Summary
Complement and Toll-like receptors (TLRs) play key roles in the host immune response and are swiftly activated by infection or other types of immunological stress. This review focuses on the capacity of complement and TLRs to engage in signaling crosstalk, ostensibly to coordinate immune and inflammatory responses through synergistic or antagonistic (regulatory) interactions. However, over-activation or dysregulation of either system may lead – often synergistically – to exaggerated inflammation and host tissue injury. Intriguingly, moreover, certain pathogens can manipulate complement-TLR crosstalk pathways in ways that undermine host immunity and favor their persistence. In the setting of polymicrobial inflammatory disease, subversion of complement-TLR crosstalk by keystone pathogens can promote dysbiosis. Knowledge of the molecular mechanisms underlying complement-TLR crosstalk pathways can, therefore, be used productively for tailored therapeutic approaches, such as, to enhance host immunity, mitigate destructive inflammation, or counteract microbial subversion of the host response.
Keywords: Complement, TLR, crosstalk, inflammation, immune evasion
Introduction
In co-evolving with the microbial world, mammalian innate immunity has developed effective sentinel mechanisms to promptly detect and respond to infections. Sentinel cells (e.g., neutrophils, macrophages, and dendritic cells) sense invading pathogens through pattern- recognition receptors (PRRs) and alert downstream innate and/or adaptive mechanisms aiming to eradicate or control the infection (1). A major PRR family is represented by the Toll-like receptors (TLRs), each member of which senses distinct types of conserved microbial structures (‘microbe-associated molecular patterns’; MAMPs), thus endowing the innate response with a degree of specificity (e.g., TLR2 responds to lipoteichoic acid, TLR3 to viral double-stranded RNA, TLR4 to lipopolysaccharide [LPS], TLR5 to flagellin and TLR9 to bacterial CpG DNA) (2, 3). The broad but distinct specificities of the TLRs as well as their ability to form heterotypic multi-receptor complexes and engage distinct intracellular signaling molecules further diversifies their recognition and signaling capacities (4, 5). These attributes of the TLRs (and other PRRs) enable the host to detect almost any type of infection, discriminate between different classes of microbes and hence mount a context-relevant immune response.
In addition to sentinel cells, innate immunity also has a humoral arm that includes a heterogeneous group of pattern-recognition molecules (PRMs), such as, collectins (e.g., mannose-binding lectin; MBL), ficolins, pentraxins and the complement component C1q (6, 7). Soluble PRMs can be released either locally by stimulated inflammatory cells or systemically following their production in liver. Although structurally heterogeneous, these molecules share evolutionarily conserved functions, such as microbial opsonization as well as activation and regulation of the complement system (8).
Historically established as a cascade of antimicrobial proteins in the blood, complement is now appreciated as a network of interacting fluid-phase and cell surface-associated molecules (PRMs, convertases and other proteases, regulators, and signaling receptors) that trigger, amplify, and regulate immunity and inflammation (9). The complement cascade is triggered by distinct mechanisms (classical, lectin, or alternative) that converge at the third component (C3) and lead to the generation of effectors with diverse functions (e.g., recruitment and activation of inflammatory cells via the C3a and C5a anaphylatoxins that activate specific G-protein-coupled receptors; microbial opsonization through C3b; and direct lysis of susceptible targeted microbes by means of the C5b-9 membrane attack complex) (9).
During an infection, complement and TLRs are rapidly activated to provide critical frontline defense and act as key mediators between innate and adaptive immunity (10). Interestingly, several microbial products, including LPS (TLR4 agonist), zymosan (TLR2/6 agonist) and CpG DNA (TLR9 agonist), can activate complement in addition to initiating TLR signaling (11, 12). Therefore, an appropriately coordinated host immune response would necessitate signaling crosstalk between TLR and complement pathways, leading to synergistic or antagonistic interactions. Synergistic pathways can enhance the sensitivity of detection, since even individually weak stimuli can potentially combine to elicit a robust immune response. Conversely, antagonistic pathways can augment the specificity of the host response by controlling it and preventing bystander tissue damage (13). Typical examples for these contrasting functions include the cooperation between TLR2 and the C-type lectin dectin-1 for effective anti-fungal immunity (14) and the homeostatic suppression of TLR-induced pro-inflammatory responses by adenosine receptors (15, 16).
This review summarizes recent literature on the biological importance of complement–TLR crosstalk pathways. Such pathways lead to diverse effects raging from reinforcement of innate immunity to exacerbation of pathologic inflammation or, conversely, regulation of unwarranted inflammation, depending on the receptors involved and the cellular context. Moreover, mechanisms that allow the interplay between complement and TLRs can be potentially exploited by certain pathogens to modulate the host response in ways that favor pathogen survival and persistence.
Regulation of immune and inflammatory responses by complement-TLR cooperation
As alluded to above, complement and TLRs are swiftly co-activated in response to microbial infection, while common microbial molecules (such as LPS and CpG DNA) can act as both TLR ligands and complement activators (9). At the cellular level, signaling crosstalk interactions between complement and TLRs have been shown in several cell types, including monocytes, macrophages, neutrophils, and dendritic cells (17–22). In vivo, the early innate immune response is shaped, to a large extent, by bidirectional crosstalk between the two systems (10).
In perhaps the first in vivo systematic study to dissect complement-TLR crosstalk pathways, the authors employed systemic administration of different TLR ligands to mice lacking decay-accelerating factor (DAF), a major membrane-associated complement inhibitor. Specifically, LPS (TLR4), zymosan (TLR2/6), and CpG oligodeoxynucleotide (TLR9) all induced significantly higher tumor necrosis factor (TNF), interleukin-1β (IL-1β), and IL-6 responses compared to the same ligands given to wild-type mice (12). Similarly, mice systemically co-treated with TLR ligands and cobra venom factor, a potent complement activator, elicited remarkably high plasma levels of proinflammatory cytokines, further supporting that complement can amplify inflammation in co-operation with TLR signaling (12). Further work revealed a critical involvement of the anaphylatoxin receptors (C3aR and C5aR1 [CD88]) in the complement-TLR synergism for enhanced production of pro-inflammatory and antimicrobial mediators (12, 23). The signaling pathways involved in complement-TLR crosstalk converge at the level of mitogen-activated protein kinases (MAPK), specifically extracellular signal-regulated kinase-1 (ERK1), ERK2 and JUN N-terminal kinase (JNK), which activate the transcriptional factors nuclear factor-κB (NF-κB) and activator protein-1 (AP-1) (12) (Figure 1). Although this synergy could potentially enhance innate immune defenses against infection, it may also contribute to inflammatory pathology. For instance, complement-TLR synergy may actually account for earlier observations that the inhibition of C5a signaling protects against sepsis induced by high-dose LPS or by cecal ligation and puncture (CLP) peritonitis (24). Moreover, the synergistic complement–TLR interaction seen in DAF-deficient mice might explain, at least in part, why DAF-deficient mice are particularly susceptible to inflammatory and autoimmune diseases (25).
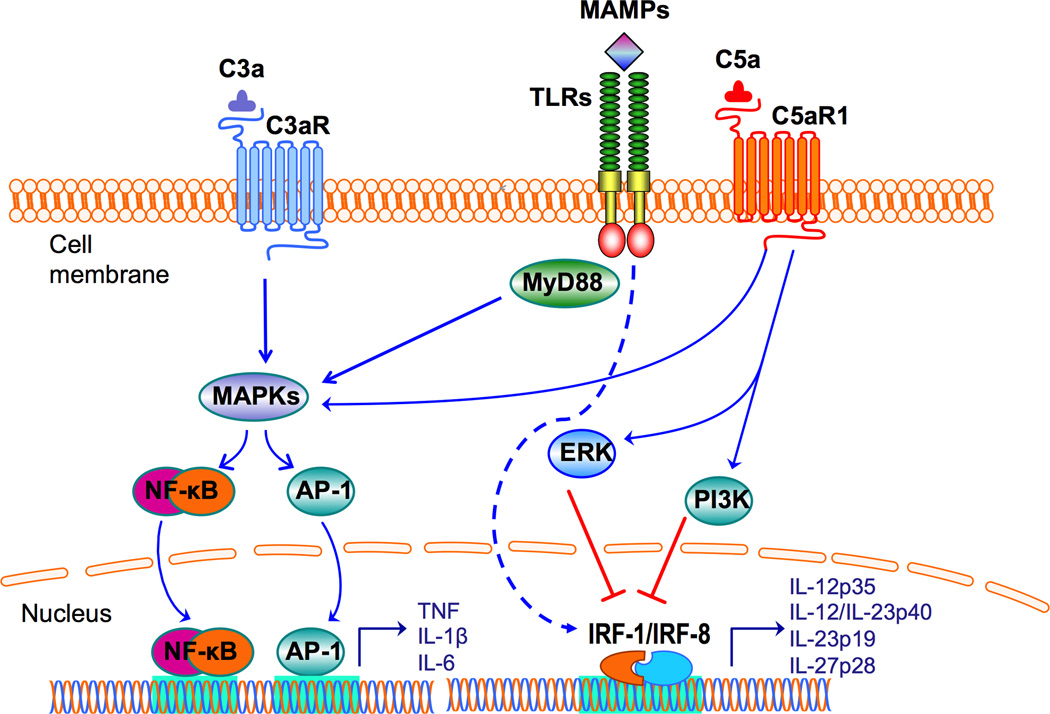
Figure 1. Synergistic and antagonistic interactions between complement and TLRs.
Complement and TLRs are co-activated in response to microbial infection. Complement anaphylatoxin receptor signaling induced by C3a or C5a synergizes with TLR signaling resulting in enhanced activation of MAPKs and transcription factors, such as NF-κB and AP-1, resulting in upregulation of proinflammatory cytokine expression. TLRs are activated by MAMPs, some of which (e.g., LPS and zymosan) can additionally co-activate complement. In contrast, complement can downregulate TLR-induced cytokines of the IL-12 family. Activation of C5aR1 by C5a suppresses TLR-induced mRNA expression of IL-12p35, IL-12/IL-23p40, IL-23p19, and IL-27p28 (hence production of bioactive IL-12, IL-23, and IL-27) in monocytes/macrophages. The underlying signaling mechanism involves induction of PI3K or ERK1/2 signaling, which in turn suppress crucial transcription factors (IRF-1 and IRF-8) that regulate these cytokines.
Complement-TLR crosstalk synergy has also been demonstrated at mucosal sites. Indeed, in the murine gingival tissue, the concomitant activation of C5aR and TLR2 by local co-injection of specific agonists (C5a and the TLR2 ligand Pam3Cys) induced significantly higher levels of TNF, IL-1β, IL-6, and IL-17A mRNA and protein than activation of each receptor alone (26). In fact, destructive periodontal inflammation appears to depend on synergy between C5aR1 and TLR2, since mice deficient in either C5aR1 or TLR2 are essentially resistant against inflammatory bone loss in the periodontium (27, 28). Consistently, treatment of mice subjected to experimental periodontitis with PMX-53, a C5aR1 antagonist, inhibits periodontal inflammation (TNF, IL-1β, IL-6, and IL-17) and bone loss, regardless of the presence of TLR2 (i.e., inflammatory bone loss can be effectively inhibited by blocking just one of the two crosstalking receptors) (26).
However, in other experimental systems, where interactions might be partially synergistic or additive, combined inhibition of complement and PRRs may be more effective than inhibition of each system alone. For instance, in a human whole-blood model, combined inhibition of complement and CD14 was shown to be more effective in blocking E. coli-induced cytokine responses than single inhibition (29, 30). CD14 lacks a transmembrane signaling domain but acts as a critical co-receptor of TLRs (mostly TLR4 and TLR2) (3), although it might also have TLR-independent effects that contribute to inflammation.
Another study in the human whole-blood model focused on interactions between complement and TLR9 signaling induced by CpG oligodeoxynucleotides, which are considered as vaccine adjuvants (11). These investigators showed that complement inhibition at C3 suppresses both DNA-backbone-mediated maturation of antigen-presenting cells (upregulation of CD40 and CD83) and DNA-sequence-specific induction of cytokines. Interestingly, a CpG oligodeoxynucleotide (CpG-2006) could trigger the classical and the alternative pathway of complement, which in turn promoted the cellular uptake of CpG-2006 (11). Therefore, the immunostimulatory function of oligodeoxynucleotides such as CpG-2006, seems to be reliant upon the combined activation of complement and TLR9.
Although C5aR1 synergizes with TLR2 for IL-17A induction in experimental mouse periodontitis (26), C5aR1 downregulates IL-17A in endotoxic shock in mice (21). It is uncertain whether this difference can be attributed to the different disease models or the different TLRs involved (TLR2 vs. TLR4). Intriguingly, C5aR1 promotes the induction of another IL-17 isoform (IL-17F) in endotoxic shock (31). In this regard, C5aR1 synergizes with TLR4 for IL-17F production in mouse macrophages via a MyD88- phosphatidylinositol-3 kinase (PI3K)-Akt pathway (31), whereas the same C5aR1-TLR4 crosstalk in the same cell type inhibits IL-17A production (21) (Figure 2). According to this study, the major source of IL-17A during endotoxemia in mice was not the ‘usual suspects’ (CD4+ T cells, γδ T cells, or NK cells) but rather CD11b+F4/80+ macrophages (21). Mechanistically, C5a was shown to activate PI3K-Akt and mitogen-activated protein kinase kinases 1/2 (MEK1/2)-ERK1/2 pathways, resulting in C5aR1 (but not C5aR2)-dependent induction of IL-10, which subsequently inhibits production of IL-17A (as well as IL-23) (21) (Figure 2). Because IL-17F has considerably reduced bioactivity as compared to IL-17A (32), C5a appears to shift the IL-17A – IL-17F balance toward the less bioactive molecule to mitigate excessive inflammation in acute conditions. However, it is currently unclear why C5aR1-induced IL-10 inhibits IL-17A preferentially over IL-17F.
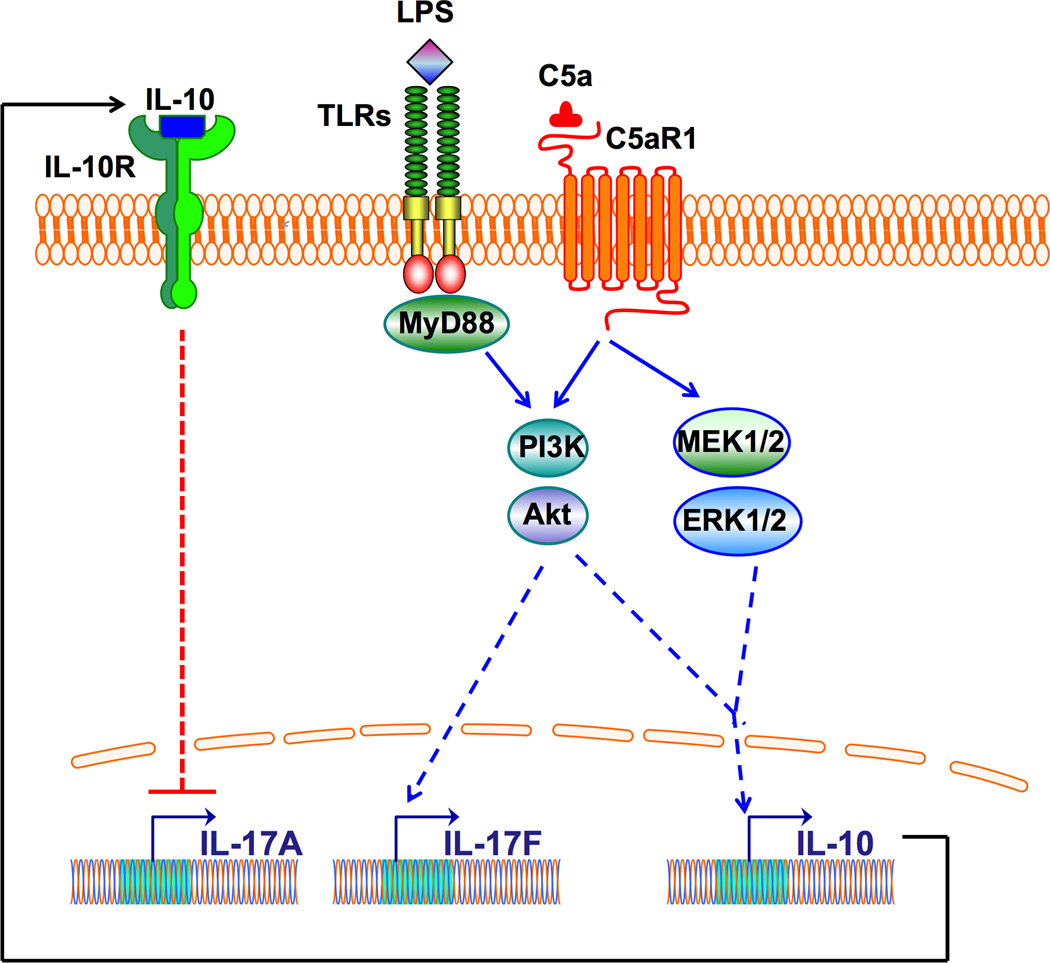
Figure 2. C5aR1 regulation of IL-17 isoforms in LPS-activated macrophages.
C5aR1 and TLR4 promote the induction of IL-17F in mouse macrophages via a MyD88-PI3K-Akt pathway. On the other hand, C5a-induced activation of C5aR1 activates PI3K-Akt and MEK1/2-ERK1/2 pathways that lead to induction of IL-10, which subsequently inhibits production of IL-17A.
The relatively recently discovered C5aR2 (also referred to as C5a-like receptor 2; GPR77) functions as an alternative high-affinity receptor for C5a (33). Owing to its inability to productively couple to G proteins, C5aR2 was originally perceived as a non-signaling decoy receptor that could compete with C5aR1 for C5a binding, thereby mitigating C5a-dependent inflammation (34, 35). Consistently, upon pulmonary immune complex injury, C5aR2-deficient mice display increased lung inflammation (as revealed by elevated TNF and IL-6 responses and neutrophil recruitment) compared to wild-type controls, although the authors did not rule out G-protein-independent anti-inflammatory signaling downstream of C5aR2 (36).
Subsequent studies indeed showed that C5aR2 might also play active, yet complex and poorly understood, roles in inflammation regulation including crosstalk interactions with TLRs (37–40). In the latter regard, C5aR2-deficient mice exhibited increased survival rates compared with wild-type controls after cecal ligation and puncture-induced sepsis (38). Rather than antagonizing C5aR1, C5aR2 synergizes with C5aR1 to cause sepsis by inducing the expression of the mobility group box 1 (HMGB1) protein (38). Interestingly, the induction of HMGB1 by LPS and C5a, or by LPS alone, is diminished in C5aR2-deficient macrophages. This finding suggests involvement of possible C5aR2–TLR4 crosstalk in the induction of HMGB1 that appears to require mitogen-activated protein MEK1/2, JNK1/2 and PI3K (38). Moreover, C5aR2 was shown to mediate C5a-induced activation of mast cells (41) and to promote atherosclerosis and neointimal plaque formation in apolipoprotein E-deficient mice (42). In contrast to these pro-inflammatory roles by C5aR2, other studies showed that C5aR2 interacts physically with and negatively regulates C5aR1 signaling in neutrophils and macrophages (39, 43), thereby providing a mechanistic basis for its reported anti-inflammatory action (36). In toto, the activities of C5aR2 appear to be dynamic and contextual depending on cell type, tissue, and disease model (44).
In macrophages, complement receptor 3 (CR3; CD11b/CD18) can regulate the signaling activity of TLRs that utilize Mal (MyD88-adaptor like; also known as Toll/IL-1R(TIR)-domain-containing adaptor protein; TIRAP) as an adaptor, i.e., TLR2 and TLR4 (45) (Figure 3). Specifically, outside-in signaling by CR3 leads to activation of ADP ribosylation factor 6 (ARF6) and induction of phosphatidylinositol-(4,5)-bisphosphate (PIP2) production by phosphatidylinositol 5-kinase (PI5K), thereby promoting the targeting of Mal to membrane-bound PIP2 through its PIP2-binding domain. Mal can subsequently facilitate the recruitment of MyD88 to either TLR2 or TLR4 for initiation of MyD88-dependent signaling (45) (Figure 3). On the other hand, an independent study showed that CR3 may negatively regulate TLR-mediated inflammatory responses in macrophages by activating Syk and promoting degradation of MyD88 and TRIF via the E3 ubiquitin ligase Cbl-b (46) (Figure 3). Moreover, CR3 activation by a small-molecule allosteric agonist was shown to induce MyD88 degradation in macrophages also downstream of TLR7 and TLR8, thereby inhibiting TLR7/8-induced production of TNF (47). Intriguingly, this regulatory effect of CR3 was abrogated in macrophages expressing a genetic variant of CR3 (specifically a missense polymorphism, R77H, of CD11b) (47), which has been identified as a risk factor in systemic lupus erythematosus (48). It could thus be suggested that this mechanism may contribute to the pathogenesis of systemic lupus erythematosus, where RNA-containing immune complexes can readily trigger TLR7/8-mediated inflammation. Taken together, the above-discussed studies indicate that CR3 can exert both positive and negative regulation of TLR signaling by controlling the localization and/or degradation of TLR adaptors, although the contextual basis of these contrasting effects is not clear.
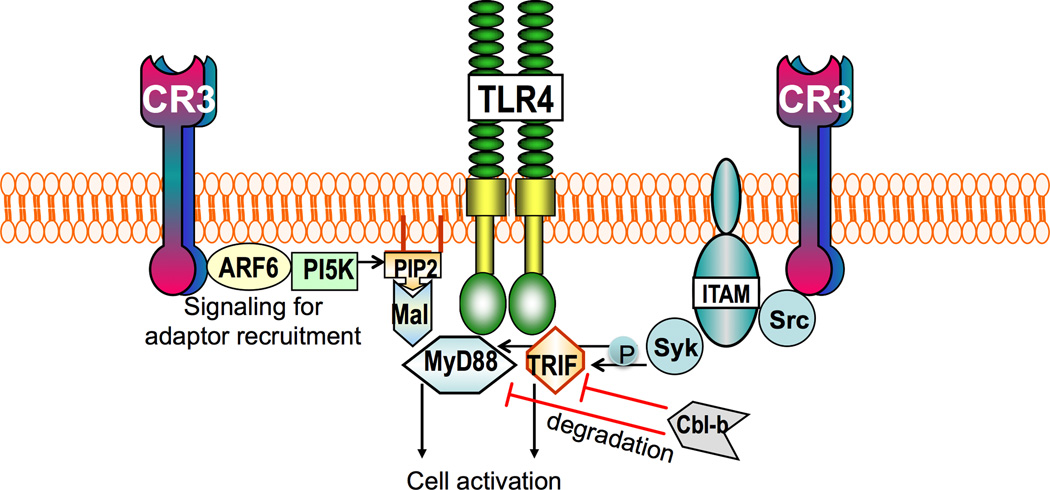
Figure 3. TLR–CR3 crosstalk pathways.
CR3 can regulate the signaling activity of TLRs that utilize Mal as an adaptor, i.e. TLR2 and TLR4. Specifically, outside-in signaling by CR3 leads to activation of ADP ribosylation factor 6 (ARF6) and induction of phosphatidylinositol-(4,5)-bisphosphate (PIP2) production by phosphatidylinositol 5-kinase (PI5K). This in turn promotes the targeting of Mal, which has PIP2-binding domain, to membrane-bound PIP2. Mal in turn facilitates the recruitment of MyD88 to either TLR2 or TLR4 to initiate pro-inflammatory signaling. Moreover, CR3 outside-in signaling stimulates ITAM-coupled activation of the tyrosine kinases Src and Syk. Syk in turn binds and phosphorylates MyD88 and TRIF, which are thereby targeted by the E3 ubiquitin ligase Cbl-b for proteolytic cleavage.
TLR regulation of expression of complement components
The previous section discussed several studies showing that complement receptors (e.g., C3aR, C5aR1, and CR3) regulate TLR-dependent responses, such as those induced by LPS (12, 21, 31, 45, 46). Reciprocally, TLR activation induces the expression of complement components, thereby potentially contributing to enhance complement activity in an inflammatory environment (49–52). For example, LPS induces robust production and release of factor B of the alternative pathway in macrophages (a major source of extra-hepatic complement synthesis) through a TLR4-TRIF pathway that leads to JNK and NF-κB activation (49). The same study showed that the double-stranded RNA analog polyI:C (a typical TLR3 agonist) also stimulates factor B production in macrophages via a JNK- and NF-κB-dependent mechanism; however, this pathway was not mediated by TLR3, suggesting the involvement of alternative receptors for polyI:C, such as the cytosolic sensors MDA-5 and RIG-I (49). An independent study showed that polyI:C induces factor B expression also in colonic epithelial cells, albeit via a TLR3-dependent mechanism (50). Importantly, the expression of factor B mRNA and protein is significantly enhanced in colonic biopsies of patients with ulcerative colitis and Crohn's disease as compared to healthy controls (50). Therefore, upon TLR stimulation, innate immune and epithelial cells can locally produce a critical component for alternative complement activation, which can in turn further amplify TLR-mediated responses. Although this positive feedback loop may contribute to host defense, the same mechanism could exacerbate pathology in diverse settings, such as inflammatory bowel disease and ischemia/reperfusion.
In the latter condition, TLRs can respond to endogenous ligands released from stressed/ischemic tissues and local production of factor B (e.g., by cardiomyocytes in the context of myocardial infarction) may potentially contribute to complement-mediated injury during ischemia (53). Intestinal ischemia/reperfusion induces the expression of factor B and C3 in the gut of wild-type but not TLR4-deficient mice, which exhibit reduced inflammation and tissue damage (52). Administration of a complement inhibitor, CR2-Crry, during reperfusion ameliorated intestinal tissue damage in wild-type mice but did not further inhibit tissue damage in TLR4-deficient mice (52). These findings suggest that ischemia/reperfusion-induced tissue damage in this model requires a crosstalk involving TLR4 regulation of local production of complement, which in turn amplifies TLR4-mediated inflammation.
A more recent study showed that, in addition to polyI:C and LPS, Pam3Cys activation of TLR2 (though not CpG activation of TLR9) also induces factor B production and release in macrophages and cardiac cells (51). Moreover, induction of polymicrobial sepsis by cecal ligation and puncture in mice was shown to increase the levels of factor B (in serum, peritoneal cavity, heart and other organs) in an MyD88-dependent manner, whereas genetic ablation of factor B reduced complement activation during sepsis, attenuated organ injury and improved survival (51). This study lends further support that factor B acts downstream of TLR activation and that bacterial sepsis is largely dependent on complement-TLR crosstalk.
Modified low-density lipoprotein (mLDL) regulates the expression and release of C3 in macrophages by acting on TLR4 and liver X receptor (54). Specifically, uptake of mLDL by macrophages results in formation of oxysterols that activate Lliver X receptor-dependent transcription of target genes including C3. Moreover, on the cell surface, mLDL interacts with CD14 and TLR4 leading to induction of MEK1/2-ERK1/2-dependent C3 mRNA expression and NF-κB-dependent C3 protein secretion. Furthermore, subsequent activation of C3 leads to C3a activation of C3aR signaling that promotes mLDL uptake by macrophages, thereby reinforcing this positive regulatory feedback loop (54). As complement, TLRs, and mLDL metabolism are involved in atherosclerosis (55), this mechanism may be a contributing factor to the development of atherosclerotic lesions.
Interestingly, TLR signaling suppresses the desensitization of GPCRs by downregulating the expression of G-protein-coupled receptor kinases, which induce GPCR phosphorylation and internalization, thereby potentially prolonging the activation of C3aR and C5aR1 (56). Moreover, TLR-induced cytokines, such as IL-6, promote the expression of C3aR and C5aR (57). In summary, TLRs regulate the expression of complement factors as well as the expression and/or activation of complement receptors, which in turn can amplify or limit TLR-dependent responses.
Subversion of innate immunity by pathogen-induced complement-TLR crosstalk
Periodontitis is a chronic inflammatory disease of the tooth-supporting tissues (periodontium) that is induced by local dysbiotic polymicrobial communities (58). These communities form on subgingival tooth sites and appear to have evolved collective strategies that enable them to persist in an inflammatory environment (59). A formidable challenge for these bacteria is to evade killing without resorting to immune suppression, as this would inhibit inflammation and hence limit their food supply, which is derived from inflammatory tissue breakdown (60). This selective pressure might be responsible for the development of some highly sophisticated microbial tactics, which represent new paradigms in immune evasion and are reviewed below.
Immune subversion by periodontal bacteria
Porphyromonas gingivalis, a low-abundance gram-negative bacterium associated with periodontitis, was shown to exert a disproportionately high impact on the dysbiotic transformation of periodontal microbial communities, thereby behaving as a keystone pathogen (61, 62). Specifically, P. gingivalis can subvert the innate host response in ways that alter the numbers and composition of the microbiota, that is, causing dysbiosis (63). The overgrowth of a subset of species, including inflammophilic pathobionts, leads to destructive periodontal inflammation and bone loss (59–61).
The manipulation of the host response by P. gingivalis is based, at least in part, on its capacity to instigate subversive crosstalk interactions between complement and TLRs. For instance, P. gingivalis can induce a C5aR1-TLR2 crosstalk in neutrophils to uncouple bacterial immune clearance from inflammation (19) (Figure 4A), which creates a nutritionally favorable environment for the bacteria as they can feed off the inflammatory spoils (e.g., degraded collagen peptides and heme-containing compounds, a source of iron) (60, 64). In addition to stimulating TLR2, P. gingivalis can directly activate C5aR1 (i.e., independently of complement activation) through the action of its gingipains that can locally cleave C5 to generate C5a ligand (27, 65). In both human and mouse neutrophils, the P. gingivalis-instigated C5aR-TLR2 signaling crosstalk triggers ubiquitination and proteasomal degradation of the TLR2 adaptor MyD88, leading to suppression of downstream antimicrobial effects that would otherwise clear this bacterium (19, 66, 67) (Figure 4A).
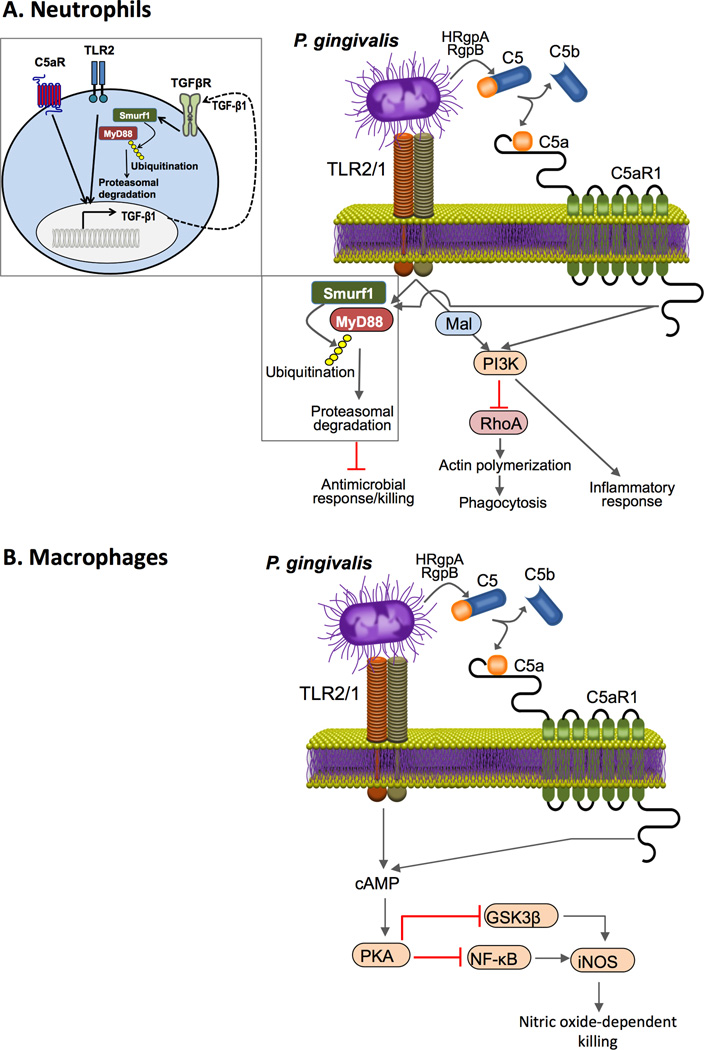
Figure 4. P. gingivalis-induced C5aR1-TLR2 crosstalk in neutrophils and macrophages.
P. gingivalis expresses ligands that activate the TLR2–TLR1 complex (TLR2/1) and enzymes (HRgpA and RgpB gingipains) with C5 convertase-like activity that generate high local concentrations of C5a ligand. The bacterium can thus co-activate C5aR and TLR2 in (A) neutrophils and (B) macrophages. In neutrophils (A), the resulting crosstalk leads to ubiquitination and proteasomal degradation of the TLR2 adaptor MyD88, thereby inhibiting a host-protective antimicrobial response. This proteolytic event requires C5aR1-TLR2-dependent release of TGF-β1, which mediates MyD88 ubiquitination via the E3 ubiquitin ligase Smurf1 (enlarged inset). Moreover, the C5aR1-TLR2 crosstalk activates PI3K, which inhibits phagocytosis through suppression of RhoA GTPase and actin polymerization, while inducing inflammatory cytokine production. In contrast to MyD88, Mal contributes to immune subversion by acting upstream of PI3K. In macrophages (B), P. gingivalis activates C5aR1 and induces intracellular Ca2+ signaling which synergistically enhances the otherwise weak cAMP responses induced by TLR2 activation alone. The resulting activation of the cAMP-dependent protein kinase A (PKA) inhibits NF-κB and glycogen synthase kinase-3β (GSK3β), thereby suppressing inducible nitric oxide synthase (iNOS)-dependent killing of the pathogen in macrophages.
Although MyD88 induces also proinflammatory signaling for NF-κB activation, the nutritionally favorable inflammatory response is not abrogated but instead mediated by an alternative TLR2 adaptor, Mal (MyD88 adaptor-like). In this pathway, Mal activates PI3K which mediates a robust inflammatory response. Indeed, genetic ablation or pharmacological inhibition of Mal or PI3K suppresses the induction of pro-inflammatory cytokines by neutrophils in vitro and in vivo (19). Moreover, P. gingivalis-induced Mal-PI3K signaling inhibits GTPase RhoA-dependent actin polymerization and hence P. gingivalis phagocytosis (19) (Figure 4A). These actions also promote the survival of bystander bacteria that are otherwise susceptible to neutrophil killing (19). Conversely, inhibition of PI3K or any of the two crosstalking receptors, C5aR1 or TLR2, in the periodontium of P. gingivalis-colonized mice promotes the elimination of P. gingivalis, reverses the increase in total microbiota counts induced earlier by P. gingivalis colonization, and blocks periodontal inflammation (19). Therefore, P. gingivalis manipulates neutrophils through distinct mechanisms that collectively promote the survival of the microbial community and the perpetuation of inflammation.
P. gingivalis induces a C5aR1-TLR2 crosstalk also in macrophages, which are thereby impaired for intracellular killing of this bacterium (68). However, the signaling mechanisms involved are completely different from those operating in neutrophils. In macrophages, the P. gingivalis C5aR1-TLR2 crosstalk leads to synergistic production of high and sustained levels of cAMP, which suppresses nitric oxide-dependent killing of P. gingivalis (68). Specifically, elevation of cAMP leads to activation of protein kinase A (PKA), which inactivates glycogen synthase kinase-3β (GSK3β) and inhibits the expression of inducible nitric oxide synthase (iNOS), hence reducing the production of nitric oxide, a potent antimicrobial molecule (68) (Figure 4B).
The P. gingivalis-induced C5aR1-TLR2 crosstalk additionally regulates cytokine expression in macrophages (27). Specifically, P. gingivalis selectively suppresses TLR2-induced IL-12p70 through a C5aR1-dependent mechanism involving ERK1/2 (Figure 1), whereas the same C5aR1-TLR2 crosstalk upregulates the production of proinflammatory cytokines (IL-1β, IL-6, and TNF), which appear to mediate inflammatory bone loss in a murine model of experimental periodontitis (27). Moreover, the ability of P. gingivalis to manipulate TLR2 activation via the C5a-C5aR1 pathway enables this microbe to inhibit the production of IL-12p70 and secondarily interferon (IFN)γ resulting in enhanced pathogen survival (27). Therefore, overall, P. gingivalis appears to inhibit both IFNγ-dependent priming of macrophages and their nitric oxide-dependent pathway for intracellular killing. The ability of complement to regulate TLR-induced IL-12 is a more general property that includes additional TLRs and IL-12-relates cytokines, such as IL-23. For instance, earlier work has shown that activation of C5aR1 in macrophages inhibits TLR4-induced mRNA expression of IL-12p35, IL-12/IL-23p40, IL-23p19 and IL-27p28, and production of IL-12, IL-23 and IL-27 proteins. The underlying mechanism involves induction of PI3K and ERK1/2 signaling, which in turn inhibit critical transcription factors (the IFN regulatory factors 1 and 8; IRF-1 and -8) that are required for expression of IL-12 family cytokines (12, 69, 70) (Figure 1). Similar but relatively attenuated inhibitory effects were observed after C3aR activation (12, 69).
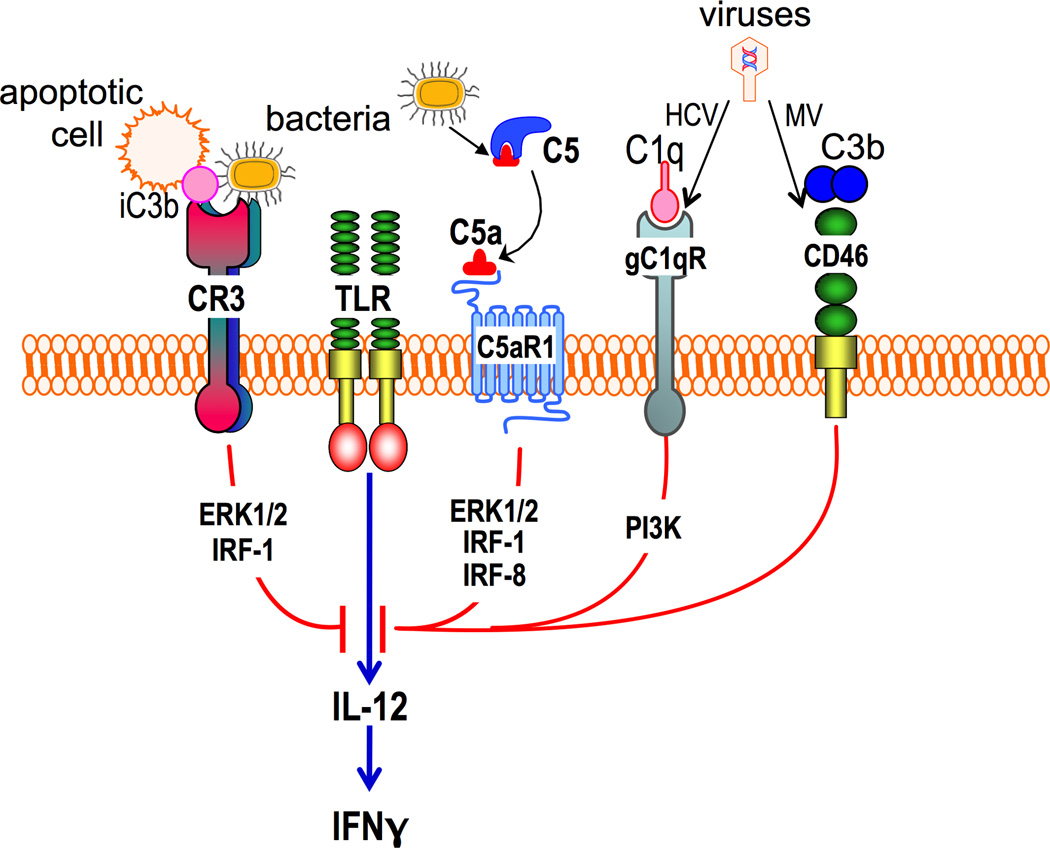
CR3 plays many and diverse roles in immunity and inflammation, including leukocyte transmigration and iC3b-mediated phagocytosis (71). Besides interacting with host molecules (iC3b, fibrinogen, and intercellular adhesion molecule-1 [ICAM-1]), CR3 can also interact with various microbial molecules, such as LPS, Bordetella pertussis filamentous hemagglutinin, Leishmania gp63, and P. gingivalis FimA fimbriae (72–76). In this regard, P. gingivalis FimA fimbriae can induce TLR2 inside-out signaling which transactivates the high-affinity conformation and hence the ligand-binding capacity of CR3 (77, 78). The interactions of CR3 on monocytes or macrophages with P. gingivalis lead to induction of proinflammatory cytokines (TNF, IL-1β, and IL-6) (75, 79) and promotion of ICAM-1-dependent monocyte transmigration across endothelial cell monolayers (80). Intriguingly, the aforementioned TLR2-CR3 crosstalk is exploited by P. gingivalis for a relatively safe entry and persistence in macrophages (81). Indeed, the intracellular survival of P. gingivalis is significantly reduced in CR3-deficient (CD11b−/−) mouse macrophages, suggesting that CR3-mediated phagocytosis of P. gingivalis prevents or ameliorates its killing (81). This finding is in line with observations that CR3 is not linked to vigorous microbicidal mechanisms, in contrast to certain other phagocytic receptors, such as Fcγ receptor III (CD16) (82–85). Indeed, in macrophages, CR3-derived phagosomes do not fuse with lysosomes as readily as CD16-derived phagosomes (86). The relatively mild post-phagocytic events downstream of CR3 are consistent with its role in the phagocytosis of iC3b-opsonized apoptotic cells, which entail minimal ‘danger’ as compared to pathogen infection (87, 88). Accordingly, upon phagocytosis of apoptotic cells, the production of IL-12 in efferocytic macrophages is suppressed (87) (Figure 5). Similarly, direct CR3 binding by P. gingivalis FimA fimbriae mitigates TLR2-induced IL-12 via outside-in signaling that induces ERK1/2-dependent inhibition of IL-12p35 and IL-12/IL-23p40 mRNA expression (89) (Figure 5). Consistent with this mechanism, CR3 blockade in a mouse peritonitis model (induced by i.p. injection of P. gingivalis) promotes IL-12-dependent clearance of P. gingivalis. Moreover, CR3-deficient mice are superior to wild-type controls in controlling P. gingivalis i.p. infection owing to elevated production of IL-12 and, secondarily, IFN-γ, a major activator of intracellular killing (89).
Figure 5. Immune evasion via complement-mediated suppression of TLR-induced IL-12 production.
The crosstalk between the indicated complement receptors (C5aR1, CR3, gC1qR and CD46) and TLRs selectively inhibits the induction of IL-12 in macrophages. Signaling molecules that have been implicated, such as ERK1/2, IRF-1, IRF-8 and PI3K, are shown downstream of the corresponding receptors. A posttranscriptional mechanism might be involved in IL-12 regulation by CD46. Activation of these complement receptors by their natural ligands likely mediates homeostatic functions. However, the same receptors can be activated by bacteria or viruses (see text for details) which can thereby downregulate TLR-induced IL-12 production and hence IFNγ to suppress cell-mediated immunity. HCV, hepatitis C virus; MV, measles virus.
Although P. gingivalis can exploit C5aR1 in neutrophils and macrophages to suppress their antimicrobial functions (19, 68), as well as bind CR3 for a safe entry into macrophages (81), the same receptors on dendritic cells do not seem to enhance the intracellular persistence of P. gingivalis (90). In stark contrast, C5aR1 promotes the intracellular killing of P. gingivalis in dendritic cells, whereas CR3 does not function as a phagocytic receptor for P. gingivalis. Similar to C5aR1, C3aR enhances the intracellular killing of P. gingivalis in dendritic cells. In contrast to C5aR1, C5aR2 is associated with increased intracellular survival of P. gingivalis in macrophages, consistent with the notion that C5aR2 can – in a certain context - downregulate the activity of C5aR1 (39, 43).
The differential effects of C5aR1 in dendritic cells as compared to macrophages might be attributed to differential regulation of the cAMP response in these two leukocyte types. As outlined above for macrophages, activation of C5aR1 leads to high levels of intracellular cAMP and thus PKA activation, which is critical for inhibiting nitric oxide-dependent killing of P. gingivalis (68). In dendritic cells, on the other hand, C5aR1 suppresses cAMP production and hence the activation of PKA (91). C3aR – which also facilitates intracellular killing of P. gingivalis in dendritic cells (90)– similarly inhibits the cAMP-PKA pathway (92). As both C3aR and C5aR1 activate Gαi protein-mediated signaling, it is not clear why the same receptors have different effects on the cAMP responses in macrophages versus dendritic cells.. However, some insights could be discussed at least at a theoretical level. Following activation of Gαi, the released Giβγ subunits regulate the production of cAMP by adenylate cyclase, either positively or negatively depending upon the specific enzyme isoform (93). The isoforms of adenylate cyclase isoforms that are positively regulated by Giβγ are different from those that are sensitive to the inhibitory action of Gαi (93). Thus, it can be reasoned that dendritic cells and macrophages express distinct isoforms of adenylate cyclase, thus C3aR- or C5aR-induced Gαi signaling has different effects on the regulation of the enzyme isoforms. Another cell type-specific difference is that whereas C5a inhibits P. gingivalis-induced IL-12p70 in macrophages (27), C5a promotes P. gingivalis-induced IL-12p70 in dendritic cells (90). The C5a-induced inhibition of IL-12p70 by P. gingivalis is mediated by ERK1/2 signaling (27), consistent with an earlier report that C5a-induced ERK1/2 signaling inhibits enterobacterial lipopolysaccharide-induced IL-12p70 in macrophages (69). Whereas C5a induces ERK1/2 signaling also in dendritic cells (94), the ERK1/2 pathway in this cell type upregulates, rather than inhibits, IL-12p70 production (95).
Despite its ability to transactivate and bind CR3 in macrophages, P. gingivalis fails to utilize CR3 as a phagocytic receptor in dendritic cells (90). The reason for this difference is not understood, although a study has suggested that CR3 cannot be readily transactivated in dendritic cells (96). Therefore, C3aR, C5aR1, and CR3, mediate cell-type-specific effects on how innate leukocytes handle P. gingivalis. Since dendritic cells are not as potent in pathogen destruction as compared to neutrophils or macrophages (97), it appears paradoxical that P. gingivalis can exploit complement receptors in neutrophils and macrophages more efficiently than it does in dendritic cells. However, given the abundance of complement cleavage products in the periodontal pocket (98), it makes sense from an evolutionary perspective that P. gingivalis developed complement-dependent evasion mechanisms against those leukocyte types that are most often encountered in its niche. Indeed, the immediate threat to P. gingivalis in its predominant niche, the periodontal pocket, is represented by neutrophils and secondarily by macrophages, which predominate in the leukocyte infiltrate of the periodontal pocket over other leukocyte types (99).
Immune subversion by other pathogens
The TLR2–CR3 crosstalk pathway may be exploited by additional pathogens. Mycobacteria and spores of Bacillus anthracis can both induce TLR2 inside-out signaling for transactivating and binding CR3, thereby promoting their uptake via CR3 (100, 101). It is thought that the ability of Mycobacterium tuberculosis to parasitize within macrophages may, in part, be reliant on its capacity to stimulate TLR2-induced CR3 uptake (101). Moreover, CR3-deficient mice display enhanced resistance to infection with B. anthracis spores. The susceptibility of wild-type mice in this model was attributed to enhanced uptake of B. anthracis spores and their carriage by the macrophages to sites of spore germination and bacterial growth (100). Upon opsonization with iC3, Francisella tularensis also uses CR3 for efficient macrophage uptake and the resulting outside-in signaling suppresses TLR2-mediated and MAPK-dependent pro-inflammatory responses, thereby promoting the pathogenesis of F. tularensis infection (102). The crosstalk of CR3 with the TLR system is bidirectional since, as discussed above, CR3 also regulates TLR signaling (45, 46). In line with this notion, a recent study has shown CR3 regulation of TLR8 responses in dendritic cells. Indeed, whereas free HIV-1 induces robust TLR8-dependent inflammatory and anti-viral responses (induction of p38, ERK, and NF-κB pathways and activation of IFN regulatory factors 1 and 7) in immature dendritic cells, iC3b-opsonized HIV interacts with CR3 leading to CR3-TLR8 crosstalk that modulates the host response in a way that enhances viral transcription (103).
In addition to CR3, gC1qR, a complement receptor for C1q, also suppresses TLR4-induced IL-12 in human monocytes (104) (Figure 5). This regulatory effect is mediated via PI3K signaling and is selective for IL-12 in that TNF, IL-6, and IL-8 are not impacted. However, this crosstalk appears to be exploited by the hepatitis C virus whose core protein acts as a ligand for gC1qR to inhibit IL-12 production and Th1 immunity (105) (Figure 5). The complement regulatory receptor CD46 also engages in a similar crosstalk with TLR4. Indeed, upon binding C3b dimers, CD46 inhibits LPS-induced IL-12 production in monocytes (106). The measles virus interacts with CD46 and thereby inhibits IL-12 production and cell-mediated immunity (106) (Figure 5). The underlying signaling mechanism is uncertain. However, a post-transcriptional mechanism was implicated in a study with human herpesvirus-6, which similarly uses CD46 as a cellular receptor to suppress TLR4-induced IL-12 (107).
Concluding remarks and outlook
The literature summarized in this review reveals an intricate interplay between complement and TLRs for regulating the expression and activation of critical components of the two systems, thereby contributing to the coordination of host immune and inflammatory responses. These bidirectional interactions range from antagonistic to synergistic (Figures 1–5) and can therefore enhance host immunity and inflammation to clear infections, or can dampen host responses to ameliorate exaggerated inflammation and tissue damage. In the latter case, future therapies for inflammatory or autoimmune diseases could focus on inhibiting either complement or TLRs, or both systems, depending on tissue or disease context (108–110). However, there may be instances where combined inhibition may have unfavorable outcomes. Indeed, although both complement and TLR2 induce inflammation in the context of renal ischemia/reperfusion (mice deficient in either factor B or TLR2 are protected from ischemic acute kidney injury), mice doubly deficient in factor B and TLR2 develop severe inflammatory tissue injury (111). These data suggest that, in this model, complement and TLR2 may also induce compensatory anti-inflammatory signals, the absence of which in the doubly deficient mice may have detrimental effects.
Some crosstalk interactions between complement and TLRs appear to be proactively instigated by pathogens ostensibly to dysregulate or modify the host response in ways that favor their persistence, often with concomitant collateral tissue damage. For instance, by inducing a C5aR1-TLR2 crosstalk, periodontal bacteria can disengage immune bacterial clearance from inflammation (Figure 4), thereby contributing to the persistence of ‘inflammophilic’ communities of pathobionts that exacerbate polymicrobial inflammatory diseases, such as periodontitis (19). In this context, novel and potentially effective approaches may be to interfere with the host signaling circuitry that is exploited for microbial subversion of the immune response.
Although this review has focused on innate immunity, complement-TLR interactions also impact on adaptive immunity. An important mechanism in this regard involves signaling crosstalk in antigen-presenting cells between C5aR1 and TLR4 which downregulates the expression of IL-12 family cytokines (IL-12, IL-23, and IL-27) (Figure 1) involved in the regulation of distinct T-cell subsets (Th1, Th2, and Th17) (12, 17, 69, 70, 112). The role of C5aR1 signaling in regulating T cell immunity in co-operation with TLRs is complex and contextual, as it can lead to different outcomes depending on the maturation stage of the antigen-presenting cell (20) or the type of crosstalking TLR (112). Moreover, cell type- and species-specific differences have been noted and reviewed elsewhere (17, 113–115)).
The complex – and still incompletely understood – crosstalk interactions of complement with TLRs (and other systems reviewed elsewhere (9)) apparently aim to fine-tune a balance between homeostatic immunity and inflammatory pathology. Future research to further dissect the molecular mechanisms of complement-TLR crosstalk and their contextual nature may contribute to the design of novel approaches to maximize the beneficial and minimize the detrimental aspects of these interactions.
Acknowledgments
The authors are supported by grants from the U.S. National Institutes of Health: DE015254, DE017138, DE021685, and DE024716 (GH); AI003040 and AI068730 (JDL) and the European Community’s Seventh Framework Programme under grant agreement number 602699 (DIREKT) (JDL).
Footnotes
The authors declare no conflicting financial interests.
REFERENCES
- 1.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 3.Beutler BA. TLRs and innate immunity. Blood. 2009;113:1399–1407. doi: 10.1182/blood-2008-07-019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Triantafilou M, Triantafilou K. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol. 2002;23:301–304. doi: 10.1016/s1471-4906(02)02233-0. [DOI] [PubMed] [Google Scholar]
- 5.Brown J, Wang H, Hajishengallis GN, Martin M. TLR-signaling networks: an integration of adaptor molecules, kinases, and cross-talk. J Dent Res. 2011;90:417–427. doi: 10.1177/0022034510381264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bottazzi B, Doni A, Garlanda C, Mantovani A. An integrated view of humoral innate immunity: pentraxins as a paradigm. Annu Rev Immunol. 2010;28:157–183. doi: 10.1146/annurev-immunol-030409-101305. [DOI] [PubMed] [Google Scholar]
- 7.Holmskov U, Thiel S, Jensenius JC. Collectins and ficolins: humoral lectins of the innate immune defense. Annu Rev Immunol. 2003;21:547–578. doi: 10.1146/annurev.immunol.21.120601.140954. [DOI] [PubMed] [Google Scholar]
- 8.Inforzato A, Bottazzi B, Garlanda C, Valentino S, Mantovani A. Pentraxins in humoral innate immunity. Adv Exp Med Biol. 2012;946:1–20. doi: 10.1007/978-1-4614-0106-3_1. [DOI] [PubMed] [Google Scholar]
- 9.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hajishengallis G, Lambris JD. Crosstalk pathways between Toll-like receptors and the complement system. Trends Immunol. 2010;31:154–163. doi: 10.1016/j.it.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mangsbo SM, et al. Complement activation by CpG in a human whole blood loop system: Mechanisms and immunomodulatory effects. J Immunol. 2009;183:6724–6732. doi: 10.4049/jimmunol.0902374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X, et al. Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood. 2007;110:228–236. doi: 10.1182/blood-2006-12-063636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hajishengallis G, Lambris JD. Microbial manipulation of receptor crosstalk in innate immunity. Nat Rev Immunol. 2011;11:187–200. doi: 10.1038/nri2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodridge HS, Underhill DM. Fungal recognition by TLR2 and dectin-1. Handb Exp Pharmacol. 2008:87–109. doi: 10.1007/978-3-540-72167-3_5. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa S, et al. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell. 2005;122:707–721. doi: 10.1016/j.cell.2005.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lukashev D, Ohta A, Apasov S, Chen JF, Sitkovsky M. Cutting edge: Physiologic attenuation of proinflammatory transcription by the Gs protein-coupled A2A adenosine receptor in vivo. J Immunol. 2004;173:21–24. doi: 10.4049/jimmunol.173.1.21. [DOI] [PubMed] [Google Scholar]
- 17.Seow V, et al. Inflammatory responses induced by lipopolysaccharide are amplified in primary human monocytes but suppressed in macrophages by complement protein C5a. J Immunol. 2013;191:4308–4316. doi: 10.4049/jimmunol.1301355. [DOI] [PubMed] [Google Scholar]
- 18.Fang C, Zhang X, Miwa T, Song WC. Complement promotes the development of inflammatory T-helper 17 cells through synergistic interaction with Toll-like receptor signaling and interleukin-6 production. Blood. 2009;114:1005–1015. doi: 10.1182/blood-2009-01-198283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maekawa T, et al. Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe. 2014;15:768–778. doi: 10.1016/j.chom.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaal A, Lissenberg-Thunnissen SN, van Schijndel G, Wouters D, van Ham SM, ten Brinke A. Crosstalk between Toll like receptors and C5a receptor in human monocyte derived DCs suppress inflammatory cytokine production. Immunobiology. 2013;218:175–180. doi: 10.1016/j.imbio.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 21.Bosmann M, Sarma JV, Atefi G, Zetoune FS, Ward PA. Evidence for anti-inflammatory effects of C5a on the innate IL-17A/IL-23 axis. FASEB J. 2011;26:1640–1651. doi: 10.1096/fj.11-199216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamada M, et al. Complement C1q regulates LPS-induced cytokine production in bone marrow-derived dendritic cells. Eur J Immunol. 2004;34:221–230. doi: 10.1002/eji.200324026. [DOI] [PubMed] [Google Scholar]
- 23.Lappegård KT, et al. Human genetic deficiencies reveal the roles of complement in the inflammatory network: lessons from nature. Proc Natl Acad Sci U S A. 2009;106:15861–15866. doi: 10.1073/pnas.0903613106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo RF, Riedemann NC, Ward PA. Role of C5a-C5aR interaction in sepsis. Shock. 2004;21:1–7. doi: 10.1097/01.shk.0000105502.75189.5e. [DOI] [PubMed] [Google Scholar]
- 25.Kim DD, Song WC. Membrane complement regulatory proteins. Clin Immunol. 2006;118:127–136. doi: 10.1016/j.clim.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Abe T, et al. Local complement-targeted intervention in periodontitis: proof-of-concept using a C5a receptor (CD88) antagonist. J Immunol. 2012;189:5442–5448. doi: 10.4049/jimmunol.1202339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang S, et al. The C5a receptor impairs IL-12-dependent clearance of Porphyromonas gingivalis and is required for induction of periodontal bone loss. J Immunol. 2011;186:869–877. doi: 10.4049/jimmunol.1003252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burns E, Bachrach G, Shapira L, Nussbaum G. Cutting Edge: TLR2 is required for the innate response to Porphyromonas gingivalis : Activation leads to bacterial persistence and TLR2 deficiency attenuates induced alveolar bone resorption. J Immunol. 2006;177:8296–8300. doi: 10.4049/jimmunol.177.12.8296. [DOI] [PubMed] [Google Scholar]
- 29.Lau C, et al. CD14 and complement crosstalk and largely mediate the transcriptional response to Escherichia coli in human whole blood as revealed by DNA microarray. PLoS One. 2015;10:e0117261. doi: 10.1371/journal.pone.0117261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brekke OL, et al. Combined inhibition of complement and CD14 abolish E. coli-induced cytokine-, chemokine- and growth factor-synthesis in human whole blood. Mol Immunol. 2008;45:3804–3813. doi: 10.1016/j.molimm.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 31.Bosmann M, et al. MyD88-dependent production of IL-17F is modulated by the anaphylatoxin C5a via the Akt signaling pathway. FASEB J. 2011;25:4222–4232. doi: 10.1096/fj.11-191205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monk PN, Scola AM, Madala P, Fairlie DP. Function, structure and therapeutic potential of complement C5a receptors. Br J Pharmacol. 2007;152:429–448. doi: 10.1038/sj.bjp.0707332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okinaga S, et al. C5L2, a nonsignaling C5a binding Protein. Biochemistry. 2003;42:9406–9415. doi: 10.1021/bi034489v. [DOI] [PubMed] [Google Scholar]
- 35.Gao H, et al. Evidence for a functional role of the second C5a receptor C5L2. Faseb J. 2005;19:1003–1005. doi: 10.1096/fj.04-3424fje. [DOI] [PubMed] [Google Scholar]
- 36.Gerard NP, et al. An anti-inflammatory function for the complement anaphylatoxin C5a-binding protein, C5L2. J Biol Chem. 2005;280:39677–39680. doi: 10.1074/jbc.C500287200. [DOI] [PubMed] [Google Scholar]
- 37.Chen NJ, et al. C5L2 is critical for the biological activities of the anaphylatoxins C5a and C3a. Nature. 2007;446:203–207. doi: 10.1038/nature05559. [DOI] [PubMed] [Google Scholar]
- 38.Rittirsch D, et al. Functional roles for C5a receptors in sepsis. Nat Med. 2008;14:551–557. doi: 10.1038/nm1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bamberg CE, et al. The C5a receptor (C5aR) C5L2 is a modulator of C5aR-mediated signal transduction. J Biol Chem. 2010;285:7633–7644. doi: 10.1074/jbc.M109.092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horst SA, Itzek A, Klos A, Beineke A, Medina E. Differential Contributions of the Complement Anaphylotoxin Receptors C5aR1 and C5aR2 to the Early Innate Immune Response against Staphylococcus aureus Infection. Pathogens. 2015;4:722–738. doi: 10.3390/pathogens4040722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pundir P, MacDonald CA, Kulka M. The Novel Receptor C5aR2 Is Required for C5a-Mediated Human Mast Cell Adhesion, Migration, and Proinflammatory Mediator Production. J Immunol. 2015;195:2774–2787. doi: 10.4049/jimmunol.1401348. [DOI] [PubMed] [Google Scholar]
- 42.Selle J, et al. Atheroprotective role of C5ar2 deficiency in apolipoprotein E-deficient mice. Thromb Haemost. 2015;114:848–858. doi: 10.1160/TH14-12-1075. [DOI] [PubMed] [Google Scholar]
- 43.Croker DE, et al. C5a2 can modulate ERK1/2 signaling in macrophages via heteromer formation with C5a1 and beta-arrestin recruitment. Immunol Cell Biol. 2014;92:631–639. doi: 10.1038/icb.2014.32. [DOI] [PubMed] [Google Scholar]
- 44.Li R, Coulthard LG, Wu MC, Taylor SM, Woodruff TM. C5L2: a controversial receptor of complement anaphylatoxin, C5a. FASEB J. 2013;27:855–864. doi: 10.1096/fj.12-220509. [DOI] [PubMed] [Google Scholar]
- 45.Kagan JC, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell. 2006;125:943–955. doi: 10.1016/j.cell.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 46.Han C, Jin J, Xu S, Liu H, Li N, Cao X. Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat Immunol. 2010;11:734–742. doi: 10.1038/ni.1908. [DOI] [PubMed] [Google Scholar]
- 47.Reed JH, et al. Complement receptor 3 influences toll-like receptor 7/8-dependent inflammation: implications for autoimmune diseases characterized by antibody reactivity to ribonucleoproteins. J Biol Chem. 2013;288:9077–9083. doi: 10.1074/jbc.M112.403303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacPherson M, Lek HS, Prescott A, Fagerholm SC. A systemic lupus erythematosus-associated R77H substitution in the CD11b chain of the Mac-1 integrin compromises leukocyte adhesion and phagocytosis. J Biol Chem. 2011;286:17303–17310. doi: 10.1074/jbc.M110.182998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaczorowski DJ, et al. Pivotal Advance: The pattern recognition receptor ligands lipopolysaccharide and polyinosine-polycytidylic acid stimulate factor B synthesis by the macrophage through distinct but overlapping mechanisms. J Leukoc Biol. 2010 doi: 10.1189/jlb.0809588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ostvik AE, et al. Mucosal toll-like receptor 3-dependent synthesis of complement factor B and systemic complement activation in inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:995–1003. doi: 10.1097/MIB.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 51.Zou L, et al. Complement factor B is the downstream effector of TLRs and plays an important role in a mouse model of severe sepsis. J Immunol. 2013;191:5625–5635. doi: 10.4049/jimmunol.1301903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pope MR, Hoffman SM, Tomlinson S, Fleming SD. Complement regulates TLR4-mediated inflammatory responses during intestinal ischemia reperfusion. Mol Immunol. 2010;48:356–364. doi: 10.1016/j.molimm.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh MV, et al. Ca2+/calmodulin-dependent kinase II triggers cell membrane injury by inducing complement factor B gene expression in the mouse heart. J Clin Invest. 2009;119:986–996. doi: 10.1172/JCI35814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mogilenko DA, et al. Modified low density lipoprotein stimulates complement C3 expression and secretion via liver X receptor and Toll-like receptor 4 activation in human macrophages. J Biol Chem. 2012;287:5954–5968. doi: 10.1074/jbc.M111.289322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hovland A, et al. The complement system and toll-like receptors as integrated players in the pathophysiology of atherosclerosis. Atherosclerosis. 2015;241:480–494. doi: 10.1016/j.atherosclerosis.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 56.Fan J, Malik AB. Toll-like receptor-4 (TLR4) signaling augments chemokine-induced neutrophil migration by modulating cell surface expression of chemokine receptors. Nat Med. 2003;9:315–321. doi: 10.1038/nm832. [DOI] [PubMed] [Google Scholar]
- 57.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 2008;8:776–787. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lamont RJ, Hajishengallis G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol Med. 2015;21:172–183. doi: 10.1016/j.molmed.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15:30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hajishengallis G. The inflammophilic character of the periodontitis-associated microbiota. Mol Oral Microbiol. 2014;29:248–257. doi: 10.1111/omi.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nat Rev Microbiol. 2012;10:717–725. doi: 10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Darveau RP. The oral microbial consortium's interaction with the periodontal innate defense system. DNA Cell Biol. 2009;28:389–395. doi: 10.1089/dna.2009.0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hajishengallis G, et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 2011;10:497–506. doi: 10.1016/j.chom.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marsh PD. Are dental diseases examples of ecological catastrophes? Microbiology. 2003;149:279–294. doi: 10.1099/mic.0.26082-0. [DOI] [PubMed] [Google Scholar]
- 65.Popadiak K, Potempa J, Riesbeck K, Blom AM. Biphasic effect of gingipains from Porphyromonas gingivalis on the human complement system. J Immunol. 2007;178:7242–7250. doi: 10.4049/jimmunol.178.11.7242. [DOI] [PubMed] [Google Scholar]
- 66.Brzezinska AA, Johnson JL, Munafo DB, Ellis BA, Catz SD. Signalling mechanisms for Toll-like receptor-activated neutrophil exocytosis: key roles for interleukin-1-receptor-associated kinase-4 and phosphatidylinositol 3-kinase but not Toll/IL-1 receptor (TIR) domain-containing adaptor inducing IFNβ (TRIF) Immunology. 2009;127:386–397. doi: 10.1111/j.1365-2567.2008.02980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burns E, Eliyahu T, Uematsu S, Akira S, Nussbaum G. TLR2-dependent inflammatory response to Porphyromonas gingivalis is MyD88 independent, whereas MyD88 is required to clear infection. J Immunol. 2010;184:1455–1462. doi: 10.4049/jimmunol.0900378. [DOI] [PubMed] [Google Scholar]
- 68.Wang M, et al. Microbial hijacking of complement-toll-like receptor crosstalk. Sci Signal. 2010;3:ra11. doi: 10.1126/scisignal.2000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hawlisch H, Belkaid Y, Baelder R, Hildeman D, Gerard C, Kohl J. C5a negatively regulates toll-like receptor 4-induced immune responses. Immunity. 2005;22:415–426. doi: 10.1016/j.immuni.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 70.la Sala A, Gadina M, Kelsall BL. G(i)-protein-dependent inhibition of IL-12 production is mediated by activation of the phosphatidylinositol 3-kinase-protein 3 kinase B/Akt pathway and JNK. J Immunol. 2005;175:2994–2999. doi: 10.4049/jimmunol.175.5.2994. [DOI] [PubMed] [Google Scholar]
- 71.Ehlers MRW. CR3: a general purpose adhesion-recognition receptor essential for innate immunity. Microbes Infect. 2000;2:289–294. doi: 10.1016/s1286-4579(00)00299-9. [DOI] [PubMed] [Google Scholar]
- 72.Diamond MS, Garcia-Aguilar J, Bickford JK, Corbi AL, Springer TA. The I domain is a major recognition site on the leukocyte integrin Mac-1 (CD11b/CD18) for four distinct adhesion ligands. J Cell Biol. 1993;120:1031–1043. doi: 10.1083/jcb.120.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McGuirk P, Mills KH. Direct anti-inflammatory effect of a bacterial virulence factor: IL-10-dependent suppression of IL-12 production by filamentous hemagglutinin from Bordetella pertussis. Eur J Immunol. 2000;30:415–422. doi: 10.1002/1521-4141(200002)30:2<415::AID-IMMU415>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 74.Russell DG, Wright SD. Complement receptor type 3 (CR3) binds to an Arg-Gly-Asp-containing region of the major surface glycoprotein, gp63, of Leishmania promastigotes. J Exp Med. 1988;168:279–292. doi: 10.1084/jem.168.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hajishengallis G, Ratti P, Harokopakis E. Peptide mapping of bacterial fimbrial epitopes interacting with pattern recognition receptors. J Biol Chem. 2005;280:38902–38913. doi: 10.1074/jbc.M507326200. [DOI] [PubMed] [Google Scholar]
- 76.Ingalls RR, Arnaout MA, Golenbock DT. Outside-in signaling by lipopolysaccharide through a tailless integrin. J Immunol. 1997;159:433–438. [PubMed] [Google Scholar]
- 77.Harokopakis E, Hajishengallis G. Integrin activation by bacterial fimbriae through a pathway involving CD14, Toll-like receptor 2, and phosphatidylinositol-3-kinase. Eur J Immunol. 2005;35:1201–1210. doi: 10.1002/eji.200425883. [DOI] [PubMed] [Google Scholar]
- 78.Hajishengallis G, Wang M, Liang S. Induction of distinct TLR2-mediated proinflammatory and proadhesive signaling pathways in response to Porphyromonas gingivalis fimbriae. J Immunol. 2009;182:6690–6696. doi: 10.4049/jimmunol.0900524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hajishengallis G, et al. Differential interactions of fimbriae and lipopolysaccharide from Porphyromonas gingivalis with the Toll-like receptor 2-centred pattern recognition apparatus. Cell Microbiol. 2006;8:1557–1570. doi: 10.1111/j.1462-5822.2006.00730.x. [DOI] [PubMed] [Google Scholar]
- 80.Harokopakis E, Albzreh MH, Martin MH, Hajishengallis G. TLR2 transmodulates monocyte adhesion and transmigration via Rac1- and PI3K-mediated inside-out signaling in response to Porphyromonas gingivalis fimbriae. J Immunol. 2006;176:7645–7656. doi: 10.4049/jimmunol.176.12.7645. [DOI] [PubMed] [Google Scholar]
- 81.Wang M, et al. Fimbrial proteins of Porphyromonas gingivalis mediate in vivo virulence and exploit TLR2 and complement receptor 3 to persist in macrophages. J Immunol. 2007;179:2349–2358. doi: 10.4049/jimmunol.179.4.2349. [DOI] [PubMed] [Google Scholar]
- 82.Lowell CA. Rewiring phagocytic signal transduction. Immunity. 2006;24:243–245. doi: 10.1016/j.immuni.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 83.Wright SD, Silverstein SC. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J Exp Med. 1983;158:2016–2023. doi: 10.1084/jem.158.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Caron E, Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science. 1998;282:1717–1721. doi: 10.1126/science.282.5394.1717. [DOI] [PubMed] [Google Scholar]
- 85.Hellwig SM, van Oirschot HF, Hazenbos WL, van Spriel AB, Mooi FR, van De Winkel JG. Targeting to Fcg receptors, but not CR3 (CD11b/CD18), increases clearance of Bordetella pertussis. J Infect Dis. 2001;183:871–879. doi: 10.1086/319266. [DOI] [PubMed] [Google Scholar]
- 86.Vieira OV, Botelho RJ, Grinstein S. Phagosome maturation: aging gracefully. Biochem J. 2002;366:689–704. doi: 10.1042/BJ20020691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim S, Elkon KB, Ma X. Transcriptional suppression of interleukin-12 gene expression following phagocytosis of apoptotic cells. Immunity. 2004;21:643–653. doi: 10.1016/j.immuni.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 88.Mevorach D, Mascarenhas JO, Gershov D, Elkon KB. Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med. 1998;188:2313–2320. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hajishengallis G, Shakhatreh MA, Wang M, Liang S. Complement receptor 3 blockade promotes IL-12-mediated clearance of Porphyromonas gingivalis and negates its virulence in vivo. J Immunol. 2007;179:2359–2367. doi: 10.4049/jimmunol.179.4.2359. [DOI] [PubMed] [Google Scholar]
- 90.Hajishengallis G, Krauss JL, Jotwani R, Lambris JD. Differential capacity for complement receptor-mediated immune evasion by Porphyromonas gingivalis depending on the type of innate leukocyte. Mol Oral Microbiol. 2016 doi: 10.1111/omi.12161. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peng Q, et al. Dendritic cell function in allostimulation is modulated by C5aR signaling. J Immunol. 2009;183:6058–6068. doi: 10.4049/jimmunol.0804186. [DOI] [PubMed] [Google Scholar]
- 92.Li K, et al. Cyclic AMP plays a critical role in C3a-receptor-mediated regulation of dendritic cells in antigen uptake and T-cell stimulation. Blood. 2008;112:5084–5094. doi: 10.1182/blood-2008-05-156646. [DOI] [PubMed] [Google Scholar]
- 93.Sunahara RK, Taussig R. Isoforms of mammalian adenylyl cyclase: Multiplicities of signaling. Mol Interv. 2002;2:168–184. doi: 10.1124/mi.2.3.168. [DOI] [PubMed] [Google Scholar]
- 94.Weaver DJ, Jr, et al. C5a receptor-deficient dendritic cells promote induction of Treg and Th17 cells. Eur J Immunol. 2010;40:710–721. doi: 10.1002/eji.200939333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baruah P, et al. C1q enhances IFN-g production by antigen-specific T cells via the CD40 costimulatory pathway on dendritic cells. Blood. 2009;113:3485–3493. doi: 10.1182/blood-2008-06-164392. [DOI] [PubMed] [Google Scholar]
- 96.Varga G, et al. Active MAC-1 (CD11b/CD18) on DCs inhibits full T-cell activation. Blood. 2007;109:661–669. doi: 10.1182/blood-2005-12-023044. [DOI] [PubMed] [Google Scholar]
- 97.Silva MT. When two is better than one: macrophages and neutrophils work in concert in innate immunity as complementary and cooperative partners of a myeloid phagocyte system. J Leukoc Biol. 2010;87:93–106. doi: 10.1189/jlb.0809549. [DOI] [PubMed] [Google Scholar]
- 98.Hajishengallis G. Complement and periodontitis. Biochem Pharmacol. 2010;80:1992–2001. doi: 10.1016/j.bcp.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Delima AJ, Van Dyke TE. Origin and function of the cellular components in gingival crevice fluid. Periodontol 2000. 2003;31:55–76. doi: 10.1034/j.1600-0757.2003.03105.x. [DOI] [PubMed] [Google Scholar]
- 100.Oliva C, Turnbough CL, Jr, Kearney JF. CD14-Mac-1 interactions in Bacillus anthracis spore internalization by macrophages. Proc Natl Acad Sci U S A. 2009;106:13957–13962. doi: 10.1073/pnas.0902392106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sendide K, Reiner NE, Lee JS, Bourgoin S, Talal A, Hmama Z. Cross-talk between CD14 and complement receptor 3 promotes phagocytosis of mycobacteria: regulation by phosphatidylinositol 3-kinase and cytohesin-1. J Immunol. 2005;174:4210–4219. doi: 10.4049/jimmunol.174.7.4210. [DOI] [PubMed] [Google Scholar]
- 102.Dai S, Rajaram MV, Curry HM, Leander R, Schlesinger LS. Fine tuning inflammation at the front door: macrophage complement receptor 3-mediates phagocytosis and immune suppression for Francisella tularensis. PLoS Pathog. 2013;9:e1003114. doi: 10.1371/journal.ppat.1003114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ellegard R, et al. Complement opsonization of HIV-1 results in decreased antiviral and inflammatory responses in immature dendritic cells via CR3. J Immunol. 2014;193:4590–4601. doi: 10.4049/jimmunol.1401781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Waggoner SN, Cruise MW, Kassel R, Hahn YS. gC1q receptor ligation selectively down-regulates human IL-12 production through activation of the phosphoinositide 3-kinase pathway. J Immunol. 2005;175:4706–4714. doi: 10.4049/jimmunol.175.7.4706. [DOI] [PubMed] [Google Scholar]
- 105.Waggoner SN, Hall CH, Hahn YS. HCV core protein interaction with gC1q receptor inhibits Th1 differentiation of CD4+ T cells via suppression of dendritic cell IL-12 production. J Leukoc Biol. 2007;82:1407–1419. doi: 10.1189/jlb.0507268. [DOI] [PubMed] [Google Scholar]
- 106.Karp CL, et al. Mechanism of suppression of cell-mediated immunity by measles virus. Science. 1996;273:228–231. doi: 10.1126/science.273.5272.228. [DOI] [PubMed] [Google Scholar]
- 107.Smith A, Santoro F, Di Lullo G, Dagna L, Verani A, Lusso P. Selective suppression of IL-12 production by human herpesvirus 6. Blood. 2003;102:2877–2884. doi: 10.1182/blood-2002-10-3152. [DOI] [PubMed] [Google Scholar]
- 108.Skjeflo EW, et al. Combined inhibition of complement and CD14 improved outcome in porcine polymicrobial sepsis. Crit Care. 2015;19:415. doi: 10.1186/s13054-015-1129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maekawa T, et al. Inhibition of pre-existing natural periodontitis in non-human primates by a locally administered peptide inhibitor of complement C3. J Clin Periodontol. 2016 doi: 10.1111/jcpe.12507. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mastellos DC, Ricklin D, Hajishengallis E, Hajishengallis G, Lambris JD. Complement therapeutics in inflammatory diseases: promising drug candidates for C3-targeted intervention. Mol Oral Microbiol. 2016;31:3–17. doi: 10.1111/omi.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Amura CR, Renner B, Lyubchenko T, Faubel S, Simonian PL, Thurman JM. Complement activation and toll-like receptor-2 signaling contribute to cytokine production after renal ischemia/reperfusion. Mol Immunol. 2012;52:249–257. doi: 10.1016/j.molimm.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bosmann M, Haggadone MD, Hemmila MR, Zetoune FS, Sarma JV, Ward PA. Complement activation product C5a is a selective suppressor of TLR4-induced, but not TLR3-induced, production of IL-27(p28) from macrophages. J Immunol. 2012;188:5086–5093. doi: 10.4049/jimmunol.1102914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kemper C, Kohl J. Novel roles for complement receptors in T cell regulation and beyond. Mol Immunol. 2013;56:181–190. doi: 10.1016/j.molimm.2013.05.223. [DOI] [PubMed] [Google Scholar]
- 114.Song WC. Crosstalk between complement and toll-like receptors. Toxicol Pathol. 2012;40:174–182. doi: 10.1177/0192623311428478. [DOI] [PubMed] [Google Scholar]
- 115.Merle NS, Noe R, Halbwachs-Mecarelli L, Fremeaux-Bacchi V, Roumenina LT. Complement System Part II: Role in Immunity. Front Immunol. 2015;6:257. doi: 10.3389/fimmu.2015.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]