Abstract
Parkinson's disease (PD) is a neurodegenerative disorder characterized by progressive motor disturbances and affects more than 1% of the worldwide population. Despite considerable progress in understanding PD pathophysiology, including genetic and biochemical causes, diagnostic approaches lack accuracy and interventions are restricted to symptomatic treatments. PD is a complex syndrome with different clinical subtypes and a wide variability in disorder course. In order to deliver better clinical management of PD patients and discovery of novel therapies, there is an urgent need to find sensitive, specific, and reliable biomarkers. The development of biomarkers will not only help the scientific community to identify populations at risk, but also facilitate clinical diagnosis. Furthermore, these tools could monitor progression, which could ultimately deliver personalized therapeutic strategies. The field of biomarker discovery in PD has attracted significant attention and there have been numerous contributions in recent years. Although none of the parameters have been validated for clinical practice, some candidates hold promise. This review summarizes recent advances in the development of PD biomarkers and discusses new strategies for their utilization.
Keywords: Parkinson's disease, Biochemical biomarkers, Imaging, Diagnosis, Progression
1. Introduction
The National Institutes of Health (NIH) defines a biomarker as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes or pharmacological responses to a therapeutic intervention” (2001). In other words, a biomarker is a tool to aid physicians, epidemiologists, and scientists in the study of human diseases by confirming a diagnosis and tracking disease progression, and may help to identify specific therapeutic targets or determine the efficacy of agents designed to influence disease progression. In general biomarkers involve measurements of biological samples (e.g., plasma, serum, cerebrospinal fluid (CSF) and biopsy) or measurements using brain imaging techniques to decipher changes in brain structure and function.
Parkinson's disease (PD) is the second most common neurodegenerative syndrome after Alzheimer's disease in the United States. It affects about 1 million Americans over age 65 and up to 10 million individuals worldwide, yet no cure exists and no disease-modifying therapies have been identified. With its prevalence expected to double within the next two decades due to the increasing age of the general population, PD is emerging as a socio-economic burden and a serious challenge for the public health system. The development of specific biomarkers that reflect disease progression and the discovery of new therapies are important for better clinical management of PD patients. As such, the establishment of reliable biomarkers is a subject of intensive investigation; the ideal biomarker being sensitive, reproducible, inexpensive, noninvasive and thoroughly validated.
2. Biomarkers in PD: from better clinical diagnosis to new therapeutics
PD is a progressive disease characterized by a complex motor disorder known as parkinsonism which is manifested by resting tremor, bradykinesia, rigidity and postural abnormalities. Additionally, many non-motor features are increasingly recognized as being integral components of the syndrome. The neuropathological hallmarks of PD are a loss of dopaminergic neurons in the substantia nigra (SN) and the formation of intraneuronal protein inclusions termed Lewy bodies (LB), which are composed primarily of alpha-synuclein (a-syn) [1]. Currently a clinical diagnosis of PD is made by the presence of motor disturbances, which remains the most important diagnostic marker. Unfortunately, due to overlap of symptoms with other neurodegenerative disorders such as multiple system atrophy and progressive supranuclear palsy, misdiagnosis is common and only autopsy can definitively confirm the disease. Moreover, motor deficits generally appear when a relatively advanced stage of neurodegeneration is present; indeed 50–60% of dopaminergic neurons in the SN are already lost prior to the clinical diagnosis, limiting the effectiveness of potential neuroprotective therapies [2]. It has become clear that PD is not just a movement disorder, but rather a complex syndrome non-motor symptoms (NMS) including olfactory deficit, sleep abnormalities, depression, autonomic dysfunction and cognitive disturbances. Indeed, more than 90% of patients experience NMS during the course of the disease [3]. Many of these NMS appear years before motor symptoms, and include gastrointestinal dysfunction, anosmia, and rapid eye movement (REM) sleep disturbances. NMS often become debilitating as the disease progresses, worsening the quality of life for PD patients. Additionally, neuropathological changes in the form of aggregated a-syn protein can be detected in peripheral tissues including skin, the olfactory bulb, and gastrointestinal tract, and predate the onset of motor symptoms [4]. At this early stage of the disease dopaminergic neurons are relatively spared. Consequently NMS and peripheral pathology might provide a window of opportunity for early neuroprotective intervention.
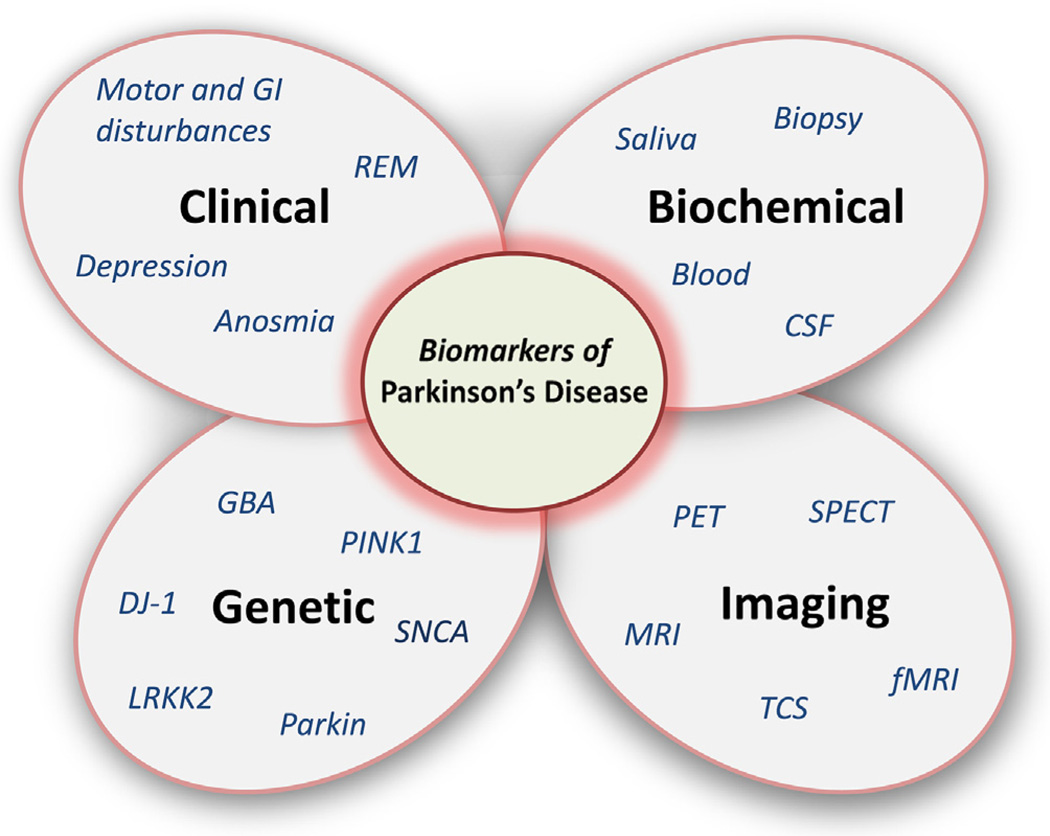
Although no specific biomarkers can be recommended in clinical practice yet, some interesting candidates exist. As mentioned earlier, PD is a complex disorder with different clinical subtypes and no clinical or pathological “gold standard” biomarker. Indeed, in the initial family in which the LRRK2 gene was discovered, all affected individuals manifested a PD phenotype. However, in the four affected individuals from this family in whom brain autopsies had already been performed, surprisingly there were four different neuropathological diagnoses described [5]. Therefore, it is unrealistic to expect that a single biomarker will fulfill all the criteria of accurate diagnosis and disease progression. Indeed, using a combination of biomarkers is the most likely rational approach (Fig. 1).
Fig. 1.
Biomarkers of Parkinson's disease: A single biomarker cannot reflect the complexity of the disorder. Clinical, laboratory, imaging, and genetic data need to be judiciously combined to accurately predict disease status and progression.
Biomarkers of PD are diverse and can be categorized into four main subgroups: clinical, biochemical, genetic, and imaging. When one group is considered alone the utility of the biomarker is often limited, but when combined and considered collectively, biomarkers for PD may be more useful. Also, multimodal assessments may be pivotal for reliable measures of progression and determination of disorder modification. In the following sections, a brief summary of existing molecular and imaging biomarkers is presented along with discussion concerning novel approaches driving the field forward.
2.1. Genetic biomarkers
The etiology of PD is not entirely understood with 90–95% of cases being idiopathic, resulting from complex interactions between genes and environmental factors. Nevertheless a clear genetic component has been identified in the last 15 years. Genetic mutations leading to familial forms of PD combine a-syn (SNCA), Parkin, PTEN-induced kinase 1 (PINK1), DJ-1, and Leucine-rich repeat kinase 2 (LRRK2), and account for 2–3% of all cases with classical parkinsonism, which is often clinically indistinguishable from idiopathic PD [6]. These discoveries were followed by population-based genome-wide association studies (GWAS) that identified an additional 20 PD risk loci. Houlden and Singleton [7] recently summarized the genetic, clinical, and pathological findings of autosomal dominant and recessive mutations and discussed the discovery of genetic risk loci for PD with an emphasis on LRRK2 and GBA (glucocerebrosidase). Indeed 5–10% of PD patients have GBA mutations, numerically making this the most important risk factor for the disease [8]. Even though this might represent a small percentage of PD cases, genetic studies are invaluable to elucidate the pathways contributing to clinical diagnosis and most importantly to identify populations at risk. Genetic causes of parkinsonism may reside in individuals for years and often decades prior to any development of symptoms, as such “gene positive at-risk” individuals comprise a unique population in which to study biomarker efficacy in reflecting disorder progression from asymptomatic to end-stage disease. In this regard, detection of multiple misregulated genes and their messenger RNA or protein expression may allow the identification of networks of interacting proteins that reflect the underlying disease process. Further, proteins associated with disease pathophysiology can be identified as candidate biomarkers.
2.2. Biochemical markers
Searching for biomarkers in body fluids and tissues provides a relatively noninvasive examination of proteins levels and other molecules specific to the disease. Investigators have evaluated various potential biomarkers in blood, saliva, cerebrospinal fluid (CSF) and biopsies. Because molecular changes in the brain are often reflected in CSF, it represents a potential source of biomarkers. The identification of proteins associated with PD has been informed by advances in genetics (Section 2.1), as witnessed in the discovery of SNCA or DJ-1 genes.
a-Syn was the first gene to be linked to PD. A direct link between a-syn and PD is strongly supported by the discovery that point mutations or multiplications of the gene cause parkinsonism. a-Syn can be detected in PD patient's CSF, saliva, serum, urine, and also in the gastrointestinal tract [9]. Seemingly discrepant findings have revealed that PD patients have significantly lower a-syn levels in CSF than control groups [10] and no association between a-syn levels and disease severity has been found. The reproducibility of the data remains challenging with the main issues being sampling protocols, blood contamination and different operating procedures. Several studies have also attempted to measure a-syn in blood cells and plasma, but so far the results have been inconsistent. Lastly, recent data have drawn attention to the presence a-syn in the gut of PD patients after gastroscopy [11]. This interesting observation requires confirmation in a larger cohort study, but it raises the possibility of in vivo a-syn histopathology improving the accuracy of PD diagnosis.
Mutations in the gene encoding DJ-1 are a rare cause of autosomal recessive PD. DJ-1 is a protein involved in many cellular functions including response to oxidative stress and is present in CSF. Elevated levels can been observed in CSF of PD patients; however conflicting data demonstrate decreased levels of DJ-1 in a large cohort of CSF samples [12]. Much like a-syn, the difference between investigations is attributable to many issues and future investigations with larger and longitudinally characterized cohorts are needed to clarify a potential correlation and the possible use of these markers. In addition to a-syn and DJ-1, other potential protein markers have been linked to an elevated or decreased risk of developing PD. Apo A1, a major component of high-density lipoprotein, appears to be reduced in plasma of PD patients, as urate, an antioxidant. Other key proteins involved in PD pathogenesis such as parkin and ubiquitin could also be investigated as potential markers.
2.3. Neuroimaging markers for PD
Several neuroimaging techniques capable of generating higher resolution data have been developed and their use has been increasingly employed to support the clinical diagnosis of PD [13]. Single photon emission tomography (SPECT), positron emission tomography (PET), magnetic resonance imaging (MRI) and transcranial sonography (TCS) allow non–invasive tracking of molecular targets of relevance to neurodegeneration. MRI and TCS can monitor structural changes in the brain that may suggest increased risk for PD, while PET and SPECT in conjunction with radioactive metabolic tracers assess function, and may support the diagnosis as well as monitor disease severity and progression. PD is associated with nigral degeneration and striatal dopamine deficiency, therefore a gold-standard biomarker is neuroimaging of the dopamine system. Three classes of radiotracers are commonly used: 1) 6-[18F] fluorodopa which primarily reflects decarboxylase activity and storage, 2) [11C]dihydrotetrabenazine (DTBZ) which reflects vesicular monoamine transporter type 2 (VMAT2), and 3), 2-beta-[11C]carbomethoxy-3-beta-4-fluorophenyltropane (CFT) reflecting membranous dopamine transporter (DAT). It is well established that a progressive reduction in binding of the ligands is observed in PD patients, reflecting neurodegeneration. PET and SPECT studies may be abnormal before any symptoms or motor signs appear and they progress as neurodegeneration continues [14]. Dopamine-based neuroimaging techniques can be selectively combined with clinical assessments to increase the certainty of a PD diagnosis. MRI is more widely available than PET or SPECT and has provided enticing clinical applications to differentiate idiopathic PD from secondary causes of parkinsonism. In a recent study using MRI techniques to measure brain iron levels, the authors observed an accumulation in the SN and caudal putamen of patients with PD evolving over a three-year period, emphasizing its potential as a biomarker of disease progression [15]. Another MRI technique, voxel-based morphometry, can reveal volume differences and changes in gray matter density related to disease [16]. This presumably might function as a surrogate of neuronal numbers. It has been shown to have some potential applications for the differential diagnosis of parkinsonian syndromes. Indeed patterns of brain atrophy in patients with and without dementia could be detected with this measurement [17]. Lastly, TCS reveals hyperechogenicity of SN in 90% of PD patients but does not appear to correlate well with disease severity or change with disease progression [18].
The use of new markers to assess the non-dopaminergic brain pathways in the pathology of both motor and non-motor symptoms in PD is also instructive. Loss of cardiac sympathetic innervation can be detected in PD and decreased uptake of the sympathetic marker I-123-metaiodobenzylguanidine (MIBG) was reported in cardiac SPECT [19]. Moreover this marker contributes to the differential diagnosis between PD and other forms of parkinsonism such as multiple system atrophy [20]. Interestingly, amyloid deposition assessed by PET imaging with the Pittsburgh Compound B (PiB), can distinguish PD patients with and without dementia. Also, neuroinflammation markers of activated microglia such as 11C-PK11195 PET have been tested with varying success [21]. Refinement of the analysis and further development of better tracers is necessary to enable accurate measurement of neuroinflammation [22]. Lastly, an imaging tracer that detects pathological accumulation of a-syn could be valuable for diagnostic accuracy and might improve our understanding of disease progression. Recently, Bagchi and colleagues [23] developed a radioligand that binds to a-syn fibrils in post-mortem PD brains. While this ligand is not currently available, such studies are paving the way for the development of novel a-syn imaging markers.
2.4. Clinical markers
The presence of clinical signs, particularly the motor features of bradykinesia, rigidity and resting tremor, is the most important diagnostic marker for PD. Bradykinesia is the physical examination finding that best correlates with nigrostriatal dopaminergic loss. Curiously, the particular anatomic neuronal circuitry damage associated with specific clinical findings, such as resting tremor, has not been identified. Why some patients initially present with resting tremor while others develop this sign later or never are unexplained. The accurate diagnosis of patients presenting with parkinsonism has plateaued in the last several decades, with evaluation after symptomatic duration of 5 years generally leading to the highest level of accuracy [24,25]. Furthermore, the detection of motor signs reflects a relatively significant stage in disease progression, as seen in functional imaging and neurochemical studies.
A number of longitudinal studies of agents thought to be potentially disease-modifying have employed clinical rating scales as the outcome measure. The DATATOP study for example, the most expensive NIH-sponsored clinical therapeutic trial at the time, used motor scores to help determine disease progression. While it was initially believed that deprenyl, the selective MAO-B inhibitor, did not have an immediate impact on clinical symptoms, the large number of patients studied led to the detection of a significant symptomatic benefit. As a consequence, while the symptomatic benefit was initially thought to potentially represent a disease modifying effect, longer follow up determined that deprenyl's symptomatic effect was the only effect that could be measured in this cohort. Because of the varying presence of many clinical signs, in conjunction with symptomatic therapies available for PD, the need to segregate symptomatic from “neuroprotective” action is a frequent challenge.
Several non-motor features including REM sleep behavioral disorder, olfactory dysfunction, depression, and bowel dysfunction often precede the classic motor features of PD, sometimes by many years. REM sleep behavior disorder in particular is associated with a higher risk for development of subsequent parkinsonism and dementia [26]. Depression, personality traits, and reduced interest in new experiences have been associated with early PD, and olfactory dysfunction is present in over 90% of patients. Smell testing is easily performed and abnormal testing is associated with greater risk for PD. Test batteries including olfaction acuity and REM sleep behavior disorder screening questionnaire have been used in several studies. These are non–invasive and low cost screening tests, but have limited specificity and sensitivity. Indeed, none of these non-motor manifestations are specific to PD pathology.
3. Towards new approaches in PD biomarker discovery
In the last decade new approaches have emerged with the application of “-omics” techniques. Proteomics, metabolomics and transcriptomics are powerful tools capable of mass analyses to identify small changes in protein, metabolites or RNA profiles in tissue or fluids from healthy and diseased individuals [27]. Those techniques are non-targeted strategies which allow identification of analytes that differ between healthy and non-healthy subjects, and also from one disease state to another. Recently, Burgos and colleagues [28] analyzed the micro RNA content in CSF from 67 PD patients and 78 controls. Their study found RNA profiles reflective of cell-based changes in pathology that could be used to assess disease progression and therapeutic efficacy. Metabolomics profiling using electrochemical coulometric array detection to search for biomarkers in plasma may also uncover potentially useful biomarkers. For example, when comparing plasma from LRRK2 mutated patients to idiopathic PD, metabolomic analysis revealed two distinct profiles [29]. Although information gained through “-omics” techniques are of potential value, issues with reproducibility of data still remain.
Despite intensive efforts and technological advances in the field of PD biomarkers, no clinical biomarker has been produced yet. There are significant issues relating to the inconsistency of results and the different detection systems used among institutions. Therefore greater attention to standardization of a given assay, as well as appropriate handling of the biosamples, should be a priority. The other limitation in the search of PD biomarkers is the heterogeneity of the disorder and the presence of shared signs and symptoms with other diseases. Furthermore, biomarkers need to take into account other stratification issues among study subjects; biomarkers may play varying roles during different stages of the disease process. And finally, a single biomarker may not be sufficient to achieve adequate sensitivity or specificity. Combining clinical or biochemical markers with imaging markers to define at-risk individuals or to identify premotor PD appears feasible. The recent work of Campbell and colleagues [30] provide an example of utilizing a combination of CSF analysis and functional MRI in 43 PD patients. The CSF a-syn level correlated with reduced sensorimotor connectivity seen in fMRI in their study.
To accelerate the research of biomarkers in this direction future effort may profit from collaborative work with standard procedures and large population-based cohort studies. In 2002 an initiative called The Parkinson's Progression Markers Initiative (PPMI), sponsored by the Michael J. Fox Foundation, and was launched. The 5-year longitudinal study involves more than 500 subjects (early PD and age-matched healthy individuals) in clinical sites from Europe, USA and Australia. Highly standardized clinical, neuroimaging, and biological data are collected and is available to the research community, making the PPMI a valuable resource for investigation, innovation, and validation of promising biomarkers.
4. Concluding remarks
The development and validation of biomarkers represents an urgent need for better clinical management of PD patients. Although much remains to be done, the search for appropriate PD biomarkers has begun to provide tools that could lead to clinical testing and have a dramatic impact upon therapeutic trial design. As discussed in this review and illustrated in Fig. 1, a single measure is unlikely to become a useful biomarker. Priority should be given to studies that allow assessment of combinations of clinical, laboratory, imaging, and genetic data, as well as seeking standardization of the assays associated with such assessments. Appropriate refinement of novel technology promises to result in the development of biomarkers that better diagnose PD and improve the efficacy of disease-modifying therapies.
References
- 1.Stefanis L. alpha-Synuclein in Parkinson's disease. Cold Spring Harb. Perspect. Med. 2012;2:a009399. doi: 10.1101/cshperspect.a009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- 3.Shulman LM, Taback RL, Bean J, Weiner WJ. Comorbidity of the nonmotor symptoms of Parkinson's disease. Mov. Disord. 2001;16:507–510. doi: 10.1002/mds.1099. [DOI] [PubMed] [Google Scholar]
- 4.Halliday GM, Del Tredici K, Braak H. Critical appraisal of brain pathology staging related to presymptomatic and symptomatic cases of sporadic Parkinson's disease. J. Neural Transm. Suppl. 2006:99–103. doi: 10.1007/978-3-211-45295-0_16. [DOI] [PubMed] [Google Scholar]
- 5.Uitti RJ, Calne DB, Dickson DW, Wszolek ZK. Is the neuropathological 'gold standard' diagnosis dead? Implications of clinicopathological findings in an autosomal dominant neurodegenerative disorder. Park. Relat. Disord. 2004;10:461–463. doi: 10.1016/j.parkreldis.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Klein C, Schlossmacher MG. Parkinson disease 10 years after its genetic revolution: multiple clues to a complex disorder. Neurology. 2007;69:2093–2104. doi: 10.1212/01.wnl.0000271880.27321.a7. [DOI] [PubMed] [Google Scholar]
- 7.Houlden H, Singleton AB. The genetics and neuropathology of Parkinson's disease. Acta Neuropathol. 2012;124:325–338. doi: 10.1007/s00401-012-1013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beavan M, McNeill A, Proukakis C, Hughes DA, Mehta A, Schapira AH. Evolution of prodromal clinical markers of Parkinson disease in a GBA mutation-positive cohort. JAMA Neurol. 2015;72:201–208. doi: 10.1001/jamaneurol.2014.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malek N, Swallow D, Grosset KA, Anichtchik O, Spillantini M, Grosset DG. Alpha-synuclein in peripheral tissues and body fluids as a biomarker for Parkinson's disease - a systematic review. Acta Neurol. Scand. 2014;130:59–72. doi: 10.1111/ane.12247. [DOI] [PubMed] [Google Scholar]
- 10.Mollenhauer B, El-Agnaf OM, Marcus K, Trenkwalder C, Schlossmacher MG. Quantification of alpha-synuclein in cerebrospinal fluid as a biomarker candidate: review of the literature and considerations for future studies. Biomark. Med. 2010;4:683–699. doi: 10.2217/bmm.10.90. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez-Ferro A, Rabano A, Catalan MJ, Rodriguez-Valcarcel FC, Fernandez Diez S, Herreros-Rodriguez J, et al. In vivo gastric detection of alpha-synuclein inclusions in Parkinson's disease. Mov. Disord. 2015;30:517–524. doi: 10.1002/mds.25988. [DOI] [PubMed] [Google Scholar]
- 12.Hong Z, Shi M, Chung KA, Quinn JF, Peskind ER, Galasko D, et al. DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson's disease. Brain. 2010;133:713–726. doi: 10.1093/brain/awq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pavese N, Brooks DJ. Imaging neurodegeneration in Parkinson's disease. Biochim. Biophys. Acta. 2009;1792:722–729. doi: 10.1016/j.bbadis.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Ravina B, Marek K, Eberly S, Oakes D, Kurlan R, Ascherio A, et al. Dopamine transporter imaging is associated with long-term outcomes in Parkinson's disease. Mov. Disord. 2012;27:1392–1397. doi: 10.1002/mds.25157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ulla M, Bonny JM, Ouchchane L, Rieu I, Claise B, Durif F. Is R2* a new MRI biomarker for the progression of Parkinson's disease? A longitudinal follow-up. PLoS One. 2013;8:e57904. doi: 10.1371/journal.pone.0057904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Summerfield C, Junque C, Tolosa E, Salgado-Pineda P, Gomez-Anson B, Marti MJ, et al. Structural brain changes in Parkinson disease with dementia: a voxel-based morphometry study. Arch. Neurol. 2005;62:281–285. doi: 10.1001/archneur.62.2.281. [DOI] [PubMed] [Google Scholar]
- 17.Beyer MK, Janvin CC, Larsen JP, Aarsland D. A magnetic resonance imaging study of patients with Parkinson's disease with mild cognitive impairment and dementia using voxel-based morphometry. J. Neurol. Neurosurg. Psychiatry. 2007;78:254–259. doi: 10.1136/jnnp.2006.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berg D, Behnke S, Seppi K, Godau J, Lerche S, Mahlknecht P, et al. Enlarged hyperechogenic substantia nigra as a risk marker for Parkinson's disease. Mov. Disord. 2013;28:216–219. doi: 10.1002/mds.25192. [DOI] [PubMed] [Google Scholar]
- 19.Taki J, Yoshita M, Yamada M, Tonami N. Significance of 123I-MIBG scintigraphy as a pathophysiological indicator in the assessment of Parkinson's disease and related disorders: it can be a specific marker for Lewy body disease. Ann. Nucl. Med. 2004;18:453–461. doi: 10.1007/BF02984560. [DOI] [PubMed] [Google Scholar]
- 20.Druschky A, Hilz MJ, Platsch G, Radespiel-Troger M, Druschky K, Kuwert T, et al. Differentiation of Parkinson's disease and multiple system atrophy in early disease stages by means of I-123-MIBG-SPECT. J. Neurol. Sci. 2000;175:3–12. doi: 10.1016/s0022-510x(00)00279-3. [DOI] [PubMed] [Google Scholar]
- 21.Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, et al. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson's disease. Neurobiol. Dis. 2006;21:404–412. doi: 10.1016/j.nbd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Bartels AL, Willemsen AT, Doorduin J, de Vries EF, Dierckx RA, Leenders KL. [11C]-PK11195 PET: quantification of neuroinflammation and a monitor of anti-inflammatory treatment in Parkinson's disease? Park. Relat. Disord. 2010;16:57–59. doi: 10.1016/j.parkreldis.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Bagchi DP, Yu L, Perlmutter JS, Xu J, Mach RH, Tu Z, et al. Binding of the radioligand SIL23 to alpha-synuclein fibrils in Parkinson disease brain tissue establishes feasibility and screening approaches for developing a Parkinson disease imaging agent. PLoS One. 2013;8:e55031. doi: 10.1371/journal.pone.0055031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajput AH, Rajput A. Accuracy of Parkinson disease diagnosis unchanged in 2 decades. Neurology. 2014;83:386–387. doi: 10.1212/WNL.0000000000000653. [DOI] [PubMed] [Google Scholar]
- 25.Adler CH, Beach TG, Hentz JG, Shill HA, Caviness JN, Driver-Dunckley E, et al. Low clinical diagnostic accuracy of early vs advanced Parkinson disease: clinicopathologic study. Neurology. 2014;83:406–412. doi: 10.1212/WNL.0000000000000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boeve BF. Idiopathic REM sleep behaviour disorder in the development of Parkinson's disease. Lancet Neurol. 2013;12:469–482. doi: 10.1016/S1474-4422(13)70054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caudle WM, Bammler TK, Lin Y, Pan S, Zhang J. Using 'omics' to define pathogenesis and biomarkers of Parkinson's disease. Expert Rev. Neurother. 2010;10:925–942. doi: 10.1586/ern.10.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burgos K, Malenica I, Metpally R, Courtright A, Rakela B, Beach T, et al. Profiles of extracellular miRNA in cerebrospinal fluid and serum from patients with Alzheimer's and Parkinson's diseases correlate with disease status and features of pathology. PLoS One. 2014;9:e94839. doi: 10.1371/journal.pone.0094839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansen KK, Wang L, Aasly JO, White LR, Matson WR, Henchcliffe C, et al. Metabolomic profiling in LRRK2-related Parkinson's disease. PLoS One. 2009;4:e7551. doi: 10.1371/journal.pone.0007551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell MC, Koller JM, Snyder AZ, Buddhala C, Kotzbauer PT, Perlmutter JS. CSF proteins and resting-state functional connectivity in Parkinson disease. Neurology. 2015;84:2413–2421. doi: 10.1212/WNL.0000000000001681. [DOI] [PMC free article] [PubMed] [Google Scholar]