Abstract
Derivatives requiring either anhydrous or aqueous reaction conditions were prepared for robust and reliable gas chromatography/mass spectrometry (GC/MS) characterization of hydroxyl, mercapto, and amino benzoic acids Methylation and trialkylsilytation are employed for blocking the acidic function. Alkyl, trimethylsilyl, acetyl, perfluoroacyl and alkoxycarbonyl derivatization groups are introduced to hydroxyl, mercapto and amino functions. The electron ionization induced fragmentation characteristics of corresponding derivatives are explained by comparing the MS1 spectra of unlabeled compounds to their 2H and 13C labeled analogs, and analysis of collision-induced dissociation data from MS2 spectra. Competing fragmentation alternatives are identified and specific decomposition processes are detailed that characterize (a) ortho isomers due to interaction or vicinal functional substituents and (b) para isomers prone to forming para quinoid type structures. Skeletal and hydrogen rearrangements typical for methyl benzoates and the blocking groups are considered when discussing diagnostically important ions. Characteristic ions produced as a result of rearrangements in ortho isomers are classified, and skeletal rearrangements required to produce para quinoid type ions specific for para isomers are noted. Key ions for structure elucidation and differentiation of isomers for derivatives of substituted benzoic acids by GC/MS are suggested.
Keywords: gas chromatography/mass spectrometry (GC/MS), “ortho effect”, “para effect”, derivatization, salicylic acid, anthranilic acid, thiosalicylic acid, alkylation, silylation, perfluoroacylation, alkoxycarbonyl derivatives
Abstract
Производные, получаемые либо и безводных, либо водных условиях реакций, были синтезированы для надеж¬ной характеристики гидрокси-, меркапто- и аминобензойных кислот методом ГХ/МС. Метилирование и три- алкилсилилирование применялись для блокирования кнслотной функции. Алкил-, триметилсилил-, ацетил-, перфторацил- и алкоксикарбонильные дериватизационные грулпы вводились для модификации гидроксиль- ¬ной, меркапто- и аминофункций. Характеристики, полученные при распаде в условиях иониэации электро- ¬нами соответствующих производных, были интерпретированы путем сравнения МС1 спектров немеченых со- ¬единений со спектрами их 2Н и 13С меченых аналогов и анализа индуцируемых соударением распадов по МЅ2 спектрам. Показаны конкурирующие альтернативные пути фрагментации, детально изучены специфические процессы распада, обусловленные взаимодействием вицинальных функциональных групп в случае орто- изо¬меров, а также образованием ионов хиноидного типа для пара-изомеров. Рассмотрены скелетные и водород- ¬ные перегруппировки, типичные для метилбензоатов и конкретных блокирующих групп. Классифицированы характеристические ионы, образующиеся в результате перегруппировок орто-изомеров, особо отмечены ске¬летные перегруппировки, реализующиеся при образовании ионов пара-хиноидного типа. Для установления структуры и дифференциации изомеров в ряду производных замещенных бензойных кислот методом ГХ/МС предложены ключевые ионы.
Introduction
Chemical modification is a valuable technique effectively used in gas chromatography/mass spectrometry (GC/MS) to extend the range of compounds amenable to analysis. The goals for chemical modifications in GC/MS vary, and the derivatization step is usually applied to modify compounds that are unsuitable for GC/MS analysis, such as compounds with thermal lability, low GC mobility and resolution, or lacking structure-specific ions in their mass spectra. Selecting suitable reaction conditions and obtaining appropriate derivatives is an important step for the analysis to achieve desired volatility for chromatographic separation, and sufficient sensitivity, selectivity and specificity under electron ionization (EI). Acquisition of reliable chromatographic and EI fragmentation patterns for derivatives of common chemicals, and knowledge of the limitations for specific derivative types are important for the successful structure elucidation of unknown materials by GC/MS. Therefore, the NIST Mass Spectrometry Data Center (NIST MSDC) continues to acquire and evaluate data for chemical modification products. The objective in increasing the number of spectra of derivatives in the NIST/NIH/EPA Mass Spectral Library [1] is to provide a comprehensive capability to identify materials through characteristic fragmentation patterns.
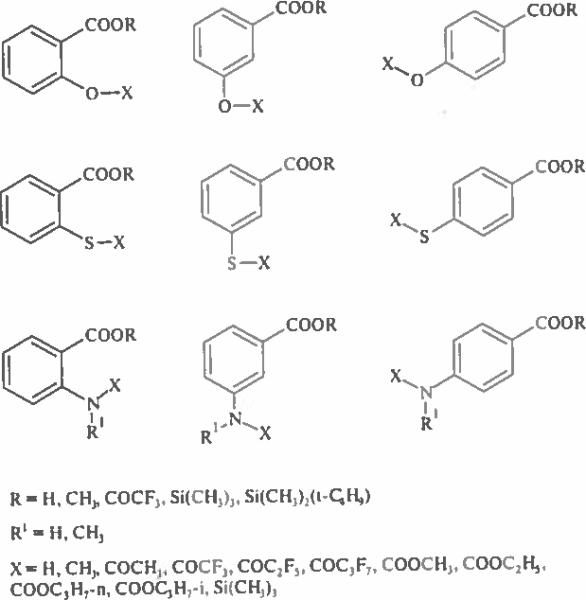
A systematic study of anthranilic, salicylic and thiosalicylic acids and their positional isomers has been carried out in continuation and amplification of efforts made by the NIST MSDC on the study of fragmentation processes of derivatives [2]. These isomeric hydroxyl, mercapto and amino benzoic acids are of practical interest because of their recognized anti-inflammatory properties, occurrence in nature as plant hormones, application in cosmetic, perfume and dye industries, and use in pharmacology as building blocks in the production of antiseptic and antifungal drugs [3–5]. Various derivatives studied in the present work are given in Fig. 1.
Figure 1.
Molecular structures of chemical modification products of salicylic, mercaptosalicylic and anthranilic acids, and their positional isomers.
Experimental1
Materials
“Puriss. p.a.” grade 2-, 3- and 4- substituted hydroxyl, mercapto and amino benzoic acids and their methyl esters were obtained from Sigma-Aldrich. The derivatization agents for alkylation (methyl, trideuteromethyl, ethyl, n-propyl and isopropyl iodides; methanol, 13C-methanol, ethanol, n-propanol and isopropanol), silylation (N,O-bis(trimethylsilyl) trifluoroacetamide/trimethylchlorosilane), N,O-bis(tert-butyldimethylsilyl)trifluoroacetamide), acylation (acetic, trifluoroacetic, pentafluoropropionic and heptafluorobutyric anhydrides), and for the synthesis of alkoxycarbonyl-derivatives (methyl, ethyl, n-propyl and isopropyl chloroformates) were of “derivatization” grade available at Sigma-Aldrich. Anhydrous pyridine, chloroform, acetonitrile, dimethylformamide, powdered sodium hydroxide and 0.1 mol L−1 hydrochloric acid solution were also commercially available at Sigma-Aldrich. “Analytical standard” grade C7-C40 saturated alkane mixture for GC calibration was purchased at Sigma-Aldrich.
Microsynthesis
Alkoxycarbonyl derivatives
The synthesis was carried out similarly to procedures described in [6]; 10 μL of alkyl chloroformate was added to a solution of 100 μL of 25 mmol L−1 aqueous hydrochloric acid, 53 μL of alcohol, 14 μL of pyridine and 1 mg of benzoic acid. Then 100 μL of chloroform containing 1 % of alkyl chloroformate (volume fraction) was added. An aliquot from the chloroform layer was separated and analyzed.
Other derivatization methods
Alkylation and trialkylsilylation were performed according to procedures described in [7–9]. Acylation was carried out according to [7, 10], and synthesis of mixed alkyl/acyl derivatives were carried out as described earlier [2].
Instrumentation and Data analysis
EI mass spectra were recorded on GC/MS systems with quadrupole analyzers (ionization energy 70 eV and ion source temperature 230 °C). Separation was achieved on a fused silica capillary column (15m, 0.25mm, 0.25 μm; non-polar stationary liquid phase: polymethylsiloxane + 5 % phenyl groups) with programming oven temperature from 150 °C to 270 °C at a rate of 10 °C min−1; the injection temperature was 270 °C. GC/MS2 data were obtained at 5, 10, 20 and 40 eV collision energies on systems with quadrupole analyzers using nitrogen as a gas for collision induced dissociation (CID). The data evaluation is based on comparison to spectra of corresponding 13C and 2H labeled analogs.
Results and discussions
Examination or EI mass spectral data of various chemical modification products (i.e. hydroxyl, mercapto and amino benzoic acids) has three objectives:
detection of characteristic “fingerprints” typical for specific classes of compounds;
determination of the consistency between a spectrum and the structure using ion thermo-chemistry along with the known ion decomposition rules to recognize possible exceptions to accepted organic mass spectrometry fragmentation rules;
documentation of fragmentation patterns useful for structure elucidation to classify and correlate reliably recognized patterns.
Comparative analysis of EI mass spectra of alkyl, trialkylsilyl, acetyl, perfluoroacyl and alkoxycarbonyl derivatives of hydroxyl, mercapto and amino benzoic acids reveals strong ortho-effects for derivatives of anthranilic, salicylic and thiosalicylic acids. Otherwise ubiquitous fragmentation of these “ortho-compounds” is almost completely suppressed by the interaction of vicinal substituents. This interaction of neighboring groups was observed early in the history of organic mass spectrometry [11]. In contrast, the EI spectra of meta- and para- isomers in these series of derivatives reflect generally similar, decomposition of M+− with some remarkable exceptions: 1,4-isomers depict para specificity leading to the formation of ions with a para quinoid-type structure. A similar para-effect was noted in the spectra of di-perfluoroacyl derivatives of bifunctional aminobenzenes [12].
para-Effect
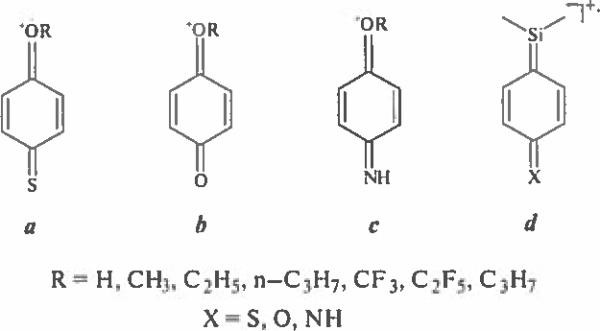
The 1,2- and 1,4-locations of functional groups in the chemical modification products of hydroxyl, mercapto and amino benzoic acids under EI may promote decomposition processes leading to the formation of stable ions containing the ortho and para quinoid structure. However, for ortho isomers this fragmentation pathway is negligible, and competing rearrangement processes dominate due to the interaction of vicinal groups. In contrast, decomposition pathways leading to ions a–d with para quinoid structure predominate for all 1,4-isomers (Scheme 1), although the routes for their formation may differ.
Scheme 1.
Characteristic ions for para isomers.
Structures of ions, their mass values and intensities of corresponding peaks in the spectra of derivatives of thiosalicylic, salicylic and anthranilic acids are given in Tabl. 1–3. Mass spectral and GCRI data for all derivatives will be included in the next release of the NIST/NIH/EPA mass spectral library (http://www.nist.gov/srd/nist1a.cfm).
Table 1.
Thiobenzoquinoid type ions in the spectra of derivatives of para-mercaptobenzoic acids.
| Compound | m/z (Rel. %) | Ion structure |
|---|---|---|
| 4-(Trifluoroacetylthio)benzoic acid | 125 (31) | HO+=C6H4=S |
| 4-(Pentafluoropropionylthio)benzoic acid | 125 (27) | |
| 4-(Heptafluorobutyrylthio)benzoic acid | 125 (24) | |
| 4-Methoxycarbonylthiobenzoic acid | 125 (58) | |
| 4-Ethoxycarbonylthiobenzoic c acid | 125 (20) | |
| 4-n-Propyloxycarbonylthiobenzoic acid | 125 (14) | |
| 4-Isopropyloxycarbonylthiobenzoic acid | 125 (13) | |
| Methyl 4-(methoxycarbonylthio)benzoate | 139 (31) | CH3O+=C6H4=S |
| Methyl 4-(ethoxycarbonylthio)benzoate | 139 (26) | |
| Methyl 4-(n-propyloxycarbonylthio)benzoate | 139 (26) | |
| Methyl 4-(isopropyloxycarbonylthio)benzoate | 139 (17) | |
| Methyl 4-(trifluoroacetylthio)benzoate | 139 (46) | |
| Methyl 4-(acetylthio)benzoate | 139 (13) | |
| Ethyl 4-(ethoxycarbonylthio)benzoate | 153 (9) | C2H5O+=C6H4=S |
| n-Propyl 4-(n-propyloxycarbonyl thio)benzoate | 167 (3) | C3H7O+=C6H4=S |
| Trifluoroacetyl 4-(trifluoroacetylthio)benzoyl anhydride | 193 (11) | CF3O+=C6H4=S |
| Trimethylsilyl 4-(trifluoroacetylthio)benzoate | 166 (12) | (CH3)2Si=C6H4=S+ |
| Trimethylsilyl 4-(pentafluoropropionylthio)benzoate | 166 (12) | |
| Trimethylsilyl 4-(heptafluorobutyrylthio)benzoate | 166 (14) | |
| t- Butyldimethylsilyl 4-(trifluoroacelylthio)benzoate | 166 (6) | |
| t-Butyldimethylsilyl 4-(pentafluoropropionylthio)benzoate | 166 (10) | |
| t-Butyldimethylsilyl 4-(heptafluorobutyrylthio)benzoate | 166 (11) | |
| Trimethylsilyl 4-(trimethylsilylthio)benzoate | 166 (12) | |
Table 3.
Benzoquinoid type ions in the spectra of derivatives of para-hydroxybenzoic acids.
| Compound | m/z (Rel. %) | Ion structure |
|---|---|---|
| 4-(Trifluoroacetyloxy)benzoic acid | 109 (10) | HO+=C6H4=O |
| 4-Hydroxybenzoic acid, N-methoxycarbonyl- | 109 (10) | |
| 4-Hydroxybenzoic acid, N-ethoxycarbonyl- | 109 (4) | |
| 4-Hydroxybenzoic acid, N-n-propyloxycarbonyl- | 109 (3) | |
| 4-Hydroxybenzoic acid, N-isopropyloxycarbonyl- | 109 (1) | |
| Methyl 4-(methoxycarbonyloxy)benzoate | 123 (8) | CH3O+=C6H4=O |
| Methyl 4-(ethoxycarbonyloxy)benzoate | 123 (5) | |
| Methyl 4-(n-propyloxycarbonyloxy)benzoate | 123 (7) | |
| Methyl 4-(isopropyloxycarbonyloxy)benzoate | 123 (6) | |
| Methyl 4-(trifluoroacetyloxy)benzoate | 123 (5) | |
| Methyl 4-(pentafluoropropionyloxy)benzoate | 123 (10) | |
| Methyl 4-(heptafluorobutyryloxy)benzoate | 123 (15) | |
| Methyl 4-(acetyloxy)benzoate | 123 (3) | |
| Trifiuoroacetyl 4-(trifluoroacetyloxy)benzoyl anhydride | 177 (1) | CF3O+=C6H4=O |
| Pentafluoropropionyl 4-(pentafluoropropionyloxy) benzoyl anhydride | 227 (.5) | C2F5O+=C6H4=O |
| Heptafluorobutyryl 4-(heptafluorobutyryloxy) benzoyl anhydride | 277 (0.4) | C3F7O+=C6H4=O |
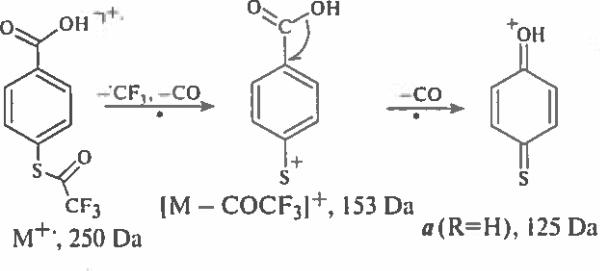
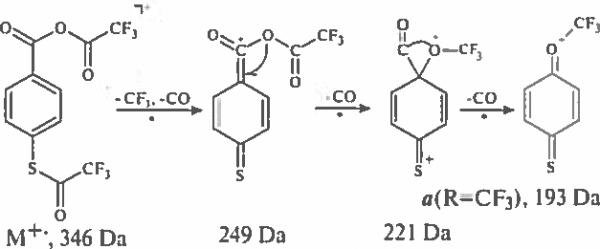
The formation of ions with a quinoid structure is demonstrated by considering the mass spectra of derivatives of 4-mercaptobenzoic acid. The simplest ion a (R=H) having a possible structure of protonated 1,4-cyclohexadien-3-thione-6-one cation at 125 Da is produced from M+− of para-(trifluoroacetylthio)benzoic acid as a result of a simple S–C bond cleavage followed by a skeletal rearrangement and a loss of carbon monoxide molecule. A two-step generation of ions a (R=H), presented in Scheme 2, is confirmed by CID data.
Scheme 2.
Formation of protonated 1,4-cyclohexadien-3-thione-6-one cation.
The methyl ester of the same derivative (methyl 4-(trifluoroacetylthio)benzoate) generates O-methyl cations a (R=CH3) via a similar route. The mass value of this ion at 139 Da is shifted to 142 Da in the spectrum of the corresponding trideuteromethyl ester indicating the presence in the cation a (R=CH3) of a methyl group originated from the carbomethoxy moiety.
O-Methyl cations a (R=CH3) are also typical for methyl 4-(alkoxycarbonylthio)benzoates (alk = methyl, ethyl, n-propyl, isopropyl), and a 1 Da mass shift is observed for this ion (from 139 Da to 140 Da) when comparing spectra of methyl and 13C-methyl 4-(alkoxycarbonylthio) benzoates (Fig. 2b, e). Ethyl 4-(ethoxycarbonylthio) benzoate and n-propyl 4-(n-propyloxycarbonylamino) benzoate are the sources for the formation of O-ethyl and O-n-propyl cations a (R=C2H5, C3H7) (Tabl. 1).
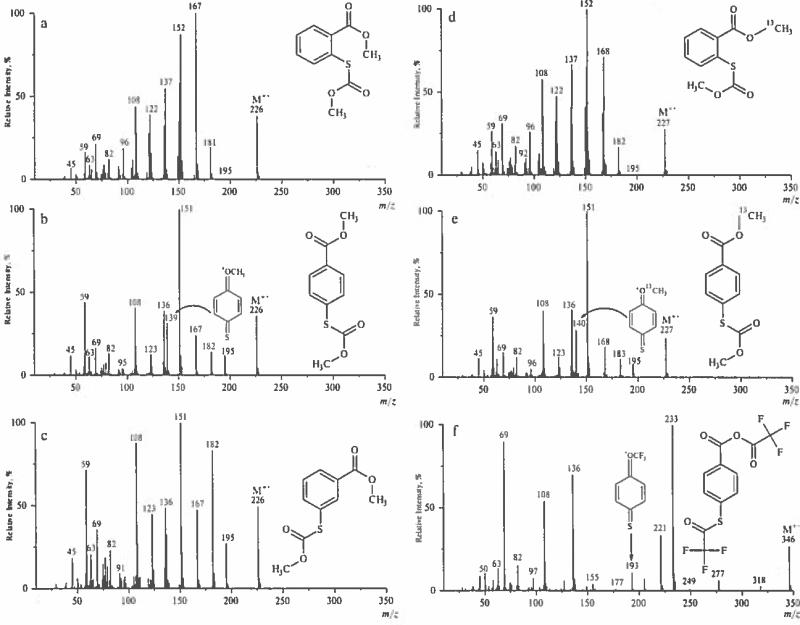
Figure 2.
Mass spectra of methyl: a – ortho-, b – para-, c – meta-S-methoxycarbonylmercaptobenzoates; 13C-methyl d – ortho-, e – para-S-methoxycarbonylmercaptobenzoates; f – anhydride of trifluoroacetic and S-trifluoroacetyllmercaptobenzoic acids.
Formation of O-trifluoromethyl cations a (R=CF3) becomes characteristic for an anhydride of trifluoroacetic acid and 4-(trifluoroacetylthio)benzoic acid (Fig. 2f. Successive loses of three carbon monoxide molecules from [M–CF3]+ ions (Scheme 3) are responsible for generation of cations a (R=CF3) as supported by CID experiments.
Scheme 3.
Elimination of three carbon monoxide molecules from [M–CF3]+ion.
Trialkylsilyl esters of 4-(perfluoroacylthio)benzoic acids form para quinoid-type ion fragments (d, X=S). However, there is a competing decomposition of the molecular ions to eliminate a methyl or a tert-butyl group from the trialkysilyl-function. The resulting [M–CH3 (or C4H9)]+ ions undergo skeletal rearrangement leading to the expulsion of carbon dioxide, and further elimination of another radical (COCF3−) violating the ‘even-electron rule’ [13, 14]. As a result 1,4-cyclohexadien-3-thion-6-dimethylsilyl radical cations [CH3)2Si=C6H4=S]+− are produced. Relative intensities of peaks of corresponding to these ions (d, X=S) in the spectra of seven various trimethylsilyl or tert-butyldimethylsilyl esters of 4-thtoperfluoroacylbenzoic acids are given in tabl. 1.
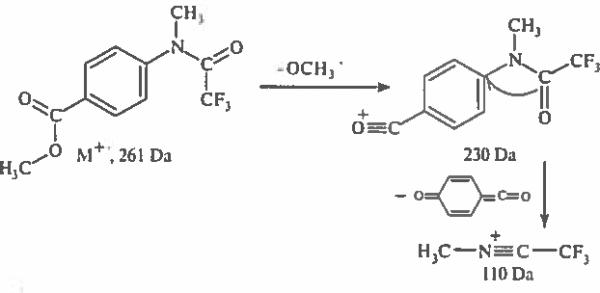
Para isomers of methyl esters of N-methyl-N-(trifluoroacetyl)aminobenzoic acid exhibit an alternative characteristic fragmentation. The N-methyl-C-trifluoroacetylnitrilium cation [CF3C=NCH3]+ at 110 Da dominates the spectrum of the para-isomer, whereas its relative intensity is 12 % and 57 % for ortho- and meta-positional isomers, respectively. The origin of this nitrilium cation has been reported earlier [15, 16], and its formation involving oxygen rearrangement is rationalized in Scheme.
The ions a, b, c and d, as well as nitrilium cations can be successfully employed for differentiation of para-isomers from their ortho- and meta- counterparts.
ortho-Effect
1,2-Location or aromatic functional groups and their interaction triggers specific decomposition reactions under EI and suppresses fragmentations characteristic for meta and para-isomers. This phenomenon is termed the ‘ortho effect’, and is well-documented [17–28]. Interactions of vicinal groups in the M+− of various derivatives of substituted benzoic acids initiate various types of hydrogen and skeletal rearrangements, and generate multiple fragmentation processes. As a result, for different derivatives within each type of benzoic acid (hydroxyl, mercapto or amino) diverse competing decomposition reactions are observed. Therefore, the ‘ortho-effect’ recorded for each derivative type is discussed separately.
Alkyl derivatives
Cations [M–CH3–H20]+ and [M–CH3–H2O–CO]+ are specific for the methyl ether or methyl salicylate (Fig. 3c,d). The M+− readily expels a methyl radical, and then 1,6-H transfers take place from the phenolic methoxy group to the carboxyl functionality; as a result, a water molecule is eliminated. The resulting cation [M–CH3–H2O]+ at 133 Da further eliminates CO giving rise to an ion at 105 Da. Similarly, the exclusive elimination of water containing the intact hydroxyl function and hydrogen from the methyl group has been confirmed by a complete analysis of MIKE spectra along with deuterium labelling studies and semi-empirical calculations for 2-methylbenzenemethanol and 2–methyl benzoic acid [14].
Figure 3.
Mass spectra of: a–meta-dimethyl-, b–para-dimethyl-, c–ortho- dimethyl-, d–ortho-di(trideuteromethyl)-hydroxybenzoic acids; e – dimethyl-, f – di(trideuteromethyl)-, g – O-methyl-S-trideuteromethyl-, h – O-trideuteromethyl-S-methyl-derivatives of ortho-mercaptobenzoic acid.
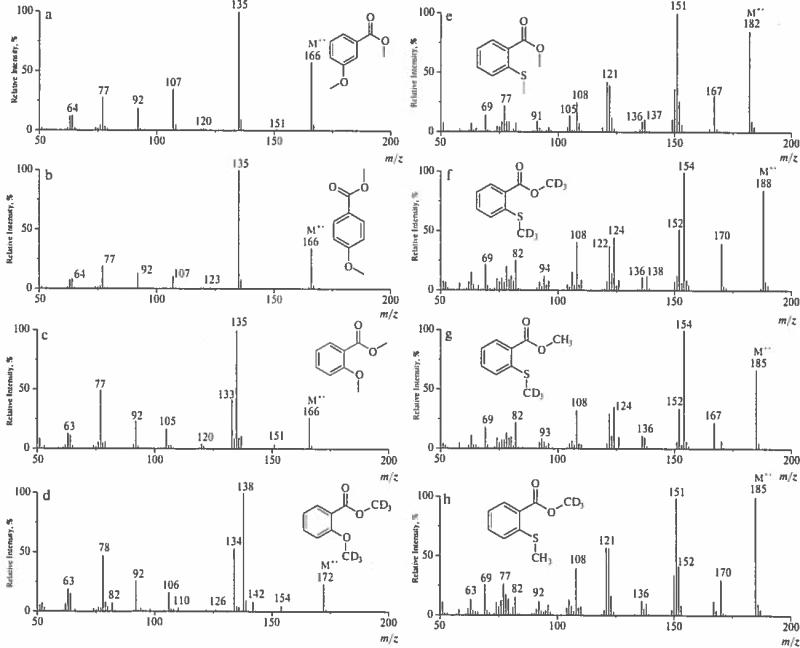
Radical-cations [M–CH3OH]+− (150 Da), [M–CH3OH-CO]+− (122 Da) and [M–CH3O–CH2O]+− (120 Da) become diagnostically important ions useful for distinction of methyl S-methylthiosalicylate, the ortho isomer, (Fig. 3e) from its meta and para counterparts. The unusual loss of a formaldehyde molecule from the [M–OCH3]+ cation is established both by CID experiments and comparison to the spectra of labeled analogs (Fig. 3f–h).
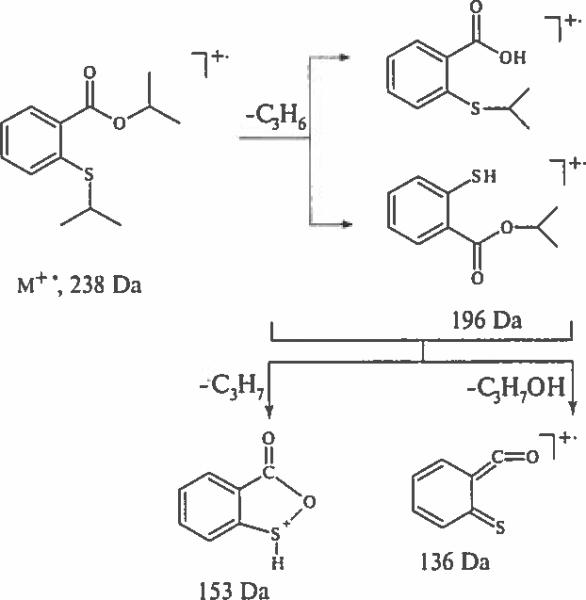
Fragmentation pathways for di-alkyl derivatives become predictable when the alkyl group is longer than methyl. Thus M+− of the di-isopropyl derivative of 2-mercaptobenzoic acid eliminates isopropene and isopropanol molecules giving rise to a base peak of an ion [M–C3H6–C3H7OH]+− at 136 Da (Scheme 5). The fragmentation of meta and para isomers is dominated by successive losses of two propene molecules, generating [M – 2C3H6]+− ions.
Scheme 5.
Decomposition of isopropyl 2-(isopropylthio) benzoate molecular ion.
Trimethylsilyl esters
Partially and completely derivatized trimethylsilyl (TMS) derivatives of hydroxyl, mercapto and amino benzoic acids, and some mixed derivatives indicate an expected dependence between the observed fragmentation pathways and the extent of functional group derivatization.
TMS hydroxyl, mercapto and aminobenzoates
Radicalcations [M–(CH3)3SiOH]+− are the key ions for distinguishing the TMS salicylates and thiosalicylates from their meta, para positional isomers, with the corresponding peaks among the most prominent in the spectra. These ions result from a 1,5-hydrogen shift followed by the elimination of trimethylsilylanol.
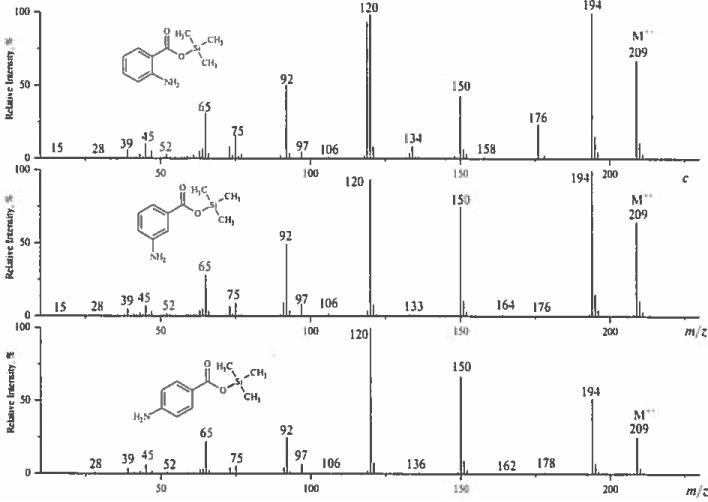
Related [M–(CH3)3SiOH]+− ions are diagnostically important for differentiation of isomeric TMS aminobenzoates. Thus, TMS anthranilate – the ortho isomer eliminates trimethylsilanol molecule and trimethylsilyloxy radical in a 1:1 ratio. Corresponding meta, para counterparts generate only [M–OSi(CH3)3]+ ions (Fig. 4). The formation of [M–OSi(CH3)3+ ions is a two-step decomposition process involving successive losses of methyl radical and neutral trimethylsilyl oxide as determined by CID experiments. In contrast, trimethylsiianol is eliminated from M+− via a single step, as a result of a 1,5 hydrogen shift rearrangement similar to the salicylate fragmentation mechanism.
Figure 4.
Mass spectra of trimethylsilyl esters of anthranilic acid and its meta- and para-isomers.
TMS O(S, NH)–TMS benzoates
Addition of TMS to both functional groups dramatically changes the major fragmentation routes of M+− of corresponding derivatives. Formation of an aromatic ring containing fragments, other than M+− and [M–CH3]+, is suppressed for di(TMS) derivatives of salicylic and thiosalicylic acids. Dominant peaks in the spectra appear at 73 Da (trimethyisilyl cation) for di-TMS salicylate, and at 147 Da (pentamethylsiloxanyl cation) for di-TMS–thiosalicylate. Ions containing silyl and aryl moieties are equally generated from M+− of di-TMS derivatives of meta and para hydroxyl and mercapto benzoic acids; prominent peaks in their spectra correspond to [M–CH3–CO2]+ ions.
TMS O(S, NH)-perfluoroacyl benzoates
Mixed derivatives formed using the perfluoroacyl group for blocking hydroxyl, mercapto or amino functions and trimethylsilyl for modification of the carboxyl group lead to generation of multiple fragmentation pathways. Ions produced as a result of vicinal group interactions and specific for ortho isomers are different for hydroxyl-, mercapto and amino benzoic acid derivatives.
Salicylates
The diagnostically important ions in the spectra of TMS O-(perfluoroacyl)salicylates formally correspond to methoxybenzoyl cation (135 Da) and a radical cation at 120 Da that is a result of -CH3 elimination from methoxybenzoyl cation.
Thiosalicylates
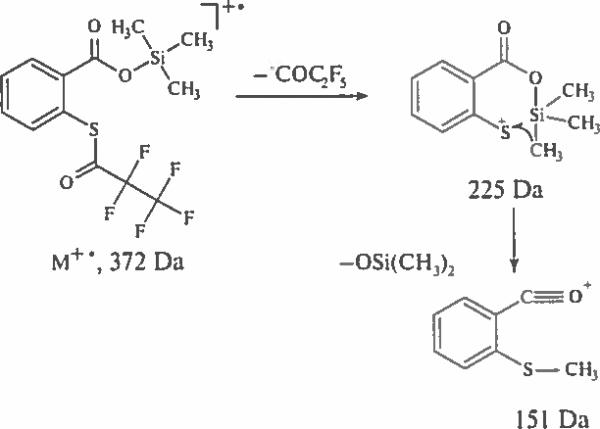
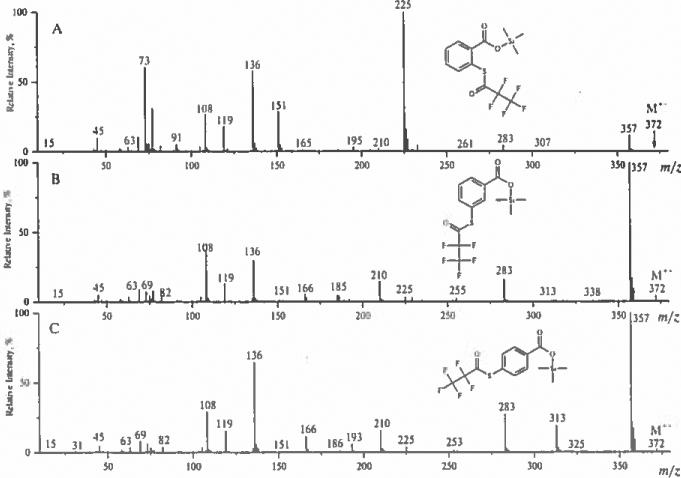
Two prominent peaks in the spectra can be successfully used for differentiation of TMS S-(perfluoroacyl)thiosalicylates from their meta and para counterparts. Thus, the spectrum of TMS S-(pentafluoropropionyl)thiosalicylate contains a base peak of [M–C2F5CO]+ ion at 225 Da and a prominent peak or [M–C2F5CO–(CH3)2SiO|+ that is a product of skeletal rearrangement and the loss of dimethylsilyl oxide from the ion at 225 Da (Scheme 6, Fig. 5).
Scheme 6.
Specific fragmentation of ortho-isomers of trimethylsilyl S-perfluoroacyl mercaptobenzoates.
Figure 5.
Mass spectra of trimethylsilyl esters of S-pentafluoropropionylthiosalicylic acid and its meta- and para-isomers.
Anthramlates
Another ion [M–CnF2n+1–(CH3)3SiOH]+ at 146 Da with maximum intensities in the spectra is characteristic for TMS O-(trifluoroacetyl)- and O-(pen-tafluoropropionyl)anthranilic acids. It can be successfully employed for differentiation of the ortho from its positional isomers.
TMS N–TMS-N-pentafluoropropionyl-benzoates
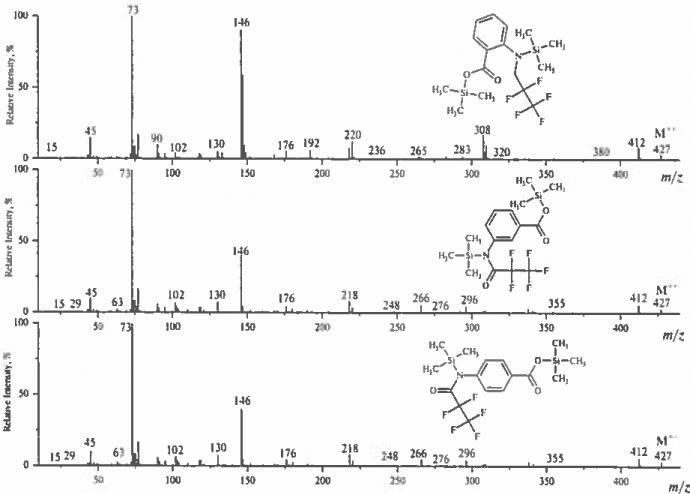
Introduction of an additional perfluoroacyl substituent to the amino group in O, N-di(trimethylsilyl)anthranilic acid molecule leads to the formation of ions more useful for structure elucidation. Thus, characteristic ions for TMS ester of N-TMS-N-pentafluoropropionylanthranilic acid are: [M–C2F5]+ and [M–C2F5-Si(CH3)4]+ (Fig. 6). They serve to differentiate the ortho- isomer from its meta- and para- counterparts.
Figure 6.
Mass spectra of trimethylsilyl esters of N-trimethylsilyl-N-pentafluoropropionylanthranilic acid and its meta- and para- isomers.
Methyl esters of acyl derivatives
Methyl esters of O-, S- or N-perfluoroacyl derivatives of benzoic acids exhibit fragmentation characteristics similar to their TMS counterparts.
Salicylates
The [M–OCH3–COCnF2n+1)+− ion at 120 Da that is generated by successive loss or two radicals dominates the spectra of methyl O-perfluoroacylsalicylates (Acyl = COCF3, COC2F5, COC3F7). A similar kind of violation of the ‘even-electron rule’ was reported [29] when a base peak of [M–30]+ was detected in the spectrum of TMS ester of methylsalicylate; it was determined that consecutive losses of two methyl radical ions resulted in the formation of this ion. The radical cations [M–OCH3–COCnF2n+1]+− can be used for distinguishing the ortho-isomer since M+− of corresponding meta and para counterparts do not exhibit these losses.
Thiosalicylates
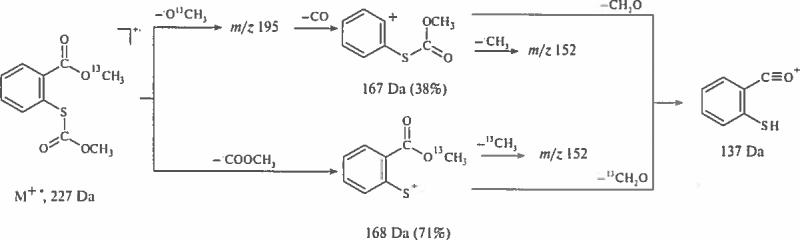
Two ions (M–COC nF2n+1–CH3]+ at 152 Da and [M–COC nF2n+1–CH2O]+ at 137 Da are sufficient for differentiation of methyl S-perfluoroacylsalicylate from its positional isomers.
Aminobenzoates
Methyl aminobenzoates may be analyzed in two ways – as N-acyl- and N-methyl-N-acyl-derivatives, since there is a possibility for the introduction of an additional alkyl to N- atom.
The presence of a base peak at 146 Da likely corresponds to the indol-2,3-dionyl cation in the spectrum of methyl N-trifluoroacetylanthranilate, and can be used for structure elucidation. This ion is a result of elimination of trifluoromelhyl radical followed by the loss of methanol.
The base peak at 132 Da, probably with a structure of indol-3-onyl cation, is characteristic of methyl N-methyl-N-trifluoroacetylanthranilate. Its corresponding molecular ion loses neutral CH3OH along with -OCH3 radical in a 1:1 ratio; elimination of methanol is possible if the N-methyl group is recognized as a potential hydrogen donor. Further, the ions at [M–CH3OH]+− eliminate -COCF3 radical producing the base peak at 132 Da.
The indol-2,3-dionyl and tndol-3-onyl cations can be employed for structure elucidation of methyl acylanthranilates.
Methoxycarbonyl derivatives
The character of 1,2-interaction between two methoxycarbonyl moieties connected to CAryl and O, S or N depends on the nature of a hetero-atom to which the methoxycarbonyl group is attached. Decomposition of methyl esters for methoxycarbonyl derivatives of hydroxyl and mercaptobenzoic acids appears more complex than their corresponding anthranilates.
Salicylates
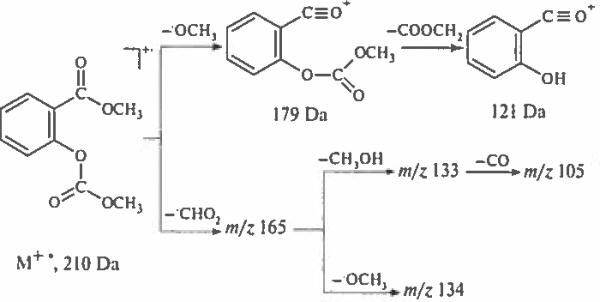
Peaks or four ions [M–HCO2]+ at 165 Da, [M–HCO2–OCH3]+− at 134 Da, [M–HCO2–CH3OH]+ at 133 Da and [M–OCH3–C2H2O2]+− at 133 Da are results of complex rearrangements (Scheme 7), These ions allow reliable differentiation of methyl methoxycarbonylsalicylate from meta- and para- isomers.
Scheme 7.
Fragmentation pathways useful for differentiation of methyl methoxycarbonylsalycilates from meta and para counterparts.
Thiosalicylates
Analysis of peak intensities and their ratios is required for differentiation of methyl thiosalicylate from its positional isomers (Fig. 2a–c). Ions specific for ortho isomers possess the following mass values: (122, 137, 150 and 152) Da. Comparative analysis of spectra for methyl thiosalicylate and 13C-methyl analog (Fig. 2d) allows differentiation of isobaric ions as denoted in Scheme 8.
Scheme 8.
Fragmentation pathways useful for differentiation of methyl S-(metoxycarbonyl) mercaptosalycilates from meta- and para- isomers.
Aminobenzoates
The key diagnostically important fragmentation of methyl N-methoxycarbonylanthranilate – the ortho isomer is associated with hydrogen rearrangement. It leads to the elimination of a neutral methanol molecule. The same derivative of the meta isomer expels both neutral methanol and a methoxy radical in a 1:2 ratio; the ortho and para-isomers eliminate only methoxy radicals. The base peaks in the spectra of all positional isomers formally correspond to the loss of hydrogen and two methoxy groups from M+−.
Meta isomerization
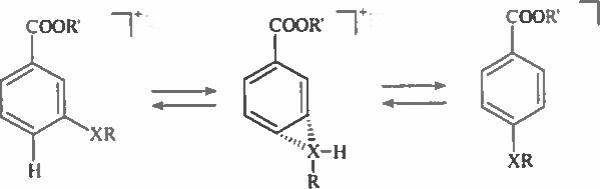
Molecular ions of meta isomers are not expected to produce ions with ortho- or para-quinoid structures, and the potential for interaction between 1,3-functional groups is unfavorable. Consequently, the fragmentation pathways for these isomers are straightforward, and decomposition of corresponding M+− is governed by well-established ion fragmentation rules, as exemplified by the EI spectra of methyl 3-methoxybenzoate (Fig. 3a), methyl 3-S-methoxycarbonylthiobenzoate (Figure 2c) and trimethylsilyl 3-pentafluoropropionylthiobenzoate (Fig. 5). However, the spectra of some derivatives contain low intensity peaks of ions characteristic for their ortho or para counterparts. Thus, a spectrum of methyl 3-S-methoxycarbonylthiobenzoate (Figure 2c) reveals a peak at 139 Da of 4% intensity vs 31 % for the para- isomer (Figure 2b). This may be a result of partial isomerism of meta- isomer to its para- counterpart under EI [30] (Scheme 9):
Scheme 9.
Possible isomerization of meta-isomer to para counterpart.
The same is true for the isomerism of meta- to ortho- there is a measureable peak at 133 Da (0.4 %) in the spectrum of methyl 3-methoxybenzoate (Fig. 3a). This peak corresponds to an ion [M–CH3–H20]+ and characteristic for the ortho isomer; the purity is controlled by GC.
Conclusion
GC/MS compatible derivatives for the identification of positional isomers of hydroxyl, mercapto and amino benzoic acids produce mass spectra with different characteristics that are useful for distinguishing among isobaric species. Methyl and trimethylsilyl esters of O, S- and N-alkyl, -trimethylsilyl, -acetyl, -perfluoroacyl and -alkoxycarbonyl derivatives of benzoic acids were analyzed and compared. General fragmentation pathways a described using the analysis of spectral data of labeled and unlabeled isotope analogs, supplemented by examination of CID data. Specific fragmentation processes for ortho isomers due to interaction of vicinal substituents are established; they mostly include additional hydrogen rearrangements. Skeletal rearrangements characteristic for para isomers are determined; the driving force for these rearrangements is the formation of stable para quinoid type ions. Knowledge of diagnostically important pathways can inform the selection of an appropriate derivative for an analysis by including the structural information required for structure differentiation. The choice of media is important as well: alkyl and acyl derivatives are prepared in anhydrous media while alkoxycarbonyl derivatives require the presence of water.
Scheme 4.
Formation of nitrilium cations.
Table 2.
Benzoquinoid type ions in the spectra of derivatives or para-aminobenzoic acids.
| Compound | m/z (Rel. %) | Ion structure |
|---|---|---|
| 4-Aminobenzoic acid, N-methoxycarbonyl- | 108 (10) | HO+=C6H4=NH |
| 4-Aminobenzoic acid, N-ethoxycarbonyl- | 108 (18) | |
| 4-Aminobenzoic acid, N- n-propyloxycarbonyl- | 108 (13) | |
| 4-Aminobenzoic acid, N-isopropyloxycarbonyl- | 108 (15) | |
| Methyl 4-(methoxycarbonylamino)benzoate | 122 (19) | CH3O+=C6H4=NH |
| Methyl 4-(ethoxycarbonylamino)benzoate | 122 (17) | |
| Methyl 4-(n-propyloxycarbonyl amino)benzoate | 122 (13) | |
| Methyl 4-(isopropyloxycarbonylamino)benzoate | 122 (10) | |
| Methyl 4-(trifluoroacetylamino)benzoate | 122 (8) | |
| Methyl 4-(pentafuoropropionylamino)benzoate | 122 (9) | |
| Methyl 4-(heptafluorobutyrylthio)benzoate | 122 (14) | |
| Methyl 4-(acetylamino)benzoate | 122 (3) | |
| Trifluoroacetyl 4-(trifluoroacetylamino)benzoyl anhydride | 176 (.8) | CF3O+=C6H4=NH |
| Pentafluoropropionyl 4-(pentafluoropropionylamino) benzoyl anhydride | 226 (.5) | C2F5O+=C6H4=NH |
| Heptafluorobutyryl 4-(heptafluorobutyrylamino) benzoyl anhydride | 276 (.5) | C3F7O+=C6H4=NH |
Acknowledgement
The authors thank prof. V.G. Zaikin, Dr. S. Markey Dr. J. A. Murray, Dr. R.A. Zangmeister and Dr. M. Lowenthal for useful reviews of the manuscript.
Footnotes
Часть I материала опубликована в Todua N.G., Tretyakov K.V., Mikaia A.I. Mass spectrometry of analytical derivatives. 1. Cyanide cations in the spectra of N-alkyl-N-perfluoroacyl-α-amino acids and their methyl esters//Eur. J. Mass Spectrom. 2015. Vol. 21, N 3. P. 183–190.
Certain commercial materials and instruments are identified in this paper in order to specify the experimental procedure adequately. Such identification is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the identified materials are necessarily the best available for the purpose.
References
- 1.NIST/NIH/EPA Mass Spectral Library . Standard Reference Database NIST 14, Standard Reference Data Program. National Institute of Standards and Technology; Gaithersburg, MD, USA.: [Google Scholar]
- 2.Todua NG, Tretyakov KV, Mikaia AI. Mass spectrometry of analytical derivatives. 1. Cyanide cations in the spectra of N-alkyl-N-perfluoroacyl-a-amino acids and their methyl esters. Eur. J. MassSpectrom. 2015;21(3):183–190. doi: 10.1255/ejms.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juurlink BHJ, Azouz HJ, AldalaU AMZ, Altinawi BMH, Ganguly P. Hydroxybenzoic acid isomers and the cardiovascular system. Nutrition J. 2014;13 doi: 10.1186/1475-2891-13-63. Article 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma S, Srivastava VK, Kumar A. Newer N-substituted anthranilic acid derivatives as potent antiinflamatory agents. Eur. J. Medicinal Chem. 2002;37(8):689–697. doi: 10.1016/s0223-5234(02)01340-5. [DOI] [PubMed] [Google Scholar]
- 5.Deng G, Zhang X, Zhang J, Quan J, Zhu W. Rapid determination or salicylic acid in plant materials by gas chromatography - mass spectrometry. Chromatographia. 2003;58(3-4):225–229. [Google Scholar]
- 6.Husck P, Sweelcy CC. Gas chromatographic separation of protein amino acids in four minutes. High. Res. Chromatogr. 1991;14(11):751–753. [Google Scholar]
- 7.Zaikin V, Halket J. A Handbook of derivatives for mass spectrometry. IMPublications; Chichester, UK: 2009. [Google Scholar]
- 8.Knapp DR. Handbook of analytical derivalization reactions. Wiley; Chichester: 1979. [Google Scholar]
- 9.Halket J, Zaikin VG. Derivatization in mass spectrometry. 3. Alkylation(arylation) Eur. J. Mass Spectrom. 2004;10(1):1–19. doi: 10.1255/ejms.619. [DOI] [PubMed] [Google Scholar]
- 10.Zaikin VG, Halket J. Derivatization in mass spectrometry 2. Acyiation. Eur. J. Mass Spectrom. 2003;9(5):421–434. doi: 10.1255/ejms.576. [DOI] [PubMed] [Google Scholar]
- 11.Teeter RM. Mass spectra of derivatives ofO-aminobcnzoic acid. Anal Chem. 1966;38(12):1736–1740. [Google Scholar]
- 12.Tretyakov KV, Todua NG, Borisov RS, Zaikin VG, Stein SE, Mikaia AI. Unique para-effect in electron ionization mass spectra of bis(pcrnuoroacyl) derivatives of bifunctional aminobenzencs. Rapid Commun. Mass Spectrom. 2010;24(17):2529–2532. doi: 10.1002/rcm.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kami M, Mandelbaum A. The ‘even-electron rule’. Org. Mass Spectrom. 1980;15(1):53–64. [Google Scholar]
- 14.Bowen RD, Harrison AG. Loss of methyl radical from some small immonium ions: Unusual violation of the even-electron rule. Org. Mass Spectrom. 1981;16(4):180–182. [Google Scholar]
- 15.Todua NG, Tretyakov KV, Borisov RS, Zhilyaev DI, Zaikin VG, Stein SE, Mikaia AI. Rapid Commun. Mass Spectrom. Vol. 25. 6: 2011. Electron ionization mass spectra of alkylated sulfabenzamides; pp. 750–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irikura KK, Todua NG. Facile smiles-type rearrangement in radical cations of N-acylarylsulfonamides and analogs. Rapid Commun. Mass Spectrom. 2014;28(7):829–834. doi: 10.1002/rcm.6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hvistendahl G, Undheim K. Mass spectrometry of onium compounds - XVII: anilinium carboxylatcs. Ortho effect in anthranilic acid derivatives. Org. Mass Spectrom. 1973;7(5):627–633. [Google Scholar]
- 18.Jariwala FB, Figus MM, Attygalle AB. Ortho effect in electron ionization mass spectrometry of N-acylanilines bearing a proximal halo substituent. J. Amer. Soc. Mass Spectrom. 2008;19(8):1114–1118. doi: 10.1016/j.jasms.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Tajima S, Azami T, Tsuchiya T. An investigation of structure of intermediate ions from several substituted alkyl benzoatesby the ortho effect and kinetic energy release. J. Mass Spectrom. Soc. Japan. 1979;72(2):247–254. [Google Scholar]
- 20.Djerassi C, Fenselau C. Mass spectrometry in structural and stereochemical problems. LXXXVI. The hydrogen transfer reactions in butyl propionate, benzoatc, and phthalate. J. Amer. Chem. Soc. 1965;87(24):5756–5762. [Google Scholar]
- 21.Attygale A, Ruzicka J, Varughese D, Sayed J. An unprecedented ortho effect in mass spectrometric fragmentation of even-electron negative ions from hydroxyphenyl carbaldehydes and ketones. Tetrahedron Letters. 2006;47(27):4601–4603. [Google Scholar]
- 22.Stambolija LJ, Stefanovic D. Deuterium isotope effects in mass spectrometry. Mechanism of formation of the [CfiHsS]+ ion in the decomposition of S-phenyl methylthiocarbamate. Org. Mass Spectrom. 1973;7(12):1415–1417. [Google Scholar]
- 23.Martens J, Praefcke K, Schwarz H. Massenspcktrometrische untersuchungen tiber ortho-Effekte und verwandte Umlagerungen bei Bcnzoe- und 2,2′-DiphensaurcDerivaien. Liebigs, Ann. Chem. 1975 [Google Scholar]
- 24.Martens J, Praefcke K, Schwarz H. Spektroskopischc untersuchungen. IX. Die analytische bedeutung des ortho-effektcs in dcr massenspektromctric: untersuchungen von benzoe- und thiobenzoesaure-derivaten. Z. Naturforsch. B. 1975;30(1):259–262. [Google Scholar]
- 25.Dankkiewicz W. Electron ionization-induced fragmentation of N-alkyl-O-nitroanUines: observation of new types of ortho effects. Eur. Mass Spectrom. 1998;4(3):167–179. [Google Scholar]
- 26.Schwarz H. Some newer aspects of mass spectrometric ortho effects. Top. Curr. Chem. 1978;73(2):231–263. [Google Scholar]
- 27.Barkow A, Pilotek S, Grutzmacher H-F. Ortho effects a mechanistic study. Eur J. Mass Spectrom. 1995;1(6):525–537. [Google Scholar]
- 28.Riley JS, Baer T, Marbury GD. Sequential ortho effects: characterization of novel [M-35|+ fragment ions in the mass spectra of 2-alkyl-4,6-dinitrophenols. Amer. Soc. Mass Spectrom. 1991;2(1):69–75. doi: 10.1016/1044-0305(91)80062-C. [DOI] [PubMed] [Google Scholar]
- 29.Krauss D, Mainx HG, Tauscher B, Btschof P. Fragmentation trimethylsilyl derivatives of 2-alkoxyphenols: a further violation of the ‘even-electron rule’. Org. Mass Spectrom. 1985;20(10):614–618. [Google Scholar]
- 30.Kuck D. Mass spectrometry of alkylbenzenes and related compounds. Part 1. Gas-phase ion chemistry of alkylbenzene radical cations. MassSpectrom. Rev. 1990;9(2):188–233. [Google Scholar]