Abstract
Objective
To evaluate the relationship between serum gonadotropin level and parameters related to insulin resistance in Korean women with polycystic ovary syndrome (PCOS).
Methods
This retrospective study included 138 women aged 18 to 35 years who were newly diagnosed with PCOS according to the Rotterdam consensus. Participants were divided into three groups based on the serum luteinizing hormone to follicle-stimulating hormone (LH/FSH) ratio in the early follicular phase: group 1 (LH/FSH <1), group 2 (1.0≤ LH/FSH >2.0), and group 3 (LH/FSH ≥2.0). The correlations between the LH/FSH ratio and various metabolic parameters were evaluated using Pearson correlation coefficients.
Results
Patients with higher LH/FSH ratios showed higher total antral follicle counts and higher total ovarian volume. In the comparison of anthropometric and biochemical parameters among the three groups, the waist to hip ratio was the only parameter that differed significantly among the groups (P=0.003). Correlation analysis revealed no significant correlations between serum LH/FSH ratios and biochemical parameters related to insulin resistance. However, after adjustments for age and body mass index, a significant correlation between total cholesterol level and serum LH/FSH ratio was observed (r=0.221, P=0.018).
Conclusion
Most parameters related to insulin resistance, with the exception of total cholesterol level, are unrelated to the inappropriate pattern of serum gonadotropin secretion in Korean women with PCOS.
Keywords: Follicle stimulating hormone, Gonadotropins, Insulin resistance, Luteinizing hormone, Polycystic ovary syndrome
Introduction
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders, occurring in 5% to 10% of women of reproductive age [1]. Currently, the diagnosis of PCOS [2] is based on the following three criteria: hyperandrogenism, oligo-anovulation, and detection of polycystic ovaries (PCO) on ultrasound. In addition to complications related to the chronic ovulatory dysfunction, metabolic disturbances such as dyslipidemia, impaired glucose tolerance, and type 2 diabetes mellitus (T2DM) can concomitantly occur with PCOS [3].
The characteristic hormonal features in addition to hyperandrogenism in PCOS include inappropriate pituitary gonadotropic hormone secretions, resulting in an increase in the luteinizing hormone (LH) level and subsequent increase in the serum LH to follicle-stimulating hormone (FSH) ratio, and reversal of the peripheral serum estradiol to estrone ratio [4]. Recently, increased serum anti-Müllerian hormone level has been recognized as a useful parameter for the diagnosis and evaluation of PCOS [5].
Inappropriate increases in LH and the LH/FSH ratio are recognized as important factors related to the persistent anovulatory state in PCOS. An inappropriate increase in LH is an important diagnostic marker for estimation of oligo- or anovulation [6], and it is partially included in the diagnostic criteria for PCOS in Japan [7], although it is not involved in the Rotterdam diagnostic criteria [2].
Common metabolic abnormalities related to PCOS are central obesity, dyslipidemia, and impaired glucose tolerance, and all of these are known to be highly associated with insulin resistance [4]. Insulin resistance is one of the most important factors involved in the pathophysiology of PCOS [8]; this is based on correlation with not only the metabolic abnormalities of PCOS but also hyperandrogenism, either directly or indirectly via induction of inappropriate ovarian steroidogenesis [1,4,8]. The manifestation of insulin resistance is one of the important features observed in obese women with PCOS and its incidence in non-obese women with PCOS is higher than that in normal women [1,4]. The prevalence of insulin resistance in women with PCOS differs by race and ethnicity [9,10,11]. The parameters used to evaluate insulin resistance include fasting glucose level, fasting insulin level, and the oral glucose tolerance test results, as well as the results obtained via the hyperinsulinemic-euglycemic clamp technique, the fasting glucose to insulin ratio (GIR), the results from homeostatic model assessment of insulin resistance (HOMA-IR), and the quantitative insulin sensitivity check index (QUICKI) [4].
A number of studies have investigated the association between serum anti-Müllerian hormone level and insulin resistance-related parameters [12,13,14,15]; however, to date, few studies have evaluated the association between serum LH level and insulin resistance with respect to PCOS [16,17]. Moreover, to the best of our knowledge no studies have assessed the relationship between serum gonadotropin level and postprandial glucose level at 1 hour (PPG1).
The aim of the present study was to conduct an in-depth analysis of the associations between early follicular phase serum pituitary gonadotropin levels and clinical and biochemical parameters related to insulin resistance in Korean women with PCOS.
Materials and methods
1. Subjects
This retrospective, observational study was approved by the institutional review board of Inje University Haeundae Paik Hospital. The study participants were Korean women aged 18 to 35 years who were diagnosed with PCOS during their first visit to Inje University Haeundae Paik Hospital between June 2010 and April 2013. All patients with PCOS were diagnosed according to the Rotterdam consensus, which required the presence of at least two out of the following three criteria: 1) oligo-anovulation; 2) clinical and/or biochemical signs of hyperandrogenism; and 3) PCO identified via ultrasonography, after exclusion of other etiologies including congenital adrenal hyperplasia, androgen-secreting tumors, Cushing’s syndrome, thyroid disease, and hyperprolactinemia [2]. Oligo or anovulation was defined as a menstrual cycle longer than 35 days. Clinical hyperandrogenism was defined by the presence of hirsutism (modified Ferriman-Gallwey score >6 [18]), and biochemical hyperandrogenism was defined as an elevated serum androgen concentration beyond the 95% confidence limits (total testosterone >0.68 ng/mL, and/or free testosterone >1.72 pg/mL) [11]. PCO detection via ultrasonography was defined using the following criteria: 1) the presence of 12 or more follicles measuring 2 to 9 mm in diameter in each ovary; and/or 2) increased ovarian volume (>10 mm3) as observed using the transrectal or transvaginal equipment [2]. Any patient with at least one follicle with a diameter of ≥10 mm on ultrasonography was excluded from the current study. Furthermore, patients who had a history of previous ovarian surgery or a suspicious ovarian malignancy and those who had been taking medications known to affect the hypothalamic-pituitary-ovarian axis within 6 months of enrollment (oral contraceptives, ovulation induction agents, glucocorticoids, or anti-androgens) were also excluded from this study. Ultimately, a total of 138 patients were enrolled in the present study.
2. Measurement of anthropometric parameters
Clinical variables such as body weight, height, body mass index (BMI), waist circumference, hip circumference, and waist to hip ratio (WHR) were assessed for all patients during the first visit to our outpatient department. BMI was calculated by dividing the body weight (kg) by the square of the body height (m2).
3. Measurement of antral follicle count and total ovarian volume
Transvaginal or transrectal sonographic examination was performed in the early follicular phase between days 2 and 4 of the menstrual cycle after spontaneous bleeding or withdrawal bleeding induced by medroxyprogesterone acetate Provera (Pfizer Inc., New York, NY, USA; 10 mg/day for 7 days) by using a Voluson S7 (GE Ultrasound Korea, Seongnam, Korea) equipped with a 7-MHz transvaginal transducer. For each ovary, the total number of visible antral follicles measuring 2 to 9 mm in diameter was counted using the continuous scanning method for both ovaries from the inner to outer margins in a longitudinal cross-section. The ovarian volume was calculated using the simplified formula for a prolate ellipsoid (0.5×length×width×thickness) [2]. The total ovarian volume was defined as the sum of both ovarian volumes, and the total antral follicle count was defined as the sum of both antral follicle counts.
4. Biochemical measurement
Blood samples were collected from all participants in tubes without anticoagulants on the same day as the ultrasound examination during the early follicular phase. Serum FSH levels were measured using an Elecsys FSH assay (Roche Diagnostics Corp., Indianapolis, IN, USA) and serum LH levels were measured using an Elecsys LH assay (Roche Diagnostics Corp.). Insulin levels were measured using an Elecsys Insulin assay (Roche Diagnostics Corp.), and fasting glucose levels and levels during a standard 2-hour 75-gram oral glucose tolerance test were measured using L-Type GluI (Wako Pure Chemical Industries, Osaka, Japan). The total cholesterol and triglyceride levels were measured using a Pureauto S (Sekisui Medical, Tokyo, Japan), and high density lipoprotein and low density lipoprotein were measured using a Cholestest (Sekisui Medical, Tokyo, Japan). Both the intraassay and interassay coefficients of variation for all measurements were less than 5%.
5. Assessment of insulin sensitivity
HOMA-IR, QUICKI, and GIR were used for assessment of insulin sensitivity. HOMA-IR was calculated by fasting glucose (mg/dL)×fasting insulin (µU/mL)/405; QUICKI was calculated as 1/[log fasting insulin+log fasting glucose]; and GIR was calculated by dividing the fasting glucose value by the fasting insulin value.
6. Statistical analysis
All data are expressed as mean±standard deviation. All statistical analyses were performed using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA). For comparisons of anthropometric and biochemical parameters among the three groups, one-way analysis of variance (ANOVA) was performed, followed by Tukey multiple comparison test for post hoc analysis. The correlations between serum gonadotropin levels and biochemical parameters related to insulin resistance were evaluated by the Pearson correlation coefficient, and partial correlation coefficients were used after adjusting for confounding factors of age and BMI. Two-tailed P-values less than 0.05 were considered significant for all analyses.
Results
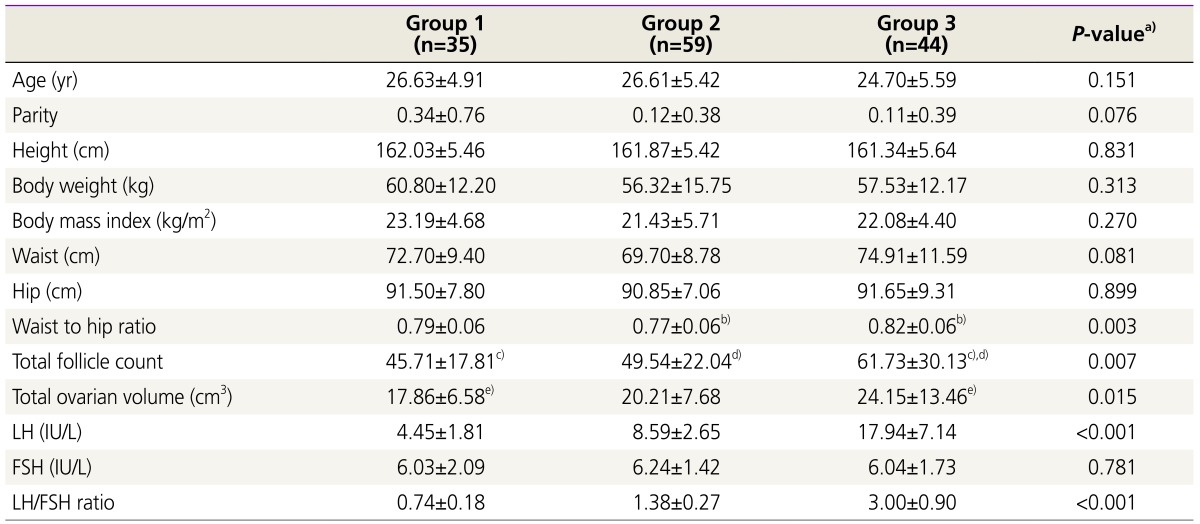
All recruited participants were divided into three groups according to the serum LH/FSH ratio: group 1 (LH/FSH ratio <1.0, n=35), group 2 (1.0≤ LH/FSH ratio <2.0, n=59), and group 3 (LH/FSH ratio ≥2.0, n=44). The comparison of the baseline clinical and hormonal characteristics of the three groups is shown in Table 1. Among the anthropometric parameters, only WHR was significantly different among the three groups. In the post hoc analysis, the mean value of the WHR of group 3 (0.82±0.06) differed significantly from that of group 2 (0.77±0.06). Patients with higher serum LH levels were more likely to show larger numbers of total antral follicles and larger total ovarian volume. Serum LH levels were significantly different among the three groups, while, serum FSH levels were not.
Table 1. Comparison of baseline clinical and hormonal characteristics among the three groups.
Values are mean±standard deviation; Group 1, LH/FSH <1.0; Group 2, 1.0≤ LH/FSH <2.0; Group 3, LH/FSH ≥2.0.
FSH, follicle-stimulating hormone; LH, luteinizing hormone.
a)ANOVA; b),c),d),e)P<0.05 by Tukey’s multiple comparison test.
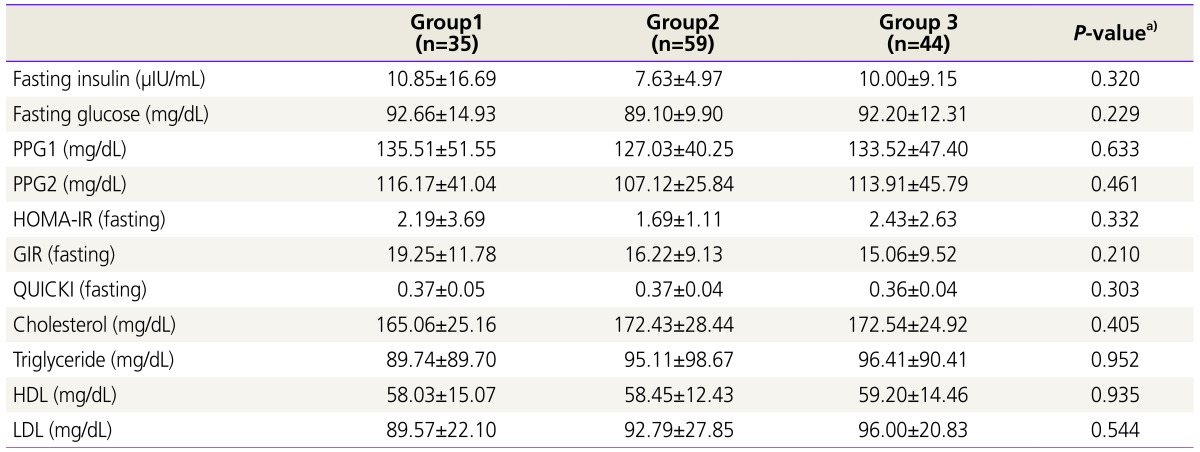
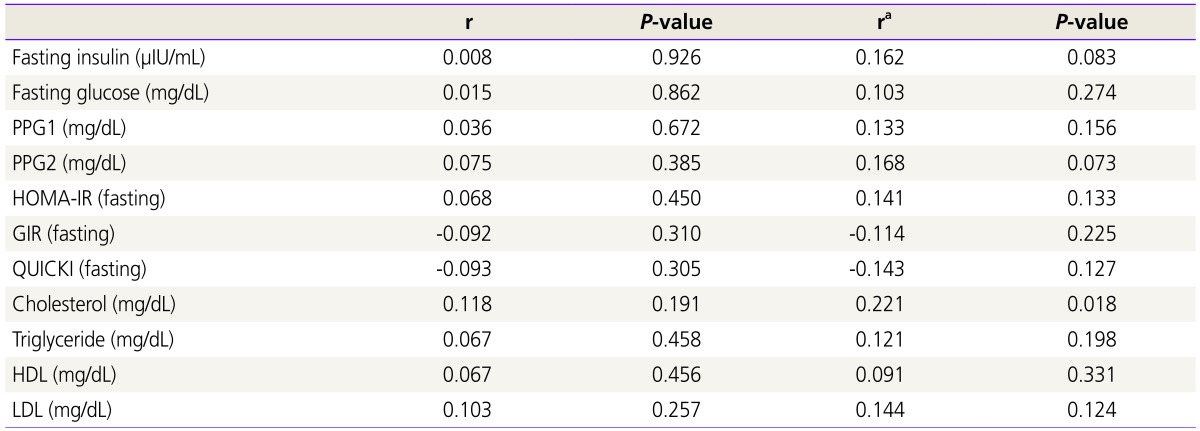
As shown in Table 2, no significant differences were observed in the biochemical metabolic parameters among the three groups. Similarly, as displayed in Table 3, no significant correlations were found between the serum LH/FSH ratio and biochemical parameters related to insulin resistance. However, after adjustments for age and BMI, blood total cholesterol level was significantly correlated with LH/FSH ratio (r=0.221, P=0.018).
Table 2. Comparison of baseline biochemical metabolic parameters among the three groups.
Values are mean±standard deviation; Group 1, LH/FSH <1.0; Group 2, 1.0≤ LH/FSH <2.0; Group 3, LH/FSH ≥2.0.
PPG1, postprandial glucose at 1 hour; PPG2: postprandial glucose at 2 hour; HOMA-IR, homeostasis model assessment of insulin resistance; GIR, glucose to insulin ratio; QUICKI, quantitative insulin sensitivity check index; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
a)ANOVA.
Table 3. Correlations between luteinizing hormone to follicle-stimulating hormone ratio and metabolic parameters among the three groups.
r, Pearson’s correlation coefficient; ra, partial correlation coefficient adjusted by age and body mass index; PPG1, postprandial glucose at 1 hour; PPG2, postprandial glucose at 2 hour; HOMA-IR, homeostasis model assessment of insulin resistance; GIR, glucose to insulin ratio; QUICKI, quantitative insulin sensitivity check index; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Discussion
Inappropriate gonadotropin secretion is one of the most characteristic hormonal features and is associated with the continuation of the anovulatory state in women with PCOS [15]. The abnormal gonadotropin secretion pattern in PCOS is characterized by increased serum LH levels and an increased LH/FSH ratio [4], and this pattern is related to increases in both the amplitude and frequency of LH secretion secondary to increased pulse frequency of hypothalamic gonadotropin-releasing hormone [8,19]. Increased frequency of gonadotropin-releasing hormone secretion favors transcription of the β-subunit of LH over the β-subunit of FSH [8], which causes the increase in the LH/FSH ratio in PCOS patients [8]. A number of studies have proposed appropriate cut-off values for the LH/FSH ratio in PCOS patients [6,20,21,22]; however, the optimal cut-off threshold remains unclear because of the varying sensitivity and specificity. Hsu et al. suggested that an LH/FSH ratio of >1 offered the best combination of sensitivity and specificity for the diagnosis of PCOS [6], while, in contrast, Papaleo et al. [22] postulated that an LH/FSH ratio of ≥2 is the characteristic feature of abnormal gonadotropin secretion in PCOS patients. In the current study, we categorized all participants into three groups according to the LH/FSH ratio: group 1 (LH/FSH ratio <1.0), group 2 (1.0≤ LH/FSH ratio <2.0), and group 3 (LH/FSH ratio ≥2.0). In this study, participants with higher LH/FSH ratios showed higher total antral follicle counts and larger total ovarian volume, and these findings are in agreement with those of previous studies [15,16,22,23,24].
In the comparison of anthropometric parameters, WHR was the only parameter that differed significantly among the three groups in our study. WHR is a well-known clinical factor that is related to hyperinsulinemia and insulin resistance. Sikka et al. [25] reported that ovarian volume positively correlates with the LH/FSH ratio and WHR, and their finding is partially consistent with our results. In contrast, a previous study conducted in Korea [16] reported that WHR was significantly lower in PCOS women with LH/FSH ratios >2, and this finding differs from the results of our study. In the present study, no significant difference was observed in BMI among the three groups. This finding is in conflict with that reported previously where a negative correlation between the LH/FSH ratio and BMI was shown [6,16]; however, it is consistent with the results observed by Carmina et al. [26]. In our study, no statistically significant differences were observed in the biochemical parameters among the three groups. In addition, in the correlation analysis, no statistically significant correlation was observed between any biochemical parameter and the serum LH/FSH ratio. However, after adjustments for age and BMI, a correlation between the total cholesterol level and the LH/FSH ratio was observed. Our finding is in disagreement with the results of Shim et al. [16], which reported that the high density lipoprotein level, not the total cholesterol level, was significantly different according to the LH/FSH ratio. It is hard to explain the rationale of the relationship between the total cholesterol level and LH/FSH ratio in the present study.
Several studies have been conducted to determine whether there is a relationship between abnormal gonadotropin secretion and insulin resistance [16,27,28,29]. Banaszewska et al. [28] reported significant differences in BMI and blood insulin levels between PCOS patients with an LH/FSH ratio >2 and those with an LH/FSH ratio <2, and in addition, most participants in the group with an LH/FSH ratio <2 demonstrated hyperinsulinemia. Kurioka et al. [27] reported that the sum of the plasma glucose and insulin levels before and at 30, 60, and 120 minutes after taking dextrose is significantly higher in those with higher LH/FSH ratios than in those with lower LH/FSH ratios. In the current study, no significant differences were observed among the three groups categorized via LH/FSH ratio in the parameters directly related to insulin resistance, including fasting glucose and insulin level, GIR, HOMA-IR, or QUICKI, and these findings are in disagreement with the results of previous studies [16,27,28].
The results of our study are quite different in many aspects from those reported by Shim et al. [16], who conducted the first study, to our knowledge, on evaluation of the association between inappropriate gonadotropin patterns and metabolic parameters in Korean women with PCOS. The authors divided 225 patients with PCOS into two groups according to LH/FSH ratio: group A (LH/FSH <2, n=160) and group B (LH/FSH >2, n=65). They reported that 2-hour postprandial glucose (PPG2), fasting insulin, and HOMA-IR were significantly higher in group A. These discrepancies in the results between the two studies are difficult to explain. One possible explanation is that most participants in our study visited the hospital for irregular menstruation or infertility and not for symptoms related to hyperandrogenism, suggesting the possibility that the number of patients with clinical or biochemical hyperandrogenism might be lower in our study compared to that of Shim et al. [16]. According to Patel et al. [29], no changes in serum LH concentration or the pattern of LH secretion or changes in reactiveness to gonadotropin-releasing hormone following injection of insulin are observed in patients with PCOS; these results are partially consistent with our results.
To the best of our knowledge, the present study is the first report in Korea on the association between abnormal serum gonadotropin levels and insulin resistance-related parameters including PPG1 and QUICKI, in addition to PPG2 and HOMA-IR. Plasma glucose concentrations peak ~60 minutes after the start of a meal in the non-diabetic population [30]. Recently, PPG1 has been regarded as a strong predictor of the future risk of T2DM comparable to PPG2 [15,31,32], and Abdul-Ghani et al. [31] even suggested that PPG1 may be more useful as a predictor of the future development of T2DM compared to fasting glucose or PPG2. QUICKI has recently become known as an accurate and useful parameter for evaluating insulin resistance [33,34]. To date, it had not been widely used for analyzing insulin resistance in individuals with PCOS.
The most important limitations of the present study are related to its small sample size and retrospective study design. The small sample size prevented us from performing subgroup analysis according to the different phenotypic subgroups of study participants with PCOS. In addition, as described in a previous study of ours [15], we did not assess the postprandial serum insulin levels at 1 and 2 hours, and we only assessed the fasting insulin level.
In conclusion, most insulin-resistance related parameters are not related to the inappropriate pattern of serum gonadotropin secretion in Korean women with PCOS. It is possible that the WHR and plasma total cholesterol level may be associated with the serum LH/FSH ratio in women with PCOS, but further prospective large-scale trials are needed to clarify this preliminary finding.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Kuzbari O, Doralis J, Peterson CM. Endocrine disorders. In: Berek JS, Novak E, editors. Berek & Novak’s gynecology. 15th ed. Philadelphia (PA): Lippincott Williams & Wilkins; 2012. pp. 1075–1080. [Google Scholar]
- 2.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Carmina E, Lobo RA. Polycystic ovary syndrome (PCOS): arguably the most common endocrinopathy is associated with significant morbidity in women. J Clin Endocrinol Metab. 1999;84:1897–1899. doi: 10.1210/jcem.84.6.5803. [DOI] [PubMed] [Google Scholar]
- 4.Fritz MA, Speroff L. Clinical gynecologic endocrinology and infertility. 8th ed. Philadelphia (PA): Lippincott Williams & Wilkins; 2011. pp. 501–518. [Google Scholar]
- 5.Lee JR, Kim SH. Anti-Mullerian hormone and female reproduction. Korean J Obstet Gynecol. 2009;52:285–300. [Google Scholar]
- 6.Hsu MI, Liou TH, Liang SJ, Su HW, Wu CH, Hsu CS. Inappropriate gonadotropin secretion in polycystic ovary syndrome. Fertil Steril. 2009;91:1168–1174. doi: 10.1016/j.fertnstert.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 7.Kubota T. Update in polycystic ovary syndrome: new criteria of diagnosis and treatment in Japan. Reprod Med Biol. 2013;12:71–77. doi: 10.1007/s12522-013-0145-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–1236. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 9.Carmina E, Lobo RA. Use of fasting blood to assess the prevalence of insulin resistance in women with polycystic ovary syndrome. Fertil Steril. 2004;82:661–665. doi: 10.1016/j.fertnstert.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 10.Lin JF, Li X, Zhu MW. Exploration of the classification of polycystic ovarian syndrome. Zhonghua Fu Chan Ke Za Zhi. 2006;41:684–688. [PubMed] [Google Scholar]
- 11.Chae SJ, Kim JJ, Choi YM, Hwang KR, Jee BC, Ku SY, et al. Clinical and biochemical characteristics of polycystic ovary syndrome in Korean women. Hum Reprod. 2008;23:1924–1931. doi: 10.1093/humrep/den239. [DOI] [PubMed] [Google Scholar]
- 12.de Kat AC, Broekmans FJ, Laven JS, van der Schouw YT. Anti-Mullerian hormone as a marker of ovarian reserve in relation to cardio-metabolic health: a narrative review. Maturitas. 2015;80:251–257. doi: 10.1016/j.maturitas.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 13.La Marca A, Orvieto R, Giulini S, Jasonni VM, Volpe A, De Leo V. Mullerian-inhibiting substance in women with polycystic ovary syndrome: relationship with hormonal and metabolic characteristics. Fertil Steril. 2004;82:970–972. doi: 10.1016/j.fertnstert.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Nardo LG, Yates AP, Roberts SA, Pemberton P, Laing I. The relationships between AMH, androgens, insulin resistance and basal ovarian follicular status in non-obese subfertile women with and without polycystic ovary syndrome. Hum Reprod. 2009;24:2917–2923. doi: 10.1093/humrep/dep225. [DOI] [PubMed] [Google Scholar]
- 15.Chun S. 1-h postprandial glucose level is related to the serum anti-Mullerian hormone level in women with polycystic ovary syndrome. Gynecol Endocrinol. 2015;31:815–818. doi: 10.3109/09513590.2015.1056143. [DOI] [PubMed] [Google Scholar]
- 16.Shim AR, Hwang YI, Lim KJ, Choi YM, Jeon YE, Seo SK, et al. Inappropriate gonadotropin secretion in polycystic ovary syndrome: the relationship with clinical, hormonal and metabolic characteristics. Korean J Obstet Gynecol. 2011;54:659–665. [Google Scholar]
- 17.Arroyo A, Laughlin GA, Morales AJ, Yen SS. Inappropriate gonadotropin secretion in polycystic ovary syndrome: influence of adiposity. J Clin Endocrinol Metab. 1997;82:3728–3733. doi: 10.1210/jcem.82.11.4377. [DOI] [PubMed] [Google Scholar]
- 18.Kim JJ, Chae SJ, Choi YM, Hwang SS, Hwang KR, Kim SM, et al. Assessment of hirsutism among Korean women: results of a randomly selected sample of women seeking pre-employment physical check-up. Hum Reprod. 2011;26:214–220. doi: 10.1093/humrep/deq303. [DOI] [PubMed] [Google Scholar]
- 19.Waldstreicher J, Santoro NF, Hall JE, Filicori M, Crowley WF., Jr Hyperfunction of the hypothalamic-pituitary axis in women with polycystic ovarian disease: indirect evidence for partial gonadotroph desensitization. J Clin Endocrinol Metab. 1988;66:165–172. doi: 10.1210/jcem-66-1-165. [DOI] [PubMed] [Google Scholar]
- 20.Cho LW, Jayagopal V, Kilpatrick ES, Holding S, Atkin SL. The LH/FSH ratio has little use in diagnosing polycystic ovarian syndrome. Ann Clin Biochem. 2006;43(Pt 3):217–219. doi: 10.1258/000456306776865188. [DOI] [PubMed] [Google Scholar]
- 21.Minakami H, Abe N, Oka N, Kimura K, Tamura T, Tamada T. Prolactin release in polycystic ovarian syndrome. Endocrinol Jpn. 1988;35:303–310. doi: 10.1507/endocrj1954.35.303. [DOI] [PubMed] [Google Scholar]
- 22.Papaleo E, Doldi N, De Santis L, Marelli G, Marsiglio E, Rofena S, et al. Cabergoline influences ovarian stimulation in hyperprolactinaemic patients with polycystic ovary syndrome. Hum Reprod. 2001;16:2263–2266. doi: 10.1093/humrep/16.11.2263. [DOI] [PubMed] [Google Scholar]
- 23.Wiser A, Shehata F, Holzer H, Hyman JH, Shalom-Paz E, Son WY, et al. Effect of high LH/FSH ratio on women with polycystic ovary syndrome undergoing in vitro maturation treatment. J Reprod Med. 2013;58:219–223. [PubMed] [Google Scholar]
- 24.Chun S. Serum luteinizing hormone level and luteinizing hormone/follicle-stimulating hormone ratio but not serum anti-Mullerian hormone level is related to ovarian volume in Korean women with polycystic ovary syndrome. Clin Exp Reprod Med. 2014;41:86–91. doi: 10.5653/cerm.2014.41.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sikka P, Gainder S, Dhaliwal LK, Bagga R, Sialy R, Sahdev S. Ultrasonography of the ovaries and its correlation with clinical and endocrine parameters in infertile women with PCOS. Int J Fertil Womens Med. 2007;52:41–47. [PubMed] [Google Scholar]
- 26.Carmina E, Orio F, Palomba S, Cascella T, Longo RA, Colao AM, et al. Evidence for altered adipocyte function in polycystic ovary syndrome. Eur J Endocrinol. 2005;152:389–394. doi: 10.1530/eje.1.01868. [DOI] [PubMed] [Google Scholar]
- 27.Kurioka H, Takahashi K, Miyazaki K. Glucose intolerance in Japanese patients with polycystic ovary syndrome. Arch Gynecol Obstet. 2007;275:169–173. doi: 10.1007/s00404-006-0241-0. [DOI] [PubMed] [Google Scholar]
- 28.Banaszewska B, Spaczynski RZ, Pelesz M, Pawelczyk L. Incidence of elevated LH/FSH ratio in polycystic ovary syndrome women with normo- and hyperinsulinemia. Rocz Akad Med Bialymst. 2003;48:131–134. [PubMed] [Google Scholar]
- 29.Patel K, Coffler MS, Dahan MH, Yoo RY, Lawson MA, Malcom PJ, et al. Increased luteinizing hormone secretion in women with polycystic ovary syndrome is unaltered by prolonged insulin infusion. J Clin Endocrinol Metab. 2003;88:5456–5461. doi: 10.1210/jc.2003-030816. [DOI] [PubMed] [Google Scholar]
- 30.American Diabetes Association. Postprandial blood glucose. American Diabetes Association. Diabetes Care. 2001;24:775–778. doi: 10.2337/diacare.24.4.775. [DOI] [PubMed] [Google Scholar]
- 31.Abdul-Ghani MA, Williams K, DeFronzo RA, Stern M. What is the best predictor of future type 2 diabetes? Diabetes Care. 2007;30:1544–1548. doi: 10.2337/dc06-1331. [DOI] [PubMed] [Google Scholar]
- 32.Abdul-Ghani MA, Lyssenko V, Tuomi T, DeFronzo RA, Groop L. Fasting versus postload plasma glucose concentration and the risk for future type 2 diabetes: results from the Botnia Study. Diabetes Care. 2009;32:281–286. doi: 10.2337/dc08-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen H, Sullivan G, Quon MJ. Assessing the predictive accuracy of QUICKI as a surrogate index for insulin sensitivity using a calibration model. Diabetes. 2005;54:1914–1925. doi: 10.2337/diabetes.54.7.1914. [DOI] [PubMed] [Google Scholar]
- 34.Hrebicek J, Janout V, Malincikova J, Horakova D, Cizek L. Detection of insulin resistance by simple quantitative insulin sensitivity check index QUICKI for epidemiological assessment and prevention. J Clin Endocrinol Metab. 2002;87:144–147. doi: 10.1210/jcem.87.1.8292. [DOI] [PubMed] [Google Scholar]