Abstract
Global warming and anthropogenic disturbances significantly influence the biosphere, tremendously increasing species extinction rates. In Central Alabama, we analyzed Drosophilidae species composition change nearly 100 years after the previous survey. We found ten Drosophilid species that were not reported during the last major biodiversity studies, two of which are invasive pests. In addition, we analyzed the influence of seasonal environmental variables characteristic of the subtropical climate zone on Drosophila abundance and biodiversity. We found a significant correlation between temperature and abundance of total Drosophila as well as for six of the seven most represented species individually, with a maximum abundance at intermediate temperatures (18–26°C). In addition, temperature was positively correlated with biodiversity of Drosophila. Precipitation produced a significant effect on the abundance of five species of Drosophila, with different optima for each species, but did not affect overall biodiversity. We concluded that in the subtropical climate zone of Central Alabama, seasonal temperature and precipitation changes produce a significant effect on Drosophila abundance and biodiversity, while local land use also impacts fly abundance, contributing to an apparent shift in species composition over the last century. We expect global climate change and other anthropogenic factors to further impact Drosophila species composition in the subtropical climate zone into the future.
Keywords: biodiversity, Drosophila, invasive species, seasonal variation, subtropical climate, urbanization
1. Introduction
In the face of global warming, we can see the influence of changes in the world's climate on the biosphere. Currently, the global species extinction rate is 100–10,000 times higher than a predicted natural rate, which suggests that a new mass extinction is underway (Ceballos et al., 2015; Pimm, Russell, Gittleman, & Brooks, 1995). A decrease in global biodiversity may cause serious consequences for the biosphere by influencing ecosystem function and reducing overall productivity of biological communities (Cardinale et al., 2006; Gamfeldt, Hillebrand, & Jonsson, 2008; Hector et al., 1999; Tilman, Wedin, & Knops, 1996). Decreases in ecosystem productivity can lead to serious implications for fishery, forestry, and other types of industries that depend on harvesting natural resources. In addition, many wild species of plants and animals potentially harbor undiscovered beneficial natural products; thus, their extinction could have negative effect on native human populations (Cooper, 2004; Newmark, 2002). Native species can also prevent or slow invasive pests’ habitat range expansion, via interspecific competition (Commar, da Conceição Galego, Ceron, & Aparecida Carareto, 2012; Hooper et al., 2005). Consequently, biodiversity surveys may reveal early colonization of pest species and native species extinction, which makes them a useful tool for natural resource management and prevention of natural habitat degradation.
The Drosophilidae family consists of about 4,000 species distributed in approximately 65 genera, including Drosophila (Brake & Bächli, 2008; da Mata, Valadão, & Tidon, 2015; Srinath & Shivanna, 2014). Members of Drosophila genus are widely used in genetic, developmental, and molecular biology studies (e.g., Guruprasad, Hegde, & Krishna, 2010; da Mata et al., 2015; Strickberger, 1962). However, relatively little is known about the ecology of Drosophila (da Mata et al., 2015; Shorrocks, 1975). Phylogenetic relationships of the Drosophila members are not fully understood, and presently Drosophila is a paraphyletic group (Robe, Valente, Budnik, & Loreto, 2005). However, several molecular and morphological studies accept that Drosophila, Sophophora, Hirtodrosophila, Mycodrosophila, Zaprionus, and Scaptomyza collectively form one monophyletic clade (Da Lage et al., 2007; van der Linde & Houle, 2008; van der Linde, Houle, Spicer, & Steppan, 2010; O'Grady & DeSalle, 2008; Remsen & O'Grady, 2002).
Drosophila has the potential to become a standard model organism for investigating the influence of climate and habitat change on the biological community, as most species have rather limited realized niches and are dependent on a particular group of host organisms for feeding and reproduction (Parsons, 1991; Poppe, Valente, & Schmitz, 2013). In addition, Drosophila are sensitive to shifts in essential climate variables (Parsons, 1991; Poppe, Valente, & Schmitz, 2013; Srinath & Shivanna, 2014). The influence of seasonal changes on Drosophila abundance and biodiversity has been surveyed mostly in tropical and temperate climatic zones (Dobzhansky & Pavan, 1950; Levitan, 1954; da Mata et al., 2015; Patterson, 1943; Shorrocks, 1975). The abundance of Drosophila species is influenced primarily by precipitation in tropical regions (Dobzhansky & Pavan, 1950; Guruprasad et al., 2010; Srinath & Shivanna, 2014; Torres & Madi‐Ravazzi, 2006), while in temperate climate regions, seasonal changes in abundance and biodiversity of Drosophila are better explained by temperature variation (da Mata et al., 2015; Patterson, 1943; Poppe, Valente, & Schmitz, 2013).
In addition, Drosophila has the potential to be a model organism for studying the influence of anthropogenic disturbance, particularly urbanization, on species biodiversity and abundance. Endemic Drosophila species are often very sensitive to natural habitat deterioration, can form very specific host associations, and are not able to adapt to human environmental modifications (Avondet, Blair, Berg, & Ebbert, 2003; Dobzhansky & Pavan, 1950; Ferreira & Tidon, 2005; van Klinken & Walter, 2001; Parsons, 1991; Shorrocks, 1975). As a result, a decrease in abundance of endemic species of Drosophila could indicate that a habitat is recently or currently being disturbed by human influence. The opposite is true for cosmopolitan species of Drosophila that are often abundant in urban environments (Avondet, Blair, Berg, & Ebbert, 2003). During a biodiversity survey in an urban area in Brazil, it was shown that exotic species of Drosophila contributed to over 90% of the total abundance in this type of environment (Ferreira & Tidon, 2005).
Surveys of wild populations of cosmopolitan Drosophila are especially important as there are two known pest Drosophilids: Drosophila suzukii and Zaprionus indianus. Both of these pest species are polyphagous and are very efficient in colonizing new types of environments (Burrack, Smith, Pfeiffer, Koeher, & Laforest, 2012; Commar et al., 2012). Drosophila suzukii originated in Asia and has recently quickly expanded its range, being first reported in the continental United States in 2008 and in Europe in 2009 (Berry, Anthony, Newfield, Ornsby, & Armstrong, 2012). In new habitats, D. suzukii broadened its host range and became a significant pest of berries and soft flesh fruits such as plums, peaches, and nectarines (Berry et al., 2012; Burrack et al., 2012). Zaprionus indianus is believed to have originated in Africa where it was not considered a significant pest (Commar et al., 2012; Joshi, Biddinger, Demchak, & Deppen, 2014). However, in 1999, Z. indianus was reported in South America, where it became a serious pest of figs (Commar et al., 2012; Vilela, 1999). In 2005, Z. indianus was reported in North America, Florida, where it continued to expand its habitat and host range (Commar et al., 2012; van der Linde et al., 2006).
To the best of our knowledge, there is very little accessible information on the influence of seasonal climate variables on abundance of Drosophila in subtropical regions, specifically Alabama. The average annual temperature for Central Alabama is 18.3°C and varies monthly from 1.6 to 33.7°C, with average annual precipitations of 1395 mm, ranging from 90 to 142 mm per month (NOAA 2014–2015). The last major Drosophila biodiversity survey reported the presence of 26 Drosophilidae species in Alabama (Sturtevant, 1916, 1918, 1921). However, the influence of climatic variables on abundance and biodiversity was not evaluated. Due to the relatively high seasonal variation in temperature and precipitation in Alabama, we hypothesized that both of these climatic variables will significantly influence Drosophila abundance and biodiversity. In addition, we hypothesized that abundance of cosmopolitan Drosophila species would be higher in urban settings than in industrial or minimally disturbed rural environments.
2. Materials and Methods
2.1. Sample collection
We collected samples from 23 sites in and around Tuscaloosa, AL (Fig. 1 and Table S1). Collection sites were chosen based on land‐use type. We sampled seven sites that are used for industrial production or storage of industrial products, eight urban parks, three nonurban parks (a biological station, an arboretum, and a state park), and five sites that did not fall into any category (a highway rest station, an apartment complex, an archeological park, a roadside, and a farm, Table S1). Latitude and longitude of each collection site were recorded with a Garmin GPS navigator (Table S1). Samples were collected from banana and mushroom traps left overnight and collected in a time range from 7:00 a.m. to 11:00 a.m. the following morning (18–24 hr collection period total) using an alcohol aspirator (Markow & O'Grady, 2005). Samples were stored in 70% ethanol at −20°C. We made 16 collection trips from July 2014 to May 2015. Collection trips were performed once per month except for the time period from August 2014 to November 2014, during which collections were performed twice per month. For the period from June 2014 to December 2014, we sampled from five to seven randomly selected sites from our 23 collection sites, then starting in January 2015, we chose six collection sites to focus on and visited them monthly.
Figure 1.
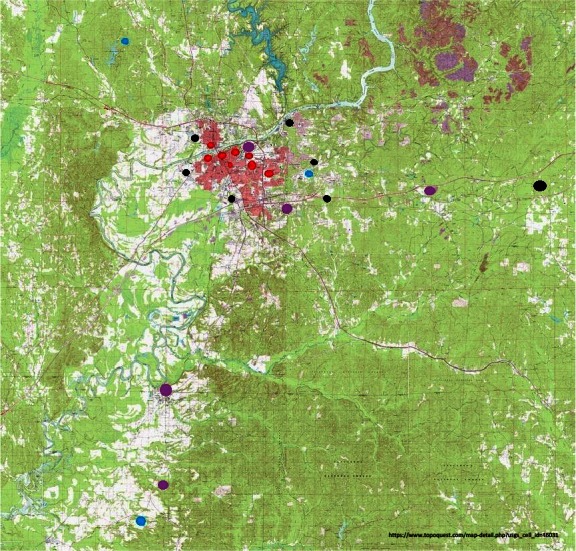
Collection sites around Tuscaloosa. Nonurban parks are marked in blue, urban parks are marked in red, sites of industrial production and industrial product storage are marked in black, and sites that do not match any of the described categories are marked in purple
2.2. Sample identification
Samples were identified based on species identification keys (Markow & O'Grady, 2005; Strickberger, 1962). DNA from representative samples of each species was extracted via DNA extraction Chelex 100 protocol (Walsh, Metzger, & Higuchi, 1991), modified by usage of one Drosophila sample per DNA extraction. Extracted DNA was PCR‐amplified for the cytochrome oxidase 1 (CO1) gene (Reed, Nyboer, & Markow, 2007) and then sent for sequencing. Morphological species identifications were confirmed with CO1 sequence similarity to Genbank reference sequences via BLAST.
2.3. Seasonal climatic data
Climate data were obtained from the Tuscaloosa weather station of the National Oceanic and Atmospheric Administration (NOAA). Average temperature was calculated via averaging daily high and low temperatures. Monthly averages were calculated via averaging daily temperature and precipitation for the 2‐day collection period and the preceding 28 days. In addition, we calculated the average climate variables for each month that preceded each collection month (56–28 days preceding).
2.4. Statistical analyses
We limited our analyses to collected representatives of Drosophila, Sophophora, Hirtodrosophila, Mycodrosophila, Zaprionus, and Scaptomyza. According to several studies, these groups form a monophyletic group: Drosophila Genus Complex (van der Linde et al., 2010; O'Grady & DeSalle, 2008; van der Linde & Houle, 2008; Da Lage et al., 2007; Remsen & O'Grady, 2002; FlyBase). All statistical analyses were performed using BiodiversityR package, version 2.5–4 according to the BiodiversityR manual (Kindt & Coe, 2005). Influence of climatic variables on the abundance of all Drosophila, Mycodrosophila, and Scaptomyza samples, as well as on each species individually was performed with negative binomial GLM of the following form:
In our work, we refer to abundance as a number of individual specimens that were collected during a single collection trip at a given site. The influence of each climatic variable on species abundance was tested with linear and quadratic models. The final single‐term model was chosen based on the lowest Akaike information criterion (AIC) value. In addition, with linear and quadratic negative binomial GLM models, we tested the influence of the precipitation average in month previous to the collection month on the abundance of mycophagous Drosophila species: D. tripunctata and D. putrida. We also tested linear and quadratic multivariate models that combined effects of temperature and precipitation. The best multivariate model was chosen based on AIC value and single‐term deletion test. The multivariate model was considered superior if it explained more deviance and has a lower AIC value than models with only one variable. Biodiversity was evaluated as species richness and the Shannon and Simpson biodiversity indices. To analyze the influence of climate variables on biodiversity, we used linear regression models of the following form:
To evaluate the influence of land use on abundance and biodiversity of Drosophila, we separated 17 of our sites into three land‐use categories: nonurban parks, urban parks, and places of industrial production. To evaluate sufficiency of the number of sampling sites for each category, rarefaction analyses were performed (Kindt & Coe, 2005). In addition, we developed a model that evaluated all of our sites for the presence or absence of six variables: garbage or trash cans, industrial or agricultural production, asphalt road, highway, railroad or airport, and residential or public catering buildings. The site's degree of anthropogenic disturbance (disturbance score) was the sum of factors present for each collection area and was then tested in a negative binomial GLM against Drosophila abundance per trap. Linear regression was used to test for correlations between biodiversity (as measured by species richness, Shannon and Simpson indices) and the disturbance score. In addition, using the negative binomial model, we tested the influence of latitude and longitude on the abundance and biodiversity of Drosophila.
3. Results
3.1. Collected species
During our collections, we found 21 Drosophilidae species. We collected and identified 14 species of named Drosophila genus: D. affinis, D. putrida, D. tripunctata, D. melanogaster, D. simulans, D. suzukii, D. busckii, D. cardini, D. euronotus, D. falleni, D. immigrans, D. macrospina, D. nigromelanica, D. robusta, and four closely related Drosophilidae species: Hirtodrosophila duncani, Mycodrosophila dimidata, Zaprionus indianus, and Scaptomyza frustfrustulifera (Fig. 2). Other Drosophilidae species that were identified but not included in statistical analyses were Scaptodrosophila latifasciaeformis, Chymomyza amoena, and Leucophenga angusta (Fig. 2), because these species fell out of the monophyletic Drosophila Genus Complex. D. affinis, D. putrida, D. tripunctata, D. melanogaster, D. simulans, D. suzukii, and D. robusta were responsible for 96.3% of the total abundance among our samples. Drosophila affinis was the most abundant species, contributing to over 42% of total Drosophila abundance (Fig. 3).
Figure 2.
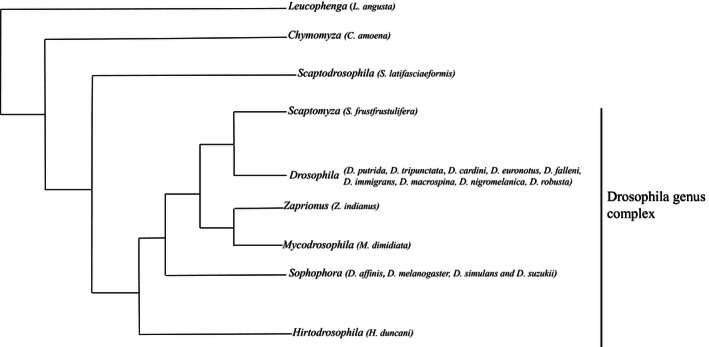
Simplified phylogenetic tree of Drosophilidae. Built on the basis of O'Grady and DeSalle (2008), and Remsen and O'Grady (2002) studies. Drosophila species that we collected in each Drosophilidae's group are shown in parentheses
Figure 3.
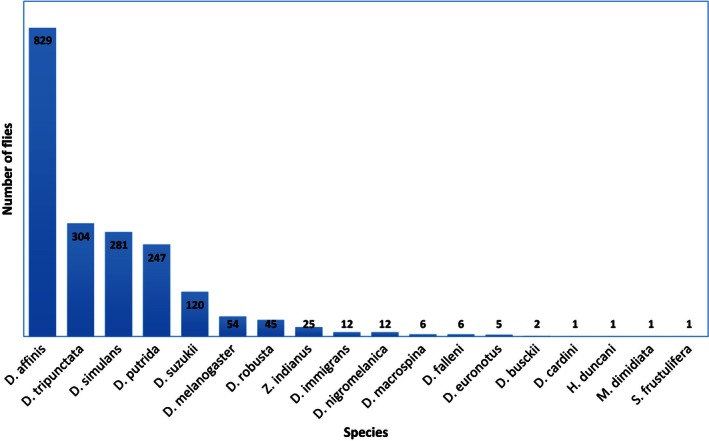
Number of individual flies per species collected from June 2014 to May 2015 and used for statistical analysis
3.2. Climatic variable influence on abundance
We analyzed the influence of climate variables on total abundance of Drosophila and closely related species, as well as on the abundance of the most represented species individually. It should be noted that in this manuscript, observed abundances of flies are the result of the combined effects of their actual numbers present in the environment, but also of their activity level and thus “catchability.” When conditions are less favorable over the short term (e.g., too cold), many species of flies become much more difficult to trap.
3.3. Overall fly abundance
Analyses of the influence of the climatic variables during the collection period, with a second‐order negative binomial GLM (sonbGLM), showed that temperature significantly influenced total number of collected samples (p = .001) with explained deviance (ED) of 43.9% (Fig. 4A) and a maximum abundance at a monthly average temperature of 21°C. We did not find any significant correlation between amount of precipitation during collection periods and total abundance of Drosophila. Combination of variables in a multivariate model did not improve the model. Taking into account monthly averages of the climatic variables, we found a significant correlation between the total number of flies and monthly average temperature (p = .001), using sonbGLM model that explained 44.5% of the deviance (Fig. 4C). Monthly average precipitation level significantly influenced total abundance of Drosophila (p = .032) and explained 21.1% of the deviance (Fig. 4D). In this case, the correlation is better explained by negative binomial GLM (nbGLM) then by sonbGLM. The combination of climatic variables in one multivariate model did not result in a better model.
Figure 4.

Influence of seasonal variation of climate variables on total Drosophila abundance. (A) Two‐day average temperature, (B) 2‐day precipitation average, (C) monthly average temperature, and (D) monthly average precipitation. Observed and predicted results are marked with black and red dots, respectively. Continuous line stands for a mean response, and dotted lines stand for confidence interval
3.4. Abundance individual species
3.4.1. Collection period climate variables
Analyzing the influence of climatic variables for the collection time period, we found a bell‐shape quadratic relation between temperature and the abundance of D. affinis (p = .01, ED = 33.5%) (Fig. 5A), D. tripunctata (p = 1.41e‐04, ED = 50.1%) (S1A), and D. putrida (p = 6.39e‐04, ED = 47.2%) (Fig. S2A). Negative binomial GLM was more efficient in explaining correlation between collection period temperature and abundance of D. simulans (p = .015, ED = 25.5%) (Fig. S3A) and D. melanogaster (p = 2.74e‐04, ED = 45.1%) (Fig. S4A). Analyzing the influence of precipitation over the collection period on the abundance of Drosophila, we found a significant negative correlation between precipitation and abundance of D. affinis (p = .009, ED = 28.3%, Fig. 5B). The negative binomial GLM was the most efficient model in explaining this correlation. The combination of climate variables in one multivariate model was appropriate in the case of D. tripunctata (p = 1.43e‐05, ED = 61.5%), D. putrida (p = 1.59e‐06, ED = 62.7%), and D. affinis (p = .002, ED = 42.6%).
Figure 5.
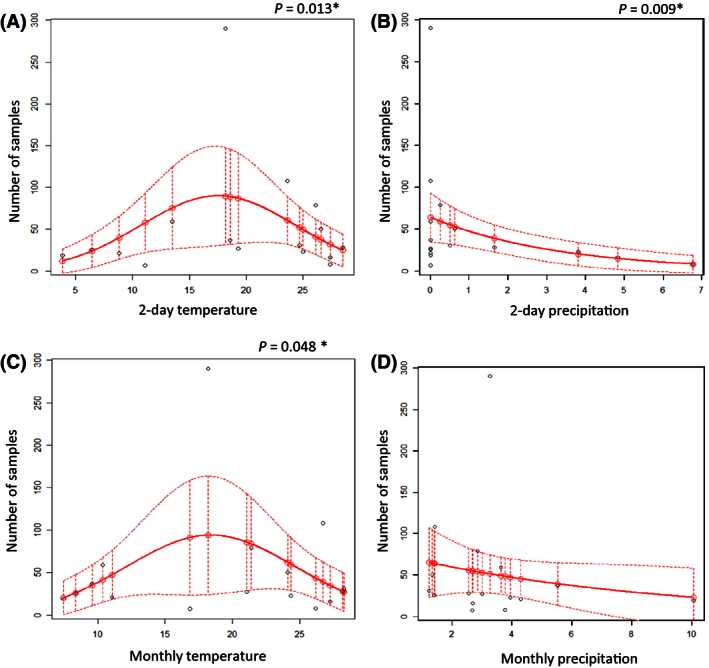
Influence of seasonal variation of climate variables on Drosophila affinis abundance. (A) Two‐day average temperature, (B) 2‐day precipitation average, (C) monthly average temperature, and (D) monthly average precipitation verses abundance. Observed and predicted results are marked with black and red dots, respectively. Continuous line stands for a mean response, and dotted lines stand for confidence interval
3.4.2. Monthly averages of climate variables
Considering monthly climate averages, we found that sonbGLM was the best model for explaining the bell‐shape correlation between temperature and abundance of D. affinis (p = .048, ED = 26%, Fig. 5C), D. tripunctata (p = 1.70e‐05, ED = 56%, Fig. S1C), D. putrida (p = .002, ED = 43.4%, Fig. S2C) D. melanogaster (p = 8.21e‐06, ED = 64.9%, Fig. S4C), and D. robusta (p = .002, ED = 48.2%, Fig. S5C). Influence of precipitation on Drosophila abundance could be best explained by the quadratic model in the cases of D. simulans (p = 2.36e‐05, ED = 58.6%, Fig. S3D), D. melanogaster (p = .04, ED = 30.2%, Fig. S4D), and D. robusta (p = 5.44e‐05, ED = 65.1%, Fig. S5D). The optimal monthly average temperature across these individual species ranged from 18 to 26°C. We found a significant positive correlation between monthly temperature average and abundance of D. simulans (p = 2.85e‐04, ED = 43.6%, Fig. S3C), as well as a negative correlation between precipitation average and abundance of D. putrida (p = .008, ED = 29%, Fig. S2D), using nbGLM. Combination of temperature and precipitation in one multivariate model resulted in a better model for D. simulans (p = 1.08e‐06, ED = 66.5%), D. robusta (p = 2.91e‐06, ED = 75%), and D. melanogaster (p = 3.45e‐06, ED = 69.9%). In addition, we did not find any significant correlation between precipitation averages during the month preceding the month of collections, with any of the models described above.
3.5. Influence of seasonal climatic variables on biodiversity
We found a variation in the number of species collected during different seasons. Species richness was highest during the fall (September–November) with 14 species, and lowest during the winter (December–February) with only five species. During spring (March–May) and summer (June–August), we found representatives of 13 and 11 Drosophila species, respectively (Table 1). There was a significant positive correlation between monthly average temperature and biodiversity indices including Shannon index (p = .001, Fig. 6A) and Simpson index (p = .0026, Fig. 6B). Monthly temperature also significantly influenced species richness, with a positive correlation (p = .0017, Fig. 6C). Average temperature for each collection period showed a similar pattern of a positive correlation with the Shannon (p = .01, Fig. 6D) and Simpson (p = .03) biodiversity indices (Fig. 6E), as well as species richness (p = .007, Fig. 6F), in positive correlations. We did not find any significant correlation between amount of precipitation and Drosophila biodiversity.
Table 1.
Species abundances by month
| Species | June | July | August | September | October | November | December | January | February | March | April | May |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drosophila affinis | 23 | 8 | 44 | 139 | 77 | 66 | 21 | 19 | 26 | 37 | 290 | 79 |
| Drosophila putrida | 1 | 3 | 72 | 69 | 14 | 2 | 1 | 0 | 1 | 2 | 48 | 34 |
| Drosophila tripunctata | 8 | 13 | 12 | 43 | 70 | 3 | 5 | 1 | 1 | 7 | 103 | 38 |
| Drosophila melanogaster | 7 | 2 | 13 | 6 | 7 | 2 | 0 | 0 | 0 | 0 | 0 | 17 |
| Drosophila simulans | 1 | 2 | 139 | 79 | 37 | 17 | 0 | 0 | 0 | 0 | 0 | 6 |
| Drosophila robusta | 6 | 5 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 14 | 16 |
| Drosophila suzukii | 2 | 5 | 18 | 12 | 24 | 13 | 13 | 3 | 12 | 1 | 17 | 0 |
| Zaprionus indianus | 0 | 0 | 0 | 8 | 12 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Drosophila busckii | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Drosophila cardini | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Drosophila euronotus | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 2 |
| Drosophila falleni | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 1 |
| Drosophila immigrans | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 6 |
| Drosophila macrospina | 0 | 0 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 |
| Drosophila nigromelanica | 0 | 7 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Hirtodrosophila duncani | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mycodrosophila dimidiata | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Scaptomyza frustulifera | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Figure 6.
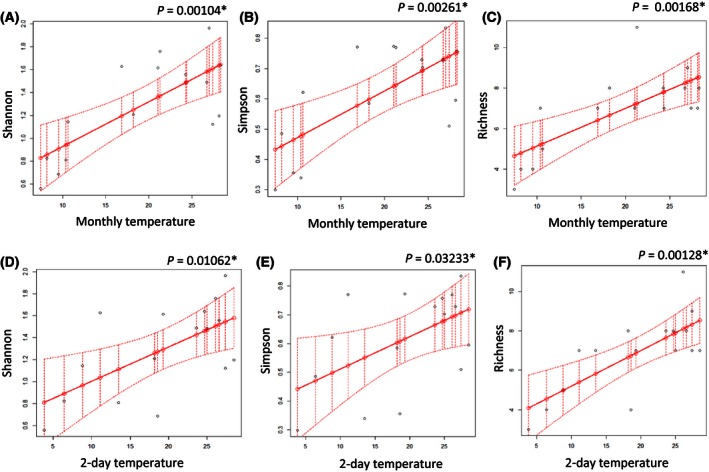
Influence of seasonal variation of climate variables on biodiversity. Monthly average temperature against: (A) Shannon index, (B) Simpson index, and (C) species richness, and 2‐day average temperature against: (D) Shannon index, (E) Simpson index, (F) species richness. Observed and predicted results are marked with black and red dots, respectively. Continuous line stands for a mean response, and dotted lines stand for confidence interval
3.6. Influence of land use on abundance and biodiversity of Drosophila
Separating our sites into three categories, industrial areas, urban parks, and nonurban parks, we found a significant correlation between land use and total abundance of Drosophila species (p = .037, Fig. 7). The analyses of most represented species individually showed a correlation between abundance of D. tripunctata (p = .008) and land‐use categories. In addition, land‐use type may influence abundance of D. putrida (p = .079). The lowest abundance of both of these mushroom‐feeding Drosophila was recorded from industrial areas. Disturbance score, which evaluated the presence or absence of disturbance factors such as garbage or trash cans, industrial or agricultural productions, asphalt roads, highway, railroads or airports, and residential or public catering buildings across all collection sites, only correlated with the abundance of D. putrida (p = .035), where greater disturbance produced lower abundances of the fly species. In contrast to abundance, we did not find any significant correlation between land use and biodiversity indices or species richness. In addition, we did not find any evidence to conclude that narrow range of latitude or longitude over the collection sites produced a significant effect on abundance or biodiversity of Drosophila.
Figure 7.
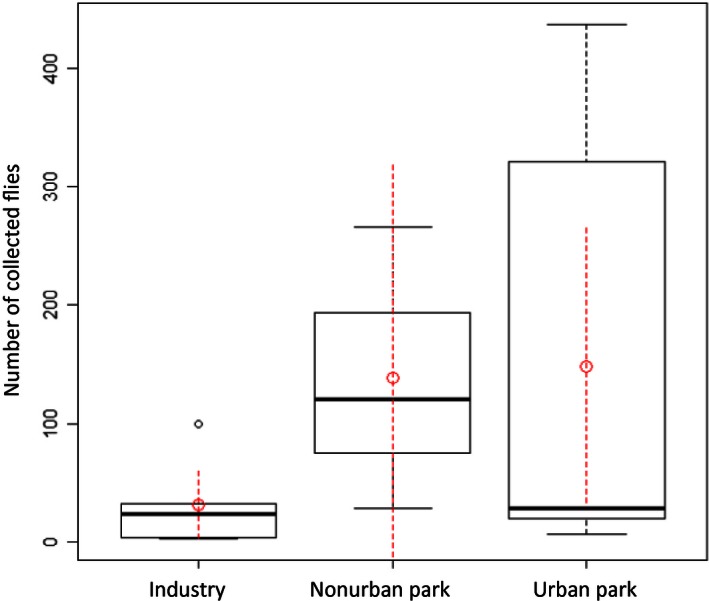
Number of individual flies collected per land‐use category. The lowest number of samples was collected in industrial zones. The highest number of samples was collected in urban park areas
4. Discussion
The previous Drosophila biodiversity studies in Alabama reported 26 Drosophilidae species (Sturtevant, 1916, 1918; Sturtevant, 1921) compared to the 18 found in this study, a difference in species richness of only five species. This difference could be due to the sampling techniques or/and sampling area choice. In previous biodiversity surveys, Sturtevant (1916, 1918), sampling was mostly performed via catching samples in a banana bait and net sweeping of fruits and mushrooms. Sturtevant and Dobzhansky (1936) noticed that at least for D. affinis subgroup species, they did not observe a significant difference in species frequencies based on the collection method. In addition, Dobzhansky and Pavan (1950) indicated that North American mycophagous Drosophila readily came to banana baits and we also found that the mycophagous flies were found on our banana baits in our study.
Sturtevant performed the Alabama collections cited in his 1918 paper throughout the year (April, June, July, October, November) between 1914 and 1916, thus covering a similar seasonal range to our own study. The Sturtevant (1918) study aimed to describe the diversity of Drosophila and other dipterous insects collected in Alabama, and the study was conducted in and around Mobile, Alabama. Mobile County and Tuscaloosa County are approximately 195 miles apart, and given the broad distribution of most of these Drosophila species (Markow & O'Grady, 2005), it is likely that many of the species should be found at both sites if they are present at all; however, further work would be needed to clarify how spatial variation influenced local species diversity changes in Mobile County specifically. Based on these observations, we believe that our sampling techniques, which were broadly similar to earlier studies, should not substantially influence the species composition difference that we have noticed between previous and current survey. However, it is still possible the differences in species presence between what was observed 100 years ago and now could be due, in part, to random variation in collecting success, while also being influenced by more deterministic factors such as climate change and urbanization of the available habitats.
We saw a significant change in species composition. Almost half of the species that we identified in this study were not reported during the last biodiversity survey: H. duncani, S. frustfrustulifera, L. angusta, Z. indianus, D. macrospina, D. nigromelanica, D. suzukii, D. euronotus, D. cardini, and D. falleni. However, before 1960 in North America, D. falleni was mistakenly lumped with D. transversa (which Sturtevant, 1918 reported finding in Alabama), and thus D. falleni might actually be a native species to Alabama and part of Sturtevant's original collections (Wheeler, 1960). Of ten previously unreported species, H. duncani, S. frustfrustulifera, and D. cardini were captured only once, which makes it difficult to ascertain their permanent presence in Alabama. Overall, it is reasonable to conclude that there has been a substantial change in Drosophilidae species diversity in Alabama over the last century.
4.1. Invasive species
Among the newly introduced Drosophilas, the most interesting was the presence of D. suzukii and Z. indianus in our collected samples. Both of these flies are recognized as invasive pests, and both were consistently present in our traps. We were able to find D. suzukii during 13 of 16 collection trips and Z. indianus in four collection trips from September to November. Drosophila suzukii was officially reported in Alabama in 2012 (Burrack et al., 2012), but there had not been a published report of Z. indianus presence in Alabama. Zaprionus indianus was first reported as a pest species in 1999 in Brazil, where it destroyed 40% of commercial fig production in the state of São Paulo (Commar et al., 2012). In the United States, Z. indianus was reported in Florida in 2006 (van der Linde et al., 2006) and Pennsylvania in 2014 (Joshi et al., 2014). It also was reported in Mexico (Lasa & Tadeo, 2015) and Canada (Renkema, Miller, Fraser, Légaré, & Hallett, 2013). Within the United States, Z. indianus was found on peach, raspberry, and blueberry farms (Biddinger, Joshi, & Demchak, 2012; Joshi et al., 2014). In Alabama, peach and blueberry production is not very substantial and valued at <4 million US dollars in 2014 (USDA). In addition, Z. indianus usually infests only damaged fruits and is considered a secondary pest (Joshi et al., 2014). Due to these reasons, Z. indianus would not be expected to become a major pest in Alabama. However, one possible concern could be an overlap of the host range of D. suzukii and Z. indianus in Alabama, which could allow Z. indianus to colonize fruits damaged by D. suzukii.
4.2. Native species
Among native species, we could see some pattern between Drosophila seasonal abundance and their host dietary types. Drosophila affinis was most abundant across all months with the exception of August. Drosophila affinis is a generalist species that feeds on tree saps, fruits, and mushrooms (Carson & Stalker, 1951; Strickberger, 1962; Sturtevant, 1916) and prefers fruits and slime fluxes for oviposition sites (Avondet, Blair, Berg, & Ebbert, 2003). Drosophila affinis has a rather wide distribution range within the United States, with sightings as far north as Maine and Quebec (Jaenike, 1978; Miller, 1958). The presence of at least one food type during all the seasons, a broad range of oviposition hosts, and the relatively high cold tolerance of D. affinis could at least partially explain its highest abundance during most of the year.
Drosophila tripunctata and D. putrida are primarily fungus feeders that choose mushrooms as their preferred breeding sites (Sturtevant, 1916; Strickberger, 1962; Avondet, Blair, Berg, & Ebbert, 2003). Drosophila tripunctata is less discriminate in food preference and can be found on rotten fruits and slime fluxes (Carson & Stalker, 1951). During our collections, both of these species were abundant in banana traps, suggesting that D. putrida could use rotten fruits as a food source in natural environments. Both of these species were found during nearly all collection months. Together, these generalist mycophagous species would make the second most abundant group of flies across our samples, which suggests that their diverse host range could be responsible for high abundance during all seasons.
Three relatively low abundance species were D. simulans, D. melanogaster, and D. robusta, and they all have a relatively narrow host range. Drosophila simulans and D. melanogaster primarily feed on rotten fruits (Strickberger, 1962; Sturtevant, 1916), and rotten fruit is also the preferred oviposition media for these species (Avondet, Blair, Berg, & Ebbert, 2003; Carson & Stalker, 1951). Drosophila simulans and D. melanogaster were absent from our traps from December to April, which could be correlated with fruit and berry season. Drosophila robusta feeds primarily on fruits, mushrooms, and tree saps (Carson & Stalker, 1951; Strickberger, 1962; Sturtevant, 1916), is very specific in choosing sites for oviposition, and in natural environments was reported to breed primarily on slime fluxes (Carson & Stalker, 1951; Avondet, Blair, Berg, & Ebbert, 2003). Abundance of D. robusta did not follow any obvious seasonal pattern, and the species was absent from our traps in September and October, and from January to March.
Based on our observations overall, we can conclude that the ability to use a broad host range for feeding and oviposition could play an important role in abundance of Drosophila species through all seasons in Alabama.
4.3. Climatic impacts on abundance and biodiversity
According to our models, monthly temperature influences abundance more than the 2‐day collection period temperature for total abundance of Drosophila, as well as abundance of D. tripunctata, D. melanogaster, D. robusta, and D. simulans. The effect of monthly temperature on total abundance was driven largely by the highly abundant D. affinis. The average amount of precipitation per month also influences total abundance and that of the most represented species of Drosophila, with the exception of D. affinis. The lack of a significant monthly precipitation effect on the D. affinis that we collected is especially interesting as D. affinis consisted of a substantial proportion of all collections, meaning that the other species were driving the variation in total abundance in response to monthly. We can conclude that difference in climate variables per month produces a more significant effect on the abundance of Drosophila than temperature and precipitation during collection days. In addition, we can see that most Drosophila species exhibit a quadratic response to a seasonal climate variation, suggesting the presence of an optimal climatic condition range for each species.
It was shown that precipitation had a positive correlation with mushroom's productivity, which might result in increased abundance of mycophagous flies (Krebs, Carrier, Boutin, Boonstra, & Hofer, 2008; Worthen & McGuire, 1990). Therefore, assuming that increased precipitation levels would facilitate fungi's fruiting body formation, we tested the influence of precipitation during the 56‐ to 28‐day period preceding the collection on the abundance of mycophagous D. tripunctata and D. putrida. However, we did not find any significant correlation. The lack of correlation can probably be explained by combination of factors in mushroom and Drosophila ecology. Worthen and McGuire (1990) observed that rainfall could produce a significant effect on the following week's mushroom abundance and noticed that an individual fungi's fruit body is often short‐lived. At 18°C, D. putrida and D. tripunctata egg to adult developmental time ranges from 14 to 15 days and might be shortened by warmer temperatures (Markow & O'Grady, 2005); thus, we might expect a spike in abundance to occur within 2–3 weeks of the increase in mushroom fruit bodies. Boulétreau (1978) described that almost half of female Drosophila melanogaster collected from natural populations were <24–36 hr old, while Roff (1980) suggested that in wild, adult Drosophila life span might be only few days; thus, any spike in abundance due to an increase in mushroom fruiting bodies might be expected to be of a short duration, on the order of a few days.
Based on the limited information about wild Drosophila life span and rate of mushrooms’ fruit body productivity increase in the response to rainfall, it is logical that monthly precipitation during the collection month would produce more effect on mycophagous Drosophila populations than precipitation in the previous month. However, surprisingly, the abundance of the two mycophagous species was actually negatively correlated with precipitation within the collection month (statistically significant for D. putrida). The lack of a clear association between host availability and mycophagous fly abundance at the monthly scale suggests that more granular analyses are needed in future studies to determine how and whether the fly abundance is influenced by the presence of mushrooms.
Temperature influences not only mature Drosophila activity but also its developmental time and larval survival (Crill, Huey, & Gilchrist, 1996; James, Azevedo, & Partridge, 1997). If temperature fluctuations during the month are out of a species’ optimal range, then fewer mature Drosophila will develop. This could generally explain the greater influence of monthly temperature on abundance of Drosophila. Interestingly, we did not find a significant correlation between abundance of D. suzukii or Z. indianus and any of the climate variables. In the case of Z. indianus, the major reason for this lack of correlation could be small sample size (25 flies) and our ability to find this pest fly only during fall months. However, D. suzukii was present most times of the year, which suggests that this pest species has physiological or behavioral adaptations to better resist differences in seasonal climate change than native Drosophila species.
Several previous studies reported no significant correlation between abundance of Drosophila and seasonal temperature variation (Guruprasad et al., 2010; Srinath & Shivanna, 2014; Torres & Madi‐Ravazzi, 2006). The reason for the difference in our results relative to theirs could be due, in part, to the different approach in statistical analyses. Most of these studies used a linear regression model. However, living organisms have an optimal range of climate conditions for their survival and reproduction that is not linear (Kindt & Coe, 2005), and different species of Drosophila exhibit different temperature tolerance (Goto & Kimura, 1998; Hoffmann, 2010; Kellermann et al., 2012). In addition, Poppe, Valente, & Schmitz, (2013) showed negative correlation between Drosophila abundance and maximum/minimum temperatures, which further suggests that Drosophila are mostly abundant in a temperature range between the extreme values. The quadratic model appears to be the most appropriate for analyzing the influence of temperature on the abundance of Drosophila species and allowed us to identify optimal condition ranges. For the most abundant species, their optimal monthly average temperature ranged from 18 to 26°C. In addition, ecological data are often over‐dispersed (Kindt & Coe, 2005), and several studies indicate that a negative binomial model, as we used in this study, and the quasi‐Poison model are more appropriate in analyses of such data (O'Hara & Kotze, 2010; Ver Hoef & Boveng, 2008).
Other possible sources of difference between our study and the past test of ecological effects on Drosophila species are the different climate zones in which the studies were carried out. Alabama exhibits subtropical climate, and the temperature range during our study was approximately 25°C. In tropical regions, the temperature range could be smaller and would not produce such significant effects (Dobzhansky & Pavan, 1950; da Mata et al., 2015). In addition, several studies took into account only temperature and precipitation levels measured during collection periods (Poppe, Valente, & Schmitz, 2013; Srinath & Shivanna, 2014; Torres & Madi‐Ravazzi, 2006). In this study, we indicated that changes in average monthly environmental variables can influence abundance of Drosophila in a more significant way than changes during the collection period. In addition, our survey found different species composition than found in other studies, and their inherent species‐specific biology could be influenced by ecological conditions in distinct ways (Hoffmann, 2010; Kellermann et al., 2012).
4.4. Land‐use impacts on abundance and biodiversity
We were able to find significant correlation between land use and abundance of Drosophila species. The highest number of samples per trap came from urban park areas and could be explained by abundance of food sources for generalist Drosophila as a result of human refuse and a relatively high amount of vegetation that could provide a shelter (Ferreira & Tidon, 2005; van Klinken & Walter, 2001). The strongest correlation between land use and individual species abundance was shown by species that tend to use mushrooms as food and breeding substrate: D. putrida and D. tripunctata. In urban areas, biodiversity of fungal communities is lower than in rural areas (Egerton‐Warburton & Allen, 2000; Newbound, Mccarthy, & Lebel, 2010), which potentially can influence abundance of mycophagous Drosophila.
In contrast to abundance measures, we found no significant correlation between Drosophila biodiversity and land use. A species accumulation curve that was made via a random accumulation method suggested that if we take into account only three sites per category (which is the maximum number of sites for nonurban parks), then the most natural environment should have had the highest number of species (Fig. 8). This is consistent with the idea that in a more undisturbed environment the number of rare Drosophila species would be higher (Parsons, 1991). Unfortunately, only the urban park zones were sampled at a sufficient number of sites to saturate species detection according to rarefaction analysis, which plots the number of species as a function of the sample's number and allows evaluation of the sufficiency of sample size (Kindt & Coe, 2005, Fig. S6).
Figure 8.
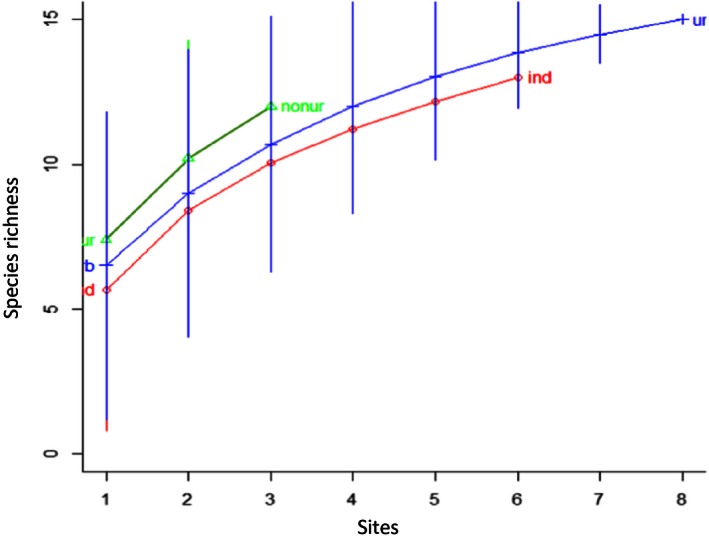
Species accumulation curves. Made with random accumulation method via 10,000 permutations for land‐use categories: Circles represent industrial areas, triangles represent nonurban parks, and crosses represent urban parks
5. Conclusion
Global climate change can cause a shift in the composition and functioning of biological communities (Cardinale et al., 2006; Parsons, 1991; Root et al., 2003), and as exotic species extend their habitat range they often replace endemic organisms (Ferreira & Tidon, 2005; Hooper et al., 2005). In this study, we analyzed biodiversity and species abundance of Drosophila in Central Alabama. Nearly half of the identified species we found were not reported during the last major biodiversity surveys (Sturtevant, 1918, 1921), which suggests a change in Alabama's Drosophila species composition overall the last 100 years. It has been shown that endemic Drosophila species are usually sensitive to climatic variable changes (Parsons, 1991), and we found a significant correlation between the most abundant endemic Drosophila species and seasonal shifts in temperature and precipitation. We also found that biodiversity overall was influenced by seasonal temperature variation. Surprisingly, analyses of invasive pest species (Z. indianus and D. suzukii) did not show any significant correlation between their abundance and seasonal climate variables, suggesting that other factors, such as human influence, drive their abundances.
Urbanization often leads to destruction of natural habitats and significant changes in biodiversity and abundance of endemic and specialist species (Ferreira & Tidon, 2005; Parsons, 1991), and in our study, we found a significant correlation between land‐use type and abundance of Drosophila. In addition, the majority of collected flies were representatives of generalist species. Our results suggest a significant change in Drosophila species composition and the absence of many historically endemic species in the subtropical region of Alabama. To better survey the whole biodiversity of Drosophila in Alabama, more collections should be performed across the state. Given the growing level of urbanization, we expect that cosmopolitan species of Drosophila such as D. simulans, D. suzukii, and especially Z. indianus will become more abundant in Alabama and could establish themselves as dominant species in urban environments.
Funding Information
National Institutes of Health (Grant/Award Number: “National Institutes of Health: 5R01GMO98856”).
Conflict of Interest
None declared.
Supporting information
Acknowledgments
We appreciate the research assistance of S. Bombin, C. Tunckanat, J. Jarnigan, N. Brown, K. MacIntyre, and K. Lowman. Helpful guidance was provided by J. Lopez‐Bautista, J. Lozier, S. Yan, and L. Rissler. Funding sources included University of Alabama Graduate School, Department of Biological Sciences, Graduate Student Association, National Institutes of Health: 5R01GMO98856.
Contributor Information
Andrei Bombin, Email: abombin@crimson.ua.edu.
Laura K. Reed, Email: lreed1@ua.edu
References
- Avondet, J. L. , Blair, R. B. , Berg, D. J. , & Ebbert, M. A. (2003). Drosophila (Diptera: Drosophilidae) response to changes in ecological parameters across an urban gradient. Environmental Entomology, 32, 347–358. doi:10.1603/0046‐225X‐32.2.347 [Google Scholar]
- Berry, J. A. , Anthony, D. , Newfield, M. , Ornsby, M. , & Armstrong, J. (2012). Pest risk assessment: Drosophila suzukii: Spotted wing drosophila (Diptera: Drosophilidae) on fresh fruit from the USA. Wellington: Ministry for Primary Industries, New Zealand Government, 46 p. [Google Scholar]
- Biddinger, D. , Joshi, N. , & Demchak, K. (2012). African fig fly: Another invasive drosophilid fly discovered in PA. Rutgers New Jersey Agricultural Experiment Station Plant & Pest Advisory, 17, 1–2. [Google Scholar]
- Boulétreau, J. (1978). Ovarian activity and reproductive potential in a natural population of Drosophila melanogaster . Oecologia, 35, 319–342. [DOI] [PubMed] [Google Scholar]
- Brake, I. , & Bächli, G. (2008). Drosophilidae (Diptera) In: Nilsson Andres N., Pape Thomas. (Ed.), World catalogue of Insects, Vol. 9 (412pp). Stenstrup, Denmark: Apollo Books. [Google Scholar]
- Burrack, H. J. , Smith, J. P. , Pfeiffer, D. G. , Koeher, G. , & Laforest, J. (2012). Using volunteer‐based networks to track Drosophila suzukii (Diptera: Drosophilidae) an invasive pest of fruit crops. Journal of Integrated Pest Management, 3(4), B1–B5. doi:10.1603/IPM12012 [Google Scholar]
- Cardinale, B. J. , Srivastava, D. S. , Duffy, J. E. , Wright, J. P. , Downing, A. L. , Sankaran, M. , & Jouseau, C. (2006). Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature, 443, 989–992. doi:10.1038/nature05202 [DOI] [PubMed] [Google Scholar]
- Carson, H. L. , & Stalker, H. D. (1951). Natural breeding sites for some wild species of Drosophila in the eastern United States. Ecology, 31, 7–330. [Google Scholar]
- Ceballos, G. , Ehrlich, P. R. , Barnosky, A. D. , García, A. , Pringle, R. M. , & Palmer, T. M. (2015). Accelerated modern human–induced species losses: Entering the sixth mass extinction. Science Advances, 1, e1400253–53. doi:10.1126/sciadv.1400253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commar, L. S. , da Conceição Galego, L. G. , Ceron, C. R. , & Aparecida Carareto, C. M. (2012). Taxonomic and evolutionary analysis of Zaprionus indianus and its colonization of Palearctic and Neotropical regions. Genetics and Molecular Biology, 35, 395–406. doi:10.1590/S1415‐47572012000300003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, E. L. (2004). Drug discovery, CAM and natural products. Evidence‐Based Complementary and Alternative Medicine, 1, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crill, W. D. , Huey, R. B. , & Gilchrist, G. W. (1996). Within‐ and between‐generation effects of temperature on the morphology and physiology of Drosophila melanogaster . Evolution, 50, 1205. doi:10.2307/2410661 [DOI] [PubMed] [Google Scholar]
- Da Lage, J. L. , Kergoat, G. J. , Maczkowiak, F. , Silvain, J. F. , Cariou, M. L. , & Lachaise, D. (2007). A phylogeny of Drosophilidae using the amyrel gene: Questioning the Drosophila melanogaster species group boundaries. Journal of Zoological Systematics and Evolutionary Research, 45(1), 47–63. doi:10.1111/j.1439‐0469.2006.00389.x [Google Scholar]
- Dobzhansky, T. , & Pavan, C. (1950). Local and seasonal variations in relative frequencies of species of Drosophila in Brazil. The Journal of Animal Ecology, 19(1), 1. doi:10.2307/1566 [Google Scholar]
- Egerton‐Warburton, L. M. , & Allen, E. B. (2000). Shifts in arbuscular mycorrhizal communities along an anthropogenic nitrogen deposition gradient. Ecological applications, 10, 484–496. [Google Scholar]
- Ferreira, L. B. , & Tidon, R. (2005). Colonizing potential of Drosophilidae (Insecta, Diptera) in environments with different grades of urbanization. Biodiversity & Conservation, 14, 1809–1821. doi:10.1007/s10531‐004‐0701‐4 [Google Scholar]
- Gamfeldt, L. , Hillebrand, H. , & Jonsson, P. R. (2008). Multiple functions increase the importance of biodiversity for overall ecosystem functioning. Ecology, 89, 1223–1231. doi:10.1890/06‐2091.1 [DOI] [PubMed] [Google Scholar]
- Goto, S. G. , & Kimura, M. T. (1998). Heat‐ and cold‐shock responses and temperature adaptations in subtropical and temperate species of Drosophila. Journal of Insect Physiology, 44, 1233–1239. doi:10.1016/S0022‐1910(98)00101‐2 [DOI] [PubMed] [Google Scholar]
- Guruprasad, B. R. , Hegde, S. N. , & Krishna, M. S. (2010). Seasonal and altitudinal changes in population density of 20 species of Drosophila in Chamundi hill. Journal of Insect Science, 10(1), 123; doi:10.1673/031.010.12301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hector, A. , Schmid, B. , Beierkuhnlein, C. , Caldeira, M. C. , Diemer, M. , Dimitrakopoulos, P. G. , Finn, J. A. , et al. (1999). Plant diversity and productivity experiments in European grasslands. Science, 286, 1123–1127. doi:10.1126/science.286.5442.1123 [DOI] [PubMed] [Google Scholar]
- Hoffmann, A. A. (2010). Physiological climatic limits in Drosophila: Patterns and implications. Journal of Experimental Biology, 213, 870–880. doi:10.1242/jeb.037630 [DOI] [PubMed] [Google Scholar]
- Hooper, D. U. , Chapin, F. S. , Ewel, J. J. , Hector, A. , Inchausti, P. , Lavorel, S. , … Schmid, B . (2005). Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecological monographs, 75(1), 3–35. [Google Scholar]
- Jaenike, J. (1978). Ecological genetics in Drosophila athabasca: Its effect on local abundance. American Naturalist, 112(984), 287–299. [Google Scholar]
- James, A. C. , Azevedo, R. B. , & Partridge, L. (1997). Genetic and environmental responses to temperature of Drosophila melanogaster from a latitudinal cline. Genetics, 146, 881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi, N. K. , Biddinger, D. J. , Demchak, K. , & Deppen, A. (2014). First report of Zaprionus indianus (Diptera: Drosophilidae) in commercial fruits and vegetables in Pennsylvania. Journal of Insect Science, 14(1), 259–59. doi:10.1093/jisesa/ieu121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellermann, V. , Overgaard, J. , Hoffmann, A. A. , Fløjgaard, C. , Svenning, J.‐C. , & Loeschcke, V. (2012). Upper thermal limits of Drosophila are linked to species distributions and strongly constrained phylogenetically. Proceedings of the National Academy of Sciences of the United States of America, 109, 16228–16233. doi:10.1073/pnas.1207553109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt, R. , & Coe, R. (2005). Tree diversity analysis: A manual and software for common statistical methods for ecological and biodiversity studies. World Agroforestry Centre, Nairobi. PMCid: PMC1156951. [Google Scholar]
- van Klinken, R. D. , & Walter, G. H. (2001). Subtropical Drosophilids in Australia can be characterized by adult distribution across vegetation type and by height above forest floor. Journal of Tropical Ecology, 17, 705–718. doi:10.1017/S0266467401001523 [Google Scholar]
- Krebs, C. J. , Carrier, P. , Boutin, S. , Boonstra, R. , & Hofer, E. (2008). Mushroom crops in relation to weather in the southwestern Yukon. Botany‐Botanique, 86, 1497–1502. [Google Scholar]
- Lasa, R. , & Tadeo, E. (2015). Invasive drosophilid pests Drosophila suzukii and Zaprionus indianus (Diptera: Drosophilidae) in Veracruz, Mexico. Florida Entomologist, 98, 987–988. doi:10.1653/024.098.0332 [Google Scholar]
- Levitan, Max. (1954). Drosophilidae in New York and New Jersey. American Midland Naturalist, 52, 453. doi:10.2307/2422247 [Google Scholar]
- van der Linde, K. , & Houle, D. (2008). A supertree analysis and literature review of the genus Drosophila and closely related genera (Diptera, Drosophilidae). Insect Systematics & Evolution, 39, 241–267. doi:10.1163/187631208788784237 [Google Scholar]
- van der Linde, K. , Houle, D. , Spicer, G. S. , & Steppan, S. J. (2010). A supermatrix‐based molecular phylogeny of the family Drosophilidae. Genetics Research, 92(01), 25–38. doi:10.1017/S001667231000008X [DOI] [PubMed] [Google Scholar]
- van der Linde, K. , Steck, G. J. , Hibbard, K. , Birdsley, J. S. , Alonso, L. M. , & Houle, D. (2006). First records of Zaprionus indianus (Diptera: Drosophilidae), a pest species on commercial fruits from Panama and the United States of America. Florida Entomologist, 89, 402–404. doi:10.1653/0015‐4040(2006)89[402:FROZID]2.0.CO;2 [Google Scholar]
- Markow, T. A. , & O'Grady, P. M. (2005). Drosophila: A guide to species identification and use. London: Academic Press. [Google Scholar]
- da Mata, R. A. , Valadão, H. , & Tidon, R. (2015). Spatial and temporal dynamics of drosophilid larval assemblages associated to fruits. Revista Brasileira De Entomologia, 59(1), 50–57. doi:10.1016/j.rbe.2015.02.006 [Google Scholar]
- Miller, D. D. (1958). Geographical distributions of the American Drosophila affinis subgroup species. American Midland Naturalist, 60(1), 52–70. [Google Scholar]
- National Oceanic and Atmospheric Administration's Climate . Retrieved from http://www.noaa.gov/climate.html (accessed 25 October 2015)
- Newbound, M. , Mccarthy, M. A. , & Lebel, T. (2010). Fungi and the urban environment: A review. Landscape and Urban Planning, 96, 138–145. [Google Scholar]
- Newmark, W. D. (2002). Conserving biodiversity in East African forests: A study of the Eastern Arc Mountains, Vol. 155. Berlin: Springer Science & Business Media. [Google Scholar]
- O'Grady, P. , & DeSalle, R. (2008). Out of Hawaii: The origin and biogeography of the genus Scaptomyza (Diptera: Drosophilidae). Biology Letters, 4, 195–199. doi:10.1098/rsbl.2007.0575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara, R. B. , & Kotze, D. J. (2010). Do not log‐transform count data. Methods in Ecology and Evolution, 1, 118–122. doi:10.1111/j.2041‐210X.2010.00021.x [Google Scholar]
- Parsons, P. A. (1991). Biodiversity conservation under global climatic change: The insect Drosophila as a biological indicator? Global Ecology and Biogeography Letters, 1(3), 77. doi:10.2307/2997493 [Google Scholar]
- Patterson, J. T. ed. (1943). Studies in the genetics of Drosophila: The Drosophilidae of the Southwest. III. Austin: University of Texas. [Google Scholar]
- Pimm, S. L. , Russell, G. J. , Gittleman, J. L. , & Brooks, T. M. (1995). The future of biodiversity. Science, 269, 347. [DOI] [PubMed] [Google Scholar]
- Poppe, J. L. , Valente, V. L. S. , & Schmitz, H. J. (2013). Population dynamics of Drosophilids in the Pampa biome in response to temperature. Neotropical Entomology, 42, 269–277. doi:10.1007/s13744‐013‐0125‐5 [DOI] [PubMed] [Google Scholar]
- Reed, L. K. , Nyboer, M. , & Markow, T. A. (2007). Evolutionary relationships of Drosophila mojavensis geographic host races and their sister species Drosophila arizonae . Molecular Ecology, 16, 1007–1022. doi:10.1111/j.1365‐294X.2006.02941.x [DOI] [PubMed] [Google Scholar]
- Remsen, J. , & O'Grady, P. (2002). Phylogeny of Drosophilinae (Diptera: Drosophilidae), with comments on combined analysis and character support. Molecular Phylogenetics and Evolution, 24, 249–264. doi:10.1016/S1055‐7903(02)00226‐9 [DOI] [PubMed] [Google Scholar]
- Renkema, J. M. , Miller, M. , Fraser, H. , Légaré, J. P. , & Hallett, R. H. (2013). First records of Zaprionus indianus Gupta (Diptera: Drosophilidae) from commercial fruit fields in Ontario and Quebec, Canada. Journal of the Entomological Society of Ontario, 144, 125–130. [Google Scholar]
- Robe, L. J. , Valente, V. L. S. , Budnik, M. , & Loreto, É. L. S. (2005). Molecular phylogeny of the subgenus Drosophila (Diptera, Drosophilidae) with an emphasis on neotropical species and groups: A nuclear versus mitochondrial gene approach. Molecular Phylogenetics and Evolution, 36, 623–640. doi:10.1016/j.ympev.2005.05.005 [DOI] [PubMed] [Google Scholar]
- Roff, D. (1980). Optimizing development time in a seasonal environment: The ‘ups and downs’ of clinal variation. Oecologia, 45, 202–208. [DOI] [PubMed] [Google Scholar]
- Root, T. L. , Price, J. T. , Hall, K. R. , Schneider, S. H. , Rosenzweig, C. , & Pounds, J. A. (2003). Fingerprints of global warming on wild animals and plants. Nature, 421, 57–60. doi:10.1038/nature01333 [DOI] [PubMed] [Google Scholar]
- Shorrocks, B. (1975). The distribution and abundance of woodland species of British Drosophila (Diptera: Drosophilidae). The Journal of Animal Ecology, 44, 851. doi:10.2307/3723 [Google Scholar]
- Srinath, B. S. , & Shivanna, N. (2014). Seasonal variation in natural populations of Drosophila in Dharwad, India. Journal of Entomology and Zoology Studies, 2(4), 35–41. [Google Scholar]
- Strickberger, M. W. (1962). Experiments in genetics with Drosophila. New York, NY and London: John Wiley & Sons Inc. [Google Scholar]
- Sturtevant, A. H. (1916). Notes on North American Drosophilidae with descriptions of twenty‐three new species. Annals of the Entomological Society of America, 9, 323–343. doi:10.1093/aesa/9.4.323 [Google Scholar]
- Sturtevant, A. H. (1918). Acalypteræ (Diptera) collected in Mobile County, Alabama. Journal of the New York Entomological Society, 26(1), 34–40. [Google Scholar]
- Sturtevant, A. H. (1921). The North American species of Drosophila (No. 301). Washington, D.C: Carnegie Institution of Washington. [Google Scholar]
- Sturtevant, A. H. , & Dobzhansky, T. (1936). Observations on the species related to new forms of Drosophila affinis, with descriptions of seven. The American Naturalist, 70, 574–584. [Google Scholar]
- Tilman, D. , Wedin, D. , & Knops, J. (1996). Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature, 379, 718–720. [Google Scholar]
- Torres, F. R. , & Madi‐Ravazzi, L. (2006). Seasonal variation in natural populations of Drosophila Spp. (Diptera) in two woodlands in the state of São Paulo, Brazil. Iheringia Série Zoologia, 96, 437–444. doi:10.1590/S0073‐47212006000400008 [Google Scholar]
- USDA/NASS 2014 State Agriculture Overview for Alabama . Retrieved from http://www.nass.usda.gov/Quick_Stats/Ag_Overview/stateOverview.php?state=ALABAMA (accessed 25 October 2015)
- Ver Hoef, J. M. , & Boveng, P. L. (2008). Quasi‐poisson vs. negative binomial regression: How should we model overdispersed count data?. Ecology, 88, 2766–2772. doi:10.1890/07‐0043.1 [DOI] [PubMed] [Google Scholar]
- Vilela, C. R. (1999). Is Zaprionus indianus Gupta, 1970 (Diptera, Drosophilidae) currently colonizing the Neotropical region. Drosophila Information Service, 82, 37–38. [Google Scholar]
- Walsh, P. S. , Metzger, D. A. , & Higuchi, R. (1991). Chelex 100 as a medium for simple extraction of DNA for PCR‐based typing from forensic material. BioTechniques, 10, 506–513. [PubMed] [Google Scholar]
- Wheeler, M. R. (1960). New species of the quinaria group of Drosophila (Diptera, Drosophilidae). The Southwestern Naturalist, 5(3), 160–164. [Google Scholar]
- Worthen, W. B. , & McGuire, T. R. (1990). Predictability of ephemeral mushrooms and implications for mycophagous fly communities. American Midland Naturalist, 124(1), 12–21. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


