Abstract
The endoplasmic reticulum (ER) is an important intracellular membranous organelle. Previous studies have demonstrated that the ER is responsible for protein folding and trafficking, lipid synthesis and the maintenance of calcium homeostasis. Interestingly, the morphology and structure of the ER were recently found to be important. Melatonin is a hormone that anticipates the daily onset of darkness in mammals, and it is well known that melatonin acts as an antioxidant by scavenging free radicals and increasing the activity of antioxidant enzymes in the body. Notably, the existing evidence demonstrates that melatonin is involved in ER homeostasis, particularly in the morphology of the ER, indicating a potential protective role of melatonin. This review discusses the existing knowledge regarding the implications for the involvement of melatonin in ER homeostasis.
Abbreviations
- AD
Alzheimer's disease
- ATF6
activating transcription factor 6
- BIP
binding immunoglobulin protein
- CCK‐8
cholecystokinin octapeptide
- Cd
cadmium
- CHOP
growth arrest‐ and DNA damage‐inducible genes 153
- CsA
cyclosporine A
- DM
diabetes mellitus
- eIF2α
eukaryotic translation initiation factor 2α
- ER
endoplasmic reticulum
- H/I
hypoxia/ischaemia
- HCC
hepatocellular carcinoma
- Hsp70
heat shock protein 70
- IP3
inositol trisphosphate
- IRE1
inositol‐requiring protein 1
- IRS
insulin receptor substrate
- LD
12‐h light:12‐h dark
- LL
continuous light
- PERK
PRKR‐like ER kinase
- RER
rough ER
- RHDV
rabbit haemorrhagic disease virus
- SER
smooth ER
- UPR
unfolded protein response
- XBP1
X‐box binding protein 1
- α‐SMA
α‐smooth muscle actin
Tables of Links
| TARGETS | |
|---|---|
| Other protein targets a | Enzymes c |
| Bax | BIP |
| Bcl‐2 | eIF2α |
| GPCRs b | IRE1 |
| Melatonin receptors | JNK |
| PERK | |
| SP1 |
| LIGANDS |
|---|
| Cadmium |
| Cyclosporine A |
| IP3 |
| Melatonin |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016) and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,b,cAlexander et al., 2015a,b,c).
Introduction
Melatonin, known as the ‘hormone of darkness’, is important in both animals (Brzezinski, 1997; Tosches et al., 2014) and plants (Arnao and Hernandez‐Ruiz, 2014). In mammals, melatonin is primarily produced by the pineal gland, displays characteristic daily and seasonal patterns of secretion and plays an important role in sleep regulation (Tosches et al., 2014; Gandhi et al., 2015). Melatonin and the melatonin receptor have been demonstrated to be associated with major depression (Hickie and Rogers, 2011), jet lag (Waterhouse et al., 2007; Sack, 2010), multiple sclerosis (Farez et al., 2015), diabetes mellitus (DM) (McMullan et al., 2013), cancer (Dauchy et al., 2014; Sigurdardottir et al., 2015; Ma et al., 2016), fibrosis in multiple organs (Hu et al., 2016) and drug addiction (Feng et al., 2013) and might represent novel treatment targets. In particular, the administration of melatonin is able to normalize the increased pro‐inflammatory profile observed during metabolic syndrome (Cano Barquilla et al., 2014), atherosclerosis (Hu et al., 2013) and sepsis (Galley et al., 2014). Furthermore, the presence of numerous transmitters originating from various sources, particularly noradrenaline, has been reported to regulate the production and secretion of melatonin in the pineal gland (Simonneaux and Ribelayga, 2003; Gonzalez et al., 2012). The melatonin produced by the pineal gland further influences the rest of the body (Borjigin et al., 1999), and it has been reported that the reproductive system is controlled by the daily rhythm in melatonin production (Tamarkin et al., 1985).
The endoplasmic reticulum (ER) has a remarkably complex structure consisting of a single bilayer with a network of sheets and dynamic tubules (Westrate et al., 2015). Numerous genetic and environmental insults have been demonstrated to disturb the ultrastructure and volume of the ER, leading to a buildup of misfolded proteins in this organelle (a condition called ER stress) and initiation of the unfolded protein response (UPR) to remove the misfolded proteins (Walter and Ron, 2011; Brodsky, 2012; Hetz, 2013; Mehnert et al., 2014; Yang et al., 2015). An abnormal ER and ER stress are emerging as key contributors to a growing list of human diseases (Tabas and Ron, 2011; Walter and Ron, 2011; Oakes and Papa, 2015), including cancer (Mahoney et al., 2011; Clarke et al., 2014), neurological disease (Roussel et al., 2013; Hetz and Mollereau, 2014) and metabolic diseases (Fu et al., 2011; Arruda et al., 2014; Yang et al., 2015). Notably, there is close relationship between ER structure and function, and defects in ER structure are associated with diseases (Westrate et al., 2015). The structure and morphology of the ER can be regulated by various factors (Chen et al., 2012; English and Voeltz, 2013), and melatonin has attracted increasing attention.
This review focuses on the relationship between melatonin and the ER. Firstly, we briefly summarize the normal structure and functions of the ER. We then discuss the existing implications for melatonin in ER homeostasis, and the regulation of ER stress by melatonin in various pathologies is subsequently introduced. The information compiled in this review should help scientists understand the involvement of melatonin in ER homeostasis.
Summary of the ER
The ER is an important intracellular membranous organelle in eukaryotic organisms that consists of an interconnected network of flattened, membrane‐enclosed sacs or tubes known as cisternae (Hu et al., 2011; Smith et al., 2011). The ER is located throughout much of the cytoplasm, and the membranes of the ER are continuous with the plasma and nuclear membranes (Giordano et al., 2013; Chung et al., 2015; Westrate et al., 2015). A close association exists between the ER and the mitochondria (Rowland and Voeltz, 2012; Arruda et al., 2014), Golgi complex (Zanetti et al., 2012; He et al., 2013) and peroxisome (van der Zand et al., 2012; Tabak et al., 2013). The ER is found in most types of eukaryotic cells, including the most primitive Giardia (Soltys et al., 1996), and can be classified into two types, namely, rough ER (RER) and smooth ER (SER), depending on the presence of protein‐manufacturing ribosomes on the surface (Reid and Nicchitta, 2015). The RER is mainly associated with protein synthesis, and the SER is mainly responsible for lipid metabolism, carbohydrate metabolism and detoxification (Friedman and Voeltz, 2011). In general, the ER is responsible for protein folding and trafficking (Rowland and Voeltz, 2012; Zanetti et al., 2012; Reid and Nicchitta, 2015), lipid synthesis (Rowland and Voeltz, 2012; Prinz, 2014) and the maintenance of calcium homeostasis (Smith et al., 2011; Stutzmann and Mattson, 2011; Rowland and Voeltz, 2012).
The correct folding of newly synthesized proteins is made possible by the involvement of several ER chaperone proteins, including protein disulfide isomerase, the heat shock protein 70 (Hsp70) family member that binds immunoglobulin protein (BIP or glucose‐regulated protein 78), calnexin, calreticulin and members of the peptidylpropyl isomerase family. Only properly folded proteins are transported from the RER to the Golgi apparatus. Numerous environmental, physiological and pathological stressors, nutrient fluctuations and chemical triggers disturb the ER protein folding environment (Zaouali et al., 2010; Brown et al., 2014; Carloni et al., 2014; Chan et al., 2015; Garcia‐Marques et al., 2015); these factors cause protein misfolding, resulting in the accumulation of misfolded or unfolded proteins, a condition defined as ER stress (Wang and Kaufman, 2014).
The UPR is a collection of signalling pathways that have evolved for the maintenance of a productive ER protein folding environment (Wang and Kaufman, 2014). The UPR comprises three parallel signalling branches: PRKR‐like ER kinase (PERK)–eukaryotic translation initiation factor 2α (eIF2α), inositol‐requiring protein 1 (IRE1)–X‐box binding protein 1 (XBP1) and activating transcription factor 6 (ATF6) (Santos et al., 2014; Wang and Kaufman, 2014). Under non‐stress conditions, BIP binds to the domains of PERK, IRE1 and ATF6 to stabilize them and prevent their activation (Ozcan et al., 2004; Cnop et al., 2012). Stress can promote the separation of the BIP from PERK, IRE1 and ATF6 and the subsequent activation of these three molecules (Figure 1). Briefly, PERK autophosphorylation promotes the phosphorylation of eIF2α to strongly inhibit mRNA translation, shuts down global protein synthesis and induces an increase in activating transcription factor 4 (ATF4). IRE1 works as an endoribonuclease by slicing the mRNA of XBP1. ATF6 is processed by membrane‐bound transcription factor site‐1 (also known as site‐1 protease, SP1) and SP2 in the Golgi complex to generate cleaved ATF6. ATF4, XBP1 and cleaved ATF6 promote genes encoding ER chaperones. Taken together, these results indicate that the triggering of ER stress and activation of the UPR might be associated with a pro‐survival or pro‐apoptotic outcome via different mechanisms, which ultimately determine the fate of cells (Santos et al., 2014). BIP, PERK, eIF2α, ATF4, growth arrest‐ and DNA damage‐inducible genes 153 (GADD153 or CHOP, whose transcription is activated by ATF4; Harding et al., 2000; Palam et al., 2011; Wang and Kaufman, 2014), IRE1, XBP1, ATF6, BIP and glucose‐regulated protein 94 (GRP94) are often used as ER stress markers.
Figure 1.
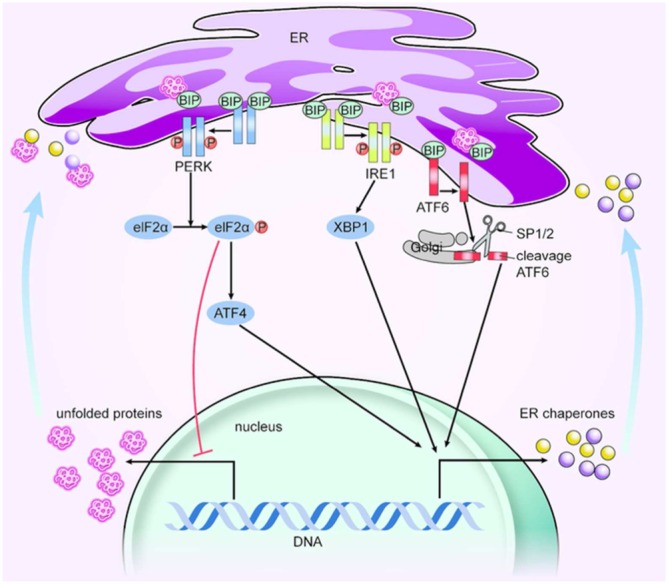
ER stress and UPR signalling pathways. Unfolded proteins (UPRs) can promote the separation of the BIP from PERK, IRE1 and ATF6. PERK autophosphorylation then promotes the phosphorylation of eIF2α to strongly inhibit mRNA translation and shut down global protein synthesis; eIF2α also causes an increase in ATF4; IRE1 works as an endoribonuclease, slicing the mRNA of XBP1; and ATF6 is processed by SP1/2 proteins in the Golgi complex to cleave ATF6. ATF4, XBP1 and cleaved ATF6 promote the transcription of genes encoding ER chaperones. In the end, unfolded proteins are decreased, but ER chaperones are activated. SP1/2, site‐1 and site‐2 protease.
Melatonin and the ER in the pineal gland
Seasonal variation in the volume densities of the ER of at least some tissues is distinct and synchronized by changes in the photoperiod regulated by the pineal gland and the melatonin rhythm (Munoz et al., 2001). This finding indicates that melatonin has implications in ER homeostasis. As an important type of indoleamine, the synthesis of melatonin and associated key enzymes in the pineal gland may be closely associated with the state of the ER, particularly the RER (Kappers, 1978; Tan et al., 2015). Higher melatonin secretion appears to be partly related to the existence of developmental changes in the morphology and quantity of pinealocytes (the functional units of the pineal gland), which are characterized by a relatively large volume of RER (Redondo et al., 2003). Due to the diurnal rhythm of pineal melatonin production, the plasma melatonin concentrations are significantly higher at 02:00 h (at night) than at 09:00 h, 14:00 h (during the day) and 21:00 h (early night), but no significant differences in the RER are discernible (Lewczuk et al., 2004). Correlated with the well‐known nocturnal enhancement in the secretion of melatonin from the pineal gland, higher relative volumes of granular ER are found at night than during the daytime in pinealocytes (Karasek et al., 1990; Swietoslawski and Karasek, 1993). This finding indicates that the ER might be closely related to the secretion of melatonin by the pineal gland.
In the pineal gland of old shrews, the parenchyma undergoes alterations that mainly affect the ER cisternae, which show increased numbers of dense bodies, the formation of many concretions and a depletion of presumed secretory products (Dekar‐Madoui et al., 2012). These changes indicate that the gradual changes in the ER cisternae that occur during aging in Crocidura russula represent a reduced pinealocyte metabolism. Various stressors, including sleep deprivation and weaning of offspring, result in changes in the physiological function and morphological character of the ER, and these changes are reversed by melatonin supplementation. Thus, in goat kids subjected to premature weaning, melatonin might contribute to improvements in the histophysiological function of the ER (Redondo et al., 2010). The mean concentrations of melatonin in the plasma of weaned goat kids are significantly lower than those in non‐weaned offspring. A quantitative ultrastructural analysis of pinealocytes showed that the relative volume of the RER in pineal glands is significantly lower in weaned goat kids than in non‐weaned goat kids; however, treatment with melatonin significantly increased the RER volume in weaned kids (Redondo et al., 2010). Additionally, as a result of sleep deprivation, many pinealocytes exhibit dilation of the cisternae of the RER and SER, and these changes are attenuated by a single injection of melatonin (Lan et al., 2001). Collectively, these findings indicate that exogenous melatonin might promote a positive feedback in its pineal production through ER regulation.
Melatonin and ER function in target glands
Exogenously administered melatonin not only affects the ER morphology of the pineal gland but also regulates that of numerous organ systems, including the reproductive organs, the parathyroid gland and cancer cells. Melatonin directly or indirectly alters the ultrastructural appearance of mouse Leydig cells and influences their secretory activity by inhibiting their capacity to secrete steroids (Reiter et al., 2009). Melatonin treatment induces an obvious reduction in the volume of the SER, RER mitochondria and Golgi complex (Redins et al., 2002). Similarly, in adult Syrian hamsters, melatonin treatment alters the morphology of the seminal vesicles and ventral prostate, which presents conspicuous secretory granules and parallel arrays of granular ER with narrow cisternae (Chow and Pang, 1989). Furthermore, the cytoplasm of the coagulating glands and dorsal prostate of non‐melatonin‐treated animals are dominated by distended cisternae of granular ER and apical blebbing of endothelial cells. The administration of melatonin induces structural modifications, reflecting a loss of functional activities or even death of accessory sex gland secretory cells and thus confirming the suppressive effects of melatonin on the reproductive structures of rodents. Although the actions of melatonin on the reproductive morphology of the hamster might be indirect due to inhibition of gonadotropin secretion (Reiter et al., 2009), melatonin also exerts direct inhibitory effects on the peripheral reproductive organs (Reiter et al., 2013a).
Melatonin might affect the secretory activity of the parathyroid gland. The in vitro melatonin treatment of these glands significantly reduces the cisternae of the RER compared with those of control parathyroid glands (Shoumura et al., 1992). These changes are hypothesized to be induced by melatonin suppressing the synthesis of parathyroid hormone in cells (Shoumura et al., 1992). In human breast cancer cells, specifically MCF‐7 cells, 4 days of exposure to melatonin resulted in the disruption of mitochondrial cristae and vesiculation of the SER (Hill and Blask, 1988). Thus, under normal conditions, the ultrastructure and physiological function of the ER in target organs are changed as a consequence of melatonin treatment.
Melatonin rectifies the abnormal appearance of the ER in target glands
The RER is decreased in volume in the neurons of patients with Alzheimer's disease (AD), and this effect is reversed by melatonin supplementation (Ling et al., 2009). Another study found that unglycosylated ER‐bound β‐APP derivatives are the predominant forms that are marginally affected by melatonin treatment (Lahiri, 1999). Under pathological conditions, melatonin rectifies not only the amount of ER, but also the ultrastructural appearance and presumed function of the ER. Similarly, dilation of the RER is observed in experimental models of acute pancreatitis (Esrefoglu et al., 2006), X‐ray‐irradiated intestine (Hussein et al., 2008) and water avoidance stress‐induced degeneration of the liver parenchyma (Contuk et al., 2005). Dilation of the ER also occurs in the testicular damage associated with hyperlipidaemia (Zhang et al., 2012). Melatonin administration prevents these changes in the ER. Methanol intoxication‐induced liver injury results in the appearance of extensive tubules of the SER, an effect that is also prevented by melatonin administration (Koksal et al., 2012).
Melatonin regulates calcium homeostasis via the ER
An elevation in the intracellular concentration of Ca2 + is considered a common pathological precursor (Santofimia‐Castano et al., 2014), and the ER is the major inositol trisphosphate (IP3)‐sensitive Ca2 + store and plays an important role in the homeostasis and function of Ca2 + (Alves et al., 2011). Cholecystokinin octapeptide (CCK‐8) induces Ca2 + mobilization in mouse, freshly isolated pancreatic acinar cells (del Castillo‐Vaquero et al., 2010). However, melatonin reduces Ca2 + release and modulates the pancreatic responses induced by CCK‐8, which might be explained by the stimulation of Ca2 + transport from cells through the plasma membrane and subsequent Ca2 + reuptake into the ER (del Castillo‐Vaquero et al., 2010; Santofimia‐Castano et al., 2014). Melatonin also exerts a dual inhibitory effect on gonadotropin‐releasing hormone‐induced increases in intracellular Ca2 + and the mobilization of Ca2 + from the ER in the pituitary gland (Vanecek, 1999; Watanabe, 1999). These studies demonstrate that the maintenance of Ca2 + in the ER, including both inhibition of its mobilization and the enhancement of its uptake by the ER, is affected by melatonin.
Melatonin also regulates the Plasmodium falciparum cell cycle via a Ca2 +‐dependent pathway (Alves et al., 2011). The Ca2 + responses to melatonin and the uncaging of IP3 are mutually exclusive in infected red blood cells (Alves et al., 2011). Melatonin promotes the generation of IP3 and opens ER‐localized IP3‐sensitive Ca2 + channels in P. falciparum (Alves et al., 2011). These data not only support the assertion that melatonin is involved in Ca2 + signalling but also provide clues regarding the mechanism underlying this effect. In addition, melatonin inhibits intracellular Ca2 + overload‐induced apoptosis. Treatments with a specific inhibitor of cytosolic Ca2 + reuptake, thapsigargin and/or a Ca2 +‐mobilizing agonist, N‐formyl‐methionyl‐leucyl‐phenylalanine, induce mitochondrial membrane depolarization, caspase activation, phosphatidylserine externalization and DNA fragmentation in leukocytes of both young and elderly volunteers; these effects, however, were far more evident in aged leukocytes (Espino et al., 2011). Notably, melatonin treatment substantially preserves the mitochondrial membrane potential, reverses caspase activation, reduces phosphatidylserine exposure and stops DNA fragmentation in leukocytes of both age groups, and these findings suggest that melatonin can delay Ca2 + overload‐induced apoptosis in aged leukocytes, particularly in those of older individuals (Espino et al., 2011).
Melatonin and ER stress
By controlling ER stress, melatonin protects against multiple disorders, including DM, liver diseases, neurological disorders, reproductive system diseases, lung diseases and chemical poisoning (Espino et al., 2011; Zha et al., 2012; Zaouali et al., 2013; Carloni et al., 2014; Romero et al., 2014; Ali and Kim, 2015; Jeong and Park, 2015; Thakor et al., 2015) (Figure 2). Interestingly, melatonin can cooperate with ER stress to promote the apoptosis of cancer cells and inhibit ER stress to attenuate chemotherapy‐associated side effects and chemoresistance.
Figure 2.
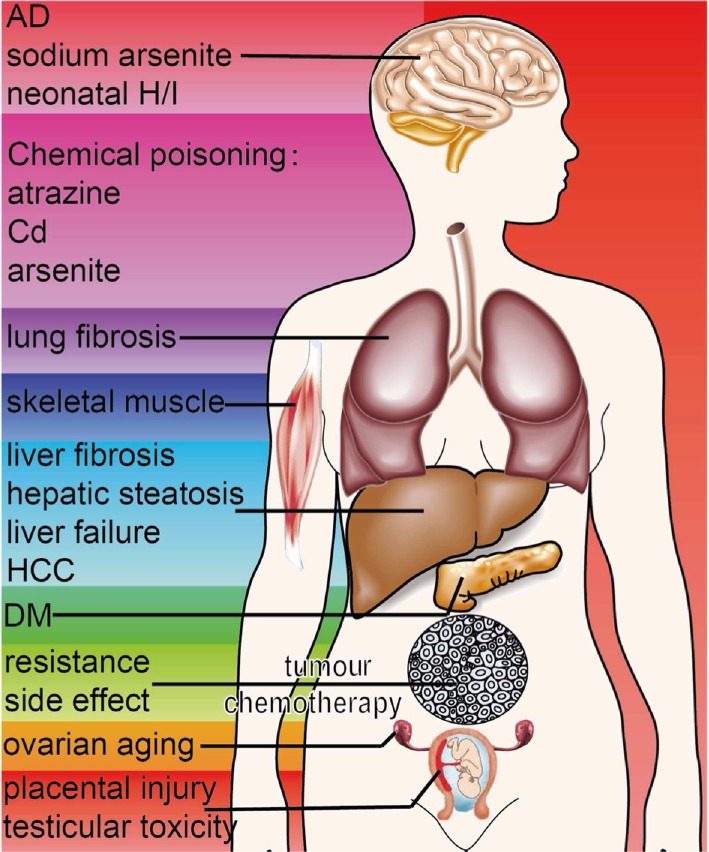
Protective effects of melatonin mediated by the regulation of ER stress. Through the regulation of ER stress, melatonin protects against DM, liver diseases, neurological disorders, reproductive system diseases, cancers, lung diseases and chemical poisoning. In most situations, melatonin exerts its protective effects by inhibiting ER stress. However, melatonin can cooperate with ER stress to promote the apoptosis of HCC.
Diabetes mellitus
ER stress has been demonstrated to cause beta cell dysfunction and death (Back and Kaufman, 2012). In rat insulinoma INS‐1E cells, the expression of insulin receptor substrate (IRS) protein is reduced under thapsigargin‐induced ER stress conditions (Yoo, 2013). Melatonin increases IRS protein expression and thereby elevates insulin secretion in a dose‐dependent manner. Melatonin is hypothesized to mediate insulin synthesis during ER stress and to reverse the results of ER stress by activating the extracellular secretion of insulin (Yoo, 2013).
Furthermore, ER stress may be a central feature of peripheral insulin resistance and DM (Ozcan et al., 2004). One of the main symptoms of DM, hyperglycaemia, is known to induce ER stress (Magierowski et al., 2013), and tunicamycin, a chemical trigger for ER stress, reduces insulin‐mediated glucose transport, supporting the role of ER stress in DM. However, melatonin pretreatment inhibits the decrease in insulin‐mediated glucose transport caused by tunicamycin (Quan et al., 2015). ER stress has been linked to insulin resistance in skeletal muscle. Tunicamycin promotes the phosphorylation of PERK in C2C12 cells and activates gene markers of ER stress, including BIP expression and the splicing of XBP1, in a time‐dependent manner (Quan et al., 2015). However, melatonin pretreatment reverses the elevation in PERK phosphorylation, and the activation of BIP expression and XBP1 splicing. Thus, melatonin inhibits the stimulatory effect of tunicamycin in ER stress and insulin resistance in skeletal muscle cells.
Liver diseases
There is plenty of evidence supporting the notion that ER stress contributes to liver diseases, including hepatic steatosis and liver fibrosis (Dara et al., 2011; Jo et al., 2013; Xiong et al., 2014; Koo et al., 2016). Challenge with tunicamycin has been observed to increase the hepatic triglyceride and intracellular calcium levels through the activation of ER stress (Kim et al., 2015). However, melatonin partially disrupts these phenomena and ameliorates ER stress‐mediated hepatic steatosis (Kim et al., 2015), further limiting non‐alcoholic fatty liver disease and its progression to irreversible complications in ob/ob mice (Stacchiotti et al., 2016).
The treatment of animals with carbon tetrachloride results in hepatic fibrosis, as evidenced by the staining of α‐smooth muscle actin (α‐SMA)‐positive cells. Moreover, increases in the expression of the ER stress chaperones CHOP, BIP and GRP94, in the mRNA levels of PERK, ATF6, ATF4, IRE1 and spliced XBP1, and in phospho‐IRE1, ATF6 and phospho‐PERK protein concentrations have been observed. Through immunohistochemical staining of α‐SMA, San‐Miguel et al. (2015) observed that melatonin significantly inhibits the UPR and ER stress and abolishes the increase in hepatic stellate cells.
Hepatocyte apoptosis is a major contributor to hepatic failure (Tunon et al., 2013). Infection with rabbit haemorrhagic disease virus (RHDV) induces fulminant hepatic failure by increasing the expression of CHOP, BIP and GRP94 and the mRNA levels of ATF6, ATF4, IRE1 and XBP1s (Tunon et al., 2013; San‐Miguel et al., 2014). Melatonin ameliorates RHDV‐induced apoptotic liver damage by attenuating ER stress and modulating the three arms of UPR signalling (Tunon et al., 2013; San‐Miguel et al., 2014). Collectively, melatonin exerts protective effects against liver diseases through the inhibition of ER stress.
Neurological disorders
Recent studies have shown the mechanisms of ER stress‐induced neuronal death (Colla et al., 2012; Mercado et al., 2013; Omura et al., 2013). Furthermore, the decreased levels of melatonin in AD patients suggest a potential relationship between melatonin and AD (Zhou et al., 2003; Rosales‐Corral et al., 2012). Concomitant with the decreased serum melatonin, rats exhibit spatial memory deficits, tau hyperphosphorylation at multiple sites and increased expression of ER stress‐related proteins, including BIP and CHOP (Ling et al., 2009). Simultaneous melatonin supplementation results in partial arrest of these molecular impairments, reduced expression of ER stress‐related proteins and inhibition of AD‐associated behaviour.
In dorsal root ganglion explants, arsenite causes neurotoxicity by elevating the levels of ATF6, ATF4, XBP1 and BIP. Melatonin supplementation suppresses arsenite‐induced ER stress and inhibits arsenite‐induced apoptosis, ultimately protecting against neurotoxicity (Lin et al., 2007; Lin et al., 2009). Furthermore, melatonin protects against ER stress and apoptosis induced by methamphetamine in the SH‐SY5Y neuroblastoma cell line (Wongprayoon and Govitrapong, 2016). In neonatal rats subjected to hypoxia/ischaemia (H/I), melatonin administration significantly reduces brain damage (Carloni et al., 2014). The UPR is strongly activated after H/I, and melatonin significantly reduces the neuronal splicing of XBP1 mRNA, the phosphorylation of eIF2α and the expression of the chaperone proteins BIP and Hsp70 observed in the brain after H/I (Carloni et al., 2014). In another study it was demonstrated that melatonin administration effectively reduces maternal LPS‐induced neonatal inflammation and related brain injury through inhibition of ER stress (Carloni et al., 2016). These findings demonstrate that the attenuation of ER stress is involved in the neuroprotective effect of melatonin against neurological disorders.
Reproductive system diseases
Maternal malnutrition has been shown to impair ovarian function, and also restricts fetal growth causing low birth weight and results in an offspring ovarian phenotype characteristic of premature ovarian aging with a reduced ovarian reserve. Thus, Chan et al. (2015) found that the reduction of adult ovarian follicles induced by early life malnutrition might be mediated by increased ovarian ER stress, resulting in elevated follicular apoptosis accompanied by reduced melatonin levels. These changes are associated with a loss of ovarian vessel density and are consistent with an accelerated ovarian aging phenotype. Thus, a decrease in melatonin might play a role in ovarian aging via activation of ER stress. However, possible protection of this process by melatonin supplementation requires further support.
The administration of LPS during pregnancy retards intrauterine growth and induces fetal death. The placenta of pregnant mice injected with LPS displays apparent ER stress, as determined by decreased BIP expression, obvious eIF2α and JNK phosphorylation and increased CHOP expression (Wang et al., 2011). Melatonin significantly alleviates LPS‐induced placental ER stress, ultimately protecting fetuses from LPS‐induced intrauterine growth restriction and fetal death (Wang et al., 2011).
Cadmium (Cd), a testicular toxicant, induces germ cell apoptosis by increasing the spliced forms of XBP1 and BIP and elevating testicular eIF2α and JNK phosphorylation. These results indicate that ER stress and the UPR pathway are activated by Cd. Melatonin almost completely inhibits the ER stress and UPR induced by Cd in the testes and protects germ cells from apoptosis (Ji et al., 2012).
Cancers
ER stress plays an important role in the development of cancer and apoptosis of cancer cells (Zha et al., 2012; Maurel et al., 2014; Wang and Kaufman, 2014). In rats with diethylnitrosamine‐induced hepatocarcinogenesis, melatonin treatment significantly increases the expression of ATF6, CHOP and BIP, which might further promote the incidence of apoptosis (Moreira et al., 2015). Other studies have shown that melatonin can cooperate with inducers of ER stress to promote cancer cell apoptosis. Significant differences in the apoptosis rate are found between HepG2 cells and HL‐7702 cells (normal human hepatocyte cells) after tunicamycin treatment. The apoptosis rate is significantly higher in HepG2 cells, and this elevated rate is accompanied by the up‐regulation of CHOP and a reduction in the Bcl‐2/Bax ratio. Co‐treatment with tunicamycin and melatonin significantly increases the apoptosis rate by elevating the levels of CHOP and reducing the Bcl‐2/Bax ratio, indicating a pro‐apoptotic effect of melatonin in hepatocellular carcinoma (HCC) through cooperation with ER stress (Zha et al., 2012). Furthermore, a pro‐apoptotic action of melatonin has been observed in other cancer cells (Bizzarri et al., 2013). Co‐treatment with melatonin and tunicamycin (induces ER stress) significantly suppresses the survival of B16F10 melanoma cells compared with treatment with melatonin alone (Kim et al., 2014). Further investigations are required to determine whether this cooperation between melatonin and ER stress exists in other tumours.
The chemoresistance of HCC, which has been widely observed and might be associated with multiple cellular responses to environmental stresses, is also reversed by melatonin (Okuyama et al., 2015). Tunicamycin pretreatment of HepG2 and SMMC‐7721 cells (two human HCC cell lines) markedly decreases the apoptosis rate induced by doxorubicin, indicating a negative role of ER stress in drug resistance. Interestingly, pretreatment with a combination of tunicamycin and melatonin significantly increases the apoptosis induced by doxorubicin, and this finding supports the hypothesis that melatonin attenuates ER stress‐induced resistance to doxorubicin in human HCC cells (Fan et al., 2013).
Cyclosporine A (CsA) is a powerful immunosuppressive drug that has a variety of side effects, including the induction of ER stress, which can further induce autophagy. In the presence of CsA, the expression of catalase is decreased compared with that found in untreated cells, and the levels of BIP and IRE1α are elevated compared with the corresponding levels in untreated cells. Co‐treatment with melatonin inhibits BIP and IRE1α expression, thereby contributing to the alleviation of CsA‐associated side effects (Yoo and Jeung, 2010). Collectively, the results show that, on the one hand, the cooperation between ER stress and melatonin promotes the apoptosis of cancer cells and that, on the other hand, the inhibition of ER stress by melatonin significantly attenuates chemoresistance and the immunosuppressive response.
Lung diseases
The chemotherapeutic agent bleomycin induces lung fibrosis, and melatonin significantly attenuates the bleomycin‐mediated epithelial–mesenchymal transition to myofibroblasts, as evidenced by its repression of α‐SMA expression (Zhao et al., 2014; Yu et al., 2015). Furthermore, melatonin markedly attenuates bleomycin‐induced BIP up‐regulation, elevation of cleaved ATF6 in the lungs and activation of pulmonary eIF2α. These findings indicate the role of ER stress in the protection against lung fibrosis induced by melatonin.
Chemical poisoning
Exposure to the herbicide atrazine adversely affects animal and human health, particularly due to its immunotoxicity. Excessive ER stress is triggered by atrazine through ATF6α, spliced XBP1 and CHOP overexpression in murine splenocytes, but this excessive ER stress is reversed by melatonin (Sharma et al., 2014). Melatonin has also been demonstrated to protect against Cd‐induced testicular toxicity (Ji et al., 2012) and arsenite‐induced neurotoxicity (Lin et al., 2007; Lin et al., 2009) through inhibition of ER stress and the UPR. Furthermore, the possible mechanisms underlying the protective effects of melatonin might also involve modification of the hepatic ER and changes in the metabolism of xenobiotic chemicals (Dhami et al., 1997).
The results from these studies provide new insights into the molecular pathways responsible for the protective effect exerted by melatonin in multiple disorders. Melatonin might be useful as a pharmacological agent that protects against various disorders and promotes optional health by regulating ER stress.
Prospects and conclusion
Melatonin is an agent that regulates protein secretion by influencing various organelles. As a functional organelle, the ER is only one of the targets of melatonin, which also include the mitochondria and Golgi complex (Redondo et al., 2010; Koksal et al., 2012; Zhang et al., 2012). Melatonin treatment could play an important role in improving the histophysiological function of the pineal gland, including the mitochondria, RER and Golgi complex, as observed in goat kids subjected to stress due to premature weaning (Redondo et al., 2010). In addition to extensive SER tubules, increased mitochondria, elevated primary lysosomes and some marked openings of the bile canaliculi are also observed during methanol intoxication, and these effects can be prevented by melatonin (Koksal et al., 2012). In the testicular damage associated with hyperlipidaemia, vacuolar degeneration of the mitochondria and dilation of the ER are observed, and the numbers of mitochondria and lipid droplets are decreased significantly in Leydig and Sertoli cells; these effects were also reversed by melatonin (Zhang et al., 2012). However, further investigations are required to determine whether melatonin specifically influences the ER.
The ER not only participates in the secretion of melatonin but can also be regulated by melatonin in the pineal gland and other tissues, including the reproductive gland, parathyroid gland, pancreas, pituitary, breast cancer cells and leukocytes. Thus, melatonin increases its activation in the pineal gland but reduces this process in other targets. The abnormal ultrastructure and volume of the ER resulting from stress can be reversed by melatonin. Furthermore, melatonin maintains calcium homeostasis via the ER (Figure 3). Thus, in addition to being a well‐known participant of melatonin production and secretion, the ER is also the intracellular regulatory target of melatonin, but the underlying mechanisms require further investigation. In particular, although the protective actions of melatonin are either mediated by its receptors or receptor‐independent (Cardinali et al., 1997; Reiter et al., 2007; Miettunen and Raevuori, 2012), whether the regulation of ER and ER stress by melatonin is mediated by its receptors still needs further investigation through pharmacological methods (Alexander et al., 2015a).
Figure 3.
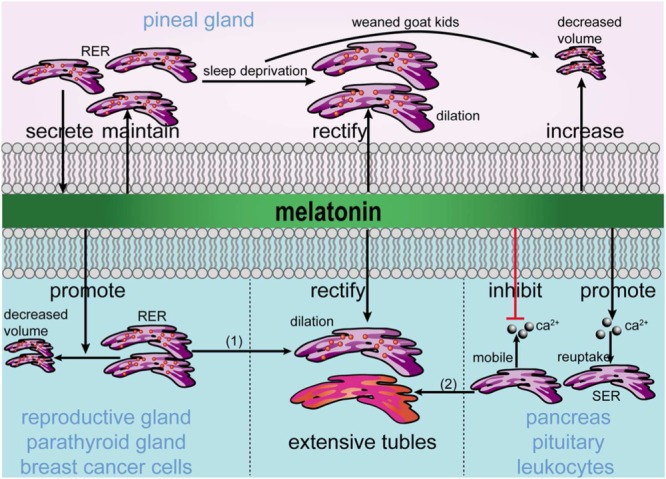
Involvement of melatonin in ER homeostasis. Melatonin is secreted by the ER and maintains the morphology and function of the ER in the pineal gland. Under stress conditions, including AD, acute pancreatitis, X‐ray‐irradiated intestines, water avoidance‐induced degeneration of the liver parenchyma and hyperlipidaemia‐induced testicular damage (indicated as 1 in the figure), the ER in the pineal gland is dilated and has a decreased volume, and these effects are rectified and increased by melatonin. In target glands and cells, melatonin inhibits the function of the ER and rectifies the decreased number and dilation of the RER and the dilation of tubules in the SER induced by stress conditions, including liver injury induced by methanol intoxication (indicated as 2 in the figure). Furthermore, melatonin inhibits the mobilization of Ca2 + from the ER but promotes the reuptake of Ca2 + into the ER.
After understanding the protective effects of melatonin, it is important to focus on its regulation. Huang et al. (2010) found that 12‐h‐light:12‐h‐dark (LD) and continuous‐light (LL) regimes have opposite effects on the plasma level of melatonin. LL regimes are characterized by increased pineal clock and decreased liver clock genes compared with LD regimes. Under LL regimes, the clock and nuclear transcription factor mRNAs, but not pineal ER stress‐related genes, do not show any daily variations in the organs studied (Huang et al., 2010). Thus, the daily routine might play an important role in maintaining the plasma level of melatonin, normal levels of expression of clock and incidence of ER stress. Regulating the levels of melatonin and ER stress by controlling the day length might serve as a basic contributor to health. Sleep deprivation is one reason for decreased melatonin, and the systemic administration of melatonin appears to constitute a potential neuroprotective treatment against neuronal damage induced by sleep deprivation (Lan et al., 2001). The potential treatment options for restoring a normal sleep cycle include the use of melatonin or its analogues (Reiter et al., 1983; Reiter et al., 2013b) and cognitive behavioural therapy. In modern life, cognitive behavioural therapy might be useful for increasing the production of melatonin and ultimately for regulating ER stress and protecting against various disorders.
Melatonin is a type of indolamine that was initially found to be produced in the pineal gland and is now known to be also synthesized in a variety of other tissues. Rigorous investigations have found that the amount of melatonin produced by enterochromaffin‐like cells in the gastrointestinal tract is approximately 400‐fold greater than that normally produced by pinealocytes (Magierowski et al., 2013; Bertrand et al., 2014). Whether melatonin from tissues other than the pineal gland can be stimulated by any means will be determined by further research. As an endogenous compound, melatonin is safe and presents little evidence of toxicity, but additional investigations are required to determine whether it interacts with other transmitters or hormones.
Author contributions
D.W. and Y.Y. designed the manuscript. W.H., C.F. and S.J. collected the data for the review. W.H., S.D. and L.Y. wrote the paper. Z.M. and W.H. illustrated the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81500263, 81200133), the China Postdoctoral Science Foundation (2015M572681), the Jiangsu Top Expert Program in Six Professions (2013‐WSN‐032, 2014‐WSN‐048), a Jiangsu Province Health Department Program grant (Z201411) and a Key Project supported by the Medical Science and Technology Development Foundation of the Nanjing Department of Health (JQX14006, YKK12056).
Hu, W. , Ma, Z. , Di, S. , Jiang, S. , Li, Y. , Fan, C. , Yang, Y. , and Wang, D. (2016) Snapshot: implications for melatonin in endoplasmic reticulum homeostasis. British Journal of Pharmacology, 173: 3431–3442. doi: 10.1111/bph.13651.
Contributor Information
Yang Yang, Email: yang200214yy@163.com.
Dongjin Wang, Email: dongjinwang210@163.com.
References
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Overview. Br J Pharmacol 172: 5729–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali T, Kim MO (2015). Melatonin ameliorates amyloid beta‐induced memory deficits, tau hyperphosphorylation and neurodegeneration via PI3/Akt/GSk3beta pathway in the mouse hippocampus. J Pineal Res 59: 47–59. [DOI] [PubMed] [Google Scholar]
- Alves E, Bartlett PJ, Garcia CR, Thomas AP (2011). Melatonin and IP3‐induced Ca2+ release from intracellular stores in the malaria parasite Plasmodium falciparum within infected red blood cells. J Biol Chem 286: 5905–5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnao MB, Hernandez‐Ruiz J (2014). Melatonin: plant growth regulator and/or biostimulator during stress? Trends Plant Sci 19: 789–797. [DOI] [PubMed] [Google Scholar]
- Arruda AP, Pers BM, Parlakgul G, Guney E, Inouye K, Hotamisligil GS (2014). Chronic enrichment of hepatic endoplasmic reticulum–mitochondria contact leads to mitochondrial dysfunction in obesity. Nat Med 20: 1427–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SH, Kaufman RJ (2012). Endoplasmic reticulum stress and type 2 diabetes. Annu Rev Biochem 81: 767–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand PP, Polglaze KE, Bertrand RL, Sandow SL, Pozo MJ (2014). Detection of melatonin production from the intestinal epithelium using electrochemical methods. Curr Pharm Des 20: 4802–4806. [DOI] [PubMed] [Google Scholar]
- Bizzarri M, Proietti S, Cucina A, Reiter RJ (2013). Molecular mechanisms of the pro‐apoptotic actions of melatonin in cancer: a review. Expert Opin Ther Targets 17: 1483–1496. [DOI] [PubMed] [Google Scholar]
- Borjigin J, Li X, Snyder SH (1999). The pineal gland and melatonin: molecular and pharmacologic regulation. Annu Rev Pharmacol Toxicol 39: 53–65. [DOI] [PubMed] [Google Scholar]
- Brodsky JL (2012). Cleaning up: ER‐associated degradation to the rescue. Cell 151: 1163–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MK, Chan MT, Zimmerman JE, Pack AI, Jackson NE, Naidoo N (2014). Aging induced endoplasmic reticulum stress alters sleep and sleep homeostasis. Neurobiol Aging 35: 1431–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezinski A (1997). Melatonin in humans. N Engl J Med 336: 186–195. [DOI] [PubMed] [Google Scholar]
- Cano Barquilla P, Pagano ES, Jimenez‐Ortega V, Fernandez‐Mateos P, Esquifino AI, Cardinali DP (2014). Melatonin normalizes clinical and biochemical parameters of mild inflammation in diet‐induced metabolic syndrome in rats. J Pineal Res 57: 280–290. [DOI] [PubMed] [Google Scholar]
- Cardinali DP, Golombek DA, Rosenstein RE, Cutrera RA, Esquifino AI (1997). Melatonin site and mechanism of action: single or multiple? J Pineal Res 23: 32–39. [DOI] [PubMed] [Google Scholar]
- Carloni S, Albertini MC, Galluzzi L, Buonocore G, Proietti F, Balduini W (2014). Melatonin reduces endoplasmic reticulum stress and preserves sirtuin 1 expression in neuronal cells of newborn rats after hypoxia–ischemia. J Pineal Res 57: 192–199. [DOI] [PubMed] [Google Scholar]
- Carloni S, Favrais G, Saliba E, Albertini MC, Chalon S, Longini M et al. (2016). Melatonin modulates neonatal brain inflammation through endoplasmic reticulum stress, autophagy, and miR‐34a/silent information regulator 1 pathway. J Pineal Res 61: 370–380. [DOI] [PubMed] [Google Scholar]
- Chan KA, Bernal AB, Vickers MH, Gohir W, Petrik JJ, Sloboda DM (2015). Early life exposure to undernutrition induces ER stress, apoptosis, and reduced vascularization in ovaries of adult rat offspring. Biol Reprod 92: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Novick P, Ferro‐Novick S (2012). ER network formation requires a balance of the dynamin‐like GTPase Sey1p and the Lunapark family member Lnp1p. Nat Cell Biol 14: 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow PH, Pang SF (1989). Ultrastructure of secretory cells of male accessory sex glands of golden hamster (Mesocricetus auratus) and effect of melatonin. Acta Anat (Basel) 134: 327–340. [DOI] [PubMed] [Google Scholar]
- Chung J, Torta F, Masai K, Lucast L, Czapla H, Tanner LB et al. (2015). INTRACELLULAR TRANSPORT. PI4P/phosphatidylserine countertransport at ORP5‐ and ORP8‐mediated ER‐plasma membrane contacts. Science 349: 428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HJ, Chambers JE, Liniker E, Marciniak SJ (2014). Endoplasmic reticulum stress in malignancy. Cancer Cell 25: 563–573. [DOI] [PubMed] [Google Scholar]
- Cnop M, Foufelle F, Velloso LA (2012). Endoplasmic reticulum stress, obesity and diabetes. Trends Mol Med 18: 59–68. [DOI] [PubMed] [Google Scholar]
- Colla E, Coune P, Liu Y, Pletnikova O, Troncoso JC, Iwatsubo T et al. (2012). Endoplasmic reticulum stress is important for the manifestations of alpha‐synucleinopathy in vivo. J Neurosci 32: 3306–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contuk G, Ercan F, Cetinel S, Cikler E, Sener G (2005). Role of melatonin in reducing water avoidance stress‐induced degeneration of the liver. Dig Dis Sci 50: 738–744. [DOI] [PubMed] [Google Scholar]
- Dara L, Ji C, Kaplowitz N (2011). The contribution of endoplasmic reticulum stress to liver diseases. Hepatology 53: 1752–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauchy RT, Xiang S, Mao L, Brimer S, Wren MA, Yuan L et al. (2014). Circadian and melatonin disruption by exposure to light at night drives intrinsic resistance to tamoxifen therapy in breast cancer. Cancer Res 74: 4099–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekar‐Madoui A, Besseau L, Magnanou E, Fons R, Ouali S, Bendjelloul M et al. (2012). Cellular aspects of aging in the pineal gland of the shrew, Crocidura russula . C R Biol 335: 9–18. [DOI] [PubMed] [Google Scholar]
- del Castillo‐Vaquero A, Salido GM, Gonzalez A (2010). Melatonin induces calcium release from CCK‐8‐ and thapsigargin‐sensitive cytosolic stores in pancreatic AR42J cells. J Pineal Res 49: 256–263. [DOI] [PubMed] [Google Scholar]
- Dhami MS, Menon M, Parke DV, Dhami MF, Afzal M (1997). Chronotoxicity as related to chronobiology. Drug Metabol Drug Interact 13: 231–260. [DOI] [PubMed] [Google Scholar]
- English AR, Voeltz GK (2013). Rab10 GTPase regulates ER dynamics and morphology. Nat Cell Biol 15: 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espino J, Bejarano I, Paredes SD, Barriga C, Reiter RJ, Pariente JA et al. (2011). Melatonin is able to delay endoplasmic reticulum stress‐induced apoptosis in leukocytes from elderly humans. Age (Dordr) 33: 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esrefoglu M, Gul M, Ates B, Selimoglu MA (2006). Ultrastructural clues for the protective effect of melatonin against oxidative damage in cerulein‐induced pancreatitis. J Pineal Res 40: 92–97. [DOI] [PubMed] [Google Scholar]
- Fan L, Sun G, Ma T, Zhong F, Lei Y, Li X et al. (2013). Melatonin reverses tunicamycin‐induced endoplasmic reticulum stress in human hepatocellular carcinoma cells and improves cytotoxic response to doxorubicin by increasing CHOP and decreasing survivin. J Pineal Res 55: 184–194. [DOI] [PubMed] [Google Scholar]
- Farez MF, Mascanfroni ID, Mendez‐Huergo SP, Yeste A, Murugaiyan G, Garo LP et al. (2015). Melatonin contributes to the seasonality of multiple sclerosis relapses. Cell 162: 1338–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng YM, Jia YF, Su LY, Wang D, Lv L, Xu L et al. (2013). Decreased mitochondrial DNA copy number in the hippocampus and peripheral blood during opiate addiction is mediated by autophagy and can be salvaged by melatonin. Autophagy 9: 1395–1406. [DOI] [PubMed] [Google Scholar]
- Friedman JR, Voeltz GK (2011). The ER in 3D: a multifunctional dynamic membrane network. Trends Cell Biol 21: 709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Yang L, Li P, Hofmann O, Dicker L, Hide W et al. (2011). Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature 473: 528–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galley HF, Lowes DA, Allen L, Cameron G, Aucott LS, Webster NR (2014). Melatonin as a potential therapy for sepsis: a phase I dose escalation study and an ex vivo whole blood model under conditions of sepsis. J Pineal Res 56: 427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi AV, Mosser EA, Oikonomou G, Prober DA (2015). Melatonin is required for the circadian regulation of sleep. Neuron 85: 1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Marques S, Randez‐Gil F, Prieto JA (2015). Nuclear versus cytosolic activity of the yeast Hog1 MAP kinase in response to osmotic and tunicamycin‐induced ER stress. FEBS Lett 589: 2163–2168. [DOI] [PubMed] [Google Scholar]
- Giordano F, Saheki Y, Idevall‐Hagren O, Colombo SF, Pirruccello M, Milosevic I et al. (2013). PI(4,5)P(2)‐dependent and Ca(2+)‐regulated ER‐PM interactions mediated by the extended synaptotagmins. Cell 153: 1494–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez S, Moreno‐Delgado D, Moreno E, Perez‐Capote K, Franco R, Mallol J et al. (2012). Circadian‐related heteromerization of adrenergic and dopamine D(4) receptors modulates melatonin synthesis and release in the pineal gland. PLoS Biol 10: e1001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M et al. (2000). Regulated translation initiation controls stress‐induced gene expression in mammalian cells. Mol Cell 6: 1099–1108. [DOI] [PubMed] [Google Scholar]
- He S, Ni D, Ma B, Lee JH, Zhang T, Ghozalli I et al. (2013). PtdIns(3)P‐bound UVRAG coordinates Golgi‐ER retrograde and Atg9 transport by differential interactions with the ER tether and the beclin 1 complex. Nat Cell Biol 15: 1206–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C (2013). The biological meaning of the UPR. Nat Rev Mol Cell Biol 14: 404. [DOI] [PubMed] [Google Scholar]
- Hetz C, Mollereau B (2014). Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat Rev Neurosci 15: 233–249. [DOI] [PubMed] [Google Scholar]
- Hickie IB, Rogers NL (2011). Novel melatonin‐based therapies: potential advances in the treatment of major depression. Lancet 378: 621–631. [DOI] [PubMed] [Google Scholar]
- Hill SM, Blask DE (1988). Effects of the pineal hormone melatonin on the proliferation and morphological characteristics of human breast cancer cells (MCF‐7) in culture. Cancer Res 48: 6121–6126. [PubMed] [Google Scholar]
- Hu J, Prinz WA, Rapoport TA (2011). Weaving the web of ER tubules. Cell 147: 1226–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Ma Z, Jiang S, Fan C, Deng C, Yan X et al. (2016). Melatonin: the dawning of a treatment for fibrosis? J Pineal Res 60: 121–131. [DOI] [PubMed] [Google Scholar]
- Hu ZP, Fang XL, Fang N, Wang XB, Qian HY, Cao Z et al. (2013). Melatonin ameliorates vascular endothelial dysfunction, inflammation, and atherosclerosis by suppressing the TLR4/NF‐kappaB system in high‐fat‐fed rabbits. J Pineal Res 55: 388–398. [DOI] [PubMed] [Google Scholar]
- Huang TS, Ruoff P, Fjelldal PG (2010). Effect of continuous light on daily levels of plasma melatonin and cortisol and expression of clock genes in pineal gland, brain, and liver in atlantic salmon postsmolts. Chronobiol Int 27: 1715–1734. [DOI] [PubMed] [Google Scholar]
- Hussein MR, Abu‐Dief EE, Kamel E, Abou El‐Ghait AT, Abdulwahed SR, Ahmad MH (2008). Melatonin and roentgen irradiation‐induced acute radiation enteritis in Albino rats: an animal model. Cell Biol Int 32: 1353–1361. [DOI] [PubMed] [Google Scholar]
- Jeong JK, Park SY (2015). Melatonin regulates the autophagic flux via activation of alpha‐7 nicotinic acetylcholine receptors. J Pineal Res 59: 24–37. [DOI] [PubMed] [Google Scholar]
- Ji YL, Wang H, Meng C, Zhao XF, Zhang C, Zhang Y et al. (2012). Melatonin alleviates cadmium‐induced cellular stress and germ cell apoptosis in testes. J Pineal Res 52: 71–79. [DOI] [PubMed] [Google Scholar]
- Jo H, Choe SS, Shin KC, Jang H, Lee JH, Seong JK et al. (2013). Endoplasmic reticulum stress induces hepatic steatosis via increased expression of the hepatic very low‐density lipoprotein receptor. Hepatology 57: 1366–1377. [DOI] [PubMed] [Google Scholar]
- Kappers JA (1978). Localization of indoleamine and protein synthesis in the mammalian pineal gland. J Neural Transm Suppl : 13–24. [PubMed] [Google Scholar]
- Karasek M, Stankov B, Lucini V, Scaglione F, Esposti G, Mariani M et al. (1990). Comparison of the rat pinealocyte ultrastructure with melatonin concentrations during daytime and at night. J Pineal Res 9: 251–257. [DOI] [PubMed] [Google Scholar]
- Kim HS, Kim TJ, Yoo YM (2014). Melatonin combined with endoplasmic reticulum stress induces cell death via the PI3K/Akt/mTOR pathway in B16F10 melanoma cells. PLoS One 9: e92627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Kang HS, Lee JH, Park JH, Jung CH, Bae JH et al. (2015). Melatonin ameliorates ER stress‐mediated hepatic steatosis through miR‐23a in the liver. Biochem Biophys Res Commun 458: 462–469. [DOI] [PubMed] [Google Scholar]
- Koksal M, Kurcer Z, Erdogan D, Iraz M, Tas M, Eren MA et al. (2012). Effect of melatonin and n‐acetylcysteine on hepatic injury in rat induced by methanol intoxication: a comparative study. Eur Rev Med Pharmacol Sci 16: 437–444. [PubMed] [Google Scholar]
- Koo JH, Lee HJ, Kim W, Kim SG (2016). Endoplasmic reticulum stress in hepatic stellate cells promotes liver fibrosis via PERK‐mediated degradation of HNRNPA1 and up‐regulation of SMAD2. Gastroenterology 150: 181–193.e188. [DOI] [PubMed] [Google Scholar]
- Lahiri DK (1999). Melatonin affects the metabolism of the beta‐amyloid precursor protein in different cell types. J Pineal Res 26: 137–146. [DOI] [PubMed] [Google Scholar]
- Lan CT, Hsu JC, Ling EA (2001). Influence of sleep deprivation coupled with administration of melatonin on the ultrastructure of rat pineal gland. Brain Res 910: 1–11. [DOI] [PubMed] [Google Scholar]
- Lewczuk B, Nowicki M, Prusik M, Przybylska‐Gornowicz B (2004). Diurnal rhythms of pinealocyte ultrastructure, pineal serotonin content and plasma melatonin level in the domestic pig. Folia Histochem Cytobiol 42: 155–163. [PubMed] [Google Scholar]
- Lin AM, Fang SF, Chao PL, Yang CH (2007). Melatonin attenuates arsenite‐induced apoptosis in rat brain: involvement of mitochondrial and endoplasmic reticulum pathways and aggregation of alpha‐synuclein. J Pineal Res 43: 163–171. [DOI] [PubMed] [Google Scholar]
- Lin AM, Feng SF, Chao PL, Yang CH (2009). Melatonin inhibits arsenite‐induced peripheral neurotoxicity. J Pineal Res 46: 64–70. [DOI] [PubMed] [Google Scholar]
- Ling ZQ, Tian Q, Wang L, Fu ZQ, Wang XC, Wang Q et al. (2009). Constant illumination induces Alzheimer‐like damages with endoplasmic reticulum involvement and the protection of melatonin. J Alzheimers Dis 16: 287–300. [DOI] [PubMed] [Google Scholar]
- Ma Z, Yang Y, Fan C, Han J, Wang D, Di S et al. (2016). Melatonin as a potential anticarcinogen for non‐small‐cell lung cancer. Oncotarget . doi:10.18632/oncotarget.8776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magierowski M, Jasnos K, Brzozowska I, Drozdowicz D, Sliwowski Z, Nawrot E et al. (2013). Melatonin as a therapeutic factor in gastric ulcer healing under experimental diabetes. Przegl Lek 70: 942–946. [PubMed] [Google Scholar]
- Mahoney DJ, Lefebvre C, Allan K, Brun J, Sanaei CA, Baird S et al. (2011). Virus‐tumor interactome screen reveals ER stress response can reprogram resistant cancers for oncolytic virus‐triggered caspase‐2 cell death. Cancer Cell 20: 443–456. [DOI] [PubMed] [Google Scholar]
- Maurel M, Samali A, Chevet E (2014). Endoplasmic reticulum stress: at the crossroads of inflammation and metabolism in hepatocellular carcinoma development. Cancer Cell 26: 301–303. [DOI] [PubMed] [Google Scholar]
- McMullan CJ, Schernhammer ES, Rimm EB, Hu FB, Forman JP (2013). Melatonin secretion and the incidence of type 2 diabetes. JAMA 309: 1388–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehnert M, Sommer T, Jarosch E (2014). Der1 promotes movement of misfolded proteins through the endoplasmic reticulum membrane. Nat Cell Biol 16: 77–86. [DOI] [PubMed] [Google Scholar]
- Mercado G, Valdes P, Hetz C (2013). An ERcentric view of Parkinson's disease. Trends Mol Med 19: 165–175. [DOI] [PubMed] [Google Scholar]
- Miettunen J, Raevuori A (2012). A meta‐analysis of temperament in axis I psychiatric disorders. Compr Psychiatry 53: 152–166. [DOI] [PubMed] [Google Scholar]
- Moreira AJ, Ordonez R, Cerski CT, Picada JN, Garcia‐Palomo A, Marroni NP et al. (2015). Melatonin activates endoplasmic reticulum stress and apoptosis in rats with diethylnitrosamine‐induced hepatocarcinogenesis. PLoS One 10: e0144517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz EM, Fogal T, Dominguez S, Scardapane L, Piezzi RS (2001). Ultrastructural and morphometric study of the Sertoli cell of the viscacha (Lagostomus maximus maximus) during the annual reproductive cycle. Anat Rec 262: 176–185. [DOI] [PubMed] [Google Scholar]
- Oakes SA, Papa FR (2015). The role of endoplasmic reticulum stress in human pathology. Annu Rev Pathol 10: 173–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyama H, Ikeda M, Kuwahara A, Takahashi H, Ohno I, Shimizu S et al. (2015). Prognostic factors in patients with hepatocellular carcinoma refractory or intolerant to sorafenib. Oncology 88: 241–246. [DOI] [PubMed] [Google Scholar]
- Omura T, Kaneko M, Okuma Y, Matsubara K, Nomura Y (2013). Endoplasmic reticulum stress and Parkinson's disease: the role of HRD1 in averting apoptosis in neurodegenerative disease. Oxid Med Cell Longev 2013: 239854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E et al. (2004). Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306: 457–461. [DOI] [PubMed] [Google Scholar]
- Palam LR, Baird TD, Wek RC (2011). Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation. J Biol Chem 286: 10939–10949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz WA (2014). The lipid trade. Nat Rev Mol Cell Biol 15: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan X, Wang J, Liang C, Zheng H, Zhang L (2015). Melatonin inhibits tunicamycin‐induced endoplasmic reticulum stress and insulin resistance in skeletal muscle cells. Biochem Biophys Res Commun 463: 1102–1107. [DOI] [PubMed] [Google Scholar]
- Redins CA, Redins GM, Novaes JC (2002). The effects of treatment with melatonin on the ultrastructure of mouse Leydig cells: a quantitative study. Braz J Biol 62: 517–523. [DOI] [PubMed] [Google Scholar]
- Redondo E, Franco A, Garcia A, Masot AJ (2010). Changes in concentrations of cortisol and melatonin in plasma, expression of synaptophysin, and ultrastructural properties of pinealocytes in goat kids in situations of stress due to early weaning: the effect of melatonin. N Z Vet J 58: 160–167. [DOI] [PubMed] [Google Scholar]
- Redondo E, Regodon S, Franco A, Masot J, Gazquez A, Cardinali DP (2003). Day‐night changes in plasma melatonin levels, synaptophysin expression and ultrastructural properties of pinealocytes in developing female sheep under natural long and short photoperiods. Histol Histopathol 18: 333–342. [DOI] [PubMed] [Google Scholar]
- Reid DW, Nicchitta CV (2015). Diversity and selectivity in mRNA translation on the endoplasmic reticulum. Nat Rev Mol Cell Biol 16: 221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter RJ, Richardson BA, Matthews SA, Lane SJ, Ferguson BN (1983). Rhythms in immunoreactive melatonin in the retina and Harderian gland of rats: persistence after pinealectomy. Life Sci 32: 1229–1236. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Rosales‐Corral SA, Manchester LC, Tan DX (2013a). Peripheral reproductive organ health and melatonin: ready for prime time. Int J Mol Sci 14: 7231–7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Manchester LC, Paredes SD, Mayo JC, Sainz RM (2009). Melatonin and reproduction revisited. Biol Reprod 81: 445–456. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Manchester LC, Pilar Terron M, Flores LJ, Koppisepi S (2007). Medical implications of melatonin: receptor‐mediated and receptor‐independent actions. Adv Med Sci 52: 11–28. [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Rosales‐Corral S, Manchester LC (2013b). The universal nature, unequal distribution and antioxidant functions of melatonin and its derivatives. Mini Rev Med Chem 13: 373–384. [DOI] [PubMed] [Google Scholar]
- Romero A, Ramos E, de Los Rios C, Egea J, Del Pino J, Reiter RJ (2014). A review of metal‐catalyzed molecular damage: protection by melatonin. J Pineal Res 56: 343–370. [DOI] [PubMed] [Google Scholar]
- Rosales‐Corral SA, Acuna‐Castroviejo D, Coto‐Montes A, Boga JA, Manchester LC, Fuentes‐Broto L et al. (2012). Alzheimer's disease: pathological mechanisms and the beneficial role of melatonin. J Pineal Res 52: 167–202. [DOI] [PubMed] [Google Scholar]
- Roussel BD, Kruppa AJ, Miranda E, Crowther DC, Lomas DA, Marciniak SJ (2013). Endoplasmic reticulum dysfunction in neurological disease. Lancet Neurol 12: 105–118. [DOI] [PubMed] [Google Scholar]
- Rowland AA, Voeltz GK (2012). Endoplasmic reticulum–mitochondria contacts: function of the junction. Nat Rev Mol Cell Biol 13: 607–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack RL (2010). Clinical practice. Jet lag. N Engl J Med 362: 440–447. [DOI] [PubMed] [Google Scholar]
- San‐Miguel B, Crespo I, Sanchez DI, Gonzalez‐Fernandez B, Ortiz de Urbina JJ, Tunon MJ et al. (2015). Melatonin inhibits autophagy and endoplasmic reticulum stress in mice with carbon tetrachloride‐induced fibrosis. J Pineal Res 59: 151–162. [DOI] [PubMed] [Google Scholar]
- San‐Miguel B, Crespo I, Vallejo D, Alvarez M, Prieto J, Gonzalez‐Gallego J et al. (2014). Melatonin modulates the autophagic response in acute liver failure induced by the rabbit hemorrhagic disease virus. J Pineal Res 56: 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santofimia‐Castano P, Ruy DC, Fernandez‐Bermejo M, Salido GM, Gonzalez A (2014). Pharmacological dose of melatonin reduces cytosolic calcium load in response to cholecystokinin in mouse pancreatic acinar cells. Mol Cell Biochem 397: 75–86. [DOI] [PubMed] [Google Scholar]
- Santos CX, Nabeebaccus AA, Shah AM, Camargo LL, Filho SV, Lopes LR (2014). Endoplasmic reticulum stress and Nox‐mediated reactive oxygen species signaling in the peripheral vasculature: potential role in hypertension. Antioxid Redox Signal 20: 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Sarkar J, Haldar C, Sinha S (2014). Melatonin reverses Fas, E2F‐1 and endoplasmic reticulum stress mediated apoptosis and dysregulation of autophagy induced by the herbicide atrazine in murine splenocytes. PLoS One 9: e108602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoumura S, Chen H, Emura S, Utsumi M, Hayakawa D, Yamahira T et al. (1992). An in vitro study on the effects of melatonin on the ultrastructure of the hamster parathyroid gland. Histol Histopathol 7: 715–718. [PubMed] [Google Scholar]
- Sigurdardottir LG, Markt SC, Rider JR, Haneuse S, Fall K, Schernhammer ES et al. (2015). Urinary melatonin levels, sleep disruption, and risk of prostate cancer in elderly men. Eur Urol 67: 191–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonneaux V, Ribelayga C (2003). Generation of the melatonin endocrine message in mammals: a review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters. Pharmacol Rev 55: 325–395. [DOI] [PubMed] [Google Scholar]
- Smith MH, Ploegh HL, Weissman JS (2011). Road to ruin: targeting proteins for degradation in the endoplasmic reticulum. Science 334: 1086–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltys BJ, Falah M, Gupta RS (1996). Identification of endoplasmic reticulum in the primitive eukaryote Giardia lamblia using cryoelectron microscopy and antibody to Bip. J Cell Sci 109 (Pt 7): 1909–1917. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacchiotti A, Favero G, Lavazza A, Golic I, Aleksic M, Korac A et al. (2016). Hepatic macrosteatosis is partially converted to microsteatosis by melatonin supplementation in ob/ob mice non‐alcoholic fatty liver disease. PLoS One 11: e0148115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzmann GE, Mattson MP (2011). Endoplasmic reticulum Ca(2+) handling in excitable cells in health and disease. Pharmacol Rev 63: 700–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swietoslawski J, Karasek M (1993). Day–night changes in the ultrastructure of pinealocytes in the Syrian hamster: a quantitative study. Endokrynol Pol 44: 81–87. [PubMed] [Google Scholar]
- Tabak HF, Braakman I, van der Zand A (2013). Peroxisome formation and maintenance are dependent on the endoplasmic reticulum. Annu Rev Biochem 82: 723–744. [DOI] [PubMed] [Google Scholar]
- Tabas I, Ron D (2011). Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol 13: 184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamarkin L, Baird CJ, Almeida OF (1985). Melatonin: a coordinating signal for mammalian reproduction? Science 227: 714–720. [DOI] [PubMed] [Google Scholar]
- Tan DX, Manchester LC, Esteban‐Zubero E, Zhou Z, Reiter RJ (2015). Melatonin as a potent and inducible endogenous antioxidant: synthesis and metabolism. Molecules 20: 18886–18906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakor AS, Allison BJ, Niu Y, Botting KJ, Seron‐Ferre M, Herrera EA et al. (2015). Melatonin modulates the fetal cardiovascular defense response to acute hypoxia. J Pineal Res 59: 80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosches MA, Bucher D, Vopalensky P, Arendt D (2014). Melatonin signaling controls circadian swimming behavior in marine zooplankton. Cell 159: 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunon MJ, San‐Miguel B, Crespo I, Laliena A, Vallejo D, Alvarez M et al. (2013). Melatonin treatment reduces endoplasmic reticulum stress and modulates the unfolded protein response in rabbits with lethal fulminant hepatitis of viral origin. J Pineal Res 55: 221–228. [DOI] [PubMed] [Google Scholar]
- van der Zand A, Gent J, Braakman I, Tabak HF (2012). Biochemically distinct vesicles from the endoplasmic reticulum fuse to form peroxisomes. Cell 149: 397–409. [DOI] [PubMed] [Google Scholar]
- Vanecek J (1999). Inhibitory effect of melatonin on GnRH‐induced LH release. Rev Reprod 4: 67–72. [DOI] [PubMed] [Google Scholar]
- Vanecek J, Watanabe K (1999). Mechanisms of melatonin action in the pituitary and SCN. Adv Exp Med Biol 460: 191–198. [DOI] [PubMed] [Google Scholar]
- Walter P, Ron D (2011). The unfolded protein response: from stress pathway to homeostatic regulation. Science 334: 1081–1086. [DOI] [PubMed] [Google Scholar]
- Wang H, Li L, Zhao M, Chen YH, Zhang ZH, Zhang C et al. (2011). Melatonin alleviates lipopolysaccharide‐induced placental cellular stress response in mice. J Pineal Res 50: 418–426. [DOI] [PubMed] [Google Scholar]
- Wang M, Kaufman RJ (2014). The impact of the endoplasmic reticulum protein‐folding environment on cancer development. Nat Rev Cancer 14: 581–597. [DOI] [PubMed] [Google Scholar]
- Waterhouse J, Reilly T, Atkinson G, Edwards B (2007). Jet lag: trends and coping strategies. Lancet 369: 1117–1129. [DOI] [PubMed] [Google Scholar]
- Westrate LM, Lee JE, Prinz WA, Voeltz GK (2015). Form follows function: the importance of endoplasmic reticulum shape. Annu Rev Biochem 84: 791–811. [DOI] [PubMed] [Google Scholar]
- Wongprayoon P, Govitrapong P (2016). Melatonin protects SH‐SY5Y neuronal cells against methamphetamine‐induced endoplasmic reticulum stress and apoptotic cell death. Neurotox Res. doi:10.1007/s12640-016-9647-z. [DOI] [PubMed] [Google Scholar]
- Xiong X, Wang X, Lu Y, Wang E, Zhang Z, Yang J et al. (2014). Hepatic steatosis exacerbated by endoplasmic reticulum stress‐mediated downregulation of FXR in aging mice. J Hepatol 60: 847–854. [DOI] [PubMed] [Google Scholar]
- Yang L, Calay ES, Fan J, Arduini A, Kunz RC, Gygi SP et al. (2015). METABOLISM. S‐Nitrosylation links obesity‐associated inflammation to endoplasmic reticulum dysfunction. Science 349: 500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo YM (2013). Melatonin‐mediated insulin synthesis during endoplasmic reticulum stress involves HuD expression in rat insulinoma INS‐1E cells. J Pineal Res 55: 207–220. [DOI] [PubMed] [Google Scholar]
- Yoo YM, Jeung EB (2010). Melatonin suppresses cyclosporine A‐induced autophagy in rat pituitary GH3 cells. J Pineal Res 48: 204–211. [DOI] [PubMed] [Google Scholar]
- Yu L, Liang H, Dong X, Zhao G, Jin Z, Zhai M et al. (2015). Reduced silent information regulator 1 signaling exacerbates myocardial ischemia–reperfusion injury in type 2 diabetic rats and the protective effect of melatonin. J Pineal Res 59: 376–390. [DOI] [PubMed] [Google Scholar]
- Zanetti G, Pahuja KB, Studer S, Shim S, Schekman R (2012). COPII and the regulation of protein sorting in mammals. Nat Cell Biol 14: 20–28. [DOI] [PubMed] [Google Scholar]
- Zaouali MA, Ben Abdennebi H, Padrissa‐Altes S, Mahfoudh‐Boussaid A, Rosello‐Catafau J (2010). Pharmacological strategies against cold ischemia reperfusion injury. Expert Opin Pharmacother 11: 537–555. [DOI] [PubMed] [Google Scholar]
- Zaouali MA, Boncompagni E, Reiter RJ, Bejaoui M, Freitas I, Pantazi E et al. (2013). AMPK involvement in endoplasmic reticulum stress and autophagy modulation after fatty liver graft preservation: a role for melatonin and trimetazidine cocktail. J Pineal Res 55: 65–78. [DOI] [PubMed] [Google Scholar]
- Zha L, Fan L, Sun G, Wang H, Ma T, Zhong F et al. (2012). Melatonin sensitizes human hepatoma cells to endoplasmic reticulum stress‐induced apoptosis. J Pineal Res 52: 322–331. [DOI] [PubMed] [Google Scholar]
- Zhang K, Lv Z, Jia X, Huang D (2012). Melatonin prevents testicular damage in hyperlipidaemic mice. Andrologia 44: 230–236. [DOI] [PubMed] [Google Scholar]
- Zhao H, Wu QQ, Cao LF, Qing HY, Zhang C, Chen YH et al. (2014). Melatonin inhibits endoplasmic reticulum stress and epithelial–mesenchymal transition during bleomycin‐induced pulmonary fibrosis in mice. PLoS One 9: e97266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JN, Liu RY, Kamphorst W, Hofman MA, Swaab DF (2003). Early neuropathological Alzheimer's changes in aged individuals are accompanied by decreased cerebrospinal fluid melatonin levels. J Pineal Res 35: 125–130. [DOI] [PubMed] [Google Scholar]


