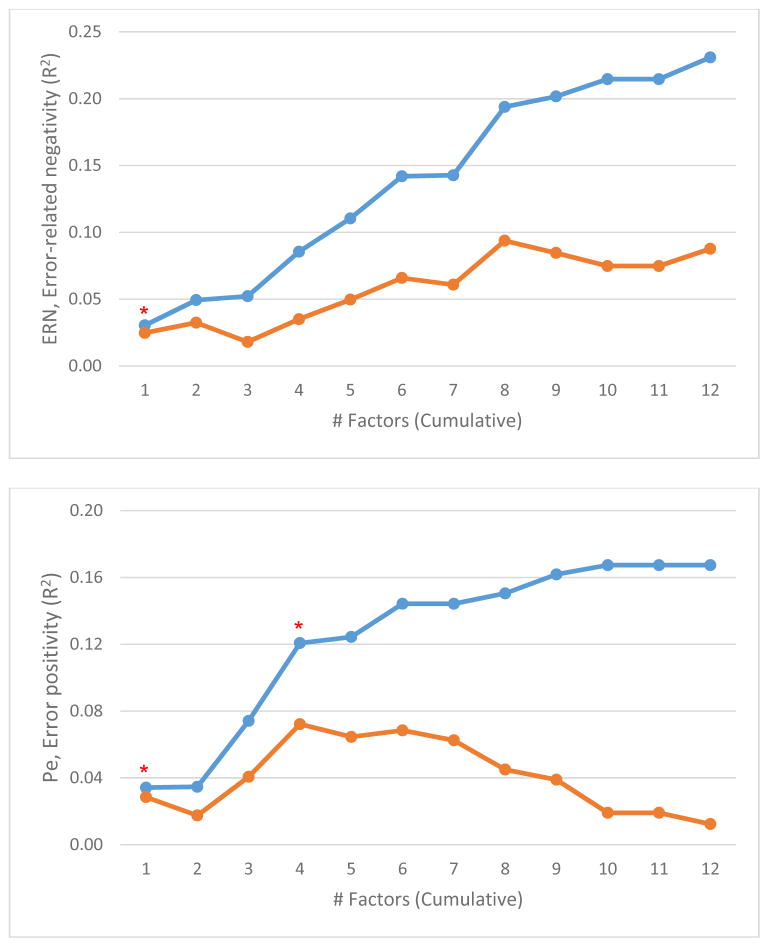
Next, we considered cross-sectional associations of symptom dimensions with measures of neural functioning. Event-related potentials were collected at Year 20, but we did not have statistical power to predict neural markers from baseline symptoms due to the smaller sample size available for neural measures (N=170). Instead, we examined links between neural markers and symptoms cross-sectionally. We scored all symptom dimensions in 20-year data according to their primary loadings in baseline analyses and then conducted regression analyses as above but with 20-year symptom dimensions as independent variables and 20-year neural markers as dependent variables (Figure 4). Data on neural correlates of individual symptoms is presented in eTable 3. Only the 1-factor model accounted for significant variance in ERN. However, both 1- and 4-factor models were significant in the analysis of Pe, and adjusted R2 peaked at four factors (.07).
Figure 4.
Symptom dimensions at Year 20 account for concurrent neural functioning. Curves show cumulative explanatory power (R2) for a given factor structure plus all simpler structures. Orange curves show the same but present adjusted R2, which controls for increase in fit due to chance, resulting from the greater number of predictors in the model. Asterisks indicate significant ΔR2 for that structure vs. all simpler structures combined (*p < .05, **p < .01, ***p < .001).
Overall, the 1-factor and 4-factor models were the most consistently informative level of the hierarchy (significant in six out of seven analyses each), the 2-factor level was uniquely informative in only half of the analyses, whereas support for the 3-, 7- and 8-factor levels was minimal (one out of seven). Thus, subsequent analyses focused on the 4-factor model. These factors were clearly interpretable as inexpressivity, apathy/asociality, reality distortion, and disorganization (Table 2). Nine symptoms loaded only weakly on the four factors and were dropped. We scored the other 40 symptoms into four scales by summing ratings according to their primary loadings.
Table 2.
Exploratory factor analysis of baseline symptoms
| Symptoms | I | II | III | IV |
|---|---|---|---|---|
| Unchanging Facial Expression | .87 | .05 | −.08 | −.07 |
| Paucity of Expressive Gestures | .87 | .03 | −.09 | −.11 |
| Decreased Spontaneous Movements | .78 | .09 | −.11 | −.11 |
| Lack of Vocal Inflections | .74 | .02 | −.01 | −.02 |
| Affective Nonresponsivity | .74 | −.08 | .05 | −.02 |
| Poor Eye Contact | .57 | −.08 | .09 | −.01 |
| Poverty of Speech | .57 | −.09 | .11 | .03 |
| Increased Latency of Response | .45 | .02 | .12 | .17 |
| Social Inattentiveness | .39 | −.02 | .24 | .30 |
| Delusions of Being Controlled | .04 | .67 | −.08 | −.01 |
| Thought Insertion | .06 | .66 | −.06 | −.04 |
| Auditory Hallucinations | −.08 | .59 | .27 | −.07 |
| Voices Commenting | −.11 | .58 | .23 | −.06 |
| Thought Withdrawal | .13 | .57 | −.09 | −.01 |
| Delusions of Mind Reading | .12 | .54 | −.10 | −.01 |
| Thought Broadcasting | .12 | .53 | −.05 | .00 |
| Voices Conversing | −.13 | .51 | .22 | −.01 |
| Delusions of Reference | −.03 | .47 | −.09 | .00 |
| Somatic or Tactile Hallucinations | −.04 | .43 | .05 | .05 |
| Olfactory Hallucinations | −.02 | .40 | .02 | −.03 |
| Visual Hallucinations | −.08 | .38 | .02 | −.03 |
| Religious Delusions | .02 | .35 | −.21 | .11 |
| Grandiose Delusions | −.04 | .31 | −.31 | .25 |
| Persecutory Delusions | −.08 | .30 | .05 | .05 |
| Somatic Delusions | −.06 | .23 | .08 | .16 |
| Delusions of Jealousy | −.08 | .06 | .05 | −.02 |
| Recreational Interests and Activities | .02 | .03 | .70 | −.16 |
| Relationships with Friends and Peers | .00 | .02 | .70 | .03 |
| Ability to Feel Intimacy and Closeness | .00 | −.02 | .63 | .12 |
| Impersistence at Work or School | .06 | .06 | .61 | .09 |
| Physical Anergia | .16 | −.02 | .58 | .03 |
| Sexual Activity | −.09 | .02 | .49 | −.11 |
| Grooming and Hygiene | .23 | −.11 | .22 | .15 |
| Delusions of Guilt or Sin | .00 | .07 | .08 | −.02 |
| Derailment | .01 | −.01 | −.04 | .68 |
| Tangentiality | −.03 | .02 | −.03 | .65 |
| Illogicality | .02 | .01 | .11 | .46 |
| Incoherence | .06 | −.07 | −.05 | .45 |
| Distractible Speech | −.03 | .00 | −.03 | .45 |
| Pressure of Speech | −.18 | −.05 | −.27 | .44 |
| Circumstantiality | −.13 | .00 | −.08 | .41 |
| Poverty of Content of Speech | .21 | −.08 | .09 | .38 |
| Clanging | .02 | −.03 | .00 | .35 |
| Social and Sexual Behavior | .04 | .09 | −.08 | .34 |
| Inappropriate Affect | −.01 | .20 | .07 | .32 |
| Aggressive and Agitated Behavior | −.12 | .02 | .11 | .27 |
| Blocking | .24 | .10 | .09 | .26 |
| Clothing and Appearance | −.01 | −.07 | .00 | .26 |
| Repetitive or Stereotyped Behavior | .06 | .08 | .04 | .14 |
| Factor correlations | I | II | III | IV |
| I | .13 | .51 | .07 | |
| II | .08 | .22 | ||
| III | .10 | |||
| IV |
Note: Factor loadings > .30 are bolded. Factor I is Inexpressivity, Factor II is Reality Distortion, Factor III is Apathy/Asociality, and Factor IV is Disorganization.
Reliability and stability of scales measuring the four dimensions
To evaluate robustness of the structure, we extracted the four factors at each time point from the 40 selected symptoms and compared these factor solutions to the baseline structure using comparability coefficients (Table 3). The structure identified at baseline was replicated (comparability coefficients for all factors > .90) in 6-month, 24-month, and 20-year data. The structure in 48-month and 10-year assessments was different, with incoherence and illogicality forming Factor III and content of Reality Distortion being spread across other dimensions. This likely is due to low levels of incoherence and illogicality observed at 48-month and 10-year, which made estimates of their association less precise; specifically the association was overestimated and this lead to structural anomalies. Of note, if the incoherence rating was removed from analyses, factor solutions at all waves resembled the baseline structure (eTable 4), but we decided to retain incoherence in the model as it performed well in the baseline and 20-year data.
Table 3.
Comparability coefficients for 4-factor solution across time points
| Baseline Factor | 6 months | 24 months | 48 months | 10 years | 20 years |
|---|---|---|---|---|---|
| Inexpressivity | .96 | .92 | .86 | .86 | .95 |
| Apathy/Asociality | .95 | .96 | .95 | .96 | .95 |
| Reality Distortion | .98 | .92 | .25 | .36 | .90 |
| Disorganization | .93 | .97 | .58 | .64 | .96 |
Note: Factor solutions were based on a pool of 40 symptoms that loaded well in baseline analysis. Comparability coefficient < .90 indicates non-negligible structural difference.
The four symptom scales were internally consistent at each wave (Cronbach’s αs > .70), although reliability was somewhat weaker for Disorganization than the other scales (Table 4). Interrater reliability also was high, with intraclass correlations of .79 for Inexpressivity, .94 for Apathy/Asociality, .95 for Reality Distortion, and .85 for Disorganization scale. The scales were clearly distinct from each other. Inexpressivity and Apathy/Asociality correlated .49 to .56 at six time points, but all other correlations were < .37.
Table 4.
Internal consistency and intercorrelations of the scales across time points
| Cronbach’s α | Correlations
|
|||
|---|---|---|---|---|
| Inexp | A/A | RD | ||
| Baseline | ||||
| Inexpressivity | .88 | |||
| Apathy/Asociality | .81 | .47 | ||
| Reality Distortion | .80 | .06 | .08 | |
| Disorganization | .72 | .00 | .00 | .20 |
| 6-months | ||||
| Inexpressivity | .91 | |||
| Apathy/Asociality | .84 | .50 | ||
| Reality Distortion | .85 | .16 | .24 | |
| Disorganization | .74 | .15 | .23 | .28 |
| 24-months | ||||
| Inexpressivity | .90 | |||
| Apathy/Asociality | .82 | .49 | ||
| Reality Distortion | .81 | .13 | .22 | |
| Disorganization | .75 | .06 | .12 | .25 |
| 48-months | ||||
| Inexpressivity | .91 | |||
| Apathy/Asociality | .83 | .51 | ||
| Reality Distortion | .83 | .01 | .24 | |
| Disorganization | .78 | .07 | .17 | .30 |
| 10-year | ||||
| Inexpressivity | .89 | |||
| Apathy/Asociality | .88 | .56 | ||
| Reality Distortion | .89 | .14 | .34 | |
| Disorganization | .80 | .16 | .33 | .47 |
| 20-year | ||||
| Inexpressivity | .90 | |||
| Apathy/Asociality | .88 | .56 | ||
| Reality Distortion | .83 | .18 | .34 | |
| Disorganization | .76 | .02 | .36 | .28 |
Note: Inexp = inexpressivity, A/A = Apathy/Asociality, RD = Reality Distortion.
Next, we compared stabilities of scales between baseline and all subsequent follow-ups (Table 5). From baseline to month 6, Inexpressivity was highly stable (r = .60), Apathy/Asociality less so (.48), Reality Distortion moderately stable (.31), and Disorganization was the least stable (.23). This relative ranking persisted across the follow-ups as stabilities declined with time but only slightly. In contrast to these convergent correlations, discriminant correlations—longitudinal associations between different scales—were much lower. Individual discriminant correlations were always smaller than convergent correlations of the two variables involved, and this difference between a discriminant correlation and a relevant convergent correlation was statistically significant (p < .05 one-tailed) in 109 of 120 comparisons, which were performed using Williams modification of the Hotelling test for two correlations involving a common variable (Kenny, 1987). Overall, the structure was not only replicable within waves, but also fairly consistent across waves. Reality Distortion and especially Disorganization were more malleable than Inexpressivity and Apathy/Asociality, but they retained their distinctiveness from the other dimensions over the 20 years.
Table 5.
Temporal relations: convergent correlations between the same dimensions and discriminant correlations between different dimensions from baseline to follow-ups
| Baseline | Follow-up | Follow-up
|
||||
|---|---|---|---|---|---|---|
| 6-month | 24-month | 48-month | 10-year | 20-year | ||
| Inexpressivity | Inexpressivity | .60 | .43 | .41 | .43 | .37 |
| Apathy/Asociality | Apathy/Asociality | .48 | .37 | .33 | .40 | .38 |
| Reality Distortion | Reality Distortion | .23 | .22 | .14 | .20 | .21 |
| Disorganization | Disorganization | .31 | .29 | .24 | .25 | .20 |
| Mean convergent | .41 | .33 | .28 | .32 | .29 | |
| Inexpressivity | Apathy/Asociality | .29 | .21 | .23 | .30 | .28 |
| Inexpressivity | Reality Distortion | .09 | .07 | .08 | .10 | .19 |
| Inexpressivity | Disorganization | .04 | −.05 | .07 | .11 | .02 |
|
| ||||||
| Apathy/Asociality | Inexpressivity | .37 | .28 | .24 | .30 | .27 |
|
| ||||||
| Apathy/Asociality | Reality Distortion | .18 | .08 | .12 | .14 | .25 |
| Apathy/Asociality | Disorganization | .14 | −.05 | .06 | .13 | .05 |
|
| ||||||
| Reality Distortion | Inexpressivity | .01 | −.01 | .00 | −.01 | .07 |
| Reality Distortion | Apathy/Asociality | .05 | −.01 | .00 | .04 | .06 |
|
| ||||||
| Reality Distortion | Disorganization | −.01 | −.04 | .03 | .03 | .11 |
|
| ||||||
| Disorganization | Inexpressivity | −.04 | −.04 | −.02 | −.05 | −.02 |
| Disorganization | Apathy/Asociality | −.02 | −.11 | −.07 | −.02 | .01 |
| Disorganization | Reality Distortion | .01 | .01 | .01 | .09 | .04 |
|
| ||||||
| Mean discriminant | .09 | .03 | .06 | .10 | .11 | |
To evaluate validity of the 4-factor structure in more detail beyond the overall evidence of incremental validity, we entered baseline scales simultaneously in a regression model to predict each of the five outcomes. Apathy/asociality was the only unique predictor of impairment in GAF, social functioning, role functioning, and life satisfaction, whereas inexpressivity was the unique predictor of low residential independence (Table 6). We also regressed Pe on the 20-year scales, as the 4-factor model showed incremental validity in accounting for Pe, and Apathy/Asociality was the only predictor of blunted Pe. We repeated these analyses controlling for covariates most likely to affect results—depression and mania symptoms contemporaneous with predictors, antipsychotic medication use contemporaneous with outcomes—and the pattern of results was unchanged (Table 6).
Table 6.
Unique effects of the four symptom scales at baseline in predicting 20-year outcomes, estimates are standardized β coefficients
| GAF | Social functioning | Role functioning | Residential independence | Life satisfaction | Pe | |
|---|---|---|---|---|---|---|
| Controlling for other symptoms of psychosis | ||||||
| Inexpressivity | −.09 | −.08 | −.03 | −.22* | −.01 | .10 |
| Apathy/Asociality | −.29* | −.32* | −.30* | −.09 | −.15* | −.31* |
| Reality Distortion | −.01 | −.02 | .01 | −.08 | −.01 | .04 |
| Disorganization | .00 | .05 | −.04 | .02 | −.01 | −.05 |
|
| ||||||
| Controlling for other symptoms of psychosis and covariatesa | ||||||
| Inexpressivity | −.08 | −.09 | .00 | −.13* | −.05 | .12 |
| Apathy/Asociality | −.26* | −.30* | −.30* | −.12 | −.15* | −.30* |
| Reality Distortion | −.01 | −.04 | −.00 | −.08 | −.02 | .04 |
| Disorganization | .03 | .07 | .00 | .03 | .04 | −.09 |
Note: GAF = Global Assessment of functioning; Pe = error positivity. Pe was regressed on 20-year symptoms, all other outcomes were regressed on baseline symptoms.
Covariates are symptoms of depression and mania contemporaneous with predictors (psychotic symptoms), and use of antipsychotic medication at Year 20.
p < .05
Discussion
This study documented a hierarchy of psychosis dimensions by analyzing a relatively comprehensive set of 49 symptoms in a first-admission sample of 628 inpatients with psychosis. All levels from one to 12 factors were interpretable, and many of them were similar to dimensions found in previous studies (Barch et al., 2013; Blanchard & Cohen, 2006; Peralta & Cuesta, 2001; Peralta et al., 2013). Analyses of validators (neural markers and long-term outcomes) suggested that relatively few dimensions were necessary to maximize validity of the symptom classification. The 4-factor model contributed to explanation and prediction of nearly all validators, whereas more complex models did not show incremental validity consistently. The four dimensions were reality distortion, disorganization, inexpressivity, and apathy/asociality. Reality distortion and disorganization are included in the widely-used 3-dimension scheme (Andreasen et al., 1995; Grube et al., 1998; Liddle, 1987; Smith et al., 1998). Decomposition of the third dimension of that model into inexpressivity and apathy/asociality is well-documented also and has been reinforced by recent advances in the assessment of negative symptoms (Kring et al., 2013; Strauss et al., 2012, 2013). Present findings lend further support to this elaboration of the seminal 3-dimension model. Of note, one-dimension and two-dimension structures also received considerable support in validator analyses. These higher-order dimensions can be easily obtained from the four factors (e.g., positive symptoms can be operationalized by compositing reality distortion and disorganization, whereas negative symptoms by compositing inexpressivity and apathy/asociality), thus the 4-dimension scheme may be considered foundational as it allows assessment of all relevant dimensions. We constructed scales to operationalize these dimensions with the SANS and SAPS items, and they showed favorable psychometric properties.
This investigation considered a wide range of long-term outcomes and consistently found incremental validity for the four symptom dimensions. Of note, GAF is a broad summary outcome, and specific outcomes examined can be considered its facets. Among specific outcomes, social and role functioning were strongly associated, but were largely distinct from each other (58% of variance was unique), which suggests that it is useful to consider both validators. With regard to neural correlates, one-dimension structure fully explained the link between ERN and psychosis, but this model accounted for only limited variance in Pe; additional explanatory power emerged at the 4-factor level. Temporal stability of the four dimensions was generally modest (higher for the negative symptom factors, lower for the positive symptom ones), likely owing to substantial evolution of the illness and effects of treatment that occurred since the first hospitalization. Importantly, stability (or convergent) correlations were always greater than discriminant correlations, which indicates that psychosis was more likely to manifest similarly over time than change presentation to a different symptom profile. This further reinforces validity of the four dimensions as well as the scales that we constructed to assess them.
Only two symptom dimensions showed independent contributions to studied outcomes and neural measures: inexpressivity and apathy/asociality, with the latter being especially informative. In fact, inexpressivity and apathy/asociality had an entirely different pattern of associations with validators, and thus their predictive power was partially obscured in models that included a single dimension of negative symptoms. The importance of apathy/asociality dimension for functional outcomes is well-established (Foussias & Remington, 2010; Strauss et al., 2013), which helps to explain why prediction of social and role functioning increased when this dimension was added to the model (i.e., in 4-factor solution). Of note, there likely is some conceptual overlap between such functional measures and apathy/asociality symptoms (Blanchard et al., 2011; Kring et al., 2013), which may explain this effect at least in part. In contrast, links of apathy/asociality to life satisfaction and Pe are less expected, and suggest that apathy/asociality is significant broadly and not solely for reasons of conceptual overlap. Even with regard to social and role functioning, ability of symptoms to predict outcomes two decades later point to utility of assessing these symptoms.
Another question with inexpressivity and apathy/asociality is whether they belong to thought disorder or detachment spectrum. Indeed, schizoid personality disorder was found to cross-load between these dimensions (Kotov et al., under review). It appears that social withdrawal, a defining feature of schizoid personality disorder, reflects two types of influences: (1) reduced pleasure from social interactions that is tapped by the detachment dimension and (2) reduced ability to initiate and maintain social interactions common in the thought disorder spectrum. Apathy/asociality and inexpressivity are thought to reflect the latter dimension, but a direct test of this possibility would require joint modeling of facets of thought disorder and detachment spectra.
The 4-dimension scheme does not map cleanly on the DSM-5 dimensional system for psychosis, which includes a single dimension for negative symptoms, and considers hallucinations and delusions as well as disorganized speech and disorganized behavior separately. Of note, the DSM-5 Psychosis workgroup reviewed structural evidence in support of the four dimensions reported here, but chose a different organization due to pragmatic considerations of coordination with formal diagnoses and ensuring clinical usability of the system (Barch et al., 2013). Further research is needed to determine whether the present 4-factor model provides sufficient detail for guiding treatment of psychotic disorders optimally or a different level of the hierarchy is superior. This can be investigated by employing treatment outcome as a validator in the present analytic approach. Of note, DSM-5 also includes dimensions of depression, mania, and impaired cognition, but they fall outside the scope of the current study.
The present findings contribute to elaboration of the HiTOP system by clarifying lower-order structure of the thought disorder spectrum. A number of faceted measures have been developed to charaterize heterogeneity within the other five spectra. To spotlight a few, the Inventory of Depression and Anxiety Symptoms (Watson et al., 2012) specifies 18 dimensions within internalizing, the Externalizing Spectrum Inventory (Krueger, Markon, Patrick, Benning, & Kramer, 2007) describes 23 dimensions within externalizing, the Personality Iventory for DSM-5 (Krueger, Derringer, Markon, Watson, & Skodol, 2012) models five dimensions within antagonism and five within detachment, and the Personality Assessment Inventory (Morey, 2007) characterizes three dimensions within somatoform. In contrast, lower-order structure of the thought disorder spectrum is less clear with numerous alternative models, and the present investigation seeks to provide a framework for organizing and evaluating these proposals. Our findings suggest that facets within the thought disorder spectrum can be effectively assessed with the SAPS and SANS using scales that we derived and evaluate here. The HiTOP group also recommended the Clinical Assessment Interview for Negative Symptoms (CAINS; Kring et al., 2013) for this task. Of note, our analyses did not include personality dimensions, which form a prominent component of the though disorder spectrum (Keyes et al., 2013; Kotov, Chang, et al., 2011; Kotov, Ruggero, et al., 2011; Markon, 2010), and thus they provide information only on the structure of signs and symptoms. Other research suggests that such traits as eccentricity, cognitive/perceptual dysregulation, and unusual beliefs and experiences need to be included to fully describe the spectrum (Wright & Simms, 2015).
With regard to the RDoC, connections of the four dimensions to this system are complex and not entirely clear at present. Inexpressivity has conceptual parallels with two RDoC constructs: production of facial communication and production of non-facial communication (Social Systems). Reality distortion has been linked to auditory perception (Cognitive Systems) and agency constructs (Social Systems) but also many others (Ford et al., 2014). Disorganization and apathy/asociality are mentioned in the RDoC matrix in connection with cognitive control (Cognitive Systems), but likely also involve other constructs, such as working memory (Cognitive Systems), approach motivation (Positive Valence Systems), and affiliation and attachment (Social Systems). Research to explicate the role of RDoC constructs in psychosis is advancing, and the hierarchical dimensional taxonomy may be able to guide this effort by suggesting particularly productive phenotypic targets.
We approached model selection from the perspective of incremental validity. A more common approach in the field is to use fit indices (e.g., BIC and AIC) to identify the best model. In a direct comparison of these strategies, we found that fit indices selected much more complex (7- and 12-factor) models, which provided a thorough account of correlations among symptoms but did not improve validity of the taxonomy beyond the four factors. It is important to recognize that not all factors that help to capture associations among items necessarily add to external validity of the model, and thus validation is an important follow-up on psychometric analyses (Barrett, 2007).
The present study also sheds light on hierarchy levels below the four dimensions. Although these levels generally did not show incremental validity in the present study, some of them may prove useful in explaining outcomes not considered here. Our hierarchical analyses built especially on a study by Peralta et al. (2013), who conducted an item-level exploratory factor analysis of a large pool of symptoms. They included content that goes beyond psychosis per se—namely, depression, mania, and catatonia—which we did not analyze and thus could not observe corresponding factors of Peralta et al. (2013). We also did not find an attention factor, likely because only one marker (“social inattentiveness”) was included in our analyses, which is insufficient to define a factor. All other dimensions of Peralta et al. (2013)—hallucinations, delusions, bizarre behavior, formal thought disorder, affective flattening, alogia, avolition-apathy, and anhedonia-asociality—emerged in our analyses. Specifically, the 8-factor solution included these dimensions, except that it had two delusions factors and a single apathy/asociality dimension. The distinction between avolition-apathy and anhedonia-asociality did emerge at the 12th level of the hierarchy, and thus all dimensions of Peralta et al. (2013) that were covered in the present symptom set were replicated.
Importantly, our selection of validators was not comprehensive. For instance, we have not examined genetic and environmental risk factors, and have not directly studied treatment outcome. Our objective was to begin the process of systematic comparison between alternative nosologic models rather than establishing one as superior. In fact, it is quite possible that ultimately different levels of the hierarchy will be found to be useful for different purposes. In this regard, the validity-based approach differs from the fit-based approach in that the former does not search for the best overall structure but for the best structure for a particular purpose. The advantage of the hierarchical approach to nosology is that it describes all possible levels of the hierarchy and enables one to use the level most informative for the given question. At present, the 4-dimension structure should be considered a useful scheme but not the only viable option.
The present study had several limitations. First, it focused on psychosis symptoms and did not consider mania and depression, which are included in some dimensional models of psychotic disorder (e.g., Barch et al., 2013; Emsley, Rabinowitz & Torreman, 2003; Peralta et al., 2013; van Os et al., 1999). This was a deliberate choice as affective symptoms fall primarily within the internalizing spectrum—and the lower-order structure of these dimensions is being mapped out in other studies (e.g., Krueger et al., 2012; Watson et al., 2012)—but ultimately dimensions comprising various spectra should be considered together to explicate the joint structure. Second, although we investigated replicability and stability of the four factors, structural analyses did not consider illness course per se, due to their focus on the early phase of the illness (first hospitalization). It will be important for future studies to capture temporal aspects of psychosis by analyzing, alongside symptoms, either characteristics of illness course (e.g., age of onset, illness duration, deteriorating vs improving course, etc) or relevant maladaptive traits that capture enduring patterns in the clinical picture. Third, the current investigation was limited to two measures, the SANS and SAPS. They are among the best established and most comprehensive instruments for assessment of psychosis symptoms, but they are not exhaustive, and future studies should seek to include multiple measures to ensure complete coverage of relevant phenotypes. Fourth, the sample size available for neural measures was modest, which prevented us from evaluating prediction of neural functioning from baseline symptoms. Nevertheless, our cross-sectional analyses were able to demonstrate the potential of symptom dimensions to advance research on neurobiology of psychosis. The 4-factor scheme, together with simpler models, was able to account for 12% of variance in Pe, which is substantial. Fifth, present analyses considered outcome at Year 20 rather than change in functioning since first hospitalization (i.e., did not control for initial functioning), because the research question was about relative merits of various symptom organizations rather than development of a prognostic algorithm. Adoption of the four symptom dimensions for prediction in clinical settings would require evaluation of the unique predictive power of these dimensions over other risk factors, and even larger sample sizes would be needed for this work. Finally, we did not control for treatment that participants received during the course of the follow-up, which likely reduced predictive power of baseline symptoms. Modeling of treatment effects in a naturalistic study is difficult and would be most feasible when treatment is controlled (all participants either receive the same regimen or are randomly assigned to different treatments), an important direction for future research.
Conclusions
The four dimensions—reality distortion, disorganization, inexpressivity, and apathy/asociality—were sufficient to capture information contained in symptom ratings that is relevant to neural abnormalities in error-processing and long-term outcomes of the illness. More general models—especially one-factor and two-factor—were associated with these validators also, and they can be easily scored from the four dimensions. These four factors have been well-documented in prior literature (e.g., Grube et al., 1998; Kring et al., 2013; Smith et al., 1998; Strauss et al., 2012, 2013), and present results provide an additional impetus for further investigation of these dimensions. Future research may reveal that a more elaborate model is needed to inform other research questions. Our aim was to facilitate this work by illustrating a validity-based technique for model comparison. This study also suggested a new scoring of the widely-used SAPS and SANS, which can improve the information provided by these instruments.
Supplementary Material
General Scientific Summary.
This study illustrates an approach to selection of factor structures that is based on external validity (prediction of validators) rather than internal validity (accounting for correlations among markers). It provides further evidence that the distinction between inexpressivity and apathy/asociality symptoms of psychosis is significant. Finally, we constructed scales to measure the identified dimensions and evaluated their psychometric characteristics.
Acknowledgments
This work was supported by the National Institutes of Health (Grant MH44801 to Evelyn J. Bromet and Grant MH094398 to Roman Kotov) and Stony Brook University (Clinical Research Scholar Award to Roman Kotov). We gratefully acknowledge the support of the participants and mental health community of Suffolk County for contributing their time and energy to this project. We are also indebted to dedicated efforts of study coordinators, interviewers for their careful assessments, and to the psychiatrists who derived the consensus diagnoses. Special thanks to Janet Lavelle for her many contributions to the study. Present findings have not been reported previously.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA school-age forms and profiles. Burlington, VA: University of Vermont Research Center for Children, Youth, and Families; 2001. [Google Scholar]
- Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City: The University of Iowa; 1983. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) Iowa City: The University of Iowa; 1984. [Google Scholar]
- Andreasen NC, Arndt S, Alliger R, Pharmed DM, Flaum M. Symptoms of schizophrenia: Methods, meanings, and mechanisms. Archives of General Psychiatry. 1995;52:341–351. doi: 10.1001/archpsyc.1995.03950170015003. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Olsen S. Negative v positive schizophrenia: definition and validation. Archives of General Psychiatry. 1982;39:789–794. doi: 10.1001/archpsyc.1982.04290070025006. [DOI] [PubMed] [Google Scholar]
- Andrews G, Goldberg DP, Krueger RF, Carpenter WT, Hyman SE, Sachdev P, Pine DS. Exploring the feasibility of a meta-structure for DSM-V and ICD-11: could it improve utility and validity? Psychological Medicine. 2009;39:1993–2000. doi: 10.1017/S0033291709990250. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: Author; 2013. [Google Scholar]
- Barch DM, Bustillo J, Gaebel W, Gur R, Heckers S, Malaspina D, … Carpenter W. Logic and justification for dimensional assessment of symptoms and related clinical phenomena in psychosis: relevance to DSM-5. Schizophrenia Research. 2013;150:15–20. doi: 10.1016/j.schres.2013.04.027. [DOI] [PubMed] [Google Scholar]
- Barrett P. Structural equation modelling: Adjudging model fit. Personality and Individual Differences. 2007;42:815–824. [Google Scholar]
- Blanchard JJ, Cohen AS. The structure of negative symptoms within schizophrenia: Implications for assessment. Schizophrenia Bulletin. 2006;32:238–245. doi: 10.1093/schbul/sbj013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard JJ, Kring AM, Horan WP, Gur R. Toward the next generation of negative symptom assessments: the collaboration to advance negative symptom assessment in schizophrenia. Schizophrenia Bulletin. 2011;37:291–299. doi: 10.1093/schbul/sbq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromet EJ, Kotov R, Fochtmann LJ, Carlson GA, Tanenberg-Karant M, Ruggero C, Chang SW. Diagnostic shifts during the decade following first admission for psychosis. American Journal of Psychiatry. 2011;168:1186–1194. doi: 10.1176/appi.ajp.2011.11010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromet EJ, Schwartz JE, Fennig S, Geller L, Jandorf L, Kovasznay B, … Rich C. The epidemiology of psychosis: The Suffolk County mental health project. Schizophrenia Bulletin. 1992;18:243–255. doi: 10.1093/schbul/18.2.243. [DOI] [PubMed] [Google Scholar]
- Brown AS, Susser ES, Jandorf L, Bromet EJ. Social class of origin and cardinal symptoms of schizophrenic disorders over the early illness course. Social Psychiatry Psychiatry and Epidemiology. 35:53–60. doi: 10.1007/s001270050008. [DOI] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. Model selection and multimodel inference: a practical information-theoretic approach. 2. New York, NY: Springer Science & Business Media; 2002. [Google Scholar]
- Carpenter WT, Bustillo JR, Thaker GK, Van Os J, Krueger RF, Green MJ. The psychoses: cluster 3 of the proposed meta-structure for DSM-V and ICD-11. Psychological Medicine. 2009;39:2025–2042. doi: 10.1017/S0033291709990286. [DOI] [PubMed] [Google Scholar]
- Carragher N, Krueger RF, Eaton NR, Markon KE, Keyes KM, Blanco C, … Hasin DS. ADHD and the externalizing spectrum: direct comparison of categorical, continuous, and hybrid models of liability in a nationally representative sample. Social Psychiatry and Psychiatric Epidemiology. 2014;49:1307–1317. doi: 10.1007/s00127-013-0770-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Minzenberg M, West R, Macdonald A. CNTRICS imaging biomarker selections: Executive control paradigms. Schizophrenia Bulletin. 2011;38:34–42. doi: 10.1093/schbul/sbr114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LA, Watson D, Reynolds S. Diagnosis and classification of psychopathology: Challenges to the current system and future directions. Annual Review of Psychology. 1995;46:121–153. doi: 10.1146/annurev.ps.46.020195.001005. [DOI] [PubMed] [Google Scholar]
- Craddock N, Owen MJ. The Kraepelinian dichotomy–going, going... but still not gone. The British Journal of Psychiatry. 2010;196:92–95. doi: 10.1192/bjp.bp.109.073429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow TJ. Molecular pathology of schizophrenia: more than one disease process? BMJ. 1980;280:66–68. doi: 10.1136/bmj.280.6207.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow TJ. The continuum of psychosis and its genetic origins. The sixty-fifth Maudsley lecture. The British Journal of Psychiatry. 1990;156:788–797. doi: 10.1192/bjp.156.6.788. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. Toward new approaches to psychotic disorders: the NIMH Research Domain Criteria project. Schizophrenia Bulletin. 2010;36:1061–1062. doi: 10.1093/schbul/sbq108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley R, Rabinowitz J, Torreman M The RIS-INT-35 Early Psychosis Global Working Group. The factor structure for the Positive and Negative Syndrome Scale (PANSS) in recent-onset psychosis. Schizophrenia Research. 2003;61:47–57. doi: 10.1016/s0920-9964(02)00302-x. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics. 1974;16:143–149. [Google Scholar]
- Everett JE, Entrekin LV. Factor comparability and the advantages of multiple group factor analysis. Multivariate Behavioral Research. 1980;15:165–180. [Google Scholar]
- Fabrigar LR, Wegener DT, MacCallum RC, Strahan EJ. Evaluating the use of exploratory factor analysis in psychological research. Psychological Methods. 1999;4:272–299. [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography Clinical Neurophysiology. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Farmer RF, Seeley JR, Kosty DB, Olino TM, Lewinsohn PM. Hierarchical organization of axis I psychiatric disorder comorbidity through age 30. Comprehensive Psychiatry. 2013;54:523–532. doi: 10.1016/j.comppsych.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn SE. Structural stability of the MMPI in adult males. Journal of Consulting and Clinical Psychology. 1986;54:703–707. doi: 10.1037//0022-006x.54.5.703. [DOI] [PubMed] [Google Scholar]
- Forbush KT, Watson D. The structure of common and uncommon mental disorders. Psychological Medicine. 2013;43:97–108. doi: 10.1017/S0033291712001092. [DOI] [PubMed] [Google Scholar]
- Ford JM, Morris SE, Hoffman RE, Sommer I, Waters F, McCarthy-Jones S, Cuthbert BN. Studying hallucinations within the NIMH RDoC framework. Schizophrenia Bulletin. 2014;40(Suppl 4):S295–S304. doi: 10.1093/schbul/sbu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Kotov R, Bromet E, Hajcak G. Beyond the broken error-related negativity: Functional and diagnostic correlates of error processing in psychosis. Biological Psychiatry. 2012;71:864–872. doi: 10.1016/j.biopsych.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foussias G, Remington G. Negative symptoms in schizophrenia: avolition and Occam’s razor. Schizophrenia Bulletin. 2010;36:359–369. doi: 10.1093/schbul/sbn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg LR. Doing it all Bass-Ackwards: The development of hierarchical factor structures from the top down. Journal of Research in Personality. 2006;40:347–358. [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, … Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders. Archives of General Psychiatry. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Grube BS, Bilder RM, Goldman RS. Meta-analysis of symptom factors in schizophrenia. Schizophrenia Research. 1998;31:113–120. doi: 10.1016/s0920-9964(98)00011-5. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman HH. Modern factor analysis. 3. Chicago: University of Chicago Press; 1976. [Google Scholar]
- Haslam N, Holland E, Kuppens P. Categories versus dimensions in personality and psychopathologyL a quantitative review of taxometric research. Psychological Medicine. 2012;42:903–920. doi: 10.1017/S0033291711001966. [DOI] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW. Meta-analysis and the science of schizophrenia: variant evidence or evidence of variants? Neuroscience & Biobehavioral Reviews. 2004;28:379–394. doi: 10.1016/j.neubiorev.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Heinrichs DW, Hanlon TE, Carpenter WT. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophrenia Bulletin. 1984;10:388–398. doi: 10.1093/schbul/10.3.388. [DOI] [PubMed] [Google Scholar]
- Ho BC, Nopoulos P, Flaum M, Arndt S, Andreasen NC. Two year outcome in first-episode schizophrenia: predictive value of symptoms for quality of life. American Journal of Psychiatry. 1998;155:1196–1201. doi: 10.1176/ajp.155.9.1196. [DOI] [PubMed] [Google Scholar]
- Jablensky A. Subtyping schizophrenia: implications for genetic research. Molecular Psychiatry. 2006;11:815–836. doi: 10.1038/sj.mp.4001857. [DOI] [PubMed] [Google Scholar]
- Johns LC, Van Os J. The continuity of psychotic experiences in the general population. Clinical Psychology Review. 2001;21:1125–1141. doi: 10.1016/s0272-7358(01)00103-9. [DOI] [PubMed] [Google Scholar]
- Kansal V, Patriciu I, Kiang M. Illness insight and neurophysiological error-processing deficits in schizophrenia. Schizophrenia Research. 2014;156:122–127. doi: 10.1016/j.schres.2014.03.023. [DOI] [PubMed] [Google Scholar]
- Kenny DA. Statistics for the social and behavioral sciences. Boston, MA: Little, Brown; 1987. [Google Scholar]
- Kim H, Eaton NR. The hierarchical structure of common mental disorders: Connecting multiple levels of comorbidity, bifactor models, and predictive validity. Journal of Abnormal Psychology. doi: 10.1037/abn0000113. (in press) [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT. A separate disease within the syndrome of schizophrenia. Archives of General Psychiatry. 2001;58:165–171. doi: 10.1001/archpsyc.58.2.165. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Survey Replication. Archives of General Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Eaton NR, Krueger RF, Skodol AE, Wall MM, Grant BF, … Hasin DS. Thought disorder in the meta-structure of psychopathology. Psychological Medicine. 2013;43:1673–1683. doi: 10.1017/S0033291712002292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Galderisi S. Deficit schizophrenia: an update. World Psychiatry. 2008;7:143–147. doi: 10.1002/j.2051-5545.2008.tb00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Chang SW, Fochtmann LJ, Mojtabai R, Carlson GA, Sedler MJ, Bromet E. Schizophrenia in the internalizing-externalizing framework: a third dimension? Schizophrenia Bulletin. 2011;37:1168–1178. doi: 10.1093/schbul/sbq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Guey LT, Bromet EJ, Schwartz JE. Smoking in schizophrenia: diagnostic specificity, symptom correlates, and illness severity. Schizophrenia Bulletin. 2010;36:173–181. doi: 10.1093/schbul/sbn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Krueger RF, Watson D, Achenbach TM, Althoff RR, Bagby M, Brown TA, Carpenter WT, Caspi A, Clark LA, Eaton NR, Forbes MK, Forbush KT, Goldberg D, Hasin D, Hyman SE, Ivanova MY, Lahey BB, Lynam DR, Markon K, Miller JD, Moffitt TE, Morey LC, Ormel J, Patrick CJ, Regier DA, Rescorla L, Robinson E, Ruggero CJ, Samuel DB, Sellbom M, Simms LJ, Skodol AE, Slade T, South SC, Tackett JL, Waldman ID, Widiger TA, Wright AGC, Zimmerman M. The Hierarchical Taxonomy Of Psychopathology (HiTOP): A dimensional alternative to traditional nosologies. doi: 10.1037/abn0000258. (submitted) [DOI] [PubMed] [Google Scholar]
- Kotov R, Leong SH, Mojtabai R, Erlanger ACE, Fochtmann LJ, Constantino E, … Bromet EJ. Boundaries of schizoaffective disorder: revisiting Kraepelin. JAMA psychiatry. 2013;70:1276–1286. doi: 10.1001/jamapsychiatry.2013.2350. [DOI] [PubMed] [Google Scholar]
- Kotov R, Ruggero CJ, Krueger RF, Watson D, Yuan Q, Zimmerman M. New dimensions in the quantitative classification of mental illness. Archives of General Psychiatry. 2011;68:1003–1011. doi: 10.1001/archgenpsychiatry.2011.107. [DOI] [PubMed] [Google Scholar]
- Kring AM, Gur RE, Blanchard JJ, Horan WP, Reise SP. The clinical assessment interview for negative symptoms (CAINS): Final development and validation. American Journal of Psychiatry. 2013;170:165–172. doi: 10.1176/appi.ajp.2012.12010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Derringer J, Markon KE, Watson D, Skodol AE. Initial construction of a maladptive personality trait model and inventory for DSM-5. Psychological Medicine. 2012;42:1879–1890. doi: 10.1017/S0033291711002674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Markon KE. Reinterpreting comorbidity: A model-based approach to understanding and classifying psychopathology. Annual Review of Clinical Psychology. 2006;2:111–133. doi: 10.1146/annurev.clinpsy.2.022305.095213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer MD. Linking antisocial behavior, substance use, and personality: an integrative quantitative model of the adult externalizing spectrum. Journal of Abnormal Psychology. 2007;116:645–666. doi: 10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Rathouz PJ, Van Hulle C, Urbano RC, Krueger RF, Applegate B, … Waldman ID. Testing structural models of DSM-IV symptoms of common forms of child and adolescent psychopathology. Journal of Abnormal Child Psychology. 2008;36:187–206. doi: 10.1007/s10802-007-9169-5. [DOI] [PubMed] [Google Scholar]
- Liddle PF. The symptoms of chronic schizophrenia. A re-examination of the positive-negative dichotomy. The British Journal of Psychiatry. 1987;151:145–151. doi: 10.1192/bjp.151.2.145. [DOI] [PubMed] [Google Scholar]
- Linscott RJ, Van Os J. An updated and conservative systematic review and meta-analysis of epidemiological evidence on psychotic experiences in children and adults: on the pathway from proneness to persistence to dimensional expression across mental disorders. Psychological Medicine. 2013;43:1133–1149. doi: 10.1017/S0033291712001626. [DOI] [PubMed] [Google Scholar]
- Luck SJ. An introduction to the event-related potential technique. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- MacCallum RC, Zhang S, Preacher KJ. On the practice of dichotomization of quantitative variables. Psychological Methods. 2003;7:19–40. doi: 10.1037/1082-989x.7.1.19. [DOI] [PubMed] [Google Scholar]
- Markon KE. Modeling psychopathology structure: A symptom-level analysis of Axis I and II disorders. Psychological Medicine. 2010;40:273–288. doi: 10.1017/S0033291709990183. [DOI] [PubMed] [Google Scholar]
- Markon KE. Epistemological pluralism and scientific development: An argument against authoritative nosologies. Journal of Personality Disorders. 2013;27:554–579. doi: 10.1521/pedi.2013.27.5.554. [DOI] [PubMed] [Google Scholar]
- Markon KE, Chmielewski M, Miller CJ. The reliability and validity of discrete and continuous measures of psychopathology: a quantitative review. Psychological Bulletin. 2011;137:856–879. doi: 10.1037/a0023678. [DOI] [PubMed] [Google Scholar]
- Markon KE, Krueger RF. Categorical and continuous models of liability to externalizing disorders: A direct comparison in NESARC. Archives of General Psychiatry. 2005;62:1352–1359. doi: 10.1001/archpsyc.62.12.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markon KE, Krueger RF, Watson D. Delineating the structure of normal and abnormal personality: An integrative hierarchical approach. Journal of Personality and Social Psychology. 2005;88:139–157. doi: 10.1037/0022-3514.88.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon DH, Fedor M, Faustman WO, Gray M, Askari N, Ford JM. Response-monitoring dysfunction in schizophrenia: an event-related brain potential study. Journal of Abnormal Psychology. 2002;111:22–41. [PubMed] [Google Scholar]
- Milev P, Ho BC, Arndt S, Andreasen NC. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. American Journal of Psychiatry. 2005;162:495–506. doi: 10.1176/appi.ajp.162.3.495. [DOI] [PubMed] [Google Scholar]
- Morey LC. Professional manual for the Personality Assessment Inventory. 2. Lutz, FL: Psychological Assessment Resources; 2007. [Google Scholar]
- Morey LC, Hopwood CJ, Markowitz JC, Gunderson JG, Grilo CM, McGlashan TH, … Skodol AE. Comparison of alternative models for personality disorders, II: 6-, 8-and 10-year follow-up. Psychological Medicine. 2012;42:1705–1713. doi: 10.1017/S0033291711002601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MT, Seal ML, Pantelis C, Phillips LJ. Evidence of a dimensional relationship between schizotypy and schizophrenia: a systematic review. Neuroscience & Biobehavioral Reviews. 2013;37:317–327. doi: 10.1016/j.neubiorev.2013.01.004. [DOI] [PubMed] [Google Scholar]
- O’Donnell BF, Salisbury DF, Niznikiewicz MA, Brenner CA, Vohs JL. Abnormalities of event-related potential components in schizophrenia. In: Luck SJ, Kappenman ES, editors. Oxford Handbook of ERP Components. New York: Oxford University Press; 2011. [Google Scholar]
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- Overbeek TJ, Nieuwenhuis S, Ridderinkhof KR. Dissociable components of error processing: on the functional significance of the Pe vis-à-vis the ERN/Ne. Journal of Psychophysiology. 2005;19:319–329. [Google Scholar]
- Pearlson GD. Etiologic, Phenomenologic, and Endophenotypic Overlap of Schizophrenia and Bipolar Disorder. Annual Review of Clinical Psychology. 2015;11:251–281. doi: 10.1146/annurev-clinpsy-032814-112915. [DOI] [PubMed] [Google Scholar]
- Peralta V, Cuesta MJ. How many and which are the psychopathological dimensions in schizophrenia? Issues influencing their ascertainment. Schizophrenia Research. 2001;49:269–285. doi: 10.1016/s0920-9964(00)00071-2. [DOI] [PubMed] [Google Scholar]
- Peralta V, Moreno-Izco L, Calvo-Barrena L, Cuesta MJ. The low-and higher-order factor structure of symptoms in patients with a first episode of psychosis. Schizophrenia Research. 2013;147:116–124. doi: 10.1016/j.schres.2013.03.018. [DOI] [PubMed] [Google Scholar]
- Perez VB, Ford JM, Roach BJ, Woods SW, McGlashan TH, Srihari VH, … Mathalon DH. Error monitoring dysfunction across the illness course of schizophrenia. Journal of Abnormal Psychology. 2012;121:372–387. doi: 10.1037/a0025487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins E, Guze SB. Establishment of diagnostic validity in psychiatric illness: its application to schizophrenia. American Journal of Psychiatry. 1970;126:983–987. doi: 10.1176/ajp.126.7.983. [DOI] [PubMed] [Google Scholar]
- Roysamb E, Kendler KS, Tambs K, Orstavik RE, Neale MC, Aggen SH, … Reichbørn-Kjennerud T. The joint structure of DSM-IV Axis I and Axis II disorders. Journal of Abnormal Psychology. 2011;120:198–209. doi: 10.1037/a0021660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo M, Levine SZ, Demjaha A, Di Forti M, Bonaccorso S, Fearon P, … Reichenberg A. Association between symptom dimensions and categorical diagnoses of psychosis: a cross-sectional and longitudinal investigation. Schizophrenia Bulletin. 2014;40:111–119. doi: 10.1093/schbul/sbt055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel SJ, Irani F, Brensinger CM, Kohler CG, Bilker WB, Ragland JD, … Gur RE. Prognostic variables at intake and long-term level of function in schizophrenia. American Journal of Psychiatry. 2006;163:433–441. doi: 10.1176/appi.ajp.163.3.433. [DOI] [PubMed] [Google Scholar]
- Smith DA, Mar CM, Turoff BK. The structure of schizophrenic symptoms: A meta-analytic confirmatory factor analysis. Schizophrenia Research. 1998;31:57–70. doi: 10.1016/s0920-9964(98)00009-7. [DOI] [PubMed] [Google Scholar]
- Strauss JS, Carpenter WT, Bartko JJ. Speculations on the processes that underlie schizophrenic symptoms and signs: III. Schizophrenia Bulletin. 1974;1:61–69. doi: 10.1093/schbul/1.11.61. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Hong LE, Gold JM, Buchanan RW, McMahon RP, Keller WR, … Kirkpatrick B. Factor structure of the brief negative symptom scale. Schizophrenia Research. 2012;142:96–98. doi: 10.1016/j.schres.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Horan WP, Kirkpatrick B, Fischer BA, Keller WR, Miski P, … Carpenter WT. Deconstructing negative symptoms of schizophrenia: avolition–apathy and diminished expression clusters predict clinical presentation and functional outcome. Journal of Psychiatric Research. 2013;47:783–790. doi: 10.1016/j.jpsychires.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart GW, Pantelis C, Klimidis S, Minas IH. The three-syndrome model of schizophrenia: Meta-analysis of an artefact. Schizophrenia Research. 1999;39:233–242. doi: 10.1016/s0920-9964(99)00019-5. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Ivleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, … Sweeney JA. Clinical phenotypes of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) American Journal of Psychiatry. 2013;170:1263–1274. doi: 10.1176/appi.ajp.2013.12101339. [DOI] [PubMed] [Google Scholar]
- Teesson M, Slade T, Mills K. Comorbidity in Australia: Findings of the 2007 National Survey of Mental Health and Wellbeing. Australian and New Zealand Journal of Psychiatry. 2009;43:606–614. doi: 10.1080/00048670902970908. [DOI] [PubMed] [Google Scholar]
- van Os J, Gilvarry C, Bale R, Van Horn E, Tattan T, White I. A comparison of the utility of dimensional and categorical representations of psychosis. Psychological Medicine. 1999;29:595–606. doi: 10.1017/s0033291798008162. [DOI] [PubMed] [Google Scholar]
- Ventura J, Subotnik KL, Gitlin MJ, Gretchen-Doorly D, Ered A, Villa KF, … Nuechterlein KH. Negative symptoms and functioning during the first year after a recent onset of schizophrenia and 8years later. Schizophrenia Research. 2015;161:407–413. doi: 10.1016/j.schres.2014.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton KE, Ormel J, Krueger RF. The dimensional nature of externlaiizng behaviors in adolescence: evidence from a direct comparison of categorical, dimensional, and hybri models. Journal of Abnormal Child Psychology. 2011;39:553–561. doi: 10.1007/s10802-010-9478-y. [DOI] [PubMed] [Google Scholar]
- Watson D. Subtypes, specifiers, epicycles, and eccentrices: towards a more parsimonious taxonomy of psychopathology. Clinical Psychology: Science and Practice. 2003a;10:233–238. [Google Scholar]
- Watson D, Clark LA. Clinical diagnosis at a cross roads. Clinical Psychology: Science and Practice. 2006;13:210–215. [Google Scholar]
- Watson D, O’Hara MW, Naragon-Gainey K, Koffel E, Chmielewski M, Kotov R, … Ruggero C. Development and validation of new anxiety and bipolar symptom scales for an expanded version of the IDAS (the IDAS-II) Assessment. 2012;19:399–420. doi: 10.1177/1073191112449857. [DOI] [PubMed] [Google Scholar]
- Westen D, Novotny CM, Thompson-Brenner H. The empirical status of empirically supported psychotherapies: assumptions, findings, and reporting in controlled clinical trials. Psychological Bulletin. 2004;130:631–663. doi: 10.1037/0033-2909.130.4.631. [DOI] [PubMed] [Google Scholar]
- Widiger TA, Samuel DB. Diagnostic categories or dimensions? A question for the Diagnostic and statistical manual of mental disorders--fifth edition. Journal of Abnormal Psychology. 2005;114:494–504. doi: 10.1037/0021-843X.114.4.494. [DOI] [PubMed] [Google Scholar]
- Widiger TA, Trull TJ. Plate tectonics in the classification of personality disorder: shifting to a dimensional model. American Psychologist. 2007;62:71–83. doi: 10.1037/0003-066X.62.2.71. [DOI] [PubMed] [Google Scholar]
- White C, Stirling J, Hopkins R, Morris J, Montague L, Tantam D, Lewis S. Predictors of 10-year outcome of first-episode psychosis. Psychological Medicine. 2009;39:1447–1456. doi: 10.1017/S003329170800514X. [DOI] [PubMed] [Google Scholar]
- Whitty P, Clarke M, McTigue O, Browne S, Kamali M, Kinsella A, … O’Callaghan E. Predictors of outcome in first-episode schizophrenia over the first 4 years of illness. Psychological Medicine. 2008;38:1141–1146. doi: 10.1017/S003329170800336X. [DOI] [PubMed] [Google Scholar]
- Wright AGC, Simms LJ. A metastructural model of mental disorders and pathological personality traits. Psychological Medicine. 2015;45:2309–2319. doi: 10.1017/S0033291715000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A, Krueger RF, Hobbs MJ, Markon KE, Eaton NR, Slade T. The structure of psychopathology: toward an expanded quantitative empirical model. Journal of Abnormal Psychology. 2013;122:281–294. doi: 10.1037/a0030133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavos HM, Freeman D, Haworth CM, McGuire P, Plomin R, Cardno AG, Ronald A. Consistent etiology of severe, frequent psychotic experiences and milder, less frequent manifestations: a twin study of specific psychotic experiences in adolescence. JAMA psychiatry. 2014;71:1049–1057. doi: 10.1001/jamapsychiatry.2014.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M, Ellison W, Young D, Chelminski I, Dalrymple K. How many different ways do patients meet the diagnostic criteria for major depressive disorder? Comprehensive Psychiatry. 2015;56:29–34. doi: 10.1016/j.comppsych.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Clark HL, Multach MD, Walsh E, Rosenstein LK, Gazarian D. Have treatment studies of depression become even less generalizable? A review of the inclusion and exclusion criteria in placebo-controlled antidepressant efficacy trials published during the past 20 years. Mayo Clinic Proceedings. doi: 10.1016/j.mayocp.2015.06.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.