Abstract
Background and Purpose
Chronic treatment can differentially impact the effects of pharmacologically related drugs that differ in receptor selectivity and efficacy.
Experimental Approach
The impact of daily nicotine treatment on the effects of nicotinic ACh receptor (nAChR) agonists was examined in two groups of rhesus monkeys discriminating nicotine (1.78 mg·kg−1 base weight) from saline. One group received additional nicotine treatment post‐session (1.78 mg·kg−1 administered five times daily, each dose 2 h apart; i.e. Daily group), and the second group did not (Intermittent group).
Key Results
Daily repeated nicotine treatment produced a time‐related increase in saliva cotinine. There was no significant difference in the ED50 values of the nicotine discriminative stimulus between the Daily and Intermittent group. Mecamylamine antagonized the effects of nicotine, whereas dihydro‐β‐erythroidine did not. Midazolam produced 0% nicotine‐lever responding. The nAChR agonists epibatidine, RTI‐36, cytisine and varenicline produced >96% nicotine‐lever responding in the Intermittent group. The respective maximum effects in the Daily group were 100, 72, 59 and 28%, which shows that the ability of varenicline to produce nicotine‐like responding was selectively decreased in the Daily as compared with the Intermittent group. When combined with nicotine, both varenicline and cytisine increased the potency of nicotine to produce discriminative stimulus effects.
Conclusion and Implications
Nicotine treatment has a greater impact on the sensitivity to the effects of varenicline as compared with some other nAChR agonists. Collectively, these results strongly suggest that varenicline differs from nicotine in its selectivity for multiple nAChR subtypes.
Abbreviations
- DHβE
dihydro‐β‐erythroidine
- nAChR
nicotinic ACh receptor
- RTI‐36
2′‐fluorodeschloroepibatidine
Tables of Links
| TARGETS | |
|---|---|
| Ligand‐gated ion channels | |
| Nicotinic acetylcholine receptors |
| LIGANDS | |
|---|---|
| DHβE | Nicotine |
| Epibatidine | Varenicline |
| Mecamylamine |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016) and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015).
Introduction
Cigarette smoking is a leading cause of respiratory disease, coronary heart disease, cancer and premature death. Many smokers struggle to quit despite knowing the risks. According to some annual estimates, only 3% who try to quit smoking without pharmacotherapy or counselling do so successfully (Le Houezec and Aubin, 2013). Pharmacotherapies that facilitate quit attempts include nicotine replacement (e.g. gum or transdermal patch), bupropion and varenicline (Wu et al., 2006). Varenicline, a low efficacy α4β2 nicotinic ACh receptor (nAChR) agonist, is reported to be more effective than nicotine replacement as a smoking cessation aid (Aubin et al., 2008).
Drug discrimination has predictive validity for drug abuse treatment. If a test drug shares discriminative stimulus effects with a drug of abuse, then that test drug has the potential to be a ‘substitution’ therapy (Schuster et al., 1981; Schuster and Johanson, 1988; McMahon, 2015). Drug discrimination has also been used to classify pharmacological mechanisms underlying the in vivo effects of nicotine, varenicline and other nAChR‐based smoking cessation aids such as cytisine, which is used in Europe to promote smoking cessation (Stolerman, 1990; Etter et al., 2008; Perkins, 2009; Smith and Stolerman, 2009). Varenicline and cytisine were shown to substitute fully for the discriminative stimulus effects of nicotine in some studies (Pratt et al., 1983; Craft and Howard, 1988; Chandler and Stolerman, 1997; Rollema et al., 2007; Cunningham et al., 2012), but to partially substitute for nicotine in other studies (Smith et al., 2007; LeSage et al., 2009; Jutkiewicz et al., 2011; Cunningham and McMahon, 2013). These results suggest that varenicline and cytisine are clinically effective because they partially or fully mimic the effects of nicotine. However, the pharmacological factors responsible for partial versus full substitution of these drugs for nicotine have not been firmly established. One possibility is that the low efficacy of varenicline and cytisine underlies partial substitution, but experimental support has not been unanimous (Chandler and Stolerman, 1997; Cunningham and McMahon, 2013).
Typically, nicotine is trained as a discriminative stimulus when administered once every 2–3 days; this has two potential limitations. Firstly, drug administration every 2–3 days might not adequately predict pharmacological mechanisms under conditions of frequent, daily administration, that is, cigarette smoking throughout the day every day. Secondly, with respect to detecting differences in efficacy, they are most likely to be evident under conditions of decreased receptor function (Kenakin, 1997), such as that resulting from chronic treatment. Although nicotine treatment can differentially modify the number and function of multiple nAChR subtypes; marked desensitization of α4β2 nAChRs is typical (Reitstetter et al., 1999; Gentry and Lukas, 2002; Quick and Lester, 2002; Picciotto et al., 2008).
The current study compared the effects of nAChR agonists differing in selectivity and efficacy at the two most widely expressed nAChR subtypes in brain (i.e. α4β2 and α7; Table 1) under two distinct conditions of nicotine treatment. As described previously (Cunningham et al., 2012), one group of rhesus monkeys discriminated nicotine (1.78 mg·kg−1) from saline in the absence of additional nicotine treatment outside of discrimination sessions. Varenicline and cytisine, but not the benzodiazepine midazolam (i.e. a non‐nAChR control), were demonstrated to fully substitute for nicotine; moreover the non‐selective nAChR antagonist mecamylamine (Bacher et al., 2009), but not the β2‐subunit selective antagonist dihydro‐β‐erythroidine (DHβE) (Harvey and Luetje, 1996; Harvey et al., 1996), antagonized the nicotine discriminative stimulus (Cunningham et al., 2012). In a second group, the same dose of nicotine (1.78 mg·kg−1) was discriminated from saline; however, this group received five additional doses of 1.78 mg·kg−1 of nicotine every 2 h for a total of 8.9 mg·kg−1·day−1 after daily discrimination sessions. Here, in a systematic replication, drugs tested previously were tested again, that is, for the first time in the monkeys receiving chronic nicotine treatment and a second time in monkeys not receiving chronic nicotine. Two additional nAChR agonists, epibatidine and a chemical analogue 2′‐fluorodeschloroepibatidine (RTI‐36) (Carroll et al., 2005), were tested. Agonists with relatively low α4β2 nAChR efficacy (e.g. varenicline and cytisine) were predicted to be less likely to substitute for nicotine under conditions of chronic as compared with intermittent nicotine treatment.
Table 1.
Published binding affinities and efficacies of nAChR ligands at α4β2 and α7 nAChRs
Methods
Subjects
Five adult rhesus monkeys (Macaca mulatta), including four male and one female, discriminated nicotine (1.78 mg·kg− 1 s.c.) from saline while receiving daily nicotine treatment (8.9 mg·kg−1·day−1). A separate group of five adult rhesus monkeys, including two males and three females, discriminated nicotine (1.78 mg·kg− 1 s.c.) from saline as described previously (Cunningham et al., 2012) but did not receive daily nicotine treatment. All monkeys were housed individually in stainless steel cages on a 14 h light/10 h dark schedule (lights on at 06:00 h). They were maintained at 95% free‐feeding weight (range 6–10.5 kg) with a diet consisting of primate chow (Harlan Teklad, High Protein Monkey Diet; Madison, WI), fresh fruit and peanuts; water was continuously available in the home cage. Monkeys were maintained, and experiments were conducted in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio and the National Institutes of Health's Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 2011).
Monkeys were removed from home cages, weighed and inspected daily for signs of illness or distress. Anaesthesia, analgesia or surgical procedures were not required for the conduct of these experiments; animals were not killed as part of this study. Experiments were conducted as humanely as possible. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015). The behavioural effects of drugs in non‐human subjects are highly predictive of psychopharmacological effects of drugs in humans and are highly sensitive to the effects of daily drug treatment on complex behavioural processes, such as those relating to the subjective effects of drugs, when such a study in humans is not feasible or ethical. Monkeys have been used extensively for studying the effects of abused drugs in drug discrimination procedures, although the use of nicotine discrimination procedures in monkeys has been relatively underutilized. The within‐subjects design used in the current study, in which all subjects experienced all treatment conditions, reduces the number of animals required to complete the study (Sidman, 1960).
Behavioural apparatus
Each monkey sat in a chair (Model R001; Primate Products, Miami, FL, USA) facing two levers and two lights (i.e. one above each lever) affixed to a stainless steel panel within ventilated, sound‐attenuating chambers. Feet were maintained in contact with brass electrodes to which a brief electric stimulus (3 mA, 250 ms) could be delivered from an a/c generator (Coulbourn Instruments, Whitehall, PA, USA). An interface (MedAssociates) connected the chambers to a computer, which controlled and recorded lever responses with Med‐PC software.
Daily nicotine treatment
Monkeys received nicotine (8.9 mg·kg−1·day−1) via multiple s.c. injections in the home cage. The pH of the nicotine solution was adjusted to 7 with buffer. Nicotine was administered daily in five separate injections of 1.78 mg·kg−1 every 2 h, that is, at 10:00 h, 12:00 h, 14:00 h, 16:00 h and 18:00 h. Monkeys did not receive nicotine or other drugs after 18:00 h and before discrimination sessions beginning at 08:00 h the following day. Monkeys that discriminated nicotine in the absence of daily nicotine treatment received saline throughout the day. However, because nicotine dose–effect functions did not vary according to whether saline was administered every 2 h after test sessions or not; repeated saline was discontinued.
Quantification of saliva cotinine
Once nicotine treatment was initiated, saliva was collected daily for eight consecutive days and discrimination sessions were not conducted so that nicotine could be administered every day at 08:00 h, that is, the time corresponding with the discrimination sessions. On Day 1, saliva was collected immediately before nicotine (1.78 mg·kg−1) was administered at 08:00 h. Additional samples were collected at 10, 30, 60, 90 and 120 min post‐injection and thereafter at 1 h intervals until 18:00 h. Subsequent doses (1.78 mg·kg−1) of nicotine were administered at 10:00 h, 12:00 h, 14:00 h, 16 00 h and 18:00 h; saliva was collected immediately before nicotine administration at those time points. For the next 7 days (i.e. days 2–8 of chronic treatment), saliva was collected twice daily, the first sample before the first daily dose of nicotine (i.e. 08:00 h) and the second sample before the last dose of the day (i.e. 18:00 h).
While monkeys were seated in chairs, a sterile, 6 inch cotton swab was inserted between the gums and the lips of the jaw on both sides of the mouth. The cotton tip was then separated from the applicator and placed into a 2 mL microcentrifuge tube containing a filter (Grace Davison Discovery Science, Deerfield, IL, USA); the tube was spun at 10 000 g for 5 min. Samples were frozen at −80°C until extraction for HPLC analysis. For sample extraction, mobile phase B (2% acetonitrile, 98% Millipore water, 2 g octane sulfonic acid salt, 13.6 g sodium acetate, pH 4.0) and internal standard (desipramine) were added to the samples and then centrifuged at 16 060 g for 5 min. Filters were then removed from the tubes, and 3.4 M perchloric acid was added; samples were again spun at 16 060 g for 5 min. The supernatant was transferred to a new microcentrifuge tube and 100 mM potassium phosphate buffer was added; Certify Bond Elut preparatory columns (130 mg) were prepared, and the samples were loaded, rinsed and eluted with dichloromethane/isopropanol/ammonium hydroxide in respective proportions of 78/20/2. Samples were then dried under nitrogen at 37°C, suspended again in 50% methanol, centrifuged at 16 060 g for 5 min and transferred to an autosampler; the injection volume was 160 μL, and the flow rate was 1 mL·min−1. The HPLC column was an Alltima C18 5 μ (150 × 4.6 mm) with UV detection (Waters 2487).
Discrimination training
Discrimination session parameters were identical for both groups of monkeys: those receiving nicotine treatment daily and those that did not. Responding was maintained under a fixed ratio 5 schedule of stimulus shock termination. Experimental sessions consisted of 1–2 cycles; the duration of a cycle was 20 min. The beginning of each cycle consisted of a 10 min time out; during a time out, the lights were not on and responding had no programmed consequence. The time out was immediately followed by a 10 min schedule of stimulus shock termination. Illumination of the lights signalled that an electric stimulus was scheduled for delivery every 10 s; however, five consecutive responses on the correct lever extinguished the lights, prevented delivery of the electric stimulus and postponed the schedule for 30 s. Incorrect responses reset the response requirement. The correct lever was determined by administration of either saline or the training dose at the beginning of a cycle. For half of the monkeys, the left lever was correct after the training dose of nicotine and the right lever was correct after saline. The assignments were reversed for the remaining monkeys. If four electric stimuli were delivered in a cycle, the experimental session was terminated.
Saline training consisted of administration of saline in the first, 20 min cycle followed by saline or sham in the second, 20 min cycle. Nicotine training consisted of administration of the training dose at the beginning of a cycle; a nicotine training cycle was preceded by 0–1 saline training cycles. The order of training with nicotine and saline varied non‐systematically across cycles. Consecutive cycles for a particular training condition did not exceed two for nicotine training or three for saline training.
Discrimination testing
The first test was conducted when, during five consecutive or six out of seven training sessions, at least 80% of the total responses occurred on the correct lever and fewer than five responses occurred on the incorrect lever prior to completion of the first ratio requirement on the correct lever. Test sessions were identical to training sessions except that five consecutive responses on either lever postponed the schedule and animals could receive various doses of nicotine or a test drug. Further tests were conducted when performance for consecutive training sessions, including both saline and nicotine training sessions, satisfied the test criteria.
To establish dose–effect functions, a dose of nicotine, epibatidine, RTI‐36, varenicline, cytisine or midazolam was administered at the beginning of a session consisting of a single cycle. The dose range was determined per individual monkey and included a dose producing less than 20% nicotine‐lever responding up to a dose that produced greater than 80% nicotine‐lever responding, decreased response rate to less than 20% control or produced observable signs of toxicity. To study the effects of nicotine in combination with another test drug, saline, mecamylamine (1 mg·kg−1), DHβE (3.2 mg·kg−1), varenicline (3.2 mg·kg−1) or cytisine (32 mg·kg−1) was administered in the first cycle followed by saline or a dose of nicotine in the second cycle. These doses of varenicline and cytisine were selected for drug combination tests because they were the largest doses that did not decrease response rate to less than 20% of control when administered alone. The order of testing of doses and drugs was non‐systematic within and between groups. In the chronic nicotine treatment group, the nicotine dose–effect function was determined twice approximately 18 months apart.
Drugs
Drugs were administered s.c. in the midscapular region of the back in a volume of 0.1–0.3 mL·kg−1; doses (mg·kg−1) were expressed as the weight of the forms listed below, with the exception of nicotine that was expressed as the weight of the free base. The following were dissolved in physiological saline: nicotine hydrogen tartrate salt, epibatidine dihydrochloride (Sigma‐Aldrich, St. Louis, MO, USA), mecamylamine hydrochloride (Waterstone Technology, Carmel, IN, USA), RTI‐36 synthesized as described previously (Carroll et al., 2005), varenicline dihydrochloride (Research Triangle Institute, Research Triangle Park, NC, USA), cytisine (Atomole Scientific, Hubei, China) and dihydro‐β‐erythroidine hydrobromide (Tocris, St. Louis, MO, USA). Midazolam was obtained in a commercially available solution of 5 mg·mL−1 in saline (Bedford Laboratories, Bedford, OH, USA).
Data analysis
Data are expressed as the mean ± SEM of values from five monkeys, unless otherwise noted. Discrimination data were calculated as a percentage of responses on the nicotine lever divided by the sum of responses on the saline and nicotine levers. Rate of responding was calculated as responses.s‐1 on both levers during illumination of the lights and excluded responses during time outs. Response rate was calculated as a percentage of control; control response rate was defined as the mean of the five saline training cycles immediately preceding the test, excluding any failed training sessions. Discrimination data for an individual subject were not included for analyses when response rate was less than 20% of the control for that subject, but all response rate data were plotted and analysed.
Potency to produce discriminative stimulus effects or changes in the rate of responding was calculated with linear regression of individual dose–effect data combined in the same analysis by means of GraphPad Prism version 5.0 for Windows (San Diego, CA, USA). Doses included for analysis were selected on an individual monkey basis and included all doses producing 20–80% nicotine‐lever responding, not more than one dose producing less than 20% nicotine‐lever responding and not more than one dose producing greater than 80% nicotine‐lever responding. Other doses were excluded from the analyses. When comparing two or more dose–effect functions, if the slopes were not significantly different from each other, then the common slope of best fit was used to calculate doses producing 50% effect (i.e. ED50 values) and corresponding 95% confidence limits. If the intercepts of two dose–effect functions were significantly different, then the corresponding potencies of the functions were considered significantly different (P < 0.05). If mean nicotine‐lever responding was not greater than 50% up to the largest dose studied in all monkeys (e.g. varenicline in the Daily nicotine group), then an F‐ratio test was used to examine whether or not the dose–response functions in the Intermittent and Daily nicotine treatment groups were significantly different from each other. To determine whether or not a drug significantly decreased response rate, linear regression was conducted with the three largest doses and an F‐ratio test examined whether or not the slope was significantly different from 0. If mean response rate was decreased to less than 50% of control, then ED50 values were calculated. The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015).
Results
Cotinine
In five monkeys that had not received nicotine for 30 days, initiation of repeated nicotine treatment produced a time‐related increase in saliva cotinine. The first dose of nicotine (1.78 mg·kg−1 s.c.) administered at 08:00 h produced 1789 ng·mL−1 cotinine in saliva at 90 min (i.e. 09:30 h; Figure 1 top). There was a further increase in cotinine with subsequent doses of nicotine administered at 10:00 h, 12:00 h, 14:00 h, 16:00 h and 18:00 h; cotinine was 18 676 ng·mL−1 saliva when measured immediately before administration of the last daily dose at 18:00 h. From Days 2–8 of daily nicotine treatment, cotinine was measured twice daily: immediately before the first nicotine dose at 08:00 h and before the last dose at 18:00 h. Cotinine exhibited a systematic pattern of relatively low amounts at 08:00 h and higher amounts at 18:00 h (Figure 1 bottom). On Day 2, cotinine was at 8235 ng·mL−1 of saliva immediately prior to the first daily dose and 15 831 ng·mL−1 saliva immediately before the last daily dose. The means of the 08:00 h and 18:00 h measurements for all Days 2–8 were 5128 and 15 320 ng·mL−1 respectively.
Figure 1.
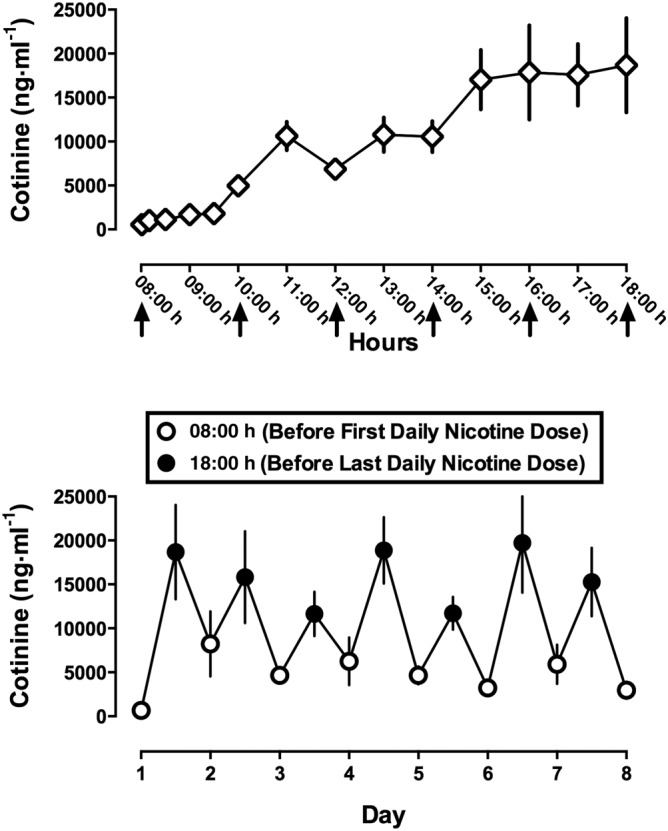
Saliva cotinine in rhesus monkeys receiving 8.9 mg·kg−1·day−1 of nicotine. Abscissae: hour of day, spanning the first to the last dose of nicotine, on the first day of daily nicotine administration (top) and consecutive days of nicotine treatment (bottom). Ordinates: mean ± SEM (n = 5) cotinine in ng·mL−1 of saliva. Nicotine was administered at a dose of 1.78 mg·kg−1 every 2 h beginning at 08 h and ending at 18 h. The arrows show time of nicotine administration. Open circles are cotinine levels immediately before the first daily dose (8 AM), and closed circles are cotinine levels immediately before the last daily dose (6 PM).
Discriminative stimulus effects of nicotine in monkeys receiving 8.9 mg·kg−1·day−1 of nicotine: effects of mecamylamine, DHβE and midazolam
For the group of monkeys (n = 5) receiving 8.9 mg·kg−1·day−1 of nicotine post‐session, the total number of training sessions required for each monkey to pass the test criteria, including both nicotine and saline training sessions, was 26, 32, 38, 71 and 88. The nicotine dose–effect functions determined at the beginning and end of this study were not significantly different from each other (F 2,23 = 1.66, P = 0.21). Nicotine dose‐dependently increased the percentage of responses on the nicotine lever to 98% at the training dose (1.78 mg·kg− 1 s.c.) (Figure 2 top, open circles). Saline resulted in 1% nicotine‐lever responding (Figure 2 top, open circle above Sal). The ED50 value (95% confidence limits) of nicotine to produce discriminative stimulus effects was 0.67 (0.50–0.90) mg·kg−1 (Table 2). Nicotine (up to 1.78 mg·kg−1) did not significantly modify response rate (F 1,13 = 2.07, P = 0.17).
Figure 2.
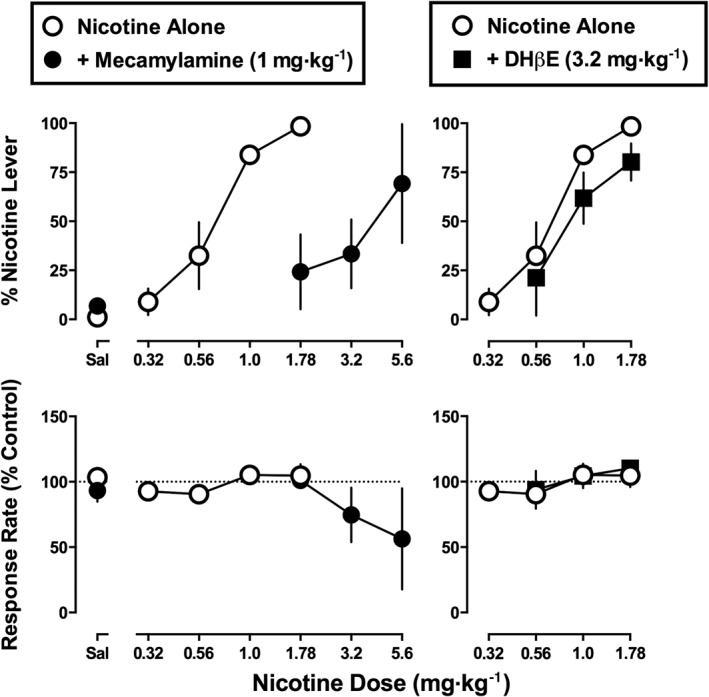
Effects of nicotine alone and in combination with 1 mg·kg−1 of mecamylamine or 3.2 mg·kg−1 of DHβE in monkeys discriminating nicotine (1.78 mg·kg−1) while receiving 8.9 mg·kg−1·day−1 of nicotine. Abscissae: saline (Sal) or dose of nicotine in mg·kg−1 body weight administered s.c. Ordinates: mean (± SEM) percentage of responding on the nicotine lever (top) and mean (± SEM) rate of responding expressed as a percentage of control (bottom). The data for nicotine alone in the corresponding left and right panels are identical. Data are the means of five monkeys per group.
Table 2.
Statistical comparison of slopes and intercepts of dose–effect functions for nicotine alone versus nicotine in combination with another test drug for discriminative stimulus effects and corresponding ED50 values in the Daily nicotine treatment group
| Test drug (dose mg·kg−1) | Slope | Intercept | ED50 (mg·kg−1) |
|---|---|---|---|
| Nicotine alone | – | – | 0.67 |
| + Mecamylamine (1) | F 1,22 = 0.60, P = 0.45 | F 1,24 = 41.4, P < 0.05a | 3.7 |
| + DHβE (3.2) | F 1,20 = 0.11, P = 0.74 | F 1,22 = 2.08 P = 016 | 0.92 |
| + Varenicline (3.2) | F 1,20 = 2.49, P = 0.13 | F 1,22 = 6.75, P < 0.05a | 0.31 |
| + Cytisine (32) | F 1,16 = 1.09, P = 0.31 | F 1,18 = 14.5, P < 0.05a | 0.19 |
The F‐ratio values compare the slope and intercept of the control nicotine dose–effect function (i.e. Nicotine alone) to the respective slope and intercept of the nicotine dose–effect function determined in the presence of another test drug.
Significant difference in potency versus nicotine alone.
Mecamylamine (1 mg·kg−1) alone produced 7% responding on the nicotine lever (Figure 2 top left, closed circle above Sal). When combined with nicotine, mecamylamine (1 mg·kg−1) produced significant antagonism; maximum drug‐lever responding was 69% at 5.6 mg·kg−1 of nicotine in combination with mecamylamine (Figure 2 top left, filled circles). The slopes of the nicotine dose–effect functions determined in the absence and presence of mecamylamine (1 mg·kg−1) were not significantly different from each other (F 1,22 = 0.60, P = 0.45). Antagonism was evidenced by a significant difference in intercept (F 1,24 = 41.4, P < 0.05); mecamylamine increased the ED50 value of nicotine 5.5‐fold to 3.7 mg·kg−1 (Table 2). Up to the training dose (1.78 mg·kg−1), nicotine alone did not significantly alter response rate. Moreover, in the presence of mecamylamine, nicotine up to a dose of 5.6 mg·kg−1 did not significantly alter response rate (F 1,9 = 3.28, P = 0.10). The nicotine dose–effect functions determined in the absence and presence of DHβE (3.2 mg·kg−1) had slopes that were not significantly different from each other (F 1,20 = 0.11, P = 0.74). DHβE did not significantly antagonize the discriminative stimulus effects of nicotine (F 1,22 = 2.08, P = 0.16); moreover, response rate was not significantly altered by DHβE in combination with nicotine (F 1,9 = 1.39, P = 0.27) (Figure 2 right).
Midazolam (0.32–3.2 mg·kg−1) produced a maximum of 1% nicotine‐lever responding up to a dose (3.2 mg·kg−1) that significantly decreased response rate (F 1,11 = 19.2, P < 0.001) (data not shown). The ED50 value (95% confidence limits) of midazolam to produce rate‐decreasing effects was 0.63 (0.33–1.0) mg·kg−1.
The effects of nicotine, epibatidine and RTI‐36 in monkeys discriminating nicotine: intermittent versus daily (8.9 mg·kg−1) nicotine treatment
Nicotine dose‐dependently increased the percentage of responses on the nicotine lever to 98% at the training dose (1.78 mg·kg−1) in five monkeys not receiving daily nicotine treatment (i.e. Intermittent group; Figure 3, top left, squares); saline produced 0% of responses on the nicotine lever. The slopes of the nicotine dose–effect functions in monkeys receiving Intermittent versus Daily nicotine treatment were not significantly different from each other (F 1,20 = 2.65, P = 0.12) (Table 3). The ED50 value (95% confidence limits) of nicotine to produce discriminative stimulus effects in the Intermittent group was 0.40 (0.21–0.79) mg·kg−1; the ED50 values of nicotine in the Intermittent versus Daily treatment groups were not significantly different from each other (F 1,22 = 2.70, P = 0.11). Nicotine did not significantly modify response rate in either the Intermittent or Daily nicotine treatment group (F 1,13 = 0.28, P = 0.60 and F 1,13 = 2.07, P = 0.17 respectively).
Figure 3.
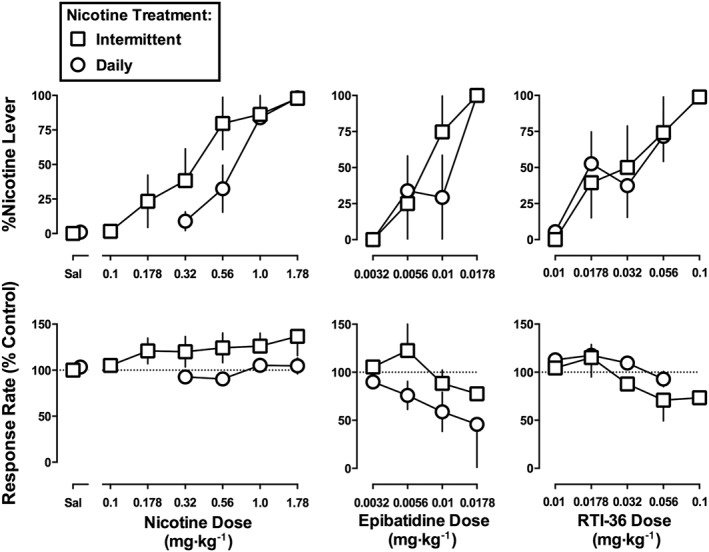
Effects of nicotine (left), epibatidine (middle) and RTI‐36 (right) in monkeys discriminating nicotine (1.78 mg·kg−1) without additional nicotine treatment (i.e. Intermittent) or while receiving 8.9 mg·kg−1·day−1 of nicotine (i.e. Daily). Abscissae: saline (Sal) or dose in mg·kg−1 body weight administered s.c. Ordinates: mean (± SEM) percentage of responding on the nicotine lever (top) and mean (± SEM) rate of responding expressed as a percentage of control (bottom). The nicotine data for the Daily group are re‐plotted from Figure 1. Data for nicotine and RTI‐36 are the means of five monkeys per group. Data for epibatidine are the means of four monkeys per group, except for the dose of 0.0178 mg·kg−1 in the Daily group, which are from two monkeys.
Table 3.
Statistical comparison of slopes and intercepts of the dose–effect functions for each test drug in the Intermittent versus Daily nicotine treatment groups for discriminative stimulus effects and corresponding ED50 values
| Test drug | Slope | Intercept | ED50 (mg·kg−1) | |
|---|---|---|---|---|
| Intermittent | Daily | |||
| Nicotine | F 1,20 = 2.65, P = 0.12 | F 1,22 = 2.70, P = 0.11 | 0.40 | 0.67 |
| Epibatidine | F 1,11 = 0.06, P = 0.82 | F 1,13 = 0.01, P = 0.92 | 0.0075 | 0.0078 |
| RTI‐36 | F 1,21 = 0.0013, P = 0.97 | F 1,23 = 0.21, P = 0.65 | 0.027 | 0.037 |
| Varenicline | N/A | N/A | 0.72 | N/Aa |
| Cytisine | F 1,16 = 1.97, P = 0.18 | F 1,18 = 1.05, P = 0.32 | 34 | 48 |
F‐ratio values compare the slopes and intercepts of the dose–effect functions for each drug determined in the absence of daily nicotine treatment (i.e. Intermittent) versus the presence of daily nicotine treatment (i.e. Daily).
Significant difference Intermittent versus Daily.
N/A, not applicable; % nicotine‐lever responding was not increased to greater than 50% in the Daily nicotine treatment group.
Epibatidine dose‐dependently increased the percentage of responses on the nicotine lever up to 100% at 0.0178 mg·kg−1 in both the Intermittent and Daily nicotine treatment groups (Figure 3, top middle). Complete dose–response functions were generated in four monkeys per group; however, the largest dose of epibatidine was tested in two of four monkeys in the Daily nicotine treatment group. The slopes of the epibatidine dose–effect functions were not significantly different from each other (F 1,11 = 0.06, P = 0.82). The ED50 values (95% confidence limits) of epibatidine to produce nicotine‐like discriminative stimulus effects were 0.0075 (0.0037–0.015) mg•kg−1 for the Intermittent group and 0.0078 (0.0042–0.014) mg·kg−1 for the Daily nicotine treatment group; these ED50 values did not significantly differ (F 1,13 = 0.01, P = 0.92). Although epibatidine dose‐dependently decreased response rate in a subset of monkeys, the effect was not significant at the group level (F 1,6 = 0.89, P = 0.38 for the Intermittent group; F 1,7 = 2.07, P = 0.41 for the Daily group). RTI‐36 dose‐dependently increased nicotine‐lever responding in both the Intermittent and Daily nicotine treatment groups to 74 and 72%, respectively, at a dose of 0.056 mg·kg−1 (Figure 3, top right). Only 4/5 monkeys in each group responded greater than 80% on the nicotine lever at 0.056 mg·kg−1 of RTI‐36. In the monkey from the Intermittent group that did not respond greater than 80% on the nicotine lever at 0.056 mg·kg−1, the next larger dose of RTI‐36 (0.1 mg·kg−1) produced 99% nicotine‐lever responding. However, because behavioural toxicity (e.g. tremor and ataxia) was observed, 0.1 mg·kg−1 was not tested further. The slopes and ED50 values calculated from the RTI‐36 dose–effect functions did not vary significantly between the Intermittent and Daily nicotine treatment groups. The ED50 values (95% confidence limits) of RTI‐36 were 0.027 (0.0064–0.12) mg·kg−1 in the Intermittent group and 0.037 (0.013–0.11) mg·kg−1 in the Daily nicotine group. RTI‐36 up to 0.056 mg·kg−1 did not significantly alter response rate in the Intermittent (F 1,8 = 4.29, P = 0.07) or the Daily group (F 1,13 = 4.03, P = 0.07).
The effects of varenicline and cytisine alone and in combination with nicotine
Varenicline dose‐dependently increased nicotine‐lever responding up to 99% at a dose of 1.78 mg·kg−1 in the Intermittent nicotine treatment group (Figure 4 top left, squares); the ED50 value of varenicline was 0.72 mg·kg−1. Varenicline, at a dose of 3.2 mg·kg−1, produced 0, 0, 0, 29 and 100% nicotine‐lever responding (mean 28%) in individual monkeys in the Daily treatment group (Figure 4 top left, circles). One monkey in the Daily group responded 100% on the nicotine lever at a larger dose of varenicline (5.6 mg·kg−1); however, because marked signs of behavioural toxicity including tremor and ataxia were observed up to 8 h post‐injection, 5.6 mg·kg−1 of varenicline was not studied further. The varenicline dose–effect functions (excluding 5.6 mg·kg−1) determined in the Intermittent and Daily groups were significantly different from each other (F 2,17 = 4.49, P < 0.05). Varenicline, up to the largest dose studied, did not significantly modify response rate in either group (P = 0.45 and P = 0.27 for Intermittent and Daily groups respectively). Cytisine, up to 56 mg·kg−1, dose‐dependently increased the percentage of responses on the nicotine lever to 96% in the Intermittent group and 59% in the Daily group (Figure 4, top right); respective ED50 values were 34 and 48 mg·kg−1. The slopes and intercepts of the cytisine dose–effect functions were not significantly different from each other (Table 3). Cytisine, up to 56 mg·kg−1, did not significantly modify response rate in either group (P = 0.63 and P = 0.51 for Intermittent and Daily groups respectively). Larger doses of cytisine were not studied to avoid adverse effects as reported in Cunningham et al. (2012).
Figure 4.
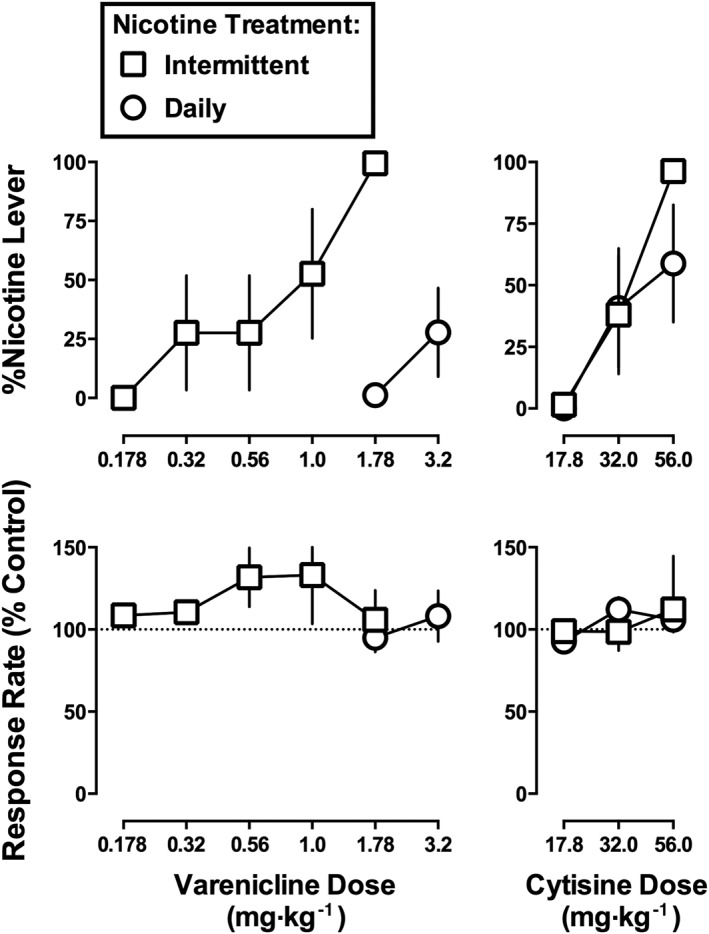
Effects of varenicline (left) and cytisine (right) in monkeys discriminating nicotine (1.78 mg·kg−1) without additional nicotine treatment (i.e. Intermittent) or while receiving 8.9 mg·kg−1·day−1 of nicotine (i.e. Daily). Abscissae: saline (Sal) or dose in mg·kg−1 body weight administered s.c. Ordinates: mean (± SEM) percentage of responding on the nicotine lever (top) and mean (± SEM) rate of responding expressed as a percentage of control (bottom). Data are the means of five monkeys per group.
In the Daily nicotine treatment group, varenicline (3.2 mg·kg−1) produced 28% nicotine‐lever responding when given alone (Figure 5, top left, triangle above Sal). When that dose of varenicline (3.2 mg·kg−1) was combined with nicotine in the Daily nicotine treatment group, the potency of nicotine to produce discriminative stimulus effects was significantly increased, that is, the ED50 value of nicotine decreased 2.2‐fold (Figure 5, top left; Table 2). Similarly, a dose of cytisine (32 mg·kg−1) that produced 59% nicotine‐lever responding on its own significantly increased the potency of nicotine to produce discriminative stimulus effects 3.5‐fold (Figure 5, top right; Table 2). Response rate was not significantly modified by the combination of varenicline (3.2 mg·kg−1) and nicotine (F 1,10 = 0.95, P = 0.35) or cytisine (32 mg·kg−1) and nicotine (F 1,5 = 1.37, P = 0.29).
Figure 5.
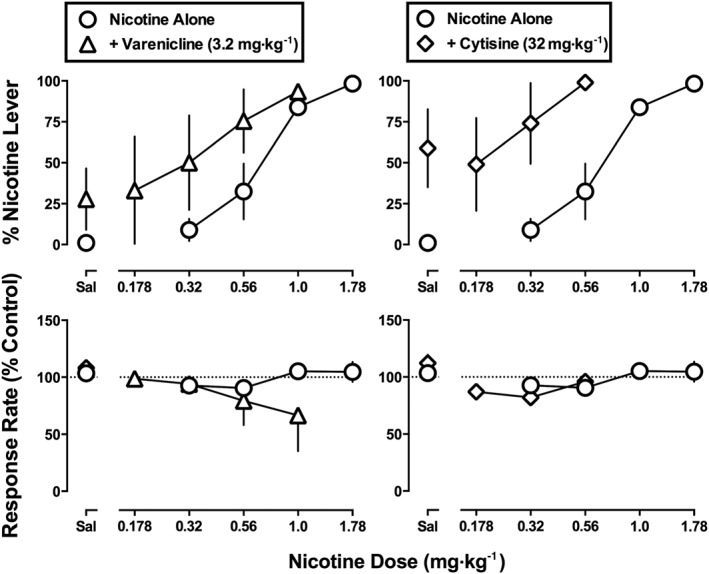
Effects of nicotine alone and in combination with 3.2 mg·kg−1 of varenicline (left) or 32 mg·kg−1 of cytisine (right) in monkeys discriminating nicotine (1.78 mg·kg−1) while receiving 8.9 mg·kg−1·day−1 of nicotine. Abscissae: saline (Sal) or dose of nicotine in mg·kg−1 body weight administered s.c. Ordinates: mean (± SEM) percentage of responding on the nicotine lever (top) and mean (± SEM) rate of responding expressed as a percentage of control (bottom). The effects of varenicline (3.2 mg·kg−1) and cytisine (32 mg·kg−1) alone are shown above Sal and are re‐plotted from Figure 4. Data for nicotine alone are re‐plotted from Figure 1. Data are the means of five monkeys per group.
Discussion
A dose of nicotine (1.78 mg·kg−1 free base) previously established as a discriminative stimulus in the absence of daily nicotine treatment in rhesus monkeys (i.e. Intermittent group; Cunningham et al., 2012) was established here as a discriminative stimulus in a separate group of monkeys that received 8.9 mg·kg−1·day−1 of nicotine post‐session during the light portion of the light–dark cycle (i.e. Daily group). Saliva cotinine from the Daily group greatly exceeded levels measured in cigarette smokers (Swan et al., 1993), yet discrimination was pharmacologically selective for nAChR agonism inasmuch as midazolam failed to substitute for nicotine in the Daily group (present results) and the Intermittent group (Cunningham et al., 2012). Mecamylamine (1 mg·kg−1) antagonized the discriminative stimulus effects of nicotine, consistent with involvement of nAChRs; however, the β2 nAChR antagonist DHβE did not antagonize the effects of nicotine in either group (present results; Cunningham et al., 2012). Epibatidine, RTI‐36 and cytisine produced nicotine‐like effects that did not differ between groups. In contrast, the effectiveness of varenicline was significantly decreased in the Daily group as compared with the Intermittent group. Varenicline and cytisine increased the potency of nicotine in both groups (present results; Cunningham et al., 2012). While these results show that chronic nicotine selectively decreases the potency of varenicline, the nature of the interactions among nAChR agonists implicates differences in selectivity for multiple nAChR subtypes.
Varenicline (Chantix) yields higher abstinence rates than nicotine replacement in cigarette smokers (Aubin et al., 2008) and has lower efficacy than nicotine at α4β2 nAChRs in vitro (Rollema et al., 2007). However, it remains unclear to what extent this pharmacological profile mediates in vivo effects. According to receptor theory (Kenakin, 1997), decreased receptor function resulting from chronic drug treatment produces a greater loss of sensitivity to the effects of a low efficacy agonist as compared with a higher efficacy agonist, assuming effects are mediated by the same receptor across different experimental conditions. The apparent loss of maximum effects for varenicline but not other nAChR agonists with higher efficacy, under conditions of chronic nicotine treatment, is compatible with a difference in efficacy. However, varenicline did not antagonize the effects of nicotine, inconsistent with actions differentiated solely upon the basis of low versus high efficacy.
Nicotine, epibatidine, RTI‐36, varenicline and cytisine have a minimum 41‐fold higher affinity for α4β2 nAChRs over homomeric α7 nAChRs (Table 1), yet the current results implicate the involvement of nAChR subtypes aside from or in addition to α4β2 nAChRs. DHβE, a selective antagonist for nAChRs containing β2 subunits in vitro (Grady et al., 2010), did not significantly antagonize the discriminative stimulus effects of nicotine. Before ruling out actions at nAChRs containing β2 subunits, one caveat is that doses of DHβE larger than 3.2 mg·kg−1 are lethal in rhesus monkeys (Cunningham et al., 2012), thereby limiting its utility as a pharmacological tool in vivo. DHβE is more selective for nAChRs containing β2 as compared with those containing β4 subunits, and replacing α3 with α4 subunits in β4‐containing nAChRs further decreases sensitivity to DHβE (Harvey and Luetje, 1996; Harvey et al., 1996). In addition to nAChRs containing β4 subunits, the current results implicate the possible involvement of α7 nAChRs in mediating the discriminative stimulus effects of nAChR agonists. However, the extent to which β4‐containing and α7 nAChRs differentially mediate the effects of varenicline as compared with nicotine and the other nAChR test drugs remains to be established.
Epibatidine and its chemical analogue RTI‐36 substituted for nicotine in both the Intermittent and Daily nicotine treatment groups. Substitution of epibatidine for nicotine is consistent with its high efficacy at nAChRs in vitro (Grady et al., 2010). The results of the current study suggest that RTI‐36 also has high efficacy. The potencies of the nAChR agonists to produce discriminative stimulus effects differed markedly, with epibatidine and RTI‐36 being 53‐ and 15‐fold more potent, respectively, than nicotine in the Intermittent group and 86‐ and 18‐fold more potent, respectively, than nicotine in the Daily group. This is comparable to relative potencies for producing other effects in other species (Rodriguez et al., 2014).
Nicotine is metabolized by CYP2A6 enzymes in the liver into 3‐hydroxycotinine and cotinine (Nakajima et al., 1996; Schoedel et al., 2003; Dempsey et al., 2004). Because cotinine has a much longer half‐life in biological fluids (16–18 h) than nicotine (1–2 h; Benowitz et al., 2009), cotinine is typically preferred over nicotine as a biomarker of daily intake in cigarette smokers (Swan et al., 1993). There is a good correlation between cotinine measured in the blood, urine and saliva (Jarvis et al., 2003). Unlike blood draws, the collection of saliva samples is noninvasive and there is no concern about the amount of sample to be collected. Unlike urination, saliva can be readily obtained at multiple time points at intervals determined by the experimenter. The amount of cotinine measured in monkeys from the Daily nicotine treatment group markedly exceeded amounts typically measured in cigarette smokers. According to one study (Swan et al., 1993), individuals who smoked up to 25 cigarettes in 17 h exhibited cotinine levels as high as 600 ng·mL−1. By comparison, cotinine measured before and after 8.9 mg·kg−1·day−1 of nicotine in the current study was 8‐ and 25‐fold larger respectively. As demonstrated previously (Cunningham et al., 2012), the training dose of nicotine produced 1128 ng of cotinine mL−1 saliva in the Intermittent group. Because published functions describing the relationship between cotinine and the number of cigarettes smoked are not linear (e.g. Swan et al., 1993), the current cotinine data cannot be easily translated into the number of smoked cigarettes in humans.
The doses of nicotine used in the current study are generally larger than doses used previously in monkeys. Nicotine administered s.c. up to 5.6 mg·kg−1 (De La Garza and Johanson, 1983; Mello and Newman, 2011) and i.v. at 0.1 mg·kg−1 (Gould et al., 2011) did not fully substitute for the discriminative stimulus effects of cocaine in rhesus monkeys. In squirrel monkeys discriminating nicotine, relatively small doses can be trained (e.g. 0.1 and 0.178 mg·kg−1; Takada et al., 1989; Desai and Bergman, 2014). Nicotine (up to 0.32 mg·kg−1) substitutes for a methamphetamine discriminative stimulus in squirrel monkeys, and these effects are antagonized by DHβE (Desai and Bergman, 2014). Nicotine is approximately threefold more potent in rhesus monkeys as compared with squirrel monkeys, based on self‐administration data (Slifer and Balster, 1985; Mello and Newman, 2011; Desai et al., 2016; Kohut and Bergman, 2016) and is approximately threefold more potent when administered i.v. versus s.c. (unpublished observations).
The pharmacology underlying the discriminative stimulus effects of nicotine varies as a function of training dose (Jutkiewicz et al., 2011; Cunningham and McMahon, 2013), with α4β2 nAChRs mediating the effects of small training doses and other nAChR subtypes mediating the effects of larger training doses. Because mecamylamine but not DHβE antagonized the discriminative stimulus effects of nicotine (1.78 mg·kg−1 s.c.) in rhesus monkeys (current results; Cunningham et al., 2012), nAChRs besides α4β2 appear to be involved. The current nicotine discrimination assays could inform upon the pharmacological mechanisms underlying nicotine‐induced aversion and dependence. Large doses of nicotine punish operant responding in monkeys (Goldberg and Spealman, 1982; Koffarnus and Winger, 2015) and produce conditioned place aversion in mice (Risinger and Oakes, 1995). Nicotine‐induced aversion and dependence are mediated by nAChR subtypes containing α5 or β4 subunits (reviewed in Fowler and Kenny, 2014), and chronic nicotine treatment results in marked decreases in the function of β2‐containing nAChRs and little or no change in function at β4‐containing nAChRs (Meyers et al., 2015). Collectively, these results suggest that discrimination of relatively large doses of nicotine in monkeys is mediated by β4‐containing nAChRs.
In summary, in monkeys discriminating nicotine, daily nicotine treatment selectively decreased the effects of varenicline relative to other nAChR agonists. Because the antagonism tests implicate β4‐containing and α7 nAChRs in mediating the effects of nicotine under the current experimental conditions, varenicline appears to have less prominent actions at these particular subtypes. These results underscore the contribution of multiple receptor subtypes to the in vivo effects of nicotine and varenicline, especially at large doses, which leads to different behavioural effects. These results suggest that the clinical effectiveness of smoking cessation aids is determined by actions at multiple nAChRs in vivo, rather than a single subtype of nAChR, such as α4β2.
Author contributions
C.S.C., M.J.M. and L.R.M. participated in research design. C.S.C. and M.J.M. conducted experiments. M.A.J. and F.I.C. contributed new reagents or analytical tools. C.S.C., M.J.M., M.A.J. and L.R.M. performed data analysis. C.S.C., M.J.M., M.A.J. and L.R.M. wrote or contributed to writing of the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
The authors are grateful to C. Rock and D. Schulze for technical assistance. This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grant DA25267].
Cunningham, C. S. , Moerke, M. J. , Javors, M. A. , Carroll, F. I. , and McMahon, L. R. (2016) Attenuated nicotine‐like effects of varenicline but not other nicotinic ACh receptor agonists in monkeys receiving nicotine daily. British Journal of Pharmacology, 173: 3454–3466. doi: 10.1111/bph.13635.
References
- Alexander SPH, Peters JA, Kelly E, Marrion N, Benson HE, Faccenda E et al. (2015). The Concise Guide to PHARMACOLOGY 2015/16: Ligand‐gated ion channels. Br J Pharmacol 172: 5870–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin HJ, Bobak A, Britton JR, Oncken C, Billing CB Jr, Gong J et al. (2008). Varenicline versus transdermal nicotine patch for smoking cessation: results from a randomised open‐label trial. Thorax 63: 717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacher I, Wu B, Shytle DR, George TP (2009). Mecamylamine – a nicotinic acetylcholine receptor antagonist with potential for the treatment of neuropsychiatric disorders. Expert Opin Pharmacother 10: 2709–2721. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Hukkanen J, Jacob P 3rd (2009). Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol 192: 29–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll FI, Ma W, Yokota Y, Lee JR, Brieaddy LE, Navarro HA et al. (2005). Synthesis, nicotinic acetylcholine receptor binding, and antinociceptive properties of 3′‐substituted deschloroepibatidine analogues. Novel nicotinic antagonists. J Med Chem 48: 1221–1228. [DOI] [PubMed] [Google Scholar]
- Chandler CJ, Stolerman IP (1997). Discriminative stimulus properties of the nicotinic agonist cytisine. Psychopharmacology (Berl) 129: 257–264. [DOI] [PubMed] [Google Scholar]
- Craft RM, Howard JL (1988). Cue properties of oral and transdermal nicotine in the rat. Psychopharmacology (Berl) 96: 281–284. [DOI] [PubMed] [Google Scholar]
- Cunningham CS, McMahon LR (2013). Multiple nicotine training doses in mice as a basis for differentiating the effects of smoking cessation aids. Psychopharmacology (Berl) 228: 321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CS, Javors MA, McMahon LR (2012). Pharmacologic characterization of a nicotine‐discriminative stimulus in rhesus monkeys. J Pharmacol Exp Ther 341: 840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Garza RD, Johanson CE (1983). The discriminative stimulus properties of cocaine in the rhesus monkey. Pharmacol Biochem Behav 19: 145–148. [DOI] [PubMed] [Google Scholar]
- Desai RI, Bergman J (2014). Methamphetamine‐like discriminative‐stimulus effects of nicotinic agonists. J Pharmacol Exp Ther 348: 478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai RI, Sullivan KA, Kohut SJ, Bergman J (2016). Influence of experimental history on nicotine self‐administration in squirrel monkeys. Psychopharmacology (Berl) 233: 2253–2263. [DOI] [PubMed] [Google Scholar]
- Dempsey D, Tutka P, Jacob P 3rd., Allen F, Schoedel K, Tyndale RF et al. (2004). Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther 76: 64–72. [DOI] [PubMed] [Google Scholar]
- Etter JF, Lukas RJ, Benowitz NL, West R, Dresler CM (2008). Cytisine for smoking cessation: a research agenda. Drug Alcohol Depend 92: 3–8. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Kenny PJ (2014). Nicotine aversion: Neurobiological mechanisms and relevance to tobacco dependence vulnerability. Neuropharmacology 76: 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry CL, Lukas RJ (2002). Regulation of nicotinic acetylcholine receptor numbers and function by chronic nicotine exposure. Curr Drug Targets CNS Neurol Disord 1: 359–385. [DOI] [PubMed] [Google Scholar]
- Grady SR, Drenan RM, Breining SR, Yohannes D, Wageman CR, Fedorov NB et al. (2010). Structural differences determine the relative selectivity of nicotinic compounds for native alpha4beta2*‐, alpha6beta2*‐, alpha3beta4*‐ and alpha7‐ nicotine acetylcholine receptors. Neuropharmacology 58: 1054–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD (1982). Maintenance and suppression of behavior by intravenous nicotine injections in squirrel monkeys. Fed Proc 41: 216–220. [PubMed] [Google Scholar]
- Gould RW, Czoty PW, Nader SH, Nader MA (2011). Effects of varenicline on the reinforcing and discriminative stimulus effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther 339: 678–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SC, Luetje CW (1996). Determinants of competitive antagonist sensitivity on neuronal nicotinic receptor beta subunits. J Neurosci 16: 3798–3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SC, Maddox FN, Luetje CW (1996). Multiple determinants of dihydro‐betaerythroidine sensitivity on rat neuronal nicotinic receptor alpha subunits. J Neurochem 67: 1953–1959. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (2011). Guide for the Care and Use of Laboratory Animals, 8th edn. Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council: Washington, DC. [Google Scholar]
- Jarvis MJ, Primatesta P, Erens B, Feyerabend C, Bryant A (2003). Measuring nicotine intake in population surveys: comparability of saliva cotinine and plasma cotinine estimates. Nicotine Tob Res 5: 349–355. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Brooks EA, Kynaston AD, Rice KC, Woods JH (2011). Patterns of nicotinic receptor antagonism: nicotine discrimination studies. J Pharmacol Exp Ther 339: 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin TP (1997). Pharmacologic Analysis of Drug‐Receptor Interaction. Lippincott‐Raven: Philadelphia. [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Winger G (2015). Individual differences in the reinforcing and punishing effects of nicotine in rhesus monkeys. Psychopharmacology (Berl) 232: 2393–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohut SJ, Bergman J (2016). Reinforcing effectiveness of nicotine in nonhuman primates: effects of nicotine dose and history of nicotine self‐administration. Psychopharmacology (Berl) 233: 2451–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Houezec J, Aubin HJ (2013). Pharmacotherapies and harm‐reduction options for the treatment of tobacco dependence. Expert Opin Pharmacother 14: 1959–1967. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Shelley D, Ross JT, Carroll FI, Corrigall WA (2009). Effects of the nicotinic receptor partial agonists varenicline and cytisine on the discriminative stimulus effects of nicotine in rats. Pharmacol Biochem Behav 91: 461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LR (2015). The rise (and fall?) of drug discrimination research. Drug Alcohol Depend 151: 284–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Newman JL (2011). Discriminative and reinforcing stimulus effects of nicotine, cocaine, and cocaine + nicotine combinations in rhesus monkeys. Exp Clin Psychopharmacol 19: 203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers EE, Loetz EC, Marks MJ (2015). Differential expression of the beta4 neuronal nicotinic receptor subunit affects tolerance development and nicotinic binding sites following chronic nicotine treatment. Pharmacol Biochem Behav 130: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, Yamamoto T, Nunoya K, Yokoi T, Nagashima K, Inoue K et al. (1996). Characterization of CYP2A6 involved in 3'‐hydroxylation of cotinine in human liver microsomes. J Pharmacol Exp Ther 277: 1010–1015. [PubMed] [Google Scholar]
- Ondachi PW, Ye Z, Castro AH, Luetje CW, Damaj MI, Mascarella SW et al. (2015). Synthesis, nicotinic acetylcholine receptor binding, in vitro and in vivo pharmacology properties of 3′‐(substituted pyridinyl)‐deschloroepibatidine analogs. Bioorg Med Chem 23: 5693–5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA (2009). Discriminative stimulus effects of nicotine in humans. Handb Exp Pharmacol 192: 369–400. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH (2008). It is not "either/or": activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol 84: 329–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt JA, Stolerman IP, Garcha HS, Giardini V, Feyerabend C (1983). Discriminative stimulus properties of nicotine: further evidence for mediation at a cholinergic receptor. Psychopharmacology (Berl) 81: 54–60. [DOI] [PubMed] [Google Scholar]
- Quick MW, Lester RA (2002). Desensitization of neuronal nicotinic receptors. J Neurobiol 53: 457–478. [DOI] [PubMed] [Google Scholar]
- Reitstetter R, Lukas RJ, Gruener R (1999). Dependence of nicotinic acetylcholine receptor recovery from desensitization on the duration of agonist exposure. J Pharmacol Exp Ther 289: 656–660. [PubMed] [Google Scholar]
- Risinger FO, Oakes RA (1995). Nicotine‐induced conditioned place preference and conditioned place aversion in mice. Pharmacol Biochem Behav 51: 457–461. [DOI] [PubMed] [Google Scholar]
- Rodriguez JS, Cunningham CS, Moura FB, Ondachi P, Carroll FI, McMahon LR (2014). Psychopharmacology (Berl) 231: 4455–4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA et al. (2007). Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology 52: 985–994. [DOI] [PubMed] [Google Scholar]
- Sala M, Braida D, Pucci L, Manfredi I, Marks MJ, Wageman CR et al. (2013). CC4, a dimer of cytisine, is a selective partial agonist at α4β2/α6β2 nAChR with improved selectivity for tobacco smoking cessation. Br J Pharmacol 168: 835–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoedel KA, Sellers EM, Palmour R, Tyndale RF (2003). Down‐regulation of hepatic nicotine metabolism and a CYP2A6‐like enzyme in African green monkeys after long‐term nicotine administration. Mol Pharmacol 63: 96–104. [DOI] [PubMed] [Google Scholar]
- Schuster CR, Johanson CE (1988). Relationship between the discriminative stimulus properties and subjective effects of drugs. Psychopharmacol Ser 4: 161–175. [DOI] [PubMed] [Google Scholar]
- Schuster CR, Fischman MW, Johanson CE (1981). Internal stimulus control and subjective effects of drugs In: Thompson T, Johanson CE. (eds). Behavioral Pharmacology of Human Drug Dependence, NIDA Research Monograph Series 37 National Institutes of Health, U.S. Department of Health and Human Services: Washington, DC, pp. 116–129. [PubMed] [Google Scholar]
- Sidman M (1960). Tactics of Scientific Research: Evaluating Experimental Data in Psychology. Authors Cooperative: Boston. [Google Scholar]
- Slifer BL, Balster RL (1985). Intravenous self‐administration of nicotine: with and without schedule‐induction. Pharmacol Biochem Behav 22: 61–69. [DOI] [PubMed] [Google Scholar]
- Smith JW, Stolerman IP (2009). Recognising nicotine: the neurobiological basis of nicotine discrimination. Handb Exp Pharmacol 192: 295–333. [DOI] [PubMed] [Google Scholar]
- Smith JW, Mogg A, Tafi E, Peacey E, Pullar IA, Szekeres P et al. (2007). Ligands selective for alpha4beta2 but not alpha3beta4 or alpha7 nicotinic receptors generalise to the nicotine discriminative stimulus in the rat. Psychopharmacology (Berl) 190: 157–170. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP (1990). Behavioural pharmacology of nicotine: implications for multiple brain nicotinic receptors. Ciba Found Symp 152: 3–22. [DOI] [PubMed] [Google Scholar]
- Swan GE, Habina K, Means B, Jobe JB, Esposito JL (1993). Saliva cotinine and recent smoking – evidence for a nonlinear relationship. Public Health Rep 108: 779–783. [PMC free article] [PubMed] [Google Scholar]
- Takada K, Swedberg MD, Goldberg SR, Katz JL (1989). Discriminative stimulus effects of intravenous l‐nicotine and nicotine analogs or metabolites in squirrel monkeys. Psychopharmacology (Berl) 99: 208–212. [DOI] [PubMed] [Google Scholar]
- Wu P, Wilson K, Dimoulas P, Mills EJ (2006). Effectiveness of smoking cessation therapies: a systematic review and meta‐analysis. BMC Public Health 6: 300. [DOI] [PMC free article] [PubMed] [Google Scholar]


