Abstract
Solanine, a naturally steroidal glycoalkaloid in nightshade (Solanum nigrum Linn.), can inhibit proliferation and induce apoptosis of tumor cells. However, the mechanism of solanine‐suppressing prostate cancer cell growth remains to be elucidated. This study investigates the inhibition mechanism of solanine on cancer development in vivo and in cultured human prostate cancer cell DU145 in vitro. Results show that solanine injection significantly suppresses the tumor cell growth in xenograft athymic nude mice. Solanine regulates the protein levels of cell cycle proteins, including Cyclin D1, Cyclin E1, CDK2, CDK4, CDK6, and P21 in vivo and in vitro. Also, in cultured DU145 cell, solanine significantly inhibits cell growth. Moreover, the administration of NAC, an active oxygen scavenger, markedly reduces solanine‐induced cell death. Blockade of P38 MAPK kinase cannot suppress reactive oxygen species (ROS), but can suppress solanine‐induced cell apoptosis. Also, inhibition of ROS by NAC inactivates P38 pathway. Taken together, the data suggest that inhibition of prostate cancer growth by solanine may be through blocking the expression of cell cycle proteins and inducing apoptosis via ROS and activation of P38 pathway. These findings indicate an attractive therapeutic potential of solanine for suppression of prostate cancer.
Keywords: Cell cycle proteins, P38, prostate cancer, reactive oxygen species, solanine
Introduction
Prostate cancer is a relatively common malignant cancer and the second leading cause of cancer‐related deaths among male patients 1. Several effective therapies are applied for the clinical treatment of prostate cancer; including surgery and radiotherapy for patients with early‐stage disease and adjuvant systemic treatments, which are effective for advanced‐stage disease 2. However, the therapies all have different side‐effect profiles and are challenged by the emergence of resistance in tumor cells 3, 4. Therefore, it is of particularly importance to develop novel therapeutic strategies to overcome these challenges.
Solanine, molecular formula C45H73NO15, a trisaccharide glycoalkaloid, is one of the main steroidal glycoalkaloids in Solanaceae family species such as nightshade and potato (Solanum tuberosum L.) 5, 6. The structure of solanine (Fig. 1A) is similar to human steroid hormones such as androgen, estrogen, progesterone, and other sex hormones. Solanine, like other alkaloids steroid, has a variety of bio‐activities, such as anti‐inflammatory, preventing asthma, antiallergic, regulate metabolism, and endocrine effects 7. Studies have demonstrated that solanine inhibits the growth of many human cancers, including human colon, liver, cervical, lymphoma, and stomach cancer 8, 9, 10. Solanine also exerts chemoprotective and chemotherapeutic effects in an animal model of breast cancer by induction of apoptosis, and inhibition of cell proliferation and angiogenesis 11. Moreover, solanine inhibits the migration and invasion of human melanoma cells, by reducing matrix metalloproteinase activities 12. Thus, solanine possesses the potential for cancer chemotherapeutic action.
Figure 1.
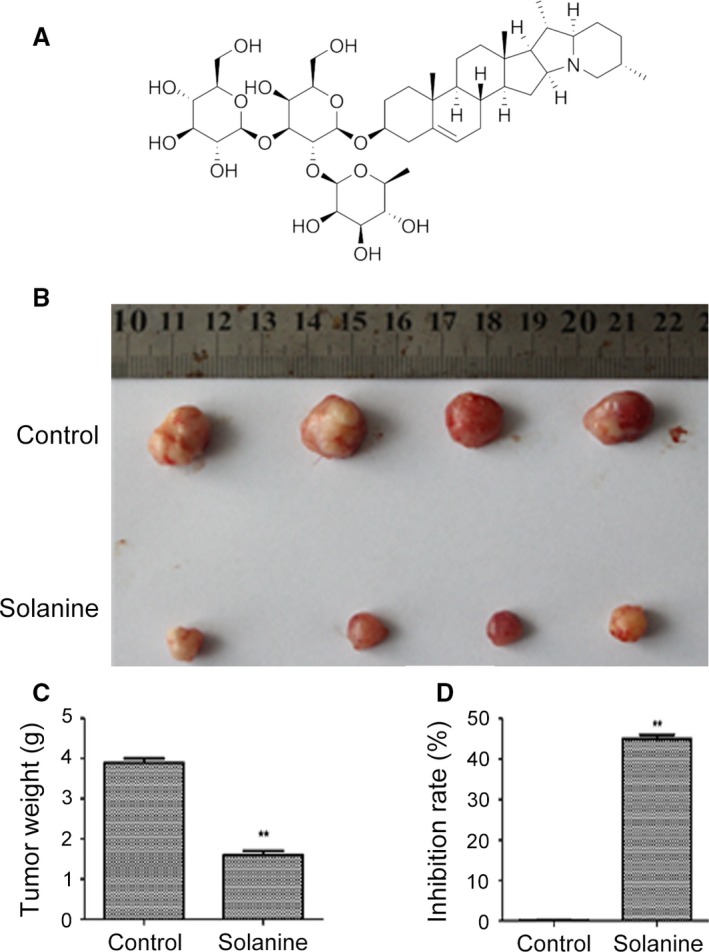
Attenuation of the tumors formed by the inoculation of DU145 cells into nude mice after the treatment with solanine. (A) Chemical formula of solanine. (B) Tumors from the two groups were shown. The statistical data of tumor weight were shown in (C) and the tumor inhibition rate were shown in (D). ** denotes P < 0.01 versus control group.
Solanine has been shown anticarcinogenic potential against various cancer cell lines, but the underlying mechanism involved in inhibition of tumor growth by solanine remains to be further elucidated. This work determines the inhibitory effect and the molecular mechanisms of solanine on tumor inhibition in vivo and in vitro. Solanine can inhibit cancer growth in vivo and can induce the apoptosis of prostate cancer cell line DU145, via the suppression of cell cycle protein expression, induction of reactive oxygen species (ROS) and activation of P38 MAPK pathway.
Methods and Materials
Ethics statement
All human and animal experiments were approved by the Jinan University. All experiments using human specimens were performed in accordance with the Declaration of Helsinki. Informed consent was received from all participated subjects prior to the study. Human tissue samples were obtained from osteosarcoma patients in the First Affiliated Hospital of Jinan University. All animal procedures followed the humane care guidelines of the Chinese National Institute of Health, and the protocols were approved by the Committee on Animal Research of Jinan University.
Cell culture
Cell lines human prostate cancer DU145 was purchased from Cell Bank of Shanghai Institute of Cell Biology, Chinese Academy of Sciences. Cells were maintained in Dulbecco's minimal essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/mL), and streptomycin (100 μg/mL) at 37°C in a humidified atmosphere with 5% CO2.
ROS detection
The intracellular ROS level was detected using an oxidation sensitive fluorescent probe (DCFH‐DA) (Beyotime, China). DCFH‐DA can be deacetylated by nonspecific esterase to form DCFH that can be oxidized by hydrogen peroxide or low‐molecular‐weight peroxides to produce the fluorescent compound 2′,7′‐dichlorofluorescein (DCF) and it is a stable fluorescent ROS‐sensitive compound and can readily diffuse into cells. In this study, DU145 cells were incubated with solanine. The cells were harvested and washed with serum‐free medium for three times. Aliquots of cells were resuspended in fresh medium without serum and loaded with DCFH‐DA (30 μmol/L) for 20 min at 37°C, according to the manufacturer's instruction. Green fluorescence density of 10,000 events was detected by flow cytometry for each sample.
MTS assay
In vitro DU145 cell proliferation assay was performed using CellTiter 96® AQueous One Solution Cell Proliferation Assay Kit (Promega, Madison, WI), according to the manufacturer's instructions. In brief, 2500 cells per well were seeded into a 96‐well plate. After respective treatments, cells were allowed to grow for 24 h. After 24 h, 20 μL of CellTiter 96 AQueous One Solution reagent was added to each well in 100 μL of total volume of media. Cells were incubated for 3–4 h, and absorbance was recorded at 490 nm using an ELISA plate reader. The absorbance is directly proportional to the number of living cells and is expressed as a relative percentage to vehicle‐treated cells.
Western blotting
As described previously 13, cells were lysed in sample buffer and subjected to sodium dodecyl sulfate–polyacrylamide gel (SDS‐PAGE), with the antibodies Cyclin D1, Cyclin E1, CDK2, CDK4, CDK6, P21, P38, p‐P38, ATF2, p‐ATF2, Bcl‐2, Bax, and GAPDH (all were from Abcam, Cambridge, UK). GAPDH was used as loading control. After washing with phosphate‐buffered saline (PBS) containing 0.1% Tween 20 for five times, the membrane was incubated the appropriate horseradish peroxidase conjugated secondary antibodies (Amersham Biosciences, Uppsala, Sweden) for 1 h and bands were detected by enhanced chemiluminescence (Amersham, Bucks, UK). In all cases, the average intensity of the pixels in a background‐selected region was calculated and subtracted from each pixel in the sample. To correct for deviation, the densitometry values obtained in the linear range of detection were normalized to those obtained for GAPDH. Statistical analysis was performed using a one‐way analysis of variance (ANOVA), followed by the Bonferroni correction.
Total RNA extraction and real‐time PCR
Total RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. For real‐time quantative PCR, equal amount of cDNA was added to SYBR premix EX Taq II (Takara, Japan) and run in Stepone real‐time PCR system (AB Applied Biosystems, Foster City, CA). The cycling program was 95°C for 5 sec and 60°C for 30 sec. Each sample was assayed in triplicates, and GAPDH was used as an endogenous control. Results were normalization to the expression of GAPDH.
Apoptosis detection by flow cytometry
Cell death via apoptosis was determined using Annexin V‐APC/7‐AAD Apoptosis Kit (BD Biosciences, San Diego, CA) and quantified by flow cytometry as described previously 14, 15. Briefly, at the end of the treatment time, DU145 cells were washed once with PBS and harvested in a 0.5% trypsin/EDTA solution at 37°C, centrifuged at 220g for 5 min and then immediately resuspended in the physiological buffer provided in the kit. Cells (1 × 105 cells/500 μL) were then maintained in the dark for 15 min at room temperature with both Annexin V, allophycocyanin conjugate (APC) and 7‐amino‐actinomycin D (7‐AAD) accordingly, after which the samples were analyzed immediately by BD Biosciences FACSCalibur flow cytometer (San Jose, CA). The results were quantified using the CellQuest software (BD Biosciences, San Jose, CA). Q2 plus Q4 area were calculated as apoptosis ratio.
Prostate tumor xenograft model
Six‐ to eight‐week‐old male nude mice were kept on a 12 h light–dark cycle with access to food and water ad libitum. Nude mice with subcutaneous DU145 prostate cancer cell xenografts (1 × 106 DU145 cells) were subjected to daily i.p. injections of solanine (5 mg/kg) 16 or vehicle for 4 weeks. After 30 days the mice were killed by ketamin injection (50 mg/kg) followed by cervical dislocation and the xenografts were dissected out and immediately snap frozen in liquid nitrogen and stored at −80°C until further processing.
Statistical analysis
All experiments were repeated at least three times, and the results are presented as the mean ± SEM. Analyses of significance were performed using Student's t‐tests or one‐way ANOVAs, followed by Bonferroni corrections. P < 0.05 was considered statistically significant.
Results
Solanine inhibits prostate cancer growth in vivo
To confirm the antitumor effect of solanine in vivo, we performed a xenograft assay of DU145 cell in athymic nude mice. After inoculated human prostate cancer DU145 cells into nude mice subcutaneously, solanine was simultaneously injected into the mice. Afterward, the same amount of solanine was injected to mice every 3 days. One month later, mice‐bearing tumors were killed and the tumors were separated. As shown in Figure 1B, tumors in solanine‐treated mice were much smaller than that in the control mice. The tumor weight was shown in Figure 1C, and the inhibition rate was shown in Figure 1D. These data indicate that solanine significantly inhibit the tumor growth in vivo.
Solanine regulates the expression of cell cycle proteins in vivo
To investigate the mechanisms of solanine regulated tumor inhibition, we detected the mRNA and protein expression levels of cell cycle proteins from the tumor tissues of xenograft mice injected with DU145 cells. As shown in Figure 2, by the performance of QPCR, we found that compared to control group, the mRNA levels of CyclinD1, CyclinE1, CDK2, CDK4, and CDK6 were significantly inhibited and the level of P21 was markedly increased. Furthermore, we performed western blotting and found the same trend as the mRNA. Compared to control group, the protein levels of CyclinD1, CyclinE1, CDK2, CDK4, and CDK6 were significantly decreased and that of P21 was increased (Fig. 3).
Figure 2.
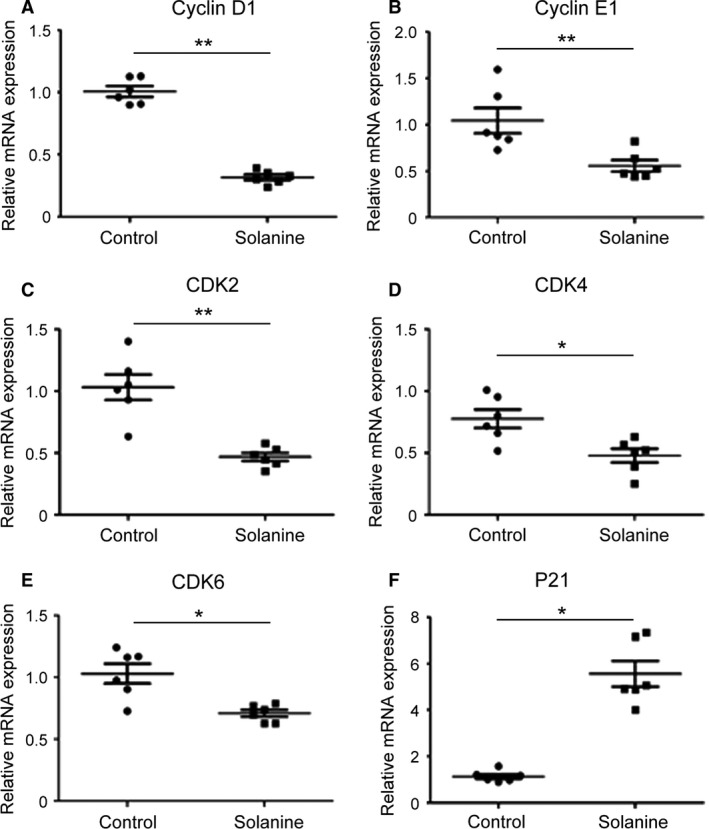
The mRNA levels in xenograft mice injected with DU145 cells. After taken the photos of these tumors in figure 1, the tissues were subjected to QPCR. The mRNA levels of Cyclin D1 (A), Cyclin E1 (B), CDK2 (C), CDK4 (D), CDK6 (E) and P21 (F) in control group were normalized to 1 (100%), the levels in solanine group were reset to relative mRNA expression to control (fold changes). * denotes P < 0.05 versus control group; ** denotes P < 0.01 versus control group.
Figure 3.
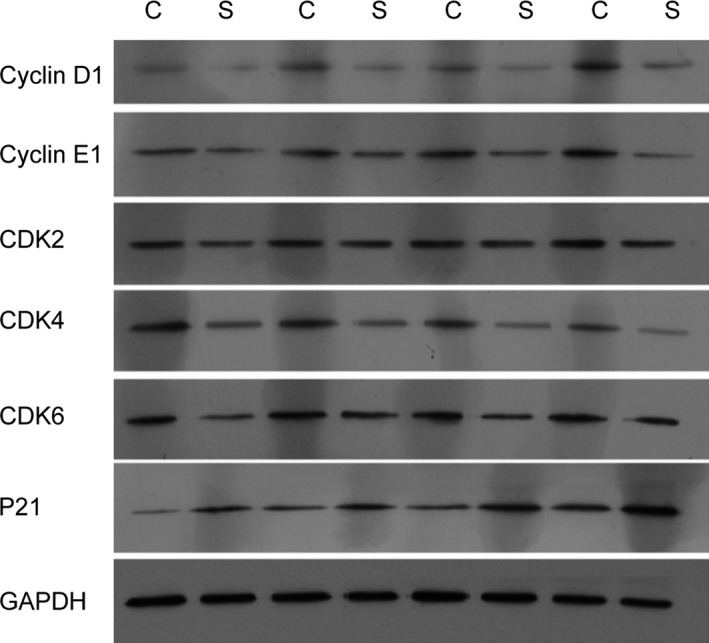
The protein levels in xenograft mice injected with DU145 cells. One month after DU145 cell injection, the tumor were separated and subjected to western blot with the indicated antibodies. C denotes the control group; S denotes the solanine group.
Solanine inhibits the cell growth of prostate cancer cell DU145
To explore the detailed mechanisms and signaling pathways of solanine‐induced cell growth, we moved our study to cultured DU145 cells in vitro. First, we need to confirm the appropriate concentration of solanine on DU145 cells, by MTS assay. Concentrations of 10–160 μmol/L solanine were used on DU145 cells. At 24 h, the calculated IC50 of solanine was 32.18 μmol/L, so the concentration of 40 μmol/L solanine can significantly inhibit the cell growth. We chose this concentration for our further study. Meanwhile, we used an active oxygen scavenger NAC (5 mM) to inhibit ROS and found that the usage of NAC significantly reversed the inhibition effect of solanine, leading to the increased use of concentration of solanine to suppress the cell growth. Concentrations lower than 80 μmol/L showed remarkable effect (Fig. 4A). These data indicated that solanine inhibited prostate cancer DU145 cell via ROS. Moreover, we determined the protein levels of CyclinD1, CyclinE1, CDK2, CDK4, CDK6, and p21 in DU145 cells. And found the same trend as that in tumor tissues of xenograft mice (Fig. 4B), indicating the consistence of mechanisms of solanine‐induced cell death in vivo and in vitro.
Figure 4.
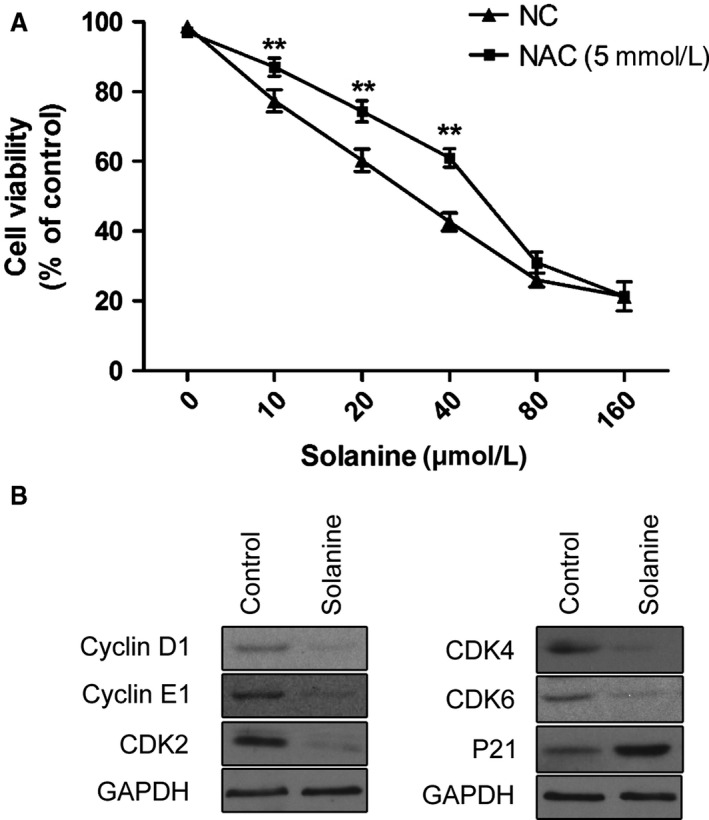
Solanine inhibit DU145 cell growth via ROS. (A) Different concentrations (10–160 μmol/L) of solanine with or without NAC (5 mmol/L), were applied on cultured DU145. After the treatment, cells were harvested and subjected to MTS. The cell viability was calculated under different treatment group. ** denotes P < 0.01 versus NC (control for NAC) group. (B) Cultured DU145 treated with solanine or control were subjected to western blot with the indicated antibodies. ROS, reactive oxygen species.
Solanine induces ROS in prostate cancer cell DU145
To further confirm the relationship of solanine and ROS, we applied flow cytometry assay to detect the ROS level under different treatment, using an oxidation‐sensitive fluorescent probe (DCFH‐DA) 17. As shown in Figure 5, the treatment of solanine significantly induced ROS, and the usage of NAC markedly erased solanine‐induced ROS level. Meanwhile, we used SB203580, an inhibitor of P38 MAPK kinase pathway, together with solanine. We found that SB203580 cannot suppress the ROS level induced by solanine. These data confirmed that solanine‐induced DU145 ROS and this cannot be inhibited by P38 inhibitor.
Figure 5.
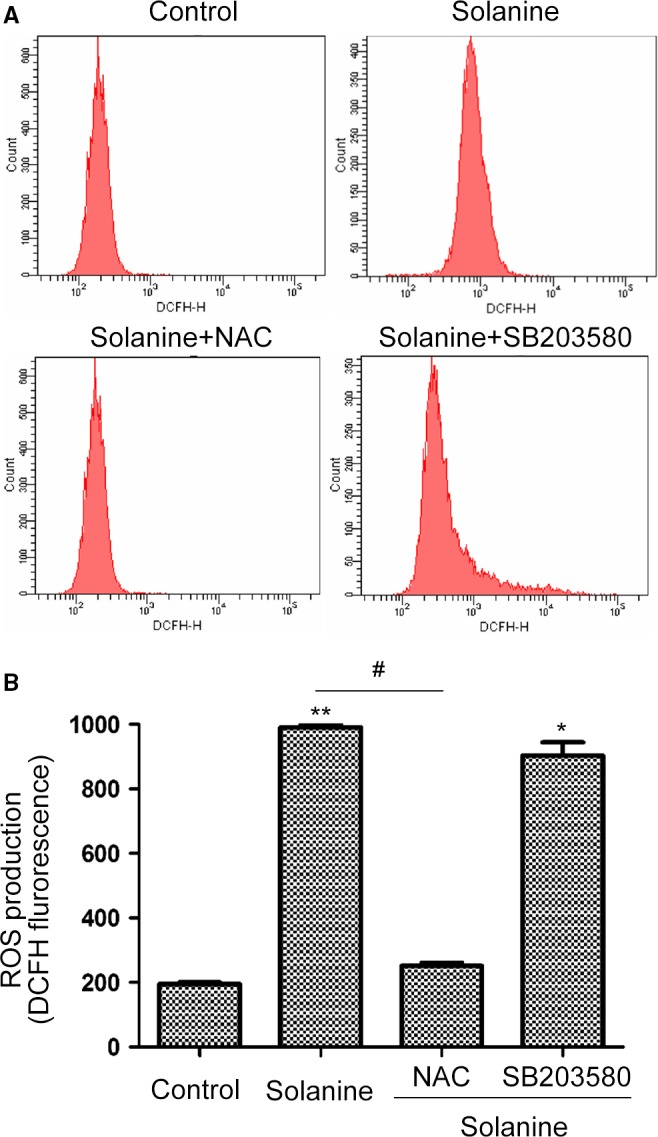
Solanine induces reactive oxygen species (ROS) in DU145 cells. (A) Cells were treated solanine with or without NAC (5 mmol/L) or SB203580 (40 μmol/L) for 24 h. Then cells were subjected to DCFH staining for the detection of ROS by flow cytometry. (B) The statistical data were shown. * denotes P < 0.05 versus control group; ** denotes P < 0.01 versus control group; # denotes P < 0.05 versus solanine group.
Solanine induces apoptosis in DU145 cells via P38 pathway downstream of ROS
After demonstrated solanine can inhibit prostate cancer growth and induce ROS in cultured DU145, we further need to know which signaling pathway was responsible. So we performed flow cytometry to detect the cell apoptosis. By the staining of Annexin V‐APC and 7‐AAD with flow cytometry, we found that solanine significantly induced the apoptosis of DU145 cells, the administration of NAC and SB203580 can markedly decrease solanine‐induced cell apoptosis (Fig. 6). Furthermore, we need to explore the signaling mechanisms underlying. By western blot, we detected the protein expression and activation of P38 pathway. As shown in Fig. 7, solanine induced the expression of phosphorylated P38, not the total level of P38, indicating the activation of P38 pathway. ATF2 is a downstream target of P38 18. We found the protein level of phosphorylated ATF2 was also increased, but not the total level. The administration of NAC and SB203580 both can suppress solanine‐induced P38/ATF2 activation. Furthermore, we determined the levels of pro and antiapoptotic proteins. As shown in Fig. 7, the level of antiapoptotic protein Bcl‐2 was decreased and the level of proapoptotic protein Bax was increased, and these proteins responded with NAC and P38 inhibitor. These data suggest that solanine induces cell apoptosis via P38 pathway, downstream of ROS induction.
Figure 6.
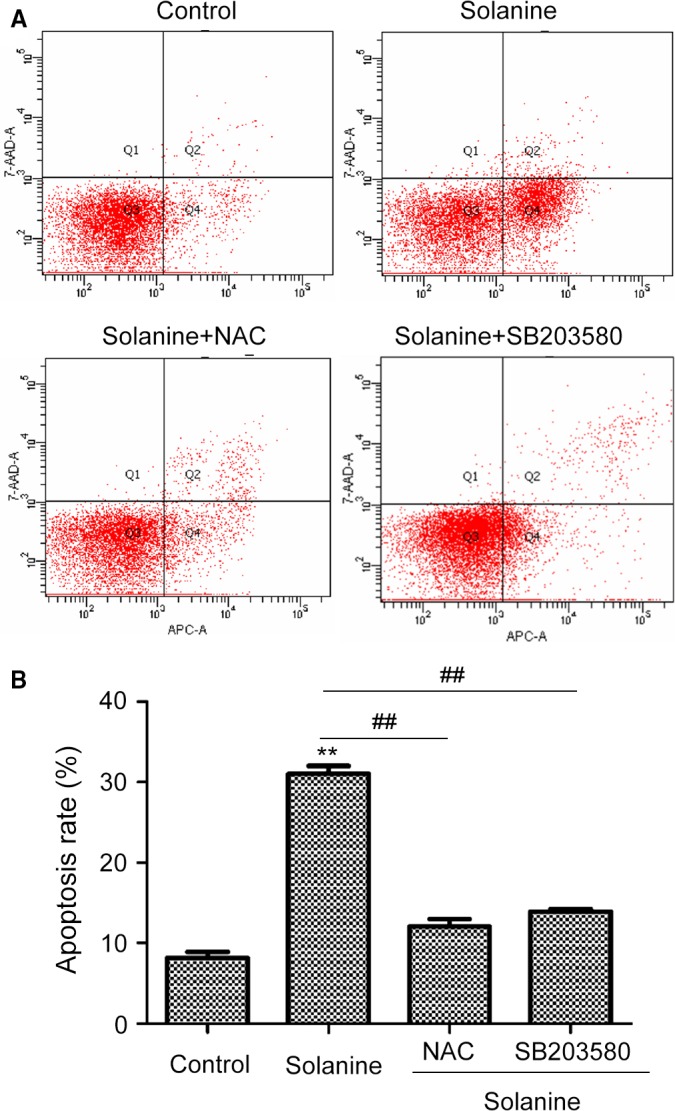
Cell apoptosis of DU145 detected by flow cytometry. (A) DU145 cells treated as the same as in Figure 5 were stained with Annexin V and 7‐AAD, then cells were subjected to flow cytometry to detect cell apoptosis. The representative scatter plots were shown in (A) and the statistical data were shown in (B). ** denotes P < 0.01 versus control group; ## denotes P < 0.01 versus solanine group.
Figure 7.

P38 activation detected by western blot in DU145 cells. DU145 cells were treated with solanine, together with or without NAC (5 mmol/L) or SB203580 (40 μmol/L) as in Figure 5. The cells were harvested and subjected to western blot with indicated antibodies. The representative scatter plots were shown in (A) and the statistical data were shown in (B). a denotes P < 0.05 versus control group; b denotes P < 0.05 versus solanine group.
Discussion
In recent years, the incidence of prostate cancer was significantly increased. Advanced primary treatment of prostate cancer is endocrine therapy, which would lead to hormonal resistance 19, 20. Thus, researches on new treatments and new drugs for prostate cancer have important significance. Solanine, an important glycoalkaloid in nightshade, exhibits anticarcinogenic potentials, inhibiting cell growth of various cancer cell lines and inducing apoptosis of human cancer cells 8, 9, 10. The present findings demonstrate that solanine suppresses tumor growth of DU145 in xenograft athymic nude mice in vivo and in cultured DU145 cell in vitro. Solanine regulates the mRNA and protein levels of many cell cycle proteins, induces cell apoptosis via the induction of ROS and the activation of P38 MAPK pathway, downstream of ROS.
The activation of CDKs (cyclin‐dependent kinases) is critical for cells entering S phase from G1, which are regulated by the periodic expression of cyclins. Cyclin D/E forms complexes with and activates CDK 2, 4, or 6, promoting cell transition from G1 to S phase 21, 22. P21, a CDK inhibitor (CKDI), regulates the negative feedback of G1/S transition, and increased expression of P21 blocks the progress of G1/S transition 23, 24. Our study of qPCR and western blots show that solanine administration can significantly downregulate the expression of Cyclin D1/E1, CDK 4 and 6, upregulate P21, indicating that solanine affects the G1/S transition signal, blocks the progress of G1/S transition, inhibits cell growth of cancer cell and tumor growth in vivo. The detailed mechanisms of how solanine influences the signal of cell cycle G1/S transition needs to be further explored. Staurosporine can cause the increasing of Ca2+ and accumulation of ROS, and induce cell apoptosis 25. So, solanine may induce ROS accumulation to damage DNA, upregulate the expression of P21, restrain the expression of c‐Myc, thus block cell G1/S progress.
ROS is important for the development and metastasis 26. Recent studies show that regulating ROS can reach the therapeutic purposes by accelerating tumor cell death 27, 28. The level of ROS in cancer cells is high, so cancer cells are always under oxidative stress, leading to the higher sensitivity than healthy cells. This provides theoretical basis for the application of pro‐oxidant drugs, such as trioxide, gemcitabine, for curing cancers 29, 30, 31, 32. ROS suppresses cancer development mainly through the following aspects: (1) promoting cell apoptosis, (2) inducing the necrosis and (3) regulating autophagic cell death 33, 34. Our data show the solanine induces ROS. Once the active oxygen scavenger NAC is used, solanine‐induced cell growth inhibition and apoptosis are all suppressed, indicating ROS plays an important role solanine‐regulated apoptosis.
P38, a MAPK kinase protein, is an important regulator of inflammation, which can be activated by physiological stress, lipopolysaccharide, osmotic stress, and UV irradiation 35, 36, 37. Lipopolysaccharide, tumor necrosis factor, platelet‐activating factor, IL‐1, and other inflammatory stimuli can induce the activation of P38 in endogenous immune cells such as monocytes, endothelial cells, and neutrophils 38, 39. P38 activation is closely related to apoptosis and P38 can increase the expression of c‐Myc, phosphorylation of P53 and activation of c‐Jun, to regulate apoptosis 40, 41. ATF2 is a commonly recognized downstream target of P38 MAPK kinase 18. P38 phosphorylates Thr‐69 and Thr‐71 of ATF2, leading to the activation of ATF2. Activated ATF2 forms dimer with c‐Jun to activate caspase‐3, thus leading the mitochondrial‐dependent apoptosis 42. We found the downregulation of Bcl‐2 and upregulation of Bax when treated with solanine. The unbalance of Bcl‐2/Bax leads to the mitochondrial membrane permeabilization of mitochondria, leading to the release of cytochrome C, and finally irreversible apoptosis 43. Moreover, our results show that after the treatment of solanine in DU145 cells, the expression level of phosphorylated P38/ATF2 is significantly upregulated, which can be blocked by the administration of P38 inhibitor SB203580 and the active oxygen scavenger NAC, indicating the P38 pathway is downstream of ROS induction in solanine‐induced apoptosis and tumor inhibition.
In summary, the data in this study demonstrates the solanine, through the suppression of cell cyclin proteins to inhibit cancer development and through the induction of ROS, promotes P38 activation to induce DU145 cell apoptosis. This data elucidates the mechanisms of solanine‐induced prostate cancer cell inhibition, providing theoretical and experimental foundation for the clinical application of solanine.
Conflict of Interest
The authors declare no conflicts of interest.
Acknowledgment
This work was supported by the Natural Science Foundation of Guangdong Province, China (2015A030310250), the Medical Scientific Research Foundation of Guangdong Province, China (A2015557) and the Guangzhou Medicine and Health Science and Technology Project (20141A010105).
Cancer Medicine 2016; 5(11):3214–3222
References
- 1. Jemal, A. , Siegel R., Xu J., and Ward E.. 2010. Cancer statistics, 2010. CA Cancer J. Clin. 60:277–300. [DOI] [PubMed] [Google Scholar]
- 2. Jani, A. B. , and Hellman S.. 2003. Early prostate cancer: clinical decision‐making. Lancet 361:1045–1053. [DOI] [PubMed] [Google Scholar]
- 3. Ganswindt, U. , Stenzl A., Bamberg M., and Belka C.. 2008. Adjuvant radiotherapy for patients with locally advanced prostate cancer–a new standard? Eur. Urol. 54:528–542. [DOI] [PubMed] [Google Scholar]
- 4. Kent, E. C. , and Hussain M. H.. 2003. Neoadjuvant Therapy for Prostate Cancer: An Oncologist's Perspective. Rev. Urol. 5(Suppl 3):S28–S37. [PMC free article] [PubMed] [Google Scholar]
- 5. Ji, Y. B. , Gao S. Y., Ji C. F., and Zou X.. 2008. Induction of apoptosis in HepG2 cells by solanine and Bcl‐2 protein. J. Ethnopharmacol. 115:194–202. [DOI] [PubMed] [Google Scholar]
- 6. Friedman, M. 2006. Potato glycoalkaloids and metabolites: roles in the plant and in the diet. J. Agric. Food Chem. 54:8655–8681. [DOI] [PubMed] [Google Scholar]
- 7. Barceloux, D. G. 2009. Potatoes, tomatoes, and solanine toxicity (Solanum tuberosum L., Solanum lycopersicum L.). Dis. Mon. 55:391–402. [DOI] [PubMed] [Google Scholar]
- 8. Lee, K. R. , Kozukue N., Han J. S., Park J. H., Chang E. Y., Baek E. J., et al. 2004. Glycoalkaloids and metabolites inhibit the growth of human colon (HT29) and liver (HepG2) cancer cells. J. Agric. Food Chem. 52:2832–2839. [DOI] [PubMed] [Google Scholar]
- 9. Friedman, M. , Lee K. R., Kim H. J., Lee I. S., and Kozukue N.. 2005. Anticarcinogenic effects of glycoalkaloids from potatoes against human cervical, liver, lymphoma, and stomach cancer cells. J. Agric. Food Chem. 53:6162–6169. [DOI] [PubMed] [Google Scholar]
- 10. Yang, S. A. , Paek S. H., Kozukue N., Lee K. R., and Kim J. A.. 2006. Alpha‐chaconine, a potato glycoalkaloid, induces apoptosis of HT‐29 human colon cancer cells through caspase‐3 activation and inhibition of ERK 1/2 phosphorylation. Food Chem. Toxicol. 44:839–846. [DOI] [PubMed] [Google Scholar]
- 11. Mohsenikia, M. , Alizadeh A. M., Khodayari S., Khodayari H., Kouhpayeh S. A., Karimi A., et al. 2013. The protective and therapeutic effects of alpha‐solanine on mice breast cancer. Eur. J. Pharmacol. 718:1–9. [DOI] [PubMed] [Google Scholar]
- 12. Lu, M. K. , Shih Y. W., Chang Chien T. T., Fang L. H., Huang H. C., and Chen P. S.. 2010. alpha‐Solanine inhibits human melanoma cell migration and invasion by reducing matrix metalloproteinase‐2/9 activities. Biol. Pharm. Bull. 33:1685–1691. [DOI] [PubMed] [Google Scholar]
- 13. Wang, L. , Li X. Y., Jiang G. F., Liang J. Z., Sun Y., and Liu W.. 2013. Reversal effect of BM‐cyclin 1 on multidrug resistance by down‐regulating MRP2 in BALB/C nude mice bearing C‐A120 cells. J. Huazhong Univ. Sci. Technolog. Med. Sci. 33:840–844. [DOI] [PubMed] [Google Scholar]
- 14. Umemura, M. , Baljinnyam E., Feske S., De Lorenzo M. S., Xie L. H., Feng X., et al. 2014. Store‐operated Ca2+ entry (SOCE) regulates melanoma proliferation and cell migration. PLoS ONE 9:e89292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaur, T. , Mukherjea D., Sheehan K., Jajoo S., Rybak L. P., and Ramkumar V.. 2011. Short interfering RNA against STAT1 attenuates cisplatin‐induced ototoxicity in the rat by suppressing inflammation. Cell Death Dis. 2:e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lv, C. , Kong H., Dong G., Liu L., Tong K., Sun H., et al. 2014. Antitumor efficacy of alpha‐solanine against pancreatic cancer in vitro and in vivo. PLoS ONE 9:e87868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li, J. J. , Tang Q., Li Y., Hu B. R., Ming Z. Y., Fu Q., et al. 2006. Role of oxidative stress in the apoptosis of hepatocellular carcinoma induced by combination of arsenic trioxide and ascorbic acid. Acta Pharmacol. Sin. 27:1078–1084. [DOI] [PubMed] [Google Scholar]
- 18. Waas, W. F. , Lo H. H., and Dalby K. N.. 2001. The kinetic mechanism of the dual phosphorylation of the ATF2 transcription factor by p38 mitogen‐activated protein (MAP) kinase alpha. Implications for signal/response profiles of MAP kinase pathways. J. Biol. Chem. 276:5676–5684. [DOI] [PubMed] [Google Scholar]
- 19. Zhang, H. Y. , Cui J., Zhang Y., Wang Z. L., Chong T., and Wang Z. M.. 2016. Isoflavones and Prostate Cancer: A Review of Some Critical Issues. Chin. Med. J. (Engl). 129:341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lei, J. H. , Liu L. R., Wei Q., Song T. R., Yang L., Meng Y., et al. 2016. Androgen‐deprivation therapy alone versus combined with radiation therapy or chemotherapy for nonlocalized prostate cancer: a systematic review and meta‐analysis. Asian J. Androl. 18:102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liao, Y. , Feng Y., Shen J., Hornicek F. J., and Duan Z.. 2016. The roles and therapeutic potential of cyclin‐dependent kinases (CDKs) in sarcoma. Cancer Metastasis Rev. 35:151–163. [DOI] [PubMed] [Google Scholar]
- 22. Simmons Kovacs, L. A. , Orlando D. A., and Haase S. B.. 2008. Transcription networks and cyclin/CDKs: the yin and yang of cell cycle oscillators. Cell Cycle 7:2626–2629. [DOI] [PubMed] [Google Scholar]
- 23. Perkins, N. D. 2002. Not just a CDK inhibitor: regulation of transcription by p21(WAF1/CIP1/SDI1). Cell Cycle 1:39–41. [PubMed] [Google Scholar]
- 24. Ball, K. L. 1997. p21: structure and functions associated with cyclin‐CDK binding. Prog. Cell. Cycle. Res. 3:125–134. [DOI] [PubMed] [Google Scholar]
- 25. Gil, J. , Almeida S., Oliveira C. R., and Rego A. C.. 2003. Cytosolic and mitochondrial ROS in staurosporine‐induced retinal cell apoptosis. Free Radic. Biol. Med. 35:1500–1514. [DOI] [PubMed] [Google Scholar]
- 26. Nishikawa, M. , Hashida M., and Takakura Y.. 2009. Catalase delivery for inhibiting ROS‐mediated tissue injury and tumor metastasis. Adv. Drug Deliv. Rev. 61:319–326. [DOI] [PubMed] [Google Scholar]
- 27. Subramani, R. , Gonzalez E., Arumugam A., Nandy S., Gonzalez V., Medel J., et al. 2016. Nimbolide inhibits pancreatic cancer growth and metastasis through ROS‐mediated apoptosis and inhibition of epithelial‐to‐mesenchymal transition. Sci. Rep. 6:19819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qin, W. , Li C., Zheng W., Guo Q., Zhang Y., Kang M., et al. 2015. Inhibition of autophagy promotes metastasis and glycolysis by inducing ROS in gastric cancer cells. Oncotarget 6:39839–39854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. You, B. R. , and Park W. H.. 2012. Arsenic trioxide induces human pulmonary fibroblast cell death via increasing ROS levels and GSH depletion. Oncol. Rep. 28:749–757. [DOI] [PubMed] [Google Scholar]
- 30. Park, W. H. 2012. MAPK inhibitors and siRNAs differentially affect cell death and ROS levels in arsenic trioxide‐treated human pulmonary fibroblast cells. Oncol. Rep. 27:1611–1618. [DOI] [PubMed] [Google Scholar]
- 31. Wu, W. , Xia Q., Luo R. J., Lin Z. Q., and Xue P.. 2015. In vitro Study of the Antagonistic Effect of Low‐dose Liquiritigenin on Gemcitabine‐induced Capillary Leak Syndrome in Pancreatic Adenocarcinoma via Inhibiting ROS‐ Mediated Signalling Pathways. Asian Pac. J. Cancer Prev. 16:4369–4376. [DOI] [PubMed] [Google Scholar]
- 32. Fiorini, C. , Cordani M., Gotte G., Picone D., and Donadelli M.. 2015. Onconase induces autophagy sensitizing pancreatic cancer cells to gemcitabine and activates Akt/mTOR pathway in a ROS‐dependent manner. Biochim. Biophys. Acta 1853:549–560. [DOI] [PubMed] [Google Scholar]
- 33. Li, Z. Y. , Yang Y., Ming M., and Liu B.. 2011. Mitochondrial ROS generation for regulation of autophagic pathways in cancer. Biochem. Biophys. Res. Commun. 414:5–8. [DOI] [PubMed] [Google Scholar]
- 34. Simon, H. U. , Haj‐Yehia A., and Levi‐Schaffer F.. 2000. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5:415–418. [DOI] [PubMed] [Google Scholar]
- 35. Woll, S. , Windoffer R., and Leube R. E.. 2007. p38 MAPK‐dependent shaping of the keratin cytoskeleton in cultured cells. J. Cell Biol. 177:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cheng, H. , Kartenbeck J., Kabsch K., Mao X., Marques M., and Alonso A.. 2002. Stress kinase p38 mediates EGFR transactivation by hyperosmolar concentrations of sorbitol. J. Cell. Physiol. 192:234–243. [DOI] [PubMed] [Google Scholar]
- 37. Chen, W. , and Bowden G. T.. 1999. Activation of p38 MAP kinase and ERK are required for ultraviolet‐B induced c‐fos gene expression in human keratinocytes. Oncogene 18:7469–7476. [DOI] [PubMed] [Google Scholar]
- 38. Arana‐Argaez, V. E. , Delgado‐Rizo V., Pizano‐Martinez O. E., Martinez‐Garcia E. A., Martin‐Marquez B. T., Munoz‐Gomez A., et al. 2010. Inhibitors of MAPK pathway ERK1/2 or p38 prevent the IL‐1{beta}‐induced up‐regulation of SRP72 autoantigen in Jurkat cells. J. Biol. Chem. 285:32824–32833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moretti, M. , Budni J., Freitas A. E., Neis V. B., Ribeiro C. M., de Oliveira Balen G., et al. 2015. TNF‐alpha‐induced depressive‐like phenotype and p38(MAPK) activation are abolished by ascorbic acid treatment. Eur. Neuropsychopharmacol. 25:902–912. [DOI] [PubMed] [Google Scholar]
- 40. Li, F. X. , Huang L. Z., Dong C., Wang J. P., Wu H. J., and Shuang S. M.. 2015. Down‐regulation of aquaporin3 expression by lipopolysaccharide via p38/c‐Jun N‐terminal kinase signalling pathway in HT‐29 human colon epithelial cells. World J. Gastroenterol. 21:4547–4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu, B. , Yuan B., Zhang L., Mu W., and Wang C.. 2015. ROS/p38/p53/Puma signaling pathway is involved in emodin‐induced apoptosis of human colorectal cancer cells. Int J Clin Exp Med. 8:15413–15422. [PMC free article] [PubMed] [Google Scholar]
- 42. Song, B. , Xie B., Wang C., and Li M.. 2011. Caspase‐3 is a target gene of c‐Jun:ATF2 heterodimers during apoptosis induced by activity deprivation in cerebellar granule neurons. Neurosci. Lett. 505:76–81. [DOI] [PubMed] [Google Scholar]
- 43. Kroemer, G. , Galluzzi L., and Brenner C.. 2007. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 87:99–163. [DOI] [PubMed] [Google Scholar]


