Abstract
AIM
To compare the assessment of cerebrovascular reserve (CVR) using CO2BOLD magnetic resonance imaging (MRI) vs positron emission tomography (PET) and single photon emission computed tomography (SPECT) as reference standard.
METHODS
Ten consecutive patients (8 women, mean age of 41 ± 26 years) with moyamoya syndrome underwent 14 pre-surgical evaluations for external-internal carotid artery bypass surgery. CVR was assessed using CO2BOLD and PET (4)/SPECT (11) with a maximum interval of 36 d, and evaluated by two experienced neuroradiologists.
RESULTS
The inter-rater agreement was 0.81 for SPECT (excellent), 0.43 for PET (fair) and 0.7 for CO2BOLD (good). In 9/14 cases, there was a correspondence between CO2BOLD and PET/SPECT. In 4/14 cases, CVR was over-estimated in CO2BOLD, while in 1/14 case, CVR was underestimated in CO2BOLD. The sensitivity of CO2BOLD was 86% and a specificity of 43%.
CONCLUSION
CO2BOLD can be used for pre-surgical assessment of CVR in patients with moyamoya syndrome and combines the advantages of absent irradiation, high availability of MRI and assessment of brain parenchyma, cerebral vessels and surrogate CVR in one stop.
Keywords: Moyamoya, Vascular reserve, CO2BOLD, Cerebrovascular reserve
Core tip: Inter-rater agreement of cerebrovascular reserve (CVR) assessment in CO2BOLD is similar to positron emission tomography (PET)/single photon emission computed tomography (SPECT); CO2BOLD has a sensitivity of 86% and specificity of 43% compared to PET/SPECT; Overall, CO2BOLD tends to over-estimate reduction in CVR compared to PET and SPECT as reference standard; Taking this over-estimation of CO2BOLD into account would further improve its sensitivity and specificity; CVR can be assessed using CO2BOLD for pre-surgical evaluation and follow-up of moyamoya syndrome patients.
INTRODUCTION
The term “moyamoya” means puff of smoke and describes the formation of collateral vessels due to high-grade stenosis of the distal internal carotid and proximal middle cerebral arteries. There are two related forms of the disease. Moyamoya disease is typically symmetric, genetically determined, prevalent notably in Japan and symptomatic at early stages in most cases[1]. In contrast, moyamoya syndrome or pattern is typically asymmetric, caused by a variety of underlying etiologies, more common in European countries with a prevalence of 3/1000000 and usually symptomatic only at later stages of the disease[2,3]. Extracranial-intracranial (EC-IC) bypass is the most important surgical intervention, notably to prevent subsequent ischemic events and secondary hemorrhage[2,4]. The challenge in the follow-up of patients with moyamoya syndrome is the selection of the optimal time-point for the surgical intervention of the EC-IC bypass, as the benefit of prevention of stroke or hemorrhage contrasts the risk of peri-surgical complications of this difficult intervention.
The assessment of the cerebrovascular reserve (CVR) was previously proposed as one option to define the optimal timing for a surgical intervention[4-7]. Nuclear medicine, notably positron emission tomography (PET) and single photon emission computed tomography (SPECT) based methods, are the established techniques for the assessment of CVR. Disadvantages of these techniques are the radiation exposure, notably during several longitudinal follow-up investigations in moyamoya syndrome patients, the relatively limited availability and high costs compared to magnetic resonance imaging (MRI). Moreover, PET or SPECT imaging does not allow for the assessment of the brain parenchyma to evaluate acute or old vascular lesions, nor the assessment of the vessels to evaluate the progression of the vascular stenosis. The assessment of CVR in MRI would therefore provide a one-stop imaging solution assessing brain structure, vessels and CVR in a widely available and radiation free technique.
The current investigation tests whether carbon dioxide (CO2) blood oxygenation level dependent (BOLD) MRI may be used for the pre-surgical assessment of surrogate CVR in moyamoya patients. In principle, the inhalation of CO2 induces a vasodilation and as a consequence a BOLD response, which can be measured by dedicated MRI sequences as known from functional MRI[8]. CO2BOLD was demonstrated to be safe and feasible in clinical neuroimaging[9] and provided interesting results for example in the assessment of patients with proximal internal carotid artery stenosis both before and after intervention[10,11] as well as for the evaluation of surrogate CVR in adult and pediatric moyamoya[12-17]. Note that CVR is historically defined as CBF increase following a vasodilator challenge. In contrast, the change in the BOLD signal following the CO2 stimulation includes contributions from CBF, cerebral blood volume, and oxygenation changes, and is thus more complex than simple CBF change. Consequently, CO2 induced BOLD change is not equal to CVR as classically defined, yet correlated as demonstrated previously[18]. We therefore use the term “surrogate” CVR in the context of CO2BOLD.
In particular, we directly compared surrogate CVR assessment based on CO2BOLD MRI using a simple nasal cannula setup vs PET/SPECT as reference standard in ten consecutive patients with moyamoya syndrome undergoing pre-surgical evaluation for EC-IC bypass.
MATERIALS AND METHODS
Participants
This retrospective study was approved by the local ethical committee and includes ten consecutive patients (8 women and 2 men with a mean age of 41 ± 26 years, see Table 1) with moyamoya syndrome undergoing 14 pre-surgical evaluations for EC-IC bypass between November 2010 and August 2014. All patients underwent pre-surgical evaluation for moyamoya syndrome with SPECT and/or PET as part of their clinical routine workup as well as MRI. For these 14 pre-surgical evaluations, we compared the CO2BOLD to the SPECT and/or PET assessments of the corresponding time point, and the maximum delay between PET/SPECT and CO2BOLD MRI was 36 d. Two cases had both SPECT and PET, but only in one case PET and SPECT were performed at the same time point. For this case, we compared the CO2BOLD the consensus of PET and SPECT.
Table 1.
Essential demographic and clinical data of the included patients
| No | Age | Gender | Diagnosis | Etiology | Treatment | PET | SPECT | BOLD |
| 1 | 53 | F | Bilateral moyamoya | Unknown | Bypass | 0 | 1 | 1 |
| 2 | 41 | F | Bilateral moyamoya | Unknown | Bypass | 1 | 2 | 3 |
| 3 | 26 | F | Left moyamoya | Unknown | Bypass | 1 | 1 | 1 |
| 4 | 15 | F | Right moyamoya | Unknown | Conservative | 0 | 1 | 1 |
| 5 | 42 | F | Bilateral moyamoya | Unknown | Bilateral bypass | 0 | 1 | 1 |
| 6 | 39 | F | Bilateral moyamoya | Unknown | Bypass | 2 | 0 | 2 |
| 7 | 51 | M | Right moyamoya | Dissection | Bypass | 0 | 1 | 1 |
| 8 | 42 | F | Right moyamoya | Unknown | Bypass | 0 | 2 | 2 |
| 9 | 46 | M | Bilateral moyamoya | Unknown | Conservative | 0 | 1 | 1 |
| 10 | 58 | F | Bilateral moyamoya | Stenosis | Bypass | 0 | 1 | 1 |
| Mean age | 41.3 | Total | 4 | 11 | 14 |
PET: Positron emission tomography; SPECT: Single photon emission computed tomography; F: Female; M: Male.
MRI
Imaging data were acquired on a clinical routine whole body 3T MR scanner (MAGNETOM Trio, Siemens Healthcare, Erlangen, Germany).
We deliberately chose a very simple CO2 challenge, which was equivalent to previous investigations[11,19]. In essence, the CO2 challenge consisted of a 9 min block-design of 1 min OFF, 2 min ON, 2 min OFF, 2 min ON, 2 min OFF (Figure 1). During the ON periods, a ready-to use mix of 7% CO2 with synthetic air (in a gas bottle outside the MR scanner room) was applied using a simple nasal cannula which was connected using a simple silicone tube. The MR technician manually started and stopped the CO2 application at 8 L/min using a standard clinical flow meter. During the OFF periods, participant inhaled normal room air. Data acquisition consisted in a standard echo echo-planar imaging (EPI) covering the entire brain with the following parameters: 74 × 74 matrix, 45 slices, voxel size 2.97 mm × 2.97 mm × 3.0 mm, TE of 30 ms, TR 3000 ms, 180 repetitions.
Figure 1.
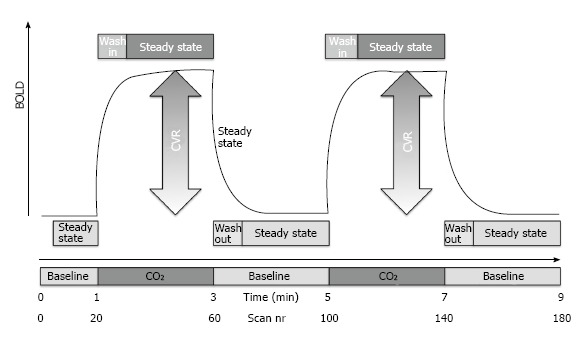
Schematic representation of the ON-OFF CO2 challenge of 7% CO2 applied via a simple nasal cannula. The magnitude of the CO2 induced change in the blood oxygenation level dependent (BOLD) response represents the cerebrovascular reserve (CVR).
A structural 3DT1 sequence was collected for spatial normalization (256 × 256 matrix, 176 slices, 1 mm × 1 mm × 1 mm, TE 2.3 ms, TR 2300 ms). Additional sequences (axial SE T2w and GRE T2*, diffusion tensor imaging DTI wit 30 directions, fluid attenuated inversion recovery FLAIR) were acquired and analyzed to rule out concomitant disease such as ischemic stroke, subdural hematomas and susceptibility artifacts from prior hemorrhage or space-occupying lesions.
PET imaging
PET imaging was performed during routine clinical assessment using the following parameters: Quantitative images were created after two intravenous injections of about 800 MBq of 15OH2 separated by at least 90 min. The injection of acetazolamide was made 20 min before the second acquisition. For patients weighing more than 60 kg, 1 g was intravenously administered, for others the dose was reduced proportionally. The acquisition was performed on a standard high-resolution PET scanner with trans-axial images of the brain reconstructed using filtered back-projection (128 × 128 matrix, 35 slices, 2.34 mm × 2.34 mm × 4.25 mm voxel size) on non–decay-corrected data. Parametric images were obtained following a previously published approach[13]. CVR maps are generated by comparison of pre- and post-acetazolamide images.
SPECT imaging
SPECT imaging was performed during routine clinical assessment using the following parameters (known as “split-dose” protocol): “Baseline images” were acquired 20 min after the intravenous injection of 300 MBq of 99m-Tc-HMPAO. Subsequently, 1000 mg of acetazolamide were administered (slow infusion over 5 min) under blood pressure and heart rate monitoring. Twenty minutes later, a second dose of 600 MBq of 99m-Tc-HMPAO was injected and “acetazolamide images” were acquired 20 min after administration. The 2 acquisitions are made on a triple head gama-camera (Toshiba Medical Systems Corporation), equipped with fan beam, low-energy, high-resolution collimators. Sixty projection over 360 degrees were acquired. Filtered back projection reconstruction was done using a 128 × 128 matrix and applying a uniform Chang attenuation correction. Equivalent to PET, CVR maps are generated by comparison of pre- and post-acetazolamide images.
CO2BOLD data post-processing
The CO2BOLD data were processed with the free FSL software (FMRIB Software Library, version 5.0.2.1; www.fmrib.ox.ac.uk/fsl). The essential processing steps include motion correction, spatial smoothing (10 mm filter size) and masking of non-brain voxels using brain extraction tool (BET, part of FSL). We then calculated parametric maps of the SURROGATE CVT response using an adapted ON-OFF regressor, which was convolved with a square basic shape corresponding to the ON-OFF CO2 inhalation. This schematic curvature was modified with a Gaussian convolution where the sigma factor was change. We calculated the maps with different models of sigma curvature to take into account delayed vascular response due to the proximal vascular stenosis. The delay was varied as 30, 45, 60, 75, 90 and 120 s to model variable speed of the CO2 induced BOLD response (Figure 2). Both readers had all 6 maps available for the comparison between BOLD and PET/SPECT and reported that they preferentially used the 90 and 120 maps because the other maps were less informative.
Figure 2.
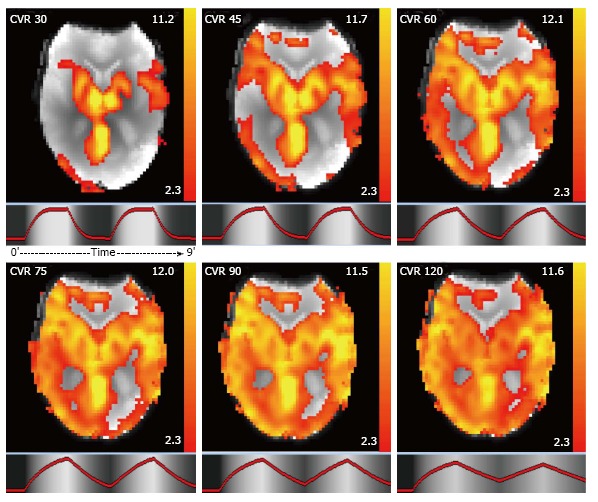
CO2BOLD cerebrovascular reserve maps in axial view. To take into account delayed vascular response due to the proximal vascular stenosis, the delay of the CO2 induced BOLD response was varied from 30, 45, 60, 75, 90 to 120 s. Units = Z value. CVR: Cerebrovascular reserve.
Visual analysis
The visual analysis of the surrogate CVT maps derived from CO2BOLD MRI, PET and SPECT images was independently performed by two neuroradiologists (Figure 3 for an example case) and the ratings were considered as independent points. All cases and all imaging modalities were independently evaluated in four vascular territories: Left/right anterior cerebral artery (ACA) and left/right middle cerebral artery (MCA) territory based on a three point rating system: 0 = no reduction in CVR, 1 = mild reduction in CVR, 2 = severe reduction in CVR.
Figure 3.
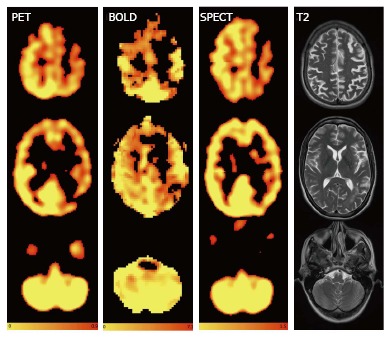
Axial images of a sample patient showing reduced cerebrovascular reserve on the left hemispheric anterior cerebral artery and middle cerebral artery territories without any diminution of the cerebrovascular reserve in the cerebellum for positron emission tomography, CO2BOLD and single photon emission computed tomography and axial T2. The readings for the territories right ACM, right anterior cerebral artery (ACA), left ACM and left ACA were 0 (normal), 0, 2 (substantial reduction), 2 for reader A and 1, 0, 2, 2 for reader B for PET, 0, 1, 2, 2 and 1, 0, 2, 2 for CO2BOLD and 1, 1, 2, 2 and 1, 1, 2, 2 for SPECT. 0 = no reduction in CVR, 1 = mild reduction in CVR, 2 = severe reduction in CVR Color-scales represent: PET: Normalized accumulated activity, dimensionless under the assumption that 1 mL of tissue weights 1 g. BOLD: Statistical Z value, SPECT: Relative counts, normalized to the mean counts in the cerebellum, dimensionless. PET: Positron emission tomography; SPECT: Single photon emission computed tomography; BOLD: Blood oxygenation level dependent.
Statistical analysis
In a first step, we calculated the inter-rater agreement between both raters separately for CO2BOLD, SPECT and PET using Cohen’s kappa statistics with quadratic weighting.
In a second step, we binarized the results in order to calculate sensitivity and specificity values of CVR for the comparison of CO2BOLD vs PET/SPECT. This analysis was done within the clinical context, i.e., the presence of at least one territory with a substantial reduction of CVR is considered as indication for a treatment. This means that the CVR for both PET/SPECT or CO2BOLD was considered “reduced” when at least one vascular territory had a significant reduction of CVR, for example substantial reduction in left MCA and mild reduction in left ACA territory. Conversely, the CVR was considered as normal when no territory had a substantial reduction of CVR, for example mild reduction in both left ACA and MCA territories. Based on this classification, we calculated sensitivity, specificity, positive predictive value, negative predictive value and accuracy.
RESULTS
Inter-rater agreement
The inter-rater agreement was 0.81 for SPECT (excellent), 0.43 for PET (fair) and 0.7 for CO2BOLD (good).
Correspondence of CVR between CO2BOLD and PET/SPECT
The correspondence of CVR assessment between CO2BOLD and PET/SPECT is illustrated in Table 2. In 9/14 cases, there was a correspondence between CO2BOLD and PET/SPECT. In 4/14 cases, Surrogate CVT was significantly reduced in CO2BOLD but not in PET/SPECT, while in 1/14 case surrogate CVT was significantly reduced in PET/SPECT but not in CO2BOLD. Consequently, CO2BOLD tends to over-estimate the reduction in surrogate CVR compared to PET/SPECT. The sensitivity of CO2BOLD was 86% and a specificity of 43%.
Table 2.
Concordance between CO2BOLD and positron emission tomography/single photon emission computed tomography
| PET/SPECT pathologic | PET/SPECT normal | ||
| CO2BOLD pathologic | 6 | 4 | PPV 60% |
| TP 43% | FP 29% | ||
| CO2BOLD normal | 1 | 3 | NPV 75% |
| FN 7% | TN 21% | ||
| SENS 86% | SPEC 43% | ACC 64% |
TP: True positive; TN: True negative; FP: False positive; FN: False negative; SENS: Sensitivity; SPEC: Specificity; PPV: Positive predictive value; NPV: Negative predictive value; ACC: Accuracy; PET: Positron emission tomography; SPECT: Single photon emission computed tomography.
DISCUSSION
The assessment of surrogate CVR using CO2BOLD and a simple nasal cannula approach in MRI in the pre-surgical evaluation and follow-up of moyamoya patients combines the advantages of: (1) absent irradiation notably in the context of serial follow-up; (2) minimal experimental setup; (3) high patient comfort; and (4) the simultaneous assessment of brain parenchyma (acute/old vascular lesions), vessels (progression of vascular stenosis) and CVR in only one imaging session.
The assessment of surrogate CVR in CO2BOLD compared to the reference standard PET/SPECT provided a good sensitivity of 86% yet a relatively low specificity of 43%. The relatively high sensitivity is of particular interest in the clinical context of pre-surgical evaluation of CVR. CO2BOLD could therefore be used as a screening modality for reduction of surrogate CVR in the pre-surgical evaluation and follow-up of moyamoya patients, and in case of a pathologic result of CO2BOLD the reference standard techniques PET or SPECT could be performed in a second step. Moreover, our results show that CO2BOLD tends to over-estimate the reduction of surrogate CVR with respect to the reference standard techniques PET or SPECT. Taking this knowledge into account during the interpretation of the CO2BOLD CVR maps could therefore further improve the sensitivity and specificity of this technique in the future. Moreover, as the CO2BOLD challenge includes contributions from CBF, cerebral blood volume, and oxygenation changes, and is thus more complex than simple CBF change assessed in PET or SPECT. Future direct comparisons of CO2BOLD vs PET or SPECT, ideally in a larger sample size or model, might assess the differences between CO2BOLD surrogate CVR and PET/SPECT CVR in more detail. The inter-rater reliability of CO2BOLD is in the range of the reference techniques PET and SPECT.
A few previous investigations already successfully CVR in moyamoya patients based on BOLD imaging. Heyn et al[12] investigated 11 patients with moyamoya using a tight-fitting facemask demonstrated good correlated of CVR maps with conventional angiography disease stage. Mandell et al[13] investigated CVR based on BOLD in several vascular diseases including 12 patients with moyamoya again using a tight facemask with a rebreathing bag and found good correlation with arterial spin labeling (ASL). A follow-up study by the same group demonstrated that preoperative CO2BOLD CVR predicts the hemodynamic effect of ECIC bypass using the same computer-controlled gas blender and a tight facemask[17]. The BOLD technique may also be used to assess treatment effect in pre- vs post-operative moyamoya disease[14]. Han et al[15] used a computer-driven gas blender and a tight facemask in thirteen pediatric moyamoya patients and were able to reliably derive CVR maps. The advantage of tight facemasks and gas blenders is not only the more accurate delivery of the CO2 stimulus, but also the possibility of assessment of the end tidal CO2 concentration for quantification. An alternative approach for the assessment of CVR is the breath-hold technique[20]. Thomas et al[16] used a breath-hold challenge in eight consecutive pediatric moyamoya patients and found that while all breath-hold challenge during general anesthesia resulted in best quality CVR maps, only 42% of studies without general anesthesia yielded best quality CVR maps. Finally, a previous study Shiino et al[18] validated CO2BOLD CVR against acetazolamide SPECT CVR in 17 healthy subjects and 10 patients with severe ICA steno-occlusive disease, including 2 patients with moyamoya disease. In summary, these previous results indicate that CVR maps can reliably be assessed using tight facemasks while a simple breath-hold technique might not be sufficient to obtain high-quality CVR maps in all patients. With respect to these previous investigations, the current study differs in two main aspects. First, we used a very simple nasal cannula for CO2 stimulation to obtain a simple experimental setup which does not interfere with the other MR imaging sequences yet still allows reliable CVR map generation. While this approach evidently is not as accurate as the use of a computer-driven gas blender and a tight facemask, we consider this as a clinically acceptable compromise to obtain CVR maps with a simple and widely applicable setup, which does not interfere with patient comfort during the acquisition of the other MRI sequences. Second, we directly compare the surrogate CVR maps to PET/SPECT as references standard.
An alternative approach for the assessment of CVR in MRI is an acetazolamide challenge during ASL imaging, which was successfully applied in several recent investigations[21-25]. The advantage of ASL is the estimation of semi-quantitative relative cerebral blood flow (relCBF) maps. However, the intravenous injection of acetazolamide is more invasive than the inhalation of CO2 via a nasal cannula in setup of the current CO2BOLD study. Advantages of CO2BOLD are the in general higher signal to noise ratio. Moreover, one BOLD sequence with ON-OFF CO2 challenge is enough, while ASL usually requires two sequences before and after intravenous acetazolamide injection, and a delay between injection and image acquisition results in an overall longer exam time. Moreover, transit delay may interfere with ASL imaging notably in the context of significant vascular stenosis in moyamoya. Future head-to-head comparison of CO2BOLD vs acetazolamide ASL are required to determine which MRI technique has the better test performance.
Limitations
One of the major limitations of the current investigation is the limited sample size of ten patients with 14 pre-surgical follow-up investigations. This sample size should however be considered in light of the very low prevalence of moyamoya syndrome of 3/1000000 in European countries[3]. Consequently, the previous related studies discussed above include 8[16], 11[12], 12[13] and 13 pediatric[15] patients with moyamoya. It is also important to note that the vasodilation induced by acetazolamide is stronger than the vasodilation induced by the inhalation of CO2 via a simple nasal cannula. Another factor, which can be discussed, is the application of the CO2 via a simple nasal cannula instead of the alternative approach of a tight face-mask that separates in-flow and out-flow. Such a tight face-mask allows for a much more precise application and monitoring of the applied CO2 concentration, however at the cost of a significantly more complex experimental setup, reduced patient comfort during the entire scanning also including anatomic MR sequences and usually increased motion artifacts. As discussed in detail before[11,19], such systems are undoubtedly more precise and appropriate notably for research settings. For example, as we cannot precisely estimate the degree of hypercapnia achieved by the CO2 challenge, which can vary from scan to scan, the current simple nasal cannula technique may not normalized CVR maps with respect to hypercapnia to reduce inter-scan variability during serial follow-up. However, the minimal experimental setup and patient comfort are in our opinion of paramount importance for potential widespread clinical applications of CO2BOLD CVR imaging and arguments in favor of the simple nasal cannula setup. As described above, the CVR assessment based on CO2BOLD was validated already in 2003[18] yet did not make the transition into widespread clinical use and instead remains limited to very few academic centers. This is probably at least in part due to fact that most previous studies use expensive and complicated computer-controlled gas blenders and tight facemasks reducing patient comfort. We deliberately take into account shortcomings with respect to the precision of CO2 application and monitoring using the nasal cannula approach, yet still obtained a CVR map of diagnostic quality in all cases. This approach was already successfully applied to study patients with vascular stenosis of the internal carotid artery[11].
The CO2BOLD technique using a simple nasal cannula approach can be used for pre-surgical assessment of surrogate CVR in patients with moyamoya syndrome and combines the advantages of absent irradiation, patient comfort, simple experimental setup, high availability of MRI and assessment of brain parenchyma, cerebral vessels and surrogate CVR in one stop.
COMMENTS
Background
Moaymoya disease may cause stroke due to a reduction in cerebral blood flow. In order to decide when and if an intervention is needed, it is necessary to assess the capacity of the brain to respond.
Research frontiers
It is important to develop new MR based techniques to replace the currently performed nuclear medicine techniques such as positron emission tomography (PET) and single photon emission computed tomography (SPECT).
Innovations and breakthroughs
The authors find that MR perfusion with COBOLD technique can reliably investigate cerebrovascular reserve (CVR).
Applications
The use of MR based COBOLD will allow to easily test the CVR inpatients with moyamoya and other diseases.
Peer-review
This is a useful study for assessing the patients with moyamoya disease.
Footnotes
Supported by The Swiss National Science Foundation grant, No. 140340.
Institutional review board statement: We declare that the current study has been approved by the University of Geneva Ethical Committee and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent statement: Individual patient consent was waived by the local ethical committee for this retrospective study.
Conflict-of-interest statement: No conflicts of interest.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: Switzerland
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: April 22, 2016
First decision: July 5, 2016
Article in press: September 22, 2016
P- Reviewer: Li YZ S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
References
- 1.Wakai K, Tamakoshi A, Ikezaki K, Fukui M, Kawamura T, Aoki R, Kojima M, Lin Y, Ohno Y. Epidemiological features of moyamoya disease in Japan: findings from a nationwide survey. Clin Neurol Neurosurg. 1997;99 Suppl 2:S1–S5. doi: 10.1016/s0303-8467(97)00031-0. [DOI] [PubMed] [Google Scholar]
- 2.Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med. 2009;360:1226–1237. doi: 10.1056/NEJMra0804622. [DOI] [PubMed] [Google Scholar]
- 3.Yonekawa Y, Ogata N, Kaku Y, Taub E, Imhof HG. Moyamoya disease in Europe, past and present status. Clin Neurol Neurosurg. 1997;99 Suppl 2:S58–S60. doi: 10.1016/s0303-8467(97)00042-5. [DOI] [PubMed] [Google Scholar]
- 4.Mesiwala AH, Sviri G, Fatemi N, Britz GW, Newell DW. Long-term outcome of superficial temporal artery-middle cerebral artery bypass for patients with moyamoya disease in the US. Neurosurg Focus. 2008;24:E15. doi: 10.3171/FOC/2008/24/2/E15. [DOI] [PubMed] [Google Scholar]
- 5.Conklin J, Fierstra J, Crawley AP, Han JS, Poublanc J, Mandell DM, Silver FL, Tymianski M, Fisher JA, Mikulis DJ. Impaired cerebrovascular reactivity with steal phenomenon is associated with increased diffusion in white matter of patients with Moyamoya disease. Stroke. 2010;41:1610–1616. doi: 10.1161/STROKEAHA.110.579540. [DOI] [PubMed] [Google Scholar]
- 6.Kang KH, Kim HS, Kim SY. Quantitative cerebrovascular reserve measured by acetazolamide-challenged dynamic CT perfusion in ischemic adult Moyamoya disease: initial experience with angiographic correlation. AJNR Am J Neuroradiol. 2008;29:1487–1493. doi: 10.3174/ajnr.A1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schubert GA, Weinmann C, Seiz M, Gerigk L, Weiss C, Horn P, Thomé C. Cerebrovascular insufficiency as the criterion for revascularization procedures in selected patients: a correlation study of xenon contrast-enhanced CT and PWI. Neurosurg Rev. 2009;32:29–35; discussion 35-36. doi: 10.1007/s10143-008-0159-z. [DOI] [PubMed] [Google Scholar]
- 8.Cohen ER, Ugurbil K, Kim SG. Effect of basal conditions on the magnitude and dynamics of the blood oxygenation level-dependent fMRI response. J Cereb Blood Flow Metab. 2002;22:1042–1053. doi: 10.1097/00004647-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Spano VR, Mandell DM, Poublanc J, Sam K, Battisti-Charbonney A, Pucci O, Han JS, Crawley AP, Fisher JA, Mikulis DJ. CO2 blood oxygen level-dependent MR mapping of cerebrovascular reserve in a clinical population: safety, tolerability, and technical feasibility. Radiology. 2013;266:592–598. doi: 10.1148/radiol.12112795. [DOI] [PubMed] [Google Scholar]
- 10.Ziyeh S, Rick J, Reinhard M, Hetzel A, Mader I, Speck O. Blood oxygen level-dependent MRI of cerebral CO2 reactivity in severe carotid stenosis and occlusion. Stroke. 2005;36:751–756. doi: 10.1161/01.STR.0000157593.03470.3d. [DOI] [PubMed] [Google Scholar]
- 11.Haller S, Bonati LH, Rick J, Klarhöfer M, Speck O, Lyrer PA, Bilecen D, Engelter ST, Wetzel SG. Reduced cerebrovascular reserve at CO2 BOLD MR imaging is associated with increased risk of periinterventional ischemic lesions during carotid endarterectomy or stent placement: preliminary results. Radiology. 2008;249:251–258. doi: 10.1148/radiol.2491071644. [DOI] [PubMed] [Google Scholar]
- 12.Heyn C, Poublanc J, Crawley A, Mandell D, Han JS, Tymianski M, terBrugge K, Fisher JA, Mikulis DJ. Quantification of cerebrovascular reactivity by blood oxygen level-dependent MR imaging and correlation with conventional angiography in patients with Moyamoya disease. AJNR Am J Neuroradiol. 2010;31:862–867. doi: 10.3174/ajnr.A1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandell DM, Han JS, Poublanc J, Crawley AP, Stainsby JA, Fisher JA, Mikulis DJ. Mapping cerebrovascular reactivity using blood oxygen level-dependent MRI in Patients with arterial steno-occlusive disease: comparison with arterial spin labeling MRI. Stroke. 2008;39:2021–2028. doi: 10.1161/STROKEAHA.107.506709. [DOI] [PubMed] [Google Scholar]
- 14.Mikulis DJ, Krolczyk G, Desal H, Logan W, Deveber G, Dirks P, Tymianski M, Crawley A, Vesely A, Kassner A, et al. Preoperative and postoperative mapping of cerebrovascular reactivity in moyamoya disease by using blood oxygen level-dependent magnetic resonance imaging. J Neurosurg. 2005;103:347–355. doi: 10.3171/jns.2005.103.2.0347. [DOI] [PubMed] [Google Scholar]
- 15.Han JS, Mikulis DJ, Mardimae A, Kassner A, Poublanc J, Crawley AP, deVeber GA, Fisher JA, Logan WJ. Measurement of cerebrovascular reactivity in pediatric patients with cerebral vasculopathy using blood oxygen level-dependent MRI. Stroke. 2011;42:1261–1269. doi: 10.1161/STROKEAHA.110.603225. [DOI] [PubMed] [Google Scholar]
- 16.Thomas B, Logan W, Donner EJ, Shroff M. Assessment of cerebrovascular reactivity using real-time BOLD fMRI in children with moyamoya disease: a pilot study. Childs Nerv Syst. 2013;29:457–463. doi: 10.1007/s00381-012-1952-0. [DOI] [PubMed] [Google Scholar]
- 17.Mandell DM, Han JS, Poublanc J, Crawley AP, Fierstra J, Tymianski M, Fisher JA, Mikulis DJ. Quantitative measurement of cerebrovascular reactivity by blood oxygen level-dependent MR imaging in patients with intracranial stenosis: preoperative cerebrovascular reactivity predicts the effect of extracranial-intracranial bypass surgery. AJNR Am J Neuroradiol. 2011;32:721–727. doi: 10.3174/ajnr.A2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiino A, Morita Y, Tsuji A, Maeda K, Ito R, Furukawa A, Matsuda M, Inubushi T. Estimation of cerebral perfusion reserve by blood oxygenation level-dependent imaging: comparison with single-photon emission computed tomography. J Cereb Blood Flow Metab. 2003;23:121–135. doi: 10.1097/01.WCB.0000037546.46809.CA. [DOI] [PubMed] [Google Scholar]
- 19.Richiardi J, Monsch AU, Haas T, Barkhof F, Van de Ville D, Radü EW, Kressig RW, Haller S. Altered cerebrovascular reactivity velocity in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2015;36:33–41. doi: 10.1016/j.neurobiolaging.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 20.Pillai JJ, Mikulis DJ. Cerebrovascular reactivity mapping: an evolving standard for clinical functional imaging. AJNR Am J Neuroradiol. 2015;36:7–13. doi: 10.3174/ajnr.A3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goetti R, O’Gorman R, Khan N, Kellenberger CJ, Scheer I. Arterial spin labelling MRI for assessment of cerebral perfusion in children with moyamoya disease: comparison with dynamic susceptibility contrast MRI. Neuroradiology. 2013;55:639–647. doi: 10.1007/s00234-013-1155-8. [DOI] [PubMed] [Google Scholar]
- 22.Noguchi T, Kawashima M, Irie H, Ootsuka T, Nishihara M, Matsushima T, Kudo S. Arterial spin-labeling MR imaging in moyamoya disease compared with SPECT imaging. Eur J Radiol. 2011;80:e557–e562. doi: 10.1016/j.ejrad.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Saida T, Masumoto T, Nakai Y, Shiigai M, Matsumura A, Minami M. Moyamoya disease: evaluation of postoperative revascularization using multiphase selective arterial spin labeling MRI. J Comput Assist Tomogr. 2012;36:143–149. doi: 10.1097/RCT.0b013e31824150dd. [DOI] [PubMed] [Google Scholar]
- 24.Sugino T, Mikami T, Miyata K, Suzuki K, Houkin K, Mikuni N. Arterial spin-labeling magnetic resonance imaging after revascularization of moyamoya disease. J Stroke Cerebrovasc Dis. 2013;22:811–816. doi: 10.1016/j.jstrokecerebrovasdis.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Zaharchuk G, Do HM, Marks MP, Rosenberg J, Moseley ME, Steinberg GK. Arterial spin-labeling MRI can identify the presence and intensity of collateral perfusion in patients with moyamoya disease. Stroke. 2011;42:2485–2491. doi: 10.1161/STROKEAHA.111.616466. [DOI] [PMC free article] [PubMed] [Google Scholar]


