Summary
During 2013, 53 reporting jurisdictions reported 5,865 rabid animals and 3 human rabies cases to the CDC, representing a 4.8% decrease from the 6,162 rabid animals and 1 human case reported in 2012. Ninety-two percent of reported rabid animals were wildlife. Relative contributions by the major animal groups were as follows: 1,898 raccoons (32.4%), 1,598 bats (27.2%), 1,447 skunks (24.7%), 344 foxes (5.9%), 247 cats (4.2%), 86 cattle (1.5%), and 89 dogs (1.5%). One human case was reported from Maryland. The infection was determined to have been transmitted via organ transplantation. Infection in the organ donor, a North Carolina resident, was retrospectively diagnosed. Both the organ donor and the organ recipient were infected with the raccoon rabies virus variant. The third human case, reported by Texas, involved a Guatemalan resident who was detained while crossing the US border. The infection was determined to be caused by a canine rabies virus variant that circulates in Central America.
Cases of animal and human rabies are reported annually within the United States, and rabies has been a nationally notifiable condition since 1944.1 Since 1960, most animal rabies cases have involved wildlife. The number of human rabies cases has steadily declined because of elimination of the canine rabies virus variant associated with domestic dogs, timely application of modern rabies biologics following suspected rabies exposure, and successful educational outreach campaigns.2,3
Rabies is a disease caused by RNA viruses in the genus Lyssavirus (family Rhabdoviridae).4 Currently, 14 species of lyssaviruses have been identified.5 However, only 1 species, Rabies virus, has been detected in the western hemisphere. All mammals are susceptible to rabies virus infection, which can occur via bites from infected animals or contamination of fresh wounds or mucous membranes with infectious material (ie, saliva or nervous tissue). Since the elimination of the canine rabies virus variant from the United States during the late 1970s, most reported rabid animals have been wildlife.6 These cases occur predominantly in reservoir species (ie, bats [order Chiroptera], foxes [Urocyon or Vulpes spp], mongooses [Herpestes javanicus], raccoons [Procyon lotor], and skunks [family Mephitidae). While spillover of rabies virus variants may occur, such reports are less frequent, and spillover infections are rarely associated with sustained transmission among nonreservoir species.2,7 Terrestrial rabies virus variants circulate in distinct geographic regions, whereas bat-associated rabies virus variants cover broad geographic regions across the range of their associated bat species.6 Molecular epidemiology suggests that there are 2 distinct lineages of circulating variants associated with canids and bats. Of the current rabies virus variants in circulation among terrestrial mammals, 6 are canine lineages (Arctic fox rabies virus variant, Arizona gray fox rabies virus variant, Texas gray fox rabies virus variant, California skunk rabies virus variant, north central skunk rabies virus variant, and mongoose rabies virus variant), and 2 are bat lineages (raccoon rabies virus variant and south central skunk rabies virus variant).2
Despite its high fatality rate once clinical signs develop, rabies is entirely preventable if postexposure prophylaxis is administered in a timely manner after a suspected rabies exposure.3 For human patients who have never received rabies vaccination, the postexposure prophylaxis series consists of immediate wound washing, infiltration of the wound with human rabies immune globulin, and administration of 4 doses of cell culture vaccine IM in the deltoid muscle on days 0, 3, 7, and 14.3,8 The postexposure prophylaxis series for patients who were previously immunized consists of 2 booster doses of rabies vaccine on days 0 and 3.3
Pre-exposure prophylaxis is recommended for individuals at higher risk of rabies exposure because of occupational hazards or recreational activity.3,9 In addition, if a person is traveling to areas in which rabies is endemic and medical care may be difficult to obtain, he or she may have pre-exposure prophylaxis administered to avoid the need for costly medical evacuation.9 Pre-exposure prophylaxis consists of administration of 3 doses of cell culture vaccine IM in the deltoid muscle on days 0, 7, and 21 or 28. Pre-exposure prophylaxis does not eliminate the need for medical care following a potential rabies exposure, but simplifies the protocol and eliminates the need for administration of human rabies immune globulin.10
This report presents an overview of rabies epidemiology and events that occurred during 2013. Summaries of rabies surveillance activities during 2013 are also provided for Canada and Mexico.
Reporting and Analysis
Rabies is primarily diagnosed in animals through application of the direct fluorescent antibody test, which requires a full cross section of the brainstem and cerebellum and thus euthanasia of the animal.11 Routine animal rabies diagnostic testing is currently performed by nearly 130 state health, agriculture, and university laboratories in the United States. In addition, the direct rapid immunohistochemistry test, which also requires brain tissue, is used to conduct targeted enhanced surveillance by the USDA Wildlife Services as part of large-scale wildlife oral rabies vaccination programs.12–14 During 2013, most reporting jurisdictions provided animal rabies diagnostic data directly to the CDC Poxvirus and Rabies Branch. However, 9 states (Arkansas, Idaho, Massachusetts, Maryland, Minnesota, New Jersey, South Dakota, Virginia, and West Virginia) used the Public Health Laboratory Information System to transmit electronic laboratory data for rabies diagnostic activity.
Annual animal rabies surveillance data, consisting of detailed information on animals submitted for rabies testing, is requested from state, city, and territorial health departments as previously described.15 Reporting jurisdictions provided denominator data on species, county, and date of testing or specimen collection for all animals tested, with the exception of California. During 2013, only data for cases with positive rabies test results were available from California at the time of reporting. Additional data requested from reporting jurisdictions included the rabies vaccination status of domestic animals, exposure history, and rabies virus variant typing results for rabid animals. Percentages of rabid animals were calculated as previously described.15 California data were removed from 2013 and preceding years in this report when ratios of rabid to submitted animals were compared between years. A total of 96,589 samples were submitted for laboratory diagnosis, of which 94,359 were considered suitable for testing (Figure 1). This represented a 5.0% decrease from the 101,699 animals found suitable for testing during 2012 (excluding California). The direct rapid immunohistochemistry test was the primary rabies diagnostic test used for 5,375 animals found suitable for testing by USDA Wildlife Services as part of active surveillance efforts. This accounted for 5.7% of all animals tested in 2013. Most counties in the United States submitted between 2 and 25 animals for rabies diagnostic testing during 2013. Animals submitted for rabies diagnostic testing were predominantly selected on the basis of abnormal behavior or visible illness or because they were involved in potential exposure incidents involving humans or domestic animals. Because animals submitted for rabies diagnostic testing were selected on the basis of these criteria and did not represent a random sample of all animals, percentages reported are not likely to be representative of the incidence of rabies within animal populations. The number of animals submitted for rabies diagnostic testing varied with interaction rates between humans and animals, local disease dynamics, and land use or laboratory submission policy changes.
Figure 1.
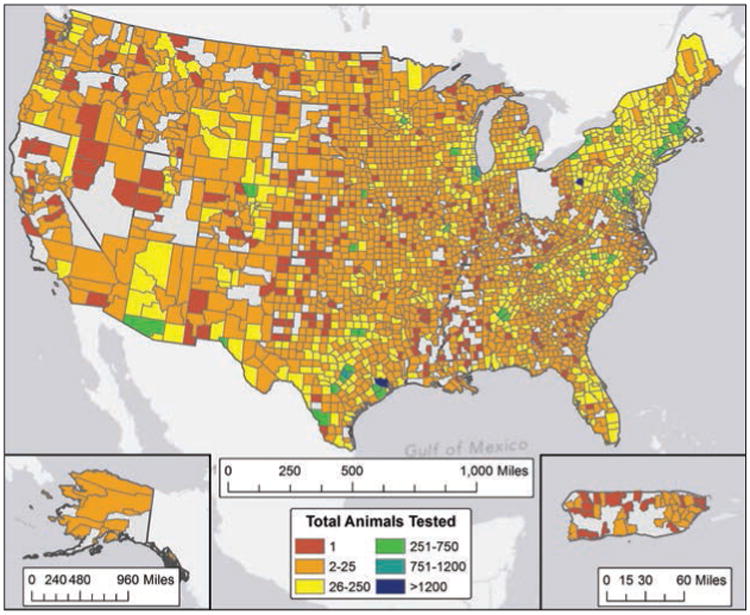
Animals submitted for rabies diagnostic testing, by county, 2013.
Submission rates were calculated on the basis of 2010 population data available from the US Census Bureau.16 Reported rabid animals were grouped by US Census regions to highlight geographic variations in animal submissions, rabies burden, and distribution of terrestrial rabies virus variants (Table 1). Geographic ranges of terrestrial reservoirs in the United States were developed by aggregating surveillance data from 2009 through 2013, and all maps were produced as previously described.15,17
Table 1.
Cases of rabies in the United States, by location, during 2013.
| Domestic animals | Wildlife | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||||||||
| Location | Reservoir | Total cases | Domestic animals | Wildlife | Cats | Cattle | Dogs | Horses and mules | Sheep and goats | Other domestic* | Raccoons | Bats | Skunks | Foxes | Other wild† | Rodents and lagomorphs‡ | Humans | % Pos 2013 | 2012 cases | Change(%) |
| Northeast | 1,483 | 94 | 1,389 | 75 | 14 | 2 | 2 | 0 | 1 | 703 | 290 | 257 | 100 | 15 | 24 | 0 | 6.34 | 1,678 | −11.62 | |
| CT | Raccoon | 150 | 5 | 145 | 3 | 2 | 0 | 0 | 0 | 0 | 84 | 22 | 30 | 7 | 0 | 2u | 0 | 7.72 | 173 | −13.29 |
| MA | Raccoon | 100 | 3 | 97 | 2 | 0 | 0 | 0 | 0 | 1a | 37 | 18 | 32 | 5 | 2e | 3v | 0 | 3.81 | 113 | −11.50 |
| ME | Raccoon | 54 | 0 | 54 | 0 | 0 | 0 | 0 | 0 | 0 | 23 | 7 | 20 | 4 | 0 | 0 | 0 | 9.15 | 88 | −38.64 |
| NH | Raccoon | 34 | 2 | 32 | 2 | 0 | 0 | 0 | 0 | 0 | 13 | 3 | 8 | 5 | 2f | 1w | 0 | 6.75 | 29 | 17.24 |
| NJ | Raccoon | 315 | 18 | 297 | 17 | 0 | 0 | 1 | 0 | 0 | 157 | 66 | 51 | 13 | 1g | 9x | 0 | 10.09 | 309 | 1.94 |
| NY | Raccoon | 335 | 15 | 320 | 9 | 6 | 0 | 0 | 0 | 0 | 147 | 82 | 50 | 31 | 7h | 3y | 0 | 6.20 | 425 | −21.18 |
| NYC | Raccoon | 56 | 1 | 55 | 1 | 0 | 0 | 0 | 0 | 0 | 46 | 4 | 4 | 0 | 1i | 0 | 0 | 11.67 | 13 | 330.77 |
| PA | Raccoon | 361 | 45 | 316 | 40 | 4 | 1 | 0 | 0 | 0 | 167 | 76 | 36 | 31 | 1j | 5z | 0 | 5.02 | 433 | −16.63 |
| RI | Raccoon | 28 | 0 | 28 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 9 | 6 | 1 | 0 | 1aa | 0 | 5.12 | 28 | 0.00 |
| VT | Raccoon | 50 | 5 | 45 | 1 | 2 | 1 | 1 | 0 | 0 | 18 | 3 | 20 | 3 | 1k | 0 | 0 | 8.70 | 67 | −25.37 |
| Midwest | 470 | 68 | 402 | 17 | 29 | 9 | 8 | 1 | 4 | 5 | 262 | 134 | 0 | 1 | 0 | 0 | 2.07 | 596 | −21.14 | |
| IA | Skunk | 12 | 2 | 10 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 6 | 4 | 0 | 0 | 0 | 0 | 0.88 | 31 | −61.29 |
| IL | None | 54 | 0 | 54 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 54 | 0 | 0 | 0 | 0 | 0 | 1.34 | 63 | −14.29 |
| IN | Skunk | 10 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 1.07 | 20 | −50.00 |
| KS | Skunk | 60 | 14 | 46 | 2 | 4 | 4 | 3 | 0 | 1b | 1 | 6 | 38 | 0 | 1l | 0 | 0 | 5.47 | 56 | 7.14 |
| MI | Skunk | 40 | 0 | 40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 40 | 0 | 0 | 0 | 0 | 0 | 1.31 | 61 | −34.43 |
| MN | Skunk | 63 | 7 | 56 | 1 | 3 | 0 | 2 | 1 | 0 | 0 | 36 | 20 | 0 | 0 | 0 | 0 | 2.86 | 72 | −12.50 |
| MO | Skunk | 40 | 6 | 34 | 2 | 0 | 3 | 1 | 0 | 0 | 0 | 13 | 21 | 0 | 0 | 0 | 0 | 2.21 | 28 | 42.86 |
| ND | Skunk | 40 | 16 | 24 | 5 | 8 | 0 | 1 | 0 | 2c | 0 | 3 | 21 | 0 | 0 | 0 | 0 | 6.70 | 75 | −46.67 |
| NE | Skunk | 33 | 13 | 20 | 3 | 7 | 1 | 1 | 0 | 1d | 0 | 6 | 14 | 0 | 0 | 0 | 0 | 3.05 | 59 | −44.07 |
| OH | None | 60 | 3 | 57 | 3 | 0 | 0 | 0 | 0 | 0 | 4 | 53 | 0 | 0 | 0 | 0 | 0 | 1.59 | 41 | 46.34 |
| SD | Skunk | 28 | 7 | 21 | 1 | 5 | 1 | 0 | 0 | 0 | 0 | 5 | 16 | 0 | 0 | 0 | 0 | 4.38 | 60 | −53.33 |
| WI | Skunk | 30 | 0 | 30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 30 | 0 | 0 | 0 | 0 | 0 | 1.43 | 30 | 0.00 |
| South | 3,265 | 269 | 2,993 | 147 | 41 | 57 | 16 | 8 | 0 | 1,187 | 651 | 905 | 225 | 9 | 16 | 3 | 7.69 | 3,135 | 4.15 | |
| AL | Raccoon | 60 | 4 | 56 | 2 | 0 | 2 | 0 | 0 | 0 | 46 | 7 | 0 | 3 | 0 | 0 | 0 | 2.28 | 54 | 11.11 |
| AR | Skunk | 151 | 8 | 143 | 3 | 1 | 3 | 1 | 0 | 0 | 0 | 26 | 117 | 0 | 0 | 0 | 0 | 11.72 | 131 | 15.27 |
| DC | Raccoon | 57 | 4 | 53 | 3 | 0 | 1 | 0 | 0 | 0 | 36 | 14 | 0 | 2 | 0 | 1ab | 0 | 14.81 | 60 | −5.00 |
| DE | Raccoon | 17 | 1 | 16 | 1 | 0 | 0 | 0 | 0 | 0 | 8 | 3 | 0 | 4 | 0 | 1ac | 0 | 12.32 | 15 | 13.33 |
| FL | Raccoon | 108 | 8 | 100 | 8 | 0 | 0 | 0 | 0 | 0 | 75 | 19 | 2 | 2 | 2m | 0 | 0 | 4.80 | 109 | −0.92 |
| GA | Raccoon | 297 | 22 | 275 | 9 | 1 | 11 | 1 | 0 | 0 | 176 | 18 | 53 | 23 | 5n | 0 | 0 | 12.33 | 373 | −20.38 |
| KY | Skunk | 16 | 3 | 13 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 7 | 6 | 0 | 0 | 0 | 0 | 2.46 | 14 | 14.29 |
| LA | Skunk | 8 | 1 | 7 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 3 | 4 | 0 | 0 | 0 | 0 | 1.43 | 4 | 100.00 |
| MD | Raccoon | 382 | 29 | 352 | 25 | 0 | 1 | 1 | 2 | 0 | 238 | 51 | 19 | 33 | 1o | 10ad | 1 | 9.50 | 325 | 17.54 |
| MS | None | 5 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 1.84 | 2 | 150.00 |
| NC | Raccoon | 385 | 31 | 353 | 20 | 4 | 5 | 0 | 2 | 0 | 207 | 28 | 56 | 62 | 0 | 0 | 1 | 8.90 | 434 | −11.29 |
| OK | Skunk | 85 | 22 | 63 | 2 | 12 | 7 | 1 | 0 | 0 | 0 | 3 | 59 | 1 | 0 | 0 | 0 | 7.34 | 81 | 4.94 |
| SC | Raccoon | 124 | 8 | 116 | 6 | 0 | 2 | 0 | 0 | 0 | 68 | 5 | 17 | 26 | 0 | 0 | 0 | 7.74 | 137 | −9.49 |
| TN | Skunk | 36 | 9 | 27 | 0 | 0 | 5 | 4 | 0 | 0 | 1 | 8 | 17 | 1 | 0 | 0 | 0 | 1.70 | 48 | −25.00 |
| TX | Skunk | 937 | 60 | 876 | 23 | 13 | 16 | 6 | 2 | 0 | 27 | 437 | 402 | 10 | 0 | 0 | 1 | 7.03 | 683 | 37.19 |
| VA | Raccoon | 506 | 49 | 457 | 37 | 9 | 1 | 0 | 2 | 0 | 247 | 16 | 135 | 55 | 0 | 4ae | 0 | 11.73 | 597 | −15.24 |
| WV | Raccoon | 91 | 10 | 81 | 8 | 1 | 0 | 1 | 0 | 0 | 58 | 1 | 18 | 3 | 1p | 0 | 0 | 9.11 | 68 | 33.82 |
| West | 596 | 20 | 576 | 8 | 2 | 7 | 3 | 0 | 0 | 3 | 395 | 151 | 19 | 8 | 0 | 0 | 6.8§ | 681 | −12.48 | |
| AK | Artic Fox | 9 | 3 | 6 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 2q | 0 | 0 | 14.06 | 6 | 50.00 |
| AZ | Skunk | 77 | 0 | 77 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 46 | 22 | 4 | 5r | 0 | 0 | 10.52 | 57 | 35.09 |
| CA | Skunk | 198 | 2 | 196 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 188 | 7 | 1 | 0 | 0 | 0 | NA§ | 252 | −21.43 |
| CO | Skunk | 187 | 9 | 178 | 5 | 1 | 0 | 3 | 0 | 0 | 3 | 66 | 102 | 7 | 0 | 0 | 0 | 9.81 | 183 | 2.19 |
| HI | None | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0 | 0.00 |
| ID | None | 26 | 0 | 26 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 26 | 0 | 0 | 0 | 0 | 0 | 6.21 | 23 | 13.04 |
| MT | Skunk | 36 | 3 | 33 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 20 | 13 | 0 | 0 | 0 | 0 | 8.29 | 25 | 44.00 |
| NM | Skunk | 11 | 1 | 10 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 6 | 3 | 1 | 0 | 0 | 0 | 2.46 | 48 | −77.08 |
| NV | None | 9 | 1 | 8 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 4.71 | 20 | −55.00 |
| OR | None | 10 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 2 | 1s | 0 | 0 | 2.50 | 17 | −41.18 |
| UT | None | 12 | 0 | 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 0 | 0 | 0 | 0 | 0 | 3.55 | 15 | −20.00 |
| WA | None | 12 | 0 | 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 0 | 0 | 0 | 0 | 0 | 2.20 | 9 | 33.33 |
| WY | Skunk | 9 | 1 | 8 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 4 | 0 | 0 | 0 | 0 | 1.83 | 26 | −65.38 |
| Caribbean | 54 | 16 | 38 | 0 | 0 | 14 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 38 | 0 | 0 | 46.20 | 73 | −26.03 | |
| PR | Mongoose | 54 | 16 | 38 | 0 | 0 | 14 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 38t | 0 | 0 | 46.20 | 73 | −26.03 |
| Total: | 5,868 | 467 | 5,398 | 247 | 86 | 89 | 31 | 9 | 5 | 1,898 | 1,598 | 1,447 | 344 | 71 | 40 | 3 | − | 6,163 | −4.79 | |
| % 2013 | 100.00 | 7.96 | 91.99 | 4.21 | 1.47 | 1.52 | 0.53 | 0.15 | 0.09 | 32.34 | 27.23 | 24.66 | 5.86 | 1.21 | 0.68 | 0.05 | ||||
| %Pos2013§ | 6.22 | 0.98 | 11.53 | 1.06 | 6.62 | 0.41 | 3.58 | 1.64 | 2.05 | 16.25 | 5.84 | 33.01 | 18.70 | 2.95 | 1.82 | 4.76 | ||||
| Total 2012 | 6,163 | 519 | 5,643 | 257 | 115 | 84 | 47 | 13 | 3 | 1,953 | 1,680 | 1,539 | 340 | 85 | 46 | 1 | ||||
| % Change | −4.79 | −10.02 | −4.34 | −3.89 | −25.22 | 5.95 | −34.04 | −30.77 | 66.67 | −2.82 | −4.88 | −5.98 | 1.18 | −16.47 | −13.04 | 200.00 | ||||
Other domestic includes
1 swine;
1 llama;
2 swine;
1 llama.
Other wild includes
2 bobcats;
2 bobcats;
1 otter;
2 bobcats, 2 deer, 1 fisher, 2 otters;
1 opossum;
1 deer;
1 bobcat;
1 coyote;
2 bobcats;
2 bobcats, 2 coyotes, 1 deer;
1 opossum;
1 bobcat;
2 wolves;
4 bobcats, 1 coyote;
1 coyote;
38 mongooses.
Rodents and lagomorphs include
1 groundhog, 1 rabbit;
3 groundhogs;
1 groundhog;
9 groundhogs;
3 groundhogs;
5 groundhogs;
1 groundhog;
1 groundhog;
1 groundhog;
10 groundhogs;
2 groundhogs, 2 marmots.
Data for animals that tested negative for rabies were unavailable and have been excluded from 2013 and 2012 data for comparison purposes.
— = Not applicable. NYC = New York City. Pos = Positive.
Total cases refers to total number of cases in domestic animals, wildlife, and humans. Reservoir refers to the major rabies virus variant terrestrial reservoir in the locality.
Variant typing was primarily performed on samples submitted for rabies diagnostic testing from areas where epizootics have occurred, that involved unusual species, or that were part of the epidemiological surveillance of the distribution of distinct rabies virus variants. If variant typing data were unavailable for rabid terrestrial animals, it was assumed that the animal was infected with the local terrestrial rabies Variant.10,18 Two methods were used to perform variant typing: the indirect fluorescent antibody test and sequencing of reverse transcription PCR amplicons. The indirect fluorescent antibody test uses a panel of monoclonal antibodies against the rabies virus nucleoprotein for antigenic variant typing. Indirect fluorescent antibody test results may distinguish between carnivore and bat rabies virus variants, but the test is less sensitive at distinguishing specific bat rabies virus variants from other bat rabies virus variants.19 Alternatively, sequencing of reverse transcription PCR amplicons can provide a more robust analysis of the phylogenetic relationships of rabies virus variants.2
The summary rabies update for Canada during 2013 was provided by the Center of Expertise for Rabies, Ottawa Laboratory, Fallowfield, and the Animal Health, Welfare and Biosecurity Division, Canadian Food Inspection Agency. Summary canine rabies data for Mexico during 2013 were provided by the Instituto de Salud del Estado de México.
Rabies in Wild Animals
Most of the rabid animals reported during 2013 consisted of wildlife (5,398/5,865 [92%]; Table 1). This represented a 4.34% decrease, compared with the number of rabid wild animals reported during 2012 (n = 5,643). Examination of data for the number of cases of rabies among various wildlife species from 1984 to 2013 showed a decrease in the number of rabid raccoons since 1993 and relatively little change in the number of rabid foxes over this period (Figure 2). Seasonal trends in numbers of reported rabies cases in wildlife were similar to those in previous years, with peaks in the numbers of reported rabid skunks and raccoons between March and April.
Figure 2.
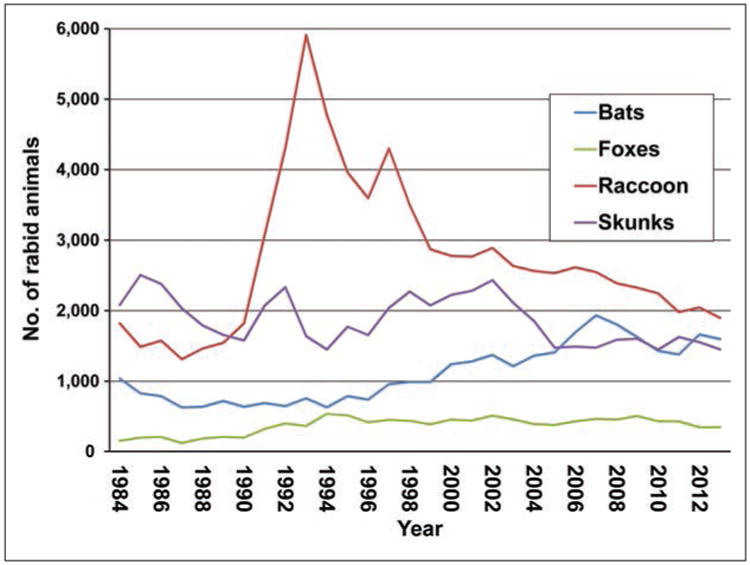
Cases of rabies among wildlife in the United States, by year and species, 1984 to 2013.
The most frequently reported rabid wildlife were raccoons (1,898 [32.34% of all cases of rabies during 2013]), bats (1,598 [27.23%]), skunks (1,447 [24.66%]), and foxes (344 [5.86%]). However, the most frequently submitted wildlife for rabies diagnostic testing were bats (n = 24,152), followed by raccoons (11,680). Although raccoons were the most commonly reported rabid wildlife species during 2013 (Figure 3), the 1,898 reported rabid raccoons represented a 2.82% decrease, compared with the 1,953 rabid raccoons reported during 2012. In addition, the 1,898 rabid raccoons reported during 2013 represented a significant decrease from the mean annual number reported in 2008 through 2012 (2,181.0; 95% CI, 2,007.5 to 2,354.5; Table 2). However, a significant increase in the prevalence of rabies among raccoons submitted for diagnostic testing was reported.
Figure 3.
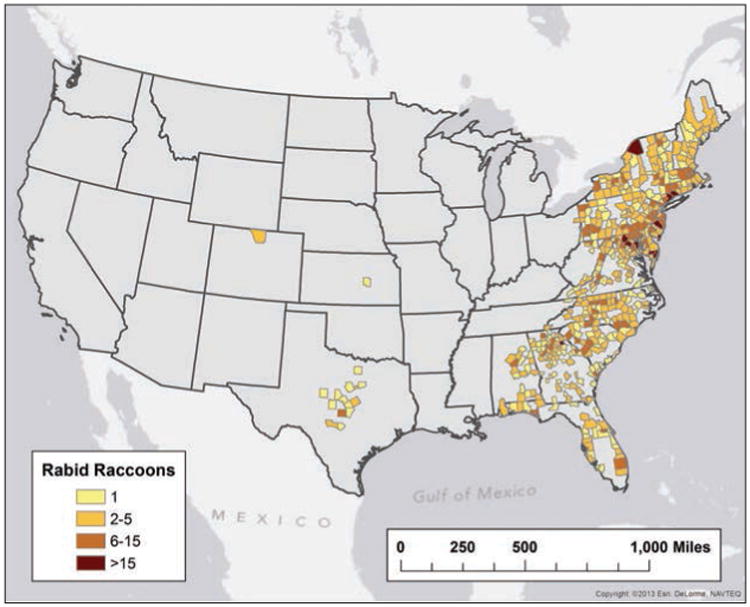
Reported cases of rabies involving raccoons, by county, 2013.
Table 2.
Numbers of animals reported to be rabid and percentages of samples tested for rabies that yielded positive results, 2008 through 2013.
| Animals | 2013 | 2008–2012 | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| No. of rabid animals | Percentage of samples with positive results* | No. of rabid animals | Percentage of samples with positive results* | |||
|
|
|
|||||
| Mean | 95% CI* | Mean | 95% CI* | |||
| Domestic animals | ||||||
| Cats | 247† | 1.06 | 291.4 | (274.2–308.5) | 1.2 | (1.1–1.3) |
| Cattle | 86 | 6.62 | 76.8 | (57.4–96.2) | 6.4 | (5.3–7.5) |
| Dogs | 89† | 75.8 | (70.0–81.6) | 0.3 | (0.3–0.4) | |
| Horses and mules | 31† | 3.81 | 39.8 | (34.0–45.6) | 4.5 | (3.8–5.2) |
| Sheep and goats | 9 | 1.64 | 10.2 | (7.5–12.9) | 2.2 | (1.6–2.8) |
| Wildlife | ||||||
| Raccoons | 1,898† | 16.25† | 2181.0 | (2,007.5–2,354.5) | 14.6 | (13.2–16.0) |
| Bats | 1,598 | 5.84 | 1584.8 | (1,429.2–1,740.4) | 5.7 | (5.5–5.9) |
| Skunks | 1,447† | 33.04† | 1561.2 | (1,499.0–1,623.4) | 29.7 | (27.2–32.2) |
| Foxes | 344† | 18.70† | 430.8 | (378.6–483.0) | 22.8 | (19.7–26.0) |
| All rabid animals | 5,865 | 6.03 | 6156.6 | (5,833.3–6,479.9) | 5.8 | (5.4–6.2) |
| Rabid domestic animals | 467† | 0.98 | 493.2 | (478.2–508.2) | 1.0 | (0.9–1.1) |
| Rabid wildlife | 5,398 | 11.17 | 5663.4 | (5,331.9–5,994.9) | 10.3 | (9.0–11.6) |
Does not include data from California.
Significantly (P < 0.05) different from mean value for 2008–2012.
Rabid bats were reported by all reporting localities within the contiguous United States during 2013; no rabid bats were reported in Alaska, Hawaii, or Puerto Rico (Figure 4). Bats were the only reported rabid animals in Idaho, Illinois, Indiana, Michigan, Mississippi, Utah, Washington, and Wisconsin during 2013. A total of 1,598 rabid bats were reported during 2013, a decrease of 4.88%, compared with the 1,680 rabid bats reported during 2012, but not significantly different from the annual numbers for 2008 through 2012. The 24,351 bats submitted for rabies testing represented 29 bat species, but genus and species data were not available for 12,446 bats (51.1%; Table 3).
Figure 4.
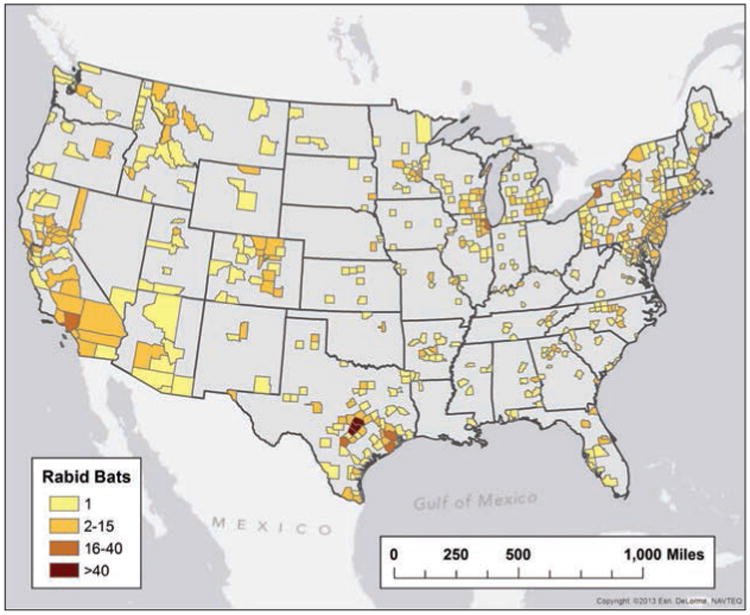
Reported cases of rabies involving bats, by county, 2013.
Table 3.
Species of bats submitted for rabies testing in the United States during 2013.
| Species (common name) | No. tested | No. positive | Percentage positive |
|---|---|---|---|
| Unspeciated | 12,446 | 1,097 | 8.8 |
| Eptesicus fuscus (big brown bat) | 10,100 | 370 | 3.7 |
| Myotis lucifigus (little brown bat) | 642 | 12 | 1.9 |
| Tadarida brasiliensis (Mexican free-tailed bat) | 248 | 29 | 11.7 |
| Lasionycteris noctivagans (silver-haired bat) | 197 | 12 | 6.1 |
| Lasiurus borealis (red bat) | 157 | 14 | 8.9 |
| Myotis species (not further speciated) | 108 | 8 | 7.4 |
| Nycticeius humeralis (evening bat) | 83 | 1 | 1.2 |
| Myotis californicus (California myotis) | 73 | 5 | 6.8 |
| Nyctinomops macrotis (big free-tailed bat) | 73 | 0 | 0.0 |
| Lasiurus cinereus (hoary bat) | 55 | 29 | 52.7 |
| Myotis yumanensis (Yuma myotis) | 50 | 2 | 4.0 |
| Myotis evotis (long-eared myotis) | 32 | 7 | 21.9 |
| Lasiurus seminolus (Seminole bat) | 11 | 2 | 18.2 |
| Antrozous pallidus (desert pallid bat) | 10 | 3 | 30.0 |
| Myotis keenii (Keen's myotis) | 10 | 1 | 10.0 |
| Perimyotis subflavus (tri-colored bat) | 10 | 0 | 0.0 |
| Myotis ciliolabrum (western small-footed bat) | 8 | 0 | 0.0 |
| Myotis volans (long-legged myotis) | 8 | 2 | 25.0 |
| Parastrellus hesperus (canyon bat) | 8 | 6 | 75.0 |
| Myotis septentrionalis (northern long-eared myotis) | 6 | 0 | 0.0 |
| Myotis thysanodes (fringed myotis) | 3 | 0 | 0.0 |
| Lasiurus intermedius (northern yellow bat) | 2 | 0 | 0.0 |
| Lasiurus xanthinus (western yellow bat) | 2 | 1 | 50.0 |
| Plecotus townsendii (Townsend's big-eared bat) | 2 | 0 | 0.0 |
| Rousettus aegyptiacus (Egyptian rousette*) | 2 | 0 | 0.0 |
| Rousettus lanosus (long-haired rousette*) | 2 | 0 | 0.0 |
| Desmodus rotundus (common vampire* bat) | 1 | 0 | 0.0 |
| Eumops perotis (western mastiff bat) | 1 | 0 | 0.0 |
| Plecotus rafinesquii (Rafinesque's big-eared bat) | 1 | 0 | 0.0 |
| Total | 24,351 | 1,601 | 6.57 |
Exotic species submitted by wildlife parks.
The 1,447 rabid skunks reported in 2013 (Figure 5) represented a 5.98% decrease, compared with the 1,539 rabid skunks reported during 2012. The prevalence of rabies among skunks submitted for rabies diagnostic testing (33.0%) was significantly higher than the annual prevalence among skunks submitted for testing from 2008 through 2012.
Figure 5.
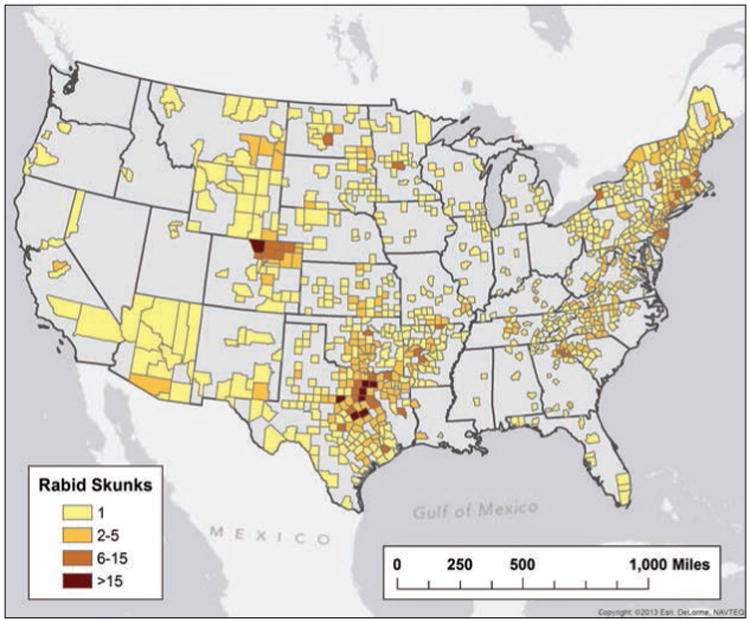
Reported cases of rabies involving skunks, by county, 2013.
A total of 344 rabid foxes were reported in 2013 (Figure 6), which represented a 1.18% increase, compared with the 340 reported during 2012. However, the number reported during 2013 was significantly lower than annual numbers for 2008 through 2012 (430.8; 95% CI, 378.6 to 483.0).
Figure 6.
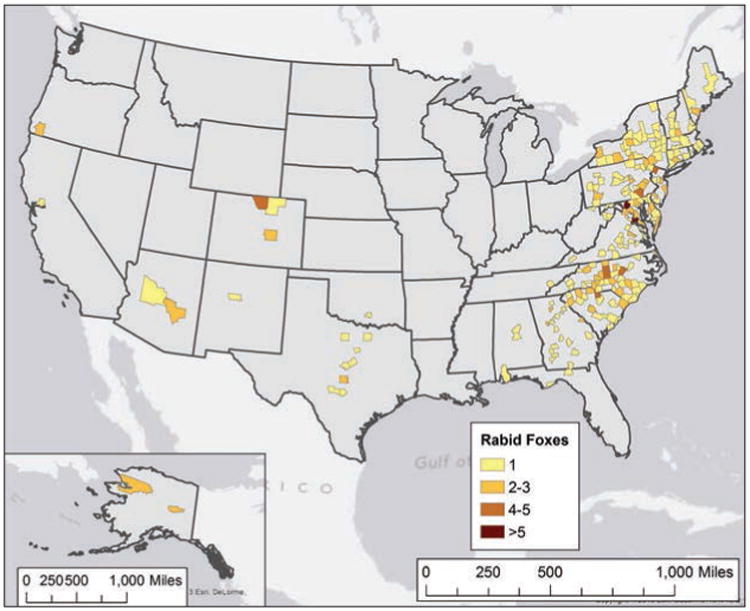
Reported cases of rabies involving foxes, by county, 2013.
In addition to cases of rabies reported among primary reservoir species, 71 cases of rabies were reported in other wildlife species. The most common were mongooses (38 [53.5%]), all of which were reported from Puerto Rico, followed by bobcats (Lynx rufus; 16 [22.8%]), coyotes (Canis latrans; 5 [7.0%]), deer (presumably Odocoileus virginianus; 4 [5.6%]), otters (not specified; 3 [4.2%]), opossums (Didelphis virginiana; 2 [2.8%]), wolves (Canis lupus; 2 [2.8%]), and a fisher (Martes pennant; 1 [1.4%]). A total of 40 rabid rodents and lagomorphs were reported in 2013. Most were groundhogs (Marmota monax; 37 [92.5%]), followed by marmots (Marmota sp; 2 [5.0%]) and a rabbit (family Leporidae; 1 [2.5%]).
Rabies in Domestic Animals
Domestic animals accounted for 7.96% (467/5,865) of all reported rabid animals in 2013. This was a 10.02% decrease, compared with the 519 rabid domestic animals reported during 2012. The most frequently reported rabid domestic animals were cats (Felis catus; 247 [52.9%]), followed by dogs (Canis lupus familiaris; 89 [19.1%]), cattle (Bos taurus; 86 [18.4%]), horses and mules (Equus spp; 31 [6.6%]), sheep and goats (Capra spp; 9 [1.9%]), and other domestic animals (3 swine and 2 llamas [1.1%]). The most frequently submitted domestic animals for rabies diagnostic testing were cats (23,264 [48.9%]) and dogs (21,274 [44.7%]), followed by cattle (1,299 [2.7%]), horses and mules (867 [1.8%]), and sheep and goats (550 [1.2%]).
Cats have represented the majority of rabid domestic animals since 1992; however, there was a significant decrease in the number of rabid cats reported in 2013, compared with annual numbers reported in 2008 through 2012 (Table 2). Pennsylvania reported the greatest number of rabid cats (40 [16.2%]), followed by Virginia (37 [15.0%]), Maryland (25 [10.1%]), Texas (23 [9.3%]), and North Carolina (20 [8.1%]; Figure 7). Vaccination history was not reported for 89.3% (20,783/23,264) of the cats submitted for testing. Of the 2,481 cats with a recorded vaccination history, 1,157 (46.6%) had no previous rabies vaccination, 883 (35.5%) had an unknown rabies vaccination status, 277 (11.2%) reportedly were up-to-date on their rabies vaccination, and 164 (6.6%) had reportedly been previously vaccinated but were not up-to-date. All 441 cats that were up-to-date or that had previously been vaccinated but were not up-to-date were negative for rabies.
Figure 7.
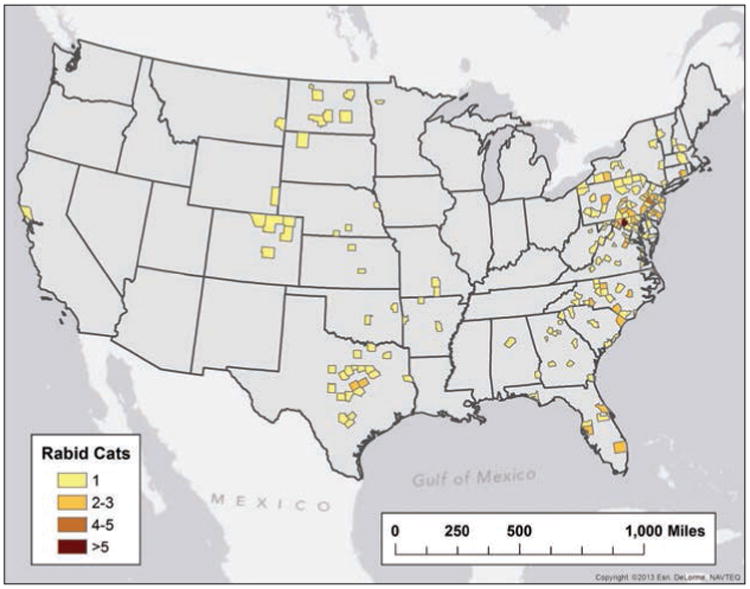
Reported cases of rabies involving cats, by county, 2013.
There were 89 rabid dogs reported during 2013, representing a 5.95% increase, compared with the 84 rabid dogs reported during 2012. This was significantly higher than the mean number of rabid dogs reported annually from 2008 through 2012 (Table 2). The prevalence of rabies among dogs submitted for diagnostic testing (0.41%) was also significantly higher than in previous years. Texas reported the greatest number of rabid dogs (16 [18.0%]), followed by Puerto Rico (14 [15.7%]), Georgia (11 [12.4%]), Oklahoma (7 [7.9%]), North Carolina (5 [5.6%]), and Tennessee (5 [5.6%]; Figure 8). Vaccination history was not reported for 87.5% (18,621/21,288) of the dogs that were tested. Among the 2,667 dogs with a recorded vaccination history, 871 (32.7%) had no previous rabies vaccinations, 869 (32.6%) had an unknown rabies vaccination status, 872 (32.7%) reportedly were up-to-date on their rabies vaccination, and 55 (2.1%) had reportedly previously been vaccinated but were not up-to-date. All 927 dogs that were up-to-date or that had previously been vaccinated but were not up-to-date were negative for rabies. One 10-month-old dog reportedly developed rabies approximately 7 months after administration of its primary dose of rabies vaccine.
Figure 8.
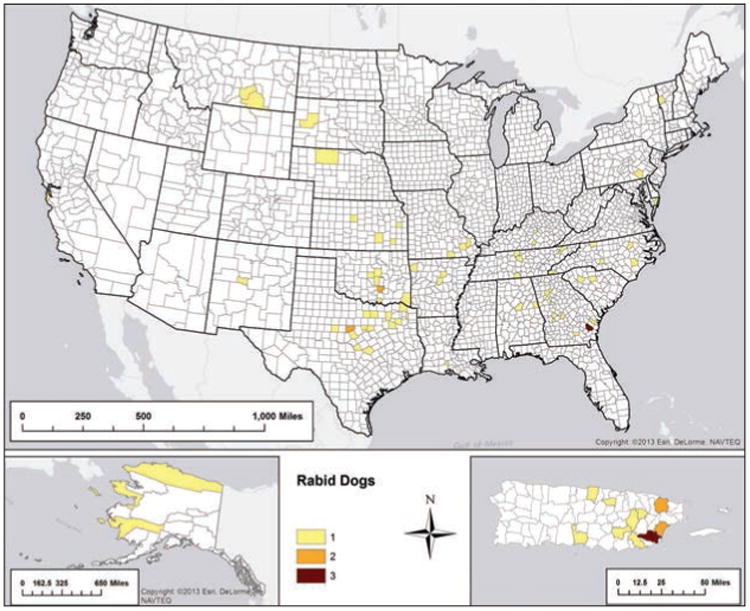
Reported cases of rabies involving dogs, by county, 2013.
Eighty-six rabid cattle were reported during 2013, representing a 25.22% decrease from the 115 rabid cattle reported during 2012, but this was not a significant change from the preceding 5-year period. The number of reported rabid horses and mules (n = 31) was significantly decreased, compared with mean annual number for the preceding 5-year period (Table 2). Compared with 2012, the number of reported rabid sheep and goats declined (9 in 2013 and 13 in 2012). A total of 5 other domestic animals (2 llamas and 3 swine) found to be rabid were reported (Table 1).
Rabid Animals by US Regions
During 2013, 1,483 (25.3%) rabid animals were reported from the Northeast region (ie, Connecticut, Maine, Massachusetts, New Hampshire, New Jersey, New York, New York City, Pennsylvania, Rhode Island, and Vermont). Overall, 6.34% of animals submitted for rabies diagnostic testing in the Northeast region were rabid (Table 1). Localities in the Northeast region submitted 46.4 animals/100,000 persons for rabies testing during 2013. Rabid raccoons (n = 703 [47.4%]) were the most commonly reported animal. The raccoon rabies virus variant was the primary terrestrial variant for all states in the Northeast region. Decreases of ≥ 10% in the number of rabid raccoons during 2013 versus 2012 were reported for 5 localities in the Northeast region of the United States (Maine, 45.2%; Vermont, 40.0%; Pennsylvania, 29.5%; Massachusetts, 22.9%; and New York, 21.0%).
In 2013, 470 (8.0%) rabid animals were reported from the Midwest region (ie, Iowa, Illinois, Indiana, Kansas, Michigan, Minnesota, Missouri, North Dakota, Nebraska, Ohio, South Dakota, and Wisconsin). Overall, 2.07% of animals submitted for rabies diagnostic testing in the Midwest region were rabid (Table 1). Within the Midwest region, 34.4 animals/100,000 persons were submitted for rabies testing during 2013. The primary terrestrial rabies virus variants within the Midwest region were associated with skunks in all of the states in this region with the exception of Illinois and Ohio. No terrestrial rabies virus variants were reported from Illinois. In addition, limited cases of raccoon rabies were reported in counties in the northeast of Ohio. No other terrestrial rabies virus variants were reported in Ohio. Decreases of ≥ 10% in the number of rabid skunks during 2013 versus 2012 were reported for 6 localities in the Midwest region (Wisconsin, 100%; Michigan, 100%; Nebraska, 60.0%; North Dakota, 58.0%; Iowa, 55.6%; and South Dakota, 55.6%).
Most of the rabid animals reported during 2013 (3,262/5,865 [55.62%]) were from the South region (ie, Alabama, Arkansas, District of Columbia, Delaware, Florida, Georgia, Kentucky, Louisiana, Maryland, Mississippi, North Carolina, Oklahoma, South Carolina, Tennessee, Texas, Virginia, and West Virginia; Table 1). The South region had the highest prevalence of rabies in animals submitted for diagnosis (7.69%) during 2013 as well as the greatest number of rabid cattle (41). Animals were submitted for rabies diagnostic testing at a rate of 36.9 animals/100,000 persons during 2013. Within this region, the primary terrestrial rabies virus variant was associated with raccoons in 10 locations (Alabama, District of Columbia, Delaware, Florida, Georgia, Maryland, North Carolina, South Carolina, Virginia, and West Virginia) and skunks in 6 (Arkansas, Kentucky, Louisiana, Oklahoma, Tennessee, and Texas); no terrestrial rabies virus variants were reported in Mississippi. A decrease of ≥ 10% in the number of rabid skunks during 2013 versus 2012 was reported in Tennessee (42.5%), where the primary terrestrial rabies virus variant was the north central skunk rabies virus variant. Increases of ≥ 10% in the number of rabid raccoons were reported in 6 states (West Virginia, 56.8%; Texas, 42.1%; Alabama, 39.4%; Maryland, 17.2%; Delaware, 14.3%; and Florida, 13.6%). Except for Texas, all states were in areas where the primary terrestrial rabies virus variant was the raccoon rabies virus variant.
A total of 596 (10.2%) rabid animals were reported from the West region (ie, Alaska, Arizona, California, Colorado, Hawaii, Idaho, Montana, New Mexico, Nevada, Oregon, Utah, Washington, and Wyoming; Table 1). Overall, 6.8% of animals submitted for rabies diagnostic testing in the West region were rabid (data for California were excluded because California did not report information on number of animals tested). In the West region, 14.3 animals/100,000 persons were submitted for rabies diagnostic testing during 2013. Most of the rabid animals were bats (395/596 [66.3%]). No terrestrial rabies virus variants circulated in 6 states in this region (Hawaii, Idaho, Nevada, Oregon, Utah, and Washington). In the remaining states, the primary terrestrial rabies virus variant was associated with skunks in 6 states (Arizona, California, Colorado, Montana, New Mexico, and Wyoming) and with Arctic foxes in 1 state (Alaska). The rabies virus variant associated with Arctic foxes was only circulating in Alaska within the United States. A decrease of ≥ 10% in the number of rabid skunks during 2013 versus 2012 was reported in California (100% decrease), New Mexico (90.9% decrease), and Wyoming (60.0% decrease). An increase in the number of rabid skunks during 2013 versus 2012 was reported in 3 localities. The primary terrestrial rabies virus variants were the south central skunk rabies virus variant in 2 of the localities (Arizona, 69.2% increase; Colorado, 15.9% increase) and the north central skunk rabies virus variant in the remaining locality (Montana, 85.7% increase).
Puerto Rico was the sole jurisdiction in the Caribbean that reported animal submission data for rabies testing. Most of the rabid animals reported from this region were mongooses, and the mongoose rabies virus variant was the primary terrestrial rabies virus variant on the island. In Puerto Rico, 3.1 animals/100,000 persons were submitted for rabies diagnostic testing during 2013.
Rabies Virus Variants
A total of 24 rabies virus variants were reported in 24 species of rabid animals during 2013. Rabies virus variant information was reported for 1,687 (28.76%) of the 5,865 reported rabid animals. A total of 1,221 animals were reported that were infected with a terrestrial rabies virus variant during 2013. Terrestrial rabies virus variants were distributed in distinct geographic ranges with a few instances of overlapping ranges (Figure 9). Most of the reported terrestrial variants were raccoon rabies virus variants (660/1,221 [54.1%]; Table 4). Of the animals infected with the raccoon rabies virus variant, 353 (53.5%) were raccoons. The remaining 307 (46.5%) cases were the result of cross-species transmission, with most involving wildlife species. The second most frequently reported terrestrial rabies virus variant was the south central skunk rabies virus variant (n = 498), and 405 (81.3%) of the rabid animals infected with the south central skunk rabies virus variant were skunks; the remaining cases were a result of spillover and primarily involved domestic animals. Nine of the 13 fox rabies virus variants were isolated from species other than foxes. One animal infected with the Texas gray fox rabies virus variant during 2013 was a cow in Concho County, Texas.
Figure 9.
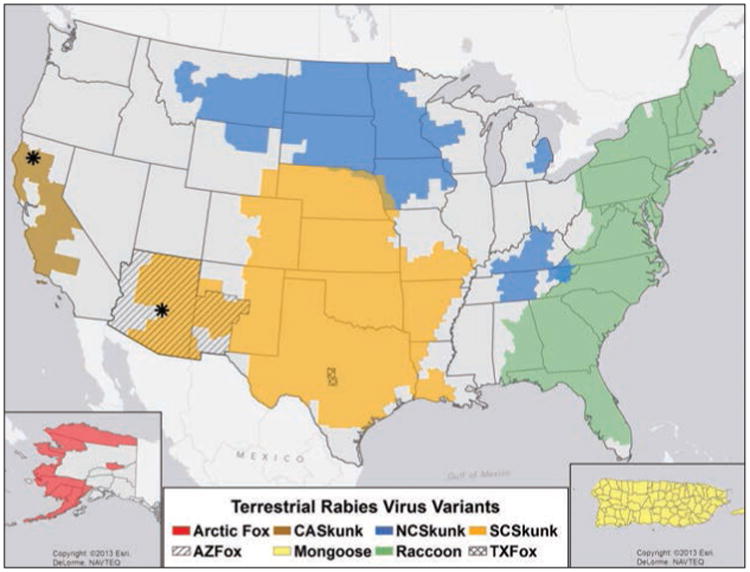
Distribution of major rabies virus variants among mesocarnivores in the United States and Puerto Rico, 2009 through 2013. *Potential host shift event. AZ = Arizona. CA = California. NC = North central. SC = South central. TX = Texas.
Table 4.
Rabies virus variants identified in rabid animals, 2013.
| Variant | Domestic animals | Wild animals | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||
| Cats | Cattle | Dogs | Hores and mules | Sheep and goats | Other domestic | Raccoon | Bats | Skunks | Foxes | Other wild | Rodents and lagomorphs | ||
| Raccoon | 57 | 10 | 7 | 2 | 2 | 0 | 353 | 0 | 155 | 65 | 5 | 4 | 660 |
| South central skunk | 23 | 12 | 17 | 6 | 2 | 0 | 24 | 0 | 405 | 9 | 0 | 0 | 498 |
| North central skunk | 2 | 3 | 7 | 4 | 0 | 2 | 1 | 0 | 31 | 0 | 0 | 0 | 50 |
| Arctic fox | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 6 |
| Arizona gray fox | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 4 | 0 | 6 |
| Texas gray fox | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Bat* | 1 | 0 | 1 | 2 | 0 | 0 | 2 | 455 | 1 | 3 | 1 | 0 | 466 |
| No variant reported | 164 | 60 | 55 | 17 | 5 | 3 | 1,518 | 1,143 | 855 | 263 | 59 | 36 | 4,178 |
| Total | 247 | 86 | 89 | 31 | 9 | 5 | 1,898 | 1,598 | 1,447 | 344 | 71 | 40 | 5,865 |
| Variant typed (%) | 33.6 | 30.2 | 38.2 | 45.2 | 44.4 | 40.0 | 20.0 | 28.5 | 40.9 | 23.5 | 16.9 | 10.0 | 28.8 |
A total of 13 rabies virus variants associated with bats were reported.
Of the 1,687 rabid animals for which rabies virus variant information was available, 466 (27.6%) were infected with rabies virus variants associated with bats (Table 4). Most of these (346 [74.2%]) were reportedly infected with the Mexican free-tailed bat (Tadarida brasiliensis) rabies virus variant. The second most commonly reported bat-associated variant was the big brown bat (Eptesicus fuscus) rabies virus variant (45; 9.7%). Cross-species transmission of bat variants was reported for 11 animals.
Rabies in Humans
Samples from 42 human patients from 24 states were submitted to the CDC for rabies diagnostic testing during 2013, and infection was confirmed in 3 (7.1%). Thirty-four cases of human rabies have been diagnosed in the United States since 2003 (Table 5); 24 (70.6%) of these patients acquired the disease in the United States or Puerto Rico. Organ or tissue transplantation was attributed as the source of infection for 5 of the 24 (20.8%) domestic cases. Seven of the 19 patients in which infection was not attributed to organ or tissue transplantation had reportedly been bitten by a bat, and 5 had reportedly had contact with a bat (although no bite was reported). Two of the remaining 7 patients were infected with the raccoon rabies virus variant, 1 was infected with the mongoose rabies virus variant (Puerto Rico), 3 were infected with a bat rabies virus variant, and 1 had an unknown rabies virus variant. Most human patients with non–transplant-acquired rabies virus infection were males (14/19 [73.7%]), and their mean age was 35.9 years (range, 8 to 70 years).
Table 5.
Cases of rabies in humans in the United States and Puerto Rico, 2003 through July 2014, by circumstances of exposure and rabies virus variant.
| Date of onset | Date of death | Reporting state | Age (y) | Sex | Exposure* | Rabies virus variant† |
|---|---|---|---|---|---|---|
| 10 Feb 03 | 10 Mar 03 | VA | 25 | M | Unknown | Raccoon, eastern United States |
| 28 May 03 | 5 Jun 03 | PR | 64 | M | Bite-Puerto Rico | Dog/mongoose, Puerto Rico |
| 23 Aug 03 | 14 Sep 03 | CA | 66 | M | Bite | Bat, Ln |
| 9 Feb 04 | 15 Feb 04 | FL | 41 | M | Bite-Haiti | Dog, Haiti |
| 27 Apr 04 | 3 May 04 | AR | 20 | M | Bite (organ donor) | Bat, Tb |
| 25 May 04 | 31 May 04 | OK | 53 | M | Liver transplant | Bat, Tb |
| 27 May 04 | 21 Jun 04 | TX | 18 | M | Kidney transplant | Bat, Tb |
| 29 May 04 | 9 Jun 04 | TX | 50 | F | Kidney transplant | Bat, Tb |
| 2 Jun 04 | 10 Jun 04 | TX | 55 | F | Arterial transplant | Bat, Tb |
| 12 Oct 04 | Survived | WI | 15 | F | Bite | Bat, unknown |
| 19 Oct 04 | 26 Oct 04 | CA | 22 | M | Unknown-El Salvador | Dog, El Salvador |
| 27 Sep 05 | 27 Sep 05 | MS | 10 | M | Contact | Bat, unknown |
| 4 May 06 | 12 May 06 | TX | 16 | M | Contact | Bat, Tb |
| 30 Sep 06 | 2 Nov 06 | IN | 10 | F | Bite | Bat, Ln |
| 15 Nov 06 | 14 Dec 06 | CA | 11 | M | Bite-Philippines | Dog, Philippines |
| 19 Sep 07 | 20 Oct 07 | MN | 46 | M | Bite | Bat, unknown |
| 16 Mar 08 | 18 Mar 08 | CA | 16 | M | Bite-Mexico | Fox, Tb related |
| 19 Nov 08 | 30 Nov 08 | MO | 55 | M | Bite | Bat, Ln |
| 25 Feb 09 | Survived | TX | 17 | F | Contact | Bat, unknown |
| 5 Oct 09 | 20 Oct 09 | IN | 43 | M | Unknown | Bat, Ps |
| 20 Oct 09 | 11 Nov 09 | MI | 55 | M | Contact | Bat, Ln |
| 23 Oct 09 | 20 Nov 09 | VA | 42 | M | Contact-India | Dog, India |
| 2 Aug 10 | 21 Aug 10 | LA | 19 | M | Bite-Mexico | Bat, Dr |
| 24 Dec10 | 10 Jan 11 | WI | 70 | M | Unknown | Bat, Ps |
| 30 Apr 11 | Survived | CA | 8 | F | Unknown | Unknown |
| 30 Jun 11 | 20 Jul 11 | NJ | 73 | F | Bite-Haiti | Dog, Haiti |
| 14 Aug 11 | 21 Aug 11 | NY | 25 | M | Contact-Afghanistan | Dog, Afghanistan |
| 21 Aug 11 | 1 Sep 11 | NC | 20 | M | Unknown (organ donor)‡ | Raccoon, eastern United States |
| 1 Sep 11 | 14 Oct 11 | MA | 40 | M | Contact-Brazil | Dog, Brazil |
| 3 Dec 11 | 19 Dec 11 | SC | 46 | F | Unknown | Tb |
| 22 Dec 11 | 23 Jan 12 | MA | 63 | M | Contact | My sp |
| 6 Jul 12 | 31 Jul 12 | CA | 34 | M | Bite | Bat, Tb |
| 31 Jan 13 | 27 Feb 13 | MD | 49 | M | Kidney transplant | Raccoon, eastern United States |
| 16 May 13 | 11 Jun 13 | TX | 28 | M | Unknown-Guatemala | Dog, Guatemala |
Data for exposure history are reported when plausible information was reported directly by the patient (if lucid or credible) or when a reliable account of an incident consistent with rabies virus exposure (eg, dog bite) was reported by an independent witness (usually a family member). Exposure histories are categorized as bite, contact (eg, waking to find bat on exposed skin) but no known bite was acknowledged, or unknown (ie, no known contact with an animal was elicited during case investigation).
Variants of the rabies virus associated with terrestrial animals in the United States and Puerto Rico are identified with the names of the reservoir animal (eg, dog or raccoon), followed by the name of the most definitive geographic entity (usually the country) from which the variant has been identified. Variants of the rabies virus associated with bats are identified with the names of the species of bats in which they have been found to be circulating. Because information regarding the location of the exposure and the identity of the exposing animal is almost always retrospective and much information is frequently unavailable, the location of the exposure and the identity of the animal responsible for the infection are often limited to deduction.
Infection was not identified until 2013, when an organ recipient developed rabies.
Dr = Desmodus rotundus. Ln = Lasionycteris noctivagans. My sp = Myotis species. Ps = Perimyotis subflavus. Tb = Tadarida brasiliensis.
Ten of the 34 human patients with rabies reported since 2003 were infected outside of the United States and its territories, representing exposures from 7 countries. Exposures were attributed to dogs (n = 6), bats (1), and foxes (1); in 2 patients, exposure history was not known. Nine of these 10 patients were male; their mean age was 31.7 years (range, 11 to 73 years).
In January 2013, a 49-year-old man presented to a Maryland emergency department with right hip pain. A diagnosis of sciatica was made, and the patient was discharged. However, he returned to the emergency department 4 days later with fever, nausea, lower extremity weakness, and pain in the right lower abdominal quadrant. He developed encephalitis and hypersalivation and died 24 days later. Samples obtained 5 days prior to the patient's death were submitted to the CDC, and rabies was confirmed, with the infecting virus typed as the raccoon rabies virus variant. No known animal exposures were identified; however, the patient had undergone a deceased-donor kidney transplant in 2011, 17 months prior to the onset of symptoms.
The kidney transplant in this patient had been obtained from a 20-year-old man who presented in August 2011 to a primary care clinic in Florida with severe nausea, vomiting, and upper extremity paresthesia. He had recently returned from a fishing trip during which he had consumed raw fish and been stung by a jellyfish. The nausea and vomiting were attributed to the consumption of raw fish, and the paresthesia was attributed to the jellyfish sting. On the fourth day after symptom onset, however, the patient developed fever, seizures, and an altered mental status; was admitted to a hospital; and was immediately sedated and intubated. He was declared brain-dead 11 days after illness onset, with the cause of death listed as severe gastroenteritis. He was determined to be eligible for organ donation, and his kidneys, heart, and liver were transplanted into 4 recipients.
In February 2013, following confirmation of rabies in the Maryland organ recipient, banked samples from the Florida organ donor were tested at the CDC, and a diagnosis of rabies confirmed. Sequence analysis found > 99.9% identity between the rabies virus isolate from the donor and the isolate from the Maryland recipient. On further investigation, the donor was identified as an active outdoorsman who, while he resided in North Carolina, had had numerous encounters with raccoons and foxes, including several bites, which were reported by friends and family. The 3 remaining organ recipients were identified and provided postexposure prophylaxis. At the time of final follow-up, they remained healthy. A multistate contact investigation of community and health-care providers who potentially could have had contact with these patients was conducted. Five hundred sixty-four people were assessed for contact with infectious materials, and postexposure prophylaxis was recommended for 58 (10.3%) because of concerns about exposure to saliva, tears, or nervous tissues from the 2 patients.
In May 2013, a 28-year-old Guatemalan national was apprehended while illegally crossing the Texas-Mexico border. Seven days after his arrest, while in Immigration and Customs Enforcement custody, the patient began to experience insomnia, anxiety, nausea, dysphagia, and hypersalivation. After 2 days of worsening symptoms, the patient was admitted to a hospital for evaluation of possible pneumomediastinum. Although the pneumomediastinum resolved without surgical intervention, the patient's mental and respiratory status deteriorated. Serum tested with an ELISA at a commercial laboratory was positive for rabies virus antibodies. Antemortem samples were sent to the CDC, where rabies was confirmed and the infecting virus was typed as a Central American canine rabies virus variant.
In Guatemala, the patient had owned a dog that died of unknown causes in 2011, but family members reported that they were unaware of any history of animal bites. No animal exposures were reported at the time of hospitalization, and autopsy revealed no evidence of bite wounds. Contact investigations identified more than 500 detainees who were potentially housed with the patient during his infectious period. The CDC coordinated with Immigration and Customs Enforcement to perform follow-up risk assessments for contacts still detained in the United States and contacted the Pan American Health Organization to notify countries to which other contacts had been returned. Two hundred sixty risk assessments were completed, and 25 (9.6%) people were recommended to receive postexposure prophylaxis because of potential exposure to infectious materials from the patient.
Summary Report of Rabies in Canada and Mexico
Canada reported 116 laboratory-confirmed rabid animals during 2013, a 17.7% decrease from the 141 rabid animals reported in 2012. Wildlife continued to be the most common rabid animals, representing 87.9% (n = 102) of all rabid animals. The remaining rabid animals consisted of cats and dogs (12 [10.3%]) and livestock (2 [1.7%]). The number of animals submitted for diagnostic testing to the Canadian Food Inspection Agency rabies laboratories decreased 10.0% from 3,851 in 2012 to 3,466 in 2013. In addition to Canadian Food Inspection Agency submissions, several provincial ministries undertook active wildlife rabies surveillance testing during 2013, and they identified 2 rabid animals (included in above totals). The first rabid raccoon identified in Canada since 2008 was reported. Variant typing determined that the rabid raccoon was infected with the western Canadian skunk rabies virus variant. No rabid wolves were reported in 2013. The number of rabid skunks decreased by 15.6%, while numbers of rabid bats and cats increased by 24.4% and 50.0%, respectively. During 2012, there was a northern epizootic in both Arctic and red foxes, but this remitted in 2013, with a 56.1% decrease in the number of rabid foxes reported. In addition, the number of rabid dogs reported decreased 43.7%, and the number of rabid equids reported remained the same as in 2012 (n = 2). No cases of rabies were reported in cattle or humans during 2013.
For the first time since 1938, no human deaths associated with rabies were reported in Mexico during 2013. Eleven rabid dogs were reported, representing an 8.3% decrease from the 12 reported in 2012. Ten cases occurred in southeast Mexico (8 in Chiapas and 2 in the Yucatan). One imported case was reported in Michoacán, although the origin of the dog was not known. Molecular typing results of the reported rabid dogs showed high similarities to isolates from the same region (Chiapas isolates showed 98% similarity to an isolate obtained from a Yucatan dog in 2002 and an isolate obtained from a Yucatan dog in 1998; the isolate reported from Michoacán corresponded to a rabies virus variant of canine lineage that appeared to be associated with skunks).
Discussion
The number of animals submitted for rabies diagnostic testing in the United States has decreased since 2009.20 Laboratory testing of animals suspected to have rabies is a critical public health service that directly influences rabies postexposure prophylaxis recommendations. Rabies exposure risk assessments remain the best way to determine whether an exposure warrants administration of rabies postexposure prophylaxis.
Vaccination of domestic animals is a critical component of a successful rabies prevention and control program and is a cost-effective means to prevent human rabies.21 Nearly half of the animals submitted for rabies diagnostic testing in the United States during 2013 were cats and dogs. Most of these animals presumably had never been vaccinated against rabies or their status was unknown. Improving vaccination coverage and documentation, ownership rates, and adherence to local regulations might reduce the number of animals involved in potential human exposures subsequently requiring rabies testing. Standardization of variables used to categorize vaccination status will aid in the identification of potential rabies vaccine failures. Domestic animals that have received only primary immunizations are considered currently vaccinated 30 days after the primary immunization; therefore, exposures during this 30-day period would be handled as though the animal were not vaccinated. Additionally, animals that develop signs of rabies during the 30-day period after primary immunization are not considered to be vaccination failures. A 12-month booster is required for both the 1-year and 3-year animal rabies vaccine. The 1-year animal rabies vaccines currently on the market are labeled for annual administration following the primary immunization, while the 3-year vaccines currently on the market are labeled for administration every 3 years following the primary immunization and a 12-month booster.
Variant typing of rabid animals emphasizes the ecological variations of rabies viruses in circulation within the United States. Increasing the rate of variant typing of rabid animals will help detect potential introductions of new rabies virus variants into the United States (eg, reintroduction of the canine rabies virus variant) or translocation of rabies virus variants inside the United States and could also improve our understanding of the transmission and possible emergence of new rabies virus variants into previously unassociated species. Although 12 bat rabies virus variants were reported from terrestrial animals during 2013, these results were not comparable to previous years' data because of changes in submission rates for variant typing. If variant typing submission protocols were standardized, this information could be used to determine whether a potential new rabies virus variant was emerging.
After > 4 years of no reported cases, the report of a rabid cow infected with the Texas gray fox rabies virus variant illustrates that the variant remains in circulation despite efforts to maintain active surveillance for this variant. An oral rabies vaccination program targeted at eliminating this variant in gray foxes has been an ongoing effort by the Texas Department of Health Services since the mid-1990s. While the oral rabies vaccination program has greatly reduced the incidence of fox rabies in the region, ongoing oral rabies vaccination will be necessary in addition to ongoing active surveillance to determine when the variant has been eliminated and vaccination efforts might be discontinued. Similarly, the recent discovery of rabid ferret-badgers in Taiwan after 50 years of presumed rabies-free status illustrates the difficulties in maintaining adequate surveillance in a wildlife species.22 Additional guidance and recommendations on appropriate rabies surveillance efforts is needed. Recommendations should include where passive and active surveillance efforts would be sufficient to accurately determine endemicity, particularly where oral rabies vaccination programs are ongoing, because this information is necessary for accurate planning.
Over the past 10 years, 5 of the 24 (21%) human rabies cases acquired domestically were the result of transplantation of organs or tissues from donor patients who died of rabies but in whom the disease was not diagnosed until rabies developed in transplant recipients. Human rabies cases in the United States are rare, with only 1 to 3 cases annually, while tens of thousands of lives are saved each year through organ donation programs.23 Universal screening of all organ and tissue donations for rabies remains impractical owing to the relative rarity of the disease and the resources needed to perform laboratory diagnostic testing. However, as part of the continued efforts to improve transplant safety, rabies should be included in the differential diagnoses for patients with unexplained viral encephalitis. Risks to organ and tissue recipients can be further reduced by the timely administration of postexposure prophylaxis if rabies antigen is later detected in donor organs or tissues. Given that 21% of domestic human rabies cases reported in recent years are due to the transplantation of organs and tissues, a more standardized approach to recognizing organ donors with unexplained infectious encephalitis and development of guidelines for the use of those organs are warranted.
Acknowledgments
The authors thank the state and territorial health and agriculture departments and laboratories for their contributions of rabies surveillance data and human case investigations, especially Laura Robinson from the Texas Department of Health and Darlene Bhavnani from the CDC Division of Global Migration and Quarantine. The authors also thank N. Kuzmina and A. Velasco-Villa from the CDC Rabies Program for assistance with diagnostic testing and viral typing; C. Paddock, D. Blau, and S. Zaki from the CDC Infectious Diseases Pathology Branch for collaborative support; Kim Knight-Picketts and Christine Fehlner-Gardiner from the Center of Expertise for Rabies, Ottawa Laboratory, Fallowfield, and from the Animal Health, Welfare and Biosecurity Division, Canadian Food Inspection Agency, for providing 2013 summary data for Canada; and Fernando Vargas Pino from the Instituto de Salud del Estado de México for providing 2013 canine rabies summary data for Mexico.
Abbreviation
- CI
Confidence interval
Footnotes
Use of trade names and commercial sources is for identification only and does not imply endorsement by the US Department of Health and Human Services. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC.
This article has not undergone peer review.
References
- 1.CDC. 2012 nationally notifiable diseases and conditions and current case definitions. Atlanta: CDC; 2012. [Google Scholar]
- 2.Velasco-Villa A, Reeder SA, Orciari LA, et al. Enzootic rabies elimination from dogs and reemergence in wild terrestrial carnivores, United States. Emerg Infect Dis. 2008;14:1849–1854. doi: 10.3201/eid1412.080876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manning SE, Rupprecht CE, Fishbein D, et al. Human rabies prevention—United States, 2008: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2008;57:1–28. [PubMed] [Google Scholar]
- 4.Orciari LRC. Rabies virus. In: Versalovic J, editor. Manual of clinical microbiology. 10th. Washington, DC: American Society for Microbiology; 2011. [Google Scholar]
- 5.International Union of Microbiological Societies Virology Division. Virus taxonomy: classification and nomenclature of viruses: ninth report of the International Committee on Taxonomy of Viruses. San Diego: Elsevier Academic Press; 2012. [Google Scholar]
- 6.Dyer JL, Wallace R, Orciari L, et al. Rabies surveillance in the United States during 2012. J Am Vet Med Assoc. 2013;243:805–815. doi: 10.2460/javma.243.6.805. [DOI] [PubMed] [Google Scholar]
- 7.Kuzmin IV, Shi M, Orciari LA, et al. Molecular inferences suggest multiple host shifts of rabies viruses from bats to mesocarnivores in Arizona during 2001–2009. [Accessed Jun 11, 2014];PLoS Pathog. 2012 8:e1002786. doi: 10.1371/journal.ppat.1002786. serial online. Available at: www.plospathogens.org/article/info%3Adoi%2F10.1371%2Fjournal.ppat.1002786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rupprecht CE, Briggs D, Brown CM, et al. Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2010;59:1–9. [PubMed] [Google Scholar]
- 9.Jentes ES, Blanton JD, Johnson KJ, et al. The global availability of rabies immune globulin and rabies vaccine in clinics providing indirect care to travelers. J Travel Med. 2014;21:62–66. doi: 10.1111/jtm.12085. [DOI] [PubMed] [Google Scholar]
- 10.Oertli EH, Wilson PJ, Hunt PR, et al. Epidemiology of rabies in skunks in Texas. J Am Vet Med Assoc. 2009;234:616–620. doi: 10.2460/javma.234.5.616. [DOI] [PubMed] [Google Scholar]
- 11.Ronald G, Powell J, Raj P, et al. Protocol for postmortem diagnosis of rabies in animals by direct fluorescent antibody testing: a minimum standard for rabies diagnosis in the United States. Atlanta: CDC: 2003. [Accessed Sep 22, 2014]. Available at: www.cdc.gov/rabies/pdf/rabiesdfaspv2.pdf. [Google Scholar]
- 12.Sidwa TJ, Wilson PJ, Moore GM, et al. Evaluation of oral rabies vaccination programs for control of rabies epizootics in coyotes and gray foxes: 1995–2003. J Am Vet Med Assoc. 2005;227:785–792. doi: 10.2460/javma.2005.227.785. [DOI] [PubMed] [Google Scholar]
- 13.Slate D, Rupprecht CE, Donovan D, et al. Attaining raccoon rabies management goals: history and challenges. Dev Biol (Basel) 2008;131:439–447. [PubMed] [Google Scholar]
- 14.Slate D, Algeo TP, Nelson KM, et al. Oral rabies vaccination in North America: opportunities, complexities, and challenges. [Accessed Jun 26, 2014];PLoS Negl Trop Dis. 2009 3:e549. doi: 10.1371/journal.pntd.0000549. serial online. Available at: www.plosntds.org/article/info%3Adoi%2F10.1371%2Fjournal.pntd.0000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanton JD, Robertson K, Palmer D, et al. Rabies surveillance in the United States during 2008. J Am Vet Med Assoc. 2009;235:676–689. doi: 10.2460/javma.235.6.676. [DOI] [PubMed] [Google Scholar]
- 16.US Census Bureau. 2010 Census summary file. Washington, DC: US Census Bureau; 2010. [Google Scholar]
- 17.Blanton JD, Dyer J, McBrayer J, et al. Rabies surveillance in the United States during 2011. J Am Vet Med Assoc. 2012;241:712–722. doi: 10.2460/javma.241.6.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McQuiston JH, Yager PA, Smith JS, et al. Epidemiologic characteristics of rabies virus variants in dogs and cats in the United States, 1999. J Am Vet Med Assoc. 2001;218:1939–1942. doi: 10.2460/javma.2001.218.1939. [DOI] [PubMed] [Google Scholar]
- 19.Smith JS, Reid-Sanden FL, Roumillat LF, et al. Demonstration of antigenic variation among rabies virus isolates by using monoclonal antibodies to nucleocapsid proteins. J Clin Microbiol. 1986;24:573–580. doi: 10.1128/jcm.24.4.573-580.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blanton JD, Palmer D, Rupprecht CE. Rabies surveillance in the United States during 2009. J Am Vet Med Assoc. 2010;237:646–657. doi: 10.2460/javma.237.6.646. [DOI] [PubMed] [Google Scholar]
- 21.Fitzpatrick MC, Hampson K, Cleaveland S, et al. Cost-effectiveness of canine vaccination to prevent human rabies in rural Tanzania. Ann Intern Med. 2014;160:91–100. doi: 10.7326/M13-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu H, Chang SS, Tsai HJ, et al. Notes from the field: wildlife rabies on an island free from canine rabies for 52 years—Taiwan, 2013. MMWR Morb Mortal Wkly Rep. 2014;63:178. [PMC free article] [PubMed] [Google Scholar]
- 23.OPTN/SRTR 2012 annual data report. Rockville, Md: Department of Health and Human Services, Health Resources and Services Administration; 2014. Organ Procurement and Transplantation Network and Scientific Registry of Transplant Recipients. [Google Scholar]


