Abstract
The proportion of men and women surviving over 65 years has been steadily increasing over the last century. In their later years, many of these individuals are afflicted with multiple chronic conditions, placing increasing pressure on healthcare systems. The accumulation of multiple health problems with advanced age is well documented, yet the causes are poorly understood. Animal models have long been employed in attempts to elucidate these complex mechanisms with limited success. Recently, the domestic dog has been proposed as a promising model of human aging for several reasons. Mean lifespan shows twofold variation across dog breeds. In addition, dogs closely share the environments of their owners, and substantial veterinary resources are dedicated to comprehensive diagnosis of conditions in dogs. However, while dogs are therefore useful for studying multimorbidity, little is known about how aging influences the accumulation of multiple concurrent disease conditions across dog breeds. The current study examines how age, body weight, and breed contribute to variation in multimorbidity in over 2,000 companion dogs visiting private veterinary clinics in England. In common with humans, we find that the number of diagnoses increases significantly with age in dogs. However, we find no significant weight or breed effects on morbidity number. This surprising result reveals that while breeds may vary in their average longevity and causes of death, their age-related trajectories of morbidities differ little, suggesting that age of onset of disease may be the source of variation in lifespan across breeds. Future studies with increased sample sizes and longitudinal monitoring may help us discern more breed-specific patterns in morbidity. Overall, the large increase in multimorbidity seen with age in dogs mirrors that seen in humans and lends even more credence to the value of companion dogs as models for human morbidity and mortality.
Keywords: aging, multimorbidity, breeds, body weight, electronic medical records
Global human populations are aging rapidly, with 17% of the population – a predicted 1.6 billion people – expected to be over the age of 65 by the year 2050 (1). Among these older individuals, a large percentage will be afflicted with multiple morbidities. Incidence of many of these morbidities, including – but not limited to – diabetes mellitus, arthritis, hypertension, osteoporosis, neurodegenerative disease, and various forms of cancer, increases with age. Furthermore, these diseases often present with each other as multiple (two or more) morbidities (1,2). However, it is currently unknown if accumulation of multiple morbidities is a consequence or a cause of biological aging and shortened longevity. The significant increase in morbidity count with age suggests that multimorbidity is a consequence of aging; however, increases in multiple chronic conditions are associated with shorter longevity (3), indicating multimorbidity as a potential cause of biological aging. Close examination of why certain diseases present together and why others do not, as well as the trajectories of disease accumulation throughout age, can offer critical insights into the biology of aging. Furthermore, multiple morbidities are often complex to manage in the clinic and undeniably result in a heavy burden on social and healthcare infrastructures currently in place (4,5). For these reasons, the phenomenon of age-related multimorbidities has long been recognized as a key area for research in the field of aging (6).
Experimental laboratory animal models are commonly used to study singular, age-related diseases. However, studies exploring how different age-related conditions present and interact with one another are difficult to perform in experimental models. As such, the genetic and physiological mechanisms underlying different multimorbidity patterns and trends have yet to be adequately addressed in current animal models. One reason for this is that most of the popular aging models (flies, yeast, and worms) are too evolutionarily distant from humans to model all aspects of individual chronic diseases, let alone multiple ones. Furthermore, although many murine models do often present with multiple morbid conditions (7), aging out large cohorts of genetically diverse mouse populations is costly, and there are no established systems known to us for diagnosing, treating, and preventing chronic conditions in mice. And finally, the environmental factors that influence aging in laboratory animals are likely to be wildly different from the environmental effects that humans experience.
In the current study, we discuss the domestic dog, Canis lupus familiaris, as a potentially powerful model for studying the age-related accumulation of morbid conditions. Dogs offer a host of advantages for studying disease, perhaps the most pronounced of which is their unique breed-based population structure. The domestic dog, which is the most phenotypically diverse mammal species on earth (8), exhibits high phenotypic heterogeneity among breeds, in addition to phenotypic and genetic homogeneity within breeds (9). Moreover, breeds of dogs tend to present with distinct patterns of diseases and causes of death (10). Dogs and humans also share many diseases that present in old age, and the quality of medical care for dogs is second only to that for humans. Furthermore, companion dogs share our environment and its associated disease risk factors in a way that can never be replicated in a laboratory. These facts make the companion dog an exciting model in which to study complex diseases.
Here, we present multimorbidity patterns across an extensive database of electronic veterinary records in dogs with the aims of 1) understanding how multimorbidity trends vary with age, body weight, and breed within a population, and 2) highlighting the translational potential of the dog model in future studies of multimorbidity and aging. We are interested in body weight because of its large influence on lifespan – small breeds tend to live longer than larger breeds (11,12). Thus, we hypothesize that both dog body weight and age will have significant effects on multimorbidity patterns, specifically that the number of morbidities will increase with increasing observed age and body weight of dogs presenting in an extensive database of electronic medical records from veterinary clinics in the UK.
Methods
Data
Multimorbidity data on a large cohort of dogs were acquired from the VetCompass database of the Royal Veterinary College in the United Kingdom (13). Data were included from dogs attending primary practice veterinary clinics in England over the course of 3.5 years, from 2009 to 2013. Some dogs visited their veterinarians several times over the period, whereas others had a single visit. All defining symptoms/diagnoses recorded for each dog during the study period were extracted, and we considered each defining symptom/diagnosis to be a morbidity for that dog. Full details of the methods used for developing the data set used for this study have been previously published (13). We removed descriptors or procedures that were not related to the health of the animal or were determined by one of the authors (KEC) to be too vague (e.g. ‘puppy vet check’, ‘ID chip insertion’, and ‘nail clip’). The total number of diagnoses for each dog was summed and treated as the morbidity score for each dog. Thus, all included diagnoses were weighted equally. While some dogs had more than one included veterinary record across the time period of data collection, only a single age was recorded for each dog.
This age was assigned by subtracting the dog’s birthdate from the last day it visited the veterinary clinic. Dogs under 1 year of age were removed from the analysis due to their constantly changing body weights. In addition, only those dogs with both body weight and age data recorded were included in the analysis. Dogs were separated into small (<10 kg), medium (10–20 kg), or large (20+ kg) body weight classes. Weight class cut-offs were based on a modified version of cut-offs previously assigned by a veterinarian in another study (14). We then chose those breeds with the largest sample sizes in the database (i.e. breeds represented by at least 50 individuals), and grouped them by breed standard into their respective weight classes: small (Jack Russell Terrier, West Highland White Terrier, Shih-Tzu, and Cavalier King Charles Spaniel), medium (Border Collie, Staffordshire Bull Terrier, Cocker Spaniel, and English Springer Spaniel), and large (Labrador Retriever, Golden Retriever, and Rottweiler). Breeds were grouped into weight classes based on American Kennel Club average weights (15). Mixed breeds, which represented the largest sized sample population, were excluded from this analysis, allowing us to determine the effects of genetic homogeneity on multimorbidity number. Ethical approval for the study was granted by the Royal Veterinary College Ethics and Welfare Committee (reference number 2015/1369).
Statistical models
Due to the discrete and over-dispersed (i.e. non-Gaussian) nature of the morbidity data, we first used a generalized linear model (GLM) with a negative-binomial distribution to discover the effects of age, body weight, and their interaction on the morbidity scores, treating all factors as fixed effects. Next, we looked among individual breeds within each different weight classes to see whether there was significant breed variation for age-related changes in multimorbidity within similar-sized dogs. Similar to our weight analysis, we ran a GLM with a negative-binomial distribution for each individual weight class for the effects of breed, age, and their interaction on morbidity score. All statistical analyses were completed in the program R (16). We used a model selection approach to identify the best distribution of the data based on Akaike’s information criterion (AIC) (17). For each candidate model, an AIC was computed using the R package fitdistrplus (18), and the model with the lowest AIC was selected as the best-supported distribution. Regressions were performed using the R package MASS (19).
Results
Distribution of multiple morbidities
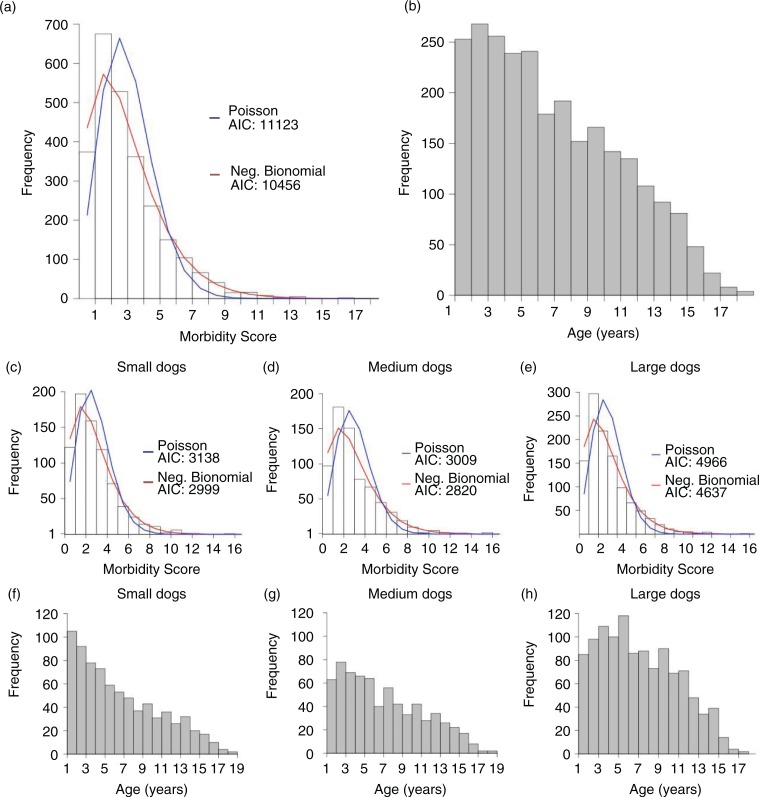
The original database consisted of 3,225 adult dogs (aged over 1 year at their final record), of which 2,586 (80.2%) had both body weight and age data, and were thus included in the analysis. The distribution of morbidity scores across all dogs in the data set more closely follow a negative-binomial distribution rather than a Poisson distribution (AICnegbinom<AICpois; Fig. 1a). Morbidity scores across dogs separated by weight class follow very similar distributions (Fig. 1c–e; Mann–Whitney test results: p>0.05 for all pairwise comparisons), suggesting that there are very few differences between differently sized dogs in terms of the number of morbidities accumulated over the lifetime.
Fig. 1.
Morbidity scores and ages of dogs in the VetCompass data set. Shown are distributions of (a) morbidity scores and (b) age of dog at veterinary visit across all dogs in the data sets; (c–e) morbidity score and (f–h) age distributions of dogs are also visualized by body weight class as labeled. Total number of animals in each weight class is as follows: nsmall=766, nmedium=692, nlarge=1,128, and ntotal=2,586.
The distributions of the ages of the dogs at the time of their visits are shown in Table 1 and Fig. 1b and f–h. In contrast to the size class-specific morbidity score distributions, the shape of age distributions varies noticeably between weight classes. Among small dogs, the greatest number of dogs seen by the veterinarian were 1 year old, whereas in medium and large dogs, that age increases (3 and 5 years old, respectively).
Table 1.
Summary statistics of distributions of ages (in years) of dogs in the VetCompass data
| Mean | Minimum | 1st quartile | Median | 3rd quartile | Max | Count | |
|---|---|---|---|---|---|---|---|
| Small dogs | 6.52 | 1.01 | 2.87 | 5.45 | 9.6 | 18.48 | 766 |
| Medium dogs | 6.95 | 1.01 | 3.36 | 6.11 | 10.16 | 18.74 | 692 |
| Large dogs | 7.07 | 1 | 3.84 | 6.53 | 9.95 | 17.52 | 1,128 |
| All dogs | 6.87 | 1 | 3.39 | 6.08 | 9.92 | 18.74 | 2,586 |
Age, but not body weight or breed, affects multiple morbidities
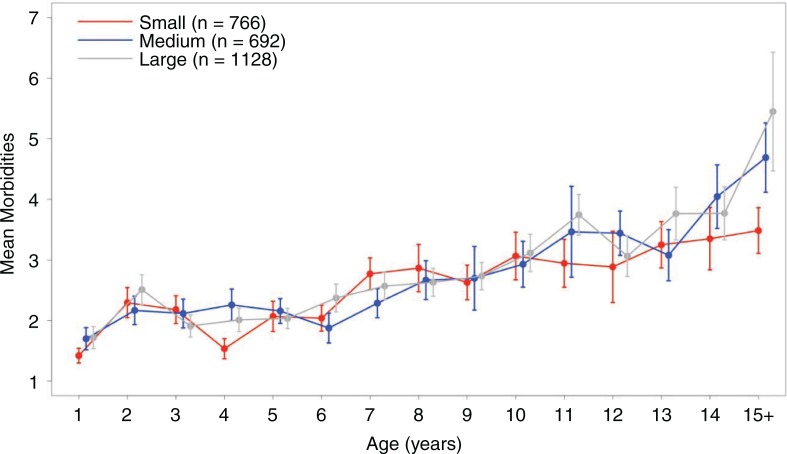
To determine whether age and/or body weight are significantly associated with multimorbidity score, we ran a GLM of morbidity score as a function of age, weight, and their interaction. As expected, there was a highly significant positive effect of age on number of diagnoses (p=2.34E-12, Table 2 and Fig. 2). This trend represents a highly significant effect of age on morbidity score across all dogs. However, failing to support the initial study hypothesis, no significant association was detected between body weight (p=0.777) and age-by-weight interactions (p=0.278) on multimorbidity score.
Table 2.
Results of linear model on age, weight, and age-by-weight interaction
| Variable | Estimate | Std. error | z | Pr(>∣z∣) |
|---|---|---|---|---|
| Intercept | 0.463 | 6.12E-02 | 7.575 | 3.59E-14 |
| Age | 5.15E-02 | 7.35E-03 | 7.013 | 2.34E-12 |
| Weight | 7.87E-04 | 2.78E-03 | 0.283 | 0.777 |
| Age*weight | 3.67E-04 | 3.39E-04 | 1.084 | 0.278 |
Fig. 2.
Age-related changes in morbidity scores for dogs by body weight class. Error bars indicate ±1 standard error. Due to small sample size, all dogs within a weight class with age ≥15 years were grouped together for visualization purposes.
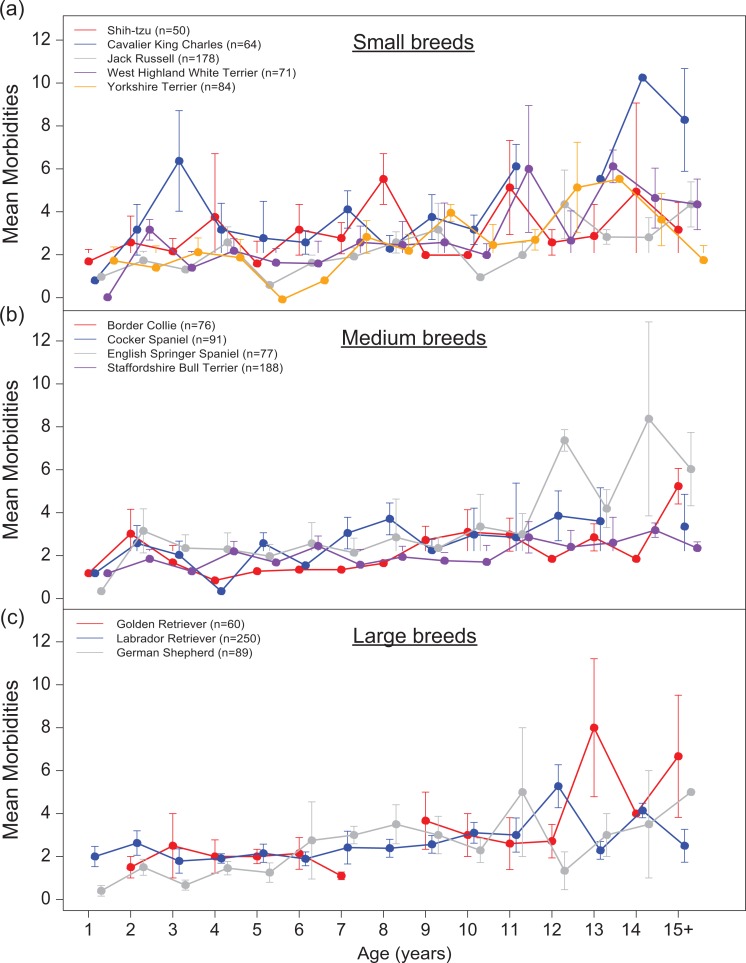
We then broke down the weight classes into individual breeds (Fig. 3). Our breed-based analysis surveyed 12 breeds comprising 1,278 dogs. In medium and large breeds, age was significantly associated with morbidity score (p<7.31E-04, Table 3), consistent with trends observed in the entire data set (Table 2). Specific breeds did not vary with respect to morbidity score in any weight class. Interestingly, no significant effects of breed, age, or their interaction were found on morbidity score in small dogs.
Fig. 3.
Age-related changes in morbidity scores for dogs by breed. Breeds are grouped by (a) small, (b) medium, and (c) large weight class. Error bars indicate ±1 standard error. Due to small sample size, all dogs within a breed with age ≥15 years were grouped together for visualization purposes.
Table 3.
Results of analysis of variance for breed and age within different weight classes
| Variable | Estimate | Std. error | t | p |
|---|---|---|---|---|
| Small breeds | ||||
| Intercept | 0.762 | 0.217 | 3.504 | 4.58E-04 |
| Age | 8.96E-05 | 6.54E-05 | 1.369 | 0.171 |
| Cavalier King Charles Spaniel | 0.182 | 0.297 | 0.613 | 0.540 |
| Jack Russell | −0.447 | 0.257 | −1.738 | 0.082 |
| West Highland White Terrier | −0.510 | 0.319 | −1.599 | 0.110 |
| Yorkshire Terrier | −0.321 | 0.285 | −1.125 | 0.261 |
| Age*Cavalier King Charles Spaniel | 4.80E-05 | 9.64E-05 | 0.498 | 0.618 |
| Age*Jack Russell | 7.41E-05 | 7.65E-05 | 0.969 | 0.333 |
| Age*West Highland White Terrier | 1.45E-04 | 9.33E-05 | 1.563 | 0.118 |
| Age*Yorkshire Terrier | 4.96E-05 | 8.63E-05 | 0.574 | 0.566 |
| Medium breeds | ||||
| Intercept | 0.302 | 0.219 | 1.379 | 0.168 |
| Age | 2.02E-04 | 6.00E-05 | 3.378 | 7.31E-04 |
| Cocker Spaniel | 0.279 | 0.280 | 0.996 | 0.319 |
| English Springer Spaniel | 0.303 | 0.283 | 1.071 | 0.284 |
| Staffordshire Bull Terrier | 0.184 | 0.252 | 0.732 | 0.464 |
| Age*Cocker Spaniel | −4.80E-05 | 8.62E-05 | −0.557 | 0.578 |
| Age*English Springer Spaniel | 4.82E-07 | 8.16E-05 | 0.006 | 0.995 |
| Age*Staffordshire Bull Terrier | −9.93E-05 | 7.59E-05 | −1.307 | 0.191 |
| Large breeds | ||||
| Intercept | 2.53E-02 | 0.280 | 0.090 | 0.928 |
| Age | 3.02E-04 | 8.14E-05 | 3.716 | 2.02E-04 |
| Labrador Retriever | 0.507 | 0.303 | 1.670 | 0.095 |
| German Shepherd | −4.52E-02 | 0.344 | −0.131 | 0.895 |
| Age*Labrador Retriever | −1.65E-04 | 9.00E-05 | −1.841 | 0.066 |
| Age*German Shepherd | −5.32E-06 | 1.06E-04 | −0.050 | 0.960 |
Discussion
We began our investigation by exploring the distribution of morbidity scores across all dogs in the VetCompass data set. We found that all morbidity scores follow a non-Poisson distribution (Fig. 1a and c–e). The Poisson distribution is used to describe events that are randomly scattered over space or time. Departures from Poisson, therefore, are often interpreted as departures from randomness and neutrality (20). Thus, much like how departures from Hardy–Weinberg equilibrium suggest the presence of forces acting upon genetic variation in a population (21), the deviations from Poisson in our data imply that accumulation of multimorbid conditions is not a random process and that other forces may be significantly influencing multimorbidity rates in dogs. At present, we are unable to identify what these external forces may be, but the question is an exciting one, that further underscores the research potential of the canine model for understanding multimorbidity.
We also found that the shapes of the morbidity distributions did not differ substantially between body weight classes of dog. This observation is intriguing, as it is well documented that large dogs have shorter lifespans than small dogs (10). Therefore, one might expect that larger breeds may have a different distribution of disease accumulation than smaller breeds. However, this does not appear to be the case, as all size classes of dogs have similar morbidity score distributions.
Of many potential factors that might influence this unexpected observation, one is the distribution of ages of presenting dogs. Despite similar distributions of morbidity scores, we discovered differences among body weight groups of dogs in the most frequent age of presentation to the veterinary practices (Fig. 1f–h). This interesting observation indicates that there may be external factors influencing the age at which dogs are most likely to present to the clinic, and those factors may vary based on size and/or breed of the animal. We need more data on the timing of disease manifestation between different size classes, and by definition, different breeds of dogs. In addition to the breed-specific differences in the age at onset of different diseases, extrinsic forces may also affect these patterns. For example, recent increases in popularity of certain small dog breeds [e.g. rising ownership of Pugs in England (22)] would result in an observed distribution skewed towards younger small dogs. In a growing population, younger dogs would make up a larger proportion of the sample population. Given the recent growth in this small, relatively long-lived breed, this demographic phenomenon could skew morbidity counts towards lower levels.
We found that across all dogs, multimorbidities increase significantly with age, but not with size (Table 2 and Fig. 2). Overall, this trend holds within breeds of different size classes as well. We know that frequencies of specific diagnoses differ among size classes of dogs (10,13). However, within our data set, the total number of morbidities for each dog is dependent almost exclusively on age with no detectable effect of weight or breed. Different breeds are known to experience different morbidities, as well as to have different causes of death (10,23). In this light, we would have expected to find that certain breeds were more prone to multiple morbidities, but our study failed to identify this association. This suggests that there is little effect of breed on the overall number of multiple morbidities, only the types of morbidities. However, replicate and controlled studies are needed to test this hypothesis more directly.
Additionally, the choice to weight each diagnosis equally may have allowed the morbidity scores to appear the same across breeds, while the actual burden of disease comprising each score differed. Existing morbidity indices for humans often weigh specific measures by their potential effect on overall function (24,25). Future clinical studies on aging in dogs could benefit greatly from developing such weighted measures.
Our breed-based analysis showed similar patterns as seen in the data set overall, with one exception. Small dog breeds failed to show a significant effect of age on morbidity score, suggesting that compared with larger dogs, small dogs may differ physiologically in a way that attenuates the effect of age on multimorbidity. This hypothesis is consistent with the observation that smaller dog breeds tend to live longer than larger breeds, as well as with the idea that smaller dogs may be aging more slowly (11,12).
Overall, our results highlight a novel and valuable application of the companion dog as a model for age-related multimorbidities in humans. We believe the key to its value, in part, lies in the dog’s ability to model non-biological variables in addition to biological ones. Biological variables, including sex, neutering, body weight, and age, are important variables that can contribute to the development of chronic disease, but they are not the only important variables. Non-biological factors include, but are not limited to, environmental, behavioral, social, and economic components and have large and complex effects on both canine and human health. For example, several previous papers employing the VetCompass database have highlighted dramatic financial and social effects from pet insurance on disease diagnosis and survival rates. Compared with non-insured dogs, insured dogs are four times more likely to be diagnosed with hyper-adrenocorticism (26), cranial cruciate disease (27), and mast cell tumor (28). Non-insured diabetic dogs have 1.7 times the hazard of death compared with insured dogs (29). Consequently, we believe that with the increase in available data, and improvements in data science methods, the scientific and medical research community can now incorporate these non-biological factors when investigating complex biological processes such as aging. Given that dogs and humans share many environmental risk factors such as the same air and water pollutants, similar levels of exercise by exercising together, and similar potential economic constraints, such as access to medical care, the potential of the dog both as a sentinel (30) and translational model is high.
Caveats
While this is the largest multimorbidity analysis in the dog to date, our analysis was not without its limitations. First, our sample size of just over 2,500 dogs is still relatively small, especially once divided by breed. To better understand age-related changes in multimorbidities, we need studies with many more dogs at various ages in each breed, and a better understanding of the distribution of the complete at-risk population in terms of age, size, and breed. Furthermore, the relatively small sample size of this data set did not allow us to look at the effects of sex and sterilization status, the latter of which significantly affects disease risk and mortality (14,31).
Second, as described above, we treated every diagnosis the dog had been given with equal weight, other than those removed for vagueness and/or irrelevance as described in the Methods section. As such, some morbidities may only have minor effects on the physiology and health of the dog. Many studies in humans have used similarly unweighted disease counts as measures of multimorbidity (25), though inclusion criteria for specific diseases can vary. Our current unweighted approach may have acted to limit our ability to detect the true differences of accumulated morbidity between small and large dogs. That is to say, while the distributions of multiple morbidities we present are similar in small and large dogs (Fig. 1c and e), conditions in large dogs could be more detrimental to overall health than those in small dogs.
Third, owners may choose to euthanize dogs before they develop their ‘maximum’ morbidity levels. The original study from which these data were derived reported that 88.9% of the recorded deaths were by euthanasia (13). This could have a strong confounding effect on certain breeds; since we know that diverse breeds differ in disease profile, some of the more debilitating and painful diseases that lead to earlier euthanasia might be over-represented among certain breeds (10,32).
Fourth, the nature of this data set does not allow us to know if these diagnoses were chronic within each dog or were only present at one specific time point. Many human studies focus specifically on multiple chronic conditions (2), which we were not able to ascertain from this population. An ideal canine study of chronic multimorbidities would follow dogs throughout life to discover the degree to which different conditions persist throughout the lifespan of the dog.
Finally, other unexamined factors likely affect the observed distribution (which might differ from the true distribution) of morbidities. For example, the probability that an owner brings a dog to clinic might be a function of the animal’s multimorbidity score, leading to ascertainment bias. Higher multimorbid individuals may be more likely to be brought to the clinic; additionally, these animals may be shorter-lived overall, leading to a right truncation of the data due to high-morbidity individuals existing in that state for a shorter time period compared with those individuals with fewer morbidities. Furthermore, individual morbidity scores can move backwards in the real world with the cure of some morbidities due to pharmacological interventions or lifestyle changes, which would not be reflected in our cross-sectional data as such. Hidden variables such as these can only be revealed and accounted for with long-term longitudinal studies – an approach that will be key to future advancement in our understanding of the causes and consequences of aging and age-related disease.
Conclusions
Here, we have presented the largest multimorbidity analysis in the companion dog to date. We find little variation in morbidity scores across dogs of varying body-weight groups despite previously published dramatic variation in causes and ages of death across weight classes and individual breeds (10,33). Furthermore, most of the variation in morbidity score patterns can be explained by age rather than the body size or breed of the dog, suggesting that age is a major, if not the most influential, risk factor for accumulation of disease in dogs, as it is in humans. Although much more investigation is needed to identify mechanisms of multimorbidity, this early study has revealed interesting insights into the architecture of morbidity across dog breeds. This study further shows that the companion dog can be an excellent model for studying disease variation and age-related decline in health.
Acknowledgements
This study was supported in part by NIH R24 AG044284 (DEP) and NIH T32 GM095421 (KJ). The authors are grateful to The Kennel Club, The Kennel Club Charitable Trust, and Dogs Trust for supporting VetCompass in the UK.
Authors’ contributions
KJ, JMH, and DEP designed the study. DGO collected the data. KEC evaluated dog diagnoses, as indicated in the Methods section. KJ and JMH analyzed the data, created the figures, and wrote the manuscript. All authors helped interpret the data and commented on the manuscript.
Conflict of interest and funding
The authors declare no conflicts of interest.
References
- 1.He W, Goodkind D, Kowal P. An aging world: 2015. U.S. Census Bureau. Washington, DC: US Government Publishing Office; 2016. p. P95. [Google Scholar]
- 2.Schneider KM, O’Donnell BE, Dean D. Prevalence of multiple chronic conditions in the United States’ Medicare population. Health Qual Life Outcomes. 2009;7:82. doi: 10.1186/1477-7525-7-82. doi: http://dx.doi.org/10.1186/1477-7525-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DuGoff EH, Canudas-Romo V, Buttorff C, Leff B, Anderson GF. Multiple chronic conditions and life expectancy: a life table analysis. Med Care. 2014;52(8):688–94. doi: 10.1097/MLR.0000000000000166. [DOI] [PubMed] [Google Scholar]
- 4.Bock JO, Konig HH, Brenner H, Haefeli WE, Quinzler R, Matschinger H, et al. Associations of frailty with health care costs – results of the ESTHER cohort study. BMC Health Serv Res. 2016;16:128. doi: 10.1186/s12913-016-1360-3. doi: http://dx.doi.org/10.1186/s12913-016-1360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Picco L, Achilla E, Abdin E, Chong SA, Vaingankar JA, McCrone P, et al. Economic burden of multimorbidity among older adults: impact on healthcare and societal costs. BMC Health Serv Res. 2016;16:173. doi: 10.1186/s12913-016-1421-7. doi: http://dx.doi.org/10.1186/s12913-016-1421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yancik R, Ershler W, Satariano W, Hazzard W, Cohen HJ, Ferrucci L. Report of the National Institute on Aging Task Force on Comorbidity. J Gerontol A Biol Sci Med Sci. 2007;62(3):275–80. doi: 10.1093/gerona/62.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Treuting PM, Linford NJ, Knoblaugh SE, Emond MJ, Morton JF, Martin GM, et al. Reduction of age-associated pathology in old mice by overexpression of catalase in mitochondria. J Gerontol A Biol Sci Med Sci. 2008;63(8):813–22. doi: 10.1093/gerona/63.8.813. [DOI] [PubMed] [Google Scholar]
- 8.Sutter NB, Bustamante CD, Chase K, Gray MM, Zhao K, Zhu L, et al. A single IGF1 allele is a major determinant of small size in dogs. Science. 2007;316(5821):112–15. doi: 10.1126/science.1137045. doi: http://dx.doi.org/10.1126/science.1137045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker HG, Kim LV, Sutter NB, Carlson S, Lorentzen TD, Malek TB, et al. Genetic structure of the purebred domestic dog. Science. 2004;304(5674):1160–4. doi: 10.1126/science.1097406. doi: http://dx.doi.org/10.1126/science.1097406. [DOI] [PubMed] [Google Scholar]
- 10.Fleming JM, Creevy KE, Promislow DE. Mortality in North American dogs from 1984 to 2004: an investigation into age-, size-, and breed-related causes of death. J Vet Intern Med. 2011;25(2):187–98. doi: 10.1111/j.1939-1676.2011.0695.x. doi: http://dx.doi.org/10.1111/j.1939-1676.2011.0695.x. [DOI] [PubMed] [Google Scholar]
- 11.Kraus C, Pavard S, Promislow DE. The size-life span trade-off decomposed: why large dogs die young. Am Nat. 2013;181(4):492–505. doi: 10.1086/669665. doi: http://dx.doi.org/10.1086/669665. [DOI] [PubMed] [Google Scholar]
- 12.Galis F, Van Der Sluijs I, Van Dooren TJM, Metz JAJ, Nussbaumer M. Do large dogs die young? J Exp Zool Part B. 2007;308b(2):119–26. doi: 10.1002/jez.b.21116. doi: http://dx.doi.org/10.1002/jez.b.21116. [DOI] [PubMed] [Google Scholar]
- 13.O’Neill DG, Church DB, McGreevy PD, Thomson PC, Brodbelt DC. Prevalence of disorders recorded in dogs attending primary-care veterinary practices in England. PLoS One. 2014;9(3):e90501. doi: 10.1371/journal.pone.0090501. doi: http://dx.doi.org/10.1371/journal.pone.0090501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffman JM, Creevy KE, Promislow DEL. Reproductive capability is associated with lifespan and cause of death in companion dogs. PLos One. 2013;8(4):e61082. doi: 10.1371/journal.pone.0061082. doi: http://dx.doi.org/10.1371/journal.pone.0061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.AKC Dog Breeds: American Kennel Club. [cited 2016 Oct 19]. Available from: http://www.akc.org/dog-breeds/
- 16.R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 17.Burnham KP, Anderson DR, Burnham KP. Model selection and multimodel inference: a practical information-theoretic approach. 2nd ed. New York: Springer; 2002. p. 488. [Google Scholar]
- 18.Delignette-Muller ML, Dutang C. fitdistrplus: an R package for fitting distributions. J Stat Softw. 2015;64(4):1–34. [Google Scholar]
- 19.Venables WN, Ripley BD. Modern applied statistics with S. 4th ed. New York: Springer; 2002. [Google Scholar]
- 20.Frank SA. The common patterns of nature. J Evol Biol. 2009;22(8):1563–85. doi: 10.1111/j.1420-9101.2009.01775.x. doi: http://dx.doi.org/10.1111/j.1420-9101.2009.01775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez S, Gaunt TR, Day INM. Hardy-Weinberg equilibrium testing of biological ascertainment for Mendelian randomization studies. Am J Epidemiol. 2009;169(4):505–14. doi: 10.1093/aje/kwn359. doi: http://dx.doi.org/10.1093/aje/kwn359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Neill DG, Darwent EC, Church DB, Brodbelt DC. Demography and health of Pugs under primary veterinary care in England. Canine Genet Epidemiol. 2016;3:5. doi: 10.1186/s40575-016-0035-z. doi: http://dx.doi.org/10.1186/s40575-016-0035-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayward JJ, Castelhano MG, Oliveira KC, Corey E, Balkman C, Baxter TL, et al. Complex disease and phenotype mapping in the domestic dog. Nat Commun. 2016;7:10460. doi: 10.1038/ncomms10460. doi: http://dx.doi.org/10.1038/ncomms10460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, Mackenzie CR. A new method of classifying prognostic co-morbidity in longitudinal-studies – development and validation. J Chron Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. doi: http://dx.doi.org/10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 25.Huntley AL, Johnson R, Purdy S, Valderas JM, Salisbury C. Measures of multimorbidity and morbidity burden for use in primary care and community settings: a systematic review and guide. Ann Fam Med. 2012;10(2):134–41. doi: 10.1370/afm.1363. doi: http://dx.doi.org/10.1370/afm.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Neill DG, Scudder C, Faire JM, Church DB, McGreevy PD, Thomson PC, et al. Epidemiology of hyperadrenocorticism among 210,824 dogs attending primary-care veterinary practices in the UK from 2009 to 2014. J Small Anim Pract. 2016;57(7):365–73. doi: 10.1111/jsap.12523. doi: http://dx.doi.org/10.1111/jsap.12523. [DOI] [PubMed] [Google Scholar]
- 27.Taylor-Brown FE, Meeson RL, Brodbelt DC, Church DB, McGreevy PD, Thomson PC, et al. Epidemiology of cranial cruciate ligament disease diagnosis in dogs attending primary-care veterinary practices in England. Vet Surg. 2015;44(6):777–83. doi: 10.1111/vsu.12349. doi: http://dx.doi.org/10.1111/vsu.12349. [DOI] [PubMed] [Google Scholar]
- 28.Mattin M, O’Neill D, Church D, McGreevy PD, Thomson PC, Brodbelt D. An epidemiological study of diabetes mellitus in dogs attending first opinion practice in the UK. Vet Rec. 2014;174(14):349. doi: 10.1136/vr.101950. doi: http://dx.doi.org/10.1136/vr.101950. [DOI] [PubMed] [Google Scholar]
- 29.Shoop SJ, Marlow S, Church DB, English K, McGreevy PD, Stell AJ, et al. Prevalence and risk factors for mast cell tumours in dogs in England. Canine Genet Epidemiol. 2015;2:1. doi: 10.1186/2052-6687-2-1. doi: http://dx.doi.org/10.1186/2052-6687-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scotch M, Odofin L, Rabinowitz P. Linkages between animal and human health sentinel data. BMC Vet Res. 2009;5:15. doi: 10.1186/1746-6148-5-15. doi: http://dx.doi.org/10.1186/1746-6148-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hart BL, Hart LA, Thigpen AP, Willits NH. Long-term health effects of neutering dogs: comparison of Labrador Retrievers with Golden Retrievers. PLoS One. 2014;9(7):e102241. doi: 10.1371/journal.pone.0102241. doi: http://dx.doi.org/10.1371/journal.pone.0102241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Neill DG, Church DB, McGreevy PD, Thomson PC, Brodbelt DC. Longevity and mortality of owned dogs in England. Vet J. 2013;198(3):638–43. doi: 10.1016/j.tvjl.2013.09.020. doi: http://dx.doi.org/10.1016/j.tvjl.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 33.Chase K, Jones P, Martin A, Ostrander EA, Lark KG. Genetic mapping of fixed phenotypes: disease frequency as a breed characteristic. J Hered. 2009;100(Suppl 1):S37–41. doi: 10.1093/jhered/esp011. doi: http://dx.doi.org/10.1093/jhered/esp011. [DOI] [PMC free article] [PubMed] [Google Scholar]