Abstract
Kidney disease is a devastating condition that affects millions of people worldwide, and its prevalence is predicted to significantly increase. The kidney is a complex organ encompassing many diverse cell types organized in a elaborate tissue architecture, making regeneration a challenging feat. In recent years, there has been a surge in the field of stem cell research to develop regenerative therapies for various organ systems. Here, we review some recent progressions in characterizing the role of renal progenitors in development, regeneration, and kidney disease in mammals. We also discuss how the zebrafish provides a unique experimental animal model that can provide a greater molecular and genetic understanding of renal progenitors, which may contribute to the development of potential regenerative therapies for human renal afflictions.
Keywords: Kidney, Renal progenitor, Nephrogenesis, Development, Nephron, Regeneration, Zebrafish, Parietal epithelial cell, Tubular progenitor cell
Core tip: The kidney is a complex organ comprised of many diverse cell types. Damage of renal cells leads to devastating kidney diseases because humans have limited abilities to regenerate these cells. Here, we explore recent research that has sought to better characterize renal progenitors during development, to identify whether renal stem cells exist in the adult kidney, and to understand the enigmatic properties of renal progenitors across diverse vertebrate species such as fish.
INTRODUCTION
The kidney is a vital organ comprised of many specific cell types that work in conjunction to maintain body fluid homeostasis[1]. Notably, this organ is responsible for regulating pH, secreting hormones, maintaining blood pressure, and controlling red blood cell numbers[2]. Each human kidney contains up to 2 million functional units called nephrons that are divided into distinct epithelial segments[3]. Nephrons are organized within an intricate tissue architecture, where they are joined to a centralized collecting duct (CD) network for waste excretion[4]. Due to the complexity of the kidney, the coordination of developmental events that create nephrons and their surrounding interstitial populations from embryonic progenitor cells remains a key question in the biomedical field.
Previous studies using the murine animal model have shown that the mammalian kidney is derived from Osr1+ cells of the intermediate mesoderm (IM) (Figure 1)[5]. The Osr1+ cells give rise to the metanephric mesenchyme (MM), which condenses to form the cap mesenchyme (CM) (Figure 1)[6]. The CM is a self-renewing renal stem cell population from which nephrons are crafted through a reiterative, coordinated process that involves inducing cohorts of CM cells to undergo a simultaneous mesenchymal-to-epithelial transition (MET) upon receiving differentiation signals from the adjacent ureteric bud (UB) (Figure 1)[7]. A pre-tubular aggregate arises from each cohort of these induced renal progenitors, which ultimately becomes an epithelialized renal vesicle (RV) (Figure 1). The activated RVs signal reciprocally to the UB to undergo branching morphogenesis, eventually forming an elaborate, arborized CD network[8]. Meanwhile, the RV structures undergo proliferation and morphogenesis, changing to form a comma-shaped body (CSB) followed by an S-shaped body (SSB) (Figure 1)[9]. The SSBs undergo further elongation and maturation, becoming an intricately segmented nephron (N) structure that connects to the CD system (Figure 1), and contains discrete glomerular, proximal, and distal regions[10].
Figure 1.
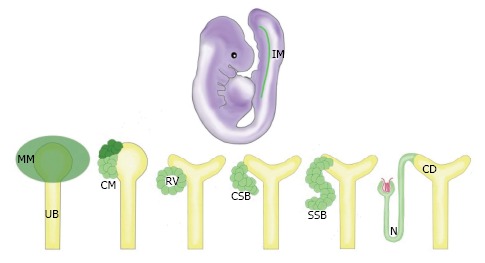
The progression of kidney organogenesis in mammals. The MM is derived from the IM. The MM condenses to form the CM, a renal progenitor population. These progenitors receive signals to self renew (dark green) or differentiate (light green). Cells receiving differentiation signals are organized into an epithelialized RV. Upon further maturation, these cells form a CSB, then an SSB, and finally the N. IM: Intermediate mesoderm; MM: Metanephric mesenchyme; UB: Ureteric bud; CM: Cap mesenchyme; RV: Renal vesicle; CSB: Comma-shaped body; SSB: S-shaped body; N: Nephron; CD: Collecting duct.
During development, nephrogenesis involves a synchronized sequence of dynamic cellular events reliant upon the replenishment of the self-renewing CM and the subsequent patterning of the renal progenitors. Interestingly, nephrogenesis in mammalian and avian species ceases either at the end of gestation or shortly after birth, while in other vertebrates such as fish, reptiles, and amphibians, nephrogenesis has been documented to occur throughout the animal’s lifetime[11,12]. Further, the mammalian kidney is believed to be an organ with relatively limited regenerative potential compared to structures such as the liver[2]. This is problematic, as kidney disease is an escalating global health issue in today’s society. Upon acute injury, however, the mammalian kidney has been observed to undergo considerable structural remodeling and repair[13-15]. Whether an endogenous adult stem cell population contributes to this process of epithelial regeneration remains controversial.
RENAL PROGENITORS DURING MAMMALIAN DEVELOPMENT
In 2008, Kobayashi et al[16] provided the first evidence of multipotent renal progenitors in the developing mammalian kidney. The investigators’ approach consisted of using transgenic mice to perform lineage tracing of Six2+ cells. It had been previously shown that the transcription factor Six2 is necessary for nephrogenesis during murine development[17]. During early stages of nephron induction, Six2+ labeled cells were observed in the CM surrounding the UB epithelium[16]. The CM progenitors receive signals from the UB to either self-renew, thus exhibiting a key stem cell attribute, or undergo MET and differentiate into distinct epithelial segments of the nephron[6]. Under the correct signals, these Six2+ cells form RV, and subsequently the SSB. This RV progenitor pool eventually gives rise to multiple epithelial cell types that comprise the nephron including proximal tubular cells, distal tubular cells, connecting tubular cells, and podocytes. Interestingly, the Six2+ progenitors did not contribute to the CD, vasculature, or interstitium. The transcriptional regulator, Osr1, is broadly expressed in the IM, and was found to be required for the formation of the Six2+ progenitor population[5]. In a separate study by Boyle et al[18] (2008) a transgenic strategy was employed to trace a renal progenitor pool expressing Cited1. Similar to the previously mentioned study[16], the Cited1+ progenitors are induced in the MM and continually contribute to nephron formation during kidney organogenesis. Over time, the self-renewing CM stem cells cease to self-renew and found a final wave of nephrons at the cortex of the metanephros[9].
In the following sections, we discuss how the maintenance of renal stem or progenitor cells in the adult kidney has been debated extensively based on a series of conflicting experimental observations. The existence of renal stem/progenitor cells has been proposed as an explanation for the observation that injuries to nephron epithelial cells can be healed through replenishment with newly proliferative cells (Figure 2). At present, however, it remains an unsettled controversy as to whether the adult mammalian kidney contains self-renewing renal progenitors or can be induced to form cells that exhibit stem cell-like behaviors in the context of renal injuries and other disease conditions.
Figure 2.
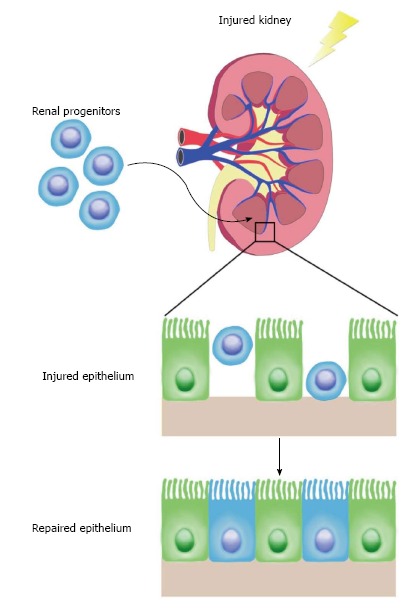
Regenerative capacity of adult renal progenitors. Proposed adult renal stem cells were isolated and transplanted into hosts with injured kidneys. These progenitors homed to the site of injury and repopulated the renal tubular epithelium robustly. This supports a model in which an adult stem cell population contributes to kidney regeneration after acute injury.
EARLY EVIDENCE FOR ADULT RENAL STEM CELLS IN MAMMALS
In 2003, Maeshima et al[19] identified progenitor-like cells scattered throughout the tubules of the adult rat kidney by utilizing in vivo BrdU labeling. Upon ischemic injury, the label-retaining cells underwent multiple cell divisions, becoming positive for proliferating cell nuclear antigen[19]. The progeny of the BrdU+ cells first expressed vimentin, a mesenchymal marker, but later began to express E-cadherin, an epithelial cell marker[19]. Collectively, results from this study suggest that label-retaining cells are renal progenitors that contribute to regeneration of the rat kidney.
In a follow-up study, Kitamura et al[20] (2005) dissected a single nephron from an adult rat kidney and isolated a cell line (rKS56) with high proliferative potential. Upon genetic analysis, the rKS56 cells expressed both developmental markers and mature tubular markers[20]. When these cells were transplanted into injured rat kidneys, they readily engrafted, restored tubules, and improved renal function[20]. These rat studies support the existence of renal adult stem cells that possess the capability to repair tissue and self-renew.
In the same year, Bussolati et al[21] (2005) discovered CD133+ progenitor cells derived from the adult human kidney. These cells expressed Pax2, which is an embryonic kidney marker, and were capable of expansion and self-renewal in vitro[21]. Interestingly, when these cells were implanted subcutaneously into immunocompromised mice, they formed tubules expressing renal epithelial markers[21]. Upon intravenous injection of CD133+ cells into mice with acute tubular injury, they homed to the kidney and assimilated into the proximal and distal tubules[21]. These data support that an adult stem cell population exists in the adult kidney and may participate in regeneration after injury.
In 2006, Dekel et al[22] isolated Sca1+Lin- multipotent progenitors that were distinct from hematopoietic stem cells from the adult mouse kidney by fluorescence-activated cell sorting. Upon transplantation of this population into mice with ischemic injured kidneys, the cells engrafted into the interstitial space and repopulated the renal tubule[22]. Because the Sca1+Lin- progenitors were able to contribute to tubule repair, this provides further evidence that may suggest the existence of resident adult renal stem/progenitor cells in mammals.
PARIETAL EPITHELIAL CELLS AS RENAL PROGENITORS
Previous findings suggest that renal progenitor cells (RPCs) are present in humans and may be the origin of podocyte replacement (Figure 3)[21,23,24]. In humans, these RPCs are a subset of parietal epithelial cells (PECs) located in Bowman’s capsule that coexpress species-specific surface markers CD133 and CD24. Under correct culture conditions, CD133+CD24+ PECs have the potential to differentiate into podocytes or tubular epithelium[24]. However, in some cases activation of RPCs can be harmful, as they have been shown to contribute to hyperplastic lesions within the glomerulus leading to degenerative disease[25].
Figure 3.
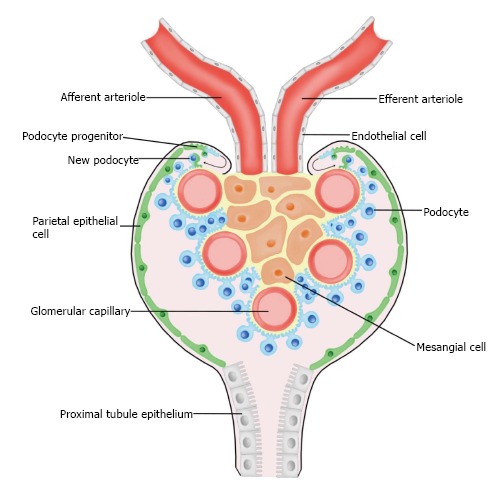
A model of podocyte maintenance and regeneration. Parietal epithelial cells (green) line the inside of Bowman’s capsule, and are suggested to serve as a renal progenitor population. Upon acute injury, parietal epithelial cells have been observed to give rise to fully differentiated podocytes (blue).
Wanner et al[26] (2014) investigated the regenerative role of RPCs during glomerular aging and injury. The researchers further characterized the function of PECs during kidney development by using a transgenic mouse system where upon administration of doxycycline, PECs become genetically labeled with membrane-tagged green fluorescent protein. Upon further analysis, mice exposed to doxycycline from days E8.5 to P28 exhibited mG-labeled cells with foot processes, indicating that PECs can give rise to fully differentiated podocytes. Then, the researchers induced acute podocyte loss in an mT/mG reporter strain of mice by utilizing an inducible diptheria toxin receptor system. In this context, only podocytes coexpressing mG and the diptheria toxin receptor are ablated. Upon flow cytometric analysis of kidneys four weeks after ablation, it was observed that there was a significant increase in the numbers of resident mT-labeled podocytes. This data illustrates how podocytes possess regenerative capacity after acute injury. Alternatively, in a unilateral nephrectomy damage context, podocyte turnover was not detected. In addition, it was observed that during aging, podocyte renewal does not occur. Taken together, these results suggest that podocyte regeneration seems to be limited to developmental and acute injury contexts. This study was the first to report that PECs can form fully differentiated podocytes, however their model does not identify the source of the new podocyte population after acute injury.
In a recent study conducted by Lasagni et al[27] (2015) the regenerative potential of these RPCs in response to podocyte injury was studied in mice. In order to examine if the generation of new podocytes influences disease outcome, an inducible transgenic mouse model (NPHS2.iCreERT2; mT/mG) was used. Upon tamoxifen administration, podocytes were genetically labeled with GFP, while all other kidney cells were labeled with TomRed. Although, after tamoxifen withdrawal, newly generated podocytes are labeled with TomRed. Mice were injected with doxorubicin to induce Adriamycin nephropathy and later biopsied, where the numbers of GFP+/Syn+ cells (pre-existing podocytes) and TomRed+/Syn+ cells (newly generated podocytes) were counted. It was found that a significant increase of newly generated podocytes occurred after injury. In addition, it was determined that remission of proteinuria in these mice is associated with the generation of new podocytes. These data suggest that RPCs may play a role in the remission of glomerular disease in mice.
Further, a model for RPC lineage tracing was established by Lasagni et al[27] (2015) using an inducible transgenic mouse line where green, yellow, cyan, or red are randomly expressed under the control of the Pax2 promoter. It was observed that Pax2+ cells localized in the parietal epithelium of the glomerulus are progenitors that give rise to podocytes during postnatal kidney development. Interestingly, nascent podocytes were labeled with different colors, indicating that these cells did not arise due to clonal division of a single progenitor. These Pax2+ RPCs were found to be responsible for podocyte regeneration in the Adriamycin nephropathy disease context. Mice with proteinuria remission exhibited abundant intraglomerular Pax2+ cells surrounding capillaries. Conversely, mice with persistent proteinuria exhibited virtually no intraglomerular Pax2+ cells. Furthermore, treating diseased animals with the GSK3 inhibitor BIO significantly increased the number of Pax2+Syn+ cells. All eight BIO-treated mice underwent proteinuria remission, where only two DMSO-treated mice exhibited proteinuria remission. Significantly, the differentiation of RPCs into podocytes can be pharmacologically driven in order to reverse glomerular disease.
RESIDENT TUBULAR PROGENITOR CELLS
In 2011, Lindgren et al[28] provided the first evidence for the existence of tubular progenitor cells in humans (Figure 4). In this study, progenitor cells were isolated from renal tissue by cell sorting for high ALDH enzymatic activity. It was observed that these progenitors were scattered throughout the proximal tubules and displayed stem cell properties such as sphere formation and anchorage-independent growth[28]. Human tubular progenitors are localized in the proximal tubule and distal convoluted tubule and possess the following expression profile: CD133+CD24+CD106-. Upon injection of these progenitors into SCID mice with acute kidney injury, these cells were able to engraft, form new tubule cells, and improve renal function[29].
Figure 4.
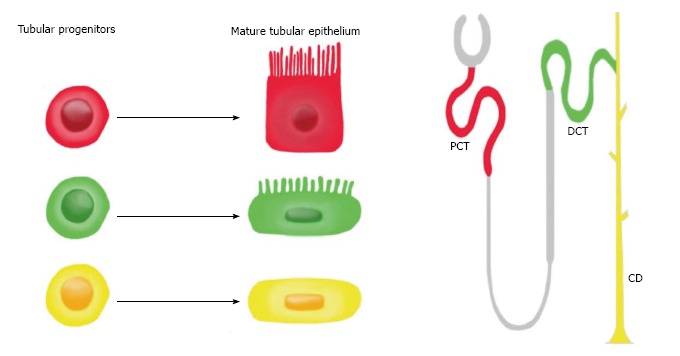
The fate of tubular progenitors. Tubular progenitors are believed to be involved in renal tissue maintenance and regeneration. This stem cell population is lineage-restricted to a specific segment of the nephron. For example, the red progenitor is predestined to become PCT, the green progenitor is predestined to become DCT, and the yellow progenitor is predestined to become CD. PCT: Proximal convoluted tubule; DCT: Distal convoluted tubule; CD: Collecting duct.
Recently, Rinkevich et al[30] (2014) sought to further characterize renal tubular progenitor involvement in development, maintenance, and regeneration. The investigators crossed Actin CrER mice with “Rainbow” mice in order to genetically trace individual epithelial cells within the adult kidney. Offspring were injected with tamoxifen at 12 wk old, and were sacrificed at varying time points for clonal analysis. After 1 mo, 2-3 cell clones were scattered throughout the renal cortex, medulla, and papillae. These singly colored clones later grew in size and contributed to existing tubules. The composition of these clones was further examined by immunostaining for segment-specific markers, where it was determined that they did not expand into different segments. These results suggest a model in which tissue-restricted progenitor cells are responsible for kidney maintenance.
In addition, the researchers performed similar clonal analysis during kidney development, where they traced embryonic renal progenitors from E13.5 to P1[30]. Resulting tubules were observed to be polyclonal, indicating several progenitor cells are present during organogenesis. Immunostaining for segment-specific markers revealed clones separately composed of proximal tubule, distal tubule, or CD fates. These results support that renal progenitors during development are lineage-restricted to a specific tubule type. Furthermore, the clonal response to acute injury was studied by performing unilateral ischemia/reperfusion to the left kidneys of adult animals. After 2 mo, single colored clones appeared restricted to specific tubule segments. In damaged areas, significant tubule regeneration was observed where clones contributed circumferentially to the entire tubule. The clones expanded longitudinally and perpendicularly within the same tubule, however they did not extend into adjacent segments of the nephron or invade into neighboring nephrons. Upon long-term fate analysis, clones maintained the identity of a single epithelial lineage. These adult renal clones were found to originate from Wnt-responsive precursors that form segment specific tubules. Harvested kidneys from transgenic animals were dissociated into single cells and cultured in Matrigel to form organoids. Each monoclonal renal sphere was comprised of a distinct epithelial cell type. Collectively, data from this study supports the existence of fate-restricted progenitors that function in maintaining and regenerating the mammalian kidney[30].
FISH AS A MODEL TO STUDY RENAL PROGENITORS AND REGENERATION
Renal progenitors exist in the adult kidney across many different vertebrate species, such as fish (Figure 5)[11]. In lower vertebrates, renal regeneration and structural remodeling occurs in response to injury due to the presence of potent renal progenitors. Interestingly, the presence of these progenitors can result in the formation of new nephrons during adult growth as well as during regeneration, in a process termed neonephrogenesis[4]. In stark contrast, mammals cease the generation of new nephrons at birth or shortly after[9]. While we have previously discussed observations that have led the hypothesis that renal progenitors may exist in the adult mammalian kidney, there are alternative views including the generation of scattered progenitors in response to injury[31]. Despite such controversies, it is well accepted that the mammalian kidney responds to resection with compensatory glomerular and tubular hypertrophy[32].
Figure 5.
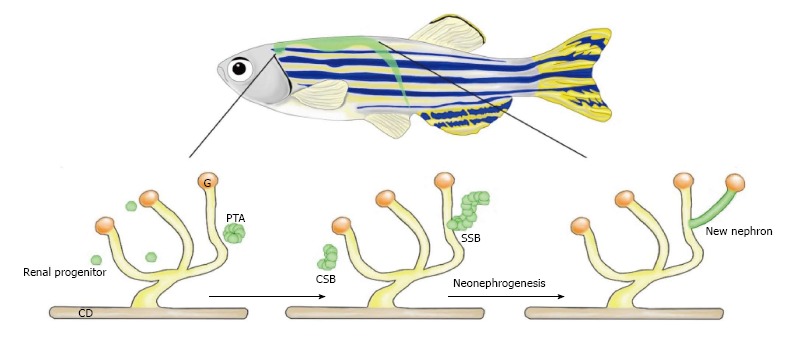
Neonephrogenesis in the adult zebrafish. Adult zebrafish possess the unique ability to generate new nephrons during adulthood. Neonephrogenesis in zebrafish mimics the cellular dynamics of nephrogenesis during mammalian kidney development. Renal progenitors cluster to create a PTA. This aggregate changes morphology as it first forms a comma-shape followed by an S-shape. The SSB differentiates into specific cell types that comprise the blood filter and tubules of a mature nephron. CD: Collecting duct; G: Glomerulus; PTA: Pre-tubular aggregate; CSB: Comma-shaped body; SSB: S-shaped body.
To date, the existence of renal progenitors capable of neonephrogenesis has been most extensively documented in a number of fish species including skate, zebrafish, dogfish, rainbow trout, catfish, goldfish, toadfish, and tilapia[33-44]. A deeper understanding of the molecular mechanisms driving neonephrogenesis in these fish may provide novel insights in the pathogenesis of human kidney diseases and potential regenerative therapeutics.
In a study by Elger et al[45] (2003) partial nephrectomy was performed to characterize kidney regeneration in Leucoraja erinacea, a species of skate[46]. Interestingly, upon resection a neonephrogenic zone was identified that resembles the mammalian embryonic metanephric kidney. This zone encompassed stem cell-like mesenchymal cells that were observed to aggregate around the CD tips. These cells proceeded to epithelialize and form cysts of varying morphologies, which appeared similar to mammalian metanephric structures such as RVs, comma-shaped bodies, and S-shaped bodies. The cysts progressively differentiated into distinct nephron segments, and vascularization of the glomerulus occurred. Neonephrogenesis not only occurred within the injured kidney, but also occurred within the uninjured contralateral kidney. Because neonephrogenesis in fish proceeds through similar stages as mammals, this suggests that genes regulating these events are conserved[38]. This study presents a possible model where renal stem cells persist in the adult kidney of skates and lower vertebrates[45].
In 2011, Diep et al[41] performed the first extensive molecular analysis of adult nephron progenitors in the adult zebrafish kidney and assessed their self-renewal capacity through transplantation studies as well. First, the researchers isolated whole-kidney marrow (WKM) cells from transgenic animals that express fluorescent reporters in the distal nephron. Upon transplant of these cells into immunocompromised, gentamicin treated recipients, many donor-derived nephrons were observed. The donor-derived nephrons were capable of blood filtration, indicating integration of the new structures in the recipient’s tissue. These results support that renal progenitors are present in the adult zebrafish kidney and are able to engraft and give rise to new nephrons after transplantation. When a mix of EGFP+ and mCherry+ WKM cells was transplanted into conditioned recipients, mosaic nephrons resulted. This indicates that multiple progenitors can contribute to an individual nephron, consistent with similar data from mouse studies[16]. In addition, serial transplantation of WKM revealed that nephron progenitors are a self-renewing population possessing substantial proliferative potential. It was determined that lhx1a+ cellular aggregates are comprised of renal progenitors, and when these aggregates are ablated, nephrogenesis is terminated. Transplantation of a single lxh1a+ cellular aggregate was sufficient to form multiple nephrons. This study illustrates lhx1a+ progenitors in adult zebrafish act comparably to the Six2+ CM cells during mammalian development. Although zebrafish lhx1a+ progenitors and mouse Six2+ progenitors possess distinct global gene expression profiles, several factors associated with renal development and stem cell potential were found to be conserved between the two cell populations. Using zebrafish as a model to elucidate molecular pathways regulating renal progenitors may be translatable in the establishment of novel stem cell therapies to treat human kidney diseases.
DISCUSSION
Chronic kidney disease (CKD) continues to be a problem that plagues our society, as it affects millions of individuals worldwide[47]. CKD can progress to end stage renal disease, which is ultimately an irreversible condition. The only treatment options for patients with end stage renal disease are organ transplant or dialysis[48]. This poses a serious problem, as the availability of donor organs is low and dialysis is not a permanent cure. In addition to CKD, a variety of developmental disorders affecting the renal and urinary tract exist[49]. Although these congenital conditions are rare, they involve severe kidney malformations that give rise to many health complications. Achieving a greater understanding of the dynamic biological mechanisms governing kidney development will unravel the mysteries of disease pathogenesis and lead to the discovery of innovative regenerative therapies.
The identification and characterization of adult renal progenitors paves the way for potential stem-cell therapies. Stem cell populations, like renal progenitors, are ideal targets for gene therapy, cell transplantation, and tissue engineering[50]. For example, it has been shown in various studies that the transplant of renal progenitors into injured rodents drives tissue repair and improves kidney functionality[20-22,27,29].
In addition to mice, zebrafish provide a unique model system to study kidney development and regeneration[51-53]. Zebrafish are incredible animals, as they are experts of kidney regeneration due to their extraordinary ability to undergo neonephrogenesis throughout their adult life, which can be induced further with well-established injury models[54-59]. Although vertebrates possess kidneys of varying organization and complexity, the genetic pathways that regulate organogenesis are highly conserved[60]. The diverse cell types that comprise the nephron are conserved across species, contributing to a growing appreciation of zebrafish as a relevant model system to study kidney development and regeneration. Furthermore, zebrafish may help identify novel genes regulating renal progenitors, neonephrogenesis, and regeneration. Future studies could determine factors essential for activating renal progenitors in adult zebrafish, which could potentially be translated to humans in order to induce these cells to facilitate tissue repair in disease contexts. The discovery of molecular mechanisms directing renal progenitor cell-fate decisions during development and regeneration holds great promise in advancing the fields of tissue engineering and stem-cell therapy.
ACKNOWLEDGMENTS
We thank the staffs of the University of Notre Dame Department of Biological Sciences, Center for Zebrafish Research and Center for Stem Cells and Regenerative Medicine for their support. We also thank the members of our research lab for helpful discussions.
Footnotes
Supported by National Institutes of Health, No. DP2 OD008470; the University of Notre Dame College of Science and Graduate School; and a generous donation to support stem cell research provided by the Gallagher Family.
Conflict-of-interest statement: Authors declare no conflict of interest for this article.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: July 1, 2016
First decision: August 5, 2016
Article in press: September 8, 2016
P- Reviewer: Bosch RJ, Ciccone MM S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
References
- 1.Dressler GR. The cellular basis of kidney development. Annu Rev Cell Dev Biol. 2006;22:509–529. doi: 10.1146/annurev.cellbio.22.010305.104340. [DOI] [PubMed] [Google Scholar]
- 2.Little MH. Regrow or repair: potential regenerative therapies for the kidney. J Am Soc Nephrol. 2006;17:2390–2401. doi: 10.1681/ASN.2006030218. [DOI] [PubMed] [Google Scholar]
- 3.Takasato M, Little MH. The origin of the mammalian kidney: implications for recreating the kidney in vitro. Development. 2015;142:1937–1947. doi: 10.1242/dev.104802. [DOI] [PubMed] [Google Scholar]
- 4.Drummond BE, Wingert RA. Insights into kidney stem cell development and regeneration using zebrafish. World J Stem Cells. 2016;8:22–31. doi: 10.4252/wjsc.v8.i2.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mugford JW, Sipilä P, McMahon JA, McMahon AP. Osr1 expression demarcates a multi-potent population of intermediate mesoderm that undergoes progressive restriction to an Osr1-dependent nephron progenitor compartment within the mammalian kidney. Dev Biol. 2008;324:88–98. doi: 10.1016/j.ydbio.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basta JM, Robbins L, Kiefer SM, Dorsett D, Rauchman M. Sall1 balances self-renewal and differentiation of renal progenitor cells. Development. 2014;141:1047–1058. doi: 10.1242/dev.095851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothenpieler UW, Dressler GR. Pax-2 is required for mesenchyme-to-epithelium conversion during kidney development. Development. 1993;119:711–720. doi: 10.1242/dev.119.3.711. [DOI] [PubMed] [Google Scholar]
- 8.Saxén L, Sariola H. Early organogenesis of the kidney. Pediatr Nephrol. 1987;1:385–392. doi: 10.1007/BF00849241. [DOI] [PubMed] [Google Scholar]
- 9.Little MH, McMahon AP. Mammalian kidney development: principles, progress, and projections. Cold Spring Harb Perspect Biol. 2012;4:a008300. doi: 10.1101/cshperspect.a008300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herzlinger D, Koseki C, Mikawa T, al-Awqati Q. Metanephric mesenchyme contains multipotent stem cells whose fate is restricted after induction. Development. 1992;114:565–572. doi: 10.1242/dev.114.3.565. [DOI] [PubMed] [Google Scholar]
- 11.Romagnani P, Lasagni L, Remuzzi G. Renal progenitors: an evolutionary conserved strategy for kidney regeneration. Nat Rev Nephrol. 2013;9:137–146. doi: 10.1038/nrneph.2012.290. [DOI] [PubMed] [Google Scholar]
- 12.Hartman HA, Lai HL, Patterson LT. Cessation of renal morphogenesis in mice. Dev Biol. 2007;310:379–387. doi: 10.1016/j.ydbio.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humes HD, Cieslinski DA, Coimbra TM, Messana JM, Galvao C. Epidermal growth factor enhances renal tubule cell regeneration and repair and accelerates the recovery of renal function in postischemic acute renal failure. J Clin Invest. 1989;84:1757–1761. doi: 10.1172/JCI114359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nony PA, Schnellmann RG. Mechanisms of renal cell repair and regeneration after acute renal failure. J Pharmacol Exp Ther. 2003;304:905–912. doi: 10.1124/jpet.102.035022. [DOI] [PubMed] [Google Scholar]
- 15.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell. 2008;3:169–181. doi: 10.1016/j.stem.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Self M, Lagutin OV, Bowling B, Hendrix J, Cai Y, Dressler GR, Oliver G. Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J. 2006;25:5214–5228. doi: 10.1038/sj.emboj.7601381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyle S, Misfeldt A, Chandler KJ, Deal KK, Southard-Smith EM, Mortlock DP, Baldwin HS, de Caestecker M. Fate mapping using Cited1-CreERT2 mice demonstrates that the cap mesenchyme contains self-renewing progenitor cells and gives rise exclusively to nephronic epithelia. Dev Biol. 2008;313:234–245. doi: 10.1016/j.ydbio.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maeshima A, Yamashita S, Nojima Y. Identification of renal progenitor-like tubular cells that participate in the regeneration processes of the kidney. J Am Soc Nephrol. 2003;14:3138–3146. doi: 10.1097/01.asn.0000098685.43700.28. [DOI] [PubMed] [Google Scholar]
- 20.Kitamura S, Yamasaki Y, Kinomura M, Sugaya T, Sugiyama H, Maeshima Y, Makino H. Establishment and characterization of renal progenitor like cells from S3 segment of nephron in rat adult kidney. FASEB J. 2005;19:1789–1797. doi: 10.1096/fj.05-3942com. [DOI] [PubMed] [Google Scholar]
- 21.Bussolati B, Bruno S, Grange C, Buttiglieri S, Deregibus MC, Cantino D, Camussi G. Isolation of renal progenitor cells from adult human kidney. Am J Pathol. 2005;166:545–555. doi: 10.1016/S0002-9440(10)62276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dekel B, Zangi L, Shezen E, Reich-Zeliger S, Eventov-Friedman S, Katchman H, Jacob-Hirsch J, Amariglio N, Rechavi G, Margalit R, et al. Isolation and characterization of nontubular sca-1+lin- multipotent stem/progenitor cells from adult mouse kidney. J Am Soc Nephrol. 2006;17:3300–3314. doi: 10.1681/ASN.2005020195. [DOI] [PubMed] [Google Scholar]
- 23.Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, et al. Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J Am Soc Nephrol. 2006;17:2443–2456. doi: 10.1681/ASN.2006010089. [DOI] [PubMed] [Google Scholar]
- 24.Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, et al. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol. 2009;20:322–332. doi: 10.1681/ASN.2008070709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smeets B, Angelotti ML, Rizzo P, Dijkman H, Lazzeri E, Mooren F, Ballerini L, Parente E, Sagrinati C, Mazzinghi B, et al. Renal progenitor cells contribute to hyperplastic lesions of podocytopathies and crescentic glomerulonephritis. J Am Soc Nephrol. 2009;20:2593–2603. doi: 10.1681/ASN.2009020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wanner N, Hartleben B, Herbach N, Goedel M, Stickel N, Zeiser R, Walz G, Moeller MJ, Grahammer F, Huber TB. Unraveling the role of podocyte turnover in glomerular aging and injury. J Am Soc Nephrol. 2014;25:707–716. doi: 10.1681/ASN.2013050452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lasagni L, Angelotti ML, Ronconi E, Lombardi D, Nardi S, Peired A, Becherucci F, Mazzinghi B, Sisti A, Romoli S, et al. Podocyte Regeneration Driven by Renal Progenitors Determines Glomerular Disease Remission and Can Be Pharmacologically Enhanced. Stem Cell Reports. 2015;5:248–263. doi: 10.1016/j.stemcr.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindgren D, Boström AK, Nilsson K, Hansson J, Sjölund J, Möller C, Jirström K, Nilsson E, Landberg G, Axelson H, et al. Isolation and characterization of progenitor-like cells from human renal proximal tubules. Am J Pathol. 2011;178:828–837. doi: 10.1016/j.ajpath.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Angelotti ML, Ronconi E, Ballerini L, Peired A, Mazzinghi B, Sagrinati C, Parente E, Gacci M, Carini M, Rotondi M, et al. Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury. Stem Cells. 2012;30:1714–1725. doi: 10.1002/stem.1130. [DOI] [PubMed] [Google Scholar]
- 30.Rinkevich Y, Montoro DT, Contreras-Trujillo H, Harari-Steinberg O, Newman AM, Tsai JM, Lim X, Van-Amerongen R, Bowman A, Januszyk M, et al. In vivo clonal analysis reveals lineage-restricted progenitor characteristics in mammalian kidney development, maintenance, and regeneration. Cell Rep. 2014;7:1270–1283. doi: 10.1016/j.celrep.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berger K, Moeller MJ. Mechanisms of epithelial repair and regeneration after acute kidney injury. Semin Nephrol. 2014;34:394–403. doi: 10.1016/j.semnephrol.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Wingert RA. Regenerative medicine for the kidney: stem cell prospects & amp; challenges. Clin Transl Med. 2013;2:11. doi: 10.1186/2001-1326-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reimschuessel R, Bennett RO, May EB, Lipsky MM. Renal histopathological changes in the golfish (Carassius auratus) after sublethal exposure to hexachlorobutadiene. Aquat Toxicol. 1989;15:169–180. [Google Scholar]
- 34.Reimschuessel R, Bennett RO, May EB, Lipsky MM. Development of newly formed nephrons in the goldfish kidney following hexachlorobutadiene-induced nephrotoxicity. Toxicol Pathol. 1990;18:32–38. doi: 10.1177/019262339001800105. [DOI] [PubMed] [Google Scholar]
- 35.Reimschuessel R, Williams D. Development of new nephrons in adult kidneys following gentamicin-induced nephrotoxicity. Ren Fail. 1995;17:101–106. doi: 10.3109/08860229509026246. [DOI] [PubMed] [Google Scholar]
- 36.Augusto J, Smith B, Smith S, Robertson J, Reimschuessel R. Gentamicin-induced nephrotoxicity and nephroneogenesis in Oreochromis nilotica, a tilapian fish. Dis Aquatic Org. 1996;26:49–58. [Google Scholar]
- 37.Salice CJ, Rokous JS, Kane AS, Reimschuessel R. New nephron development in goldfish (Carassius auratus) kidneys following repeated gentamicin-induced nephrotoxicosis. Comp Med. 2001;51:56–59. [PubMed] [Google Scholar]
- 38.Reimschuessel R. A fish model of renal regeneration and development. ILAR J. 2001;42:285–291. doi: 10.1093/ilar.42.4.285. [DOI] [PubMed] [Google Scholar]
- 39.Watanabe N, Kato M, Suzuki N, Inoue C, Fedorova S, Hashimoto H, Maruyama S, Matsuo S, Wakamatsu Y. Kidney regeneration through nephron neogenesis in medaka. Dev Growth Differ. 2009;51:135–143. doi: 10.1111/j.1440-169X.2009.01090.x. [DOI] [PubMed] [Google Scholar]
- 40.Zhou W, Boucher RC, Bollig F, Englert C, Hildebrandt F. Characterization of mesonephric development and regeneration using transgenic zebrafish. Am J Physiol Renal Physiol. 2010;299:F1040–F1047. doi: 10.1152/ajprenal.00394.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diep CQ, Ma D, Deo RC, Holm TM, Naylor RW, Arora N, Wingert RA, Bollig F, Djordjevic G, Lichman B, et al. Identification of adult nephron progenitors capable of kidney regeneration in zebrafish. Nature. 2011;470:95–100. doi: 10.1038/nature09669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCampbell KK, Springer KN, Wingert RA. Analysis of nephron composition and function in the adult zebrafish kidney. J Vis Exp. 2014;90:e51644. doi: 10.3791/51644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCampbell KK, Springer KN, Wingert RA. Atlas of Cellular Dynamics during Zebrafish Adult Kidney Regeneration. Stem Cells Int. 2015;2015:547636. doi: 10.1155/2015/547636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McKee RA, Wingert RA. Zebrafish Renal Pathology: Emerging Models of Acute Kidney Injury. Curr Pathobiol Rep. 2015;3:171–181. doi: 10.1007/s40139-015-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elger M, Hentschel H, Litteral J, Wellner M, Kirsch T, Luft FC, Haller H. Nephrogenesis is induced by partial nephrectomy in the elasmobranch Leucoraja erinacea. J Am Soc Nephrol. 2003;14:1506–1518. doi: 10.1097/01.asn.0000067645.49562.09. [DOI] [PubMed] [Google Scholar]
- 46.Drummond I. The skate weighs in on kidney regeneration. J Am Soc Nephrol. 2003;14:1704–1705. doi: 10.1097/01.asn.0000072725.30005.03. [DOI] [PubMed] [Google Scholar]
- 47.Tomino Y. Pathogenesis and treatment of chronic kidney disease: a review of our recent basic and clinical data. Kidney Blood Press Res. 2014;39:450–489. doi: 10.1159/000368458. [DOI] [PubMed] [Google Scholar]
- 48.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 49.Yosypiv IV. Congenital anomalies of the kidney and urinary tract: a genetic disorder? Int J Nephrol. 2012;2012:909083. doi: 10.1155/2012/909083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pleniceanu O, Harari-Steinberg O, Dekel B. Concise review: Kidney stem/progenitor cells: differentiate, sort out, or reprogram? Stem Cells. 2010;28:1649–1660. doi: 10.1002/stem.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ebarasi L, Oddsson A, Hultenby K, Betsholtz C, Tryggvason K. Zebrafish: a model system for the study of vertebrate renal development, function, and pathophysiology. Curr Opin Nephrol Hypertens. 2011;20:416–424. doi: 10.1097/MNH.0b013e3283477797. [DOI] [PubMed] [Google Scholar]
- 52.Gerlach GF, Wingert RA. Kidney organogenesis in the zebrafish: insights into vertebrate nephrogenesis and regeneration. Wiley Interdiscip Rev Dev Biol. 2013;2:559–585. doi: 10.1002/wdev.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Swanhart LM, Cosentino CC, Diep CQ, Davidson AJ, de Caestecker M, Hukriede NA. Zebrafish kidney development: basic science to translational research. Birth Defects Res C Embryo Today. 2011;93:141–156. doi: 10.1002/bdrc.20209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hentschel DM, Park KM, Cilenti L, Zervos AS, Drummond I, Bonventre JV. Acute renal failure in zebrafish: a novel system to study a complex disease. Am J Physiol Renal Physiol. 2005;288:F923–F929. doi: 10.1152/ajprenal.00386.2004. [DOI] [PubMed] [Google Scholar]
- 55.Cianciolo Cosentino C, Roman BL, Drummond IA, Hukriede NA. Intravenous microinjections of zebrafish larvae to study acute kidney injury. J Vis Exp. 2010;42:pii: 2079. doi: 10.3791/2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kamei CN, Liu Y, Drummond IA. Kidney Regeneration in Adult Zebrafish by Gentamicin Induced Injury. J Vis Exp. 2015;102:e51912. doi: 10.3791/51912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou W, Hildebrandt F. Inducible podocyte injury and proteinuria in transgenic zebrafish. J Am Soc Nephrol. 2012;23:1039–1047. doi: 10.1681/ASN.2011080776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang J, McKee M, Huang HD, Xiang A, Davidson AJ, Lu HA. A zebrafish model of conditional targeted podocyte ablation and regeneration. Kidney Int. 2013;83:1193–1200. doi: 10.1038/ki.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kroeger PT, Wingert RA. Using zebrafish to study podocyte genesis during kidney development and regeneration. Genesis. 2014;52:771–792. doi: 10.1002/dvg.22798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Phillips JB, Westerfield M. Zebrafish models in translational research: tipping the scales toward advancements in human health. Dis Model Mech. 2014;7:739–743. doi: 10.1242/dmm.015545. [DOI] [PMC free article] [PubMed] [Google Scholar]


