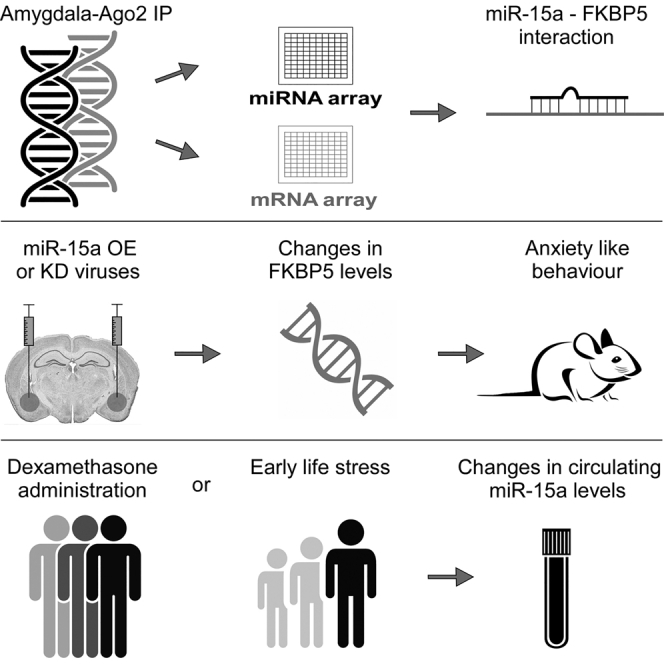
Summary
MicroRNAs are important regulators of gene expression and associated with stress-related psychiatric disorders. Here, we report that exposing mice to chronic stress led to a specific increase in microRNA-15a levels in the amygdala-Ago2 complex and a concomitant reduction in the levels of its predicted target, FKBP51, which is implicated in stress-related psychiatric disorders. Reciprocally, mice expressing reduced levels of amygdalar microRNA-15a following exposure to chronic stress exhibited increased anxiety-like behaviors. In humans, pharmacological activation of the glucocorticoid receptor, as well as exposure to childhood trauma, was associated with increased microRNA-15a levels in peripheral blood. Taken together, our results support an important role for microRNA-15a in stress adaptation and the pathogenesis of stress-related psychopathologies.
Keywords: amygdala, FKBP5, microRNA-15a, chronic stress, anxiety, early life stress, Ago2, stress-related psychopathologies, stress adaption, childhood trauma
Graphical Abstract
Highlights
-
•
miR-15a levels are elevated in the amygdala-Ago2 complex following chronic stress
-
•
miR-15a targets FKBP51 and affects behavioral responses to stressful challenges
-
•
miR-15a is elevated in peripheral human blood following dexamethasone exposure
-
•
miR-15a is elevated in peripheral human blood of patients exposed to childhood trauma
Volk et al. reveal an important role for microRNA-15a in coping with chronic stress, with amygdala-specific manipulation affecting behavioral responses to stressful challenge. Individuals exposed to childhood trauma exhibit increased levels of miR-15a in their peripheral blood, suggesting a target for the treatment of stress-related psychopathologies.
Introduction
Recent studies have linked microRNA (miRNA) expression or biogenesis dysregulation to various psychiatric disorders, including anxiety and depression (Dias et al., 2014b, Issler and Chen, 2015, Issler et al., 2014, Lopez et al., 2014, O’Connor et al., 2012, Volk et al., 2014). However, changes in miRNA expression levels do not necessarily reflect their immediate activity; it is only when a specific miRNA, in the canonical pathway, has matured and been incorporated into the RNA-induced silencing complex (RISC) in the presence of argonaute RISC catalytic component 2 (Ago2) that it becomes truly active (Meister et al., 2004) as a result of its association with its target mRNA.
The amygdala plays a pivotal role in regulating the behavioral responses to stressful challenges (Dunsmoor and Paz, 2015, Duvarci and Pare, 2014, Johansen et al., 2011, Lüthi and Lüscher, 2014, Maren and Holmes, 2016). Recently, regulation of some amygdalar functions and stress-related behaviors has been attributed to miRNAs. miR-34c is involved in regulating stress-induced anxiety (Haramati et al., 2011) and miR-34a in fear memory consolidation (Dias et al., 2014a). Furthermore, miR-19b plays an important role in memory consolidation following stress by regulating the adrenergic receptor beta 1 (Volk et al., 2014).
In this study, we investigated Ago2-associated miRNAs and transcripts in the amygdala of mice subjected to a chronic social defeat stress. This chronic social stress paradigm consists of 10 consecutive days of short physical encounters between a C57BL/6 mouse and an aggressive ICR (CD1) mouse (Golden et al., 2011, Krishnan et al., 2007). The repeated exposure to stress is considered a model for the induction of chronic stress (Elliott et al., 2010, Elliott et al., 2016, Issler et al., 2014), as well as depression-like behavior (Hollis and Kabbaj, 2014, Malatynska and Knapp, 2005) in mice. Molecular analysis and behavioral studies demonstrate that miR-15a is recruited to the Ago2 complex following chronic stress and is an essential regulator of an intact behavioral response to chronic stress.
Results
miR-15a and FKBP51 mRNA Are Associated with Ago2 in the Amygdala following Chronic Stress
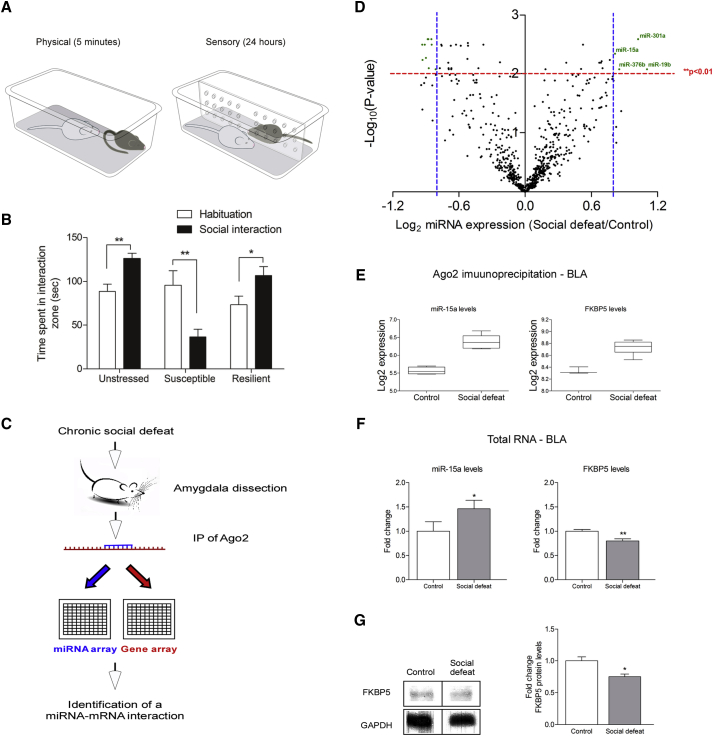
To identify miRNAs that are involved in the regulation of the behavioral response to chronically stressful challenges, we immunoprecipitated the Ago2 complex in tissue obtained from the amygdala of mice 8 days after completion of the chronic social defeat stress. Mice were subjected to the chronic social defeat stress paradigm for 10 consecutive days (Figure 1A), following which they were subjected to a social avoidance test to categorize them as being either “susceptible” or “resilient” to the chronic social defeat stress (Figure 1B). The RNA from the Ago2 complex of three groups—susceptible, resilient, and control—was extracted and analyzed in parallel using two distinct microarray platforms; a miRNA and a mRNA expression array (Figure 1C). Initially, we hypothesized that we would observe changes in the miRNA population of the Ago2 complex in the amygdala not only between stressed and control mice but also between susceptible and resilient mice. However, our analysis revealed that no significant changes were detected between the susceptible and resilient mice. For this reason, we combined the two groups of mice into one that is referred to in the text as “social defeat.” Analysis of the miRNA array revealed four miRNAs that were upregulated and ten that were downregulated (Figure 1D; Figure S1A). A parallel analysis on the gene array revealed a small number of mRNAs that were either upregulated or downregulated in the Ago2 complex immunoprecipitation (IP) following social defeat (Figure S1B). When we analyzed the mRNAs that were changed following social defeat, we focused on stress-associated genes that were previously described to be expressed in the amygdala. This is the reason we focused on FK506 binding protein 51 (FKBP51) and miR-15a. FKBP51 has been previously linked to the pathogenesis of posttraumatic stress disorder and depression (Binder et al., 2008, Klengel et al., 2013, Lekman et al., 2008, Zannas et al., 2016). miR-15a levels were raised 1.8-fold (p = 0.0002) in the array following exposure to chronic stress (Figure 1E). Interestingly, a parallel increase in FKBP51 mRNA (p = 0.001), a predicted target of miR-15a, was observed in the Ago2 complex (Figure 1E). Whereas the levels of miR-15a were also elevated (p = 0.039) in the total RNA levels of the amygdala tissue, FKBP51 levels were, as expected, decreased (p = 0.008) (Figure 1F), supporting the possibility that FKBP51 is directly downregulated by miR-15a in the amygdala. Consistently, the protein levels of FKBP51 were measured, and a reduction of 25% in its levels was observed (p = 0.014) (Figure 1G). Interestingly, the levels of miR-15a were also elevated by 60% in the plasma of mice subjected to chronic stress (p = 0.047), whereas the levels of miR-124, an abundant brain miRNA, were unchanged (Figures S2A and S2B), implicating miR-15a as a possible marker for chronic stress exposure. These experiments led us to focus on miR-15a and FKBP51 and address their involvement in mediating chronic stress cellular processes.
Figure 1.
miR-15a Is Elevated following Chronic Stress and Potentially Regulates FKBP51
(A) Schematic illustration of the social defeat paradigm. C57 mice are subjected to 5 min of physical contact (left) with an aggressive ICR mouse, followed by sensory contact for 24 hr (right).
(B) Social avoidance test. Unstressed mice spend more time in the interaction zone following introduction of an unfamiliar mouse, t(16) = −3.657, p = 0.002. Susceptible mice spend less time in the interaction zone following introduction of an unfamiliar mouse, t(16) = 3.133, p = 0.006. Resilient mice spend more time in the interaction zone following introduction of an unfamiliar mouse, t(16) = −2.358, p = 0.031. Data are represented as mean ± SEM.
(C) Extracts of the amygdalae of mice subjected to social defeat were used for immunoprecipitation (IP) with anti-Ago2 antibody. The bound RNA was analyzed on a miRNA array (four control arrays, n = 12 animals; six social defeat arrays, n = 18 animals) and a gene expression array (three control arrays, n = 9 animals; six social defeat arrays, n = 18 animals).
(D) Log2 miRNA expression analysis. Four miRNAs were elevated, and ten miRNAs were decreased in the amygdala Ago2 complex following social defeat.
(E) miR-15a levels were elevated in the Ago2 precipitate, t(7) = 7.147, p = 0.0002; as was FKBP51 mRNA, t(7) = 5.352, p = 0.0011.
(F) miR-15a was also elevated in total RNA extracted from mice amygdalae (n = 5) following social defeat, t(8) = 2.46, p = 0.039; whereas FKBP51 levels (n = 5) were decreased, t(8) = 3.531, p = 0.008. Data are represented as mean ± SEM.
(G) FKBP51 protein levels following social defeat. FKBP51 protein levels are downregulated in chronically stressed mice compared to control, t(6) = 3.049, p = 0.014. Data are represented as mean ± SEM.
Error bars represent mean ± SEM. ∗p < 0.05; ∗∗p < 0.01.
See also Figures S1 and S2.
miR-15a Transcription Regulation
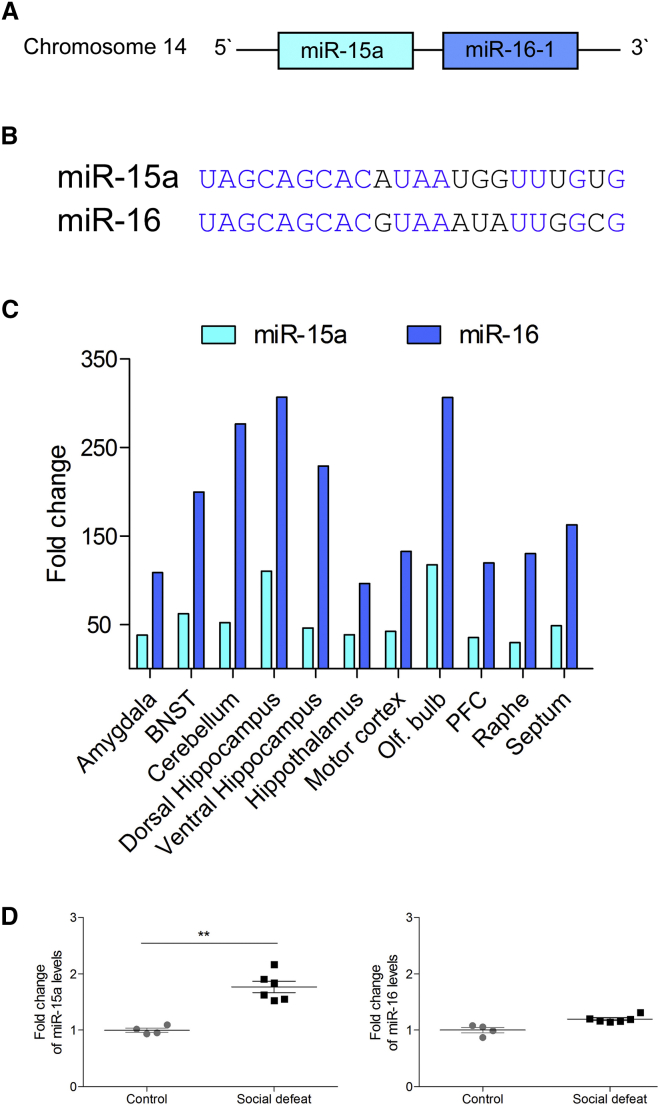
miR-15a is located on chromosome 14 as part of a cluster with miR-16-1 (Figure 2A), indicating that these two miRNAs are cotranscribed. Previous studies have demonstrated that the promoter for miR-15a and miR-16-1 is likely to be the promoter for DLEU2, a non-coding gene that contains the transcript for miR-15a (Zhang et al., 2012). Although both miR-15a and miR-16-1 share a seed sequence, their mature miRNA sequence differs in several nucleotides (Figure 2B). In addition, the total levels of miR-16 in most brain areas appear to be higher than that of miR-15a (Figure 2C), possibly since miR-16 has two copies in the genome (miR-16-1 on chromosome 14 and miR-16-2 on chromosome 3), which both give rise to a similar mature form of miR-16, whose genomic origin is indistinguishable. Importantly, the elevation in miR-15a levels observed in our Ago2 IP is specific for this miRNA and not for miR-16 (Figure 2D), implying miR-15a specificity at the level of the Ago2 complex formation.
Figure 2.
miR-15a and miR-16-1 Are Differentially Expressed following Chronic Stress
(A) Schematic illustration of the miR-15a and miR-16-1 transcript.
(B) Alignment of the mature sequence of miR-15a and miR-16.
(C) The distribution of miR-15a and miR-16 in different brain regions. Olf., olfactory; PFC, prefrontal cortex.
(D) Comparison of the amygdala Ago2 IP results of miR-15a and miR-16. Data are represented as mean ± SEM. ∗∗p < 0.01.
FKBP51 Is a Confirmed Target of miR-15a
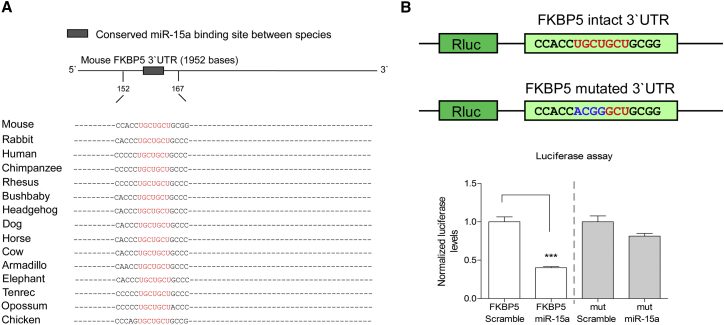
Consistent with direct targeting of FKBP51 by miR-15a, the seed sequence for miR-15a binding at the 3′ UTR of FKBP51 is highly conserved (Figure 3A). Moreover, a luciferase assay, in which a construct containing luciferase followed by the 3′ UTR of FKBP51 was constructed and transfected into Huh7 cells expressing either miR-15a or a scramble control for it, showed a robust specific reduction in normalized luciferase levels (p < 0.001; Figure 3B). Importantly, this reduction was abolished when the miR-15a seed sequence was mutated (Figure 3B). These results support a regulatory role for miR-15a in directly controlling FKBP51 levels.
Figure 3.
FKBP51 Is Regulated by miR-15a In Vitro
(A) Schematic illustration of FKBP51 3′ UTR indicating the conserved seed match for miR-15a.
(B) Luciferase assay with luciferase fused to the 3′ UTR of FKBP51 containing an intact or a control of a mutated (mut) seed site for miR-15a in the presence of miR-15a or control scramble miR (n = 6) showed a 50% decrease in luciferase levels, t(10) = 9.083, p = 0.000. This decrease was abolished when the intact FKBP51 3′ UTR contained a miR-15a mutated seed. Data are represented as mean ± SEM. ∗∗∗p < 0.001
Overexpression of miR-15a in the Basolateral Amygdala Does Not Affect Anxiety-like Behavior
To examine whether increased levels of miR-15a in the amygdala are sufficient to mimic the behavioral effects associated with chronic stress exposure, we designed, constructed, and produced lentiviruses overexpressing the precursor of miR-15a or a scramble miR sequence as a control (Figure S3A). The degree of infection and the levels of miR-15a expression were verified using qPCR on RNA samples extracted from amygdala punches obtained from mice injected with these viruses into the basolateral amygdala (BLA). The treated mice showed an approximately 2-fold increase in the level of amygdalar miR-15a compared to scramble control (p < 0.001; Figure S3B), which is similar to the elevated levels of miR-15a observed following exposure to chronic stress (Figure 1F). To assess the stress-related behavioral changes of mice expressing higher levels of miR-15a, mice were injected bilaterally into the BLA with either miR-15a-overexpressing or control-scrambled viruses under basal or chronic stress conditions (Figures S3C–S3E). Behavioral assessment of the injected mice indicated no significant changes between mice overexpressing miR-15a or a control scramble miR in the open-field test, or in the elevated plus maze (EPM) test, under baseline (Figures S4A and S4C) or chronic stress (Figures S4B and S4D) conditions. In addition, no changes were observed in the locomotor activity or total time traveled in the open field test of mice overexpressing miR-15a compared to control scramble miR under basal (Figures S4E–S4G) or chronic stress conditions (Figures S4H–S4J). Therefore, we concluded that overexpression of miR-15a in the BLA is not sufficient to mimic the behavioral effects associated with exposure to chronic social defeat.
Reduced Levels of miR-15a in the BLA Increases Anxiety-like Behavior following Exposure to Chronic Stress
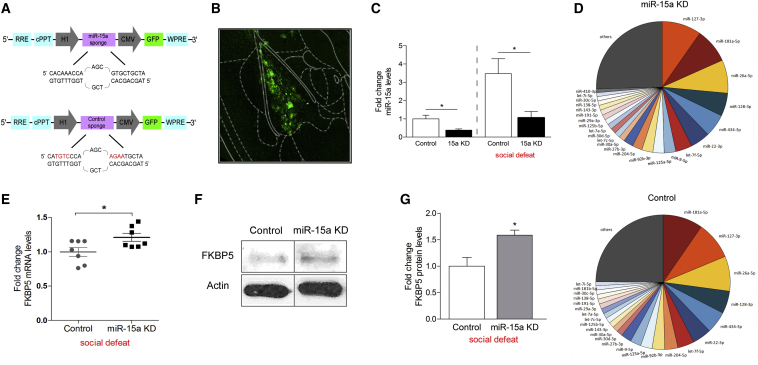
Next, we assessed the requirement of endogenous amygdalar miR-15a levels for the behavioral responses under baseline and chronic stress conditions. We designed, constructed, and produced a viral vector containing multiple binding sites for miR-15a (miR-15a Sponge), which enabled the knockdown (KD) of miR-15a levels in the BLA (Figure 4A). The control sponge viral construct was generated by specifically mutating 4 bp on each side of the bulge of the sponge (Figure 4A). Injection of the miR-15a KD or control sponge viruses into the BLA of mice, regardless of their exposure to chronic social defeat, resulted in an approximately 2.5-fold reduction in the levels of miR-15a in the BLA under basal conditions (p = 0.019) and following social defeat (p = 0.012) (Figures 4B and 4C). Whereas a reduction was observed independently of chronic stress, the absolute levels of miR-15a were higher in the chronic social defeat group compared to controls (Figure 4C). This supports our initial observation regarding elevation in miR-15a levels in the amygdala following chronic social defeat. The BLA of an additional group of mice was injected with miR-15a sponge and a control sponge virus (Figure S5A), and RNA was extracted. The levels of miR-15a were confirmed to be reduced by 40% (p = 0.009) using real-time PCR (Figure S5B). These samples were also sequenced using the Illumina TruSeq Small RNA Library Preparation Kit, and no significant changes were observed in the 25 most abundant miRNAs (Figure 4D), thus verifying a specific KD for miR-15a. As expected from our luciferase assay, FKBP51 mRNA levels were elevated in the BLA of mice injected with the miR-15a KD virus (p = 0.033; Figure 4E). The protein levels of FKBP51 were increased by approximately 50% (p = 0.022; Figures 4F and 4G) following injection of miR-15a KD or control viruses.
Figure 4.
KD of miR-15a in the BLA Results in Increased FKBP51 Levels in the BLA
(A) Schematic illustration of lentiviral GFP-labeled constructs of control and sponge used to knock down miR-15a.
(B) Representative microscope image of virally infected basolateral amygdala (BLA) of a 10-week-old mouse following injection of lentiviral miR-15a KD with enlargement of the BLA region that corresponds to the injection site (Paxinos and Franklin, 2001).
(C) Left: decreased miR-15a levels in the BLA (n = 4) of mice injected with miR-15a KD relative to control under basal conditions, t(6) = 3.175, p = 0.019; or (right) following social defeat, t(6) = 3.528, p = 0.012. Data are represented as mean ± SEM.
(D) miRNA sequencing data. No differences are observed in the top 25 most abundant miRNA from mice injected with miR-15a KD virus compared with the control virus.
(E) Elevated FKBP51 levels in the BLA (n = 7) of mice injected with miR-15a KD relative to control, t(12) = −2.413, p = 0.033. Data are represented as mean ± SEM.
(F and G) FKBP51 protein levels following miR-15a KD virus injection to the BLA. FKBP51 protein levels are upregulated in the BLA of mice injected with miR-15a KD virus compared with control virus, t(6) = −3.060, p = 0.022. Data are represented as mean ± SEM.
∗p < 0.05.
See also Figures S3 and S5.
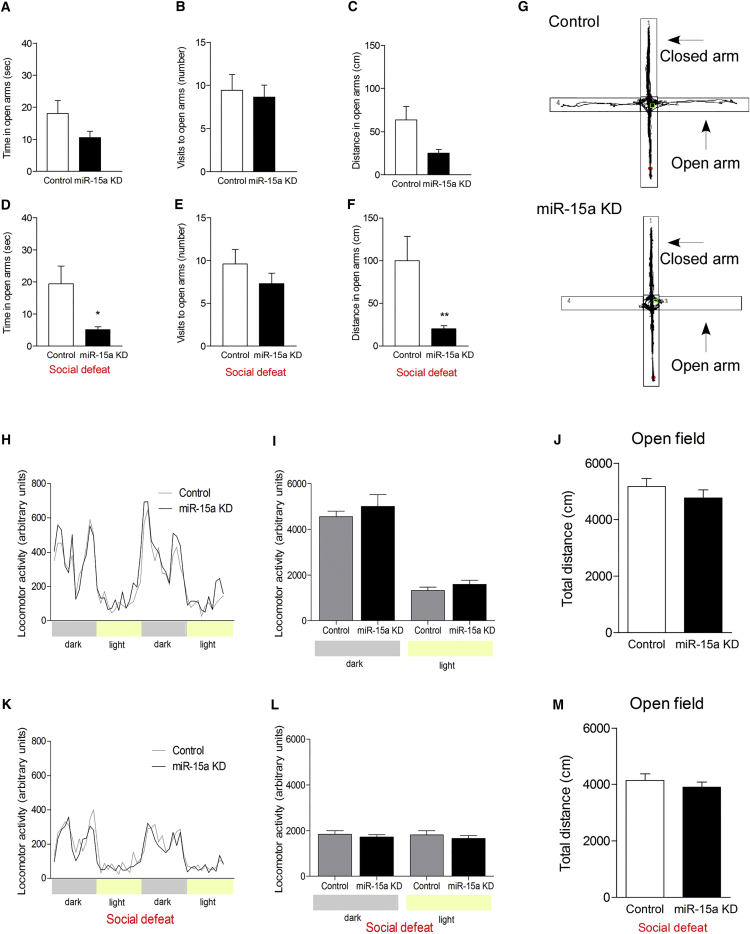
Next, we assessed the miR-15a KD mice for anxiety-like behavior using the EPM test. Under baseline conditions (in which mice were not exposed to chronic social defeat), a tendency toward main effect was observed (p = 0.058). No significant changes were observed in the time spent in the open arms, number of visits to the open arms, or distance traveled in the open arms between the miR-15a KD and control groups (Figures 5A–5C). Similarly, the locomotor activity and the total distance traveled in the open field test showed no differences between these groups (Figures 5H–5J). Intriguingly, however, following chronic social defeat, a main effect between the behavior of miR-15a KD and control mice was observed (p = 0.026). Mice with miR-15a KD spent significantly less time in the open arms (p = 0.009) (Figures 5D and 5G) and traveled less distance in the open arms relative to controls (p = 0.002; Figures 5F and 5G, (asterisks indicate significance following correction with Bonferroni correction for multiple testing). No differences were observed in the number of visits to the open arms, the locomotor activity between the groups, or the total distance traveled in the open field test (Figures 5E and 5K–5M). These results demonstrate that KD of miR-15a levels in the amygdala specifically impaired the recovery and behavioral response of mice following their exposure to chronic stress. In the open field test, miR-15a KD mice spent less time in the center of the arena (p = 0.032), but no changes were observed in the distance traveled in the center or the number of visits to the center (Figure S5C). Following chronic social defeat, miR-15a KD and control mice spent similarly less time in the center of the arena, suggesting a “floor effect.” However, miR-15a KD mice showed a tendency to travel for less distance in the center of the arena (p = 0.060) and made fewer visits to the center of the arena (p = 0.041) (Figure S5D). These results are in accordance with Hartmann et al. (2015), who observed induced anxiety-related behavior following overexpression of FKBP51 in the BLA. Moreover, Attwood et al. (2011) showed that silencing of FKBP51 levels in the BLA by injection of lentiviral short hairpin RNA led to a reduction in anxiety levels in the EPM. Taken together, the present data suggest that amygdalar miR-15a levels are functionally important in regulating the behavioral response to challenge and suggest that this effect is mediated, at least in part, via a reduction of FKBP51 levels.
Figure 5.
Mice with Virally Mediated Reduced Levels of BLA-miR-15a Exhibit Increased Anxiety-like Behavior
(A–C) Results from the elevated plus maze (EPM) test of mice injected with miR-15a KD or control viruses (ns = 11 and 12, respectively) showing a tendency for differences, F(3, 19) = 2.971, p = 0.058. No significant differences were observed in the time spent in the open arms (A), the number of visits to the open arms (B), or the distance traveled in the open arms (C), according to Bonferroni correction for multiple testing. Data are represented as mean ± SEM.
(D–F) Mice injected with miR-15a KD or control viruses (ns = 10 and 9, respectively) that were also subjected to social defeat showed different behavior in the EPM, F(3, 15) = 4.08, p = 0.026; with a significant decrease in the time (D) (U = 13, p = 0.009) and distance (F) (U = 7, p = 0.002) spent in the open arms of the EPM (corrected according to Bonferroni correction for multiple testing). No changes were observed in the number of visits to the open arms (E). Data are represented as mean ± SEM.
(G) Representative tracking in the EPM of control mice (upper panel) relative to miR-15a KD mice (lower panel).
(H–M) No changes were observed in the locomotor activity and total distance traveled in the open field test between miR-15a KD and control mice under basal conditions (H–J) or following social defeat (K–M). Data in (I)–(J), (L), and (M) are represented as mean ± SEM.
∗p < 0.05; ∗∗p < 0.01.
See also Figures S4 and S5.
miR-15a Is Regulated by Glucocorticoids and Trauma in Human Samples
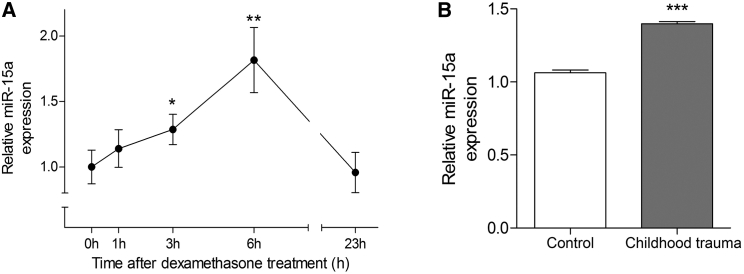
To examine the potential parallel role of miR-15a in the human stress response, we first analyzed miR-15a expression levels in RNA extracted from peripheral blood cells of young healthy male subjects following administration of the glucocorticoid receptor (GR) agonist dexamethasone (1.5 mg orally [p.o.]). We observed a significant upregulation of miR-15a at 3 and 6 hr post-treatment (Figure 6A), indicating that miR-15a is potentially regulated by activation of the stress hormone system in humans. In addition, we performed miRNA analyses on peripheral blood cells of subjects with childhood trauma and control subjects matched for age and gender with no history of early life stress. We found that the levels of miR-15a were significantly higher by 32% in subjects exposed to childhood trauma as compared to control subjects who were not exposed (p = 0.000, Figure 6B). Taken together, these results support a functional association between the blood levels of miR-15a and psychiatric impairment.
Figure 6.
miR-15a Level in Human Peripheral Blood Is Higher following Acute Dexamethasone Treatment and Exposure to Childhood Trauma
(A) Relative miR-15a levels in peripheral blood cells of young healthy male subjects following 1.5 mg dexamethasone treatment. Repeated-measures ANOVA: F(4, 22) = 4.42, p = 0.009. ∗p < 0.05; ∗∗p < 0.01. A significant upregulation of miR-15a was observed 3 hr post-treatment, t(25) = −2.240, p = 0.034; and 6 hr post-treatment, t(25) = −3.487, p = 0.002.
(B) Levels of miR-15a in blood of subjects exposed to childhood trauma as compared to subjects not exposed, t(29.715) = −13.776, p = 0.000. Data are represented as mean ± SEM.
Discussion
The present study reveals an important role for amygdalar miR-15a in regulating the behavioral responses to chronic stressful challenges. miR-15a levels are significantly increased in the amygdala of mice subjected to chronic stress, and amygdala-specific KD of miR-15a changes the behavioral responses to chronic stressful challenges. A target of miR-15a, FKBP51, identified in our studies, has been implicated in a number of stress-related psychiatric disorders (Binder, 2009, Zannas et al., 2016). FKBP51 is part of the immunophilin protein family and is known to play a role in GR transcriptional activation following the elevation of cortisol (Gillespie et al., 2009). Manipulation of FKBP51 levels in the BLA, using small interfering RNA (Attwood et al., 2011), or of its overexpression, using a viral vector (Hartmann et al., 2015), has been linked to changes in anxiety-like behavior. FKBP51, which is strongly implicated in a number of stress-related psychiatric disorders and is currently a leading target for pharmacological manipulation for the treatment of various psychopathologies, is robustly regulated both in vitro and in vivo by miR-15a. Importantly, miR-15a is upregulated by pharmacological activation of the stress response in humans by dexamethasone treatment, as well as exposure to early adverse life events. Therefore, miR-15a might represent an important target for the treatment of stress-related psychopathologies.
Our results imply that, in the chronic stress response, miR-15a and its target FKBP51 represent major components for the following reasons: Although miR-15a is bioinformatically predicted to target other stress- and depression/anxiety-related transcripts, such as GILZ or Sgk1 (Anacker et al., 2013, Thiagarajah et al., 2014), the mRNA levels of these genes were unchanged in our Ago2 IP array (data not shown), supporting the specificity of the assay. In the present study, we focused exclusively on miR-15a regulation of FKBP51 due to the reported involvement of this gene in stress-response regulation and stress-linked psychopathologies (Hartmann et al., 2012, Hartmann et al., 2015, Scharf et al., 2011). Furthermore, FKBP51 mRNA was detected in the Ago2 complex, implicating a direct binding to the RNAi machinery. Finally, a significant decrease in FKBP51 levels was observed in the total RNA samples that concomitantly exhibited elevated miR-15a levels. Nevertheless, it is important to note that FKBP51 is not the only predicted target of miR-15a and that the changes observed in anxiety-like behavior after knocking down the levels of miR-15a in vivo are not mediated merely by affecting the levels of FKBP51.
Although BLA-specific overexpression of miR-15a resulted in no significant behavioral changes, knocking down miR-15a in the BLA caused an anxiogenic phenotype following exposure to chronic stress. The regulation of miR-15a following exposure to chronic stress and the observed anxiogenic phenotype in the BLA-miR-15a KD mice following chronic stress exposure may suggest that miR-15a is specifically involved in regulating the behavioral responses to repeated or chronic stressful exposure. Nevertheless, miR-15a may potentially be important also in regulating anxiety levels, regardless of the stress history of the mice, and its effect may be amplified by stress. The lack of an anxiolytic phenotype in the BLA-miR-15a-overexpressing mice could be explained either by lack of spatial specificity, meaning that the overexpression of miR-15a was not induced in endogenously relevant BLA neurons, or by a possible “ceiling effect,” in which increasing levels of miR-15a on top of its endogenous stress-induced elevation is not effective because the stress response has already reached its full capacity. However, preventing the elevation of endogenous miR-15a in the BLA by its KD resulted in a failure of the mice to mount the required behavioral response when exposed to a chronic stressful challenge.
Finally, the elevation of miR-15a in two distinct human stress-linked scenarios—namely, administration of dexamethasone to healthy subjects as well as individuals exposed to childhood trauma—strongly suggests its involvement in human stress conditions. Collectively, the preclinical and human translational results presented in the present study strongly suggest that alterations in miR-15a levels are associated with the behavioral response to chronic or repeated stressful challenges and may be relevant in the pathogenesis of adverse life events and stress-linked psychiatric disorders such as anxiety. Targeting miR-15a levels might prove to be beneficial in the treatment of these conditions.
Experimental Procedures
See also the Supplemental Experimental Procedures.
Chronic Social Defeat Stress
10-week old C57BL/6J male mice were subjected to a chronic social defeat stress protocol, as previously described (Krishnan et al., 2007). Briefly, the mice were placed randomly in a home cage of an aggressive ICR mouse and allowed to physically interact for 5 min. During this time, the ICR mouse attacked the intruder mouse and the intruder displayed subordinate posturing. A perforated clear Plexiglas divider was then placed between the animals, and they remained in the same cage for 24 hr to allow sensory contact. The procedure was then repeated with an unfamiliar ICR mouse for each of the next 10 days. The animal protocols were approved by the Institutional Animal Care and Use Committee (IACCU) of the Weizmann Institute of Science.
IP of Ago2 Protein, RNA Purification, and Microarray
Pools of three amygdalae taken from three mice from the same treatment group (either social defeat, n = 18, or control, n = 12) were immunoprecipitated using magnetic protein G beads (Dynabeads, Invitrogen/Life Technologies) and Ago2 monoclonal antibody (WAKO Chemicals).
RNA from the Ago2 IP samples was isolated and analyzed on an Affymetrix miRNA 2.0 array (enriched RNA protocol) and an Affymetrix Mouse Gene 1.0 ST array.
Cloning of 3′ UTRs into Psicheck2 Luciferase Expression Plasmid
The 3′ UTR sequence of FKBP51 was PCR amplified from mouse genomic DNA. This mutation replaced the first 4 nt in the miR-15a seed sequence of FKBP51.
Design, Construction, and Validation of miR-15a Lentiviruses
The miR-15a overexpression vector was cloned following the human synapsin promoter. The miR scramble control was purchased from GeneCopoeia. The H1-miR-15a sponge KD and its control were designed according to Lin et al. (2011).
Stereotactic Intracranial Injections
A computer-guided stereotaxic instrument and a motorized nanoinjector (Angle Two Stereotaxic Instrument, myNeuroLab, Leica Biosystems) were used as described previously (Elliott et al., 2010, Kuperman et al., 2010, Regev et al., 2012).
Behavioral Assessments
All behavioral assessments were performed during the dark (active) phase following habituation to the test room for 2 hr before each test.
Open-Field Test
The open-field test was performed in a 50 × 50 × 22-cm white box, lit to 120 lux. The mice were placed in the box for 10 min. Locomotion in the box was quantified using a video tracking system (VideoMot2; TSE Systems).
EPM Test
The apparatus in this test is designed as a plus sign and contains two barrier walls and two open arms. During the 5-min test, which is performed in relative darkness (6 lux), data are scored using a video tracking system (VideoMot2, TSE Systems).
Homecage Locomotion
Homecage locomotion was assessed using the InfraMot system (TSE Systems). Measurements of general locomotion consisted of two light and two dark cycles in the last 48 hr, collected at 10-min intervals.
Statistics
Data are expressed as mean ± SEM and were performed using the Statistical Package for the Social Sciences (SPSS) software.
Human Studies: Dexamethasone
Dexamethasone-unstimulated peripheral blood samples were drawn at 12:00 p.m. followed by oral administration of 1.5 mg dexamethasone. Subsequently, stimulated samples were collected at 1:00 p.m., 3:00 p.m., 6:00 p.m., and at 11:00 a.m. the following day.
Author Contributions
Conceptualization, N.V. and A. Chen; Methodology, N.V. and A. Chen; Investigation, N.V., J.C.P., M.E., A.S.Z., and N.C.; Writing – Original Draft, N.V., J.C.P., E.B.B., and A. Chen; Writing – Review and Editing, N.V., J.C.P., E.B.B., and A. Chen; Funding Acquisition, E.B.B. and A. Chen; Supervision, A. Cattaneo, E.B.B., and A. Chen.
Acknowledgments
A. Chen is the head of the Max Planck - Weizmann Laboratory for Experimental Neuropsychiatry and Behavioral Neurogenetics. We thank Mr. Sharon Ovadia for his devoted assistance with animal care and Dr. Jessica Keverne for professional English editing, formatting, and scientific input. This work is supported by: The Max Planck Foundation; an FP7 grant from the European Research Council (260463); a research grant from the Israel Science Foundation (1351/12); research support from Roberto and Renata Ruhman; Bruno and Simone Licht; Estate of Toby Bieber; the Henry Chanoch Krenter Institute for Biomedical Imaging and the Irving I. Moskowitz Foundation; and the I-CORE Program of the Planning and Budget Committee and the Israel Science Foundation (grant no. 1916/12). The human studies were supported by the Behrens-Weise Foundation (to E.B.B.) and a European Research Council starting grant (grant number 281338, GxE molmech) within the FP7 framework (to E.B.B.).
Published: November 8, 2016
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and five figures and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2016.10.038.
Accession Numbers
The accession numbers for the data reported in this paper are GEO: GSE87488, GSE87489, and GSE87533.
Supplemental Information
References
- Anacker C., Cattaneo A., Musaelyan K., Zunszain P.A., Horowitz M., Molteni R., Luoni A., Calabrese F., Tansey K., Gennarelli M. Role for the kinase SGK1 in stress, depression, and glucocorticoid effects on hippocampal neurogenesis. Proc. Natl. Acad. Sci. USA. 2013;110:8708–8713. doi: 10.1073/pnas.1300886110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwood B.K., Bourgognon J.M., Patel S., Mucha M., Schiavon E., Skrzypiec A.E., Young K.W., Shiosaka S., Korostynski M., Piechota M. Neuropsin cleaves EphB2 in the amygdala to control anxiety. Nature. 2011;473:372–375. doi: 10.1038/nature09938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder E.B. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34(Suppl 1):S186–S195. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Binder E.B., Bradley R.G., Liu W., Epstein M.P., Deveau T.C., Mercer K.B., Tang Y., Gillespie C.F., Heim C.M., Nemeroff C.B. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias B.G., Goodman J.V., Ahluwalia R., Easton A.E., Andero R., Ressler K.J. Amygdala-dependent fear memory consolidation via miR-34a and Notch signaling. Neuron. 2014;83:906–918. doi: 10.1016/j.neuron.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias C., Feng J., Sun H., Shao N.Y., Mazei-Robison M.S., Damez-Werno D., Scobie K., Bagot R., LaBonté B., Ribeiro E. β-catenin mediates stress resilience through Dicer1/microRNA regulation. Nature. 2014;516:51–55. doi: 10.1038/nature13976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor J.E., Paz R. Fear generalization and anxiety: behavioral and neural mechanisms. Biol. Psychiatry. 2015;78:336–343. doi: 10.1016/j.biopsych.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Duvarci S., Pare D. Amygdala microcircuits controlling learned fear. Neuron. 2014;82:966–980. doi: 10.1016/j.neuron.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott E., Ezra-Nevo G., Regev L., Neufeld-Cohen A., Chen A. Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nat. Neurosci. 2010;13:1351–1353. doi: 10.1038/nn.2642. [DOI] [PubMed] [Google Scholar]
- Elliott E., Manashirov S., Zwang R., Gil S., Tsoory M., Shemesh Y., Chen A. Dnmt3a in the medial prefrontal cortex regulates anxiety-like behavior in adult mice. J. Neurosci. 2016;36:730–740. doi: 10.1523/JNEUROSCI.0971-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie C.F., Phifer J., Bradley B., Ressler K.J. Risk and resilience: genetic and environmental influences on development of the stress response. Depress. Anxiety. 2009;26:984–992. doi: 10.1002/da.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S.A., Covington H.E., 3rd, Berton O., Russo S.J. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramati S., Navon I., Issler O., Ezra-Nevo G., Gil S., Zwang R., Hornstein E., Chen A. MicroRNA as repressors of stress-induced anxiety: the case of amygdalar miR-34. J. Neurosci. 2011;31:14191–14203. doi: 10.1523/JNEUROSCI.1673-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann J., Wagner K.V., Liebl C., Scharf S.H., Wang X.D., Wolf M., Hausch F., Rein T., Schmidt U., Touma C. The involvement of FK506-binding protein 51 (FKBP5) in the behavioral and neuroendocrine effects of chronic social defeat stress. Neuropharmacology. 2012;62:332–339. doi: 10.1016/j.neuropharm.2011.07.041. [DOI] [PubMed] [Google Scholar]
- Hartmann J., Wagner K.V., Gaali S., Kirschner A., Kozany C., Rühter G., Dedic N., Häusl A.S., Hoeijmakers L., Westerholz S. Pharmacological inhibition of the psychiatric risk factor FKBP51 has anxiolytic properties. J. Neurosci. 2015;35:9007–9016. doi: 10.1523/JNEUROSCI.4024-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis F., Kabbaj M. Social defeat as an animal model for depression. ILAR J. 2014;55:221–232. doi: 10.1093/ilar/ilu002. [DOI] [PubMed] [Google Scholar]
- Issler O., Chen A. Determining the role of microRNAs in psychiatric disorders. Nat. Rev. Neurosci. 2015;16:201–212. doi: 10.1038/nrn3879. [DOI] [PubMed] [Google Scholar]
- Issler O., Haramati S., Paul E.D., Maeno H., Navon I., Zwang R., Gil S., Mayberg H.S., Dunlop B.W., Menke A. MicroRNA 135 is essential for chronic stress resiliency, antidepressant efficacy, and intact serotonergic activity. Neuron. 2014;83:344–360. doi: 10.1016/j.neuron.2014.05.042. [DOI] [PubMed] [Google Scholar]
- Johansen J.P., Cain C.K., Ostroff L.E., LeDoux J.E. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klengel T., Mehta D., Anacker C., Rex-Haffner M., Pruessner J.C., Pariante C.M., Pace T.W., Mercer K.B., Mayberg H.S., Bradley B. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat. Neurosci. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V., Han M.H., Graham D.L., Berton O., Renthal W., Russo S.J., Laplant Q., Graham A., Lutter M., Lagace D.C. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Kuperman Y., Issler O., Regev L., Musseri I., Navon I., Neufeld-Cohen A., Gil S., Chen A. Perifornical Urocortin-3 mediates the link between stress-induced anxiety and energy homeostasis. Proc. Natl. Acad. Sci. USA. 2010;107:8393–8398. doi: 10.1073/pnas.1003969107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekman M., Laje G., Charney D., Rush A.J., Wilson A.F., Sorant A.J., Lipsky R., Wisniewski S.R., Manji H., McMahon F.J., Paddock S. The FKBP5-gene in depression and treatment response--an association study in the STAR∗D Cohort. Biol. Psychiatry. 2008;63:1103–1110. doi: 10.1016/j.biopsych.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q., Wei W., Coelho C.M., Li X., Baker-Andresen D., Dudley K., Ratnu V.S., Boskovic Z., Kobor M.S., Sun Y.E., Bredy T.W. The brain-specific microRNA miR-128b regulates the formation of fear-extinction memory. Nat. Neurosci. 2011;14:1115–1117. doi: 10.1038/nn.2891. [DOI] [PubMed] [Google Scholar]
- Lopez J.P., Lim R., Cruceanu C., Crapper L., Fasano C., Labonte B., Maussion G., Yang J.P., Yerko V., Vigneault E. miR-1202 is a primate-specific and brain-enriched microRNA involved in major depression and antidepressant treatment. Nat. Med. 2014;20:764–768. doi: 10.1038/nm.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüthi A., Lüscher C. Pathological circuit function underlying addiction and anxiety disorders. Nat. Neurosci. 2014;17:1635–1643. doi: 10.1038/nn.3849. [DOI] [PubMed] [Google Scholar]
- Malatynska E., Knapp R.J. Dominant-submissive behavior as models of mania and depression. Neurosci. Biobehav. Rev. 2005;29:715–737. doi: 10.1016/j.neubiorev.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Maren S., Holmes A. Stress and fear extinction. Neuropsychopharmacology. 2016;41:58–79. doi: 10.1038/npp.2015.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister G., Landthaler M., Patkaniowska A., Dorsett Y., Teng G., Tuschl T. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol. Cell. 2004;15:185–197. doi: 10.1016/j.molcel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- O’Connor R.M., Dinan T.G., Cryan J.F. Little things on which happiness depends: microRNAs as novel therapeutic targets for the treatment of anxiety and depression. Mol. Psychiatry. 2012;17:359–376. doi: 10.1038/mp.2011.162. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Franklin K.B.J. Second Edition. Academic Press; 2001. The Mouse Brain in Stereotaxic Coordinates. [Google Scholar]
- Regev L., Tsoory M., Gil S., Chen A. Site-specific genetic manipulation of amygdala corticotropin-releasing factor reveals its imperative role in mediating behavioral response to challenge. Biol. Psychiatry. 2012;71:317–326. doi: 10.1016/j.biopsych.2011.05.036. [DOI] [PubMed] [Google Scholar]
- Scharf S.H., Liebl C., Binder E.B., Schmidt M.V., Müller M.B. Expression and regulation of the Fkbp5 gene in the adult mouse brain. PLoS ONE. 2011;6:e16883. doi: 10.1371/journal.pone.0016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajah A.S., Eades L.E., Thomas P.R., Guymer E.K., Morand E.F., Clarke D.M., Leech M. GILZ: Glitzing up our understanding of the glucocorticoid receptor in psychopathology. Brain Res. 2014;1574:60–69. doi: 10.1016/j.brainres.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Volk N., Paul E.D., Haramati S., Eitan C., Fields B.K., Zwang R., Gil S., Lowry C.A., Chen A. MicroRNA-19b associates with Ago2 in the amygdala following chronic stress and regulates the adrenergic receptor beta 1. J. Neurosci. 2014;34:15070–15082. doi: 10.1523/JNEUROSCI.0855-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannas A.S., Wiechmann T., Gassen N.C., Binder E.B. Gene-stress-epigenetic regulation of FKBP5: clinical and translational implications. Neuropsychopharmacology. 2016;41:261–274. doi: 10.1038/npp.2015.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Chen X., Lin J., Lwin T., Wright G., Moscinski L.C., Dalton W.S., Seto E., Wright K., Sotomayor E., Tao J. Myc represses miR-15a/miR-16-1 expression through recruitment of HDAC3 in mantle cell and other non-Hodgkin B-cell lymphomas. Oncogene. 2012;31:3002–3008. doi: 10.1038/onc.2011.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.