Abstract
Objective
The management of cervical intraepithelial neoplasia (CIN) and early invasive cancer of the uterine cervix is very difficult to approach, especially in case of young woman who wants to preserve her fertility. Conization of the cervix may have various kinds of disadvantage. The objective of this clinical retrospective study is to investigate the therapeutic effects and clinical efficacy of photodynamic therapy (PDT) including combined chemo-photodynamic therapy in patients with pre-malignant CIN and malignant invasive cervical cancer.
Methods
Total number of PDT trial case was 50 cases and total number of patient was 22 patients who registered to PDT clinic. We used photogem sensitizer and 632 nm diode laser in early two cases. After then we performed PDT using photofrin sensitizer and 630 nm diode laser in other cases. We used flat-cut, microlens, cylindrical diffuser, and interstitial type optic fibers in order to irradiate the lesions. 240 J/cm2 energy was irradiated to the lesions.
Results
CIN 2 were 4 cases (18.2%) and CIN 3 were 15 (68.2%) and invasive cervical cancer were 3 (13.6%). Complete remission (CR) was found in 20 patients (91%). One case of 19 patients with CIN lesion recurred at 18 months after PDT treatment. CR was found in 18 cases in the patients with CIN lesions (95%). CR was found in 2 cases in the patients with invasive cervical cancer (67%).
Conclusion
Our data showed that CR rate was fantastic in CIN group (95%). This study suggests that PDT can be recommended as new optimistic management modality on the patients with pre-malignant CIN lesions including carcinoma in situ and relatively early invasive cancer of the uterine cervix. Combined chemo-photodynamic therapy is essential in case of invasive cervical cancer. For the young age group who desperately want to preserve their fertility and have a healthy baby, PDT can be a beacon of hope.
Keywords: Cervical intraepithelial neoplasia, Combined chemo-photodynamic therapy, Invasive cervical cancer, Photodynamic therapy
Introduction
Cervical cancer is fourth most common female cancer worldwide. Cancer statistics in 2012 in World Health Organization showed an estimated 528,000 new patients and an estimated 266,000 deaths from cervical cancer. Cervical cancer deaths were accounted for 7.5% of all female cancer deaths. Especially cervical cancer deaths (87%) occur in the less developing or developing regions [1].
Most standard therapeutic methods of cervical neoplasia were based on the focal destruction; conization, loop electrosurgical excision procedure (LEEP), cryotherapy, electrosurgery, Semm coagulation, laser ablation, and total hysterectomy. These conventional therapeutic modality have many disadvantages; cervical stenosis, infertility due to cervical factor, danger of abortion, the possibility of incompetent cervix, premature rupture of membrane or preterm delivery, and low birth weight infant, etc. And so the optimal therapeutic modality of pre-cancerous lesions and early invasive cancer is still not clear in fertility preserving cervical cancer groups [2]. Radical trachelectomy is known as one of fertility preserving therapeutic modality. But it also has some serious complications such as deep vein thrombosis, pulmonary embolism, and preterm birth. Moreover in patients with clinically high-risks and refusing surgery, non-operative alternative therapeutic modalities should be needed to reduce or cure disease. In these reasons, non-invasive therapeutic modality for cervical neoplasia is trying clinical approach.
Developments of science have had a clue in alternative therapeutic modalities such as photodynamic therapy (PDT), immune therapy, and oncolytic viral therapy. In these alternative modalities, the basic principle of PDT was introduced in BC 3000 in India, Greek, and Egypt. Dougherty, et al. reported the effectiveness of PDT in 1975. However it was much later in 1980 that photo irradiation therapy was studied for cancer treatment [3].
PDT is principally a new treatment modality (both curative and palliative) of destroying malignant tumors and premalignant lesions based on photodynamical damage to tumor cells [4]. PDT uses the photosensitizer, optic fiber with tip, and non-thermal laser light with selected 600 to 700 nm wave length. In Korean gynecology, it had been introduced in the late 1990’s and began to be used clinically in the early 2000’s [5].
PDT has advantages of normal tissue preservation, relatively less pain and bleeding tendency, combination with other therapeutic or adjuvant therapy, repeatability, and improving quality of life, etc. [6]. On the other hand, PDT also has disadvantages of photosensitive side effects: inconvenience, and relatively high cost, etc. Light penetration depth in PDT is known to be limited, up to less than one-third of an inch, and it is difficult to cover large areas [7,8,9]. So combined chemophotodynamic therapy (CCPDT) is necessary to the invasive cervical cancer.
Objective of study is to investigate comprehensively the therapeutic effects and clinical efficacy of PDT including CCPDT in cervical intraepithelial neoplasia (CIN) and invasive cervical cancer.
Materials and methods
1. Patients
From October 2005 to December 2011, retrospective study was designed. Total number of PDT trial case was 50 cases and total number of patient was 22 patients who registered to PDT clinic in Dankook University Hospital. To select PDT clinical trial patients, selection criteria were as follows: (1) high-grade cervical intraepithelial lesions, (2) early stage cervical cancer without metastatic lesions, (3) strong desire for fertility preservation, (4) no surgical management desire, (5) fully awareness of PDT treatment, and (6) no allergic reaction for porphyrin and light. Before PDT, Papanicolaou (Pap) cytology, HPV DNA test, cervicography, and histology were examined. Pelvic computed tomography was examined on selected cases. Work-up study for staging was performed in patients with invasive cervical cancer.
2. Treatment
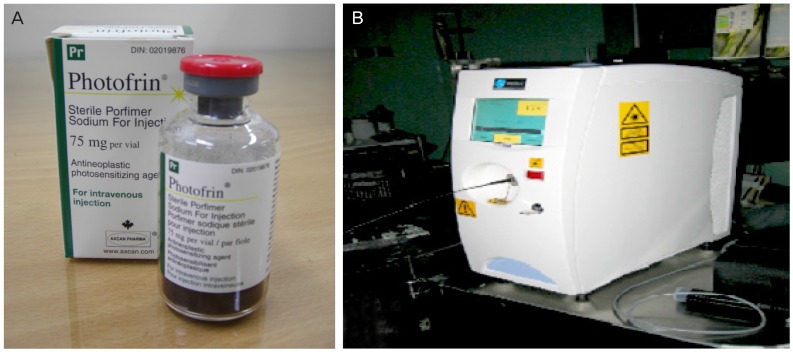
We used photogem sensitizer and 632 nm (Biolitec, Ceralas, Germany) diode laser in early two cases. After then we performed PDT using photofrin sensitizer and 630 nm (Diomed 630 nm PDT laser; Diomed, Cambridge, UK) diode laser in other cases (Fig. 1). In admission state, photosensitizer 2.0 mg/kg was injected intravenously in a dark room. At 48 hours after injection, PDT was performed in lithotomy position, under general anesthesia.
Fig. 1. Porfimer photosensitizer (A: Photofrin, Axcan Pharma Inc., Canada/USA; INMEX Corp., Seoul, Korea) and non-thermal laser device (B: Diomed 630 nm PDT laser, Diomed, Cambridge, UK; LitePharmTech Co., Seoul, Korea).
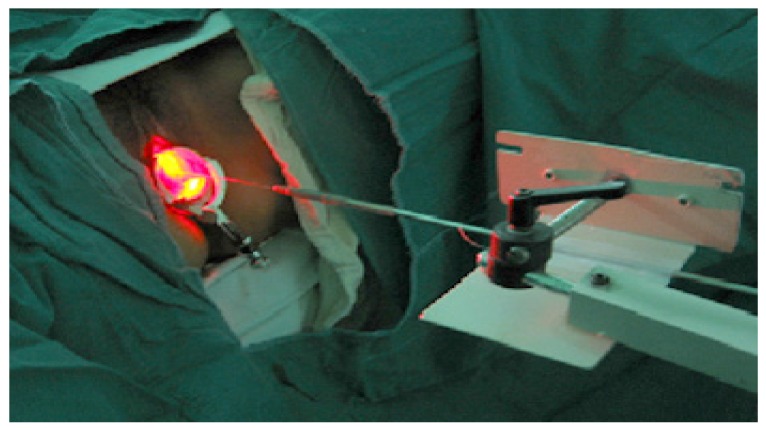
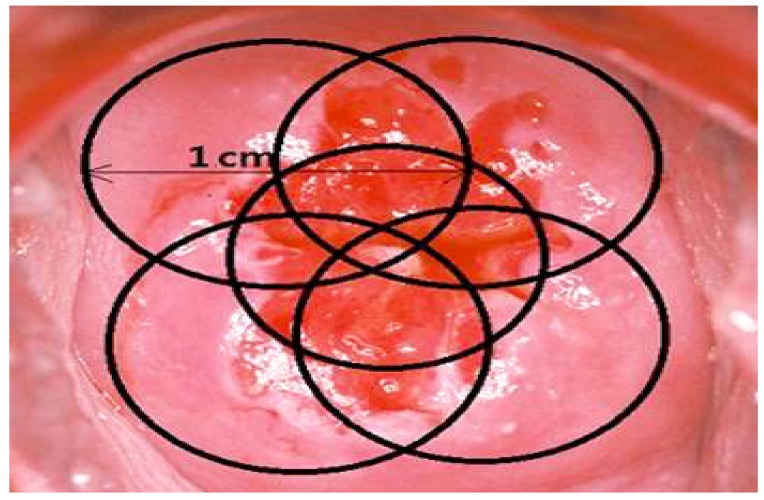
We used flat-cut, microlens, cylindrical diffuser, and interstitial type optic fibers in order to irradiate the lesions. 240 J/cm2 (400 mW for 600 seconds) energy was irradiated to the lesions. Exocervical lesions were irradiated in diameter of 1 cm with flat-cut and 2 cm with microlens. Irradiation of endocervix was performed with 3 or 4 cm cylindrical diffuser. Especially cancerous tissues were irradiated with interstitial type (Figs. 2,3,4).
Fig. 2. Photodynamic laser irradiation therapy directly into the lesions (exocervix, endocervix, and the core of cancer tissue).
Fig. 3. Schematic figure of the photodynamic therapy irradiation in exocervix. The divided 5 parts (2, 5, 7, 11 o’clock directions and center of the cervix, 1 cm in diameter) were irradiated, each part using 240 J/cm2 with 400 mW for 600 seconds with flat-cut optic fiber.
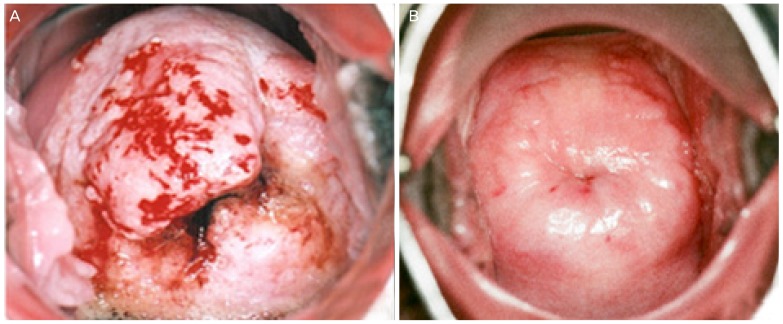
Fig. 4. The pre-photodynamic therapy cervicographic figures of the cervix in a 32-year-old married woman (para 2-0-0-2) with carcinoma in situ histologic lesion showed broad acetowhitening and mosaic lesion with punctuation at 8 to 1 o’clock direction (A). The post-photodynamic therapy cervicographic figures cervicography at 16 months after photodynamic therapy shows nearly normal appearance (B).
Dosimetry of PDT laser irradiation is as followings:
The photodynamic reaction ∝ energy (J/cm2)×activated drug concentration
Energy (J/cm2)=power density (W/cm2)×time of exposure (sec)
In CCPDT, at 45 hours after sensitizer injection, carboplatin 75 mg/m2 was administered. At 48 hours after sensitizer injection PDT was performed. PDT or CCPDT were repeated on 2 days after the 1st PDT or CCPDT. The 2nd cycles of CCPDT were performed at 1 or 2 months after the 1st cycle of CCPDT according to the condition of the cervix in case of the patients with invasive cervical cancer (Fig. 5).
Fig. 5. The pre-combined chemo-photodynamic therapy (CCPDT) cervicographic figures of the cervix in a 30-year-old cohabited woman (para 0-0-0-0) with invasive barrel-shaped endocervical cancer (FIGO [International Federation of Gynecology and Obstetrics] stage 1B1) showed acetowhitening and slightly bulging lesions with partly bloody increased vessels in all around the exocervix (A). The post-CCPDT cervicographic figures at 62 months after 1st CCPDT shows also normal and beautiful appearance. Pap cytology was negative and human papillomavirus DNA test was negative (B).
Patients were monitored for toxicity for 3 months after discharge. All patients were educated to avoid sunlight for preventing any possible toxicity. When the patient goes out of the house, sun-shield items should be applied in order to avoid direct light.
3. Response
In terms of remission, no definitive criteria have been determined in PDT. RECIST (Response Evaluation Criteria In Solid Tumors) 1.1 guideline was ordinarily adapted as remission criteria in this clinical study [10]. Complete remission (CR) is defined as complete disappearance of the pathologic lesions in histology. Partial response (PR) is defined if the pathologic tissue is decreased at least 30% from initial lesions. Stable disease is defined if the pathologic tissue is between decreased less than 30% and increased less than 20% from initial lesions. Progressive disease (PD) is defined if the pathologic tissue is increased more than 20% from initial lesions. Recurrence is defined if the pathologic tissue develops again at least 4 months after CR.
Results
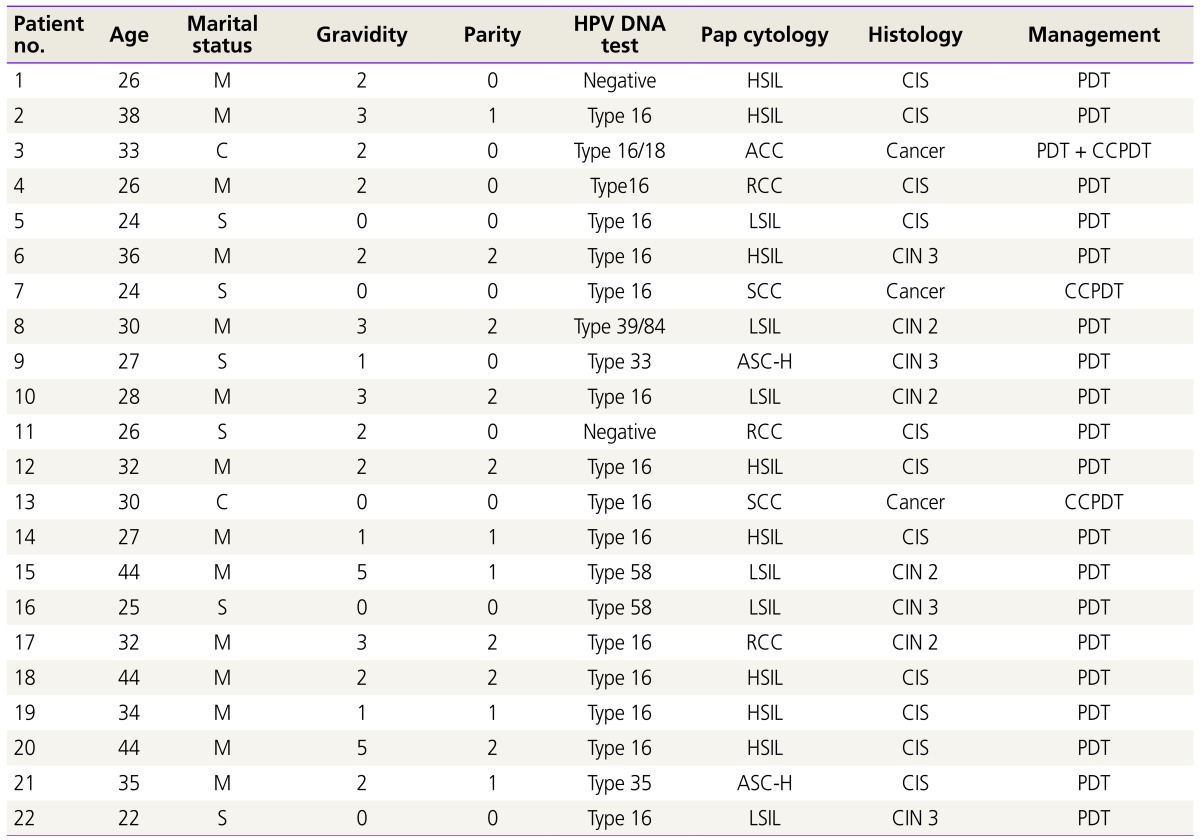
We have performed total 50 cases of PDT irradiation including 10 cases of CCPDT in 22 patients. PDT or CCPDT were repeated on 2 days after the 1st PDT or CCPDT. The 2nd cycles of CCPDT were performed at 1 or 2 months after the 1st cycle of CCPDT according to the condition of the cervix in case of the patients with invasive cervical cancer, in order to increase the cure rate. We performed 2 cases of CCPDT in the 3rd patient, 4 cases of CCPDT in the 7th patient, and 4 cases of CCPDT in the 13th patient (Table 1). The mean age was 31.2 years. The mean age in carcinoma in situ (CIS) group was 32.4 years. The mean gravidity was 2. And the mean parity was 1.1 in CIN group. The mean gravidity was 0. And the mean parity was 0 in invasive cervical cancer group (Table 1).
Table 1. Clinical features in all patients.
HPV, human papilloma virus; Pap, Papanicolaou; M, married; HSIL, high-grade squamous intraepithelial lesion; CIS, carcinoma in situ; PDT, photodynamic therapy; C, cohabit; ACC, adenocarcinoma; CCPDT, combined chemo-photodynamic therapy; RCC, reactive cellular change; S, single; LSIL, low-grade squamous intraepithelial lesion; CIN, cervical intraepithelial neoplasia; SCC, squamous cell carcinoma; ASC-H, atypical squamous cells-exclude high-grade lesions.
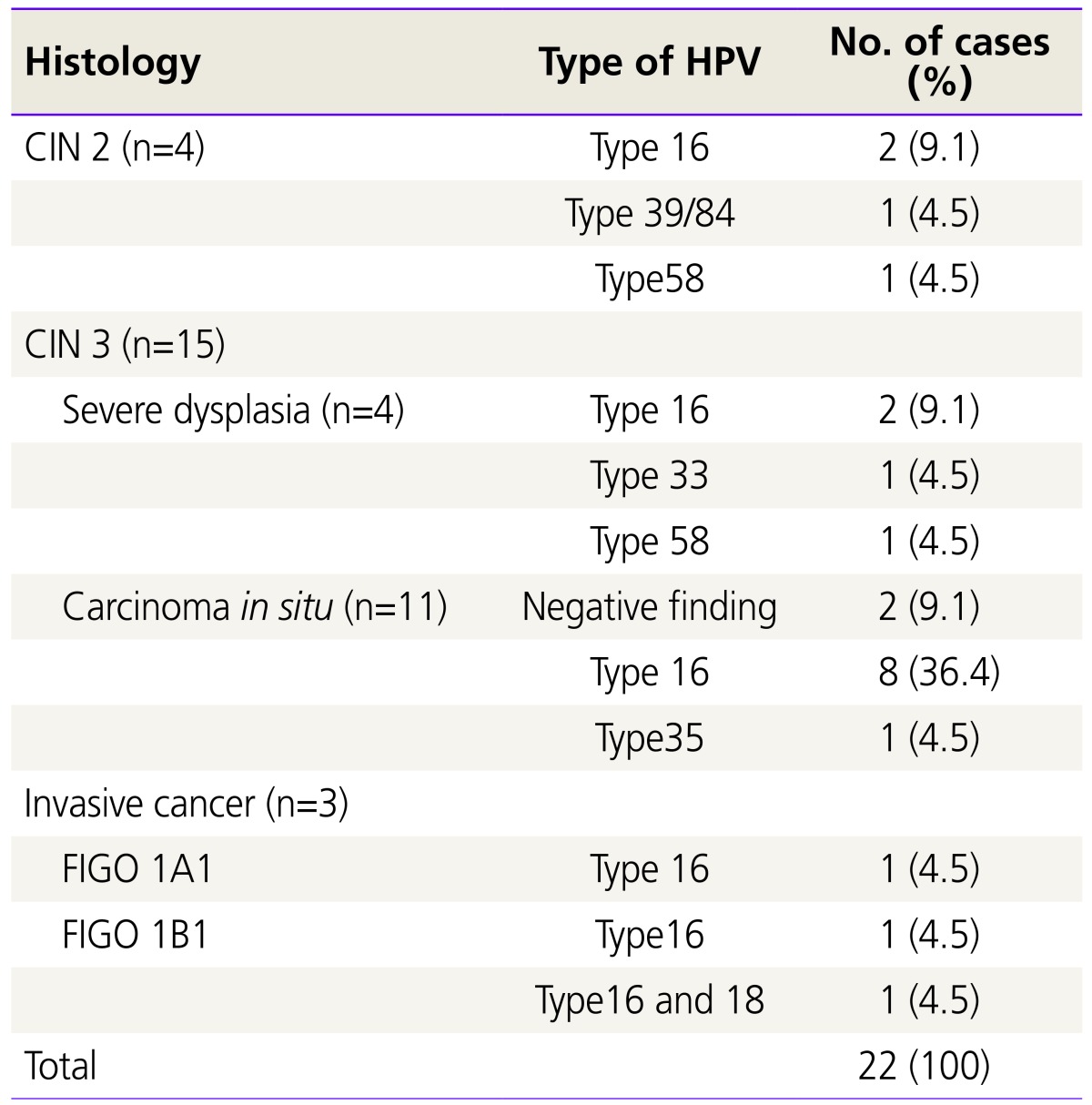
CIN 2 were 4 cases (18.2%) and CIN 3 were 15 (68.2%) and invasive cervical cancer were 3 (13.6%). There were 15 (68.2%) positive HPV 16 DNA test diagnoses: 2 in CIN 2, 10 in CIN 3, and 3 in invasive cervical cancer. There were 2 negative HPV DNA test diagnoses in CIN 3. There was 1 positive HPV 33 DNA test diagnosis in CIN 3. There was 1 positive HPV 35 DNA test diagnosis in CIN 3. In 1 patient, combined HPV infection (HPV 39 and 84 DNA) was revealed. There were 2 positive HPV 58 DNA test diagnoses: 1 in CIN 2, and 1 in CIN 3 (Table 2).
Table 2. Histology according to oncogenic types of HPV.
HPV, human papillomavirus; CIN, cervical intraepithelial neoplasia; FIGO, International Federation of Gynecology and Obstetrics.
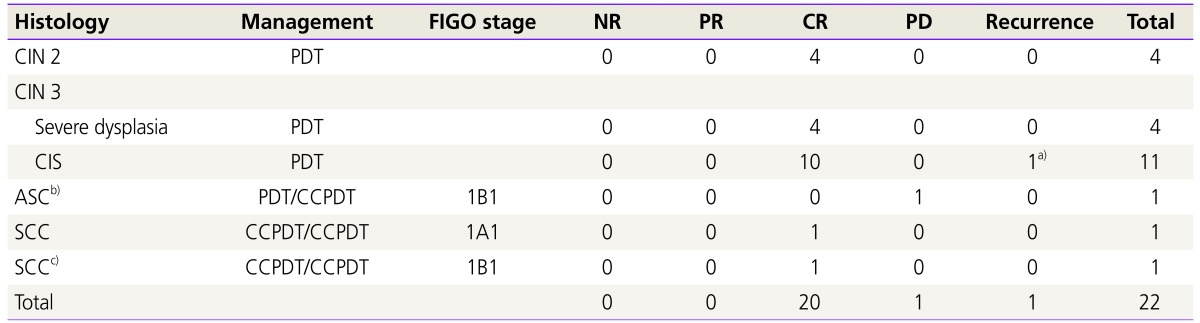
CR was found in 20 patients (91%). One case of 19 patients with CIN lesion recurred at 18 months after PDT treatment. In her large conization after PDT, pathologic diagnosis reported ‘moderate to severe dysplasia’. Complete remission was found in 18 cases in the patients with CIN lesions (95%).
Three patients with invasive cervical cancer have shown the histologic diagnoses of the followings: 1 case of adenosquamous cell carcinoma and 2 cases of squamous cell carcinoma. The patient with adenosquamous cell carcinoma progressed to the worse lesion at 4 months after PDT treatment. Radical hysterectomy and bilateral pelvic and para-aortic lymphadenectomy were performed. CR was found in 2 cases in the patients with invasive cervical cancer (67%) (Table 3).
Table 3. Results of PDT in patients with cervical intraepithelial neoplasia and invasive cervical cancer.
PDT, photodynamic therapy; FIGO, International Federation of Gynecology and Obstetrics; NR, no response; PR, partial response; CR, complete remission; PD, progressive disease; CIN, cervical intraepithelial neoplasia; CIS, carcinoma in situ; ASC, adenosquamous cell carcinoma; CCPDT, combined chemo-photodynamic therapy; SCC, squamous cell carcinoma.
a)The case of recurrence after complete remission; b)The case of progression after partial remission; c)The case of CCPDT with interstitial type method.
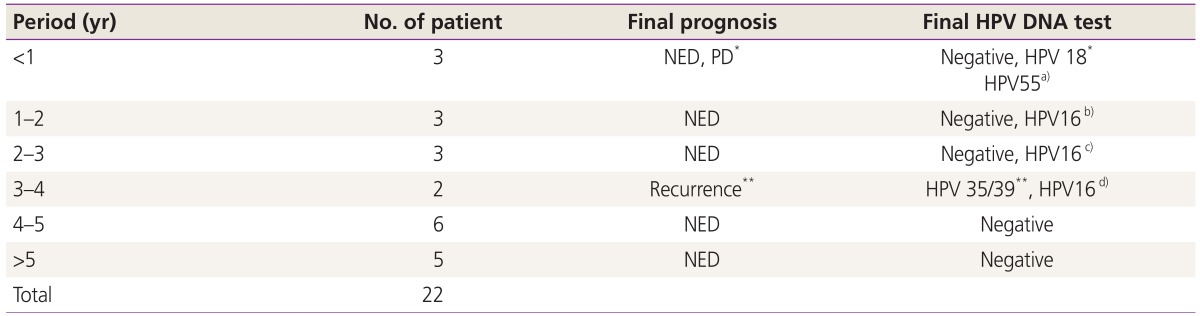
Table 4 shows the follow-up and clinical managements after PDT. Follow-up period ranged from 1 year to 9 years. One case of adenosquamous cell carcinoma showed PD after PR. And 1 case of squamous cell carcinoma in situ showed recurrence after CR. In that case recurrence seemed to happen at 18 months after PDT.
Table 4. Follow-up period after phodynamic therapy.
HPV, human papillomavirus; NED, no evidence of disease; PD, progressive disease.
*The case of progressive disease after partial remission in invasive cancer group; **The case of recurrence after complete remission in CIN group. This case also revealed negative Papanicolaou cytology and positive HPV 35 DNA test at 10 months after PDT. Her initial diagnosis was positive HPV 35 DNA test.
a)The case of negative Papanicolaou cytology and a new-type positive HPV 55 DNA test in CIN group at 8 months after PDT. Her initial diagnoses were positive HPV 66 and 70 DNA tests; b),c),d)The cases of negative Papanicolaou cytology and positive HPV DNA test in CIN group.
Any significant photosensitizer-related side effect was not observed in all cases after PDT. Even though, we found some mild post-PDT side effects such as focal edema and burning sensation etc., which generally decreased with symptomatic treatments.
Discussion
Globally the age specific incidence rate of cervical cancer is not uniform. Changing sexual culture, uterine cervical neoplasia has been increasing among young women. Major concern of oncologist is still conventional therapy rather than alternative therapy for fertility preservation. From 1970 to 2000, the mean age of first delivery increased by 3.5 years (from 21.4 to 24.9 years). Also in the America, birth rate is growing up to 31% in 35 to 39-year-old women and 51% in 40 to 45-year-old women between 1990 and 2002. In childbearing age group, oncologist has challenges about necessity for both fertility preservation and high cervical neoplasia cure rate [11,12,13].
Three main components are required in PDT; a photosensitizer, oxygen and light source of a certain wave length. PDT has stepwise mechanisms. First, when photosensitizer is applied with systemic or topical route, it has remained with a ground state in targeted lesion. The harmless visible light of certain wave length is irradiated in the lesion or whole body, and so photosensitizer absorbs wave length, transferred into excited state. In excited state, photosensitizer triggers the oxygen in cell, to produce cytotoxic reactive oxygen species, free radicals and singlet oxygen. The oxygen materials produced via these procedures induce cellular toxicity and kill malignant or pre-malignant cells by apoptosis and/or necrosis. Except infiltration mechanism, it also closes down the tumor microvasculature and stimulates the host immune system. With multifactorial mechanisms, PDT makes many advantages; less invasive, lack of intrinsic or acquired resistance mechanism. It may also have various therapeutic options for combination therapy, high selectivity to tumor site, repeated treatment option, patient customized therapy, simultaneous multiple treatment, organ function sparing, and improving quality of life, etc. [14,15,16,17].
Our data of this study are consistent with results of other studies. We performed total of 50 PDT cases including 10 CCPDT cases in 22 patients. CR was found in 20 patients (91%). CR was 95% in CIN group and was 68% in invasive cancer group. Three patients had 4 successful pregnancies and delivered 4 healthy live births. The 1st PDT had been performed about 9 years ago to the patient with CIS of the cervix. And she delivered a son at 21 months after PDT and a healthy daughter at 50 months after PDT. The other patient with CIS delivered a healthy son at 23 months after PDT.
The patient with recurrence delivered a healthy son at 14 months after PDT. The recurrence-case revealed high-grade squamous intraepithelial lesion Pap cytology and positive HPV 35 and 39 DNA test diagnoses at 34 months after PDT. Her initial diagnosis was positive HPV 35 DNA test. Therefore we had performed an extensively large conization procedure to the cervix at 35 months after PDT. Histology revealed the diagnoses of ‘moderate to severe dysplasia and negative resection margins’. Magnetic resonance imaging examination at 39 months after PDT showed negative findings. No patient had any PDT-related complication during pregnancy and delivery.
Muroya et al. [18] reported that they have performed PDT on 131 cases (95 CIS, 31 dysplasia, 1 vulva dysplasia, 3 squamous cell carcinomas, microinvasion, and 1 CIS + endocervical adenocarcinoma, microinvasion). Of these cases, 127 became CR (96.9%). The first CR case was 10 years ago and no recurrence has been observed yet.
Lee et al. [19] showed that 52 young patients with cervical intraepithelial neoplasia were treated via PDT with photogem and diode laser 635 nm. Cure rate of cervical intraepithelial neoplasia was 92.3%, and eradication rate of HPV infection was 83.3%. In the patients treated via LEEP therapy, cure rate was 96.0% and eradication rate of HPV infection was 81%. They concluded that the cure rates and eradication of HPV infection of PDT were comparable with LEEP therapy in the management of cervical intraepithelial neoplasia.
Han et al. [20] reported that 10 high-grade squamous intraepithelial lesion patients between 23 to 32 years of age were treated with PDT using photogem and diode laser 632 nm. Eight patients were negative in triple combined test of ThinPrep Pap cytology, cervicography, and an HPV DNA test with single PDT. The dual PDT patients showed negative results in triple combined test. Yamaguchi et al. [21] reported that 105 cases with cervical intraepithelial neoplasia treated with PDT using photofrin and Excimer dye laser or a YAG-OPO laser with 630 nm. One hundred and three cases cured after PDT (98%) and 3 cases was recurred.
Di Saia and Creasman [22] reported that the surgical treatment including cold-knife excision, electrocautery, cryosurgery and laser ablation achieved high success rates between 90% and 98%. Our results were comparable with the above reports. Therefore PDT may have replaced to conventional surgical treatment for CIN.
In this non-invasive CIN group, the cause of recurrence seems to be two factors. The original pathologic slides showed some controversial diagnosis about microinvasion, in addition to squamous cell carcinoma in situ with glandular extension of the cervix. We think that secondary cause was her special constitution for photosensitive drug. In the case of usual PDT, the sun light allergy and photosensitivity should be avoided for 6 to 12 weeks after PDT because of allergy and photosensitivity. But this patient avoided the sun light for only 10 days.
With invasive cervical cancer group, one case of 3 patients progressed to the worse lesion at 4 months after PDT treatment. Radical hysterectomy and bilateral pelvic & para-aortic lymphadenectomy were performed. She visited our PDT clinic at 2 months after initial punch biopsy (adenocarcinoma) and at 5 weeks after LEEP conization (adenosquamous carcinoma and positive resection margins). Adenosquamous cell-type might be resistant to PDT rather than squamous cell-type carcinoma. Then we had not gotten the plenty of experience of PDT including CCPDT. In this case, we think that adenosquamous cell carcinoma might progress after partial response. Now CCPDT should be essential in any case of invasive cervical cancer. In some case of invasive cancer we could get the utmost effects with interstitial-type CCPDT.
Serious adverse effects associated with PDT were not observed. Some patients had mild edema and burning sensation which had been probably caused by photosensitivity. And these were kept under control with conservative treatment. Recently several photosensitizers, which can provoke less generalized photosensitivity, shorter half-life period, and better tumor selectivity have actively been investigated in the world.
More than 99% of cervical cancers contain one or more of the approximately 15 HPV genotypes that have been associated with the development of cervical cancer. As approximately 50% to 60% of these cancers contain HPV 16, and another 10% to 20% contain HPV 18. Persistent infection with high-risk HPV types, especially 16 and 18, is strongly predictive of cervical neoplasia and cancer. HPV 16 is also the single most commonly identified HPV type in high-grade CIN (CIN 2, 3), as well as among women in the general population [23].
The data of this study are consistent with results of other studies. The data of this study showed that mean age was 31years old. Of 22 patients, CIN 2 were 4 cases (18.2%) and CIN 3 were 15 cases (68.2%) and invasive cervical cancer were 3 cases (13.6%). Of 22 patients, there were 15 (68.2%) positive HPV 16 DNA test diagnoses: 2 in CIN 2, 10 in CIN 3, and 3 in invasive cervical cancer.
On the basis of 1-year period after PDT, the cure rate of HPV was 90.9%. Two cases of 22 patients showed positive HPV DNA test diagnoses: recurrence case in CIN group and PD case in invasive cervical cancer. Recurrence case revealed negative Pap cytology and positive HPV 35 DNA test at 10 months after PDT. Her initial diagnosis was positive HPV 35 DNA test. PD case revealed adenocarcinoma positive Pap cytology and positive HPV 18 DNA test at 4 months after PDT. Her initial diagnoses were positive HPV 16 and 18 DNA tests. 1 case in CIN group showed negative Pap cytology and a new-type HPV 55 DNA test at 8 months after PDT. Her initial diagnoses were positive HPV 66 and 70 DNA tests. Other 3 patients in CIN group have showed negative Pap cytology and positive HPV 16 DNA test: at 16, 35, and 45 months after PDT, respectively. They are in state of no evidence of disease.
Lee et al. [19] reported that cure rate of cervical intraepithelial neoplasia was 92.3%, and eradication rate of HPV infection was 83.3% in PDT group. In the LEEP group, cure rate was 96% and eradication rate of HPV infection was 81%. They concluded that the cure rates and eradication of HPV infection of PDT were comparable with those of LEEP therapy in the management of cervical intraepithelial neoplasia. This study showed that cure rate was 95% and eradication rate of HPV infection also was 95%.
Negativity for both cytology and HPV DNA test has a very high negative predictive value in CIN 2 and the worse. And so, women negative by both cytology and HPV DNA testing could be rescreened after 3 years [24,25].
Nowadays some studies reported improved PDT technique or a combination therapy. PDT based on bioluminescence resonance energy transfer (BRET) suggests more advanced photosensitizer. BRET uses internal bioluminescence light source which is triggered with low wave length of light. When BRET come with day light or ultraviolet light, it will increase the efficiency of PDT in deep tissue by stimulating internal light source [26]. Also another study reported protease-mediated PDT, dual-targeted activatable PDT, gold nanorod-AlPcS4 complex for near-infrared fluorescence imaging and photothermal/PDT of cancers in vivo [27], Smart dual-functional warhead for folate receptor-specific activatable imaging and PDT [28].
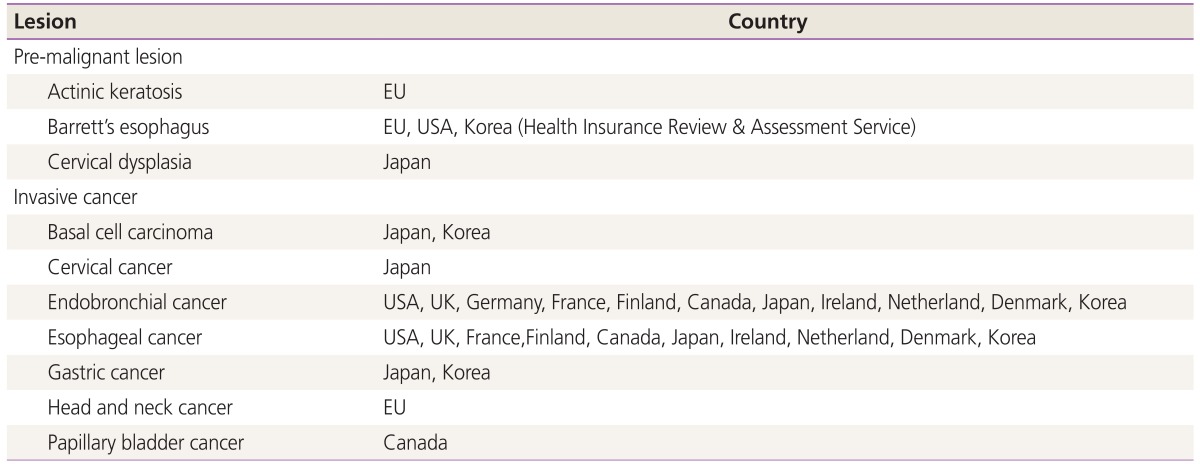
Based on this study, successful pregnancies are possible after PDT without significant adverse effects to the human body for a long follow-up period. However application of PDT is very difficult because of the following reasons especially on the gynecologic field in Korea. First, the medical cost of PDT is very expensive. Now Korean government-oriented health insurance does not permit or cover the medical cost of PDT application in gynecologic patients with pre-malignant or malignant diseases. Only the medical cost of basal cell carcinoma, Barrett’s esophagus, endobronchial cancer, esophageal cancer, and gastric cancer can be covered by government-oriented health insurance in Korea. The other private insurances do not cover the medical cost of PDT at all. Second, photofrin is only photosensitizer approved by Food and Drug Administration. Therefore the prices of photofrin have gone up continuously. Third, there are a few university hospitals to perform PDT on the gynecologic field. Fourth, PDT also has disadvantages of photosensitive side effects, inconvenience, and suffering for a relatively long period (2 to 3 months) due to long half-life period of photofrin photosensitizer (Table 5).
Table 5. Insurance indication for photodynamic therapy in the world.
Anyway, first of all in order to apply PDT actively in Korea, both government-oriented health insurance and the other private insurances can cover the medical cost of PDT in gynecologic patients with pre-malignant or malignant diseases as soon as possible.
In conclusion, our data showed that complete remission rate was fantastic in CIN group (95%). This study suggests that PDT can be recommended as new optimistic management modality on the patients with pre-malignant CIN lesions including CIS and relatively early invasive cancer of the uterine cervix. CCPDT is essential in case of invasive cervical cancer.
For the young age group who desperately want to preserve their fertility and have a healthy baby, PDT can be a beacon of hope.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.International Agency for Research on Cancer. World cancer report 2014 [Internet] Lyon: International Agency for Research on Cancer; [cited 2016 Sep 20]. Available from: http://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports/World-Cancer-Report-2014. [Google Scholar]
- 2.Park CH. Photodynamic therapy in uterine cervix cancer. Dankook Med J. 2009;10:39–47. [Google Scholar]
- 3.Ackroyd R, Kelty C, Brown N, Reed M. The history of photodetection and photodynamic therapy. Photochem Photobiol. 2001;74:656–669. doi: 10.1562/0031-8655(2001)074<0656:thopap>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Park CH. Gynecological cancer and photodynamic therapy. J Korean Photodyn Assoc. 2007;4:10–18. [Google Scholar]
- 5.Park CH, Park JW, Kwon MS, Ahn JC, Chung PS, Rhee CK, et al. Shanghai Laser Medical Association of Chinese Medical Association. A case of squamous cell carcinoma in situ of the uterine cervix cured by photodynamic therapy; Proceedings of the 11th World Congress of the International Photodynamic Association; 2007 March 28-31; Shanghai, China. Beijing: Chinese Medical Association; 2007. p. 158. [Google Scholar]
- 6.Allison RR, Cuenca R, Downie GH, Randall ME, Bagnato VS, Sibata CH. PD/PDT for gynecological disease: a clinical review. Photodiagnosis Photodyn Ther. 2005;2:51–63. doi: 10.1016/S1572-1000(05)00033-5. [DOI] [PubMed] [Google Scholar]
- 7.Cho HC, Park CH. The anticancer effect of photodynamic therapy using ALA and 632 nm diode laser on cervical cancer [dissertation] Cheonan: Dankook University; 2006. [Google Scholar]
- 8.Choi MC, Jung SG, Park H, Lee SY, Lee C, Hwang YY, et al. Fertility preservation by photodynamic therapy combined with conization in young patients with early stage cervical cancer: a pilot study. Photodiagnosis Photodyn Ther. 2014;11:420–425. doi: 10.1016/j.pdpdt.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Kim JS, Chung PS. The antitumor effect and mechanism of photodynamic therapy using 9-hydropheophorbide-a and 670 nm diode laser on HT-3 cervical cancer cell [dissertation] Cheonan: Dankook University; 2006. [Google Scholar]
- 10.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Park CH. Photodynamic therapy using photofrin 630 nm PDT laser in woman with recurrent squamous cell carcinoma in situ of the uterine cervix. J Korean Photodyn Assoc. 2007;4:163–170. [Google Scholar]
- 12.Tao XH, Guan Y, Shao D, Xue W, Ye FS, Wang M, et al. Efficacy and safety of photodynamic therapy for cervical intraepithelial neoplasia: a systemic review. Photodiagnosis Photodyn Ther. 2014;11:104–112. doi: 10.1016/j.pdpdt.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Hopper C. Photodynamic therapy: a clinical reality in the treatment of cancer. Lancet Oncol. 2000;1:212–219. doi: 10.1016/s1470-2045(00)00166-2. [DOI] [PubMed] [Google Scholar]
- 14.Huang Z. A review of progress in clinical photodynamic therapy. Technol Cancer Res Treat. 2005;4:283–293. doi: 10.1177/153303460500400308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.PDT Korea. Handbook of clinical photodynamic therapy. 2nd ed. Seoul: PDT Research Center; 2001. [Google Scholar]
- 16.Korean Photodynamic Therapy Association. Photodynamic therapy. Seoul: Korea Medicine; 2007. [Google Scholar]
- 17.Brown SB, Brown EA, Walker I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004;5:497–508. doi: 10.1016/S1470-2045(04)01529-3. [DOI] [PubMed] [Google Scholar]
- 18.Muroya T, Kawasaki K, Suehiro Y, Kunugi T, Umayahara K, Akiya T, et al. Application of PDT for uterine cervical cancer. Diagn Ther Endosc. 1999;5:183–190. doi: 10.1155/DTE.5.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee C, Kim J, Jeong CH, Na YJ, Kim IH, Lee SY, et al. Photodynamic therapy in the management of cervical intraepithelial neoplasia. Korean J Gynecol Oncol Colposc. 2004;15:85–91. [Google Scholar]
- 20.Han SJ, Song CH, An TK, Lee BR, An YS. The effect of photodynamic therapy on HSIL with hematoporphyrin (HpD), especially in young women. J Korean Photodyn Assoc. 2004;1:101–107. [Google Scholar]
- 21.Yamaguchi S, Tsuda H, Takemori M, Nakata S, Nishimura S, Kawamura N, et al. Photodynamic therapy for cervical intraepithelial neoplasia. Oncology. 2005;69:110–116. doi: 10.1159/000087812. [DOI] [PubMed] [Google Scholar]
- 22.Di Saia PJ, Creasman WT. Preinvasive disease of the cervix. In: Di Saia PJ, Creasman WT, editors. Clinical gynecological oncology. 4th ed. St. Louis (MO): Mosby Year Book; 1992. pp. 1–36. [Google Scholar]
- 23.Park CH, Kim JK. Detection of human papillomavirus type 16 and 18 by PCR in patients with cervical neoplasia. Korean J Obstet Gynecol. 2010;53:894–904. [Google Scholar]
- 24.Park CH. Cervical cytology. 1st ed. Cheonan: Hanyoung; 2004. pp. 61–96. [Google Scholar]
- 25.Park CH. False-negative results of conventional Papanicolaou cervical cytology in women with cervical conization. Korean J Gynecol Oncol. 2008;19:40–47. [Google Scholar]
- 26.Yao H, Zhang Y, Xiao F, Xia Z, Rao J. Quantum dot/bioluminescence resonance energy transfer based highly sensitive detection of proteases. Angew Chem Int Ed Engl. 2007;46:4346–4349. doi: 10.1002/anie.200700280. [DOI] [PubMed] [Google Scholar]
- 27.Kines R, Cuburu N, Kobayashi H, MacDougall J, de los Pinos E, Schiller J. HPV based photodynamic therapy: a new approach for anti-cancer therapy (VAC12P.1019) J Immunol. 2014;192(1 Supplement):206.8. [Google Scholar]
- 28.Kim J, Tung CH, Choi Y. Smart dual-functional warhead for folate receptor-specific activatable imaging and photodynamic therapy. Chem Commun (Camb) 2014;50:10600–10603. doi: 10.1039/c4cc04166f. [DOI] [PubMed] [Google Scholar]