Abstract
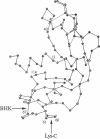
The residues that are indispensable for the ribonucleolytic activity of angiogenin are also known to be essential for its angiogenic activity. We now demonstrate that residues in another region of the protein, devoid of catalytic residues, are additionally required for angiogenesis. Endoproteinase Lys-C or a baby hamster kidney cell protease cleaves angiogenin at the peptide bond either between Lys-60 and Asn-61 or between Glu-67 and Asn-68, respectively. The two polypeptide fragments resulting from either cleavage remain linked by disulfide bonds. These two derivatives and des-(Asn61-Glu67)-angiogenin--in which both bonds are cleaved--retain their ribonucleolytic activities toward tRNA, 18S and 28S rRNA, and dinucleoside phosphates but are no longer angiogenic on the chicken embryo chorioallantoic membrane. Further, their capacity to elicit a second messenger response in endothelial cells is greatly decreased. Moreover, none of these three derivatives inhibit angiogenin-induced angiogenesis. This contrasts with two active site mutants of angiogenin. These results identify the residues from 60 to 68 as a region of angiogenin that is part of a cell-surface receptor binding site [see accompanying manuscript: Hu, G.-F., Chang, S.-I., Riordan, J.F. & Vallee, B.L. (1991) Proc. Natl. Acad. Sci. USA 88, 2227-2231] and serve as the basis for a dual site model of the organogenic activity of angiogenin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badet J., Soncin F., Guitton J. D., Lamare O., Cartwright T., Barritault D. Specific binding of angiogenin to calf pulmonary artery endothelial cells. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8427–8431. doi: 10.1073/pnas.86.21.8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beintema J. J., Schüller C., Irie M., Carsana A. Molecular evolution of the ribonuclease superfamily. Prog Biophys Mol Biol. 1988;51(3):165–192. doi: 10.1016/0079-6107(88)90001-6. [DOI] [PubMed] [Google Scholar]
- Bicknell R., Vallee B. L. Angiogenin activates endothelial cell phospholipase C. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5961–5965. doi: 10.1073/pnas.85.16.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell R., Vallee B. L. Angiogenin stimulates endothelial cell prostacyclin secretion by activation of phospholipase A2. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1573–1577. doi: 10.1073/pnas.86.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond M. D., Strydom D. J. Amino acid sequence of bovine angiogenin. Biochemistry. 1989 Jul 11;28(14):6110–6113. doi: 10.1021/bi00440a057. [DOI] [PubMed] [Google Scholar]
- Esmon N. L., Owen W. G., Esmon C. T. Isolation of a membrane-bound cofactor for thrombin-catalyzed activation of protein C. J Biol Chem. 1982 Jan 25;257(2):859–864. [PubMed] [Google Scholar]
- Fett J. W., Strydom D. J., Lobb R. R., Alderman E. M., Bethune J. L., Riordan J. F., Vallee B. L. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry. 1985 Sep 24;24(20):5480–5486. doi: 10.1021/bi00341a030. [DOI] [PubMed] [Google Scholar]
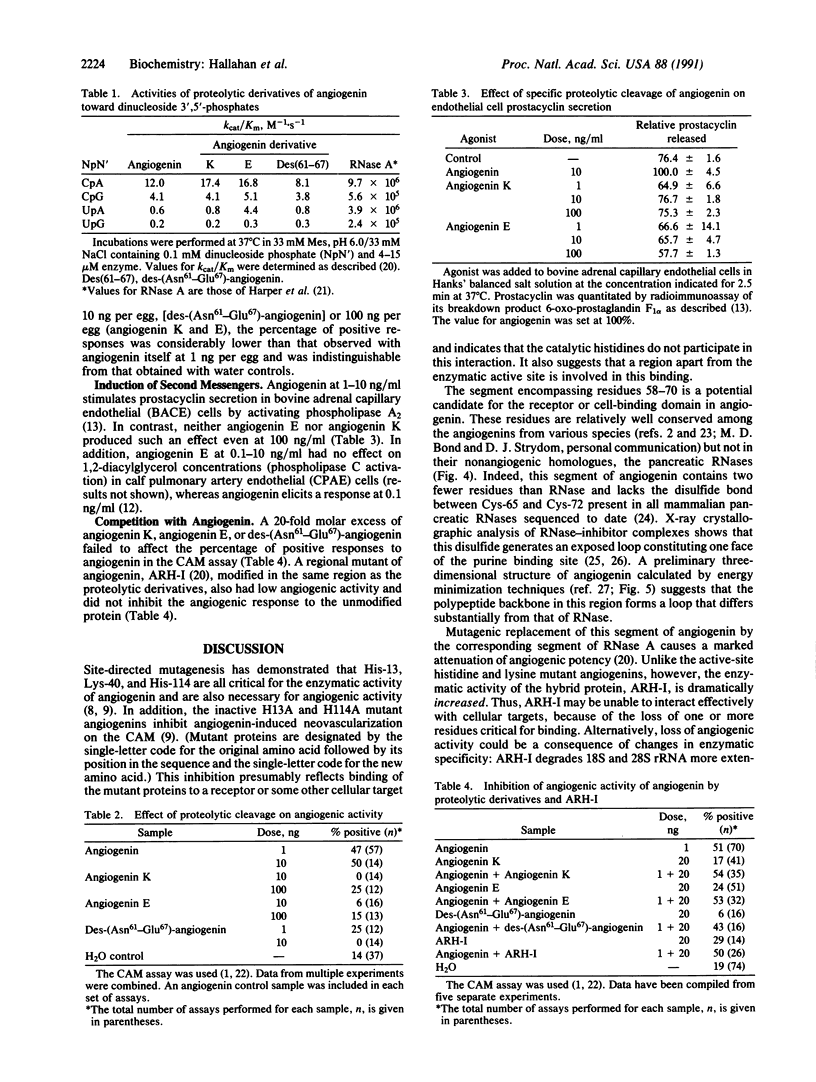
- Harper J. W., Fox E. A., Shapiro R., Vallee B. L. Mutagenesis of residues flanking Lys-40 enhances the enzymatic activity and reduces the angiogenic potency of angiogenin. Biochemistry. 1990 Aug 7;29(31):7297–7302. doi: 10.1021/bi00483a020. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Vallee B. L. A covalent angiogenin/ribonuclease hybrid with a fourth disulfide bond generated by regional mutagenesis. Biochemistry. 1989 Feb 21;28(4):1875–1884. doi: 10.1021/bi00430a067. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Vallee B. L. Conformational characterization of human angiogenin by limited proteolysis. J Protein Chem. 1988 Aug;7(4):355–363. doi: 10.1007/BF01024885. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Vallee B. L. Mutagenesis of aspartic acid-116 enhances the ribonucleolytic activity and angiogenic potency of angiogenin. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7139–7143. doi: 10.1073/pnas.85.19.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath W. F., Jr, Moore F., Bicknell R., Vallee B. L. Modulation of mitogenic stimuli by angiogenin correlates with in vitro phosphatidylinositol bisphosphate synthesis. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2718–2722. doi: 10.1073/pnas.86.8.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G. F., Chang S. I., Riordan J. F., Vallee B. L. An angiogenin-binding protein from endothelial cells. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2227–2231. doi: 10.1073/pnas.88.6.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knighton D., Ausprunk D., Tapper D., Folkman J. Avascular and vascular phases of tumour growth in the chick embryo. Br J Cancer. 1977 Mar;35(3):347–356. doi: 10.1038/bjc.1977.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurachi K., Davie E. W., Strydom D. J., Riordan J. F., Vallee B. L. Sequence of the cDNA and gene for angiogenin, a human angiogenesis factor. Biochemistry. 1985 Sep 24;24(20):5494–5499. doi: 10.1021/bi00341a032. [DOI] [PubMed] [Google Scholar]
- Kurachi K., Rybak S. M., Fett J. W., Shapiro R., Strydom D. J., Olson K. A., Riordan J. F., Davie E. W., Vallee B. L. Expression of human angiogenin in cultured baby hamster kidney cells. Biochemistry. 1988 Aug 23;27(17):6557–6562. doi: 10.1021/bi00417a054. [DOI] [PubMed] [Google Scholar]
- Moore F., Riordan J. F. Angiogenin activates phospholipase C and elicits a rapid incorporation of fatty acid into cholesterol esters in vascular smooth muscle cells. Biochemistry. 1990 Jan 9;29(1):228–233. doi: 10.1021/bi00453a031. [DOI] [PubMed] [Google Scholar]
- Palmer K. A., Scheraga H. A., Riordan J. F., Vallee B. L. A preliminary three-dimensional structure of angiogenin. Proc Natl Acad Sci U S A. 1986 Apr;83(7):1965–1969. doi: 10.1073/pnas.83.7.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak S. M., Vallee B. L. Base cleavage specificity of angiogenin with Saccharomyces cerevisiae and Escherichia coli 5S RNAs. Biochemistry. 1988 Apr 5;27(7):2288–2294. doi: 10.1021/bi00407a007. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Fox E. A., Riordan J. F. Role of lysines in human angiogenin: chemical modification and site-directed mutagenesis. Biochemistry. 1989 Feb 21;28(4):1726–1732. doi: 10.1021/bi00430a045. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Harper J. W., Fox E. A., Jansen H. W., Hein F., Uhlmann E. Expression of Met-(-1) angiogenin in Escherichia coli: conversion to the authentic less than Glu-1 protein. Anal Biochem. 1988 Dec;175(2):450–461. doi: 10.1016/0003-2697(88)90569-6. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Riordan J. F., Vallee B. L. Characteristic ribonucleolytic activity of human angiogenin. Biochemistry. 1986 Jun 17;25(12):3527–3532. doi: 10.1021/bi00360a008. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Vallee B. L. Human placental ribonuclease inhibitor abolishes both angiogenic and ribonucleolytic activities of angiogenin. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2238–2241. doi: 10.1073/pnas.84.8.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro R., Vallee B. L. Site-directed mutagenesis of histidine-13 and histidine-114 of human angiogenin. Alanine derivatives inhibit angiogenin-induced angiogenesis. Biochemistry. 1989 Sep 5;28(18):7401–7408. doi: 10.1021/bi00444a038. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Weremowicz S., Riordan J. F., Vallee B. L. Ribonucleolytic activity of angiogenin: essential histidine, lysine, and arginine residues. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8783–8787. doi: 10.1073/pnas.84.24.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Clair D. K., Rybak S. M., Riordan J. F., Vallee B. L. Angiogenin abolishes cell-free protein synthesis by specific ribonucleolytic inactivation of ribosomes. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8330–8334. doi: 10.1073/pnas.84.23.8330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strydom D. J., Fett J. W., Lobb R. R., Alderman E. M., Bethune J. L., Riordan J. F., Vallee B. L. Amino acid sequence of human tumor derived angiogenin. Biochemistry. 1985 Sep 24;24(20):5486–5494. doi: 10.1021/bi00341a031. [DOI] [PubMed] [Google Scholar]
- Wodak S. Y. The structure of cytidilyl(2',5')adenosine when bound to pancreatic ribonuclease S. J Mol Biol. 1977 Nov;116(4):855–875. doi: 10.1016/0022-2836(77)90275-3. [DOI] [PubMed] [Google Scholar]
- Xiao Y., Bicknell R., Vallee B. L. Angiogenin depresses aortic smooth muscle cell cAMP by a pertussis toxin sensitive mechanism. Biochem Biophys Res Commun. 1989 Sep 15;163(2):902–907. doi: 10.1016/0006-291x(89)92307-3. [DOI] [PubMed] [Google Scholar]