Abstract
Chemokines are a group of low molecular weight peptides. Their major function is the recruitment of leukocytes to inflammation sites, but they also play a key role in tumor growth, angiogenesis, and metastasis. In the last few years, accumulated experimental evidence supports that monokine induced by interferon (IFN)‐gamma (CXCL9), a member of CXC chemokine family and known to attract CXCR3‐ (CXCR3‐A and CXCR3‐B) T lymphocytes, is involved in the pathogenesis of a variety of physiologic diseases during their initiation and their maintenance. This review for the first time presents the most comprehensive summary for the role of CXCL9 in different types of tumors, and demonstrates its contradictory role of CXCL9 in tumor progression. Altogether, this is a useful resource for researchers investigating therapeutic opportunities for cancer.
Keywords: Cancer, CXCL9, therapy, tumor promoter, tumor suppressor
Introduction
The development of malignant tumors is dependent on the tumor microenvironment, where chemokines and their receptors are important participants 1. Chemokine families are defined as small (8–15 kD) proteins that induce chemotaxis, tissue extravasation and, in some instances, modulate the functional properties of different leukocytes. Chemokines can be subdivided into four classes according to the number and spacing of two conserved N‐terminal cysteine residues, consisting of the C, C‐C, C‐X‐C, and C‐X3‐C families 2. Chemokine CXCL9 is a member of the CXC family and has an important role in the chemotaxis of immune cells. It is secreted by various cell types including immune cells (T lymphocytes, NK cells 3, dendritic cells 4, macrophages 5, eosinophils 6, etc.), and non‐immune cells (hepatic stellate cells 7, preadipocytes, thyrocytes 8, endothelial cell, tumor cells, and fibroblasts 9, etc). CXCL9 has a versatile and controversial role in tumors, and accumulating evidence suggests that CXCL9 is closely associated with the prognosis of tumor patients. Here, the role of CXCL9 in cancer development was reviewed, as well as the molecular mechanisms leading to aberrant expression of CXCL9 in cancer and the potential clinical applications of CXCL9 in diagnosis, prognosis, and cancer treatment.
Regulation of CXCL9
The transcriptional regulation of CXCL9 is a multistep process involving many transcription factors (Fig. 1), of which signal transducer and activator of transcription (STAT1) and nuclear factor κB (NF‐κB) are two most well‐characterized members 10. Both the gene mutation of STAT1 11 and the blocking of the Janus‐activated kinase (JAK)/STAT‐1 pathway 12, 13 can reduce CXCL9 expression induced by IFN‐γ. Moreover, CXCL9 expression can be suppressed by reducing the levels of components of the STAT1‐IRF‐1(IRF‐1, Interferon regulatory factor) transcriptional activation pathway by Porphyromonas gingivalis that leads to the immune function decline 14. Lipopolysaccharide (LPS) and D‐galactosamine could induce the phosphorylation of STAT1 and enhance the transcription of CXCL9 leading to the enhancement of liver inflammation, and even liver apoptosis and injury 15. In this process, VSL#3 (a mixture of eight different probiotic bacteria) can specifically reduce the phosphorylation of STAT‐1 15, 16. STAT‐1 can also be activated by IL‐27 accompanied with IFN‐γ. Then CXCL9 induced by STAT‐1 finally affects the liver inflammation 17. In addition, our previous work by Xia et al. identified that HBx protein can induce the CXCL9 transcription by activating NF‐κB that binds to its promoter, and CXCL9 promotes the migration of leukocytes in liver with HBV infection 18. Unlike STAT1, phosphorylation of NF‐κB could not be suppressed by VSL#3 16. STAT1 and NF‐κB can cooperatively regulate the expression of CXCL9, and this transcriptional synergy could cause the enhanced recruitment of RNA polymerase II complex to the promoter via simultaneous interaction of CBP with STAT1 and NF‐κB 10.
Figure 1.
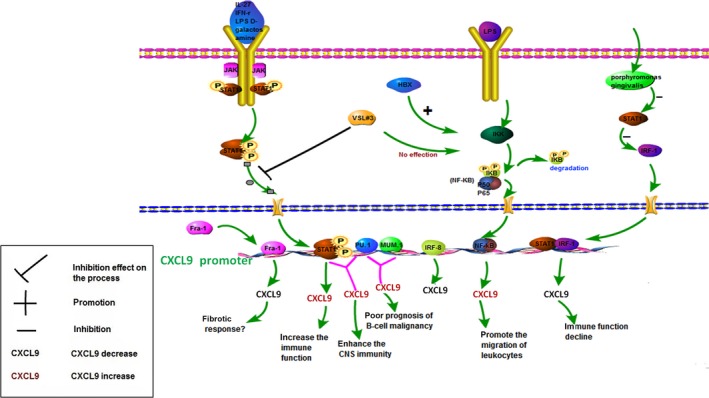
Role and regulation of CXCL9 in cancer. CXCL9 expression could be induced by IFN‐γ, IL‐27, D‐galactosamine, and so on, through JAK/STAT1, PU.1, MUM1, NF‐ kB, and Fra‐1 (direct binding to CXCL9 promoter), and Egr‐1 (not clear). Also, CXCL9 showed a key role on immune function, such as chemotaxis of leukocytes, B‐cells, and T‐cells. STAT1, signal transducer and activator of transcription; JAK, Janus‐activated kinase; PU.1, Myeloid Transcription Factor PU.1; MUM1, multiple myeloma oncogene 1; Egr‐1, Early growth response‐1; Fra‐1, Fos‐related antigen 1.
Besides STAT1 and NF‐κB, myeloid transcription factor PU.1 (PU.1) is also involved in regulating the CXCL9 gene transcription 19, 20. PU.1 is a cell‐specific nuclear transcription factor and a key transcription activator of the gene encoding CXCL9 in response to IFN‐γ. In microglia of the central nervous system (CNS), both STAT1 and PU.1 constitutively bind to the CXCL9 gene promoter. However, only STAT1 binds to the CXCL9 gene promoter in astrocytes, and this binding can be counteracted by the ectopic expression of PU.1 19. Besides, studies also showed that IRF‐8 can bind to the CXCL9 gene promoter induced by IFN‐γ and this binding was accompanied by decreasing PU.1 binding 20. In the B‐cell lymphoma/leukemia, multiple myeloma oncogene 1 (MUM1) can upregulate the expression of CXCL9 by activating its promoter in cooperation with PU.1, leading to poor prognosis of B‐cell malignancy 21.
Early growth response‐1 (Egr‐1), a zinc‐finger transcription factor, also correlates with the expression of CXCL9 in the invading macrophages and accumulation of NK cells in Lewis lung carcinoma 22. Nevertheless, this phenomenon is not observed for all types of tumors and whether Egr‐1 directly regulates CXCL9 or other Egr family members take part in this process is not clearly understood.
CXCL9 is also associated with human hepatic fibrosis and anti‐fibrosis in mice 23, 24. Fos‐related antigen 1 (Fra‐1) represses CXCL9 expression by direct promoter binding in hepatocytes and affects the fibrotic response to some extent 25.
Receptor CXCR3
CXCR3, a G protein‐coupled receptor, binds to C‐X‐C motif chemokines including CXCL9, CXCL10, CXCL11, CXCL4, and CXCL4L1 26. CXCR3 is highly expressed on T cells, NK cells, and subsets of B cells, and also on epithelial cells, endothelial cells, fibroblasts etc 27, 28. Increasing evidence shows that the abnormal expression of CXCR3 has a significant impact on immune response, inflammation, tumor development, angiogenesis etc 29, 30, 31, 32. There are three spliced variants of CXCR3 in humans, including CXCR3‐A, CXCR3‐B, and CXCR3‐alt, of which only CXCR3‐alt could bind CXCL11. CXCR3‐A and CXCR3‐B are the primary variants, however, their role in physiological functions resembles a double‐edged sword in. In general, CXCR3‐A enhances cell proliferation, chemotaxis, and metastasis, while CXCR3‐B suppresses cell growth, angiogenesis, migration, and promotes apoptosis 26. CXCR3 regulates several signaling pathways, such as MAPK, phospholipase C, and PI3K 33, 34, 35.
CXCL9 as a Tumor Suppressor
A summary of the increasing evidence about the effects of CXCL9 on tumor suppression is exhibited in Table 1.
Table 1.
CXCL9 as a tumor suppressor
| Type of cancer | CXCL9 Expression | Source | Sample Number | Prognosisa | Ref |
|---|---|---|---|---|---|
| NSCLC | Low | Tumor cells | 109 | No relation | 38 |
| High | Tumor cells | 12 | Might be good | 37 | |
| High | Tumor cells | 90 | Good | 36 | |
| Breast cancer | High | Tumor cells | 60 | Good | 42 |
| High | Tumor cells | 1058 | Good | 43 | |
| CTCL | High (early), low (advanced) | Tumor cells | 9 (early), 13 (advanced) | Might be good | 49 |
| High | Tumor cells | 11 | Might be good | 48 | |
| Colorectal cancer | Low | Tumor cells | 196 | Poor | 55 |
| High | Tumor cells | 130 | Good | 54 | |
| UC‐Ca | High | Serum | 10 | Might be good | 56 |
| Melanoma | High | Tumor cells | 113 | Might be good | 60 |
| High | Tumor cells | 44 | Might be good | 61 | |
| Ovarian cancer | High | TCs, macrophages | 85 | Might be good | 66 |
| GC(lymphocyte‐rich) | High | stromal cells, a few TCs | 42 | Might be good | 68 |
| GC | High | mononuclear cells | 22 | Might be good | 69 |
| Ewing sarcoma | High | tumor and stromal cells | 20 | No relation | 70 |
| Cutaneous tumor | High | Tumor cells | 42 | Might be good | 71 |
NSCLC, Non‐small‐cell lung cancer; CTCL, Cutaneous T‐cell lymphoma; UC‐Ca, ulcerative colitis‐associated cancer; RCC, Renal cell carcinoma; TCs, Tumor cells; GC, Gastric carcinoma.
The response as “Good” means good prognosis of cancer patients, good response to tumor therapy, or reduction of tumor burden.
Lung cancer
There is no precise staging system that predicts the prognosis of early‐stage non‐small‐cell lung cancer (NSCLC). Addison et al. found that high protein levels of ELR‐ (Glu‐Leu‐Arg) chemokine CXCL9 existed in 90 human NSCLC tissues. Moreover, they showed that either recombinant human cytokine CXCL9 (rhCXCL9) or gene transfer of CXCL9 inhibited tumor‐derived angiogenesis, suppressing tumor growth and metastasis, which counteracted the angiogenic role of ELR+ chemokine (such as IL‐8 and epithelial neutrophil activating protein 78) partly 36. Metodieva et al. also showed a high expression level of CXCL9 in 12 NSCLC patients 37. However, a study by Kowalczuk et al. also showed that CXCL9 expression was low in 109 NSCLC tumor tissues, but it could not influence both overall and disease‐free survival 38. In addition, other studies have also demonstrated that Egr‐1 deficiency 22, IL‐7 39, CCL21 40, and myeloid‐derived suppressor cells depletion 41 reduced tumor burden by upregulating CXCL9 and CXCL10 expression, which played an anti‐angiogenic role and attracted tumor macrophages, CD4 and CD8+ T lymphocytes, and NK cells. Also, IFN‐γ, interleukin‐12 (IL‐12), and granulocyte macrophage colony‐stimulating factor were involved in the reduction of tumor burden caused by CCL21 40.
Breast cancer
It is recognized that CXCL9 is significantly associated with lymphocytes infiltration and chemotherapy response in human breast cancer (BC) patients 42, 43, 44. Bronger et al. discovered that a predominantly high mRNA expression of CXCL9 was observed in breast cancer cells in 60 BC tissues 42. Denkert et al. also suggested that the high expression levels of T‐cell‐related markers CD3D and CXCL9 caused a significantly increased pathologic complete response rate (pCR) in 1,058 pretherapeutic BC tissues from two neoadjuvant anthracycline/taxane‐based studies 43. Thakur et al. suggested that the recombined human cytokines IFN‐γ, CXCL9, and CXCL10 could decrease myeloid‐derived suppressor cells (MDSC) population, and might suppress MDSC differentiation 45. Walsern et al. found that in a breast cancer murine model, CXCL9‐expressing tumor cells inhibited local tumor growth and lung metastases via host NK cells, and large numbers of CD4+CXCR3+ and CD8+CXCR3+ host T cells 46. However, Fulton et al. found that the capacity of CXCL9 to inhibit local tumor growth was completely abolished by the depletion of T cells but not compromised by the loss of NK cells, for the reason, T cells inhibited the growth of primary and metastatic tumors, while NK cells controlled transiting tumor cells 47.
Lymphoma
It is reported that CXCL9 plays a significant role in lymphoma because of its chemotaxis on immunocytes. Tensen et al. showed that CXCL9 and CXCL10 mRNA, not IL‐8, were highly expressed in 11 patients with cutaneous T‐cell lymphoma (CTCL), and correlated with increased CD4+ T cells infiltration, not CD8+ T cells 48. CXCL9 was also reported to be significantly elevated in nine early CTCL compared to the normal skin or 13 advanced CTCL skin 49. Przewoznik et al. showed that the overexpression of CXCL9 and CXCL10 revealed a significant correlation with increased NK cells and their migration in late B‐cell lymphoma stages, which were prerequisites for the potential tumor therapy of adoptive NK‐cell transfer 50. In Addition, IL‐12 and Th1‐derived IFN‐γ exerted antitumor effects through the inhibitory effects of endogenous CXCL9 and CXCL10 on tumor vasculature in human Burkitt's lymphoma 51 and in B‐cell lymphoma 52, respectively.
Colorectal cancer
Colorectal cancer (CRC) is one of the most prevalent tumor types worldwide. Tumor‐infiltrating T cells are crucial for anti‐tumor immunity 53. Wu et al. showed that CXCL9 was highly expressed in 130 patients’ tumor tissues using PCR and IHC testing, and correlated this with clinic‐pathological features, such as tumor metastasis and differentiation. Moreover, high expression level of CXCL9 predicted a better overall survival 54. Mlecnik et al. found that high expression of CX3CL1, CXCL9, and CXCL10 were correlated with significantly high density of CD8+ T cells, while CXCL9 and CXCL10 attracted memory CD8+ T cells and macrophages, and CX3CL1 attracted effector‐activated cytotoxic T cells and TH1 cells. All of them and the adhesion molecules (ICAM1, VCAM1, MADCAM1) were associated with prolonged disease‐free survival (DFS) 53. Chaput et al. showed that CXCL9 was significantly decreased in tumor tissues from a tissue microarray, consisted of 196 consecutive patients with stage II‐III CRC, which indicated worse relapse‐free survival 55. Watanabe et al. showed that CXCL9 expression was higher in ulcerative colitis‐associated colorectal cancer (UC‐Ca) of 10 patients than in UC‐NonCa of 43 patients, which would improve the accuracy of UC‐Ca diagnosis (positive value 83%, negative value 100%) when combined with 19 other cancer‐related genes, such as cytochrome P450, family 27 B1 (CYP27B1), and Runt‐related transcription factor 3 (RUNX3) 56. A study conducted by Akeus et al. demonstrated endogenous CXCL9 and CXCL10 were selectively increased, followed by accumulation of CXCR3+ conventional T cells, in Treg‐cell‐depleted tumors, which indicated that targeting Treg cells and upregulating CXCL9 and CXCL10 might be a potential immunotherapy 57.
Melanoma
Tumor‐infiltrating T lymphocytes represent improved prognosis in primary 58 and metastatic melanomas 59. Bedognetti et al. found that CXCL9, CXCL10, CXCL11, and CCL5 were all significantly associated with overall response to therapy in 142 metastatic melanoma patients 60. Moreover, Harlin et al. found that in 44 biopsies of melanoma, highly expressed chemokines, including CXCL9, CCL2, CCL3, CCL4, CCL5, and CXCL10, correlated significantly with CD8+ T‐cell recruitment and migration, which predicted good prognosis for cancer patients 61. Using metastatic‐like melanoma model, Clancy‐Thompson et al. observed that endogenous CXCL9 and CXCL10 were correlated with lungs bearing minimal metastasis lesions by accumulation of CD8+ T cells in a CXCR3‐ and host IFN‐γ‐dependent manner, while it can be suppressed, partly, by adenosine signaling in the tumor microenvironment 62. Deng et al. found that endogenous chemokines CXCL9, CXCL16, CCL12, CCL4, and CCL2 could be significantly increased when DNA methyltransferase 3a that promoted melanoma metastasis and growth was depleted in vivo 63.
Other tumors
In renal cell carcinoma, endogenous CXCL9 was closely implicated in the antitumor effects that were produced by IL‐2 64 and IL‐12 (also producing CXCL10) 65 by inhibiting tumor angiogenesis and infiltration of CD8+ T lymphocytes, respectively. In ovarian cancer, synergistic effect of tumor‐associated IL‐17 and Th17 cells 66, as well as IL‐18‐primed “helper” NK cells 67, induced the production of endogenous CXCL9 and CXCL10 that were directly correlated with tumor‐infiltrating CD8+ T cells. The former also attracted the NK cells, and the latter also induced CCL5 that was involved in the T‐cells infiltration 66. In gastric carcinoma, endogenous CXCL9 could promote antitumor immunity exerted by T cells, and CXCL10, occasionally, also participated in this process 68, 69. In 20 Ewing sarcoma patients, Berghuis reported that CXCL9, CXCL10, and CCL5 that is highly expressed by tumor and stromal cells were correlated positively with accumulated CD8+ T cells. However, CXCL9 was not related to patient survival rate 70. Moreover, CXCL9 was significantly increased in cutaneous tumor patients, and played a critical role in CXCR3+ T cells (including CD4+ and CD8+ T cells) and NK‐mediated tumor immune‐surveillance and suppression 71, 72. Some other IFN‐regulated proteins (MxA, IDO) were also involved in the recruitment 71.
CXCL9 as a Tumor Promoter
An increasing body of evidences has been demonstrated that CXCL9 acts as a tumor promoter in multiple types of cancer (summarized in Table 2).
Table 2.
CXCL9 as a tumor promoter HCC
| Type of cancer | CXCL9 expression | Source | Sample number | Prognosisa | Ref |
|---|---|---|---|---|---|
| HCC | High | Epithelial cells | 40 | Poor | 76 |
| Lung cancer | High | Serum | 526 | Poor | 77 |
| High | Tumor cells | 40 | No relation | 78 | |
| BC (HR+) | High | Serum | 40 | Might be poor | 79 |
| High | Serum | 120 | Poor | 80 | |
| FL (chemotherapy) | High | Serum | 392 | Poor | 81 |
| PCNSL | High | Macrophages, pericytes | 22 | Might be poor | 82 |
| LAHS | High | Serum | 15 | Poor | 83 |
| THRLBL | High | Macrophage | 8 | Poor | 84 |
| ENKL | High | Tumor cells | 7 | Might be poor | 85 |
| Melanoma | High | TuECs | 29 | Might be poor | 87 |
| NPC | High | Serum, tumor cells | 205 (serum)/86 (tissue) | Poor/No relation | 91 |
| OSCC | High | Serum, tumor cells | 181 (serum)/50(tissue) | Poor/Poor | 92 |
| Cervical cancer | High | Serum | 1057 | Might be poor | 93 |
| CLL | High | Serum | 84 | Poor | 94 |
| Prostate cancer | High | Tumor cells | 20 | Poor | 95 |
| Glioblastoma | High | Tumor cells | 44 | Might be poor | 97 |
| CNS GCTs | High | Tumor cells | 103 | Might be poor | 98 |
Hepatocellular carcinoma; BC, Breast cancer; FL, Follicular lymphoma; THRLBCL, TAM of T cell/histiocyte‐rich large B cell lymphoma; PCNSL, Primary central nervous system lymphoma; ENKL, Extranodal natural killer/T‐cell lymphoma; LAHS, Lymphoma‐associated hemophagocytic syndrome; TuECs, tumor endothelial cells; NPC, Nasopharyngeal carcinoma; CNS GCTs, primary central nervous system germ cell tumors, OSCC, Oral cavity squamous cell carcinoma; CLL, Chronic lymphocytic leukemia.
The response as “Good” means good prognosis of cancer patients, good response to tumor therapy, or reduction of tumor burden.
Hepatocellular carcinoma
Hepatocellular carcinoma (HCC) causes the third most common cancer mortality due to early metastasis and recurrence 73. Ding et al. in our team showed that CXCL9 promoted the migration and invasion abilities of CD133+ liver cancer cells, and its receptor isoform CXCR3‐A upregulated invasion abilities via activation of p‐ERK1/2‐MMP2/MMP9 pathway induced by rhCXCL9 74. Lan et al. in our team also demonstrated that rhCXCL9 enhanced the invasion ability of hepatocellular carcinoma through the upregulation of phosphatidylinositol‐3, 4, 5‐trisphosphate RAC Exchanger 2 (PREX2) 75. In addition, Liu et al. found that there was pronounced expression of CXCL9, CXCL10, and CXCL11, induced by IL‐17, at the HCC invading edge in 40 patient tissues, which were correlated with the recruitment of CXCR3+ B cells. CXCR3+ B cells could trigger the polarization of protumorigenic M2b macrophages in an IgG‐dependent manner and were positively associated with early recurrence in HCC patients 76.
Lung cancer
Although CXCL9 is considered as an anti‐tumor factor according to some reports, it could also promote the tumor development as follows. Shiels et al. conducted two independent nested case–control studies (the discovery study and the replication study). The results showed that CXCL9 was associated with lung cancer risk in the replication study, and remained associated with it more than 6 years prior to diagnosis in pooled analyses. Their research suggested that CXCL9 has an etiologic role in lung cancer 77. On the contrary, Nakanishi et al. showed that in 40 lung cancer patients, although CXCL9 was highly upregulated in tumor tissues, no significant relationship between CXCL9 expression and DFS or lower risk of postsurgical recurrence was observed 78.
Breast cancer
Metastasis of breast cancer (BC) remains a challenge for the therapeutic management. Ejaeidi et al. showed that the levels of CXCL9, CXCL10, and CXCL11 were markedly high in 40 HR+ (hormone receptor) metastatic BC patients’ sera when compared to HR—patients and healthy controls. These three chemokines in sera, especially CXCL10, played an important role in BC development through activation of survivin, β‐catenin, mitogen‐activated protein kinase phosphatase 1 (MKP‐1), and matrix metalloproteinase‐1 (MMP‐1) 79. Ruiz‐Garcia et al. found that among 120 BC patients, the serum concentration of CXCL9 was higher in cancer patients compared with normal volunteers, and the difference between ER‐negative BC samples and normal volunteers was statistically significant. However, it was not different between ER‐positive BC and normal volunteers. When a cutoff of 1000 pg/mL for CXCL9 was selected, the sensitivity of ER‐negative BC diagnosis was 27% and specificity was 90%, and the positive predictive value was 76%. In addition, when CXCL9 combined with fibronectin 1 (Fibronectin 1 > 200 pg/mL as positive; 150 < fibronectin 1 < 200 pg/mL and CXCL9 concentrations >1000 pg/mL as positive), the sensitivity for BC diagnosis was 53% and specificity was 97%. The positive predictive value was up to 96% 80.
Lymphoma
CXCL9 could also be of great importance for enhancing the understanding of metastasis of lymphoma. Mir et al. demonstrated that in 209 follicular lymphoma patients receiving chemotherapy, elevated serum levels of CXCL9 predicted a shorter median event‐free survival (EFS). When a separate study of 183 patients was combined in a meta‐analysis, the results still showed that CXCL9 was still significantly associated with shorter EFS 81. Venetz et al. found that in primary CNS lymphoma, perivascular CXCL9 correlated with CD8+, CD4+, and Foxp3+ T cells infiltration, and formed heterocomplexes with CXCL12 in vivo. Moreover, rhCXCL9 enhanced rhCXCL12‐induced migration of CXCR3+/CXCR4+/CD8+ T cells and malignant B cells in vitro. These results highlighted the importance of regions expressing chemokines in tumor development 82. Maruoka et al. demonstrated that CXCL9 and CXCL10 (5000 pg/mL and 500 pg/mL as the cutoff levels, respectively) were significantly useful for the early diagnosis and therapeutic outcomes of Lymphoma‐associated hemophagocytic syndrome (LAHS). They also could distinguish LAHS from sepsis and, furthermore, severe from moderate/mild LAHS, and B‐cell‐type from T/NK cell‐type LAHS 83. Many other studies have delineated that CXCL9 was probably involved in tumor‐associated macrophages polarization 84, tumor cell motility 85, and the survival of H‐RS cells 86.
Melanoma
Amatschek et al. found that low concentration of rhCXCL9 was able to induce melanoma cell migration, conversely at high concentration. And rhCXCL9 enhanced the breakdown of endothelial cells monolayer during transendothelial migration of tumor cells 87. Moreover, RhCXCL9 facilitated the intracellular actin polymerization, cell adhesions (phosphorylation of focal adhesion kinase and paxillin), and cell survival. RhCXCL9 could also increase the intracellular calcium concentration, an early biochemical events in response to chemokines. Endogenous CXCL9 and CXCL10, upregulated by complete Freund's adjuvant in draining lymph nodes, promoted the metastasis of tumor cells to the lymph nodes 88. Jehs et al. reported that uveal melanoma cell lines that were cocultured with activated T cells resulted in an upregulation of chemokines in the supernatant, such as CXCL9, CXCL10, CXCL11, CCL2, CCL5, and VEGF. In turn, these cytokines could generate a tumor‐promoting inflammatory microenvironment 89.
Head and neck cancer
The head and neck cancer (HNC) is the seventh most common malignancy worldwide, of which squamous cell carcinoma (SCC) is the most common type 90. In a research study that included sera from 205 nasopharyngeal carcinoma (NPC) patients and 231 healthy individuals, and 86 NPC tumor samples, Hsin et al. identified that CXCL9 serum concentrations correlated significantly with tumor stages, nodal stages, overall stages, 5‐year overall survival and DFS in NPC patients, as well as EBV DNA load. However, the immunohistochemical results showed no association between CXCL9 overexpression and clinic‐pathological characteristics 91. Chang et al. found that CXCL9 expression was significantly higher in 50 tumor samples than the normal samples, as well as in the sera of 181 oral cavity SCC patients than the 231 healthy individuals. Serum CXCL9 levels were correlated with pT status, pathological overall stages, tumor depths, and positive bone invasion. Moreover, the results also indicated that higher CXCL9 serum levels predicted a poor prognosis of patient's overall survival and DFS 92.
Other cancers
In cervical cancer, Zhi et al. detected that serum CXCL9 was significantly increased at invasive International Federation of Gynecology and Obstetrics stages II, and III in a total of 1057 women, as compared to the noninvasive stage. This suggested that CXCL9 was involved in the development of cervical cancer 93. In chronic lymphocytic leukemia, Yan et al. reported that high serum levels of chemokines CL1 (CXCL9, CXCL10, CXCL11, CCL3, CCL4, CCL19, IL‐5, IL‐12, and IFN‐γ) correlated with shorter overall survival, suggesting that high levels of CXCL9 might predict a poor prognosis 94. In prostate cancer (PCa), Hu et al. reported that PCa cells could secrete CXCL9 more than the normal cells, and CD4+ T cells recruited by endogeneous CXCL9, consequently, promoted PCa metastasis via modulation of FGF11/miRNA‐541/AR/MMP9 signaling 95. Liu et al. showed that recombinant mouse and human CXCL9 and CXCL10 facilitated proliferation of murine and human gliomaspheres, suggesting that they may promote tumorigenesis 96. In addition, studies also demonstrate that CXCL9 is highly upregulated in gliolastoma 97 and primary pediatric CNS germ cell tumor (germinoma type) 98.
CXCL9 in cancer therapy
Accumulating evidence indicates that manipulation of the tumor microenvironment, which involves CXCL9, could enhance the therapeutic efficacy of strategies via tumor‐specific T cells (summarized in Table 3).
Table 3.
CXCL9 in cancer therapy
| Type of cancer | Treatment | CXCL9 expression | Prognosisa | Target of CXCL9 | Ref |
|---|---|---|---|---|---|
| Lung cancer | IL‐7/IL‐7Rα‐Fc | Up | Good | M1 macrophages | 99 |
| IL‐7 | Up | Good | anti‐angiogenisis | 100 | |
| MIG plus cisplatin | Up | Good | see Note 1 | 101 | |
| Breast cancer | COX‐2 deficiency | Up | Good | CD4+Th cells, CD8+CTL | 102 |
| PGE2/ COX inhibitors | Down/Up | Good | NK cells, T cells | 42 | |
| CMF | Up | Good | See Note 2 | 44 | |
| Lapatinib, doxorubicin | Up | Good | CD8+ T cells | 104 | |
| Melanoma | ATRA, polyI:C | Up | Good | APCs | 106 |
| IL‐2 | Up | Good | TILs | 60 | |
| HNC | IL‐12 | Up | Good | CD4+ T not CD8+ T cells | 107 |
| INF‐ α | UP | Good | anti‐angiogenisis | 108 | |
| CLL | αDC1 | Up | Good | NK, NKT, CD8+T cells | 109 |
| RCC | IL‐2 | Up | Good | anti‐angiogenisis | 110 |
| Genital carcinoma | Imiquimod | Up | Good | CD8+ CTL | 111 |
| Sarcoma | OX40L‐Fc | Up | Good | type 1 T‐cell | 112 |
| Cutaneous melanoma | temozolomide | Up | Good | Growth inhibition | 113 |
ATRA, all‐transretinoic acid; IL‐12, interleukin‐12; COX‐2, cyclooxygenase 2; CTL, cytotoxic T lymphocytes; polyI:C, polyinosinic:polycytidylic acid PGE2; prostaglandin E2; CMF, cyclophosphamide, methotrexate and 5‐fluorouracile; APCs, antigen‐presenting cells; TILs, tumor‐infiltrating lymphocytes; HNC, Head and neck cancer; CLL, Chronic lymphocytic leukaemia; RCC, Renal cell carcinoma; βDC1, tumor‐loaded α‐type 1‐polarized dendritic cells cocktail (IL‐1β/TNF‐α/IFN‐α/IFN‐γ/poly‐I:C); OX40L‐Fc, OX40 ligand–Fc fusion protein; Note1, anti‐angiogenisis, apoptosis, and CTL activity; Note 2, the target of CXCL9 was not found in the article, but CXCL9 could be a predictive factor.
The response as “Good” means good prognosis of cancer patients, good response to tumor therapy, or reduction of tumor burden.
Lung cancer
Although inflammatory responses always occurred with the progression of tumor growth and invasion, cancer cells could escape the cytotoxic effects via immune tolerance. Andersson et al. found that IL‐7/IL‐7Rα‐Fc treatment induced M1 macrophages, reduced tumor burden, and prolonged survival time in mice bearing lung cancer. Depletion of endogenous CXCL9, CXCL10, or IFN‐γ abrogated IL‐7/IL‐7Rα‐Fc‐mediated antitumor activity through reduction of T cells infiltration 99. A further evaluation of genetic immunotherapy by Sharma et al. provided evidence that adenovirus vector expressing interleukin (IL)‐7 (DC‐AdIL‐7) reduced the tumor burden via the increase of IFN‐γ and IL‐12 as well as the antiangiogenic endogenous chemokines CXCL10 and CXCL9, and the decrease of immunosuppressive cytokines TGF‐β and VEGF 100. Additionally, Zhang et al. showed that the combination regime of plasmid‐borne CXCL9 gene therapy plus low‐dose cisplatin augmented the antitumor efficacy by enhancing the tumor anti‐angiogenesis and apoptosis or CTL activity. These effects were shown in Lewis lung carcinoma (LL/2c) murine models, as well as in the colon carcinoma (CT26) murine models 101.
Breast cancer
Several evidences demonstrate the close relationship between CXCL9 and medicine in BC. The cyclooxygenase‐2 (COX‐2) and its pro‐inflammatory products, prostaglandin E2 (PGE2), are strongly implicated in a range of human cancers including BC in a lymphocyte‐dependent manner 102. Markosyan et al. found that compared to wild‐type mice, ErbB2‐transgenic mice, deficient in mammary epithelial cells COX‐2, showed enhanced immune surveillance by recruiting more CD4+ Th cells and CD8+ CTL, which were coincident with intratumoral CXCL9 influx, a key T‐cell chemoattractant 103. Bronger et al. discovered that there existed inverse correlation between endogenous CXCL9 concentration and COX overexpression in BC tissues. CXCL9 and CXCL10 could be inhibited by PGE2, and induced by unselective COX inhibitors. However, the expected increase of CXCL9 was only observed at low concentrations of COX‐2‐specific inhibitor celecoxib, and decreased by high concentrations 42. Specht et al. discovered that CXCL9, and intersectin 2 (ITSN2) in BC tissues were significantly associated with prolonged DFS in 70 patients with cyclophosphamide, methotrexate, and 5‐fluorouracile (CMF)‐chemotherapy. Furthermore, the results by multivariate Cox analysis showed that the CXCL9/ITSN2 or CXCL9/FLJ22028 (Hypothetical protein FLJ22028 (NM_024854)) ratios could be independent predictive factors of DFS 44. Hannesdóttir et al. showed that the lapatinib and doxorubicin enhanced the expression of Stat1‐dependent endogenous chemokines CXCL9, CXCL10, and CXCL11 (importance for attracting CD8+ T cells), and reduced tumor‐associated macrophages (TAMs), consequently augmenting the antitumor immune response 104.
Melanoma
The incidence of melanoma has increased significantly, but there are no effective treatments yet 105. Szabo et al. reported that combination of all‐transretinoic acid (ATRA) and polyinosinic:polycytidylic acid (polyI:C) in melanoma cell lines upregulated the expression level of IL‐1β, IL‐6, IFN‐β, CXCL10, CXCL9, CXCL8, and CXCL1 more than treatments with either ATRA or polyI:C separately, which was mediated by toll‐like receptor 3 and MDA5. In this study, they found CXCL9 and CXCL10 to attract activated T lymphocytes 106. Bedognetti et al. found that CXCL9, CXCL10, CXCL11, and CCL5 were all highly expressed in metastatic melanoma patients, and this was associated with responsiveness to adoptive therapy and IL‐2 treatment 60.
Head and neck cancer
IL‐12 has been known as an effective cytokine for cancer treatment. Using the SCCVII tumor model, Li et al. found that intratumoral and intramuscular injection of IL‐12 gene by electroporation upregulated CXCL9 expression by 15‐fold and 6‐fold, respectively. They also detected that CXCL9 gene injection significantly promoted the infiltration of CD4+ T cells in the tumors, not CD8+ T cells, indicating that CXCL9 played a crucial role in the IL‐12 antitumor efficacy 107. Dorsey et al. showed that intratumoral IFN‐α DNA electroporation caused 50% of tumor eradication rate and more than doubled the survival time when compared with the controls, of which upregulated CXCL9 and CXCL10 had a pivotal role through inhibiting angiogenesis 108.
Other cancers
In chronic lymphocytic leukemia, Gustafsson et al. detected that tumor‐loaded α‐type 1‐polarized dendritic cells cocktail (IL‐1β/TNF‐α/IFN‐α/IFN‐γ/poly‐I:C;αDC1) showed a better tumor therapeutic efficiency by producing more NK cell‐, NKT cell, and CD8 + T cell‐recruiting chemokines CXCL9, CXCL10, and CXCL11 in the culture supernatants, as compared with the “standard” cocktail (IL‐1β/TNF‐α/IL‐6/PGE2;PGE2DCs), indicating the key role of the three chemokines in cancer treatment 109. In renal cell carcinoma (RCC), it was reported that high‐dose IL‐2 treatment in 20 patients elevated the plasma levels of CXCR3 ligands (CXCL9, CXCL10, and CXCL11), sequentially, forming an angiostatic environment 110. In external genital carcinoma, Soong et al. found that imiquimod, a toll‐like receptor 7 agonist, induced local expression of CXCL9 and CXCL10 leading to CXCR3+ CD8+ CTL accumulation in the cervicovaginal tract, and enhanced potent antitumor efficacy in the orthotropic cervical cancer model when combined with intramuscular CRT/E7 vaccination 111. In Sarcoma, Pardee et al. identified that OX40 ligand–Fc fusion protein (OX40L‐Fc), a novel tumor necrosis factor receptor family member, promoted anti‐tumor activity in most mice by rendering the tumor microenvironment permissive to type 1 T‐cell infiltration and increase of vascular cell adhesion molecule (VCAM‐1) and CXCL9 expression, upregulated by CD31+ vascular endothelial cells 112. In cutaneous melanoma, Hong et al. discovered that in temozolomide‐treated mice CCL5, CXCL9, and CXCL10 were significantly upregulated, which was followed by T‐cell infiltration, enhanced tumor control, and this prolonged overall survival 113.
Concluding remarks
Chemokines play a divergent role in controlling the growth and metastasis of malignant tumors. Certain chemokines enhance nonspecific or specific host immunity against tumor implantation, while others could promote tumor growth, metastasis, or neovascularization in tumor tissues. So far, doubts about the role of CXCL9 still exist in tumors, even in the same type of tumor. CXCL9 could promote cancer metastasis via enhanced migration and invasion of tumor cells 74, and breaking of the endothelial cells monolayer 87. However, as a tumor suppressor, it mainly recruited tumor‐infiltrating CD8+ T cells and NK cells 62, and inhibited tumor angiogenesis 36. However, in the section discussing “CXCL9 in cancer therapy” in this review, all the research conducted so far has suggested that high expression level of CXCL9 might be an important target for anti‐cancer therapies. In addition, CXCL9 has been identified as a candidate biomarker in breast cancer 80 and nasopharyngeal carcinoma 91. Therefore, a joint effort among researchers could provide a pave the path to further understand the role of CXCL9 in therapeutic purposes. The complex role of CXCL9 in tumor might be due to the following reasons: First, CXCL9 plays an important role in tumor immunity. CXCL9 could recruit not only CTL, inhibiting tumor development, but also other host immune cells, such as regulatory T cells (Tregs), tumor‐associated macrophages, and MDSC, which mediate immune tolerance in tumors 42, 43, 46, 47. Role of CXCL9 might be dependent on the cancer immune stage, as discussed by Cancer Immunoediting theory that immunity efficacy was different at different stages 114. Second, the contradictory role of CXCL9 might be associated with its receptor's splice variants CXCR3‐A and CXCR3‐B, as they always showed a counteracting role in tumor progression. CXCL9/CXCR3‐A could promote tumor migration and invasion via PI3K 33, MAPK 74, pathways and so on, but CXCL9/CXCR3‐B could inhibit endothelial cells proliferation 115 and tumor angiogenesis 36, which might be mediated by VEGF/VEGFR2 (KDR) and its downstream phospholipase Cγ, p‐JNK, and p‐ERK 24. Lastly, the different functions of CXCL9 were closely related with the many cell types (stated above) secreting it, and its concentration in the tumor microenvironment. CXCL9 will draw more researchers’ interests and attention in the future because of its contradictory and key effects on tumor initiation and development. Nevertheless, a more detailed characterization and mechanism of the role of CXCL9 in tumor biology is desperately required, as it may improve cancer treatment and possibly lead to clinical applications in cancer prognosis, diagnosis, and therapy.
Conflict of Interest
None declared.
Cancer Medicine 2016; 5(11):3246–3259
References
- 1. Klemm, F. , and Joyce J. A.. 2015. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 25:198–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Muller, M. , Carter S., Hofer M. J., and Campbell I. L.. 2010. Review: the chemokine receptor CXCR3 and its ligands CXCL9, CXCL10 and CXCL11 in neuroimmunity–a tale of conflict and conundrum. Neuropathol. Appl. Neurobiol. 36:368–387. [DOI] [PubMed] [Google Scholar]
- 3. Smit, M. J. , Verdijk P., van der Raaij‐Helmer E. M., Navis M., Hensbergen P. J., Leurs R., et al. 2003. CXCR3‐mediated chemotaxis of human T cells is regulated by a Gi‐ and phospholipase C‐dependent pathway and not via activation of MEK/p44/p42 MAPK nor Akt/PI‐3 kinase. Blood 102:1959–1965. [DOI] [PubMed] [Google Scholar]
- 4. Muthuswamy, R. , Urban J., Lee J. J., Reinhart T. A., Bartlett D., and Kalinski P.. 2008. Ability of mature dendritic cells to interact with regulatory T cells is imprinted during maturation. Cancer Res. 68:5972–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ikeda, A. , Aoki N., Kido M., Iwamoto S., Nishiura H., Maruoka R., et al. 2014. Progression of autoimmune hepatitis is mediated by IL‐18‐producing dendritic cells and hepatic CXCL9 expression in mice. Hepatology 60:224–236. [DOI] [PubMed] [Google Scholar]
- 6. Tworek, D. , Kuna P., Mlynarski W., Gorski P., Pietras T., and Antczak A.. 2013. MIG (CXCL9), IP‐10 (CXCL10) and I‐TAC (CXCL11) concentrations after nasal allergen challenge in patients with allergic rhinitis. Arc. Med. Sci. 9:849–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holt, A. P. , Haughton E. L., Lalor P. F., Filer A., Buckley C. D., and Adams D. H.. 2009. Liver myofibroblasts regulate infiltration and positioning of lymphocytes in human liver. Gastroenterology 136:705–714. [DOI] [PubMed] [Google Scholar]
- 8. Antonelli, A. , Ferrari S. M., Fallahi P., Frascerra S., Santini E., Franceschini S. S., et al. 2009. Monokine induced by interferon gamma (IFNgamma) (CXCL9) and IFNgamma inducible T‐cell alpha‐chemoattractant (CXCL11) involvement in Graves’ disease and ophthalmopathy: modulation by peroxisome proliferator‐activated receptor‐gamma agonists. J. Clin. Endocrinol. Metabol. 94:1803–1809. [DOI] [PubMed] [Google Scholar]
- 9. Vandercappellen, J. , Van Damme J., and Struyf S.. 2008. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 267:226–244. [DOI] [PubMed] [Google Scholar]
- 10. Hiroi, M. , and Ohmori Y.. 2003. The transcriptional coactivator CREB‐binding protein cooperates with STAT1 and NF‐kappa B for synergistic transcriptional activation of the CXC ligand 9/monokine induced by interferon‐gamma gene. J. Biol. Chem. 278:651–660. [DOI] [PubMed] [Google Scholar]
- 11. Staab, J. , Herrmann‐Lingen C., and Meyer T.. 2013. Clinically relevant dimer interface mutants of STAT1 transcription factor exhibit differential gene expression. PLoS ONE 8:e69903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chung, E. Y. , Kim B. H., Hong J. T., Lee C. K., Ahn B., Nam S. Y., et al. 2011. Resveratrol down‐regulates interferon‐gamma‐inducible inflammatory genes in macrophages: molecular mechanism via decreased STAT‐1 activation. J. Nutr. Biochem. 22:902–909. [DOI] [PubMed] [Google Scholar]
- 13. Chung, E. Y. , Roh E., Kwak J. A., Lee H. S., Lee S. H., Lee C. K., et al. 2010. alpha‐Viniferin suppresses the signal transducer and activation of transcription‐1 (STAT‐1)‐inducible inflammatory genes in interferon‐gamma‐stimulated macrophages. J. Pharmacol. Sci. 112:405–414. [DOI] [PubMed] [Google Scholar]
- 14. Jauregui, C. E. , Wang Q., Wright C. J., Takeuchi H., Uriarte S. M., and Lamont R. J.. 2013. Suppression of T‐cell chemokines by Porphyromonas gingivalis. Infect. Immun. 81:2288–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim, W. H. , Hong F., Radaeva S., Jaruga B., Fan S., and Gao B.. 2003. STAT1 plays an essential role in LPS/D‐galactosamine‐induced liver apoptosis and injury. Am. J. Physiol. Gastrointest. Liver Physiol. 285:G761–G768. [DOI] [PubMed] [Google Scholar]
- 16. Mariman, R. , Tielen F., Koning F., and Nagelkerken L.. 2014. The probiotic mixture VSL#3 dampens LPS‐induced chemokine expression in human dendritic cells by inhibition of STAT‐1 phosphorylation. PLoS ONE 9:e115676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Basset, L. , Chevalier S., Danger Y., Arshad M. I., Piquet‐Pellorce C., Gascan H., et al. 2015. Interleukin‐27 and IFNgamma regulate the expression of CXCL9, CXCL10, and CXCL11 in hepatitis. J. Mol. Med. 93:1355–1367. [DOI] [PubMed] [Google Scholar]
- 18. Xia, L. M. , Huang W. J., Wu J. G., Yang Y. B., Zhang Q., Zhou Z. Z., et al. 2009. HBx protein induces expression of MIG and increases migration of leukocytes through activation of NF‐kappaB. Virology 385:335–342. [DOI] [PubMed] [Google Scholar]
- 19. Ellis, S. L. , Gysbers V., Manders P. M., Li W., Hofer M. J., Muller M., et al. 2010. The cell‐specific induction of CXC chemokine ligand 9 mediated by IFN‐gamma in microglia of the central nervous system is determined by the myeloid transcription factor PU.1. J. Immunol. 185:1864–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kanno, Y. , Levi B. Z., Tamura T., and Ozato K.. 2005. Immune cell‐specific amplification of interferon signaling by the IRF‐4/8‐PU.1 complex. J. Interferon Cytokine Res. 25:770–779. [DOI] [PubMed] [Google Scholar]
- 21. Uranishi, M. , Iida S., Sanda T., Ishida T., Tajima E., Ito M., et al. 2005. Multiple myeloma oncogene 1 (MUM1)/interferon regulatory factor 4 (IRF4) upregulates monokine induced by interferon‐gamma (MIG) gene expression in B‐cell malignancy. Leukemia 19:1471–1478. [DOI] [PubMed] [Google Scholar]
- 22. Caso, G. , Barry C., and Patejunas G.. 2009. Dysregulation of CXCL9 and reduced tumor growth in Egr‐1 deficient mice. J. Hematol. Oncol. 2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wasmuth, H. E. , Lammert F., Zaldivar M. M., Weiskirchen R., Hellerbrand C., Scholten D., et al. 2009. Antifibrotic effects of CXCL9 and its receptor CXCR3 in livers of mice and humans. Gastroenterology 137:309–319. , 319 e301‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sahin, H. , Borkham‐Kamphorst E., Kuppe C., Zaldivar M. M., Grouls C., Al‐samman M., et al. 2012. Chemokine Cxcl9 attenuates liver fibrosis‐associated angiogenesis in mice. Hepatology 55:1610–1619. [DOI] [PubMed] [Google Scholar]
- 25. Hasenfuss, S. C. , Bakiri L., Thomsen M. K., Hamacher R., and Wagner E. F.. 2014. Activator Protein 1 transcription factor Fos‐related antigen 1 (Fra‐1) is dispensable for murine liver fibrosis, but modulates xenobiotic metabolism. Hepatology 59:261–273. [DOI] [PubMed] [Google Scholar]
- 26. Billottet, C. , Quemener C., and Bikfalvi A.. 2013. CXCR3, a double‐edged sword in tumor progression and angiogenesis. Biochim. Biophys. Acta 1836:287–295. [DOI] [PubMed] [Google Scholar]
- 27. Loetscher, M. , Gerber B., Loetscher P., Jones S. A., Piali L., Clark‐Lewis I., et al. 1996. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T‐lymphocytes. J. Exp. Med. 184:963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Van Raemdonck, K. , Van den Steen P. E., Liekens S., Van Damme J., and Struyf S.. 2015. CXCR3 ligands in disease and therapy. Cytokine Growth Factor Rev. 26:311–327. [DOI] [PubMed] [Google Scholar]
- 29. Lu, B. , Humbles A., Bota D., Gerard C., Moser B., Soler D., et al. 1999. Structure and function of the murine chemokine receptor CXCR3. Eur. J. Immunol. 29:3804–3812. [DOI] [PubMed] [Google Scholar]
- 30. Zhu, G. , Yan H. H., Pang Y., Jian J., Achyut B. R., Liang X., et al. 2015. CXCR3 as a molecular target in breast cancer metastasis: inhibition of tumor cell migration and promotion of host anti‐tumor immunity. Oncotarget 6:43408–43419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Groom, J. R. , and Luster A. D.. 2011. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol. Cell Biol. 89:207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wadwa, M. , Klopfleisch R., Adamczyk A., Frede A., Pastille E., Mahnke K., et al. 2016. IL‐10 downregulates CXCR3 expression on Th1 cells and interferes with their migration to intestinal inflammatory sites. Mucosal Immunol. 9:1263–1277. [DOI] [PubMed] [Google Scholar]
- 33. Bonacchi, A. , Romagnani P., Romanelli R. G., Efsen E., Annunziato F., Lasagni L., et al. 2001. Signal transduction by the chemokine receptor CXCR3: activation of Ras/ERK, Src, and phosphatidylinositol 3‐kinase/Akt controls cell migration and proliferation in human vascular pericytes. J. Biol. Chem. 276:9945–9954. [DOI] [PubMed] [Google Scholar]
- 34. Shahabuddin, S. , Ji R., Wang P., Brailoiu E., Dun N., Yang Y., et al. 2006. CXCR3 chemokine receptor‐induced chemotaxis in human airway epithelial cells: role of p38 MAPK and PI3K signaling pathways. Am. J. Physiol. Cell Physiol. 291:C34–C39. [DOI] [PubMed] [Google Scholar]
- 35. Martins, V. L. , Vyas J. J., Chen M., Purdie K., Mein C. A., South A. P., et al. 2009. Increased invasive behaviour in cutaneous squamous cell carcinoma with loss of basement‐membrane type VII collagen. J. Cell Sci. 122:1788–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Addison, C. L. , Arenberg D. A., Morris S. B., Xue Y. Y., Burdick M. D., Mulligan M. S., et al. 2000. The CXC chemokine, monokine induced by interferon‐gamma, inhibits non‐small cell lung carcinoma tumor growth and metastasis. Hum. Gene Ther. 11:247–261. [DOI] [PubMed] [Google Scholar]
- 37. Metodieva, S. N. , Nikolova D. N., Cherneva R. V., Dimova I. I., Petrov D. B., and Toncheva D. I.. 2011. Expression analysis of angiogenesis‐related genes in Bulgarian patients with early‐stage non‐small cell lung cancer. Tumori 97:86–94. [DOI] [PubMed] [Google Scholar]
- 38. Kowalczuk, O. , Burzykowski T., Niklinska W. E., Kozlowski M., Chyczewski L., and Niklinski J.. 2014. CXCL5 as a potential novel prognostic factor in early stage non‐small cell lung cancer: results of a study of expression levels of 23 genes. Tumour Biol. 35:4619–4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Andersson, A. , Yang S. C., Huang M., Zhu L., Kar U. K., Batra R. K., et al. 2009. IL‐7 promotes CXCR3 ligand‐dependent T cell antitumor reactivity in lung cancer. J. Immunol. 182:6951–6958. [DOI] [PubMed] [Google Scholar]
- 40. Yang, S. C. , Batra R. K., Hillinger S., Reckamp K. L., Strieter R. M., Dubinett S. M., et al. 2006. Intrapulmonary administration of CCL21 gene‐modified dendritic cells reduces tumor burden in spontaneous murine bronchoalveolar cell carcinoma. Cancer Res. 66:3205–3213. [DOI] [PubMed] [Google Scholar]
- 41. Srivastava, M. K. , Zhu L., Harris‐White M., Kar U. K., Huang M., Johnson M. F., et al. 2012. Myeloid suppressor cell depletion augments antitumor activity in lung cancer. PLoS ONE 7:e40677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bronger, H. , Kraeft S., Schwarz‐Boeger U., Cerny C., Stockel A., Avril S., et al. 2012. Modulation of CXCR3 ligand secretion by prostaglandin E2 and cyclooxygenase inhibitors in human breast cancer. Breast Cancer Res. 14:R30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Denkert, C. , Loibl S., Noske A., Roller M., Muller B. M., Komor M., et al. 2010. Tumor‐associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J. Clin. Oncol. 28:105–113. [DOI] [PubMed] [Google Scholar]
- 44. Specht, K. , Harbeck N., Smida J., Annecke K., Reich U., Naehrig J., et al. 2009. Expression profiling identifies genes that predict recurrence of breast cancer after adjuvant CMF‐based chemotherapy. Breast Cancer Res. Treat. 118:45–56. [DOI] [PubMed] [Google Scholar]
- 45. Thakur, A. , Schalk D., Sarkar S. H., Al‐Khadimi Z., Sarkar F. H., and Lum L. G.. 2012. A Th1 cytokine‐enriched microenvironment enhances tumor killing by activated T cells armed with bispecific antibodies and inhibits the development of myeloid‐derived suppressor cells. Cancer Immunol. Immunother. 61:497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Walser, T. C. , Ma X., Kundu N., Dorsey R., Goloubeva O., and Fulton A. M.. 2007. Immune‐mediated modulation of breast cancer growth and metastasis by the chemokine Mig (CXCL9) in a murine model. J. Immunother. 30:490–498. [DOI] [PubMed] [Google Scholar]
- 47. Fulton, A. , Miller F., Weise A., and Wei W. Z.. 2006. Prospects of controlling breast cancer metastasis by immune intervention. Breast Dis. 26:115–127. [DOI] [PubMed] [Google Scholar]
- 48. Tensen, C. P. , Vermeer M. H., van der Stoop P. M., van Beek P., Scheper R. J., Boorsma D. M., et al. 1998. Epidermal interferon‐gamma inducible protein‐10 (IP‐10) and monokine induced by gamma‐interferon (Mig) but not IL‐8 mRNA expression is associated with epidermotropism in cutaneous T cell lymphomas. J. Invest. Dermatol. 111:222–226. [DOI] [PubMed] [Google Scholar]
- 49. Miyagaki, T. , Sugaya M., Suga H., Morimura S., Ohmatsu H., Fujita H., et al. 2012. Low herpesvirus entry mediator (HVEM) expression on dermal fibroblasts contributes to a Th2‐dominant microenvironment in advanced cutaneous T‐cell lymphoma. J. Invest. Dermatol. 132:1280–1289. [DOI] [PubMed] [Google Scholar]
- 50. Przewoznik, M. , Homberg N., Naujoks M., Potzl J., Munchmeier N., Brenner C. D., et al. 2012. Recruitment of natural killer cells in advanced stages of endogenously arising B‐cell lymphoma: implications for therapeutic cell transfer. J. Immunother. 35:217–222. [DOI] [PubMed] [Google Scholar]
- 51. Kanegane, C. , Sgadari C., Kanegane H., Teruya‐Feldstein J., Yao L., Gupta G., et al. 1998. Contribution of the CXC chemokines IP‐10 and Mig to the antitumor effects of IL‐12. J. Leukoc. Biol. 64:384–392. [DOI] [PubMed] [Google Scholar]
- 52. Haabeth, O. A. , Lorvik K. B., Hammarstrom C., Donaldson I. M., Haraldsen G., Bogen B., et al. 2011. Inflammation driven by tumour‐specific Th1 cells protects against B‐cell cancer. Nat. Commun. 2:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mlecnik, B. , Tosolini M., Charoentong P., Kirilovsky A., Bindea G., Berger A., et al. 2010. Biomolecular network reconstruction identifies T‐cell homing factors associated with survival in colorectal cancer. Gastroenterology 138:1429–1440. [DOI] [PubMed] [Google Scholar]
- 54. Wu, Z. , Huang X., Han X., Li Z., Zhu Q., Yan J., et al. 2016. The chemokine CXCL9 expression is associated with better prognosis for colorectal carcinoma patients. Biomed. Pharmacother. 78:8–13. [DOI] [PubMed] [Google Scholar]
- 55. Chaput, N. , Svrcek M., Auperin A., Locher C., Drusch F., Malka D., et al. 2013. Tumour‐infiltrating CD68 + and CD57 + cells predict patient outcome in stage II‐III colorectal cancer. Br. J. Cancer 109:1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Watanabe, T. , Kobunai T., Yamamoto Y., Ikeuchi H., Matsuda K., Ishihara S., et al. 2011. Predicting ulcerative colitis‐associated colorectal cancer using reverse‐transcription polymerase chain reaction analysis. Clin. Colorectal Cancer 10:134–141. [DOI] [PubMed] [Google Scholar]
- 57. Akeus, P. , Langenes V., Kristensen J., von Mentzer A., Sparwasser T., Raghavan S., et al. 2015. Treg‐cell depletion promotes chemokine production and accumulation of CXCR3(+) conventional T cells in intestinal tumors. Eur. J. Immunol. 45:1654–1666. [DOI] [PubMed] [Google Scholar]
- 58. Clemente, C. G. , Mihm M. C. Jr, Bufalino R., Zurrida S., Collini P., and Cascinelli N.. 1996. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer 77:1303–1310. [DOI] [PubMed] [Google Scholar]
- 59. Erdag, G. , Schaefer J. T., Smolkin M. E., Deacon D. H., Shea S. M., Dengel L. T., et al. 2012. Immunotype and immunohistologic characteristics of tumor‐infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 72:1070–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bedognetti, D. , Spivey T. L., Zhao Y., Uccellini L., Tomei S., Dudley M. E., et al. 2013. CXCR3/CCR5 pathways in metastatic melanoma patients treated with adoptive therapy and interleukin‐2. Br. J. Cancer 109:2412–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Harlin, H. , Meng Y., Peterson A. C., Zha Y., Tretiakova M., Slingluff C., et al. 2009. Chemokine expression in melanoma metastases associated with CD8+ T‐cell recruitment. Cancer Res. 69:3077–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Clancy‐Thompson, E. , Perekslis T. J., Croteau W., Alexander M. P., Chabanet T. B., Turk M. J., et al. 2015. Melanoma induces, and adenosine suppresses, CXCR3‐Cognate chemokine production and T‐cell infiltration of lungs bearing metastatic‐like disease. Cancer Immunol. Res. 3:956–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Deng, T. , Kuang Y., Wang L., Li J., Wang Z., and Fei J.. 2009. An essential role for DNA methyltransferase 3a in melanoma tumorigenesis. Biochem. Biophys. Res. Commun. 387:611–616. [DOI] [PubMed] [Google Scholar]
- 64. Pan, J. , Burdick M. D., Belperio J. A., Xue Y. Y., Gerard C., Sharma S., et al. 2006. CXCR3/CXCR3 ligand biological axis impairs RENCA tumor growth by a mechanism of immunoangiostasis. J. Immunol. 176:1456–1464. [DOI] [PubMed] [Google Scholar]
- 65. Tannenbaum, C. S. , Tubbs R., Armstrong D., Finke J. H., Bukowski R. M., and Hamilton T. A.. 1998. The CXC chemokines IP‐10 and Mig are necessary for IL‐12‐mediated regression of the mouse RENCA tumor. J. Immunol. 161:927–932. [PubMed] [Google Scholar]
- 66. Kryczek, I. , Banerjee M., Cheng P., Vatan L., Szeliga W., Wei S., et al. 2009. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood 114:1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wong, J. L. , Berk E., Edwards R. P., and Kalinski P.. 2013. IL‐18‐primed helper NK cells collaborate with dendritic cells to promote recruitment of effector CD8+ T cells to the tumor microenvironment. Cancer Res. 73:4653–4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ohtani, H. , Jin Z., Takegawa S., Nakayama T., and Yoshie O.. 2009. Abundant expression of CXCL9 (MIG) by stromal cells that include dendritic cells and accumulation of CXCR3+ T cells in lymphocyte‐rich gastric carcinoma. J. Pathol. 217:21–31. [DOI] [PubMed] [Google Scholar]
- 69. Eck, M. , Schmausser B., Scheller K., Brandlein S., and Muller‐Hermelink H. K.. 2003. Pleiotropic effects of CXC chemokines in gastric carcinoma: differences in CXCL8 and CXCL1 expression between diffuse and intestinal types of gastric carcinoma. Clin. Exp. Immunol. 134:508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Berghuis, D. , Santos S. J., Baelde H. J., Taminiau A. H., Egeler R. M., Schilham M. W., et al. 2011. Pro‐inflammatory chemokine‐chemokine receptor interactions within the Ewing sarcoma microenvironment determine CD8(+) T‐lymphocyte infiltration and affect tumour progression. J. Pathol. 223:347–357. [DOI] [PubMed] [Google Scholar]
- 71. Wenzel, J. , Tomiuk S., Zahn S., Kusters D., Vahsen A., Wiechert A., et al. 2008. Transcriptional profiling identifies an interferon‐associated host immune response in invasive squamous cell carcinoma of the skin. Int. J. Cancer 123:2605–2615. [DOI] [PubMed] [Google Scholar]
- 72. Gorbachev, A. V. , Kobayashi H., Kudo D., Tannenbaum C. S., Finke J. H., Shu S., et al. 2007. CXC chemokine ligand 9/monokine induced by IFN‐gamma production by tumor cells is critical for T cell‐mediated suppression of cutaneous tumors. J. Immunol. 178:2278–2286. [DOI] [PubMed] [Google Scholar]
- 73. El‐Serag, H. B. 2011. Hepatocellular carcinoma. N. Engl. J. Med. 365:1118–1127. [DOI] [PubMed] [Google Scholar]
- 74. Ding, Q. , Xia Y., Ding S., Lu P., Sun L., and Liu M.. 2016. An alternatively spliced variant of CXCR3 mediates the metastasis of CD133+ liver cancer cells induced by CXCL9. Oncotarget 7:14405–14414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lan, X. , Xiao F., Ding Q., Liu J., Liu J., Li J., et al. 2014. The effect of CXCL9 on the invasion ability of hepatocellular carcinoma through up‐regulation of PREX2. J. Mol. Histol. 45:689–696. [DOI] [PubMed] [Google Scholar]
- 76. Liu, R. X. , Wei Y., Zeng Q. H., Chan K. W., Xiao X., Zhao X. Y., et al. 2015. Chemokine (C‐X‐C motif) receptor 3‐positive B cells link interleukin‐17 inflammation to protumorigenic macrophage polarization in human hepatocellular carcinoma. Hepatology 62:1779–1790. [DOI] [PubMed] [Google Scholar]
- 77. Shiels, M. S. , Katki H. A., Hildesheim A., Pfeiffer R. M., Engels E. A., Williams M., et al. 2015. Circulating inflammation markers, risk of lung cancer, and utility for risk stratification. J. Natl Cancer Inst. 107: pii: djv199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nakanishi, T. , Imaizumi K., Hasegawa Y., Kawabe T., Hashimoto N., Okamoto M., et al. 2006. Expression of macrophage‐derived chemokine (MDC)/CCL22 in human lung cancer. Cancer Immunol. Immunother. 55:1320–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ejaeidi, A. A. , Craft B. S., Puneky L. V., Lewis R. E., and Cruse J. M.. 2015. Hormone receptor‐independent CXCL10 production is associated with the regulation of cellular factors linked to breast cancer progression and metastasis. Exp. Mol. Pathol. 99:163–172. [DOI] [PubMed] [Google Scholar]
- 80. Ruiz‐Garcia, E. , Scott V., Machavoine C., Bidart J. M., Lacroix L., Delaloge S., et al. 2010. Gene expression profiling identifies fibronectin 1 and CXCL9 as candidate biomarkers for breast cancer screening. Br. J. Cancer 102:462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mir, M. A. , Maurer M. J., Ziesmer S. C., Slager S. L., Habermann T., Macon W. R., et al. 2015. Elevated serum levels of IL‐2R, IL‐1RA, and CXCL9 are associated with a poor prognosis in follicular lymphoma. Blood 125:992–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Venetz, D. , Ponzoni M., Schiraldi M., Ferreri A. J., Bertoni F., Doglioni C., et al. 2010. Perivascular expression of CXCL9 and CXCL12 in primary central nervous system lymphoma: T‐cell infiltration and positioning of malignant B cells. Int. J. Cancer 127:2300–2312. [DOI] [PubMed] [Google Scholar]
- 83. Maruoka, H. , Inoue D., Takiuchi Y., Nagano S., Arima H., Tabata S., et al. 2014. IP‐10/CXCL10 and MIG/CXCL9 as novel markers for the diagnosis of lymphoma‐associated hemophagocytic syndrome. Ann. Hematol. 93:393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hartmann, S. , Tousseyn T., Doring C., Fluchter P., Hackstein H., Herreman A., et al. 2013. Macrophages in T cell/histiocyte rich large B cell lymphoma strongly express metal‐binding proteins and show a bi‐activated phenotype. Int. J. Cancer 133:2609–2618. [DOI] [PubMed] [Google Scholar]
- 85. Yu, J. B. , Zhang Y. C., Yang Q. P., Wang X. L., Tang Y., Zhao S., et al. 2013. Invasion‐associated genes identified by gene expression profiling in extranodal natural killer/T‐cell lymphoma, nasal type. Leuk. Lymphoma 54:90–98. [DOI] [PubMed] [Google Scholar]
- 86. Birgersdotter, A. , Baumforth K. R., Porwit A., Sjoberg J., Wei W., Bjorkholm M., et al. 2009. Inflammation and tissue repair markers distinguish the nodular sclerosis and mixed cellularity subtypes of classical Hodgkin's lymphoma. Br. J. Cancer 101:1393–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Amatschek, S. , Lucas R., Eger A., Pflueger M., Hundsberger H., Knoll C., et al. 2011. CXCL9 induces chemotaxis, chemorepulsion and endothelial barrier disruption through CXCR3‐mediated activation of melanoma cells. Br. J. Cancer 104:469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kawada, K. , Sonoshita M., Sakashita H., Takabayashi A., Yamaoka Y., Manabe T., et al. 2004. Pivotal role of CXCR3 in melanoma cell metastasis to lymph nodes. Cancer Res. 64:4010–4017. [DOI] [PubMed] [Google Scholar]
- 89. Jehs, T. , Faber C., Juel H. B., Bronkhorst I. H., Jager M. J., and Nissen M. H.. 2014. Inflammation‐induced chemokine expression in uveal melanoma cell lines stimulates monocyte chemotaxis. Invest. Ophthalmol. Vis. Sci. 55:5169–5175. [DOI] [PubMed] [Google Scholar]
- 90. Torre, L. A. , Bray F., Siegel R. L., Ferlay J., Lortet‐Tieulent J., and Jemal A.. 2015. Global cancer statistics, 2012. CA Cancer J. Clin. 65:87–108. [DOI] [PubMed] [Google Scholar]
- 91. Hsin, L. J. , Kao H. K., Chen I. H., Tsang N. M., Hsu C. L., Liu S. C., et al. 2013. Serum CXCL9 levels are associated with tumor progression and treatment outcome in patients with nasopharyngeal carcinoma. PLoS ONE 8:e80052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chang, K. P. , Wu C. C., Fang K. H., Tsai C. Y., Chang Y. L., Liu S. C., et al. 2013. Serum levels of chemokine (C‐X‐C motif) ligand 9 (CXCL9) are associated with tumor progression and treatment outcome in patients with oral cavity squamous cell carcinoma. Oral Oncol. 49:802–807. [DOI] [PubMed] [Google Scholar]
- 93. Zhi, W. , Ferris D., Sharma A., Purohit S., Santos C., He M., et al. 2014. Twelve serum proteins progressively increase with disease stage in squamous cell cervical cancer patients. Int. J. Gynecol. Cancer 24:1085–1092. [DOI] [PubMed] [Google Scholar]
- 94. Yan, X. J. , Dozmorov I., Li W., Yancopoulos S., Sison C., Centola M., et al. 2011. Identification of outcome‐correlated cytokine clusters in chronic lymphocytic leukemia. Blood 118:5201–5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hu, S. , Li L., Yeh S., Cui Y., Li X., Chang H. C., et al. 2015. Infiltrating T cells promote prostate cancer metastasis via modulation of FGF11–>miRNA‐541–>androgen receptor (AR)–>MMP9 signaling. Mol. Oncol. 9:44–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Liu, C. , Luo D., Reynolds B. A., Meher G., Katritzky A. R., Lu B., et al. 2011. Chemokine receptor CXCR3 promotes growth of glioma. Carcinogenesis 32:129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sreekanthreddy, P. , Srinivasan H., Kumar D. M., Nijaguna M. B., Sridevi S., Vrinda M., et al. 2010. Identification of potential serum biomarkers of glioblastoma: serum osteopontin levels correlate with poor prognosis. Cancer Epidemiol. Biomarkers Prev. 19:1409–1422. [DOI] [PubMed] [Google Scholar]
- 98. Wang, H. W. , Wu Y. H., Hsieh J. Y., Liang M. L., Chao M. E., Liu D. J., et al. 2010. Pediatric primary central nervous system germ cell tumors of different prognosis groups show characteristic miRNome traits and chromosome copy number variations. BMC Genom. 11:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Andersson, A. , Srivastava M. K., Harris‐White M., Huang M., Zhu L., Elashoff D., et al. 2011. Role of CXCR3 ligands in IL‐7/IL‐7R alpha‐Fc‐mediated antitumor activity in lung cancer. Clin. Cancer Res. 17:3660–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Sharma, S. , Batra R. K., Yang S. C., Hillinger S., Zhu L., Atianzar K., et al. 2003. Interleukin‐7 gene‐modified dendritic cells reduce pulmonary tumor burden in spontaneous murine bronchoalveolar cell carcinoma. Hum. Gene Ther. 14:1511–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zhang, R. , Tian L., Chen L. J., Xiao F., Hou J. M., Zhao X., et al. 2006. Combination of MIG (CXCL9) chemokine gene therapy with low‐dose cisplatin improves therapeutic efficacy against murine carcinoma. Gene Ther. 13:1263–1271. [DOI] [PubMed] [Google Scholar]
- 102. Howe, L. R. 2007. Inflammation and breast cancer. Cyclooxygenase/prostaglandin signaling and breast cancer. Breast Cancer Res. 9:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Markosyan, N. , Chen E. P., Evans R. A., Ndong V., Vonderheide R. H., and Smyth E. M.. 2013. Mammary carcinoma cell derived cyclooxygenase 2 suppresses tumor immune surveillance by enhancing intratumoral immune checkpoint activity. Breast Cancer Res. 15:R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hannesdottir, L. , Tymoszuk P., Parajuli N., Wasmer M. H., Philipp S., Daschil N., et al. 2013. Lapatinib and doxorubicin enhance the Stat1‐dependent antitumor immune response. Eur. J. Immunol. 43:2718–2729. [DOI] [PubMed] [Google Scholar]
- 105. Sharma, P. , Wagner K., Wolchok J. D., and Allison J. P.. 2011. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat. Rev. Cancer 11:805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Szabo, A. , Osman R. M., Bacskai I., Kumar B. V., Agod Z., Lanyi A., et al. 2012. Temporally designed treatment of melanoma cells by ATRA and polyI: C results in enhanced chemokine and IFNbeta secretion controlled differently by TLR3 and MDA5. Melanoma Res. 22:351–361. [DOI] [PubMed] [Google Scholar]
- 107. Li, S. , Xia X., Mellieon F. M., Liu J., and Steele S.. 2004. Candidate genes associated with tumor regression mediated by intratumoral IL‐12 electroporation gene therapy. Mol. Ther. 9:347–354. [DOI] [PubMed] [Google Scholar]
- 108. Li, S. , Xia X., Zhang X., and Suen J.. 2002. Regression of tumors by IFN‐alpha electroporation gene therapy and analysis of the responsible genes by cDNA array. Gene Ther. 9:390–397. [DOI] [PubMed] [Google Scholar]
- 109. Gustafsson, K. , Junevik K., Werlenius O., Holmgren S., Karlsson‐Parra A., and Andersson P. O.. 2011. Tumour‐loaded alpha‐type 1‐polarized dendritic cells from patients with chronic lymphocytic leukaemia produce a superior NK‐, NKT‐ and CD8 + T cell‐attracting chemokine profile. Scand. J. Immunol. 74:318–326. [DOI] [PubMed] [Google Scholar]
- 110. Reckamp, K. L. , Figlin R. A., Moldawer N., Pantuck A. J., Belldegrun A. S., Burdick M. D., et al. 2007. Expression of CXCR3 on mononuclear cells and CXCR3 ligands in patients with metastatic renal cell carcinoma in response to systemic IL‐2 therapy. J. Immunother. 30:417–424. [DOI] [PubMed] [Google Scholar]
- 111. Soong, R. S. , Song L., Trieu J., Knoff J., He L., Tsai Y. C., et al. 2014. Toll‐like receptor agonist imiquimod facilitates antigen‐specific CD8 + T‐cell accumulation in the genital tract leading to tumor control through IFNgamma. Clin. Cancer Res. 20:5456–5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Pardee, A. D. , McCurry D., Alber S., Hu P., Epstein A. L., and Storkus W. J.. 2010. A therapeutic OX40 agonist dynamically alters dendritic, endothelial, and T cell subsets within the established tumor microenvironment. Cancer Res. 70:9041–9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hong, M. , Puaux A. L., Huang C., Loumagne L., Tow C., Mackay C., et al. 2011. Chemotherapy induces intratumoral expression of chemokines in cutaneous melanoma, favoring T‐cell infiltration and tumor control. Cancer Res. 71:6997–7009. [DOI] [PubMed] [Google Scholar]
- 114. Teng, M. W. , Galon J., Fridman W. H., and Smyth M. J.. 2015. From mice to humans: developments in cancer immunoediting. J. Clin. Investig. 125:3338–3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Romagnani, P. , Annunziato F., Lasagni L., Lazzeri E., Beltrame C., Francalanci M., et al. 2001. Cell cycle‐dependent expression of CXC chemokine receptor 3 by endothelial cells mediates angiostatic activity. J. Clin. Investig. 107:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]


