Abstract
Cell signaling pathways regulate much in the life of a cell: from shuttling cargo through intracellular compartments and onto the cell surface, how it should respond to stress, protecting itself from harm (environmental insults or infections), to ultimately, death by apoptosis. These signaling pathways are important for various aspects of the immune response as well. However, not much is known in terms of the participation of cell signaling pathways in Ag presentation--a necessary first step in the activation of innate and adaptive T cells. In this brief review, I will discuss the known signaling molecules (and pathways) that regulate how Ags are presented to T cells and the mechanism(s) if identified. Studies in this area have important implications in vaccine development and new treatment paradigms against infectious diseases, autoimmunity and cancer.
Introduction of Cell Signaling Pathways
What allows cells to respond to stimuli in specific ways? A priori, one can envisage a stimulus and a cellular response. Unfortunately, in between that stimulus and response is a black box. An external or internal event triggers within a cell a cascade (linear or branched), resulting in the phosphorylation and/or dephosphorylation of specific proteins. As a consequence, these cell signaling pathways have effects upon or within the cell that results in a specific response. For example, the activation of these pathways can stimulate cell migration or arrest. This can be due to the polymerization or depolymerization of cytoskeletal proteins, or the rearrangement of proteins or intracellular compartments. A potential effect can also result in activation-induced cell death.
Studies to dissect the specific paths (i.e., proteins that are phosphorylated or dephosphorylated and consequent effects on “downstream” intracellular proteins) followed by cell signaling pathways, have helped us understand many ways in which cells react to the environment— including infections by pathogens. As this is a Brief Review in The Journal of Immunology, I will focus my comments on cell signaling pathways that control the presentation of Ag to conventional and unconventional T cells.
Why would cell signaling pathways even be important for the immune system? Certainly, APCs need to be able to capture and internalize Ag in various forms--and by various routes (1-5). This could be by phagocytosis, pinocytosis, infection by a pathogen, or even taken from within a cell as an endogenous protein or lipid. Ag-loaded molecules need to be able to be transported intracelluarly along the cytoskeletal network, to their ultimate expression on the cell surface for recognition by T cells. That “movement” needs direction—this is provided by cell signaling pathways.
APCs mainly utilize three pathways of Ag presentation; Ags are presented by MHC or MHC-like molecules (1-5). The first pathway involves MHC class I molecules (2, 6-18). In the cytosol, endogenously-synthesized polypeptide chains are threaded into a barrel-like structure called the proteasome, which contains a variety of proteolytic enzyme activities. As the polypeptide is cleaved, a diverse array of peptides consisting of approximately 9 – 15 amino acids is generated. These peptides are then delivered into the endoplasmic reticulum (ER) by the transporter associated with Ag processing (TAP). Upon being loaded onto peptide-receptive MHC class I molecules, ER resident peptidases cleave amino terminal amino acids, resulting in the 8 – 9 amino acid peptides that are usually found associated with MHC class I molecules. The peptide-loaded MHC class I molecules are then transported through the Golgi to the cell surface, where they are recognized by CD8+ T cells. The second Ag presentation pathway involves MHC class II molecules (17, 19-31). Rather than being synthesized intracellularly within an APC, Ags are taken up by phagocytosis (or pinocytosis) and delivered to late endocytic compartments where they are processed into longer (e.g., 15 – 20) peptide-sized fragments than those loaded onto MHC class I molecules. Initially, MHC class II molecules are complexed with the invariant chain (Ii). The CLIP portion of the Ii prevents peptide loading until the MHC class II molecules traffic to late endocytic compartments (e.g., MIIC). It is here that an antigenic peptide replaces the Ii chain's CLIP. The newly-loaded MHC class II molecule is then transported to the cell surface where it is recognized by CD4+ T cells. The third Ag presentation pathway does not involve peptide Ag presentation. CD1 molecules are MHC class I-like molecules that generally present lipid Ags to invariant, relatively oligoclonal or even diverse T cells. These lipids can be of microbial origin or from mammalian cells themselves (5, 32). The CD1 family of Ag presenting molecules consists of two members (based on the human CD1 molecules): Group 1 includes CD1a, CD1b and CD1c molecules, whereas Group 2 consists of CD1d as its sole member (33). Each of these molecules differ somewhat in how they traffic intracellular and thereby how they acquire Ag.
For the sake of completeness, I will note there are the very interesting MHC class I-like MR1 molecules, which present microbial vitamin B-derived metabolites to a novel T cell subpopulation called MAIT [(mucosal-associated invariant T cells; ref. (34, 35)] Because so little is known about the cell signaling pathways regulating their function, they will not be discussed here.
The presentation of specific Ags to T cells by cell-to-cell interactions, results in the production of various cytokines that, in turn, stimulate the APCs. For T cells to be stimulated by APCs, cell signaling pathways within the APCs themselves need to be activated; this results in the proper Ags being processed and loaded onto the appropriate Ag presenting molecule to be expressed on the surface. This is absolutely required for Ag-specific T cells to be activated and perform their effector cell functions.
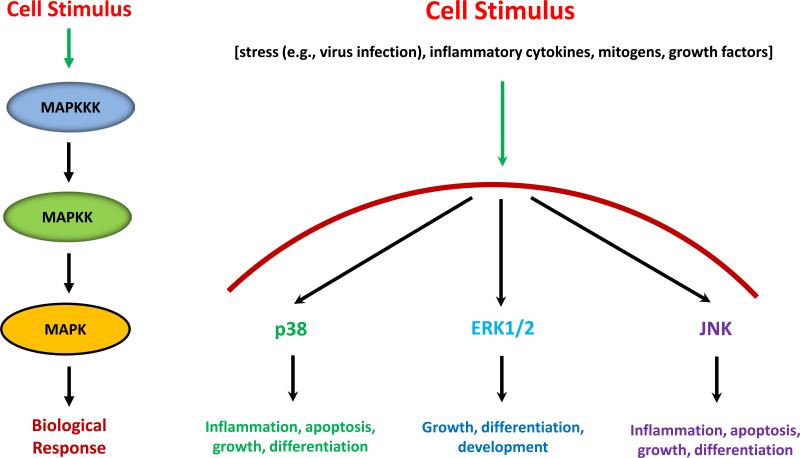
Which are the most widely studied cell signaling pathways that control classical (i.e., MHC class I and II) and non-classical (e.g., CD1d) Ag presentation and the mechanism/s (if known) by which they do this? One of the best understood cell signaling pathway family is that mediated by the MAPK (Fig. 1; left side). Following a cell stimulus, a MAPKKK is phosphorylated which, in turn, phosphorylates a MAPKK. Finally, the MAPKK activates the MAPK which then is transported into the nucleus to mediate its function. The MAPK family consists of three main members: p38, ERK and JNK [reviewed in (36)]. Upon receiving a cell stimulus, such as infection with a pathogen, exposure to inflammatory cytokines, etc., the resultant activation of the MAPK pathways results in inflammation, cell growth, differentiation or apoptosis. Consequently, the activation of MAPK pathways has the potential to impact the ability of a host's immune cells to appropriately respond to an infection. Of these three pathways, p38 has been studied the most in terms of aspects relating to Ag processing and presentation.
FIGURE 1.
Activation of the MAPK family of cell signaling molecules. There are three main families of MAPK: p38, ERK and JNK. Each has important roles in the response of a cell to a stimulus. On the left indicates the normal process of events: A cell stimulus results in the activation of a MAP3K (MAPKKK), which in turn phosphorylates a MAP2K (MAPKK). The MAPKK activates the MAPK via phosphorylation which leads to a biological response. The specific biological responses mediated by the individual MAPKs are indicated on the right.
Role for p38 on APC maturation and function
The MAPK p38 can be phosphorylated by two upstream kinases: MKK3 and MKK6 (37). For macrophage (MΦ) maturation (20) and production of IL-12 (38), p38 is required via its stimulation by MKK3 (38). This occurs because MKK3 indirectly activates the IL-12 p40 promoter through its phosphorylation of p38 (39). In MΦ, anisomycin (a p38 activator) enhances LPS-induced IL-12 production; this is blocked by the p38-specific inhibitor, SB203580 (39). Blocking p38 also impairs DC/T cell clustering, which can, of course, reduce effector T cell activation (39). Interestingly, p38 inhibits (whereas ERK promotes) the differentiation of monocytes into DCs (40). As will be mentioned below, p38 and ERK have similar reciprocal control over CD1d-mediated Ag presentation (41-43). Unstimulated DCs have a basal level of p38 that is enhanced following stimulation with anisomycin, a drug that activates both p38 and JNK (44). In both human (44) and mouse (45) DCs, activation of TLR4 results in the phosphorylation of p38. TLRs have a variety of known effects on APCs; these are discussed later in this review. The other MAPKK that can activate p38, MKK6, increases the APC activity of Langerhans cells by stimulating the NF-κB pathway (46).
The MAPK p38 has been shown to be important for Ag presentation by classical MHC class I and II molecules. Ag uptake is increased in DCs treated with cyclophin A, which results in p38 activation (47). p38 (and ERK) activation by osteoprotegerin ligand increases costimulatory molecule expression (48), readying APCs for interaction with Ag-specific T cells. In the context of Ag presentation by MHC class I, p38 can help--or hinder. For example, p38 phosphorylation following CD40L engagement results in the activation of DCs and expansion of HIV-specific memory CD8+ T cells (49). It was presumed that the expansion of these T cells is MHC class I-dependent; however, it has recently been shown that SIV peptides can be presented to CD8+ T cells by MHC class II molecules (50). I indicated above that TLR engagement activates p38 in DCs (44, 45); this also results in enhanced Ag presentation by DCs to antitumor T cells via MHC class I molecules (51). In contrast, there is increased Ag uptake for cross-presentation by MHC class I molecules in a Japanese Encephalitis Virus model when p38 is inhibited (7); thus, p38 appears to be a negative regulator in this model.
p38 activation in DCs (52) and its induction by fragments of the food product gliadin (53), increases costimulatory and MHC class II molecule expression, which, in turn, enhances Ag presentation to CD4+ T cells. This is in contrast to one report, in which the role of p38 in MHC class II-mediated Ag presentation by multiple myeloma patient DC was studied. In that system, p38 activation inhibited Ag presentation by MHC class II molecules, concomitant with reduced costimulatory molecules and MHC class II expression on the cell surface (54).
We have done extensive analyses on signal transduction pathways that can regulate lipid Ag presentation by CD1d. It is well known that viruses (along with other external stimuli) activate p38 in cells (43). When we analyzed the role for p38 in regulating CD1d-mediated Ag presentation following infection with vaccinia virus or vesicular stomatitis virus, we found it was inhibitory; blocking p38 actually increased Ag presentation to natural killer T (NKT) cells (42, 43). However, this does not only occur in a virus infection. Inducing apoptosis in CD1d+ APCs stimulates p38 activation; not surprisingly, these cells are poorer stimulators of NKT cells (41). Interestingly, another group has looked at Ag presentation by Group 1 CD1 molecules. As we have observed with CD1d, p38 is inhibitory in that system as well (55). Notably, p38 has an NKT cell-intrinsic negative effect, as treatment of iNKT cells with a p38-specific inhibitor impairs their stimulation by the CD1d-binding (and NKT cell-stimulating) glycolipid, α-galactosylceramide [α-GalCer; ref. (56)] or by an anti-CD3-specific mAb (57). Therefore, p38 can affect both the APC and T cell sides of a host's immune response.
Participation of JNK
As indicated above, JNK has effects on many of the same cellular functions as p38, although there is not total overlap (37). Certainly, analyses of JNK in DCs have reported that, like for p38, anisomycin induces JNK in human monocyte-derived DCs, although at higher concentrations (39). Unlike that for p38, at baseline, there is no detectable JNK in human DCs (39), nor does TLR4 activation stimulate JNK in murine DCs (45). Does JNK have any role in Ag processing and presentation? It has been shown that JNK2-deficient mice generate more CD8+ T cells in response to IL-2 (58) and more antiviral CD8+ cells are found in these mice upon infection with lymphocytic choriomeningitis virus (59). This suggests that JNK can have a negative effect on MHC class I-mediated Ag presentation; alternatively (or additionally), this could be T cell-intrinsic. In line with those two reports, we have found that JNK2 is a negative regulator of Ag presentation by CD1d (Liu et al., manuscript in preparation). Studies on the JNK signaling pathway in the control of Ag presentation by classical and/or non-classical MHC molecules are still limited to date.
Role of ERK
There are a few reports that have asked whether the ERK MAPK pathway is important for MΦ or DC maturation, or if it can be activated in either cell population. For example, one report suggested that LPS or GM-CSF induces ERK in macrophages, albeit with different kinetics [the latter induces ERK activation much more quickly—5 min vs. 15 min, respectively; ref. (60)]. LPS activates ERK in MΦ (44, 61); however, it apparently does not do so in DCs (62). In terms of MΦ maturation, it is both ERK- and p38-dependent (20). In contrast, ERK promotes, but p38 impairs, the differentiation of human monocytes into DCs (40). We have reported that, in the context of lipid Ag presentation by CD1d, ERK is a positive regulator (43).
As indicated above, a number of cytokines can activate MAPK signaling, with an impact on APC maturation or Ag presentation abilities. In some cases, such as with TGF-β, even though that cytokine can activate MAPKs, which negatively regulate CD1d-mediated Ag presentation [(41-43); Liu, et al., in preparation], the ultimate effect on Ag presentation by CD1d in the TGF-β model appears to be (at least) p38-independent (21).
Regulation of Ag presentation by protein kinase C (PKC) isoforms
The protein kinase C (PKC) family members are ser/thr kinases that consist of three main subgroups: 1. Conventional/Classical [(PKCα, β (I and II)], and γ; 2. Atypical (PKC ζ and λ/ι); and 3. Novel (δ, ε and θ) [reviewed in (63)]. Within these subgroups are 10 kinases that play distinct roles in the regulation of gene expression and cell proliferation. The activation of PKCs modifies them from a quiescent cytosolic form, to an active, membrane-associated form (64). Beyond their role as cytoplasmic signal transduction molecules, there is also evidence to suggest they can serve as nuclear kinases as well. In terms of the control of Ag presentation, PKC isoforms have been shown to promote Ag presentation by MHC class II molecules. In particular, the use of PKC activators such as bryostatin, which activates PKC-α, δ and ι, or PKC activation by phorbol esters and ionomycin, increases the surface expression of MHC class II molecules and thereby increases Ag presentation (27, 65, 66). The use of PKC-specific (i.e., PKC-α and/or –δ) inhibitors by our laboratory and others, further supports the positive correlation between PKC-δ activation and Ag presentation by MHC class II molecules (23, 27, 67). Interestingly, we have found that Ag presentation by CD1d, which is an MHC class I-like molecule that traffics intracellularly more like MHC class II molecules, is promoted by PKC-δ (67). In contrast to MHC class II and CD1d, Ag presentation by MHC class I molecules is not impacted by PKC-δ (27, 67).
TLRs and Ag processing and presentation
In general, when one thinks about what constitutes the innate immune response, the first thing that often comes to mind is a response via Toll-like receptors [TLRs; reviewed in (68)]. The TLR family of molecules consists of up to 13 structurally similar molecules in mammals, which are homologues of the Drosophila toll gene product (69). TLRs are molecules on the cell surface and/or in intracellular (e.g., endocytic) compartments (i.e., TLR3, TLR7/8, TLR9), to which microbial or viral products bind; this results in the activation of various cell signaling pathways, including MAPK as well as other kinases (70). The majority of TLRs (TLR3 is the exception) use the MyD88 adaptor molecule for signaling down the TLR activation cascade (68, 70). These responses can have important effects on Ag presentation by classical MHC molecules (17), as well as by CD1d (71-76). Globally, the binding of a ligand to its specific TLR can regulate immunodominance; this has been shown in the context of the Toxoplasma gondii-encoded protein, profilin, via MHC class II molecules (77). In contrast, TLR7/8 agonists can impair the differentiation of monocytes into DCs (78). Thus, activation via TLRs can promote (or inhibit) APC function.
In terms of MHC class I-mediated Ag presentation, TLR engagement can enhance (or have a minimal effect on) overall surface MHC class I expression (79, 80). However, ligands for TLR2 and other TLRs can impair conventional Ag presentation by MHC class I (80, 81). TLR signals can also have very important regulatory (albeit not direct) roles in triggering the delivery of MHC class I molecules to phagosomes for cross-presentation. These signals result in the IκB-kinase 2-mediated phosphorylation of phagosome-associated SNAP23, which stabilizes SNARE complexes, ultimately facilitating cross-presentation (11). Similar to endogenous Ag presentation by MHC class I, cross-presentation can also be reduced upon TLR engagement (7, 15, 18). Alternatively, cross-presentation can be enhanced via the binding of TLR2- (13, 82), TLR4- (18), TLR7- (8) or TLR9-specific agonists (83). Differences in terms of the activation vs. inhibition of cross-presentation are likely due to the timing of APC exposure to the TLR ligands, as compared to the cross-presented Ags.
Ag presentation by MHC class II molecules is also differentially affected by the engagement of distinct TLRs. Interestingly, in some cases, it seems to be a means of immune evasion by a pathogen. For example, 19 and 24 kDa lipoproteins from Mycobacterium tuberculosis can inhibit both MHC class II expression and Ag processing via TLR2 signaling (25, 84, 85). In contrast, a variety of TLR ligands (including those for TLR2), can upregulate MHC class II molecules on microglia (29). Ligands for TLR1/2, 4, 7 and 9 were shown to enhance the ability of APCs to present Ag-85B of Bacillus Calmette-Guerin (BCG) to CD4+ T cells specific for that Ag, via the upregulation of MHC class II molecules (86). Moreover, the stimulation of TLR signaling and delivery of a TLR-specific ligand (in this case, the TLR4 ligand, LPS) into phagosomes, can contribute to the generation of peptide/MHC class II complexes; this is as a means to segregate self vs. non-self peptides in a phagosome-autonomous manner (22). This delivery of TLR4 ligands is due to adaptor protein-3 (AP-3)-dependent transport of TLR4 from endosomes to phagosomes (87). Sometimes, MHC class II-mediated responses--in this case, flagellin-specific CD4+ T cell responses--can be enhanced in a TLR5-dependent (but nonconventional TLR signaling pathway) manner (26). In that report, DCs from MyD88-deficient mice could increase flagellin-specific CD4+ T cells in a TLR5-dependent manner that was comparable to DCs from wildtype mice (26). This response is regulated by CD103-CD11b+ DCs (88). Type I interferons can work with TLR ligand-mediated signals in order to generate type B peptide/MHC class II complexes (pMHC); type B pMHC are complexes formed in early endosomes from exogenous peptides (89). In fact, type I interferons are very important for such peptide generation, as DCs deficient in the receptor for type I interferons are impaired in their ability to generate type B pMHC (89). However, in the context of DC maturation, whereas TLR ligands can inhibit MHC class II synthesis and presence in intracellular compartments, type I interferons can prevent this from occurring (30).
The activation of TLR signaling pathways by TLR-specific ligands has also been investigated in terms of its effect on the CD1d/NKT cell axis. Mimicking APC stimulation of NKT cells (i.e., a panel of murine NKT cell hybridomas) using anti-CD3 and IFN-α upregulates TLRs on the cell surface (75). Moreover, the exposure of NKT cells to a variety of TLR ligands enhances NKT cell production of IFN-γ, IL-4 and TNF-α (75). However, another report indicated that although human NKT cells express all TLRs (except TLR8), they are not activated when directly exposed to TLR ligands; yet, they are stimulated when TLR ligands are added to total PBMCs (73). The differences could simply be due to the fact that these studies analyzed mouse NKT cell hybridomas (75), as opposed to normal human NKT cells from PBMCs (73). Culture of murine mononuclear cells (MNCs) with the CD1d-binding glycolipid, α-GalCer, and poly I:C (a TLR3 ligand), resulted in NKT cell activation in a model of airway inflammation (76). Certainly, the effects observed in that report could be NKT cell- and/or APC-specific. Murine bone marrow-derived DCs (BMDCs) exposed to LPS or infected with Salmonella typhimurium were able to stimulate NKT cells at a high level. This suggests that APC TLR4 signaling enhanced Ag presentation by CD1d molecules (71). Additionally, TLR4 has been shown to work with Nod1 and Nod2, two members of the Nod-like receptor family that are cytosolic pattern recognition receptors. Here, LPS treatment of BMDCs resulted in IFN-γ production by NKT cells (74). An in vivo infection with S. typhimurium (which contains LPS) can activate iNKT cells (71, 72), but does so without the need for a CD1d-presented lipid Ag (72).
JAK/STAT pathway effects on MHC and CD1d
The JAK/STAT signaling pathway is activated by most cytokines involved in the development and regulation of the host's immune response (90). There are four receptor-associated JAKs that, upon phosphorylation of the receptor they are bound to, recruit one of seven STATs to the receptor to be phosphorylated by that JAK. This process then results in the dimerization of the phosphorylated STATs which are translocated into the nucleus, where they regulate the expression of a variety of genes (91).
JAK/STAT signaling upon activation by IFN-γ is critical for MHC gene expression and, ultimately, expression of MHC class I and class II molecules on the cell surface (90, 91). Of importance, it has been shown that phosphorylated STAT1 facilitates human MHC locus chromatin remodeling, as a first step for the subsequent expression of HLA genes (92). Impairing the ability of IFN-γ to stimulate JAK/STAT signaling and thereby preventing MHC gene expression, is one mechanism by which viruses can evade recognition by the host's immune response (16). For example, MHC class I and/or class II molecules can be targeted by herpesviruses (19, 28, 93) or influenza virus (31). In each of these cases, it is likely that a virus-encoded protein(s) prevented IFN-γ-induced activation of the JAK/STAT pathway. Similarly, immune evasion by solid tumors can be due to an impairment of JAK/STAT signaling that prevents the upregulation of MHC class I molecules (12, 14) or TAP1 expression (9).
In contrast to the immune evasion examples presented above, activation of JAK/STAT signaling can have beneficial effects. For example, IL-10, which activates a JAK1/STAT3 complex, inhibits DC maturation by mesenchymal stem cells (94). A case where this could be important is in the prevention of NKT cell-mediated colitis. Here, crosslinking CD1d on colonic epithelial cells results in STAT3 activation; the transcription of the il-10 gene (by STAT3) and consequent IL-10 production controls inflammation in those cells (95). Recently, we showed that STAT3 is essential for CD1d-mediated Ag presentation, due to its ability to regulate the transcription of UDP glucose ceramide glycosyltransferase, an enzyme involved in the first step of glycosphingolipid biosynthesis—from which the natural ligands of CD1d are derived (96). The critical nature of STAT3 in the CD1d/NKT cell axis is evident in patients with loss-of-function mutations in STAT3. Those patients have a significantly reduced number of NKT cells (97).
Rho kinase and the actin cytoskeleton in CD1d-mediated Ag presentation
Rho GTPases are well known for mediating intracellular protein traffic [reviewed in (98)]. This is due to their control over the generation of F-actin. Thus, Rho GTPases activate the Rho kinase (ROCK) which then phosphorylates the LIM kinase (LIMK), which then activates cofilin. Normally, cofilin, in its nonphosphorylated form, prevents the polymerization of actin. The phosphorylation of cofilin by LIMK permits F-actin formation (99, 100). We found that F-actin actually impairs Ag presentation by CD1d (101). When we disrupted the actin cytoskeleton with cytochalasin D, Ag presentation was enhanced. Thus, ROCK is a negative regulator of CD1d-mediated Ag presentation. Interestingly, we found that ROCK actually promoted Ag presentation by MHC class II molecules (101). This was another example in which we have found specific cell signaling pathways that differentially regulate Ag presentation by MHC class II vs. CD1d (21, 96, 101).
Knowing that ROCK impairs Ag presentation by CD1d via the polymerization of actin is only part of the mechanism by which this occurs. How does actin actually do it? Recently, it has been reported that actin segregates CD1d nanoclusters on the cell surface via direct interaction between actin and the CD1d cytoplasmic tail (102). This interaction keeps them at a certain density on the surface. Allowing the formation of larger nanoclusters (by blocking the ability of CD1d's cytoplasmic tail to bind to actin) results in enhanced NKT cell activation—exactly the same observation we made when the actin cytoskeleton was disrupted in our system by cytochalasin D (101).
Cell signaling pathways and disease: can these be used as targets in terms of immunotherapy?
For the most part, cell signaling pathways appear to be important in inflammatory diseases (including cancer), which have a clear--or likely--immune component, where Ag processing and presentation would come into play. Of the MAPK, upstream ERK pathway components (i.e., Ras-Raf-ERK) are more involved. Either Ras or Raf contribute to malignant transformation and are thereby targeted with Ras- or Raf-specific inhibitors (103-106). Moreover, blocking ERK in esophageal and gastric cancers, or in melanoma (in the context of immunotherapy), results in an increase in MHC class I molecules (107-109).
For the p38 MAPK, inflammation has been shown to increase tumor-induced (and immunosuppressive) myeloid-derived suppressor cells (MDSC); the activation of p38 is believed to be important for MDSC survival (110). For the treatment of rheumatoid arthritis, p38 inhibitors have been considered (111). Moreover, p38 is also believed to be a culprit in the development of acquired immune deficiency syndrome and HIV-associated neurocognitive disorders in HIV patients, which could have an Ag presentation component (112).
An important aspect of p38 signaling needs to be taken into account when considering using inhibitors against that MAPK: there is reciprocal cross-talk between p38 and STAT3 [reviewed in (113)]. In the context of the CD1d/NKT cell axis, this could be critical in the disease that one is attempting to mitigate, as p38 is a negative (whereas STAT3 is a positive) regulator of lipid Ag presentation by CD1d (41-43, 96, 97, 101).
The JAK/STAT pathway (in particular, STAT3), is also important in cancer and inflammation. In a recent study of patients with non-refractory Hodgkin's lymphoma being treated using a PD-1 blockade immunotherapy paradigm, phosphorylated STAT3 was detected in the nucleus of Reed-Sternberg cells (the presence of which is necessary for the diagnosis of Hodgkin's lymphoma) (114). In Behcet's disease, an inflammatory disorder resulting in vasculitis, STAT3 is activated (115). In contrast, there appears to be attenuated IFN-α-induced STAT3 signaling in DCs from patients with Crohn's disease and IL-10 induces enhanced STAT3 activation, which could impact the DC's ability to present Ag (116). Relating Crohn's disease effects to the various cell signaling pathways in this brief review, there appears to be an association with an ICOSL loss-of-function mutation resulting in reduced cell signaling in these patients (117); this likely impacts immune homeostasis.
Lastly, signaling via TLRs have been predominantly associated with infectious diseases (118-124); however, a variety of single-nucleotide polymorphisms in human TLRs have not only been associated with infectious and inflammatory (including autoimmune) diseases, but also with cancer (121, 123-126). As such, TLRs have been looked at as potential targets for immune-based therapy against infectious diseases (118, 119), as well as sepsis-associated pathology (118). Moreover, another approach that is being considered is the specific targeting of TLR-associated adaptors that are negative regulators of TLR signaling (127). This is reminiscent of the CTLA-4 and inhibitory receptor programmed cell death-1 (PD-1) targeting of antitumor T cells that has recently shown promise in clinical trials with cancer patients (128, 129). This approach effectively removes inhibitory signals coming from the APCs to the effector T cells.
Conclusions
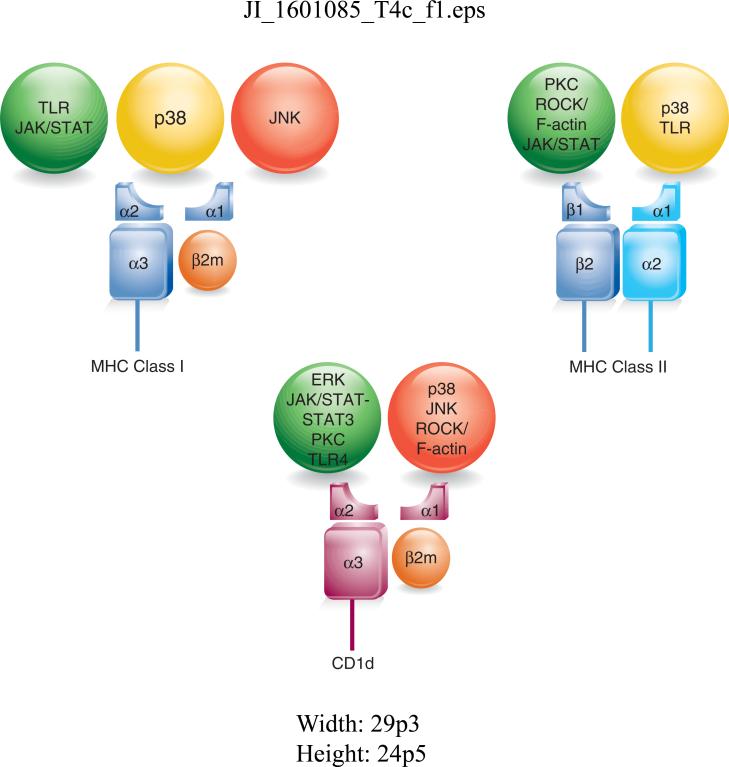
Although cell signaling pathways have been studied for quite a number of years, very little has been focused on Ag processing and presentation, and how they affect APCs and/or the T cells that recognize them. Nonetheless, Figure 2 summarizes what I have described in this Brief Review in terms of how these cell signaling pathways affect MHC class I, MHC class II and/or CD1d. To help the readers, I have also included a table (Table 1), indicating the reports cited here, that have studied these specific cell signaling pathways and Ag presentation. As is clear from the work done so far, the cell signaling pathways described above have at least some impact on various components of a host's immune response; these could potentially be exploited for adding new weapons to the arsenal against infectious and autoimmune diseases, as well as cancer. We have just begun to scratch the surface.
FIGURE 2.
Summary of the known effects of cell signaling pathways on Ag presentation by MHC class I, MHC class II and CD1d molecules. The effects are indicated as a traffic light analogy: green is a positive (or upregulating) effect, red is a negative (or downregulating) effect; those pathways in the yellow light suggest some ambiguity for the effects of those pathways (e.g., some are positive; some are negative—it depends on the context).
Table 1.
Reports addressing Ag presentation via cell signaling pathways*
| Cell Signaling Pathway | Effects on Ag presentation by: | ||
|---|---|---|---|
| MHC I | MHC II | CD1d | |
| p38 | 7; 47-49; 51 | 52-54 | 21; 42,43 |
| ERK | 47, 48 | 47, 48 | 43 |
| JNK | 58, 59 | Liu, et al.** | |
| PKC | 23, 27, 65-67 | 67 | |
| ROCK | 101 | 101 | |
| TLR | 7, 8, 13, 15, 17, 18, 51, 79-83 | 17, 22, 25, 26, 30, 77, 84-86, 88, 89 | 71-76 |
| JAK/STAT | 9, 12, 14, 16, 19, 28, 31, 90-93 | 16, 19, 28, 31, 90-93 | 96, 97 |
The table indicates the reference numbers cited in this review that describe a role for the various cell signaling pathways in Ag presentation
Liu, et al. (manuscript in preparation)
Acknowledgements
I would like to thank the members of my laboratory, past and present, who have made many important contributions to our understanding of how cell signaling pathways regulate Ag presentation by CD1d. For this Brief Review, I would like to thank Jean Liu and Abhirami Iyer for their careful reading of the manuscript (and also to Abhi for her idea regarding a summary figure for this review).
Work from my laboratory described here has been supported by grants from the National Institutes of Health and Department of Defense.
Abbreviations used in this article
- α-GalCer
α-galactosylceramide
- BCG
Bacillus Calmette-Guerin
- BMDC
bone marrow-derived dendritic cells
- LIMK
Lim kinase
- MΦ
macrophage
- MAIT cells
mucosal-associated invariant T cells
- MDSC
myeloid-derived suppressor cells
- MNCs
mononuclear cells
- NKT
natural killer T
- PD-1
programmed cell death-1
- PKC
protein kinase C
Footnotes
Disclosures
The author has no financial conflict of interest
References
- 1.Blum JS, Wearsch PA, Cresswell P. Pathways of antigen processing. Annu Rev Immunol. 2013;31:443–473. doi: 10.1146/annurev-immunol-032712-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Springer S. Transport and quality control of MHC class I molecules in the early secretory pathway. Curr Opin Immunol. 2015;34:83–90. doi: 10.1016/j.coi.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Mintern JD, Macri C, Villadangos JA. Modulation of antigen presentation by intracellular trafficking. Curr Opin Immunol. 2015;34:16–21. doi: 10.1016/j.coi.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Adiko AC, Babdor J, Gutierrez-Martinez E, Guermonprez P, Saveanu L. Intracellular Transport Routes for MHC I and Their Relevance for Antigen Cross-Presentation. Front Immunol. 2015;6:335. doi: 10.3389/fimmu.2015.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McEwen-Smith RM, Salio M, Cerundolo V. CD1d-dependent endogenous and exogenous lipid antigen presentation. Curr Opin Immunol. 2015;34:116–125. doi: 10.1016/j.coi.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Ackerman AL, Cresswell P. Regulation of MHC class I transport in human dendritic cells and the dendritic-like cell line KG-1. J Immunol. 2003;170:4178–4188. doi: 10.4049/jimmunol.170.8.4178. [DOI] [PubMed] [Google Scholar]
- 7.Aleyas AG, Han YW, Patil AM, Kim SB, Kim K, Eo SK. Impaired cross-presentation of CD8alpha+ CD11c+ dendritic cells by Japanese encephalitis virus in a TLR2/MyD88 signal pathway-dependent manner. Eur J Immunol. 2012;42:2655–2666. doi: 10.1002/eji.201142052. [DOI] [PubMed] [Google Scholar]
- 8.Crespo MI, Zacca ER, Nunez NG, Ranocchia RP, Maccioni M, Maletto BA, Pistoresi-Palencia MC, Moron G. TLR7 triggering with polyuridylic acid promotes cross-presentation in CD8alpha+ conventional dendritic cells by enhancing antigen preservation and MHC class I antigen permanence on the dendritic cell surface. J Immunol. 2013;190:948–960. doi: 10.4049/jimmunol.1102725. [DOI] [PubMed] [Google Scholar]
- 9.Heise R, Amann PM, Ensslen S, Marquardt Y, Czaja K, Joussen S, Beer D, Abele R, Plewnia G, Tampe R, Merk HF, Hermanns HM, Baron JM. Interferon alpha signalling and its relevance for the upregulatory effect of transporter proteins associated with antigen processing (TAP) in patients with malignant melanoma. PLoS One. 2016;11:e0146325. doi: 10.1371/journal.pone.0146325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacAry PA, Lindsay M, Scott MA, Craig JI, Luzio JP, Lehner PJ. Mobilization of MHC class I molecules from late endosomes to the cell surface following activation of CD34-derived human Langerhans cells. Proc Natl Acad Sci U S A. 2001;98:3982–3987. doi: 10.1073/pnas.071477498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nair-Gupta P, Baccarini A, Tung N, Seyffer F, Florey O, Huang Y, Banerjee M, Overholtzer M, Roche PA, Tampe R, Brown BD, Amsen D, Whiteheart SW, Blander JM. TLR signals induce phagosomal MHC-I delivery from the endosomal recycling compartment to allow cross-presentation. Cell. 2014;158:506–521. doi: 10.1016/j.cell.2014.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez T, Mendez R, Del Campo A, Jimenez P, Aptsiauri N, Garrido F, Ruiz-Cabello F. Distinct mechanisms of loss of IFN-gamma mediated HLA class I inducibility in two melanoma cell lines. BMC Cancer. 2007;7:34. doi: 10.1186/1471-2407-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen KY, Song YC, Chen IH, Leng CH, Chen HW, Li HJ, Chong P, Liu SJ. Molecular mechanisms of TLR2-mediated antigen cross-presentation in dendritic cells. J Immunol. 2014;192:4233–4241. doi: 10.4049/jimmunol.1302850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Svane IM, Engel AM, Nielsen M, Werdelin O. Interferon-gamma-induced MHC class I expression and defects in Jak/Stat signalling in methylcholanthrene-induced sarcomas. Scand J Immunol. 1997;46:379–387. doi: 10.1046/j.1365-3083.1997.d01-141.x. [DOI] [PubMed] [Google Scholar]
- 15.Weck MM, Grunebach F, Werth D, Sinzger C, Bringmann A, Brossart P. TLR ligands differentially affect uptake and presentation of cellular antigens. Blood. 2007;109:3890–3894. doi: 10.1182/blood-2006-04-015719. [DOI] [PubMed] [Google Scholar]
- 16.Zhou F. Molecular mechanisms of viral immune evasion proteins to inhibit MHC class I antigen processing and presentation. Int Rev Immunol. 2009;28:376–393. doi: 10.1080/08830180903013034. [DOI] [PubMed] [Google Scholar]
- 17.Nair P, Amsen D, Blander JM. Co-ordination of incoming and outgoing traffic in antigen-presenting cells by pattern recognition receptors and T cells. Traffic. 2011;12:1669–1676. doi: 10.1111/j.1600-0854.2011.01251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner CS, Cresswell P. TLR and nucleotide-binding oligomerization domain-like receptor signals differentially regulate exogenous antigen presentation. J Immunol. 2012;188:686–693. doi: 10.4049/jimmunol.1102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abendroth A, Slobedman B, Lee E, Mellins E, Wallace M, Arvin AM. Modulation of major histocompatibility class II protein expression by varicella-zoster virus. J Virol. 2000;74:1900–1907. doi: 10.1128/jvi.74.4.1900-1907.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andreae S, Buisson S, Triebel F. MHC class II signal transduction in human dendritic cells induced by a natural ligand, the LAG-3 protein (CD223). Blood. 2003;102:2130–2137. doi: 10.1182/blood-2003-01-0273. [DOI] [PubMed] [Google Scholar]
- 21.Bailey JC, Iyer AK, Renukaradhya GJ, Lin Y, Nguyen H, Brutkiewicz RR. Inhibition of CD1d-mediated antigen presentation by the transforming growth factor-beta/Smad signalling pathway. Immunology. 2014;143:679–691. doi: 10.1111/imm.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440:808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 23.Chen YW, Lang ML, Wade WF. Protein kinase C-alpha and -delta are required for FcalphaR (CD89) trafficking to MHC class II compartments and FcalphaR-mediated antigen presentation. Traffic. 2004;5:577–594. doi: 10.1111/j.1600-0854.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- 24.Delamarre L, Holcombe H, Mellman I. Presentation of exogenous antigens on major histocompatibility complex (MHC) class I and MHC class II molecules is differentially regulated during dendritic cell maturation. J Exp Med. 2003;198:111–122. doi: 10.1084/jem.20021542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gehring AJ, Dobos KM, Belisle JT, Harding CV, Boom WH. Mycobacterium tuberculosis LprG (Rv1411c): a novel TLR-2 ligand that inhibits human macrophage class II MHC antigen processing. J Immunol. 2004;173:2660–2668. doi: 10.4049/jimmunol.173.4.2660. [DOI] [PubMed] [Google Scholar]
- 26.Letran SE, Lee SJ, Atif SM, Uematsu S, Akira S, McSorley SJ. TLR5 functions as an endocytic receptor to enhance flagellin-specific adaptive immunity. Eur J Immunol. 2011;41:29–38. doi: 10.1002/eji.201040717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Majewski M, Bose TO, Sille FC, Pollington AM, Fiebiger E, Boes M. Protein kinase C delta stimulates antigen presentation by Class II MHC in murine dendritic cells. Int Immunol. 2007;19:719–732. doi: 10.1093/intimm/dxm034. [DOI] [PubMed] [Google Scholar]
- 28.Miller DM, Rahill BM, Boss JM, Lairmore MD, Durbin JE, Waldman JW, Sedmak DD. Human cytomegalovirus inhibits major histocompatibility complex class II expression by disruption of the Jak/Stat pathway. J Exp Med. 1998;187:675–683. doi: 10.1084/jem.187.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 30.Simmons DP, Wearsch PA, Canaday DH, Meyerson HJ, Liu YC, Wang Y, Boom WH, Harding CV. Type I IFN drives a distinctive dendritic cell maturation phenotype that allows continued class II MHC synthesis and antigen processing. J Immunol. 2012;188:3116–3126. doi: 10.4049/jimmunol.1101313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uetani K, Hiroi M, Meguro T, Ogawa H, Kamisako T, Ohmori Y, Erzurum SC. Influenza A virus abrogates IFN-gamma response in respiratory epithelial cells by disruption of the Jak/Stat pathway. Eur J Immunol. 2008;38:1559–1573. doi: 10.1002/eji.200737045. [DOI] [PubMed] [Google Scholar]
- 32.Kain L, Costanzo A, Webb B, Holt M, Bendelac A, Savage PB, Teyton L. Endogenous ligands of natural killer T cells are alpha-linked glycosylceramides. Mol Immunol. 2015;68:94–97. doi: 10.1016/j.molimm.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Rhijn I, Godfrey DI, Rossjohn J, Moody DB. Lipid and small-molecule display by CD1 and MR1. Nat Rev Immunol. 2015;15:643–654. doi: 10.1038/nri3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Bourhis L, Mburu YK, Lantz O. MAIT cells, surveyors of a new class of antigen: development and functions. Curr Opin Immunol. 2013;25:174–180. doi: 10.1016/j.coi.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 35.McWilliam HE, Birkinshaw RW, Villadangos JA, McCluskey J, Rossjohn J. MR1 presentation of vitamin B-based metabolite ligands. Curr Opin Immunol. 2015;34:28–34. doi: 10.1016/j.coi.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Arthur JS, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol. 2013;13:679–692. doi: 10.1038/nri3495. [DOI] [PubMed] [Google Scholar]
- 37.Rincon M, Flavell RA, Davis RA. The JNK and P38 MAP kinase signaling pathways in T cell-mediated immune responses. Free Radic Biol Med. 2000;28:1328–1337. doi: 10.1016/s0891-5849(00)00219-7. [DOI] [PubMed] [Google Scholar]
- 38.Lu HT, Yang DD, Wysk M, Gatti E, Mellman I, Davis RJ, Flavell RA. Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. EMBO J. 1999;18:1845–1857. doi: 10.1093/emboj/18.7.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bunyard P, Handley M, Pollara G, Rutault K, Wood I, Chaudry M, Alderman C, Foreman J, Katz DR, Chain BM. Ribotoxic stress activates p38 and JNK kinases and modulates the antigen-presenting activity of dendritic cells. Mol Immunol. 2003;39:815–827. doi: 10.1016/s0161-5890(02)00262-6. [DOI] [PubMed] [Google Scholar]
- 40.Xie J, Qian J, Yang J, Wang S, Freeman ME, 3rd, Yi Q. Critical roles of Raf/MEK/ERK and PI3K/AKT signaling and inactivation of p38 MAP kinase in the differentiation and survival of monocyte-derived immature dendritic cells. Exp Hematol. 2005;33:564–572. doi: 10.1016/j.exphem.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 41.Khan MA, Sriram V, Renukaradhya GJ, Du W, Gervay-Hague J, Brutkiewicz RR. Apoptosis-induced inhibition of CD1d-mediated antigen presentation: different roles for caspases and signal transduction pathways. Immunology. 2008;125:80–90. doi: 10.1111/j.1365-2567.2008.02823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Renukaradhya GJ, Khan MA, Shaji D, Brutkiewicz RR. Vesicular stomatitis virus matrix protein impairs CD1d-mediated antigen presentation through activation of the p38 MAPK pathway. J Virol. 2008;82:12535–12542. doi: 10.1128/JVI.00881-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Renukaradhya GJ, Webb TJ, Khan MA, Lin YL, Du W, Gervay-Hague J, Brutkiewicz RR. Virus-induced inhibition of CD1d1-mediated antigen presentation: reciprocal regulation by p38 and ERK. J Immunol. 2005;175:4301–4308. doi: 10.4049/jimmunol.175.7.4301. [DOI] [PubMed] [Google Scholar]
- 44.Ardeshna KM, Pizzey AR, Devereux S, Khwaja A. The PI3 kinase, p38 SAP kinase, and NF-kappaB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood. 2000;96:1039–1046. [PubMed] [Google Scholar]
- 45.Rescigno M, Martino M, Sutherland CL, Gold MR, Ricciardi-Castagnoli P. Dendritic cell survival and maturation are regulated by different signaling pathways. J Exp Med. 1998;188:2175–2180. doi: 10.1084/jem.188.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jorgl A, Platzer B, Taschner S, Heinz LX, Hocher B, Reisner PM, Gobel F, Strobl H. Human Langerhans-cell activation triggered in vitro by conditionally expressed MKK6 is counterregulated by the downstream effector RelB. Blood. 2007;109:185–193. doi: 10.1182/blood-2006-05-022954. [DOI] [PubMed] [Google Scholar]
- 47.Bharadwaj U, Zhang R, Yang H, Li M, Doan LX, Chen C, Yao Q. Effects of cyclophilin A on myeloblastic cell line KG-1 derived dendritic like cells (DLC) through p38 MAP kinase activation. J Surg Res. 2005;127:29–38. doi: 10.1016/j.jss.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 48.Seshasayee D, Wang H, Lee WP, Gribling P, Ross J, Van Bruggen N, Carano R, Grewal IS. A novel in vivo role for osteoprotegerin ligand in activation of monocyte effector function and inflammatory response. J Biol Chem. 2004;279:30202–30209. doi: 10.1074/jbc.M403968200. [DOI] [PubMed] [Google Scholar]
- 49.Yu Q, Kovacs C, Yue FY, Ostrowski MA. The role of the p38 mitogen-activated protein kinase, extracellular signal-regulated kinase, and phosphoinositide-3-OH kinase signal transduction pathways in CD40 ligand-induced dendritic cell activation and expansion of virus-specific CD8+ T cell memory responses. J Immunol. 2004;172:6047–6056. doi: 10.4049/jimmunol.172.10.6047. [DOI] [PubMed] [Google Scholar]
- 50.Hansen SG, Sacha JB, Hughes CM, Ford JC, Burwitz BJ, Scholz I, Gilbride RM, Lewis MS, Gilliam AN, Ventura AB, Malouli D, Xu G, Richards R, Whizin N, Reed JS, Hammond KB, Fischer M, Turner JM, Legasse AW, Axthelm MK, Edlefsen PT, Nelson JA, Lifson JD, Fruh K, Picker LJ. Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science. 2013;340:1237874. doi: 10.1126/science.1237874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turnis ME, Song XT, Bear A, Foster AE, Gottschalk S, Brenner MK, Chen SY, Rooney CM. IRAK-M removal counteracts dendritic cell vaccine deficits in migration and longevity. J Immunol. 2010;185:4223–4232. doi: 10.4049/jimmunol.0903507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park SY, Kim Y. Surfactin inhibits immunostimulatory function of macrophages through blocking NK-kappaB, MAPK and Akt pathway. Int Immunopharmacol. 2009;9:886–893. doi: 10.1016/j.intimp.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 53.Palova-Jelinkova L, Rozkova D, Pecharova B, Bartova J, Sediva A, Tlaskalova-Hogenova H, Spisek R, Tuckova L. Gliadin fragments induce phenotypic and functional maturation of human dendritic cells. J Immunol. 2005;175:7038–7045. doi: 10.4049/jimmunol.175.10.7038. [DOI] [PubMed] [Google Scholar]
- 54.Wang S, Hong S, Yang J, Qian J, Zhang X, Shpall E, Kwak LW, Yi Q. Optimizing immunotherapy in multiple myeloma: Restoring the function of patients' monocyte-derived dendritic cells by inhibiting p38 or activating MEK/ERK MAPK and neutralizing interleukin-6 in progenitor cells. Blood. 2006;108:4071–4077. doi: 10.1182/blood-2006-04-016980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gagliardi MC, Teloni R, Giannoni F, Mariotti S, Remoli ME, Sargentini V, Videtta M, Pardini M, De Libero G, Coccia EM, Nisini R. Mycobacteria exploit p38 signaling to affect CD1 expression and lipid antigen presentation by human dendritic cells. Infect Immun. 2009;77:4947–4952. doi: 10.1128/IAI.00607-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 57.Stuart JK, Bisch SP, Leon-Ponte M, Hayatsu J, Mazzuca DM, Maleki Vareki S, Haeryfar SM. Negative modulation of invariant natural killer T cell responses to glycolipid antigens by p38 MAP kinase. Int Immunopharmacol. 2010;10:1068–1076. doi: 10.1016/j.intimp.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 58.Conze D, Krahl T, Kennedy N, Weiss L, Lumsden J, Hess P, Flavell RA, Le Gros G, Davis RJ, Rincon M. c-Jun NH(2)-terminal kinase (JNK)1 and JNK2 have distinct roles in CD8(+) T cell activation. J Exp Med. 2002;195:811–823. doi: 10.1084/jem.20011508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arbour N, Naniche D, Homann D, Davis RJ, Flavell RA, Oldstone MB. c-Jun NH(2)-terminal kinase (JNK)1 and JNK2 signaling pathways have divergent roles in CD8(+) T cell-mediated antiviral immunity. J Exp Med. 2002;195:801–810. doi: 10.1084/jem.20011481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Valledor AF, Comalada M, Xaus J, Celada A. The differential time-course of extracellular-regulated kinase activity correlates with the macrophage response toward proliferation or activation. J Biol Chem. 2000;275:7403–7409. doi: 10.1074/jbc.275.10.7403. [DOI] [PubMed] [Google Scholar]
- 61.Monick MM, Carter AB, Flaherty DM, Peterson MW, Hunninghake GW. Protein kinase C zeta plays a central role in activation of the p42/44 mitogen-activated protein kinase by endotoxin in alveolar macrophages. J Immunol. 2000;165:4632–4639. doi: 10.4049/jimmunol.165.8.4632. [DOI] [PubMed] [Google Scholar]
- 62.Hacker H, Mischak H, Hacker G, Eser S, Prenzel N, Ullrich A, Wagner H. Cell type-specific activation of mitogen-activated protein kinases by CpG-DNA controls interleukin-12 release from antigen-presenting cells. EMBO J. 1999;18:6973–6982. doi: 10.1093/emboj/18.24.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lim PS, Sutton CR, Rao S. Protein kinase C in the immune system: from signalling to chromatin regulation. Immunology. 2015;146:508–522. doi: 10.1111/imm.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Igumenova TI. Dynamics and Membrane Interactions of Protein Kinase C. Biochemistry. 2015;54:4953–4968. doi: 10.1021/acs.biochem.5b00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Do Y, Hegde VL, Nagarkatti PS, Nagarkatti M. Bryostatin-1 enhances the maturation and antigen-presenting ability of murine and human dendritic cells. Cancer Res. 2004;64:6756–6765. doi: 10.1158/0008-5472.CAN-03-4002. [DOI] [PubMed] [Google Scholar]
- 66.Zhao D, Amria S, Hossain A, Sundaram K, Komlosi P, Nagarkatti M, Haque A. Enhancement of HLA class II-restricted CD4+ T cell recognition of human melanoma cells following treatment with bryostatin-1. Cell Immunol. 2011;271:392–400. doi: 10.1016/j.cellimm.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brutkiewicz RR, Willard CA, Gillett-Heacock KK, Pawlak MR, Bailey JC, Khan MA, Nagala M, Du W, Gervay-Hague J, Renukaradhya GJ. Protein kinase C delta is a critical regulator of CD1d-mediated antigen presentation. Eur J Immunol. 2007;37:2390–2395. doi: 10.1002/eji.200737124. [DOI] [PubMed] [Google Scholar]
- 68.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 69.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 70.McGuire VA, Arthur JS. Subverting Toll-Like Receptor Signaling by Bacterial Pathogens. Front Immunol. 2015;6:607. doi: 10.3389/fimmu.2015.00607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 72.Holzapfel KL, Tyznik AJ, Kronenberg M, Hogquist KA. Antigen-dependent versus -independent activation of invariant NKT cells during infection. J Immunol. 2014;192:5490–5498. doi: 10.4049/jimmunol.1400722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moreno M, Mol BM, von Mensdorff-Pouilly S, Verheijen RH, de Jong EC, von Blomberg BM, van den Eertwegh AJ, Scheper RJ, Bontkes HJ. Differential indirect activation of human invariant natural killer T cells by Toll-like receptor agonists. Immunotherapy. 2009;1:557–570. doi: 10.2217/imt.09.30. [DOI] [PubMed] [Google Scholar]
- 74.Selvanantham T, Escalante NK, Cruz Tleugabulova M, Fieve S, Girardin SE, Philpott DJ, Mallevaey T. Nod1 and Nod2 enhance TLR-mediated invariant NKT cell activation during bacterial infection. J Immunol. 2013;191:5646–5654. doi: 10.4049/jimmunol.1301412. [DOI] [PubMed] [Google Scholar]
- 75.Villanueva AI, Haeryfar SM, Mallard BA, Kulkarni RR, Sharif S. Functions of invariant NK T cells are modulated by TLR ligands and IFN-alpha. Innate Immun. 2015;21:275–288. doi: 10.1177/1753425914527327. [DOI] [PubMed] [Google Scholar]
- 76.Vultaggio A, Nencini F, Pratesi S, Petroni G, Romagnani S, Maggi E. Poly(I:C) promotes the production of IL-17A by murine CD1d-driven invariant NKT cells in airway inflammation. Allergy. 2012;67:1223–1232. doi: 10.1111/j.1398-9995.2012.02876.x.. [DOI] [PubMed] [Google Scholar]
- 77.Yarovinsky F, Kanzler H, Hieny S, Coffman RL, Sher A. Toll-like receptor recognition regulates immunodominance in an antimicrobial CD4+ T cell response. Immunity. 2006;25:655–664. doi: 10.1016/j.immuni.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 78.Assier E, Marin-Esteban V, Haziot A, Maggi E, Charron D, Mooney N. TLR7/8 agonists impair monocyte-derived dendritic cell differentiation and maturation. J Leukoc Biol. 2007;81:221–228. doi: 10.1189/jlb.0705385. [DOI] [PubMed] [Google Scholar]
- 79.Siddiqui S, Alatery A, Kus A, Basta S. TLR engagement prior to virus infection influences MHC-I antigen presentation in an epitope-dependent manner as a result of nitric oxide release. J Leukoc Biol. 2011;89:457–468. doi: 10.1189/jlb.0610357. [DOI] [PubMed] [Google Scholar]
- 80.Tobian AA, Potter NS, Ramachandra L, Pai RK, Convery M, Boom WH, Harding CV. Alternate class I MHC antigen processing is inhibited by Toll-like receptor signaling pathogen-associated molecular patterns: Mycobacterium tuberculosis 19-kDa lipoprotein, CpG DNA, and lipopolysaccharide. J Immunol. 2003;171:1413–1422. doi: 10.4049/jimmunol.171.3.1413. [DOI] [PubMed] [Google Scholar]
- 81.Aleyas AG, Han YW, George JA, Kim B, Kim K, Lee CK, Eo SK. Multifront assault on antigen presentation by Japanese encephalitis virus subverts CD8+ T cell responses. J Immunol. 2010;185:1429–1441. doi: 10.4049/jimmunol.0902536. [DOI] [PubMed] [Google Scholar]
- 82.Santone M, Aprea S, Wu TY, Cooke MP, Mbow ML, Valiante NM, Rush JS, Dougan S, Avalos A, Ploegh H, De Gregorio E, Buonsanti C, D'Oro U. A new TLR2 agonist promotes cross-presentation by mouse and human antigen presenting cells. Hum Vaccin Immunother. 2015;11:2038–2050. doi: 10.1080/21645515.2015.1027467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kool M, Geurtsvankessel C, Muskens F, Madeira FB, van Nimwegen M, Kuipers H, Thielemans K, Hoogsteden HC, Hammad H, Lambrecht BN. Facilitated antigen uptake and timed exposure to TLR ligands dictate the antigen-presenting potential of plasmacytoid DCs. J Leukoc Biol. 2011;90:1177–1190. doi: 10.1189/jlb.0610342. [DOI] [PubMed] [Google Scholar]
- 84.Noss EH, Pai RK, Sellati TJ, Radolf JD, Belisle J, Golenbock DT, Boom WH, Harding CV. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. J Immunol. 2001;167:910–918. doi: 10.4049/jimmunol.167.2.910. [DOI] [PubMed] [Google Scholar]
- 85.Gehring AJ, Rojas RE, Canaday DH, Lakey DL, Harding CV, Boom WH. The Mycobacterium tuberculosis 19-kilodalton lipoprotein inhibits gamma interferon-regulated HLA-DR and Fc gamma R1 on human macrophages through Toll-like receptor 2. Infect Immun. 2003;71:4487–4497. doi: 10.1128/IAI.71.8.4487-4497.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bakhru P, Sirisaengtaksin N, Soudani E, Mukherjee S, Khan A, Jagannath C. BCG vaccine mediated reduction in the MHC-II expression of macrophages and dendritic cells is reversed by activation of Toll-like receptors 7 and 9. Cell Immunol. 2014;287:53–61. doi: 10.1016/j.cellimm.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mantegazza AR, Guttentag SH, El-Benna J, Sasai M, Iwasaki A, Shen H, Laufer TM, Marks MS. Adaptor protein-3 in dendritic cells facilitates phagosomal toll-like receptor signaling and antigen presentation to CD4(+) T cells. Immunity. 2012;36:782–794. doi: 10.1016/j.immuni.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Atif SM, Uematsu S, Akira S, McSorley SJ. CD103-CD11b+ dendritic cells regulate the sensitivity of CD4 T-cell responses to bacterial flagellin. Mucosal Immunol. 2014;7:68–77. doi: 10.1038/mi.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Strong BS, Unanue ER. Presentation of type B peptide-MHC complexes from hen egg white lysozyme by TLR ligands and type I IFNs independent of H2-DM regulation. J Immunol. 2011;187:2193–2201. doi: 10.4049/jimmunol.1100152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.O'Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36:542–550. doi: 10.1016/j.immuni.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.O'Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med. 2013;368:161–170. doi: 10.1056/NEJMra1202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Christova R, Jones T, Wu PJ, Bolzer A, Costa-Pereira AP, Watling D, Kerr IM, Sheer D. P-STAT1 mediates higher-order chromatin remodelling of the human MHC in response to IFNgamma. J Cell Sci. 2007;120:3262–3270. doi: 10.1242/jcs.012328. [DOI] [PubMed] [Google Scholar]
- 93.Miller DM, Cebulla CM, Sedmak DD. Human cytomegalovirus inhibition of major histocompatibility complex transcription and interferon signal transduction. Curr Top Microbiol Immunol. 2002;269:153–170. doi: 10.1007/978-3-642-59421-2_10. [DOI] [PubMed] [Google Scholar]
- 94.Liu WH, Liu JJ, Wu J, Zhang LL, Liu F, Yin L, Zhang MM, Yu B. Novel mechanism of inhibition of dendritic cells maturation by mesenchymal stem cells via interleukin-10 and the JAK1/STAT3 signaling pathway. PLoS One. 2013;8:e55487. doi: 10.1371/journal.pone.0055487. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 95.Olszak T, Neves JF, Dowds CM, Baker K, Glickman J, Davidson NO, Lin CS, Jobin C, Brand S, Sotlar K, Wada K, Katayama K, Nakajima A, Mizuguchi H, Kawasaki K, Nagata K, Muller W, Snapper SB, Schreiber S, Kaser A, Zeissig S, Blumberg RS. Protective mucosal immunity mediated by epithelial CD1d and IL-10. Nature. 2014;509:497–502. doi: 10.1038/nature13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Iyer AK, Liu J, Gallo RM, Kaplan MH, Brutkiewicz RR. STAT3 promotes CD1d-mediated lipid antigen presentation by regulating a critical gene in glycosphingolipid biosynthesis. Immunology. 2015;146:444–455. doi: 10.1111/imm.12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wilson RP, Ives ML, Rao G, Lau A, Payne K, Kobayashi M, Arkwright PD, Peake J, Wong M, Adelstein S, Smart JM, French MA, Fulcher DA, Picard C, Bustamante J, Boisson-Dupuis S, Gray P, Stepensky P, Warnatz K, Freeman AF, Rossjohn J, McCluskey J, Holland SM, Casanova JL, Uzel G, Ma CS, Tangye SG, Deenick EK. STAT3 is a critical cell-intrinsic regulator of human unconventional T cell numbers and function. J Exp Med. 2015;212:855–864. doi: 10.1084/jem.20141992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–529. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 99.Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 100.Bernard O. Lim kinases, regulators of actin dynamics. Int J Biochem Cell Biol. 2007;39:1071–1076. doi: 10.1016/j.biocel.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 101.Gallo RM, Khan MA, Shi J, Kapur R, Wei L, Bailey JC, Liu J, Brutkiewicz RR. Regulation of the actin cytoskeleton by Rho kinase controls antigen presentation by CD1d. J Immunol. 2012;189:1689–1698. doi: 10.4049/jimmunol.1101484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Torreno-Pina JA, Manzo C, Salio M, Aichinger MC, Oddone A, Lakadamyali M, Shepherd D, Besra GS, Cerundolo V, Garcia-Parajo MF. The actin cytoskeleton modulates the activation of iNKT cells by segregating CD1d nanoclusters on antigen-presenting cells. Proc Natl Acad Sci U S A. 2016;113:E772–781. doi: 10.1073/pnas.1514530113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 104.Panka DJ, Atkins MB, Mier JW. Targeting the mitogen-activated protein kinase pathway in the treatment of malignant melanoma. Clin Cancer Res. 2006;12:2371s–2375s. doi: 10.1158/1078-0432.CCR-05-2539. [DOI] [PubMed] [Google Scholar]
- 105.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 106.Leicht DT, Balan V, Kaplun A, Singh-Gupta V, Kaplun L, Dobson M, Tzivion G. Raf kinases: function, regulation and role in human cancer. Biochim Biophys Acta. 2007;1773:1196–1212. doi: 10.1016/j.bbamcr.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mimura K, Shiraishi K, Mueller A, Izawa S, Kua LF, So J, Yong WP, Fujii H, Seliger B, Kiessling R, Kono K. The MAPK pathway is a predominant regulator of HLA-A expression in esophageal and gastric cancer. J Immunol. 2013;191:6261–6272. doi: 10.4049/jimmunol.1301597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hu-Lieskovan S, Mok S, Homet Moreno B, Tsoi J, Robert L, Goedert L, Pinheiro EM, Koya RC, Graeber TG, Comin-Anduix B, Ribas A. Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF(V600E) melanoma. Sci Transl Med. 2015;7:279ra241. doi: 10.1126/scitranslmed.aaa4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bradley SD, Chen Z, Melendez B, Talukder A, Khalili JS, Rodriguez-Cruz T, Liu S, Whittington M, Deng W, Li F, Bernatchez C, Radvanyi LG, Davies MA, Hwu P, Lizee G. BRAFV600E co-opts a conserved MHC class I internalization pathway to diminish antigen presentation and CD8+ T-cell recognition of melanoma. Cancer Immunol Res. 2015;3:602–609. doi: 10.1158/2326-6066.CIR-15-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ostrand-Rosenberg S, Sinha P, Chornoguz O, Ecker C. Regulating the suppressors: apoptosis and inflammation govern the survival of tumor-induced myeloid-derived suppressor cells (MDSC). Cancer Immunol Immunother. 2012;61:1319–1325. doi: 10.1007/s00262-012-1269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schett G, Zwerina J, Firestein G. The p38 mitogen-activated protein kinase (MAPK) pathway in rheumatoid arthritis. Ann Rheum Dis. 2008;67:909–916. doi: 10.1136/ard.2007.074278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Medders KE, Kaul M. Mitogen-activated protein kinase p38 in HIV infection and associated brain injury. J Neuroimmune Pharmacol. 2011;6:202–215. doi: 10.1007/s11481-011-9260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bode JG, Ehlting C, Haussinger D. The macrophage response towards LPS and its control through the p38(MAPK)-STAT3 axis. Cell Signal. 2012;24:1185–1194. doi: 10.1016/j.cellsig.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 114.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH, Zhu L, Grosso JF, Kim SY, Timmerman JM, Shipp MA, Armand P. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tulunay A, Dozmorov MG, Ture-Ozdemir F, Yilmaz V, Eksioglu-Demiralp E, Alibaz-Oner F, Ozen G, Wren JD, Saruhan-Direskeneli G, Sawalha AH, Direskeneli H. Activation of the JAK/STAT pathway in Behcet's disease. Genes Immun. 2015;16:170–175. doi: 10.1038/gene.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nieminen JK, Niemi M, Sipponen T, Salo HM, Klemetti P, Farkkila M, Vakkila J, Vaarala O. Dendritic cells from Crohn's disease patients show aberrant STAT1 and STAT3 signaling. PLoS One. 2013;8:e70738. doi: 10.1371/journal.pone.0070738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hedl M, Lahiri A, Ning K, Cho JH, Abraham C. Pattern recognition receptor signaling in human dendritic cells is enhanced by ICOS ligand and modulated by the Crohn's disease ICOSLG risk allele. Immunity. 2014;40:734–746. doi: 10.1016/j.immuni.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Savva A, Roger T. Targeting toll-like receptors: promising therapeutic strategies for the management of sepsis-associated pathology and infectious diseases. Front Immunol. 2013;4:387. doi: 10.3389/fimmu.2013.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mifsud EJ, Tan AC, Jackson DC. TLR Agonists as Modulators of the Innate Immune Response and Their Potential as Agents Against Infectious Disease. Front Immunol. 2014;5:79. doi: 10.3389/fimmu.2014.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cervantes JL, Hawley KL, Benjamin SJ, Weinerman B, Luu SM, Salazar JC. Phagosomal TLR signaling upon Borrelia burgdorferi infection. Front Cell Infect Microbiol. 2014;4:55. doi: 10.3389/fcimb.2014.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Skevaki C, Pararas M, Kostelidou K, Tsakris A, Routsias JG. Single nucleotide polymorphisms of Toll-like receptors and susceptibility to infectious diseases. Clin Exp Immunol. 2015;180:165–177. doi: 10.1111/cei.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Song B, Zhang Y, Chen L, Zhou T, Huang W, Zhou X, Shao L. The role of Toll-like receptors in periodontitis. Oral Dis. 2016 doi: 10.1111/odi.12468. [DOI] [PubMed] [Google Scholar]
- 123.Marques CP, Maor Y, de Andrade MS, Rodrigues VP, Benatti BB. Possible evidence of systemic lupus erythematosus and periodontal disease association mediated by Toll-like receptors 2 and 4. Clin Exp Immunol. 2016;183:187–192. doi: 10.1111/cei.12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Trejo-de la OA, Hernandez-Sancen P, Maldonado-Bernal C. Relevance of single-nucleotide polymorphisms in human TLR genes to infectious and inflammatory diseases and cancer. Genes Immun. 2014;15:199–209. doi: 10.1038/gene.2014.10. [DOI] [PubMed] [Google Scholar]
- 125.Mohammad Hosseini A, Majidi J, Baradaran B, Yousefi M. Toll-Like Receptors in the Pathogenesis of Autoimmune Diseases. Adv Pharm Bull. 2015;5:605–614. doi: 10.15171/apb.2015.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Medvedev AE. Toll-like receptor polymorphisms, inflammatory and infectious diseases, allergies, and cancer. J Interferon Cytokine Res. 2013;33:467–484. doi: 10.1089/jir.2012.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ve T, Gay NJ, Mansell A, Kobe B, Kellie S. Adaptors in toll-like receptor signaling and their potential as therapeutic targets. Curr Drug Targets. 2012;13:1360–1374. doi: 10.2174/138945012803530260. [DOI] [PubMed] [Google Scholar]
- 128.Page DB, Postow MA, Callahan MK, Allison JP, Wolchok JD. Immune modulation in cancer with antibodies. Annu Rev Med. 2014;65:185–202. doi: 10.1146/annurev-med-092012-112807. [DOI] [PubMed] [Google Scholar]
- 129.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]