Abstract
Objective
Endothelial dysfunction is associated with arterial stiffness in large arteries. The purpose of this study was to investigate the association between coronary endothelial dysfunction, coronary artery compliance and wall shear stress in patients with early atherosclerosis.
Methods
Coronary endothelial function was assessed according to responses to intracoronary acetylcholine in 120 patients without significant coronary stenosis. Acceleration of peak velocity (ACC), which is inversely related to coronary artery compliance, was derived from coronary flow velocity spectra, and wall shear rate (WSR) was calculated. Measurements were performed at baseline and after intracoronary nitroglycerin in order to eliminate the contribution of vascular smooth muscle tone to coronary artery compliance.
Results
In all patients, heart rate significantly increased (72±1 to 77±1 bpm, p<0.01) and mean arterial pressure decreased (97±2 to 93±1 mm Hg, p<0.01) after nitroglycerin. Coronary blood flow (CBF) and resistance were not significantly changed, but the diastolic to systolic velocity ratio increased significantly (2.15±0.08 to 5.36±0.61, p<0.01). Patients with abnormal endothelial function (n=70) had a higher WSR at baseline (559±41 vs 440±26 s−1, p<0.05) and after nitroglycerin (457±41 vs 339±29 s−1, p<0.05), and a higher ACC after nitroglycerin (3.9±0.4 vs 2.8±0.4 m/s2, p<0.05) than patients with normal function (n=50).
Conclusions
The current study demonstrates that intracoronary nitroglycerin does not contribute to an increase of CBF but alters the phasic coronary flow pattern. Furthermore, early coronary atherosclerosis characterised by endothelial dysfunction is associated with a decrease in coronary artery compliance and an increase in wall shear stress. Therefore, coronary wall properties are affected early in the atherosclerosis process.
The endothelium regulates vascular tone and growth by releasing endothelium-derived vasodilators and vasoconstrictors.1 Previous studies have demonstrated that endothelial dysfunction is a marker of early atherosclerosis and is associated with a greater risk of future cardiovascular events.2, 3 In addition, arterial stiffness may also predict cardiovascular morbidity or mortality even in healthy individuals.4, 5 Previous studies have also suggested that endothelial dysfunction is associated with increased stiffness of large systemic vessels such as the radial, brachial and common carotid arteries.6–8 Saito et al9 have demonstrated the relationship between large arterial stiffness and coronary flow velocity reserve using transthoracic echocardiography. However, no detailed data describe the association between coronary endothelial function and mechanical properties, such as stiffness.
Pulse wave velocity, augmentation index and cross-sectional compliance have been used to determine arterial stiffness and compliance,4–9 and the phasic coronary blood flow (CBF) measurements obtained by intracoronary Doppler guide-wire can be used to assess coronary artery compliance. In the absence of significant epicardial coronary artery stenosis, the acceleration of peak velocity (ACC) is determined by three major factors; the pressure gradient driving blood flow, coronary vascular resistance and the compliance of the coronary artery.10 Therefore, ACC may reflect the changes in vascular compliance as long as the other two factors do not change remarkably. Coronary artery compliance is in turn influenced by the stiffness of the arterial wall and the degree of vascular smooth muscle contribution to the resting vascular tone.11 The administration of intra-coronary nitroglycerin results in maximal epicardial endothelium-independent vasodilation and thus eliminates the contribution of vasomotor tone to coronary artery compliance. The main determinant of coronary artery compliance thus becomes the endogenous stiffness of the arterial wall.
The current study was designed to test the hypothesis that coronary endothelial dysfunction was associated with alterations in the elastic properties of the coronary artery wall in patients with early coronary atherosclerosis.
METHODS
Study population
The Mayo Clinic Institutional Review Board approved the current study. One hundred and twenty consecutive patients who were referred to cardiac catheterisation for evaluation of coronary artery disease and who had angiographically epicardial coronary diameter stenosis less than 30% were included in the current study. Exclusion criteria included a history of myocardial infarction, percutaneous coronary intervention, coronary artery bypass graft surgery, unstable angina pectoris, or variant angina, ejection fraction of 50% or less, valvular heart disease, peripheral vascular disease, uncontrolled hypertension, or significant endocrine, hepatic, renal, or inflammatory disease. Some of the subjects of this study were included in our previous studies.12, 13
Protocol of study
After diagnostic angiography and the exclusion of significant obstructive coronary artery stenosis, endothelium-dependent and endothelium-independent coronary vasoreactivity were assessed as previously described.3 In brief, a 0.014-inch Doppler guidewire (Flowire; Volcano Inc, Rancho Cordova, CA USA) within a coronary infusion catheter (Ultrafuse; SciMed Life System, Natick, MA, USA) was positioned in the midportion of the left anterior descending coronary artery. Then, intracoronary bolus injections of incremental doses (18–60 μg) of adenosine were administered until maximal hyperaemia was achieved in order to determine the coronary flow reserve (CFR). Subsequently, intracoronary acetylcholine at increasing concentrations (10−6, 10−5 and 10−4 mol/l) was selectively infused for 3 min at each concentration into the left anterior descending coronary artery. Haemodynamic data, Doppler measurements and coronary angiography were obtained after each infusion. The infusion was terminated when the largest molar concentration of acetylcholine (10−4 mol/l) was reached. Coronary angiography and intracoronary Doppler data were analysed according to our previous studies.3 After acetylcholine infusion, intracoronary nitroglycerin (200 μg) was administered to achieve maximal vasodilation and eliminate the component of epicardial vascular tone on coronary artery compliance,14 and adenosine was injected again to determine the endothelium-independent CFR under conditions of epicardial vasodilation.
Assessments of coronary endothelial function and vascular compliance
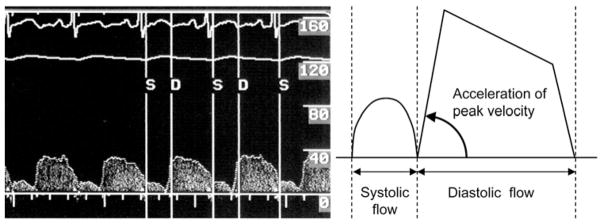
The coronary artery diameter (CAD) was measured by a blinded investigator in the segment 5 mm distal to the tip of the Doppler wire using a computer-based image analysis system. Average peak velocity (APV), ACC and the diastolic to systolic velocity ratio (DSVR) were automatically derived from the Doppler flow velocity spectra (figure 1). CBF was determined as π(CAD/2)2 × (APV/2)3 and wall shear rate (WSR) using the equation: WSR = 4CBF/π(CAD/2).3, 15 Coronary vascular resistance was obtained as the mean arterial pressure divided by CBF. Furthermore, the percentage change in ACC was calculated as (ACC after nitroglycerin – ACC at baseline)/ACC at baseline × 100. Percentage changes in DSVR and WSR were also calculated. Endothelium-dependent CFR was calculated as the percentage change in CBF in response to acetylcholine, and endothelium-independent CFR was obtained as APV after adenosine injection divided by APV before adenosine injection both at baseline and after nitroglycerin. In line with our previous studies, endothelial dysfunction was defined as endothelium-dependent CFR of 1.5 or less or an increase in CAD of 20% or less in response to the maximum dose of acetylcholine.3
Figure 1.
Coronary blood flow image derived from the Doppler guidewire in left anterior descending coronary artery. The schematic on the right panel shows the component of the curve, which is used to calculate acceleration of peak velocity (ACC).
Statistical analysis
Values are expressed as mean±SE. The χ2 test was used to compare the incidence of categorical variables, and continuous variables were compared between two groups by the unpaired Student’s t test. The paired t test was used to compare the continuous variables between baseline and after acetylcholine or nitroglycerin in each group. Statistical analysis was performed with JMP version 7.0 (SAS Institute). A value of p<0.05 was considered significant.
RESULTS
Patient characteristics
Fifty patients had normal endothelial function and 70 had abnormal endothelial function (table 1). There was a statistically significantly greater number of patients taking statins in the endothelial dysfunction group. There were no significant differences in risk factors between the groups, although the dysfunction group had a large fraction of patients with diabetes mellitus and current smokers.
Table 1.
Patient characteristics
| Normal endothelial function (n=50) | Abnormal endothelial function (n=70) | |
|---|---|---|
| Age, years | 49±2 | 49±1 |
| Male/female | 22/28 | 27/43 |
| Body mass index, kg/m2 | 28.6±0.9 | 30.0±0.8 |
| Hypertension, n (%) | 23 (46) | 32 (46) |
| Diabetes mellitus, n (%) | 2 (4) | 10 (14) |
| Dyslipidaemia, n (%) | 29 (58) | 36 (51) |
| Current smoker, n (%) | 5 (10) | 10 (14) |
| Calcium blocker, n (%) | 12 (24) | 24 (34) |
| Nitrate, n (%) | 18 (36) | 32 (46) |
| Statin use, n (%) | 12 (24) | 29 (41)* |
| LDL-cholesterol, mg/dl | 106±5 | 105±4 |
| HDL-cholesterol, mg/dl | 49±2 | 48±2 |
| Triglycerides, mg/dl | 126±11 | 145±12 |
Values are mean±SE.
p<0.05 versus normal endothelial function.
HDL, high density lipoprotein; LDL, low density lipoprotein.
Effects of intracoronary nitroglycerin
The effects of intracoronary nitroglycerin on systemic and coronary artery haemodynamics in all patients are shown in table 2. Intracoronary nitroglycerin resulted in a significant increase in heart rate (p<0.01) and a decrease in mean arterial pressure (p<0.01). The coronary cross-sectional area had significantly increased (p<0.01), but coronary resistance and blood flow did not change significantly. The administration of nitro-glycerin resulted in an increase in DSVR (p<0.01) and ACC (p<0.01) and in a decrease in WSR (p<0.01) (table 2, figure 2).
Table 2.
Systemic haemodynamics and CBF in response to intracoronary nitroglycerin
| Baseline | After nitroglycerin | |
|---|---|---|
| Heart rate, bpm | 72±1 | 77±1† |
| Mean arterial pressure, mm Hg | 97±2 | 93±1† |
| Cross-sectional area, mm2 | 4.3±0.2 | 5.2±0.2† |
| CBF, ml/min | 53.7±3.0 | 57.3±3.3 |
| Coronary resistance, mm Hg/ml/min | 2.37±0.12 | 2.38±0.15 |
| CFR | 3.1±0.1 | 3.8±0.1† |
| APV, cm/s | 26.2±1.1 | 23.4±1.2* |
| ACC, m/s2 | 2.3±0.2 | 3.4±0.3† |
| DSVR | 2.15±0.08 | 5.36±0.61† |
| WSR, 1/s | 510±27 | 409±27† |
All values are mean±SE.
p<0.05 versus baseline.
p<0.01 versus baseline.
ACC, acceleration of peak velocity; APV, average peak velocity; CBF, coronary blood flow; CFR, coronary flow reserve; DSVR, diastolic to systolic velocity ratio; WSR, wall shear rate.
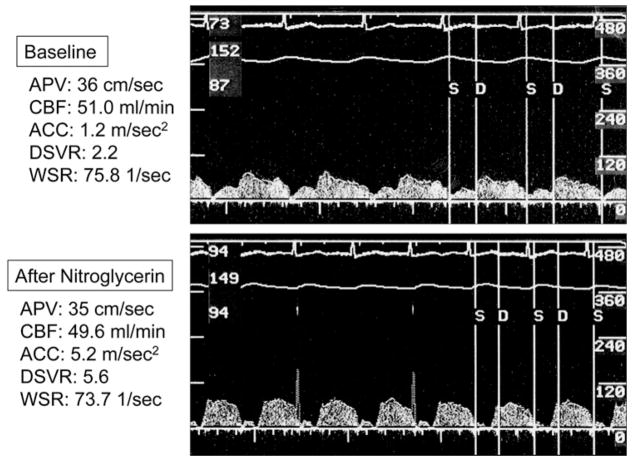
Figure 2.
Changes in coronary flow pattern after intracoronary nitroglycerin. Images of coronary flow obtained at baseline (top panel) and after nitroglycerin (bottom panel). Coronary blood flow (CBF) did not change after nitroglycerin, but acceleration of peak velocity (ACC) and the diastolic to systolic velocity ratio (DSVR) increased. APV, average peak velocity; WSR, wall shear rate.
Relationship between coronary endothelial dysfunction and vascular compliance
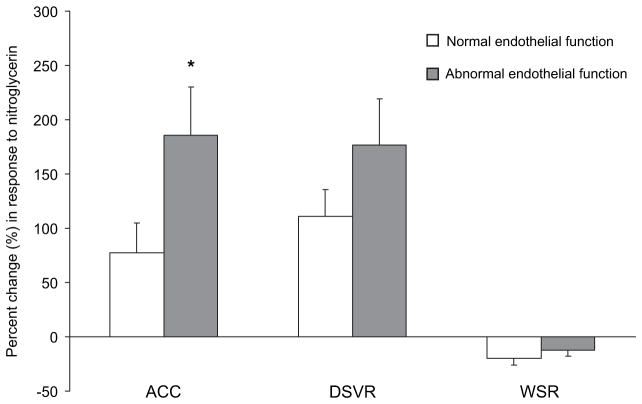
The largest molar concentration of acetylcholine (10−4 mol/l) led to a significant increase in CBF in both groups (table 3). However, CBF in the normal endothelial function group was approximately twice as large as that in the abnormal function group (p<0.01). The cross-sectional area significantly decreased in both groups, but coronary resistance decreased only in the normal endothelial function group. ACC was not significantly changed in both groups. When compared with patients with normal endothelial function in response to intracoronary nitroglycerin, patients with abnormal function had significantly higher DSVR and WSR at baseline and after nitroglycerin (p<0.05 for all) (table 4). Heart rate, mean arterial pressure, cross-sectional area, CFR, DSVR and WSR showed the same response to the administration of nitroglycerin in both groups. ACC showed no significant change in patients with normal function (p=0.206), but increased significantly in the abnormal function group (p<0.01). Consequently, ACC was significantly higher in patients with abnormal function after nitroglycerin. Furthermore, there was a significant difference in the percentage change only in ACC (figure 3).
Table 3.
Responses to acetylcholine in patients with normal or abnormal endothelial function
| Endothelial function | ||||
|---|---|---|---|---|
|
| ||||
| Baseline | After acetylcholine | |||
|
|
|
|||
| Normal | Abnormal | Normal | Abnormal | |
| Heart rate, bpm | 71±2 | 72±2 | 69±2 | 70±2 |
| Mean arterial pressure, mm Hg | 100±2 | 95±2 | 99±2 | 98±2 |
| Cross-sectional area, mm2 | 4.6±0.3 | 4.0±0.3 | 4.2±0.3* | 2.5±0.2† § |
| CBF, ml/min | 56.2±4.9 | 52.0±3.9 | 123.8±11.4† | 65.3±8.3* § |
| Coronary resistance, mm Hg/ml/min | 2.29±0.17 | 2.41±0.17 | 1.08±0.08† | 2.83±0.29§ |
| CFR | 3.0±0.1 | 3.1±0.1 | ||
| APV, cm/s | 24.2±1.0 | 27.6±1.4 | 59.0±2.8† | 55.0±3.9† |
| ACC, m/s2 | 2.3±0.3 | 2.4±0.2 | 2.1±0.3 | 2.1±0.3 |
| DSVR | 1.96±0.07 | 2.29±0.12‡ | 1.72±0.08† | 1.71±0.06† |
| WSR, 1/s | 440±26 | 559±41‡ | 1130±73† | 1703±169† § |
All values are mean±SE.
p<0.05 versus baseline.
p<0.01 versus baseline.
p<0.05 versus normal endothelial function.
p<0.01 versus normal endothelial function.
ACC, acceleration of peak velocity; APV, average peak velocity; CBF, coronary blood flow; CFR, coronary flow reserve; DSVR, diastolic to systolic velocity ratio; WSR, wall shear rate.
Table 4.
Effects of intracoronary nitroglycerin on coronary haemodynamics in patients with normal or abnormal endothelial function
| Endothelial function | ||||
|---|---|---|---|---|
|
| ||||
| Baseline | After nitroglycerin | |||
|
|
|
|||
| Normal | Abnormal | Normal | Abnormal | |
| Heart rate, bpm | 71±2 | 72±2 | 75±2† | 78±2† |
| Mean arterial pressure, mm Hg | 100±2 | 95±2 | 95±2† | 91±2 |
| Cross-sectional area, mm2 | 4.6±0.3 | 4.0±0.3 | 5.7±0.3† | 4.9±0.3† |
| CBF, ml/min | 56.2±4.9 | 52.0±3.9 | 59.4±5.1 | 55.8±4.3 |
| Coronary resistance, mm Hg/ml/min | 2.29±0.17 | 2.41±0.17 | 2.22±0.17 | 2.52±0.24 |
| CFR | 3.0±0.1 | 3.1±0.1 | 3.9±0.2† | 3.7±0.2† |
| APV, cm/s | 24.2±1.0 | 27.6±1.4 | 21.3±1.5 | 24.8±1.7 |
| ACC, m/s2 | 2.3±0.3 | 2.4±0.2 | 2.8±0.4 | 3.9±0.4† ‡ |
| DSVR | 1.96±0.07 | 2.29±0.12‡ | 4.05±0.45† | 6.27±0.97† ‡ |
| WSR, 1/s | 440±26 | 559±41‡ | 339±29† | 457±41* ‡ |
All values are mean±SE.
p<0.05 versus baseline.
p<0.01 versus baseline.
p<0.05 versus normal endothelial function.
ACC, acceleration of peak velocity; APV, average peak velocity; CBF, coronary blood flow; CFR, coronary flow reserve; DSVR, diastolic to systolic velocity ratio; WSR, wall shear rate.
Figure 3.
Change in coronary haemodynamics parameters (%) in response to intracoronary nitroglycerin in patients with normal (white bars) and abnormal (grey bars) endothelial function. *p<0.05 versus normal endothelial function. ACC, acceleration of peak velocity; DSVR, diastolic to systolic velocity ratio; WSR, wall shear rate.
DISCUSSION
The current study demonstrates for the first time that early coronary atherosclerosis characterised by endothelial dysfunction is associated with a decrease in coronary artery compliance and an increase in coronary shear stress. These observations may be consistent with an interaction between the coronary endothelium and the mechanical properties of coronary artery wall and shear stress in humans. In addition, the current study demonstrates that intracoronary nitroglycerin does not significantly affect CBF or coronary vascular resistance, but alters the phasic CBF.
Association between coronary endothelial dysfunction and artery compliance
Previous studies have demonstrated that increased vascular stiffness in the large arteries is associated with cardiovascular events4, 5 and with endothelial dysfunction.6–8 Furthermore, Wilkinson et al16 have shown that an infusion of acetylcholine significantly reduced iliac pulse wave velocity, and that an infusion of NG-monomethyl-L-arginine, which inhibits nitric oxide synthase, restrained the effect of acetylcholine on the reduction in iliac pulse wave velocity. These results suggest that endothelium-derived nitric oxide contributes to the regulation of arterial stiffness.16, 17 However, few studies have addressed the relationship between coronary endothelial function and the mechanical properties of the coronary vascular wall, such as stiffness. ACC may serve as a marker or parameter for the assessment of coronary artery stiffness, and ACC in the normal function group was equal to that in the abnormal function group after the administration of acetylcholine. However, the cross-sectional area decreased in both groups and vasomotor tone contributed to coronary artery compliance. Therefore, ACC after acetylcholine might not reflect the elastic property of the coronary artery wall. ACC is determined by three major factors: the pressure gradient driving blood flow; coronary vascular resistance and coronary artery compliance.10 As both groups of patients in our study had similar mean arterial pressures, CBF and coronary vascular resistance after intracoronary nitroglycerin, the major difference in ACC between the two groups was most likely due to the difference in coronary artery compliance, which may reflect endogenous vascular elastic properties. This difference became significant after the administration of intracoronary nitroglycerin, which mediates the endothelium-independent relaxation of vascular smooth muscle. This relaxation eliminates the component of vasomotor tone and allows the assessment of coronary artery wall stiffness. A less compliant artery is stiffer and the pressure wave is propagated faster,18 resulting in an increase in ACC. The increase in vascular stiffness leads to a decrease in the capacitance of arteries and attenuates the ability to transduce the pulsatile blood flow derived from the heart to a steady flow propagating during diastole. In coronary arteries, impairment of coronary compliance may result in diminished systolic CBF. This mechanism might explain the significant difference in DSVR between the groups. Our results suggest that early coronary atherosclerosis characterised by endothelial dysfunction is associated with a reduction in coronary artery compliance. This finding extends previous studies, which demonstrated the association between endothelial dysfunction and the compliance of larger, non-coronary arterial vessels.6–8 To our knowledge, this is the first study to demonstrate the relationship between early atherosclerosis detected by endothelial dysfunction and coronary artery compliance.
Local haemodynamic forces, such as shear stress, produce nitric oxide, which is recognised as an anti-atherogenic factor19 and influences endothelial homeostasis.1 Furthermore, previous studies have shown that a localised decrease in shear stress promotes the production of atherogenesis factors, while physiological levels of shear stress are atheroprotective.20 In this study, WSR was greater in the abnormal endothelial function group than in the normal group. In early atherosclerosis, increased shear stress may lead to positive remodelling without a marked increase in plaque.20 As atherosclerosis continues to progress, increased shear stress may be associated with an accumulation of macrophages, which facilitate plaque rupture.21 Furthermore, a recent study demonstrated that in advanced plaque, an accumulation of oxidised low-density lipoprotein was observed at the upstream of plaque where increased shear stress prevailed.22 Two mechanisms may explain the significant difference in WSR between the two groups. One explanation is that increased shear stress may lead to an increase in oxidised low-density lipoprotein even in early atherosclerosis, and oxidised low-density lipoprotein may impair nitric oxide synthase.23 The other explanation is that a long-term increase in shear stress may induce the downregulation of acetylcholine receptors and reduce endothelial nitric oxide synthase protein expression,24 thereby impairing the response to acetylcholine administration in the abnormal function group.
Pharmacological interventions in arterial stiffness and wall shear stress
Previous studies have demonstrated that several drugs, such as statins,25 ACE inhibitors or angiotensin receptor blockers,26 and peroxisome proliferator-activated receptor agonists,27 may reduce non-coronary arterial stiffness in some clinical settings. In some studies, the improvement in arterial stiffness was demonstrated after short-term medication.25, 26 It is unlikely that structural changes occurred. Therefore, it may be speculated that the improvement in endothelial dysfunction contributes to the reduction in vascular stiffness. The effects of these drugs on coronary artery compliance is unknown; however, these drugs improve endothelial dysfunction. Furthermore, we have demonstrated the relationship between coronary endothelial dysfunction and coronary artery compliance. These drugs may thus also have the ability to improve coronary artery compliance. On the other hand, the effects of these drugs on wall shear stress remain unclear, although Box et al28 have demonstrated that statins decrease wall shear stress in the carotid artery.
Effects of intracoronary nitroglycerin administration on systemic and coronary haemodynamics
Nitroglycerin is one of the most widely used agents in the treatment of cardiovascular disease, and its effects on the alleviation of symptoms are thought to be derived from two main mechanisms. One is its direct effect to increase CBF and the other indirect effect is a reduction in oxygen demand of myocardium due to a decrease in preload and afterload. In the current study, we observed that CBF did not increase significantly following intracoronary nitroglycerin, and coronary resistance was unchanged although the cross-sectional area increased significantly. Previous studies containing small numbers of subjects demonstrated that CBF and subendomyocardium flow did not significantly increase after intracoronary nitroglycerin,29, 30 underscoring our results. These results support the notion that nitroglycerin dilates conduit arteries without affecting smaller resistance-regulating arterioles.14, 31 This may be explained by the mechanism as follows: CBF and resistance are mainly regulated by the coronary microcirculation. In order for nitroglycerin to exert its effect, it has to undergo enzymatic conversion to nitric oxide. However, this enzyme is present only in the large arteries but not in the coronary microcirculation.14
Furthermore, nitroglycerin altered the phasic CBF pattern; consequently, DSVR significantly increased by increasing the percentage of blood flow that occurs during diastole in the current study. Previous studies reported that DSVR increased after intracoronary29 and intravenous nitroglycerin,32 and these were concluded by two mechanisms: a decrease in systolic flow due to myocardial contraction, allowing intracavitary systolic pressure to overcome the decreased systolic coronary pressure in response to nitroglycerin, and an increase in diastolic flow due to the reduction in vascular resistance.32 However, coronary resistance was unchanged after nitroglycerin in our study. Another plausible explanation for this change is that diastolic flow increased due to a reduction in the extravascular pressure in the subendomyocardium by the decrease in left ventricular end-diastolic pressure in response to nitroglycerin.29 In the current study, mean arterial pressure decreased significantly after nitro-glycerin. This result was compatible with previous studies showing significant decreases in mean arterial pressure30, 32 and double product after nitroglycerin administration.32 It may thus be speculated that nitroglycerin relieves ischaemic chest pain not by increasing CBF, but by decreasing oxygen expenditure of the myocardium, and by preferentially shifting blood flow to diastole.
Clinical implications
The current study focused on patients with early coronary atherosclerosis characterised by endothelial dysfunction and no significant coronary artery stenosis. Our findings suggest that this early phase atherosclerosis may already result in a decrease in coronary artery compliance and an increase in wall shear stress. In this setting, patients may be more likely to impair coronary circulation and develop structural changes. The ability to detect these changes in coronary physiology using the methods employed in this study may thus allow the development of preventive measures or intervention with medical therapy before progression to advanced atherosclerosis.
Study limitations
The current study has several limitations. First, we indirectly assessed coronary artery compliance using the Doppler guide-wire-derived ACC of blood flow, which is related to coronary artery compliance but is also influenced by coronary vascular resistance and perfusion pressure. As these two factors were similar in both groups, we attributed the difference in ACC to changes in coronary artery compliance. Second, in the study WSR was calculated using an equation that is directly proportional to APV and inversely proportional to CAD, not by direct measurement.15 The abnormal function group had a slightly smaller CAD and higher APV than the normal group, although these differences did not reach statistical significance. Consequently, a significant difference might occur in WSR between the two groups. Third, subjects consisted of patients who had angiographically epicardial coronary diameter stenosis less than 30% as early atherosclerosis in the current study. However, we did not evaluate the lesion using intravascular ultrasound. Therefore, subjects might include some patients with more advanced coronary atherosclerosis. Fourth, there is a slight difference in baseline characteristics between the two groups. Patients with abnormal endothelial function were more likely to use statins. However, statins in fact improve endothelial function and vascular stiffness and probably did not influence the results of the current study. Finally, in the current study we used one dose of nitroglycerin based on previous in-vitro studies,14 clinical practice and to prevent any systemic homodynamic effects. Therefore, we can not rule out that in some cases we did not achieve maximal epicardial vasodilation.
CONCLUSION
We have demonstrated that coronary endothelial dysfunction is associated with a decrease in coronary artery compliance and an increase in coronary artery shear stress. The results of our study may thus support a potential role for the coronary endothelium in the regulation of coronary vascular properties and shear stress. Furthermore, we observed that nitroglycerin did not significantly contribute to the increase in CBF and the decrease in coronary vascular resistance.
Acknowledgments
Funding: This work was supported by National Institutes of Health grants nos HL-03621 and HL-77131, Radi Medical Systems and the Mayo Foundation.
Footnotes
Competing interests: None.
Ethics approval The Mayo Clinic Institutional Review Board approved the current study.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–75. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 2.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 3.Suwaidi JA, Hamasaki S, Higano ST, et al. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–54. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 4.Mattace-Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–63. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 5.Willum-Hansen T, Staessen JA, Torp-Pedersen C, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–70. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 6.McEniery CM, Wallace S, Mackenzie IS, et al. Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension. 2006;48:602–8. doi: 10.1161/01.HYP.0000239206.64270.5f. [DOI] [PubMed] [Google Scholar]
- 7.Nigam A, Mitchell GF, Lambert J, et al. Relation between conduit vessel stiffness (assessed by tonometry) and endothelial function (assessed by flow-mediated dilatation) in patients with and without coronary heart disease. Am J Cardiol. 2003;92:395–9. doi: 10.1016/s0002-9149(03)00656-8. [DOI] [PubMed] [Google Scholar]
- 8.Tounian P, Aggoun Y, Dubern B, et al. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet. 2001;358:1400–4. doi: 10.1016/S0140-6736(01)06525-4. [DOI] [PubMed] [Google Scholar]
- 9.Saito M, Okayama H, Nishimura K, et al. Possible link between large artery stiffness and coronary flow velocity reserve. Heart. 2008;94:e20. doi: 10.1136/hrt.2007.126128. [DOI] [PubMed] [Google Scholar]
- 10.Cohn JN, Finkelstein S, McVeigh G, et al. Noninvasive pulse wave analysis for the early detection of vascular disease. Hypertension. 1995;26:503–8. doi: 10.1161/01.hyp.26.3.503. [DOI] [PubMed] [Google Scholar]
- 11.Bank AJ, Wang H, Holte JE, et al. Contribution of collagen, elastin, and smooth muscle to in vivo human brachial artery wall stress and elastic modulus. Circulation. 1996;94:3263–70. doi: 10.1161/01.cir.94.12.3263. [DOI] [PubMed] [Google Scholar]
- 12.Yang EH, McConnell JP, Lennon RJ, et al. Lipoprotein-associated phospholipase A2 is an independent marker for coronary endothelial dysfunction in humans. Arterioscler Thromb Vasc Biol. 2006;26:106–11. doi: 10.1161/01.ATV.0000191655.87296.ab. [DOI] [PubMed] [Google Scholar]
- 13.Lavi S, Prasad A, Yang EH, et al. Smoking is associated with epicardial coronary endothelial dysfunction and elevated white blood cell count in patients with chest pain and early coronary artery disease. Circulation. 2007;115:2621–7. doi: 10.1161/CIRCULATIONAHA.106.641654. [DOI] [PubMed] [Google Scholar]
- 14.Sellke FW, Myers PR, Bates JN, et al. Influence of vessel size on the sensitivity of porcine coronary microvessels to nitroglycerin. Am J Physiol. 1990;258:H515–20. doi: 10.1152/ajpheart.1990.258.2.H515. [DOI] [PubMed] [Google Scholar]
- 15.Reneman RS, Arts T, Hoeks AP. Wall shear stress – an important determinant of endothelial cell function and structure – in the arterial system in vivo. Discrepancies with theory. J Vasc Res. 2006;43:251–69. doi: 10.1159/000091648. [DOI] [PubMed] [Google Scholar]
- 16.Wilkinson IB, Qasem A, McEniery CM, et al. Nitric oxide regulates local arterial distensibility in vivo. Circulation. 2002;105:213–17. doi: 10.1161/hc0202.101970. [DOI] [PubMed] [Google Scholar]
- 17.Schmitt M, Avolio A, Qasem A, et al. Basal NO locally modulates human iliac artery function in vivo. Hypertension. 2005;46:227–31. doi: 10.1161/01.HYP.0000164581.39811.bd. [DOI] [PubMed] [Google Scholar]
- 18.Glasser SP, Arnett DK, McVeigh GE, et al. Vascular compliance and cardiovascular disease: a risk factor or a marker? Am J Hypertens. 1997;10:1175–89. doi: 10.1016/s0895-7061(97)00311-7. [DOI] [PubMed] [Google Scholar]
- 19.Ziegler T, Silacci P, Harrison VJ, et al. Nitric oxide synthase expression in endothelial cells exposed to mechanical forces. Hypertension. 1998;32:351–5. doi: 10.1161/01.hyp.32.2.351. [DOI] [PubMed] [Google Scholar]
- 20.Stone PH, Coskun AU, Kinlay S, et al. Effect of endothelial shear stress on the progression of coronary artery disease, vascular remodeling, and in-stent restenosis in humans: in vivo 6-month follow-up study. Circulation. 2003;108:438–44. doi: 10.1161/01.CIR.0000080882.35274.AD. [DOI] [PubMed] [Google Scholar]
- 21.Dirksen MT, van der Wal AC, van den Berg FM, et al. Distribution of inflammatory cells in atherosclerotic plaques relates to the direction of flow. Circulation. 1998;98:2000–3. doi: 10.1161/01.cir.98.19.2000. [DOI] [PubMed] [Google Scholar]
- 22.Segers D, Helderman F, Cheng C, et al. Gelatinolytic activity in atherosclerotic plaques is highly localized and is associated with both macrophages and smooth muscle cells in vivo. Circulation. 2007;115:609–16. doi: 10.1161/CIRCULATIONAHA.106.636415. [DOI] [PubMed] [Google Scholar]
- 23.Hein TW, Liao JC, Kuo L. oxLDL specifically impairs endothelium-dependent, NO-mediated dilation of coronary arterioles. Am J Physiol Heart Circ Physiol. 2000;278:H175–83. doi: 10.1152/ajpheart.2000.278.1.H175. [DOI] [PubMed] [Google Scholar]
- 24.Vaziri ND, Wang XQ. cGMP-mediated negative-feedback regulation of endothelial nitric oxide synthase expression by nitric oxide. Hypertension. 1999;34:1237–41. doi: 10.1161/01.hyp.34.6.1237. [DOI] [PubMed] [Google Scholar]
- 25.Maki-Petaja KM, Booth AD, Hall FC, et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol. 2007;50:852–8. doi: 10.1016/j.jacc.2007.04.076. [DOI] [PubMed] [Google Scholar]
- 26.Mahmud A, Feely J. Reduction in arterial stiffness with angiotensin II antagonist is comparable with and additive to ACE inhibition. Am J Hypertens. 2002;15:321–5. doi: 10.1016/s0895-7061(01)02313-5. [DOI] [PubMed] [Google Scholar]
- 27.Ryan KE, McCance DR, Powell L, et al. Fenofibrate and pioglitazone improve endothelial function and reduce arterial stiffness in obese glucose tolerant men. Atherosclerosis. 2007;194:e123–30. doi: 10.1016/j.atherosclerosis.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Box FM, van der Grond J, de Craen AJ, et al. Pravastatin decreases wall shear stress and blood velocity in the internal carotid artery without affecting flow volume: results from the PROSPER MRI study. Stroke. 2007;38:1374–6. doi: 10.1161/01.STR.0000260206.56774.aa. [DOI] [PubMed] [Google Scholar]
- 29.Goto M, Flynn AE, Doucette JW, et al. Effect of intracoronary nitroglycerin administration on phasic pattern and transmural distribution of flow during coronary artery stenosis. Circulation. 1992;85:2296–304. doi: 10.1161/01.cir.85.6.2296. [DOI] [PubMed] [Google Scholar]
- 30.Voudris V, Avramides D, Gatzov P, et al. The effect of rapid decreases of blood pressure by different mechanisms on coronary flow and flow reserve in normal coronary arteries. Am J Hypertens. 2003;16:1000–5. doi: 10.1016/j.amjhyper.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 31.Macho P, Vatner SF. Effects of nitroglycerin and nitroprusside on large and small coronary vessels in conscious dogs. Circulation. 1981;64:1101–7. doi: 10.1161/01.cir.64.6.1101. [DOI] [PubMed] [Google Scholar]
- 32.Kawabata T, Fujii T, Hiro T, et al. Vasodilator responses of coronary conduit and resistance arteries to continuous nitroglycerin infusion in humans: a Doppler guide wire study. J Cardiovasc Pharmacol. 2000;36:764–9. doi: 10.1097/00005344-200012000-00012. [DOI] [PubMed] [Google Scholar]