Abstract
A reliable protocol has been established for in vitro propagation of Artemisia nilagirica var. nilagirica (Indian wormwood), a valuable medicinal plant from India. A highly proliferating organogenic callus was obtained on Murashige and Skoog (MS) medium supplemented with 2.5 µM IAA when nodal explants were cultured on MS medium supplemented with various growth regulators. Further, highest regeneration frequency (83.3 %) of adventitious shoots was observed, when the callus was sub-cultured on MS medium supplemented with 6-benzylaminopurine (BAP; 2.5 µM) along with 7.5 µM 2-isopentenyl adenine (2-iP). An optimal of 10.16 ± 2.24 shoots were regenerated on medium supplemented with 2.5 µM BAP + 7.5 µM 2-iP. Quarter strength MS medium supplemented with 10 µM IBA was effective for rooting of the shoots. Ex-vitro plants were normal and were established successfully. Cytological and molecular marker studies showed that regenerated plants showed genetic stability in micro-propagated plants.
Keywords: Artemisia nilagirica, Genetic fidelity, Indian wormwood, Medicinal plant, Micropropagation, Multiple shoot regeneration
Introduction
Artemisia nilagirica var. nilagirica (C.B. Clarke) pamp. is commonly known as ‘Indian Wormwood’ belongs to the family Asteraceae and is an aromatic perennial herb growing in the hilly regions of India. A. nilagirica var. nilagirica is a valuable medicinal plant, widely used in the Indian traditional medicine for the treatment of depression, diabetics, epilepsy, insomnia and stress (Walter et al. 2003). It is also used as antihelmintic, antiseptic, antispasmodic, cholagogue, digestive, expectorant, purgative and stimulant. The bioactive compounds isolated from A. nilagirica such as flavonoids, sesquiterpene lactones have been reported to have antimicrobial, cytotoxic, and insecticidal properties (Naik et al. 2014; Paneerselvam et al. 2012). Essential oils borneol, caryophyllene oxide, camphor, 2-hexane-1-ol, β-thujone, thujanol, myrtenol and lynalyl acetate, α-pinene, β-pinene, limonene, linalool, γ-gurijunene, germacrane D and farnesol have been isolated from Artemisia nilagirica (Haider et al. 2010; Sati et al. 2013) and majority of these essential oil possess medicinal and pharmacological properties (Abad et al. 2012). The plant is also used traditionally in the preparation of insecticide (Bhattacharjee 2000).
Artemisia nilagirica is a promising species for extraction of an anti-malarial drug, artemisinin (Shukla et al. 2015), and this plant has been overexploited by pharmaceutical industry in India and thus natural populations are dwindling over the years. Habitat destruction for agricultural purposes and low seed viability is a major concern for the propagation of these plants. Plant tissue culture techniques are useful for mass multiplication of threatened medicinal plants (Rout et al. 2000; Krishnan et al. 2011). Consequently, the present investigation is concerned to establish axenic cultures of medicinal plant Artemisia nilagirica var. nilagirica for the in vitro propagation of this plant using nodal explants and determining the genetic fidelity of the regenerated plants.
Materials and methods
Plant material and explants establishment
Nodal explants (1.5–2 cm) were obtained from young branches of the stock plants of Artemisia nilagirica var. nilagirica (C.B. Clarke) pamp. cultivated in the Botanical Garden, Karnatak University, India. Nodal explants were washed thoroughly under running tap water using detergent 10 % (v/v) Tween-20 (Sigma, USA) for 5 min, then surface sterilized using 1 % (w/v) bavistin for 3 min, followed by 0.1 % (w/v) aqueous mercuric chloride (HgCl2) (Sigma, USA) for 2–3 min followed 95 % alcohol for 20–30 s and then 2–3 washes of sterile distilled water laminar flow chamber. The cut edges of the explants were trimmed and cultured on Murashige and Skoog (1962) medium supplemented with 3 % (w/v) sucrose and 2.5, 5.0, 7.5 and 10 µM Indole-3-acetic acid (IAA), Indole-3-butyric acid (IBA), 1-napthalene acetic acid (NAA) and 2,4-dichlorophenoxy acetic acid (2,4-D) (Himedia, India) individually. The pH of the media were adjusted to 5.8 with 1 N HCl or 1 N NaOH before gelling with 0.8 % agar and autoclaving. The medium (15 ml each) was dispensed into culture tubes (Himedia, India), plugged tightly with non-absorbent cotton and autoclaved at 15 psi and 121 °C for 15 min. The explants were cultured on the medium vertically and the cultures were incubated at 25 ± 2 °C under 16/8 (light/dark) photoperiod of 50 µmol m−2 s−1 irradiance with cool florescent tubes and with 60 % relative humidity. All the subsequent subcultures were carried out at 4 week intervals.
Multiple shoot regeneration from callus
An organogenic callus from nodal explants obtained on MS medium supplemented with 2.5 µM IAA was used for the regeneration of shoots. To evaluate the growth potential of this callus for shoot induction, the callus was excised from the explants, divided into small pieces (0.5 × 0.5 cm). The excised callus was transferred into MS medium containing 2.5 µM BAP in combination with Kn, 2-iP and TDZ (2.5, 5.0, 7.5 and 10 µM) and on MS basal medium. For each treatment 6 replicates were maintained for 6 weeks.
Rooting of shoots
Individual shoots (2–3 cm in length) were cultured onto half and quarter strength MS medium containing 3 % (w/v) sucrose and 0.8 % (w/v) agar, supplemented with different concentrations of IAA, IBA and NAA (2.5, 5.0, 7.5 and 10 µM) for root induction. One set was maintained as control without addition of any auxins.
Acclimatization of regenerated plants
Plants (3–5 cm in height) with 6–8 expanded leaves and well developed roots were transplanted to plastic cups containing cocopeat and perlite (1:1) and cocopeat and vermiculite (1:1) and a mixture of vermiculite, cocopeat and perlite (1:1:1) and were maintained in growth chambers wherein temperature was 24 ± 2 °C, relative humidity 80 %, irradiance of 100 µmol m−2 s−1 for 16-h photoperiod. The plants were irrigated with Hoagland solution twice in a week. After 4 weeks plants were transferred to pots containing soil, sand and farmyard manure (1:1:1) and maintained in greenhouse.
Data collection and statistical analysis
Randomised block design was used for setting up the experiments and each experiment was repeated 2 times with 6 replicates each. Percentage of responding explants, number of shoots per explant, shoot length, number of roots per shoot, root length, survival percentage in different potting mixture, plant height after hardening were observed and recorded.
Thirty regenerated plants from tissue culture were selected randomly and their qualitative and quantitative morphological traits were studied in detail. Morphological characters like vegetative and inflorescence were studied and recorded accordingly. The analysis of variance (ANOVA) was carried out to detect the difference between the treatment means and was compared using Duncan’s Multiple Range Test (DMRT) at 5 % probability level using SPSS software version 16.0.
Genetic fidelity analysis
Cytological analysis
Root tips from actively growing mother plant and 10 randomly selected 5 month old micropropagated plants were pre-treated with 0.05 % Colchicine (Himedia, India) solution for 3 h at room temperature. The root tips were then fixed in Carnoy’s fluid (absolute ethanol + glacial acetic acid; 3:1 v/v) for 24 h. The apices were then immersed in aceto-orceine: HCl mixture (9:1) and squash preparations were made for cytological analysis. The cytological images were taken using Leica DM750 microscope equipped with Leica EC3 camera (Leica Microsystems, Switzerland) using software LAS EZ (version 2.0) software.
DNA extraction and ISSR analysis
Isolation of genomic DNA from fresh leaves of mother and micropropagated plants of Artemisia nilagirica var. nilagirica were carried out using CTAB method (Murray and Thompson 1980). The DNA samples were initially screened for amplification using 15 inter-simple sequence repeats (ISSR) primers (UBC series, University of British Columbia, Canada). The primers which were able to amplify the genomic DNA were used for screening to analyze genetic variation between micro propagated plants with their mother plant. The 20 μl reaction mixture contained 20 ng genomic DNA, 10X Taq buffer with 15 mM MgCl2, 1 mM dNTPs, 5 pmol of primer and 1 U Taq polymerase. The ISSR-PCR conditions were set to 94 °C for 3 min followed by 45 cycles each of 94 °C for 45 s, 45 °C for 30 s and 72 °C for 45 s and followed by final extension of 72 °C for 7 min in a thermocycler (Eppendorf, Hamburg, Germany). Amplification products were separated using 1.5 % agarose gel prepared in 0.5 x TAE buffer, stained with ethidium bromide and visualized under UV gel documentation system.
Results and discussion
Establishment of cultures and callus induction
Nodal explants cultured on MS medium supplemented with all the auxins used at all concentrations exhibited callus induction at the lower cut end of the explants, which was in touch with medium after 2–4 weeks in culture. The frequency of callus induction was optimum (90 %) with the explants cultured on MS medium supplemented with 2.5 µM of IAA (Fig. 1a) and growth of the callus was vigorous. Callus was also induced from the nodal explants on medium supplemented with 2.5, 5.0, 7.5 and 10 µM NAA, 2, 4-D and IBA, however, growth of the callus was not vigorous. The callogenetic response is also reported in various other species of Artemisia, Nin et al. (1996) reported effective caulogenesis from shoot tip explants in Artemisia absinthium on medium supplemented with BAP and NAA. In Artemisia pallens, a semiorganized green callus with shoot buds on medium containing BAP and IAA and an unorganised callus on medium containing BAP and 2, 4-D from germinated seedlings has been reported by Benjamin et al. (1990). Nodal explants cultured on MS basal medium remained green for 3 weeks, and then turned brown and did not show any kind of morphogenic response. Similarly Nin et al. (1996) observed death of shoot tip explants on growth regulator free medium within few days of culturing in Artemisia absinthium.
Fig. 1.
Effect on plant growth regulators on micropropagation of Artemisia nilagirica var. nilagirica a Callus initiation from base of nodal explants (Bar = 0.3 cm); b Shoot initiation from callus (Bar = 0.5 cm); c Multiple shoot proliferation from callus (Bar = 0.9 cm); d Elongated shoot shoots with roots (Bar = 1.25 cm)
Induction of multiple shoots from callus
The organogenic ability of the callus was observed by the application of 2.5 µM BAP in combination with Kn, 2-iP and TDZ (2.5, 5.0, 7.5 and 10 µM). After 2 weeks of culture 2.5 µM BAP in combination with Kn, 2-iP and TDZ (2.5, 5.0, 7.5 and 10 µM) showed development of multiple shoots on the surface of callus. Callus cultured on cytokinin supplemented medium initially turned green and developed shoot buds on the surface of callus within 2 weeks of culture. These shoot buds developed into shoots in another 2 weeks of culture (Fig. 1b); entire callus exhibited shoot proliferation (more than 100 shoots) by the end of 6 weeks (Fig. 1c). Medium supplemented with 2.5 µM BAP + 7.5 µM 2-iP showed highest regeneration frequency (83.3 %) with highest number of shoots (10.16 ± 2.24; Table 1). Effect of different cytokinins on multiple shoot regeneration from callus cultures was previously reported in Jatropha curcas (Maharana et al. 2012) and Notahapodytes nimmoniana (Dandin and Murthy 2012a). BAP has been described as a beneficial growth regulator for induction of shoots in other Artemisa species (Sujatha and Ranjitha Kumari 2007; Umer Sharief and Jagadish Chandra 1991). In Artemisia vulgaris it has been observed that shooting response was increased when BAP (4.44 µM) and Kn (2.32 µM) combination rather than BAP (4.44–13.32 µM) or Kn (0.46–13.92 µM) (Sujatha and Ranjitha Kumari 2007). In the present study, MS medium supplemented with BAP in combination with 2-iP yielded optimal shoot regeneration. Similar to the present results, callus mediated shoot regeneration was reported from seedling explants of Artemisia annua (Fulzele et al. 1991; Ferreira and Janick 1996) and Artemisia pallens (Benjamin et al. 1990) on cytokinin supplemented medium.
Table 1.
Effect of cytokinins supplemented to MS medium on multiple shoot induction of Artemisia nilagirica var. nilagirica
| Growth regulator (µM) | Frequency of response (%) | Mean no. of shoots* | Mean shoot length* |
|---|---|---|---|
| Control | 0.00d | 0.00 ± 0.00b | 0.00 ± 0.00b |
| BAP (2.5) + Kn | |||
| 2.5 | 0.00d | 0.00 ± 0.00b | 0.00 ± 0.00b |
| 5.0 | 16.65c | 1.16 ± 1.16b | 0.11 ± 0.11b |
| 7.5 | 16.65c | 1.33 ± 1.33b | 0.17 ± 0.17b |
| 10.0 | 16.65c | 0.83 ± 0.83b | 0.13 ± 0.13b |
| BAP (2.5) + TDZ | |||
| 2.5 | 16.65c | 0.50 ± 0.50b | 0.16 ± 0.16b |
| 5.0 | 33.33b | 1.00 ± 0.63b | 0.22 ± 0.13b |
| 7.5 | 0.00d | 0.00 ± 0.00b | 0.00 ± 0.00b |
| 10.0 | 0.00d | 0.00 ± 0.00b | 0.00 ± 0.00b |
| BAP (2.5) + 2-iP | |||
| 2.5 | 33.33b | 2.66 ± 1.97b | 0.35 ± 0.22b |
| 5.0 | 0.00d | 0.00 ± 0.00b | 0.00 ± 0.00b |
| 7.5 | 83.33a | 10.16 ± 2.24a | 6.13 ± 1.53a |
| 10.0 | 0.00d | 0.00 ± 0.00b | 0.00 ± 0.00b |
* Each value represents the mean ± standard error. Mean Data recorded after 6 weeks of culture. Treatment means followed by different letters in their superscript are significantly different from each other (p < 0.05) according to Duncan’s multiple range test
Rooting of shoots
Individual shoots were excised from the culture and inoculated on half and quarter strength medium with auxins viz. NAA, IAA and IBA. There was significant variation in root number and root length with three auxins used for rooting of shoots (Table 2). NAA and IAA were found to be more adequate in inducing more number of roots on quarter strength MS medium (Fig. 1d). However, the optimal medium for rooting was having quarter the concentration of MS salts in 10 µM IBA. The primary and secondary roots started developing within 2 weeks of culture. About 83.33 % of the regenerated shoots cultured on this medium developed an average of 58.66 roots with maximum root length of 4.17 cm after 6 weeks (Table 2). In agreement with our finding, IBA was reported as a potential hormone for the induction of adventitious roots (Liu et al. 2003; Shen et al. 2010). Shoots cultured on higher salt strength (half strength) formed roots initially but later formation of callus with roots after 3 weeks of culture, therefore, quarter salt strength is adequate for root induction. Similar positive effect of lower salt strength of media on rhizogenesis has been reported earlier in Feronia limonia (Hiregoudar et al. 2005), Cercis canadensis (Mackey et al. 1995), Syzygium cuminii (Jain and Babbar 2000).
Table 2.
Effect of auxins supplemented to MS medium on root induction from the shoots of Artemisia nilagirica var. nilagirica
| MS + Growth regulator | Frequency of response (%) | Mean no. of roots/shoot* | Root length* |
|---|---|---|---|
| Control | 66.65c | 2.33 ± 0.84ef | 1.95 ± 0.65cde |
| Half strength MS + NAA | |||
| 2.5 | 100.00a | 9.66 ± 1.47cd | 10.01 ± 1.44a |
| 5 | 100.00a | 7.50 ± 2.18de | 6.71 ± 1.34b |
| 7.5 | 100.00a | 10.50 ± 1.66cd | 4.95 ± 0.77bc |
| 10 | 100.00a | 11.00 ± 2.97bcd | 4.01 ± 0.89bcd |
| Half strength MS + IAA | |||
| 2.5 | 100.00a | 18.00 ± 3.23a | 5.89 ± 1.00b |
| 5 | 100.00a | 16.16 ± 2.24ab | 4.95 ± 1.06bc |
| 7.5 | 100.00a | 14.50 ± 1.43abc | 4.64 ± 0.69b |
| 10 | 100.00a | 7.83 ± 2.25d | 4.06 ± 0.55bcd |
| Half strength MS + IBA | |||
| 2.5 | 33.33f | 1.16 ± 0.98f | 1.61 ± 1.03de |
| 5 | 50.00d | 1.66 ± 0.76f | 2.03 ± 0.92cde |
| 7.5 | 83.33b | 2.16 ± 0.60ef | 2.68 ± 0.71cde |
| 10 | 0.00 g | 0.00 ± 0.00f | 0.00 ± 0.00e |
| Control | 66.65c | 1.00 ± 0.57b | 1.93 ± 1.55bc |
| Quarter strength MS + NAA | |||
| 2.5 | 100.00a | 8.00 ± 3.51b | 0.92 ± 0.45c |
| 5 | 100.00a | 9.33 ± 1.76b | 1.14 ± 0.24bc |
| 7.5 | 100.00a | 13.00 ± 5.13b | 1.82 ± 0.18bc |
| 10 | 100.00a | 4.66 ± 0.88b | 1.06 ± 0.04bc |
| Quarter strength MS + IAA | |||
| 2.5 | 100.00a | 6.66 ± 0.88b | 1.13 ± 0.38bc |
| 5 | 100.00a | 9.33 ± 2.33b | 1.78 ± 0.44bc |
| 7.5 | 100.00a | 4.66 ± 1.20b | 1.19 ± 0.46bc |
| 10 | 100.00a | 7.00 ± 3.05b | 1.81 ± 0.24bc |
| Quarter strength MS + IBA | |||
| 2.5 | 100.00a | 4.33 ± 1.85b | 1.37 ± 0.97bc |
| 5 | 100.00a | 4.33 ± 1.20b | 1.16 ± 0.08bc |
| 7.5 | 66.65c | 52.33 ± 12.91a | 3.10 ± 0.50ab |
| 10 | 83.33b | 58.66 ± 6.35a | 4.17 ± 0.64a |
* Each value represents the mean ± standard error. Data recorded after 6 weeks of culture. Treatment means followed by different letters in their superscript are significantly different from each other (p < 0.05) according to Duncan’s multiple range test
Acclimatization of rooted plantlets
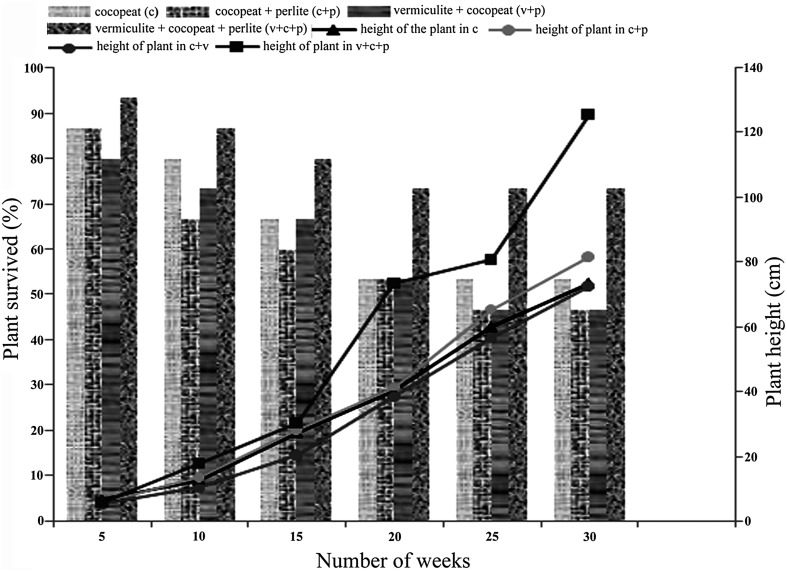
Successful transfer of in vitro grown plantlets to green house or field conditions is a crucial aspect in micropropagation (Hazarika 2003). Plantlets with well developed roots were transferred to various potting mixtures for hardening in plastic cups. The plantlets were initially maintained under controlled conditions (Fig. 2a) and later (after 4 weeks), the plantlets were transferred to green house where day-night temperatures were 34 and 23 °C (Fig. 2b). In all the potting mixtures 100 % survival was seen for initial 4 weeks; however, it reduced to 93 and 73.3 % respectively after 5 and 10 weeks after acclimatization. The initial growth in plant height was 6.05 cm during first 5 weeks of acclimatization. However the plant reached height up to 1.25 m after 30 weeks (Figs. 2c, 3).
Fig. 2.
Acclimatization of plantlets. a In vitro regenerated plantlets transferred to plastic cups containing potting mixtures (after 4 weeks; Bar = 3.1 cm); b Established plant transferred to pots in green house garden (after 8 weeks; Bar = 6.7 cm); c Plants grown up to 1.25 m (after 30 weeks; Bar = 15.9 cm)
Fig. 3.
The frequency of ex-vitro survival and growth of acclimatized plant in different potting mixtures
Morphologically the regenerated plants showed similar characters as that of the mother plant (Table 3). The studied vegetative characters of the regenerated plants were similar with that of the mother plant. Both mother and in vitro plants flowered at the same time of the year during November to March. The in vitro raised plants produced inflorescences within 1 year of planting and the studied ray and disc floret characters of in vitro plants were also found to be similar with that of mother plant. However, the length of the seeds in vitro raised plants was slightly larger and had a slightly higher germination rate (64 %) compared to that of mother plant (56 %) after 2 months of harvest. Similar stability in the morphology of in vitro grown Aloe vera was reported by Das et al. (2015).
Table 3.
Morphological characteristics of A. nilagirica var. nilagirica
| Characters | Mother plant* | In vitro regenerated plant* |
|---|---|---|
| Stem color | Green | Green |
| Length ratio of apical segment of mid stem leaf | 8.60 ± 0.42 | 9.51 ± 0.61 |
| Breadth ratio of apical segment of mid stem leaf | 2.86 ± 0.16 | 2.30 ± 0.19 |
| No. of serrations on apical segment of mid stem leaf | 8.33 ± 0.66 | 10.00 ± 1.00 |
| No. of leaves in top 20 cm of inflorescence | 8.50 ± 1.44 | 8.50 ± 0.86 |
| Leaf thickness (mm) | 0.80 ± 0.08 | 0.82 ± 0.08 |
| Leaf width (cm) | 15.22 ± 1.01 | 14.72 ± 1.73 |
| Leaf length (cm) | 20.46 ± 1.30 | 21.68 ± 1.35 |
| Capitulum shape | Elliptic | Elliptic |
| Length of capitulum (mm) | 2.67 ± 0.11 | 2.60 ± 0.10 |
| Breadth of capitulum (mm | 1.95 ± 0.05 | 1.82 ± 0.08 |
| Outer involucre bract smaller than disc | Smaller | Smaller |
| Disc floret fertile or sterile | Fertile | Fertile |
| No. of ray florets/inflorescence | 9.50 ± 0.28 | 9.75 ± 1.03 |
| No. of disc florets/inflorescence | 10.50 ± 0.64 | 10.75 ± 0.47 |
| Length of disc floret (mm) | 1.86 ± 0.08 | 1.83 ± 0.08 |
| Length of ray floret (mm) | 1.57 ± 0.08 | 1.54 ± 0.09 |
| Length of anther (mm) | 1.12 ± 0.02 | 1.10 ± 0.00 |
| Length of stigma in ray floret (mm) | 0.45 ± 0.02 | 0.47 ± 0.04 |
| Length of seed (mm) | 0.93 ± 0.03 | 1.05 ± 0.13 |
| Breadth of seed (mm) | 0.40 ± 0.01 | 0.42 ± 0.02 |
| Flowering time | November–March | November–March |
* Each value represents the mean ± Standard Error
Genetic fidelity analysis
Cytological analysis
Cytological analysis revealed that there was no change in the chromosome number of micropropagated plants. All the cells in the mother plant and micropropagated plant root tips revealed same number of chromosomes 2n = 54 (Fig. 4), showing genetic stability of the micropropagated plants. Similar reports showing the genetic stability of micropropagated plants was reported in Centaurea ultreiae and Nepenthes khasiana (Mallon et al. 2010; Devi et al. 2013).
Fig. 4.
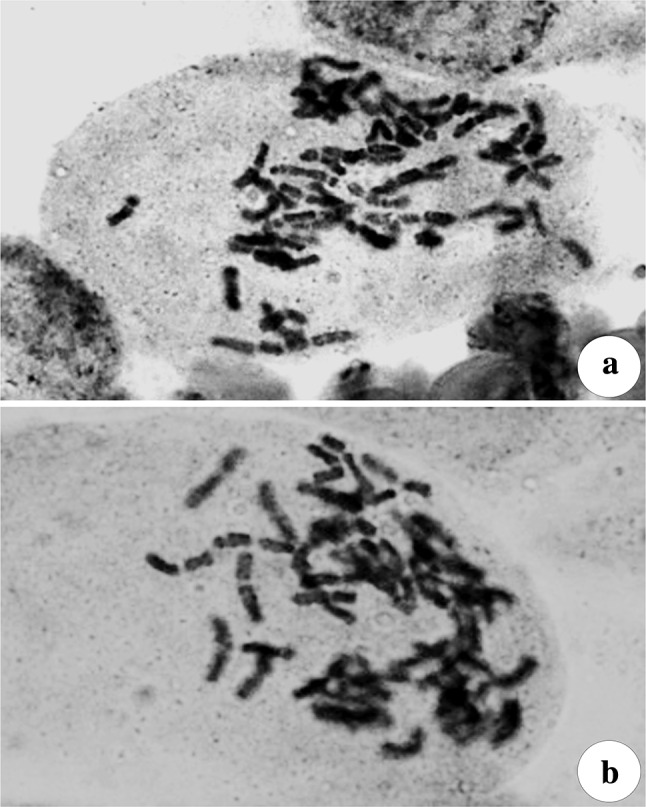
Chromosome complements of Artemisia nilagirica var. nilagirica
ISSR analysis
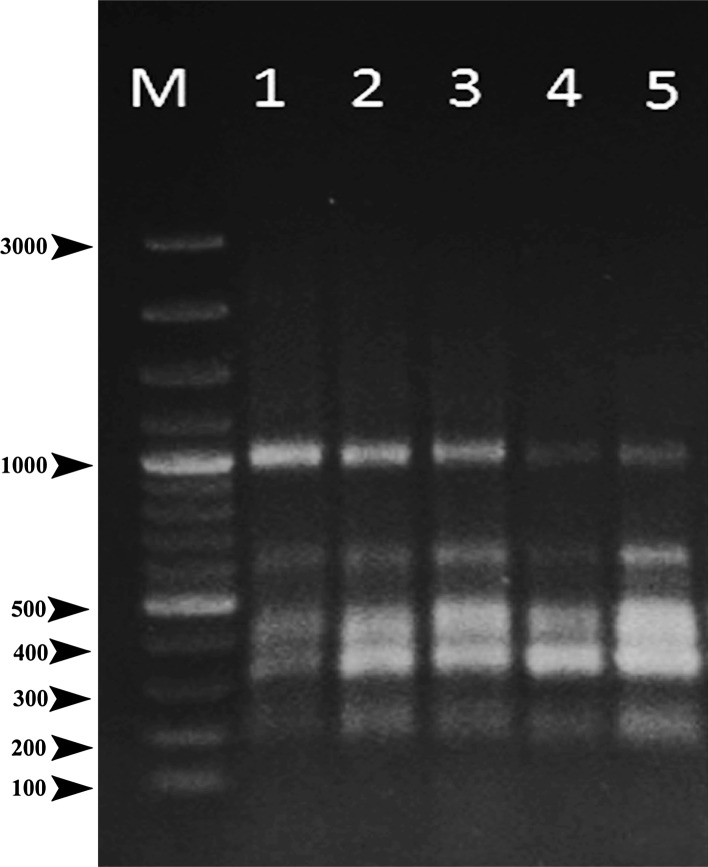
Clonal fidelity of in vitro regenerated Artemisia nilagirica var. nilagirica was tested using ISSR primers. Out of 15 ISSR primers screened only 5 primers could amplify the genomic DNA and produced monomorphic bands. Primer UBC 853 produced 5 monomorphic bands (Fig. 5), while UBC 823 produced eight monomorphic bands in both in vitro regenerated and the mother plants. Thus, confirming the genetic stability of regenerants. Likewise, molecular markers were successfully used to reveal the genetic fidelity of micropropagated plants in Andrographis paniculata (Dandin and Murthy 2012b), Atremisia absinthium (Kour et al. 2014) and Spilanthes oleracea (Dandin et al. 2014).
Fig. 5.
ISSR marker profile (obtained with the UBC-853 primers) of in vitro-regenerated plantlets and mother plant of Artemisia nilagirica var. nilagirica. Lane M DNA markers (1.0 kbp ladder); Lane 1 ISSR profile of a mother plant. Lanes 2–5, ISSR profiles of four separate regenerated plantlets
In conclusion, a reliable in vitro plant regeneration method has been developed for the Artemisia nilagirica var. nilagirica (Indian Wormwood). An optimal shoot regenerated from the nodal derived callus on MS medium supplemented 2.5 µM BAP + 7.5 µM 2-iP and maximum rooting occurred in shoots cultured on quarter strength MS medium supplemented with 10 µM IBA. Morphological, cytological studies and ISSR marker analysis showed genetic stability of regenerants. This protocol can be used for mass scale production of this important medicinal plant which could be used for plantation program and used as stock material for pharmaceutical uses.
Acknowledgments
This work was supported partially by BRNS project (BRNS Project No. 2013/35/6/BRNS/20) and DST-PURSE-Phase-2 program.
Authors' contribution
HNM designed the experiment, SS, KSJ, JJ, and SHM conducted the experiment. All the authors together written and approved the manuscript.
Compliance with ethical standards
Conflict of interest
Authors declare that they have no conflict of interest.
References
- Abad MJ, Bedoya LM, Apaza L, Bermejo P. The Artemisia L. Genus: a review of bioactive essential oils. Molecules. 2012;17:2542–2566. doi: 10.3390/molecules17032542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin BD, Sipahimaalani AT, Heble MR. Tissue culture of Artemisia pallens: organogenesis, terpenoid production. Plant Cell Tissue Organ Cult. 1990;21:159–164. doi: 10.1007/BF00033436. [DOI] [Google Scholar]
- Bhattacharjee S. Hand book of medicinal plants. Jaipur: Pointer Publishers; 2000. p. 43. [Google Scholar]
- Dandin VS, Murthy HN. Enhanced in vitro multiplication of Nothapodytes nimmoniana Graham using semisolid and liquid cultures and estimation of camptothecin in the regenerated plants. Acta Physiol Plant. 2012;24:1381–1386. doi: 10.1007/s11738-012-0934-x. [DOI] [Google Scholar]
- Dandin VS, Murthy HN. Regeneration of Andrographis paniculata Nees: analysis of genetic fidelity and andrographolide content in micropropagated plants. Afr J Biotechnol. 2012;11:12464–12471. [Google Scholar]
- Dandin VS, Naik PM, Murthy HN, Park SY, Lee EJ, Paek KY. Rapid regeneration and analysis of genetic fidelity and scopoletin contents of micropropagated plants of Spilanthes oleracea L. J Hort Sci Biotechnol. 2014;89:79–85. [Google Scholar]
- Das A, Moquammel Haque SK, Ghosh B, Nandagopal K, Jha TB. Morphological and genetic characterization field grown plants of Aloe vera L. Plant Tissue Cult Biotechnol. 2015;25:231–246. doi: 10.3329/ptcb.v25i2.26257. [DOI] [Google Scholar]
- Devi SP, Kumaria S, Rama Rao S, Tandon P. In vitro propagation of clonal fidelity of Nepenthes khasiana Hook. F.: a medicinal insectivorous plant of India. Acta Physiol Plant. 2013;35:2813–2820. doi: 10.1007/s11738-013-1314-x. [DOI] [Google Scholar]
- Ferreira JFS, Janick J. Roots as an enhancing factor for the production of artemisinin in shoot cultures of Artemisia annua. Plant Cell Tissue Organ Cult. 1996;44:211–217. doi: 10.1007/BF00048526. [DOI] [Google Scholar]
- Fulzele DP, Sipahimaalani AT, Heble MR. Tissue culture of Artemisia annua: organogenesis and Artemisinin production. Phytother Res. 1991;5:149–153. doi: 10.1002/ptr.2650050402. [DOI] [Google Scholar]
- Haider F, Kumar N, Naqui AA, Bagchi GD. Oil constituents of Artemisia nilagirica var. septentrionalis growing at different altitudes. Nat Prod Commun. 2010;5:1959–1960. [PubMed] [Google Scholar]
- Hazarika BN. Acclimatization of tissue-cultured plants. Curr Sci. 2003;85:1704–1712. [Google Scholar]
- Hiregoudar LV, Ashok Kumar HG, Murthy HN. In vitro cultgure of Feronia limonia (L.) Swingle from hypocotyls and intermodal explants. Biol Plant. 2005;49:41–45. doi: 10.1007/s10535-005-1045-y. [DOI] [Google Scholar]
- Jain N, Babbar SB. Recurrent production of plants of black palm, Syzygisum cumnii (L.) Skeels, a myrtaceous fruit tree, from in vitro cultured seedling explants. Plant Cell Rep. 2000;19:519–524. doi: 10.1007/s002990050766. [DOI] [PubMed] [Google Scholar]
- Kour B, Kour G, Kaul S, Dhar MK. In vitro mass multiplication and assessment of genetic stability of in vitro raised Artemisia absinthium L. Plants using ISSR and SSAP molecular markers. Adv Bot. 2014 [Google Scholar]
- Krishnan PN, Decruse SW, Radha RK. Conservation of medicinal plants of Western Ghats, India and its sustainable utilization through in vitro technology. n Vitro Cell Develop Bio-Plant. 2011;47(1):110–122. doi: 10.1007/s11627-011-9344-9. [DOI] [Google Scholar]
- Liu CZ, Murch SJ, El-Demerdash M, Saxena PK. Regeneration of the Egyptian medicinal plant Artemisia judaica L. Plant Cell Rep. 2003;21:525–530. doi: 10.1007/s00299-002-0561-x. [DOI] [PubMed] [Google Scholar]
- Mackey WA, Tipton JL, Thompson GA. Micropropagation of Mexican redbud, Cercis canadensis var. Mexicana. Plant Cell Tissue Organ Cult. 1995;43:295–299. [Google Scholar]
- Maharana SB, Mahato V, Behera M, Mishra RR, Panigrahi J. In vitro regeneration from node and leaf explants of Jatropha curcans L. and evaluation of genetic fidelity through RAPD markers. Indian J Biotechnol. 2012;11:280–287. [Google Scholar]
- Mallon R, Rodriguez-Oubina J, Gonzalez ML. In vitro propagation of the endangered plant Centaurea ultreiae: assessment of genetic stability by cytological studies, flow cytometry and RAPD analysis. Plant Cell Tissue Organ Cult. 2010;101:31–39. doi: 10.1007/s11240-009-9659-y. [DOI] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant. 1962;15:437–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Murray MG, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucleic Acid Res. 1980;8:4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik SK, Mohanty S, Padhi A, Pati R, Sinawane A. Evaluation of antibacterial and cytotoxic activity of Artemisia nilagirica and Murraya koenigii leaf extracts against mycobacteria and macrophages. BMC Complement Altern Med. 2014;14:87. doi: 10.1186/1472-6882-14-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nin S, Morosi E, Schhiff S, Bennici A. Callus cultures of Artemisia absinthium L. Initiation, growth optimizaiton and organogenesis. Plant Cell Tissue Organ Cult. 1996;45:67–72. doi: 10.1007/BF00043430. [DOI] [Google Scholar]
- Paneerselvam C, Murugan K, Kovendan K, Kumar PM. Mosquito larvicidal, pupicidal, adulticidal, and repellent activity of Artemisia nilagirica (Family: Compositae) against Anopheles stephensi and Aedes aegypti. Parasitol Res. 2012;111:2241–2251. doi: 10.1007/s00436-012-3073-9. [DOI] [PubMed] [Google Scholar]
- Rout GR, Samantaray S, Das P. In vitro manipulation and propagation of medicinal plants. Biotech Advan. 2000;18(2):91–120. doi: 10.1016/S0734-9750(99)00026-9. [DOI] [PubMed] [Google Scholar]
- Sati SC, Sati N, Ahluwlia V, Walia S, Sati OP. Chemical composition and antifungal activity of Artemisia nilagirica essential oil growing in northern hilly area of India. Nat Prod Res. 2013;27:45–48. doi: 10.1080/14786419.2011.650636. [DOI] [PubMed] [Google Scholar]
- Shen X, Castle WS, Gmitter FG., Jr In vitro shoot proliferation and root induction of the shoot tip explants from mature male plants of Casuarina cunninghamiana Miq. HortScience. 2010;45:797–800. [Google Scholar]
- Shukla V, Pala Z, Alok A, Desai N. Screening of different Artemisia spp. from Western Ghats of Maharashtra for an anti-malarial compound-Artemisinin. Am J Plant Sci. 2015;6:1619–1632. doi: 10.4236/ajps.2015.69162. [DOI] [Google Scholar]
- Sujatha G, Ranjitha Kumari BD. Effect of phytohormones on micropropagation of Artemisia vulgaris L. Acta Physiol Plant. 2007;29:189–195. doi: 10.1007/s11738-006-0023-0. [DOI] [Google Scholar]
- Umer Sharief MD, Jagadish Chandra KS. Micropropagation of Davana (Artemisia pallens Wall.) In: Praksh J, Pierik RLM, editors. Horticulture-new technologies and applications. Dordrech: Kluwer; 1991. pp. 258–263. [Google Scholar]
- Walter HL, Memory PF, Elvin L. Medical botany: plants affecting human health. 2. Hiboken: Wiley; 2003. p. 345. [Google Scholar]