Abstract
Background
The effect of the interaction between type 2 diabetes and dyslipidemia on inflammation and lipid peroxidation (LPO) has not been assessed.
Aim
To investigate whether diabetes coupled with dyslipidemia alters oxidative metabolism leading to increased LPO products and inflammatory status.
Methods
100 patients were divided into four groups based upon diabetic and dyslipidemic status: poorly controlled diabetes with dyslipidemia (DM-PC/D), well-controlled diabetes with dyslipidemia (DM-WC/D), normoglycemic individuals with dyslipidemia (NG/D), and normoglycemic individuals without dyslipidemia (NG/ND). Plasma was evaluated for an LPO product (MDA), antioxidant levels and inflammatory cytokines.
Results
Diabetics presented significantly higher levels of LPO (p < 0.05) and the DM-PC/D had higher levels of proinflammatory cytokines and MDA in the plasma in comparison with normoglycemics (p < 0.05). Interestingly IL1-β, IL-6, and TNF-α in DM-WC/D were not statistically different from those in DM-PC/D. Normoglycemic individuals with dyslipidemia presented significantly increased levels of IL-6 and TNF-α when compared to normoglycemic without dyslipidemia (p < 0.05). MDA levels were also positively correlated with the presence of DM complications (r = 0.42, p < 0.01).
Conclusions
These findings show that dyslipidemia is associated with an increased inflammatory status, even in well-controlled diabetics and in normoglycemics. Our results suggest that lipid metabolism and peroxidation are important for the development of inflammation, which is elevated in several complications associated with diabetes.
Keywords: Lipid peroxidation, Diabetes mellitus type 2, Cytokines, Dyslipidemia, Inflammation
1. Introduction
Diabetes is a group of metabolic diseases that leads to impaired wound healing (Goova et al., 2001), higher susceptibility to infections (Hirsch et al., 2008), atherosclerosis (Halliwell, 2000), micro- and macrovascular alterations (Ahmed, 2005; Mooradian, 2009; Velazquez, Winocour, Kesteven, Alberti, & Laker, 1991) even in diabetic patients with optimized glycaemic control (Chello et al., 2009). Inflammation is an underlying cause of several co-morbidities associated with poorly controlled diabetes (Mirza et al., 2012) and several studies have demonstrated high levels of circulating inflammatory cytokines such as interleukin-6 and tumor necrosis factor α (TNF-α) (Mirza et al., 2012; Pickup, Chusney, Thomas, & Burt, 2000; Rytter et al., 2009; Vinik, 2005; Wisse, 2004) in type 2 diabetic patients.
Several mechanisms can contribute to the systemic hyperinflammatory status in diabetes, including dyslipidemia that modulates the function and activity of myeloid cells, and the increase of oxidative metabolism with enhanced production of reactive oxygen species (ROS) (Millar, Phan, & Tibbles, 2007). As a defense mechanism cells produce antioxidants (AOs) that prevent or limit oxidative tissue injury (Chapple & Matthews, 2007; Stosic-Grujicic, Miljkovic, Cvetkovic, Maksimovic-Ivanic, & Trajkovic, 2004). Overproduction of ROS may result in an imbalance between ROS and AOS leading to oxidative stress and damage (Chapple & Matthews, 2007; Niedowicz & Daleke, 2005) that can contribute to injury of the host tissue by several mechanisms, including DNA damage, peroxidation of lipid membranes, oxidation of enzymes and stimulation of proinflammatory cytokines (Devaraj, Venugopal, Singh, & Jialal, 2005; Li, Huang, & Shen, 2014).
In spite of the biological rationale, there is paucity of information on the relationship between metabolic control in diabetes mellitus and the status of lipid metabolism (dyslipidemia), lipid peroxidation and antioxidant capacity as modulators of systemic inflammation in the same individual. The aim of this study is to determine whether type 2 diabetes, dyslipidemia and increased lipid peroxidation are specifically associated with aggravated systemic inflammation.
2. Materials and methods
2.1. Subjects
The present cross-sectional study, approved by the Human Research Ethics Committee of the School of Dentistry at Araraquara (UNESP–Univ. Estadual Paulista, Araraquara, Brazil; Protocol number 50/06), was conducted according to the ethical principles of the Declaration of Helsinki. Data collection was carried out between May 2009 and November 2010. All volunteers were informed about the aims and methods of this study, and they provided their written consent to participate.
Patients with type 2 diabetes mellitus (T2DM) were recruited from local outpatient diabetic clinics, regional community hospitals, and physicians' offices. Normoglycemic individuals were consecutively selected from the list of patients who voluntarily sought dental treatment at the School of Dentistry at Araraquara, Univ. Estadual Paulista (UNESP), Brazil, at the same time as the DM2 group.
A simple questionnaire was applied initially to all subjects to identify the patients that were eligible to be enrolled in the study, considering the inclusion/exclusion criteria. All study participants ranged in age from 35 to 60 years and had similar socio-economic level. None of the patients were pregnant or were current or former smokers. Patients taking antibiotic, nonsteroidal anti-inflammatory, hormones or hypolipidemic drugs such as statins or fibrates, were excluded from the study. Particular care was taken to exclude subjects with history of anemia and diseases known to influence lipoprotein metabolism, such as hypothyroidism.
A power analysis based on a pilot study determined that at least 25 patients in each group would be sufficient to detect a 3-unit difference in MDA levels or a 2.4-unit difference in an inflammatory cytokine with 90% power and 95% confidence interval. The population was divided into four groups based upon diabetic and dyslipidemic status: poorly controlled diabetics with dyslipidemia (DM-PC/D), well-controlled diabetics with dyslipidemia (DM-WC/D), normoglycemic individuals with dyslipidemia (NG/D), and healthy individuals (NG/ND). Post hoc power calculation (at the 95% significance level, p = 0.05) showed that all comparisons had a power > 0.8 except for hsCRP (0.56), triglycerides (0.65), and abdominal circumference (0.18).
2.2. Clinical record and physical evaluation
After enrolling in the study, all participants answered a face-to-face structured questionnaire about socio-demographic data, personal and family medical history, and use of medications. A trained examiner collected information from diabetic patients regarding time since diabetes onset, medication used to control glucose, and the presence of complications associated with diabetes. Subjects completed a physical examination including anthropometric data such as abdominal circumference (cm), hip (cm), waist (cm), height (m), weight (kg) and body mass index (BMI).
2.3. Laboratory measurements: metabolic control and lipoprotein profile
Blood samples were collected after a 12-h overnight fast to evaluate blood cell counts (white and red series), fasting plasma glucose (mg/dl) by modified Bondar and Mead method, glycated hemoglobin (HbA1c) by enzymatic immunoturbidimetry, insulin levels by the chemiluminescence immunoassay, high-sensitivity C-reactive protein by the nephelomeric method and lipid profile (total cholesterol (TC), triglycerides (TGs), and HDL) by enzymatic methods. LDL was determined by the Friedewald formula. The same laboratory performed all analyses.
To avoid the inclusion of individuals with transitory dyslipidemia, the cutoff points used were the highest values according to the National Cholesterol Educational Program (NCEP) Adult Treatment III (ATP III) (NCEP, 2002): TC ≥ 240 mg/dl, LDL ≥ 160 mg/dl, HDL ≤ 40 mg/dl, and TGs ≥ 200 mg/dl. Metabolic control was considered as inadequate when HbA1c ≥ 8.5% and these patients were included in the DM-PC/D Group (Diabetes Poorly Controlled with Dyslipidemia). When HbA1c < 7.0% the metabolic control was considered as adequate and these patients were included in the DM-WC/D (Diabetes Well-controlled with Dyslipidemia). The homeostasis model assessment of insulin resistance (HOMA IR) was calculated by the formula: fasting insulin concentration (µU/ml) × fasting glucose concentration (mmol/L) divided by 22.5 (Matthews et al., 1985).
2.4. Sample collection
Blood samples were collected in tubes with EDTA centrifuged at 3000 × g for 10 min at 4 °C, promptly aliquoted and stored at −80 °C until the analyses. The following assays were performed by professionals unaware of the experimental groups.
2.5. Analysis of cytokines in plasma
Eleven cytokines (interleukin-1β [IL-1β], IL-2, -4, -5, -6, -7, -8, -10, -12 (p70) and -13 and tumor necrosis factor-alpha [TNF-α]) were measured in plasma samples by multiplex beads (Bio-Plex system, Bio-Rad Laboratories, Hercules, CA, USA), following the manufacturer's instructions.
2.6. Lipid peroxidation and antioxidant assays
2.6.1. MDA assay
The assessment of MDA in plasma was determined by HPLC (Shimadzu, Tokyo, Japan) with a reverse-phase HPLC column (C18; 4.6 × 150 mm) (Phenomenex, Torrance, CA, USA) and compared with MDA standard curves. Plasma samples were prepared as described previously (Hong, Yeh, Chang, & Hu, 2000).
2.6.2. Antioxidant assay
The levels of total antioxidant capacity of plasma were evaluated with a commercially available colorimetric kit (Cayman Chemical Company®, Ann Arbor, MI, USA) following the manufacturer's instructions. The absorbance at 750 nm was measured by spectrophotometry using a plate reader and the results were expressed as Trolox (mM).
2.7. Statistical analysis
The distribution and normality of the variables were evaluated by the D'Agostino–Pearson test. Subsequently, the general characteristics of each group were described as mean and standard deviation (SD); the cytokines expression in plasma were expressed as median (25%/75% quartiles) in Table 1 and as mean and standard deviation (SD) in Fig. 2. The differences between the groups for parametric data were evaluated by ANOVA followed by Bonferroni's post-test, and non-parametric data were evaluated by the Kruskall–Wallis test, followed by Dunn's post-test. Correlations were analyzed by the Pearson rank test adjusting for age, gender, and BMI. To determine the influence of lipid peroxidation and diabetic status on the expression of plasma cytokines, separate multivariable logistic and linear regression models were built for each independent variable. Variables found to be associated with the independent and dependent variables, and thus possible confounding factors, were included in the models. Moreover, regardless of their statistical significance, all variables known to have biologic linkage with the outcomes were retained. Based on previous studies (Carey et al., 2004; Fentoglu et al., 2009), correlation and regression models were adjusted for age, gender, BMI, and triglycerides, considered important as possible confounders in establishing the relationship among the variables of interest. The significance level was set at α = 0.05. All analyses were carried out with SPSS software, IBM version 19.
Table 1.
Characteristics of the sample: demographic, physical, laboratory, antioxidants and cytokines data.
| DM-PC/D (DM ≥ 8.5%/with dyslip) |
DM-WC/D (DM < 7.0%/with dyslip) |
NG/D (normoglycemic/with dyslip) |
NG/ND (normoglycemic/without dyslip) |
|
|---|---|---|---|---|
| Gender (F/M) | 14/11 | 15/10 | 14/11 | 15/10 |
| Age | 47.8 (± 7.7) | 50.3 (± 6.7) | 49.1 (± 7.9) | 45.6 (± 5.3) |
| BMI (m/kg2) | 30.8 (± 4.1)# | 30.9 (± 4.1)# | 28.5 (± 4.0) | 27.6 (± 6.9) |
| Abdominal circumference | 103.7 (± 14.3) | 107.9 (± 10.0)#,§ | 98.2 (± 10.7) | 98.1 (± 18.0) |
| Waist/hip ratio | 1.0 (± 0.1) | 1.0 (± 0.1) | 0.9 (± 0.1) | 0.9 (± 0.1) |
| Fasting glucose (mg/dL) | 233.4 (± 76.6)†,‡ | 137.7 (± 43.5)‡ | 90.2 (± 6.1) | 91.0 (± 7.5) |
| HbA1c (%) | 10.7 (± 1.8)** | 6.5 (± 0.7)§,# | 5.5 (± 0.7) | 5.2 (± 0.6) |
| Insulin (U/L) | 17.7 (± 14.5)# | 16.2 (± 7.8)# | 12.5 (± 8.3) | 12.1 (± 13.7) |
| HOMA IR Index | 9.8 (± 8.5)‡ | 5.4 (± 2.7)§,# | 2.8 (± 1.8) | 2.8 (± 3.4) |
| Total cholesterol (mg/dL) | 244.0 (± 38.5)## | 247.3 (± 44.3)## | 244.8 (± 41.9)## | 173.9 (± 18.4) |
| HDL cholesterol (mg/dL) | 45.9 (± 9.8) | 47.2 (± 10.6) | 50.0 (± 11.3) | 48.9 (± 13.4) |
| LDL cholesterol (mg/dL) | 153.6 (± 39.0)## | 150.8 (± 45.1)## | 157.0 (± 43.2)## | 105.5 (± 17.8) |
| Triglycerides (mg/dL) | 218.1 (± 94.2)## | 246.2 (± 108.4)## | 189.1 (± 82.7)## | 94.1 (± 37.8) |
| Hs C-Reactive Protein (mg/dL) | 0.4 (± 0.3) | 0.7 (± 0.5)# | 0.4 (± 0.3) | 0.4 (± 0.6) |
| White blood Cells (mm3) | 7.403 (± 1.687)## | 7.144 (± 1.932) | 6.886 (± 1.814) | 5.903 (± 1.503) |
| Red blood cells (× 106) | 4.9 (± 0.4) | 4.7 (± 0.5) | 4.9 (± 0.4) | 4.6 (± 0.5) |
| Antioxidants (mM) | 0.60 (± 0.20) | 0.66 (± 0.18) | 0.66 (± 0.21) | 0.69 (± 0.18) |
| IL-1β (pg/ml) | 0.48 (0.39/0.82)§,## | 0. (0.39/0.60)## | 0.41 (0.32/0.45) | 0.35 (0.29/0.38) |
| IL-2 (pg/ml) | 0.39 (0.0/0.71) | 0.39 (0.22/0.49) | 0.26 (0.0/0.48) | 0.33 (0.22/0.42) |
| IL-4 (pg/ml) | 0.67 (0.44/1.0)# | 0.42 (0.22/1.0) | 0.44 (0.26/0.82) | 0.37 (0.15/0.45) |
| IL-5 (pg/ml) | 1.28 (1.17/1.64)# | 1.36 (1.09/1.5) | 1.2 (1.04/1.4) | 1.14 (1.0/1.28) |
| IL-6 (pg/ml) | 1.57 (1.27/2.04)# | 1.57 (1.19/1.8)# | 1.41 (1.2/1.8)# | 1.01 (0.54/1.3) |
| IL-7 (pg/ml) | 1.14 (1.01/1.36) | 1.14 (1.0/1.4) | 1.06 (0.9/1.4) | 1.0 (0.85/1.2) |
| IL-8 (pg/ml) | 2.54 (2.0/3.0)## | 2.16 (1.84/2.82)# | 2.25 (1.8/2.5) | 1.88 (1.4/2.1) |
| IL-10 (pg/ml) | 0.71 (0.6/0.8) | 0.74 (0.6/1.0) | 0.71 (0.63/0.82) | 0.64 (0.6/0.7) |
| IL-12 (p70) (pg/ml) | 0.99 (0.3/1.1) | 0.90 (0.7/1.0) | 0.90 (0.3/1.0) | 0.90 (0.7/1.1) |
| IL-13 (pg/ml) | 1.93 (1.3/2.9) | 1.85 (1.2/2.5) | 2.0 (1.4/2.9) | 1.7 (1.3/2.5) |
| TNF-α (pg/ml) | 5.68 (4.5/7.0)§,## | 5.0 (4.0/6.2)## | 4.56 (3.9/4.1)# | 2.68 (1.5/4.2) |
Data are presented as mean SD or median (25th/75th percentile).
p < 0.05 in relation to group 4;
p < 0.0001 in relation to group 4;
p < 0.05 in relation to group 3;
p < 0.05 in relation to group 2;
p < 0.0001 in relation to groups 3 and 4;
p < 0.0001 in relation to the other groups (Kruskal–Wallis Test; α = 5%).
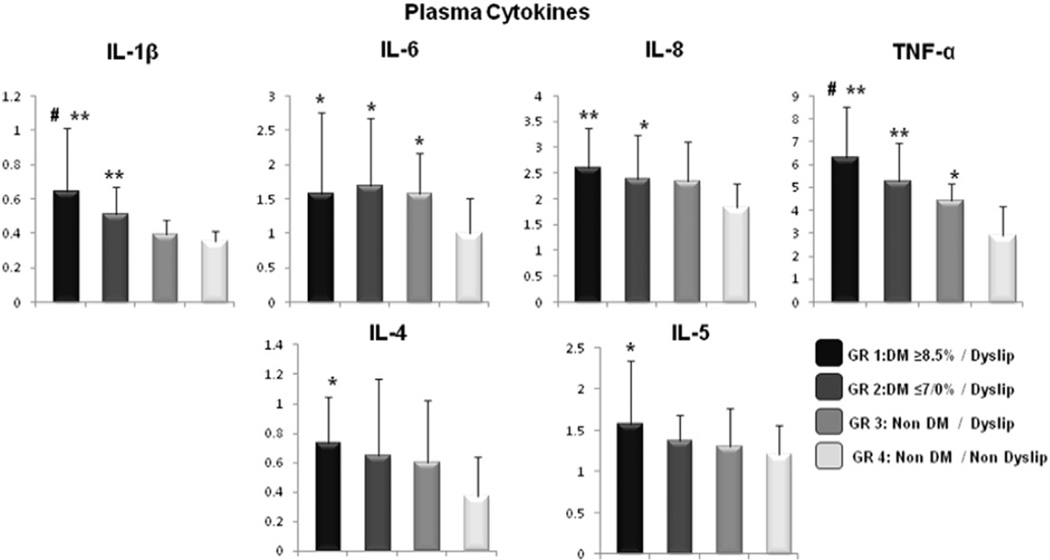
Fig. 2.
Cytokines’ expression in plasma (pg/ml). Data are presented as mean and standard deviation (SD) (**p < 0.0001 in relation to group 4; *p < 0.05 in relation to group 4; #p < 0.05 in relation to group 3; Kruskall–Wallis Test; α = 5%).
3. Results
3.1. Sample population
The general characteristics of the sample population are shown in Table 1. From the screened patients, 100 were identified as meeting the study inclusion criteria; of these, 25 patients were assigned to one of four different groups. There was no statistically significant difference among the participants with respect to gender, age and socioeconomic status. In relation to physical evaluation, all individuals were overweight and the diabetic groups were obese and presented higher values of BMI (p < 0.05), waist/hip proportion and abdominal circumference. The groups with diabetes had significantly increased levels of fasting glucose, HbA1c, and insulin resistance compared with normoglycemic patients. Based on ATP III (NCEP, 2002) definition of dyslipidemia, groups DM-PC/D, DM-WC/D and NG/D were dyslipidemic presenting increased levels of total cholesterol, LDL cholesterol, and triglycerides compared with group NG/ND (healthy individuals) (p < 0.05). The evaluation of total and differential counting of cells (white and red series) showed a statistical difference between poorly controlled diabetic/dyslipidemic patients regarding white blood cells (total leucocytes, neutrophils and lymphocytes) and healthy individuals (p < 0.05). There was no difference in time since diabetes onset (years) between poorly controlled/dyslipidemic (6.5 ± 4.5) and well-controlled/dyslipidemic diabetics (5.5 ± 7.1). Regarding diabetic complications, 60% of the poorly controlled diabetics and 40% of the well-controlled diabetics presented at least one complication related to type 2 DM. This difference was significant (p < 0.05), with the most common complication being retinopathy, followed by nephropathy. A total of 17 patients from DM-PC/D were treated with oral antidiabetic agents, 1 patient was treated with insulin therapy, and 7 were treated with the combination of insulin and antidiabetic agents. From the group DM-WC/D, 12 patients were treated with oral antidiabetic agents, 1 was treated with insulin therapy, 4 were treated with the combination of insulin and antidiabetic agents and 8 were treated with no drug therapy.
3.2. Lipid peroxidation levels
3.2.1. Plasma malondialdehyde (MDA)
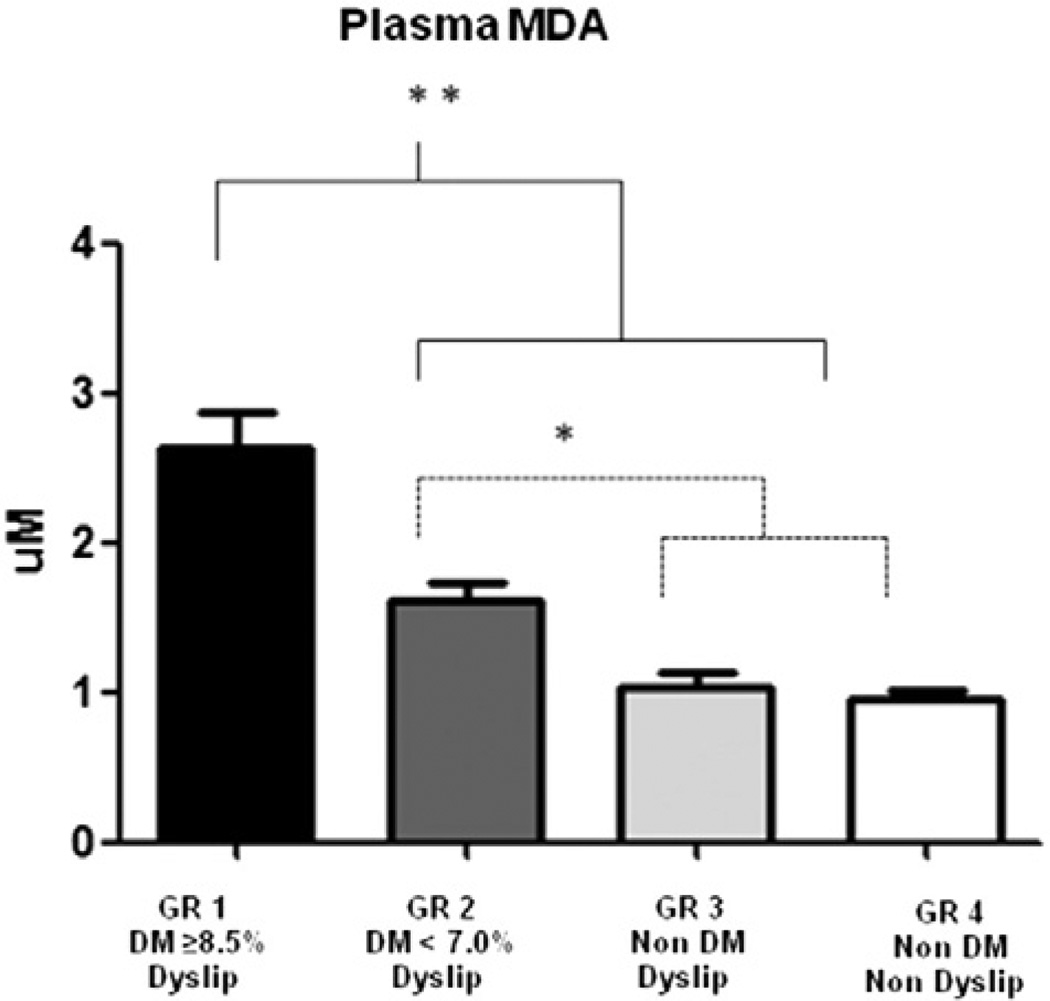
Plasma MDA levels were slightly increased in all dyslipidemic patients, regardless of their glycemic status. The highest level of plasma MDA was observed in poorly controlled diabetics with dyslipidemia who had about 2.9 times higher MDA levels when compared with those in the normoglycemic groups (p < 0.05) and approximately 1.6 times higher MDA levels when compared with well-controlled diabetics with dyslipidemia (p < 0.05) (Fig. 1). Well-controlled diabetics with dyslipidemia (DM-WC/D) presented significantly increased levels of MDA (p < 0.05) when compared with those in the normoglycemic groups (NG/D and NG/ND). Normoglycemic individuals with dyslipidemia had no increase in MDA levels compared to normoglycemic individuals without dyslipidemia (p > 0.05) indicating that the presence of hyperglycemia played a critical role in the increased MDA.
Fig. 1.
Lipid Peroxidation expression in plasma: Levels of MDA (µM). Data are presented as mean SD (**p < 0.0001 in relation to groups 2, 3 and 4; *p < 0.05 in relation to groups 3 and 4; ANOVA Test; α = 5%).
3.2.2. Antioxidant assay results
The levels of total antioxidant capacity of plasma were expressed as mean (± SD) and showed increased values in the groups without type 2 diabetes (NG/ND: 0.69 (± 0.18) and NG/D: 0.66 (± 0.21)) and in the well-controlled diabetics (DM-WC/D: 0.66 (± 0.18)). The group with poorly controlled diabetes with dyslipidemia (DM-PC/D), presented the lowest levels of plasma antioxidant capacity. However this difference was not significantly different between any of the groups (p > 0.05) (Table 1).
3.3. Systemic inflammation
Overall, the expression of proinflammatory cytokines gradually increased from NG/ND, NG/D, DM-WC/D, DM-PC/D (p < 0.05) indicating that diabetes significantly enhances the systemic level of inflammation. The cytokines’ expression in plasma were expressed as median (25%/75% quartiles) in Table 1 and as mean and standard deviation (SD) in Fig. 2. The cytokines that are typically considered adipokines such as IL-6 and TNF-α were also significantly increased in the groups with dyslipidemia in comparison with the group without dyslipidemia.
3.4. Statistical analysis
3.4.1. Correlation analysis
Table 2 shows the adjusted correlation among diabetes status, lipoprotein profile, anthropometric and lipid peroxidation markers, and plasma cytokine expression taking into account the effect of age, gender and BMI. There was a wide range of moderate positive correlations observed among diabetic status, lipid peroxidation markers and expression of proinflammatory cytokines. Among the most significant correlations were IL1-β with the following: fasting glucose (0.48), HbA1c (0.40) and MDA (0.50); all of which were significant (p < 0.0001). Moderate positive significant correlation was also observed among TNF-α and: fasting glucose (0.50), HbA1c (0.50), triglycerides (0.35) and MDA (0.44) (p < 0.0001). Diabetes was significantly associated with lipid peroxidation markers, and there was a strong positive correlation observed between MDA and HbA1c (r = 0.643, p < 0.0001), suggesting that poor metabolic control is associated with higher expression of MDA. The presence of complications related to type 2 diabetes mellitus (T2DM), with the most common complication being retinopathy, followed by nephropathy was positively correlated with HbA1c levels (r = 0.45, p < 0.01), MDA (r = 0.42, p < 0.01), diabetes duration (r = 0.47, p < 0.001) and TNF-α (r = 0.35, p < 0.05). Significant positive correlations (p < 0.05) were observed between insulin resistance (HOMA IR) and IL-6 (0.21), TNF-α (0.25) and between triglycerides and IL-1 (0.20), IL-6 (0.24) and TNF-α (0.35). Besides, a significant positive correlation was observed between adipocytokines and lipoprotein profile, such as IL-6 and triglycerides (0.24), IL-6 and total cholesterol (0.25) and TNF-α and triglycerides (0.35).
Table 2.
Adjusted correlation between plasma cytokines and diabetes status, lipoprotein profile, physical parameters, lipid peroxidation levels markers.
| Parameter | IL-1β | IL-4 | IL-5 | IL-6 | IL-8 | IL-10 | TNF-α | |
|---|---|---|---|---|---|---|---|---|
| Diabetic statusa | Fasting glucose (mg/dL) | 0.48** | 0.30** | 0.24* | 0.17 | 0.32** | 0.15 | 0.50** |
| HbA1c | 0.40** | 0.21* | 0.25* | 0.19 | 0.17 | 0.09 | 0.50** | |
| HOMA IR index | 0.18 | 0.12 | 0.04 | 0.21* | 0.15 | − 0.02 | 0.25* | |
| Lipoprotein profilea | Total Cholesterol (mg/dL) | 0.20* | 0.05 | 0.12 | 0.25* | 0.02 | − 0.03 | 0.16 |
| HDL-cholesterol (mg/dL) | − 0.15 | 0.04 | − 0.01 | − 0.17 | − 0.03 | − 0.11 | − 0.08 | |
| LDL-cholesterol (mg/dL) | 0.14 | − 0.01 | 0.04 | 0.21* | − 0.05 | − 0.01 | 0.07 | |
| Triglycerides (mg/dL) | 0.20* | 0.10 | 0.15 | 0.24* | 0.16 | 0.01 | 0.35** | |
| Physical Parametersa | BMI | 0.04 | 0.03 | − 0.01 | 0.08 | − 0.01 | − 0.03 | 0.05 |
| Wais/hip proportion | 0.15 | 0.10 | 0.11 | 0.17 | 0.20* | 0.12 | 0.14 | |
| Abdominal circunf | 0.06 | 0.05 | 0.001 | 0.12 | 0.08 | 0.02 | 0.04 | |
| Lipid peroxidation markera | MDA (µM) | 0.50** | 0.25* | 0.28* | 0.16 | 0.25* | 0.08 | 0.44** |
p < 0.05;
p < 0.0001 (r; Pearson's correlation coefficient; α = 5%).
HbA1c, glycated hemoglobin; HOMA IR, homeostatasis model assessment of insulin resistance.
Adjusted for age, gender and BMI.
The possible linkage among inflammation, diabetes, and lipid peroxidation is demonstrated by a multiple regression analysis using separate models for independent variables. Lipid peroxidation markers and diabetic status were directly related to the expression of inflammatory cytokines, especially IL-1β (β = 0.40) and TNF-α (β = 0.50). IL-6 was significantly related to lipoprotein profile rather than diabetic or inflammatory status (Table 3).
Table 3.
Multiple Regression Linear model with the influence of lipid peroxidation, diabetes status, lipoprotein profile and physical parameters on plasma cytokines.
| Independent variables (separate models) | IL-1βa β | IL-6a β | IL-8a β | TNF-αa β | IL-4a β | |
|---|---|---|---|---|---|---|
| LPO parameter | MDA PL | 0.50** | 0.15 | 0.25* | 0.44** | 0.24* |
| Diabetes status | Fasting glucose | 0.50** | 0.17 | 0.11 | 0.47 ** | 0.28* |
| HbA1c | 0.40** | 0.17 | 0.18 | 0.50** | 0.20* | |
| HOMA IR | 0.2 | 0.2 | 0.17 | 0.13 | − 0.01 | |
| Insulin | 0.01 | 0.23* | 0.01 | 0.27* | 0.12 | |
| Lipoprotein profile | Total Chol | 0.2 | 0.26* | 0.03 | 0.17 | 0.05 |
| LDL | 0.14 | 0.21* | − 0.04 | 0.008 | − 0.003 | |
| TGL | 0.21 | 0.24* | 0.18 | 0.37** | 0.10 | |
| Physical parameters | BMI | 0.04 | 0.08 | − 0.005 | 0.051 | 0.03 |
| Abdominal circ | 0.12 | 0.19 | 0.33 | − 0.004 | 0.09 |
Bold type indicates that the association reached statistical significance
p < 0.05;
p < 0.0001.
β partial standardized regression coefficient. 95%CI.
LPO, lipid peroxidation; MDA PL, malondialdehyde in plasma; HbA1c (glycated hemoglobin); HOMA IR, Insulin resistance.
Adjusted for age, gender and BMI.
3.4.2. Regression analysis
To further define the influence of lipid peroxidation, poor metabolic control and lipoprotein profile on increased levels of systemic inflammation, a logistic regression was performed taking into account the effects of age, gender and BMI. There was an approximate 3.2-, 2.0-, 1.9- and 2.6-fold increase in the odds of having elevated levels of IL-1β, IL-6, IL-8, and TNF-α, respectively, among those patients who have higher plasmatic levels of MDA (p < 0.05). In addition, increased levels of HbA1c were associated with a ~ 1.5-fold increase in the odds ratio of high of IL-1β, IL-8, and TNF-α (p < 0.05). In respect to lipoprotein profile and the relationship with increased expression of inflammatory cytokines, only elevated triglycerides were associated with a ~ 1.5-fold increase in the odds of high of IL-1β, IL-6, TNF-α and IL-4 (p < 0.05) and abdominal circumference increased the odds of having elevated IL-8 and TNF-α (Table 4).
Table 4.
Multivariate Logistic Regression model with the influence of lipid peroxidation, diabetes status, lipoprotein profile and physical parameters on plasma cytokines.
| IL-1βa OR (95% CI) | IL-6a OR (95% CI) | IL-8a OR (95% CI) | TNF-αa OR (95% CI) | IL-4a OR (95% CI) | ||
|---|---|---|---|---|---|---|
| LPO parameter | MDA PL | 3.17** (1.72–5.83) | 1.9* (1.15–3.11) | 1.87* (1.15–3.06) | 2.57** (1.46–4.53) | 1.91* (1.15–3.15) |
| Diabetes status | Fasting glucose | 1.01* (1.01–1.02) | 1.00 (1.00–1.01) | 1.12* (1.00–1.02) | 1.01* (1.00–1.01) | 1.21* (1.0–1.01) |
| HbA1c | 1.31* (1.09–1.58) | 1.16 (0.97–1.38) | 1.22* (1.02–1.45) | 1.41** (1.15–1.73) | 1.19* (1.0–1.42) | |
| HOMA IR | 1.12* (1.02–1.24) | 1.02 (0.95–1.10) | 1.1* (1.0–1.21) | 1.04 (0.96–1.12) | 1.07 (0.09–1.16) | |
| Insulin | 1.00 (0.98–1.03) | 1.0 (0.98–1.04) | 1.01 (0.98–1.04) | 1.02 (0.99–1.05) | 1.0 (0.97–1.03) | |
| Lipoprotein profile | Total Chol | 1.00 (0.99–1.01) | 1.0 (1.00–1.02) | 1.0 (0.99–1.01) | 1.01 (1.00–1.02) | 1.0 (1.0–1.01) |
| LDL | 1.00 (0.99–1.91) | 1.0 (1.00–1.01) | 1.0 (0.99–1.01) | 1.0 (0.99–1.01) | 1.0 (1.0–1.01) | |
| TGL | 1.11* (1.00–1.01) | 1.2* (1.00–1.01) | 1.0 (1.00–1.01) | 1.06* (1.00–1.10) | 1.12* (1.0–1.01) | |
| Physical parameters | BMI | 0.97 (0.89–1.07) | 1.1 (1.00–1.18) | 0.98 (0.90–1.06) | 1.04 (0.96–1.13) | 1.04 (0.96–1.13) |
| Abdominal circ | 1.06 (1.00–1.14) | 1.05 (0.99–1.12) | 1.11* (0.96–1.07) | 1.0* (1.00–1.10) | 1.02 (0.96–1.08) |
Bold type indicates that the association reached statistical significance
p < 0.05;
p < 0.0001.
LPO, lipid peroxidation; MDA PL, malondialdehyde in plasma; HbA1c (glycated hemoglobin); HOMA IR, Insulin resistance.
Adjusted for age, gender and BMI.
4. Discussion
In the present study we investigated whether type 2 diabetes mellitus (T2DM) coupled with dyslipidemia was associated with altered oxidative metabolism and increased lipid peroxidation and whether they were linked to greater systemic inflammation. Our results indicate that diabetes associated with dyslipidemia, even in well-controlled patients, was associated with increased lipid peroxidation. Furthermore, dyslipidemia and increased lipid peroxidation were strongly correlated with higher levels of systemic inflammation.
Previous studies evaluated cytokines in plasma of patients with diabetes (Arnalich et al., 2000; Mirza et al., 2012; Pickup et al., 2000) and their association with diabetic complications (Liu et al., 2010; Ridker et al., 2000). However to the best of our knowledge, this is the first study to establish a relationship between type 2 diabetes mellitus (T2DM) and dyslipidemia and their impact on lipid peroxidation levels and systemic inflammation. Elevated proinflammatory cytokines (IL-1β, IL-6, IL-8, TNF-α) were detected in diabetic patients and the presence of complications, such as retinopathy and nephropathy, was positively associated with increased levels of TNF-α. The anti-inflammatory cytokine IL-4 was also elevated in diabetic patients. Interestingly, the levels of proinflammatory cytokines (IL1-β, Il-6, TNF-α) in well-controlled diabetics with dyslipidemia were not statistically different from those of poorly controlled diabetics with dyslipidemia. These findings suggest that dyslipidemia might be one of the driving forces for elevated systemic inflammation even in well-controlled diabetics.
It is known that obesity promotes dyslipidemia and may impact insulin action via additional pathways, including the release of leptin, IL-6 and TNF-α (Vinik, 2005). In the present study, diabetic individuals were considered obese if they had BMI > 30 and a large waist circumference compared to healthy patients. Abdominal circumference statistically increased the odds of having elevated IL-8 and TNF-α. Besides, patients with type 2 diabetes had an increased insulin resistance index (HOMA IR) compared to healthy patients and significant associations were observed between insulin resistance (HOMA IR) and cytokines (IL-6 and TNF-α). This fact is in accordance with previous studies that demonstrated the association between insulin resistance and the increased expression of inflammatory cytokines (Vinik, 2005; Wisse, 2004).
The pathogenesis of diabetic dyslipidemia is not completely elucidated, although it is recognized that lipid abnormalities are a primary feature of metabolic syndrome and can precede the clinical manifestation of hyperglycemia (Davi et al., 2003; Ginsberg, 1991; Haffner, 2002; Kreisberg, 1998). It is important to note that even with the well-established effect of dyslipidemia on type 2 diabetes (Chisolm, Irwin, & Penn, 1992; Haffner, 2002; Krauss & Siri, 2004; Kreisberg, 1998) several studies with diabetic populations do not take into consideration the serum lipid characteristics of the population (Davi et al., 2003; Esposito et al., 2002; Liu et al., 2010; Pandey, Mishra, & Rizvi, 2010; Pickup et al., 2000) or the level of dyslipidemia (Cakatay, 2005; Esposito et al., 2002; Hussein et al., 2007; Mirza et al., 2012; Nakhjavani et al., 2010; Van Guilder, Hoetzer, Greiner, Stauffer, & Desouza, 2006). In the present study we evaluated the lipid profile of the whole population and included in dyslipidemic groups only patients with values consistent with an established dyslipidemia (NCEP, 2002). There was an elevated inflammatory status in normoglycemic dyslipidemic individuals (NG/D). This group presented significantly increased levels of IL-6 and TNF-α when compared to normoglycemic without dyslipidemia (NG/ND), suggesting that dyslipidemia itself can increase the release of proinflammatory cytokines. In addition, a positive correlation was observed between adipocytokines, IL-6 and TNF-α and triglycerides and total cholesterol.
A limitation of many studies evaluating diabetic individuals is the lack of attention concerning metabolic and dyslipidemic status, not separating the patients based on dyslipidemia and controlled/poorly controlled type 2 diabetes mellitus, based on recent and updated HbA1c levels (Cakatay, 2005; Gambino et al., 2004; Hussein et al., 2007; Kostolanska, Jakus, & Barak, 2009; Rytter et al., 2009). We analyzed the DM patients as separate groups based on the quality of metabolic control. As expected we observed a significant increase in lipid peroxidation and proinflammatory cytokines in poorly controlled diabetics when compared to the other groups, consistent with other reports (Chisolm et al., 1992; Colas et al., 2010; Dennis et al., 2010; Gambino et al., 2004; Hussein et al., 2007; Mirza et al., 2012; Nishigaki, Hagihara, Tsunekawa, Maseki, & Yagi, 1981; Nishimura, Iwamoto, & Soga, 2007; Sundaram et al., 1996). However, analysis of the data considering the metabolic status and lipid profile allowed us to observe novel findings related to the impact of dyslipidemia on the level of systemic inflammation in well-controlled diabetics.
Well-controlled diabetics are usually considered at a lower risk to develop cardiovascular disease and diabetic complications when compared to patients with inadequate metabolic control (American Diabetes, A, 2015; NCEP, 2002). For this reason lipid profile and levels of acute phase proteins are not usually evaluated in these patients. Interestingly, in the present study, well-controlled diabetics with dyslipidemia (DM-WC/D) showed significantly the highest levels of high sensitivity C-reactive protein. This unexpected finding could probably be due to the fact that this group also presented significantly increased measures of abdominal circumference (Hak et al., 1999; Lakka, Lakka, Salonen, Kaplan, & Salonen, 2001; Mendall et al., 2000). However, in the present study the statistical analysis did not show significant correlation between C-reactive protein and abdominal circumference after the adjustment for age, gender and BMI. The DM-WC/D group also showed high levels of lipid peroxidation and proinflammatory cytokines (IL-1β, IL-6, IL-8, TNF-α). Thus, the inflammatory and lipid peroxidation levels of the well-controlled diabetics were more similar to those of the poorly controlled diabetics and markedly higher than to those of the normoglycemic group with dyslipidemia. This shows the importance of better evaluation of these patients even if they are considerated well-controlled diabetics and also suggests that increased lipid peroxidation and systemic inflammation may represent a possible risk for the development of complications and imbalance in the metabolic control. In support to this possibility, other studies have also emphasized the hypothesis that lipid peroxidation contributes to the initiation and progression of diabetes (Giugliano, Ceriello, & Paolisso, 1996; Van Dam et al., 1995) and that oxidative stress can be an important early event in the pathogenesis of complications secondary to diabetes (Sasaki & Inoguchi, 2012) and may indicate an underlying subclinical pathology despite the good metabolic control (Cakatay, 2005). We add to these findings that lipid peroxidation is positively associated with complications related with type 2 diabetes and also with systemic inflammation, particularly IL-1β and TNF-α.
The cross-sectional design of the present study limited the interpretation of our findings. Thus, the temporal sequence showing whether diabetes coupled with dyslipidemia precedes the increased systemic inflammation or vice versa is not known. Nonetheless, the present study intended to assess the direction of the association as to whether diabetes coupled with dyslipidemia leads to increase in systemic inflammation. In an effort to minimize the influence of lifestyle behaviors and the effects of confounding factors, particular care was taken to use strict inclusion criteria, having subjects of similar socioeconomic level, age and gender-matched, who were not currently taking medication that could influence inflammatory and oxidative markers (i.e., statins). Nevertheless, our study suggests that increased lipid peroxidation aggravates systemic inflammation even in well-controlled diabetics and normoglycemic dyslipidemic patients.
5. Conclusion
In conclusion, our study suggests that lipid peroxidation may represent an additive effect to the metabolic imbalance associated with type 2 diabetes, increased systemic inflammation and the development of diabetes-associated complications. In this sense, dyslipidemia may have a relevant impact on systemic inflammation via altered oxidative metabolism leading to increased lipid peroxidation even in well-controlled diabetics.
Acknowledgments
This study was supported by financial support from the São Paulo State Research Support Foundation (FAPESP) (grant 2007/08362-8), by the Coordination for Improvement of Higher Education Personnel of the Brazilian Ministry of Education (CAPES), and by grants from the National Institute of Health (DE017732) and National Institute of Dental and Craniofacial Research (NIDCR) (K23 DE025313). A. P. M. Loureiro received fellowships from CNPq.
Footnotes
Conflict of interest: There is no conflict of interest.
References
- Ahmed N. Advanced glycation endproducts—Role in pathology of diabetic complications. Diabetes Research and Clinical Practice. 2005;67:3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- American Diabetes, A. Standards of medical care in diabetes—2015 abridged for primary care providers. Clinical Diabetes. 2015;33:97–111. doi: 10.2337/diaclin.33.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnalich F, Hernanz A, Lopez-Maderuelo D, Pena JM, Camacho J, Madero R, et al. Enhanced acute-phase response and oxidative stress in older adults with type II diabetes. Hormone and Metabolic Research. 2000;32:407–412. doi: 10.1055/s-2007-978662. [DOI] [PubMed] [Google Scholar]
- Cakatay U. Protein oxidation parameters in type 2 diabetic patients with good and poor glycaemic control. Diabetes & Metabolism. 2005;31:551–557. doi: 10.1016/s1262-3636(07)70230-6. [DOI] [PubMed] [Google Scholar]
- Carey AL, Bruce CR, Sacchetti M, Anderson MJ, Olsen DB, Saltin B, et al. Interleukin-6 and tumor necrosis factor-alpha are not increased in patients with type 2 diabetes: Evidence that plasma interleukin-6 is related to fat mass and not insulin responsiveness. Diabetologia. 2004;47:1029–1037. doi: 10.1007/s00125-004-1403-x. [DOI] [PubMed] [Google Scholar]
- Chapple IL, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontology 2000. 2007;2000(43):160–232. doi: 10.1111/j.1600-0757.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- Chello M, Spadaccio C, Lusini M, Covino E, Blarzino C, De Marco F, et al. Advanced glycation end products in diabetic patients with optimized glycaemic control and their effects on endothelial reactivity: Possible implications in venous graft failure. Diabetes/Metabolism Research and Reviews. 2009;25:420–426. doi: 10.1002/dmrr.966. [DOI] [PubMed] [Google Scholar]
- Chisolm GM, Irwin KC, Penn MS. Lipoprotein oxidation and lipoprotein-induced cell injury in diabetes. Diabetes. 1992;41(Suppl. 2):61–66. doi: 10.2337/diab.41.2.s61. [DOI] [PubMed] [Google Scholar]
- Colas R, Pruneta-Deloche V, Guichardant M, Luquain-Costaz C, Cugnet-Anceau C, Moret M, et al. Increased lipid peroxidation in LDL from type-2 diabetic patients. Lipids. 2010;45:723–731. doi: 10.1007/s11745-010-3453-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davi G, Chiarelli F, Santilli F, Pomilio M, Vigneri S, Falco A, et al. Enhanced lipid peroxidation and platelet activation in the early phase of type 1 diabetes mellitus: Role of interleukin-6 and disease duration. Circulation. 2003;107:3199–3203. doi: 10.1161/01.CIR.0000074205.17807.D0. [DOI] [PubMed] [Google Scholar]
- Dennis RJ, Maldonado D, Rojas MX, Aschner P, Rondon M, Charry L, et al. Inadequate glucose control in type 2 diabetes is associated with impaired lung function and systemic inflammation: A cross-sectional study. BMC Pulmonary Medicine. 2010;10:38. doi: 10.1186/1471-2466-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj S, Venugopal SK, Singh U, Jialal I. Hyperglycemia induces monocytic release of interleukin-6 via induction of protein kinase c-{alpha} and -{beta} Diabetes. 2005;54:85–91. doi: 10.2337/diabetes.54.1.85. [DOI] [PubMed] [Google Scholar]
- Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: Role of oxidative stress. Circulation. 2002;106:2067–2072. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- Fentoglu O, Oz G, Tasdelen P, Uskun E, Aykac Y, Bozkurt FY. Periodontal status in subjects with hyperlipidemia. Journal of Periodontology. 2009;80:267–273. doi: 10.1902/jop.2009.080104. [DOI] [PubMed] [Google Scholar]
- Gambino R, Uberti B, Alemanno N, Pisu E, Pagano G, Cassader M. In vivo oxidizability of LDL in type 2 diabetic patients in good and poor glycemic control. Atherosclerosis. 2004;173:103–107. doi: 10.1016/j.atherosclerosis.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Ginsberg HN. Lipoprotein physiology in nondiabetic and diabetic states. Relationship to atherogenesis. Diabetes Care. 1991;14:839–855. doi: 10.2337/diacare.14.9.839. [DOI] [PubMed] [Google Scholar]
- Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19:257–267. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- Goova MT, Li J, Kislinger T, Qu W, Lu Y, Bucciarelli LG, et al. Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice. The American Journal of Pathology. 2001;159:513–525. doi: 10.1016/S0002-9440(10)61723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffner SM. Lipoprotein disorders associated with type 2 diabetes mellitus and insulin resistance. The American Journal of Cardiology. 2002;90:55i–61i. doi: 10.1016/s0002-9149(02)02634-6. [DOI] [PubMed] [Google Scholar]
- Hak AE, Stehouwer CD, Bots ML, Polderman KH, Schalkwijk CG, Westendorp IC, et al. Associations of C-reactive protein with measures of obesity, insulin resistance, and subclinical atherosclerosis in healthy, middle-aged women. Arteriosclerosis, Thrombosis, and Vascular Biology. 1999;19:1986–1991. doi: 10.1161/01.atv.19.8.1986. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oral inflammation and reactive species: A missed opportunity? Oral Diseases. 2000;6:136–137. doi: 10.1111/j.1601-0825.2000.tb00324.x. [DOI] [PubMed] [Google Scholar]
- Hirsch T, Spielmann M, Zuhaili B, Koehler T, Fossum M, Steinau HU, et al. Enhanced susceptibility to infections in a diabetic wound healing model. BMC Surgery. 2008;8:5. doi: 10.1186/1471-2482-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YL, Yeh SL, Chang CY, Hu ML. Total plasma malondialdehyde levels in 16 Taiwanese college students determined by various thiobarbituric acid tests and an improved high-performance liquid chromatography-based method. Clinical Biochemistry. 2000;33:619–625. doi: 10.1016/s0009-9120(00)00177-6. [DOI] [PubMed] [Google Scholar]
- Hussein OA, Gefen Y, Zidan JM, Karochero EY, Luder AS, Assy NN, et al. LDL oxidation is associated with increased blood hemoglobin A1c levels in diabetic patients. Clinica Chimica Acta. 2007;377:114–118. doi: 10.1016/j.cca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Kostolanska J, Jakus V, Barak L. HbA1c and serum levels of advanced glycation and oxidation protein products in poorly and well controlled children and adolescents with type 1 diabetes mellitus. Journal of Pediatric Endocrinology & Metabolism. 2009;22:433–442. doi: 10.1515/jpem.2009.22.5.433. [DOI] [PubMed] [Google Scholar]
- Krauss RM, Siri PW. Dyslipidemia in type 2 diabetes. Medical Clinics of North America. 2004;88:897–909. doi: 10.1016/j.mcna.2004.04.004. (x) [DOI] [PubMed] [Google Scholar]
- Kreisberg RA. Diabetic dyslipidemia. The American Journal of Cardiology. 1998;82:67U–73U. doi: 10.1016/s0002-9149(98)00848-0. (discussion 85 U–86 U) [DOI] [PubMed] [Google Scholar]
- Lakka TA, Lakka HM, Salonen R, Kaplan GA, Salonen JT. Abdominal obesity is associated with accelerated progression of carotid atherosclerosis in men. Atherosclerosis. 2001;154:497–504. doi: 10.1016/s0021-9150(00)00514-1. [DOI] [PubMed] [Google Scholar]
- Li J, Huang M, Shen X. The association of oxidative stress and proinflammatory cytokines in diabetic patients with hyperglycemic crisis. Journal of Diabetes and its Complications. 2014;28:662–666. doi: 10.1016/j.jdiacomp.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Liu J, Shi B, He S, Yao X, Willcox MD, Zhao Z. Changes to tear cytokines of type 2 diabetic patients with or without retinopathy. Molecular Vision. 2010;16:2931–2938. [PMC free article] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Mendall MA, Strachan DP, Butland BK, Ballam L, Morris J, Sweetnam PM, et al. C-reactive protein: Relation to total mortality, cardiovascular mortality and cardiovascular risk factors in men. European Heart Journal. 2000;21:1584–1590. doi: 10.1053/euhj.1999.1982. [DOI] [PubMed] [Google Scholar]
- Millar TM, Phan V, Tibbles LA. ROS generation in endothelial hypoxia and reoxygenation stimulates MAP kinase signaling and kinase-dependent neutrophil recruitment. Free Radical Biology & Medicine. 2007;42:1165–1177. doi: 10.1016/j.freeradbiomed.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Mirza S, Hossain M, Mathews C, Martinez P, Pino P, Gay JL, et al. Type 2-diabetes is associated with elevated levels of TNF-alpha, IL-6 and adiponectin and low levels of leptin in a population of Mexican Americans: A cross-sectional study. Cytokine. 2012;57:136–142. doi: 10.1016/j.cyto.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooradian AD. Dyslipidemia in type 2 diabetes mellitus. Nature Clinical Practice. Endocrinology & Metabolism. 2009;5:150–159. doi: 10.1038/ncpendmet1066. [DOI] [PubMed] [Google Scholar]
- Nakhjavani M, Khalilzadeh O, Khajeali L, Esteghamati A, Morteza A, Jamali A, et al. Serum oxidized-LDL is associated with diabetes duration independent of maintaining optimized levels of LDL-cholesterol. Lipids. 2010;45:321–327. doi: 10.1007/s11745-010-3401-8. [DOI] [PubMed] [Google Scholar]
- Ncep. Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- Niedowicz DM, Daleke DL. The role of oxidative stress in diabetic complications. Cell Biochemistry and Biophysics. 2005;43:289–330. doi: 10.1385/CBB:43:2:289. [DOI] [PubMed] [Google Scholar]
- Nishigaki I, Hagihara M, Tsunekawa H, Maseki M, Yagi K. Lipid peroxide levels of serum lipoprotein fractions of diabetic patients. Biochemical Medicine. 1981;25:373–378. doi: 10.1016/0006-2944(81)90096-x. [DOI] [PubMed] [Google Scholar]
- Nishimura F, Iwamoto Y, Soga Y. The periodontal host response with diabetes. Periodontology 2000. 2007;2000(43):245–253. doi: 10.1111/j.1600-0757.2006.00171.x. [DOI] [PubMed] [Google Scholar]
- Pandey KB, Mishra N, Rizvi SI. Protein oxidation biomarkers in plasma of type 2 diabetic patients. Clinical Biochemistry. 2010;43:508–511. doi: 10.1016/j.clinbiochem.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Pickup JC, Chusney GD, Thomas SM, Burt D. Plasma interleukin-6, tumour necrosis factor alpha and blood cytokine production in type 2 diabetes. Life Sciences. 2000;67:291–300. doi: 10.1016/s0024-3205(00)00622-6. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000;101:2149–2153. doi: 10.1161/01.cir.101.18.2149. [DOI] [PubMed] [Google Scholar]
- Rytter E, Vessby B, Asgard R, Johansson C, Sjodin A, Abramsson-Zetterberg L, et al. Glycaemic status in relation to oxidative stress and inflammation in well-controlled type 2 diabetes subjects. The British Journal of Nutrition. 2009;101:1423–1426. doi: 10.1017/s0007114508076204. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Inoguchi T. The role of oxidative stress in the pathogenesis of diabetic vascular complications. Diabetes and Metabolism Journal. 2012;36:255–261. doi: 10.4093/dmj.2012.36.4.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stosic-Grujicic SD, Miljkovic DM, Cvetkovic ID, Maksimovic-Ivanic DD, Trajkovic V. Immunosuppressive and anti-inflammatory action of antioxidants in rat autoimmune diabetes. Journal of Autoimmunity. 2004;22:267–276. doi: 10.1016/j.jaut.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Sundaram RK, Bhaskar A, Vijayalingam S, Viswanathan M, Mohan R, Shanmugasundaram KR. Antioxidant status and lipid peroxidation in type II diabetes mellitus with and without complications. Clinical Science (London, England) 1996;90:255–260. doi: 10.1042/cs0900255. [DOI] [PubMed] [Google Scholar]
- Van Dam PS, Van Asbeck BS, Erkelens DW, Marx JJ, Gispen WH, Bravenboer B. The role of oxidative stress in neuropathy and other diabetic complications. Diabetes/Metabolism Reviews. 1995;11:181–192. doi: 10.1002/dmr.5610110303. [DOI] [PubMed] [Google Scholar]
- Van Guilder GP, Hoetzer GL, Greiner JJ, Stauffer BL, Desouza CA. Influence of metabolic syndrome on biomarkers of oxidative stress and inflammation in obese adults. Obesity (Silver Spring) 2006;14:2127–2131. doi: 10.1038/oby.2006.248. [DOI] [PubMed] [Google Scholar]
- Velazquez E, Winocour PH, Kesteven P, Alberti KG, Laker MF. Relation of lipid peroxides to macrovascular disease in type 2 diabetes. Diabetic Medicine. 1991;8:752–758. doi: 10.1111/j.1464-5491.1991.tb01695.x. [DOI] [PubMed] [Google Scholar]
- Vinik AI. The metabolic basis of atherogenic dyslipidemia. Clinical Cornerstone. 2005;7:27–35. doi: 10.1016/s1098-3597(05)80065-1. [DOI] [PubMed] [Google Scholar]
- Wisse BE. The inflammatory syndrome: The role of adipose tissue cytokines in metabolic disorders linked to obesity. Journal of the American Society of Nephrology. 2004;15:2792–2800. doi: 10.1097/01.ASN.0000141966.69934.21. [DOI] [PubMed] [Google Scholar]