Abstract
Photodynamic therapy (PDT) is a promising treatment in cancer therapy, with a photosensitizer activated by visible light. Aloe‐emodin (AE) is a promising photosensitive agent. In this study, the photosensitizing effects and possible mechanisms of AE‐PDT in MG63 cells were evaluated. The efficiency of AE‐PDT was analyzed by MTT assay. The mode of cell death was investigated by Hoechst 33,342 staining and flow cytometer. The intracellular distribution of AE was detected with confocal microscopy. The formation of reactive oxygen species (ROS) was detected by DCFH‐DA. The mitochondrial membrane potential (MMP) was measured by Rhodamine 123. The expression of proteins including cytochrome c, caspase‐3, ‐9, and ‐12, CHOP and GRP78 was detected by western blot. Apoptosis is the primary mode of cell death in our study, which occurs in a manner of depending on AE concentration and irradiation dose. Confocal microscopy showed that AE was primarily localized on the mitochondria and endoplasmic reticulum (ER) of MG63 cells. AE‐PDT resulted in rapid increases of intracellular ROS production, which reached a peak at 2 h, followed by declining of mitochondrial membrane potential, releasing of cytochrome c from mitochondria into the cytoplasm, and up‐regulation of caspase‐3, ‐9, and ‐12, CHOP and GRP78. These results suggest that death of MG63 cells induced by AE‐PDT is triggered by ROS. Meanwhile, Mitochondria and ER serve as the subcellular targets, which are responsible for AE‐PDT‐induced death of MG63 cells.
Keywords: Aloe emodin, endoplasmic reticulum, mitochondrial, osteosarcoma, photodynamic therapy
Introduction
Osteosarcoma is one of the most common malignant tumors of the bone 1. Surgery, radiotherapy, and high‐dose chemotherapy have improved survival rate over the past several decades 2. However, the 5‐year survival rates are still no more than 60%. Drug resistance and occurrence of second malignancies remain serious problems 3. Thus, there is an urgent requirement to develop novel approaches to deal with osteosarcoma 4.
Photodynamic therapy (PDT) is an anticancer modality utilizing the generation of singlet oxygen and other reactive oxygen species (ROS), after irradiating photosensitizer with visible light 5, 6. Aloe‐emodin (AE) is an active component from the root and rhizome of Rheum palmatum, which has been reported as a novel photosensitizer and played important role in many kind of carcinomas 7, 8, 9. Our study is going to explore the effect of AE‐PDT on osteosarcoma cell line MG63 and the possible mechanisms.
Materials and Methods
Photosensitizer, chemicals, and cell line
Aloe‐emodin (C34H36N4O3, 95% of purity) was purchased from Jiangxi Tiangong Technology (Jiangxi, China). A stock solution (100 mmol/L) was made in dimethyl sulfoxide (DMSO), filtered with 0.2 μm PTFE syringe filter (Alltech Association, Inc., Deerfield, IL), then stored in the dark at −20°C. Fetal bovine serum (FBS), DMEM, antibiotics penicillin‐streptomycin‐neomycin (PSN) and trypsin were supplied by Gibco (Grand‐Island, NY). Caspase‐3 and cytochrome c were purchased from CST (Danvers, MA), caspase‐9, caspase‐12 and C/EBP homologous protein (CHOP) were purchased from Immunoway Biotechnology (Newark, DE), GRP78 and β‐actin were supplied from Santa Cruz Biotechnology (Santa Cruz, CA). MTT, Hoechst 33342, Rhodamine 123 were obtained from Sigma (St Louis, MA). Annexin V‐ PI apoptosis kit was from Keygen Biotech (Nanjing, Jiangsu, China). The light‐emitting diode (LED) light was purchased from Chongqingjingyu Laser Biological Company (Chongqing, China). MG63 cells were obtained from the American Type Culture Collection (ATCC) and cultured in DMEM supplemented with 10% FBS and antibiotics. Cell cultures were incubated at 37°C in a humidified 5% CO2 incubator. Cells in growth period were used for the next experiments.
PDT protocol
Cells were inoculated into cell culture plates and placed into an incubator for 24 h. Then the cells were divided into four groups—A: 0.01% DMSO, treated as blank control; B: pure AE group; C: pure light group; D: AE‐PDT group. The steps below were conducted in the dark. Group A and C were cultured with DMEM medium free of FBS. Group B and D were incubated with DMEM medium free of FBS, but with AE (10 μmol/L). Six hours later, the medium was replaced by a culture medium with FBS (10%). Cells in group C and D were exposed to LED light (430 nm, 40 mW/cm2) for 30–120 sec to obtain an energy density of 1.2–4.8 J/cm2, respectively. After irradiation, the cells were recultivated in an incubator according to the time point required.
Cytotoxicity assay
After 24 h of PDT, the cytotoxicity was assessed by the MTT assay. The medium was removed and the cells were washed twice with PBS gently. MTT dilution was added to each well and then the plate was softly mixed and placed back to the incubator for 4 h. The optical density (OD) was obtained by an iEMS Analyzer at wavelengths of 570 nm. Then the percentage of cytotoxicity was calculated according to the following equation: cytotoxicity (%) = (OD control group − OD treatment group)/OD control group × 100%.
Measurement of ROS
Cellular ROS contents were measured by DCFH‐DA (Invitrogen, Paisley, UK) staining. Briefly, MG 63 cells (2 × 105 cells/plate) were grown on 6‐well plates for 24 h. Groups dividing and the incubation methods were the same as depicted in the PDT protocol. Cells in the AE‐PDT group were then cultured with 10 μmol/L DCFH‐DA solution, lacking LED light for 10 min, and then irradiated. Thereafter followed immediately, the cells were detected, using 488 nm for excitation and 525 nm for emission at different time points post‐irradiation.
Apoptosis measured by flow cytometer
Apoptotic cells were analyzed with an Annexin V/PI apoptosis kit, using flow cytometry. The cells (2 × 105 cells/well) were inoculated into 6‐well plates for 24 h. Six hours after treatment, the MG 63 cells were harvested and stained with Annexin V‐FITC and PI (50 mg/mL) according to the manual, then analyzed by Flow Cytometer (Becton Dickinson, San Jose, CA) immediately. The percentage of cells in the different regions was calculated for comparison.
Nuclear staining
MG63 cells (1 × 104 cells/plate) were grown on 24‐well plates for 24 h. The dividing and the incubation and methods were the same as depicted above. After 12 h of PDT, the cells were washed three times with PBS and fixed with 4% paraformaldehyde for 10 min. Subsequently, the fixed cells were washed thrice with PBS and stained with Hoechst 33342 (2 μg/mL) for 20 min, then observed with fluorescence microscope (Olympus 1X71; Olympus, Tokyo, Japan). The condensation and fragmentation of nuclei were considered to be representative of apoptosis.
Subcellular localization of AE
During the last 30 min of AE incubation, cells were co‐loaded with Mito‐Tracker green or ER‐Tracker green, at a final concentration of 500 nmol/L, to tag the mitochondria and ER, respectively. An overlapped fluorescence from organelle probe and AE was observed with confocal microscopy (TCS SP5, Leica, Heidelberg, Baden‐Wurttemberg state, German).
Mitochondrial membrane potential
Rhodamine 123 was used to measure MMP. In brief, suspended the cells of each group with 1 mL PBS and loaded with 1 μmol/L rhodamine 123 for 30 min. Flow cytometry (Becton Dickinson, San Jose, CA) was used to observe the alteration of MMP.
Western blotting analysis
Western blotting was carried out to observe the protein expressions level. Briefly, at various time points after PDT, cells in AE‐PDT group and control groups were collected and lysed using radio‐immunoprecipitation assay (RIPA) buffer (Kaiji Bio Co., Nanjing, China). The following steps were just as we depicted in our previous study 26.
Results
Cytotoxicity of AE‐PDT in the MG63 cells
The cytotoxic effect of AE‐ PDT at varied concentrations and energy densities are shown in Figure 1, from which drug‐ and light‐dose–response curves were observed. The results showed that there was no significant dark cytotoxicity when the concentration of AE was lower than 20 μmol/L, but there was apparent cytotoxicity when the concentration was over 50 μmol/L. The results are consistent with the former finding 10, 11. Apparently, AE‐PDT on MG63 cells got an IC50 at 10 μmol/L and 4.8 J/cm2, as shown in Figure 1. Therefore, AE concentration of 10 μmol/L and energy density of PDT at 4.8 J/cm2 were selected for our next experiments.
Figure 1.
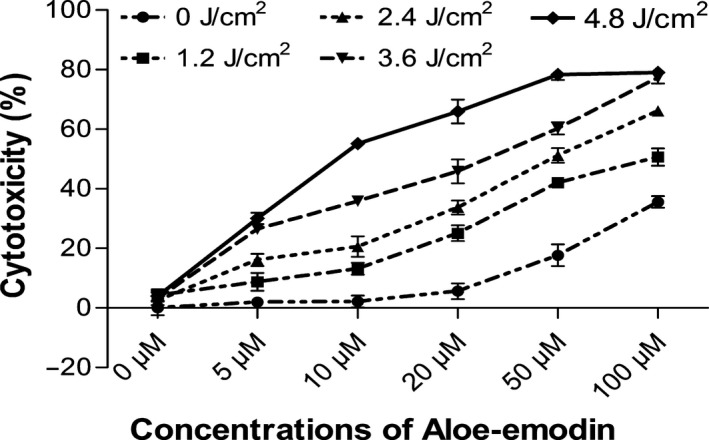
The cytotoxicity of AE‐PDT on human MG63 cells. Drug‐ and light‐dose‐dependent effect on cell cytotoxicity after incubation with Aloe‐emodin for 6 h, followed by irradiation. Data correspond to mean values ± SD from at least three different experiments.
Apoptosis and necrosis of MG63 cells induced by AE‐PDT
Cells of AE‐PDT group and control groups (pure AE group, pure light group, and the blank control group) were stained by Hoechst 33,342. Marked morphological changes were observed at 12 h post‐PDT. Density increasing, condensation, coagulation, and fragmentation of the nuclear chromatin were observed, as well as typical apoptotic bodies revealed in the AE‐PDT group, as shown in Figure 2A. While in the other control groups, the nucleus demonstrated intactly rounded/elliptical, no cell apoptosis was observed.
Figure 2.
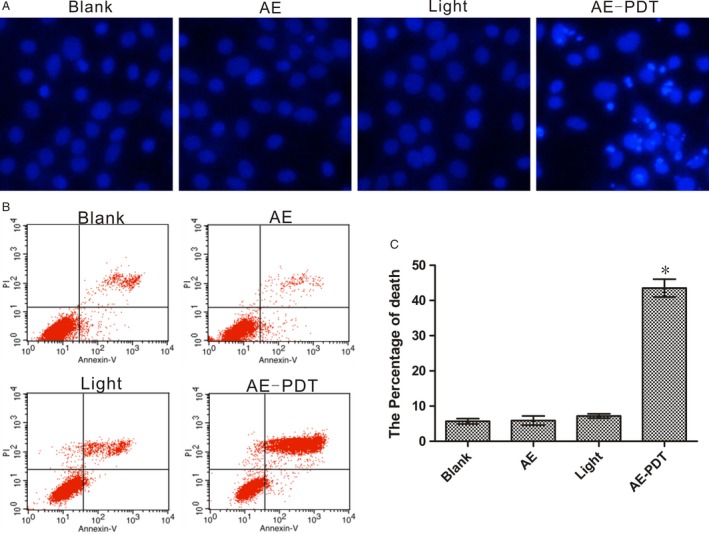
AE‐PDT‐induced death on MG63 cells. (A) Cell apoptosis revealed by Hoechst 33,342 staining of fragmented nuclei of apoptotic cells. (B) Quantified analysis of the extent and mode of cell death percentage by flow cytometer. *P < 0.01, AE‐PDT group versus the three control groups. Values are mean ± SD of three independent determinations.
Annexin V/PI staining was utilized to assess the percentage of the apoptotic and necrotic cell induced by AE‐PDT. Data showed that the MG‐63 cells in AE‐PDT group exhibited an apparent increase in both early apoptosis and late apoptosis/necrosis, while the differences in controls were not significant (Fig. 2B and C).
Subcellular localization of AE
Mito‐tracker and ER‐tracker were used in this study to unfold the subcellular localization of AE in the MG63 cells. Overlapping regions (yellow) were regarded as the subcellular localization of AE. The results showed that the fluorescence images of the mitochondria probe as well as the ER probe were partially overlapping with the AE (Fig. 3).
Figure 3.
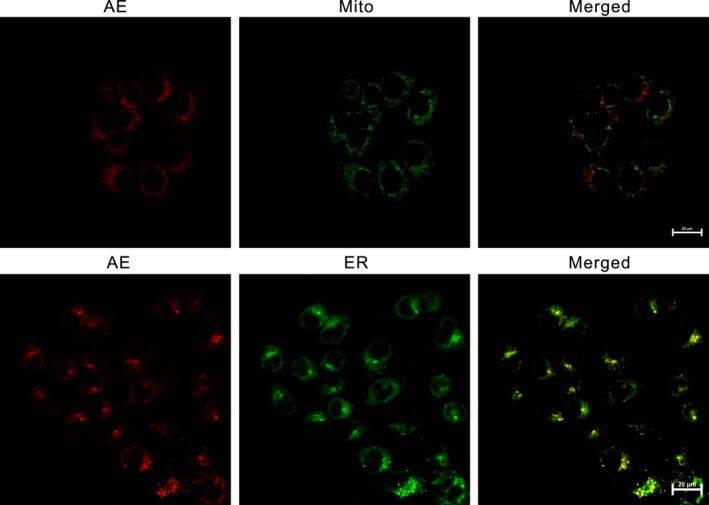
Detected subcellular localization of Aloe‐emodin (AE) by confocal microscopy. Red fluorescent corresponds to AE, green fluorescent shows Mito‐Tracker‐stained mitochondria or ER‐Tracker‐stained endoplasmic reticulum, yellow fluorescent indicates colocalization of red and green fluorescence.
Formation of ROS
In our experiment, ROS formation initiated by AE‐PDT was monitored. The production of general ROS dramatically increased at 30 min after AE‐PDT. Thereafter, the intensity peaked at 2 h, but attenuated at 3 h following PDT (Fig. 4A).
Figure 4.
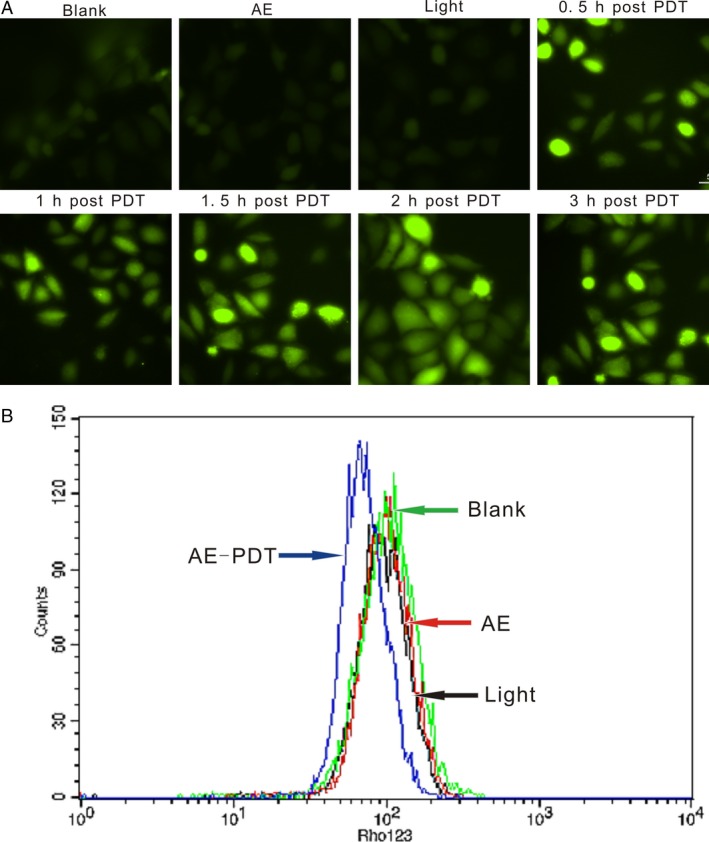
(A) Formation of reactive oxygen species (ROS) was detected by DCFH‐ DA staining. (B) Collapse of mitochondrial membrane potential was detected by Rhodamine 123.
Decline of mitochondrial membrane potential
Data from Flow cytometric with Rhodamine 123 staining have showed that there were significant changes of MMP in the MG63 cells 3 h after LED irradiation, and the decline of MMP in AE‐PDT group was observed. However, the differences in control groups were not significant (Fig. 4B).
Effect of AE‐PDT on mitochondrial‐related protein expression
We have found that AE co‐located on mitochondrial in MG63 cells, so we detected whether the related proteins were activated after AE‐PDT. As shown in Figure 5, the amount of cytosolic cytochrome c was significantly increased at 1 h after PDT. In addition, we found that the expression of caspase‐9 and caspase‐3 demonstrated an early increase and a late decrease pattern, with the peak at 6 and 9 h after PDT, respectively.
Figure 5.
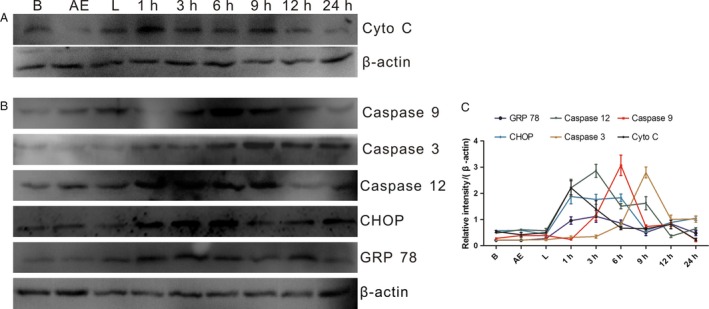
The proteins Expression of Cytochrome c, Caspase 9, 12, 3, CHOP and GRP 78. (A) Effect of Aloe‐emodin (AE) on mitochondrial‐related protein expression; (B) Effect of AE‐PDT on ER stress‐related protein expression. (C) Quantitative analysis of protein expression according to panels (A and B). The vertical axis represents the protein expression relative to β‐actin. Data represent means ± SDs (n = 3). B means blank control group, AE means single AE group, L means single light group, 1–24 h means 1–24 h post‐PDT.
Effect of AE‐PDT on ER stress‐related protein expression
At the same time, we found that AE co‐located on the ER too. Therefore, we detected the expression of ER stress‐related proteins. Data showed that the expression of caspase‐12, Grp78, and CHOP was upregulated after AE‐PDT (Fig. 5), which revealed that AE‐PDT exerted ER stress on MG63 cells.
Discussion
Aloe‐emodin is a novel anthraquinone derivative extracted from traditional Chinese medicinal plants. Previous studies have demonstrated that AE have antitumor effects with dose‐dependent cytotoxicity 7, 8, 9, 11, 12. However, the genotoxicity limits its application in large dosage 13. Fortunately, AE has been found to be one photosensitizer, which displayed stronger anticancer effect than 5‐aminolevulinic acids with a low concentration 14. Lee et al. 14 have demonstrated that the cytoskeletal alteration was potentially involved in anoikis induced by AE‐PDT in human lung carcinoma H460 cells. Our previous study also demonstrated that AE‐PDT produced significant photocytotoxicity and induced apoptosis in tumors 15, 16. However, the molecular mechanism of AE‐PDT is still not completely clear.
In this study, we also confirmed the marked photocytotoxicity of AE to MG63 cells. As shown by Figure 1, cytotoxicity of AE‐PDT occurred in a manner dependent on concentration and irradiation dose. Furthermore, apoptosis remained the principal mode of MG63 cell death. At the same time, we found these results were consistent with the changes in intracellular ROS, which was produced rapidly and significantly after AE‐PDT (Fig. 4). These results indicate that the cytotoxicity of MG63 cell after PDT is due to the generation of intracellular ROS.
Because of its short life and limited diffusion distance of ROS, the primary site of photodamage coincides with the subcellular localization of the sensitizer 17. Mitochondrial, ER, lysosme, and golgiosome have been reported to be chosen by some sensitizers as subcellular localizations, which is largely dependent on the photosensitizer characteristics as well as the cell type 18. As a new photosensitizer, the subcellular localization of AE was seldom depicted previously. However, in this current study, AE was found to localize mainly in the mitochondria as well as the ER, which prompted us to further examine mitochondrial and ER stress‐associated apoptosis (Fig. 3).
Mitochondria play a significant role in supplying cell energy and regulating cell apoptosis and are considered to be an important target organelle during the PDT 19, 20, 21. Generally, PDT induces high levels of ROS, which have proved to be effective in disrupting the mitochondrial permeability transition pore (MPTP),resulting in immediate collapse of mitochondrial membrane potential (MMP). Following the collapse of the MMP, apoptotic factors (such as cytochrome c) are released and triggers the mitochondrial apoptosis cascade. At last, cell death occurred 22, 23, 24, 25. In our study, a rapid decline of MMP was observed after PDT, which was followed by the release of cytochrome c, caspase‐9, and caspase‐3. We further found that the peak expression of caspase‐9 was observed at 6 h post‐PDT, while caspase‐3 peaked around 9–12 h after PDT (Fig. 5), then, followed with a decreasing trend. The results might because of that caspase proteins were cleaved into small fragments during the apoptotic process.
Endoplasmic reticulum is a cellular organelle that has essential roles in multiple cellular processes and has a great cross‐talk with mitochondria. To seek the effect of ER stress on apoptosis of MG63 cells induced by AE‐PDT, the protein expression of caspase‐12, CHOP, and GRP78 were monitored. All the three factors experienced essential components in ER‐induced apoptosis, and they were considered to be ER stress markers as well as the key regulators of ER stress response signaling 11, 27, 28, 29. In this study, the activation of caspasew‐12, CHOP and GRP78 shared the same response pattern, we observed the overexpression of caspase‐12 after AE‐PDT with peak expression at 3 h, CHOP with peak expression at 6–12 h (Fig. 5), while GRP78 with peak expression at 6 h. Taken together, these results suggested the initiation of the ER‐stress‐specific cascade of apoptosis.
In conclusion, AE‐PDT triggers death of MG63 cells mainly by provoked mitochondrial damage as well as the ER stress, which was supported by the main localization of AE in the mitochondria and ER of MG63 cells.
Conflict of Interest
We declare that we have no conflict of interest in relation to this article.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81101692, 81572634) and the Natural Science Foundation Project of CQ CSTC (2011BB5136).
Cancer Medicine 2016; 5(11):3186–3193
Y.‐S. O. and D.‐Q. B. are co‐corresponding authors, they contributed equally to this work.
Contributor Information
Yun‐Sheng Ou, Email: ouyunsheng2001@163.com.
Ding‐Qun Bai, Email: baidingqun2014@163.com.
References
- 1. Mirabello, L. , Troisi R. J., and Savage S. A.. 2009. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer 115:1531–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dai, X. , Ma W., He X., and Jha R. K.. 2011. Review of therapeutic strategies for osteosarcoma, chondrosarcoma, and Ewing's sarcoma. Med. Sci. Monit. 17:RA177–RA190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim, S. Y. , and Helman L. J.. 2009. Strategies to explore new approaches in the investigation and treatment of osteosarcoma. Cancer Treat. Res. 152:517–528. [DOI] [PubMed] [Google Scholar]
- 4. Hattinger, C. M. , Pasello M., Ferrari S., Picci P., and Serra M.. 2010. Emerging drugs for high‐grade osteosarcoma. Expert Opin. Emerg. Drugs 15:615–634. [DOI] [PubMed] [Google Scholar]
- 5. Galluzzi, L. , Kepp O., and Kroemer G.. 2012. Enlightening the impact of immunogenic cell death in photodynamic cancer therapy. EMBO J. 31:1055–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park, J. J. 2012. Photodynamic therapy: establishing its role in palliation of advanced esophageal cancer. Korean J. Intern. Med. 27:271–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pecere, T. , Gazzola M. V., Mucignat C., Parolin C., Vecchia F. D., Cavaggioni A., et al. 2000. Aloe‐emodin is a new type of anticancer agent with selective activity against neuroectodermal tumors. Cancer Res. 60:2800–2804. [PubMed] [Google Scholar]
- 8. Chiu, T. H. , Lai W. W., Hsia T. C., Yang J. S., Lai T. Y., Wu P. P., et al. 2009. Aloe‐emodin induces cell death through S‐phase arrest and caspase‐dependent pathways in human tongue squamous cancer SCC‐4 cells. Anticancer Res. 29:4503–4511. [PubMed] [Google Scholar]
- 9. Lin, K. Y. , and Uen Y. H.. 2010. Aloe‐emodin, an anthraquinone, in vitro inhibits proliferation and induces apoptosis in human colon carcinoma cells. Oncol. Lett. 1:541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yan, M. , Zhang L. Y., Sun L. X., Jiang Z. Z., and Xiao X. H.. 2006. Nephrotoxicity study of total rhubarb anthraquinones on Sprague Dawley rats using DNA microarrays. J. Ethnopharmacol. 107:308–311. [DOI] [PubMed] [Google Scholar]
- 11. Zhu, S. , Jin J., Wang Y., Ouyang Z., Xi C., Li J., et al. 2012. The endoplasmic reticulum stress response is involved in apoptosis induced by aloe‐emodin in HK‐2 cells. Food Chem. Toxicol. 50:1149–1158. [DOI] [PubMed] [Google Scholar]
- 12. Lee, M. S. , and Lee G. M.. 2001. Effect of hypoosmotic pressure on cell growth and antibody production in recombinant Chinese hamster ovary cell culture. Cytotechnology 36:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zaffaroni, M. , Mucignat C., Pecere T., Zagotto G., Frapolli R., D'Incalci M., et al. 2003. High‐performance liquid chro‐matographic assay for the determination of Aloe Emodin in mouse plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 796:113–119. [DOI] [PubMed] [Google Scholar]
- 14. Lee, H. Z. , Yang W. H., Hour M. J., Wu C. Y., Peng W. H., Bao B. Y., et al. 2010. Photodynamic activity of aloe‐emodin induces resensitization of lung cancer cells to anoikis. Eur. J. Pharmacol. 648:50–58. [DOI] [PubMed] [Google Scholar]
- 15. Chen, Q. , Tian S., Zhu J., Li K. T., Yu T. H., Yu L. H., et al. 2016. Exploring a novel target treatment on breast cancer: aloe‐emodin mediated photodynamic therapy induced cell apoptosis and inhibited cell metastasis. Anticancer Agents Med. Chem. 16:763–7016. [DOI] [PubMed] [Google Scholar]
- 16. Li, K. T. , Duan Q. Q., Chen Q., He J. W., Tian S., Lin H. D., et al. 2016. The effect of aloe emodin‐encapsulated nanoliposome‐mediated r‐caspase‐3 gene transfection and photodynamic therapy on human gastric cancer cells. Cancer Med. 5:361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buytaert, E. , Dewaele M., and Agostinis P.. 2007. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochim. Biophys. Acta 1776:86–107. [DOI] [PubMed] [Google Scholar]
- 18. Robertson, C. A. , Evans D. H., and Abrahamse H.. 2009. Photodynamic therapy (PDT): a short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol., B 96:1–8. [DOI] [PubMed] [Google Scholar]
- 19. Wen, S. , Zhu D., and Huang P.. 2013. Targeting cancer cell mitochondria as a therapeutic approach. Future Med. Chem. 5:53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hilf, R. 2007. Mitochondria are targets of photodynamic therapy. J. Bioenerg. Biomembr. 39:85–89. [DOI] [PubMed] [Google Scholar]
- 21. Zhang, S. , Yang L., Ling X., Shao P., Wang X., Edwards W. B., et al. 2015. Tumor mitochondria‐targeted photodynamic therapy with a translocator protein (TSPO)‐specific photosensitizer. Acta Biomater. 28:160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tang, P. M. , Chan J. Y., Au S. W., Kong S. K., Tsui S. K., Waye M. M., et al. 2006. Pheophorbide a, an active compound isolated from Scutellaria barbata, possesses photodynamic activities by inducing apoptosis in human hepatocellular carcinoma. Cancer Biol. Ther. 5:1111–1116. [DOI] [PubMed] [Google Scholar]
- 23. Lai, J. C. , Lo P. C., Ng D. K., Ko W. H., Leung S. C., Fung K. P., et al. 2006. BAM‐SiPc, a novel agent for photodynamic therapy, induces apoptosis in human hepatocarcinoma HepG2 cells by a direct mitochondrial action. Cancer Biol. Ther. 5:413–418. [DOI] [PubMed] [Google Scholar]
- 24. Li, P. , Nijhawan D., Budihardjo I., Srinivasula S. M., Ahmad M., Alnemri E. S., et al. 1997. Cytochrome c and dATP‐dependent formation of Apaf‐1/caspase‐9 complex initiates an apoptotic protease cascade. Cell 91:479–489. [DOI] [PubMed] [Google Scholar]
- 25. Lemasters, J. J. , Nieminen A. L., Qian T., Trost L. C., Elmore S. P., Nishimura Y., et al. 1998. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim. Biophys. Acta 1366:177–196. [DOI] [PubMed] [Google Scholar]
- 26. Chung, P. S. , He P., Shin J. I., Hwang H. J., Lee S. J., and Ahn J. C.. 2009. Photodynamic therapy with 9‐hydroxypheophorbide alpha on AMC‐HN‐3 human head and neck cancer cells: induction of apoptosis via photoactivation of mitochondria and endoplasmic reticulum. Cancer Biol. Ther. 8:1343–1351. [DOI] [PubMed] [Google Scholar]
- 27. Mihailidou, C. , Papazian I., Papavassiliou A. G., and Kiaris H.. 2010. CHOP‐dependent regulation of p21/waf1 during ER stress. Cell. Physiol. Biochem. 25:761–766. [DOI] [PubMed] [Google Scholar]
- 28. Szegezdi, E. , Logue S. E., Gorman A. M., and Samali A.. 2006. Mediators of endoplasmic reticulum stress‐induced apoptosis. EMBO Rep. 7:880–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Firczuk, M. , Gabrysiak M., Barankiewicz J., Domagala A., Nowis D., Kujawa M., et al. 2013. GRP78‐targeting subtilase cytotoxin sensitizes cancer cells to photodynamic therapy. Cell Death Dis. 4:e741. [DOI] [PMC free article] [PubMed] [Google Scholar]


