Abstract
Background
Vector control remains the mainstay to effective malaria management. The negative implications following persistent application of synthetic insecticides geared towards regulation of mosquito populations have necessitated prospection for ecofriendly effective chemistries. Plant-derived compounds have the potential to control malaria-transmitting mosquito populations. Previously, Agerantum conyzoides extracts have demonstrated toxicity effects on disease-transmitting mosquitoes. However, their efficacy in controlling Afrotropical malaria vectors remains unclear. Herein, the toxicity and growth disruption activities of crude methanolic leaf extract of A. conyzoides on Anopheles gambiae sensu stricto and An. arabiensis larvae were assessed.
Methods
Late third (L3) instars of An. gambiae s.s and An. arabiensis larvae were challenged with increasing doses of crude methanolic extract of A. conyzoides. The larval mortality rates were recorded every 24 h and the LC50 values determined at their associated 95% confidence levels. ANOVA followed by Post-hoc Student-Newman-Keuls (SNK) test was used to compare results between treatment and control groups. Phytochemical profiling of the extract was performed using standard chemical procedures.
Results
Treatment of larvae with the methanolic extract depicted dose-dependent effects with highest mortality percentages of ≥ 69% observed when exposed with 250 ppm and 500 ppm for 48 h while growth disruption effects were induced by sublethal doses of between 50–100 ppm for both species. Relative to experimental controls, the extract significantly reduced larval survival in both mosquito species (ANOVA, F(8,126) = 43.16776, P < 0.001). The LC50 values of the extract against An. gambiae s.s ranged between 84.71–232.70 ppm (95% CI 81.17–239.20), while against An. arabiensis the values ranged between 133.46–406.35 ppm (95% CI 131.51–411.25). The development of the juvenile stages was arrested at pupal-larval intermediates and adult emergence. The presence of alkaloids, aglycone flavonoids, triterpenoids, tannins and coumarins can partly be associated with the observed effects.
Conclusion
The extract displayed considerable larvicidal activity and inhibited emergence of adult mosquitoes relative to experimental controls, a phenomenon probably associated with induced developmental hormone imbalance. Optimization of the bioactive compounds could open pathways into vector control programmes for improved mosquito control and reduced malaria transmission rates.
Keywords: Agerantum conyzoides, Malaria, Anopheles gambiae s.s, Anopheles arabiensis, Larvicidal activity, Vector control, Growth disruption
Background
The operational scale up of indoor residual spraying (IRS), long-lasting insecticide treated nets (LLINs) and artemisinin-based combined therapy (ACT) over the last decade progressively declined transmission rates of malaria to vulnerable children and pregnant women [1]. An estimate of 69% fewer malaria cases has been reported in sub-Saharan Africa between 2001–2015 following the widespread deployment of the three key interventions [1]. However, evolution of resistance to the active ingredients of these tools and little consideration of developing new complementary compounds targeting the ever changing behavioral traits of Afrotropical malaria vectors have greatly challenged efforts geared to bring malaria under control [2–4]. Nevertheless, vector control forms the integral platform of integrated malaria management aimed to reduce malaria reproduction rate to less than 1 [5, 6].
Larviciding, a less-practiced component of integrated vector management (IVM) and larval source management (LSM), appears a promising approach of suppressing both indoor and outdoor feeding mosquito populations [7–9]. Impressive stories from Brazil and Egypt following its impactful malaria eradication motivates its revival [10] with current operation in Kenya [11], The Gambia [8], Burkina Faso, Benin [12] and Tanzania [13]. Low immobility, confinement to shallow water bodies, susceptibility to chemical attacks and less chances of developing resistance favor this vector control approach [14, 15]. Additionally, manipulation and/or modification of larval habitat bio-physicochemical parameters negatively influence vector competence of resultant mosquitoes suggesting a feasible target of mosquito control [16, 17]. For millennia, mosquito control has considerably relied on chemicals that inevitably reduced environmental quality and facilitated emergence of resistant mosquito strains a phenomenon that has limited their continued reliance, prompting for alternative chemistries [18].
One feasible way of averting the aforementioned drawbacks is prospecting for novel compounds with less environmental impacts and selectively toxic to target arthropods [19, 20]. In addition to being a rich source of bioactive pharmacophores, plants produce allelochemicals with great potential of controlling crop pests and disease-transmitting vectors [21, 22]. Among these are essential oils documented to repel nuisance human biting mosquitoes in addition to inducing toxicity to developing juveniles [23–25]. Non-volatiles, for instance, Azadirachtin and its derivatives from neem tree and plant-based ecdysteroidal analogs potentially inhibit larval development and adult emergence terminating insect metamorphosis immaturely [25, 26]. Additionally, these compounds induce growth disruption effects resulting into mortalities and non-viable females incapable of lineage progression [27–29]. Taken together, plant-derived compounds are promising sources of effective insecticides with meager chances of resistance development afforded by multimodal targets [30, 31].
Agerantum conyzoides L. is an Asteraceae herbaceous weed that grows in many countries worldwide. Ethnopharmacological surveys of this polyherbal plant have documented biological activities such as analgesic, anti-inflammatory, purgative, febrifuge, anti-asthmatic, antibacterial, antifungal, antispasmodic, anti-diarrhoeic, headache relief, antihelmintic and nematicidal [32, 33]. Phytochemically, the plant contain various bioactive compounds including alkaloids, coumarins, flavonoids, tannins and essential oils [34, 35]. Of considerable interest, the plant extracts have shown detrimental effects on survival, development and adult emergence of mosquitoes such as Aedes albopictus [36], Culex quinquefasciatus [37], Aedes aegypti and Anopheles stephensi [38] which has been attributed to possibility of compounds with anti-juvenile hormone activity. However, effectiveness of the plant extracts to control the principal Afrotropical malaria vectors An. gambiae sensu stricto and An. arabiensis remain obscure. Therefore, we sought to evaluate the larvicidal and developmental disruption effects of A. conyzoides against An. gambiae s.s and An. arabiensis. Our findings demonstrate for the first time to the best of our knowledge that, the methanolic leaf extract of A. conyzoides had considerable larvicidal and development inhibition activities in a dose-dependent manner against Afrotropical malaria vectors. In addition, we identified alkaloids, aglycone flavonoids, triterpenoids, tannins and coumarins as phytochemicals that were associated with the observed bioactivities.
Methods
Collection of plant material
Ethnobotanical survey was conducted to identify the existing gaps of A. conyzoides based on chemotaxonomic criterion. A. conyzoides leaf samples were collected from Shinyalu in Kakamega County of western Kenya in September, 2015. The plant was identified by Mr. Thomas Mbasi an ethnobotanical specialist at Kakamega Forest Reserve, and a voucher specimen deposited at the same institution. The leaf samples were packaged in a non-sterile adsorbent paper and transported to the laboratory for processing, extraction and bioactivity assays.
Preparation of crude leaf extracts
The leaves were shade-dried at room temperature to a constant weight and ground into a fine powder using an electric miller (Retsch Muhle, Haan, Germany). A 100 g of the leaf powder was subjected to methanol (Sigma Aldrich, St. Louis, USA) (2 L) extraction using soxhlet extraction technique for 8–10 h. After cooling to room temperature, the resultant extract was concentrated using a rotary evaporator (Laborota 4000 efficient, Heidolph, Germany) at a temperature of 40 °C under vacuum and stored at −20 °C until required for larvicidal bioassays.
Phytochemical profiling
The principal bioactive secondary metabolites including alkaloids, terpenoids, steroids, aglycone flavonoids, tannins and coumarins were assayed using standard procedures [39].
Mosquito colony culture
The experiments were carried out with An. gambiae s.s and An. arabiensis larvae from a colony maintained at the International Centre of Insect Physiology and Ecology (icipe) Insect Mass Rearing Unit. The larvae were separately reared under laboratory conditions of water temperature (28 ± 2 °C), relative humidity of 55–60% and 12:12 h (light: dark) photoperiod. The larvae were reared in large plastic pans (37 × 31 × 6 cm) with distilled water at densities of 200–300 per pan and supplemented with artificial diet Tetramin® fish meal (Tetra GmbH, Melle, Germany). The rearing water was replaced with fresh water and diet after every two days. Pupae were held in plastic cups and transferred into standard 30 × 30 × 30 cm rearing cages. Emergent adults were provided with 10% sucrose solution contained in a glass tube (2 × 8 cm) connected to a paper tube as a wick. Female mosquitoes were blood-fed on restrained Swiss albino mice about 4–5 days post-emergence and provided with oviposition plastic containers (11.5 cm in diameter and ~ 6.2 cm in depth, lined interiorly with a piece of filter paper as oviposition site) for egg collection 2–3 days after blood meal. The eggs were air-dried under insectarium conditions ready for colony cycle maintenance.
Larvicidal bioassays
The bioassays were conducted in accordance with the World Health Organization guidelines for testing larvicides [40] and adopted by Nyamoita et al., [41]. Crude methanolic extract (250 mg, 125 mg, 50 mg and 25 mg) was separately dissolved in 1 ml of analytical grade ethanol (Fisher Scientific, Loughborough, UK) and diluted with 499 ml of distilled water to make a 500 ml stock solution. This was then dispensed into five beakers each 100 ml to make the required concentrations of 500 ppm, 250 ppm, 100 ppm and 50 ppm, respectively. The bioassays were performed with batches of 20 (n = 20) late third instar larvae (L3) of An. gambiae and An. arabiensis per beaker. The assays were replicated five times and ran simultaneously yielding a total of 100 larvae for each dosage. The control was set up with 1 ml of ethanol diluted in 499 ml distilled water and dispensed into five beakers. The larvae were fed on TetraMin® fish meal (Tetra GmbH, Melle, Germany) during the testing period. Larval mortality (at higher doses) and morphological defects (at lower doses) were monitored at intervals of 24 h until the death of the last larva or emergence of an adult. Larvae were considered dead if they remained irresponsive within a span of two minutes when gently probed with a pipette. The number of the dead larvae was expressed as average percentage mortality for each concentration relative to negative controls.
Statistical analysis
The corrected larval mortality was expressed as % mean ± S.D of experimental replicates for each dosage of the extract. Dose-responses were analyzed by non-linear regression and half-maximal lethal concentrations (LC50) estimated at their associated 95% confidence levels using R software version 3.2.3 [42]. Significant differences between treatment means were established with analysis of variance (ANOVA) followed by Student-Newman-Keuls (SNK) test and p values of less than 0.05 considered statistically significant. Graphs were designed using GraphPad Prism version 7.01 for Windows (GraphPad Software, San Diego, California, USA).
Results
Phytochemical analysis
Qualitative analysis of A. conyzoides leaf extract revealed presence of alkaloids, aglycone flavonoids, triterpenoids, coumarins and tannins (Table 1).
Table 1.
Phytochemical constituents of crude leaf extract of A. conyzoides
| Phytochemical constituent | Absence/presence |
|---|---|
| Alkaloids | + |
| Flavonoids | + |
| Tannins | + |
| Coumarins | + |
| Terpenoids | + |
+ (Presence), − (Absence)
Effect of the crude extracts on larval survival
The toxicity of crude methanolic extract of A. conyzoides against late 3rd instars of An. gambiae s.s and An. arabiensis was evaluated. The toxicity of the extract was demonstrated to be dose-dependent with high doses of 250 ppm and 500 ppm showing ≥ 69% larval mortality at 48 h post-exposure compared to the lower doses of 50 ppm and 100 ppm which gave < 50% larval mortality (Table 2). Maximum larval mortality (100%) was recorded at 500 ppm on exposure to An. gambiae s.s larvae for 48 h with only 88% attained against An. arabiensis. A 100% larval survival was noted in the negative control group for the entire analysis period. Relative to controls, the extract significantly reduced survival rates of An. gambiae s.s (ANOVA, F(4,70) = 115.5534, P < 0.001) and An. arabiensis larvae (ANOVA, F(4,70) = 31.7382, P < 0.001). There was significant susceptibility difference between the two mosquito species to the extract (ANOVA, F(8,111) = 25.6398, P < 0.001). A time- and dose-dependent reduction in survival rates of extract-challenged mosquito larvae was demonstrated in Fig. 1.
Table 2.
Mean larval mortality evoked by the A. conyzoides extract at different concentrations against 3rd instar larvae of An. gambiae s.s and An. arabiensis
| Time | % mean mortality ± S.Da | Lethal concentration (ppm) | |||||
|---|---|---|---|---|---|---|---|
| 500 ppm | 250 ppm | 100 ppm | 50 ppm | Control | LC50 | 95% CI | |
| An. gambiae s.s larvae | |||||||
| 24 h | 84 ± 11.94 | 64 ± 17.82 | 35 ± 8.37 | 0 ± 0.00 | 0 ± 0.00 | 232.70 | 228.85–239.20 |
| 48 h | 100 ± 0.00 | 93 ± 8.37 | 65 ± 23.45 | 19 ± 12.94 | 0 ± 0.00 | 84.31 | 81.17–90.88 |
| 72 h | 100 ± 0.00 | 93 ± 8.37 | 65 ± 23.45 | 19 ± 12.94 | 0 ± 0.00 | 84.31 | 81.17–90.88 |
| An. arabiensis larvae | |||||||
| 24 h | 60 ± 15.41 | 27 ± 10.37 | 20 ± 2.24 | 0 ± 0.00 | 0 ± 0.00 | 406.35 | 403.56–411.25 |
| 48 h | 88 ± 5.70 | 69 ± 15.97 | 36 ± 6.52 | 24 ± 9.62 | 0 ± 0.00 | 133.46 | 131.51–136.16 |
| 72 h | 88 ± 5.70 | 93 ± 5.70 | 68 ± 10.37 | 49 ± 10.25 | 0 ± 0.00 | 133.46 | 131.51–136.16 |
Data expressed as % mean mortality ± standard deviation (S.D), LC50- lethal concentration that killed 50% of mosquito larvae population, CI Confidence Interval
aMortality means are significantly different at p ≤ 0.05 (Student-Newman-Keuls test)
Fig. 1.
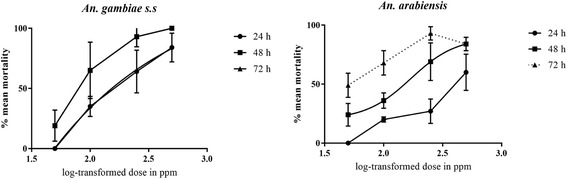
Dose-response curves for An. gambiae s.s and An. arabiensis larvae to A. conyzoides extract for 24 h, 48 h and 72 h post exposure. Doses are log-transformed and each point on the plots represents percentage mean (± S.D) larval mortality of 5 replicates for each dose of the extract
Developmental disruption effects
Besides lethality of high doses, the extract remarkably accelerated the growth of larvae into pupae resulting into incomplete melanization and abnormal dead larval-pupal intermediates as depicted in Fig. 2. At sublethal doses of 50 ppm and 100 ppm, molting continued normally but the development of the immature stages was greatly affected. Microscopic examination of the dead immature stages at 25× magnification revealed morphological defects evident as:- abnormal dead larval-pupal intermediates and emergent adults with mouthparts and wings folded within the pupal exuvium (Fig. 2). The adults that luckily emerged from the extract-treated water were unable to escape from the pupal caste and died on the surface of test solution. Overall, the extract at sublethal doses induced prolonged larval phase duration by 7 more days prior to pupation relative to negative controls (2 days).
Fig. 2.

Development disruption effects of A. conyzoides extract to An. gambiae s.s and An. arabiensis. a Demelanized An. gambiae s.s larvae (b) Abnormal An. gambiae s.s larval-pupal intermediate (c) Arrested adult emergence in An. gambiae (d) Abnormal An. arabiensis larval-pupal intermediate (e) Failed adult emergence in An. arabiensis (f) An. gambiae s.s control larvae (g) Normal An. gambiae s.s larval-pupal intermediate (h) An. arabiensis control larvae (i) Normal An. arabiensis larval-pupal intermediate (Light microscopy visualization conducted at magnification 25×)
Discussion
In search for better insecticides to replace or complement the synthetic insecticides and alleviate resistance pressure on malaria vectors, scientists have turned interests into nature for alternative controls. Many plants have been reported around the globe to have bioactivity against mosquitoes and their multiple targets of actions against mosquitoes assure effectiveness as alternative bio-insecticides. The toxic efficacy of these botanicals against various mosquito vectors vary depending on different factors such as the part of the plant used, method of extraction adopted, solvent used, geographical locality the plant was obtained, the concentration of the extract used and photosensitivity of some plant compounds [43].
In the current study, we challenged late third (L3) instar larvae of An. gambiae s.s and An. arabiensis with crude extract of A. conyzoides to evaluate their responses. Our data demonstrate that the extract had detrimental effects on both survival and development of An. gambiae s.s and An. arabiensis in a dose-dependent response manner. High dosages of 250 ppm and 500 ppm evoked acute toxicity to the developing larvae while the sublethal doses of 50 and 100 ppm induced developmental disruptions as shown by Fig. 2(b-e). The toxic effect of the plant extract could be attributed to its bioactive phytochemical constituents (Table 1). The LC50 values of the plant extract have shown significant potential of controlling An. gambiae s.s and An. arabiensis. It has been previously reported that the plant extract had larvicidal activity against Ae. albopictus [36], C. quinquefasciatus [37], Ae. aegypti and An. stephensi [38] which is similar to our data though slight variation was noted. This could be attributed to the solvents used for extraction, susceptibility differences of mosquito vectors used and geographical differences of the plant. Methanolic extract of A. conyzoides leaves was used in the present study and found effective against An. gambiae s.s and An. arabiensis larvae.
Moreover, phytochemicals extracted from many plant species have been reported to show growth inhibiting effects on the various developmental stages of different mosquito species [27–29, 40, 43]. Various pre-emergent effects such as prolongation of larval instar and pupae durations, inhibition of larval and pupal molting, morphological abnormalities and mortality may occur especially during molting and melanization processes. Developmental disruption effects induced by the plant extracts can be associated with disturbed hormonal balance or interference in chitin synthesis during the molting process. Our data recorded morphological effects on An. gambiae s.s and An. arabiensis where the immature stages failed to transform into a normal adult leading to eventual death (Fig. 2c and e). Similar results on morphological abnormalities were reported on An. stephensi, Ae. aegypti and C. quinquefasciatus exposed to A. conyzoides extract. The phenomenon has been reported by Okunade, [35] as a result of perturbation of hormonal homeostasis by precocene-3,4-epoxide, a metabolite generated by cytochrome P450s in the insect body [44]. The metabolite may either antagonize or agonize the biosynthesis and subsequent release of juvenile hormone, the regulator of insect metamorphosis.
Studies carried out by Nyamoita et al., [41], Nathan et al., [45] and Nathan et al., [46] reported that in addition to their lethality, the secondary metabolites of the botanicals used resulted in protracted larval phase, disrupted growth and malformation of the exoskeleton. Although there was no elongation of gut as observed in [29] and [47], incomplete melanization process was observed in larvae and some pupae examined under light microscopy (Fig. 2). Our data corroborated with that obtained by Ndung’u et al., [28] where limonoids from methanolic extracts of the root of Turraea mombassana Hiern (Meliaceae) resulted in larval and pupal morphological deformities in An. gambiae s.s due to incomplete melanization. Similarly, exposure of Anopheles stephensi to extracts of Melia azedarch resulted in similar observations [45]. Also, compounds from Azadirachta indica and Melia volkensii (Meliceae) extracts induced growth disruption effects to mosquito larvae besides feeding deterrence and toxicity [48]. Studies performed by Govindachari et al., [49], Martinez and Van Emden, [50] and Nathan et al., [51] confirmed the above effects of Azadirachtin on insects. Elsewhere, dichloromethane extract of Hyptis brevis (Lamiaceae) displayed strong growth inhibition on Spodoptera littoralis larvae by arresting metamorphosis [52]. The same phenomenon has been reported by Cespedes et al., [53].
Several plant species produce a myriad of bioactive chemicals as part of defenses against herbivory attacks majorly classified as volatile compounds (essential oils) and non-volatiles. The non-volatiles include the alkaloids, flavonoids, terpenoids, glucosinolates, cyanogenic glycosides, phenolic acids among others [31]. Majority of these non-volatiles particularly phytoecdysteriods, phytojuvenoids and anti-juvenile hormones act as insect growth regulators (IGRs) reducing survival rates and development of insects upon ingestion [54]. Previous reports indicate various insecticidal compounds isolated and identified from A. conyzoides extracts such as steroids, flavonoids, coumarins, pyrrolizidine alkaloids, triterpenoids, and chromenes [36, 55–57]. In this regard, phytochemical analysis revealed presence of main compounds such as alkaloids, terpenoids (e.g. precocene I and precocene II) [56], flavones (e.g. ageconyflavones A, B and C) [57], coumarins and tannins which equally agree with these reports. All these compounds may act in a concerted manner to nonspecifically induce toxicity to insects. More specifically, precocenes (terpenoids) have been reported to be anti-juvenile hormone, accelerating the development of insects and inducing dwarfness associated with low survival rates [43]. Phytochemicals that agonize or antagonize the effects of insect development hormones have been reported to be good bio-pesticides [53]. These compounds disrupt the normal metabolism of the insect hormones during the development of the juveniles leading to failure of adult emergence [55].
Two important insect developmental hormones that interplay are 20-hydroxyecdysone (20-E) and juvenile hormone (JH) [58]. It is the balance in levels of these two hormones that define the outcome of each developmental transition [59]. Ligand-binding to the insect juvenile receptor complex disrupt insect endocrine signaling and regulation causing abnormal development and lethality [21]. The accumulation of these plant compounds above threshold levels disrupt the insects’ developmental progression culminating into premature death or failure to emerge as a normal adult [60]. The active compounds from A. conyzoides extract induced toxicity and growth inhibition effects to developing mosquito larvae and could potentially be isolated for formulating effective mosquito control agents. Further, identification of molecular targets, ligand docking and simulation assays accompanied by field applications could be pursued for improved mosquito control.
Conclusions
The findings of our study showed promising larvicidal and development disrupting effects of A. conyzoides extract on the main Afrotropical malaria vectors, An. gambiae s.s and An. arabiensis. Phytochemicals present within the extract including alkaloids, aglycone flavonoids, triterpenoids, tannins and coumarins were associated with the observed experimental effects. They have potential of being used as insecticides for controlling mosquito populations around human dwellings by targeting the immature stages. Noteworthy, prior to commercial application of this botanical larvicide, factors such as safety of non-targets and beneficial organisms, efficacy in actual field conditions, and residual half-life must be put into consideration.
Acknowledgements
The authors are grateful to International Centre of Insect Physiology and Ecology (icipe) for providing the experimental insects and necessary facilities to perform this study successfully. We extend our gratitude to Mr. Thomas Mbasi for identifying the plant under the study.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Authors’ contributions
JMM, SNN, CC, RMM conceived and designed experiments. JMM carried out experiments. JMM, CC, RMM provided reagents/materials and analysis tools. JMM, SNN analyzed data. JMM wrote first manuscript draft. All authors read and approved the final version.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Abbreviations
- ACT
Artemisinin-based combined therapy
- icipe
International Centre of Insect Physiology and Ecology
- IRS
Indoor residual spraying
- IVM
Integrated vector management
- LLINs
Long-lasting insecticide treated nets
- LSM
Larval source management
Contributor Information
Jackson Mbithi Muema, Email: Jackson_mbithi@yahoo.com.
Sospeter Ngoci Njeru, Email: hicogn@gmail.com.
Céline Colombier, Email: celine135@hotmail.fr.
Rose Muthoni Marubu, Email: rmarubu@icipe.org.
References
- 1.WHO World malaria report 2015. World Heal Organ. 2015;2015:1–280. [Google Scholar]
- 2.Ranson H, Lissenden N. Insecticide resistance in African Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 2016;32:187–96. doi: 10.1016/j.pt.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Russell TL, Beebe NW, Cooper RD, Lobo NF, Burkot TR. Successful malaria elimination strategies require interventions that target changing vector behaviours. Malar J. 2013;12:1–5. doi: 10.1186/1475-2875-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nkya TE, Akhouayri I, Kisinza W, David J-P. Impact of environment on mosquito response to pyrethroid insecticides: facts, evidences and prospects. Insect Biochem Mol Biol. 2013;43:407–16. doi: 10.1016/j.ibmb.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 5.MalERA A research agenda for malaria eradication: vector control. PLoS Med. 2011;8:e1000401. doi: 10.1371/journal.pmed.1000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karunamoorthi K. Vector control: a cornerstone in the malaria elimination campaign. Clin Microbiol Infect. 2011;17:1608–16. doi: 10.1111/j.1469-0691.2011.03664.x. [DOI] [PubMed] [Google Scholar]
- 7.Tusting LS, Thwing J, Sinclair D, Fillinger U, Gimnig J, Bonner KE, et al. Mosquito larval source management for controlling malaria. Cochrane Database Syst. Rev. 2013;8:CD008923–CD008923. [DOI] [PMC free article] [PubMed]
- 8.Majambere S, Pinder M, Fillinger U, Ameh D, Conway DJ, Green C, et al. Is mosquito larval source management appropriate for reducing malaria in areas of extensive flooding in the Gambia? A cross-over intervention trial. Am J Trop Med Hyg. 2010;82:176–84. doi: 10.4269/ajtmh.2010.09-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker K, Lynch M. Contributions of Anopheles larval control to malaria suppression in tropical Africa: review of achievements and potential. Med Vet Entomol. 2007;21:2–21. doi: 10.1111/j.1365-2915.2007.00674.x. [DOI] [PubMed] [Google Scholar]
- 10.Killeen GF, Fillinger U, Kiche I, Gouagna LC, Knols BGJ. Eradication of Anopheles gambiae from Brazil: lessons for malaria control in Africa? Lancet Infect Dis. 2002;2:618–27. doi: 10.1016/S1473-3099(02)00397-3. [DOI] [PubMed] [Google Scholar]
- 11.Fillinger U, Lindsay SW. Larval source management for malaria control in Africa: myths and reality. Malar J. 2011;10:10–1186. doi: 10.1186/1475-2875-10-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dambach P, Traore I, Becker N, Kaiser A, Sie A, Sauerborn R. EMIRA: ecologic malaria reduction for Africa–innovative tools for integrated malaria control. Glob Health Action. 2014;7:25908. doi: 10.3402/gha.v7.25908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maheu-Giroux M, Castro MC. Cost-effectiveness of larviciding for urban malaria control in Tanzania. Malar J. 2014;13:1–12. doi: 10.1186/1475-2875-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Killeen GF, Fillinger U, Knols BGJ. Advantages of larval control for African malaria vectors: low mobility and behavioural responsiveness of immature mosquito stages allow high effective coverage. Malar J. 2002;1:1–7. doi: 10.1186/1475-2875-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Killeen GF, Tanner M, Mukabana WR, Kalongolela MS, Kannady K, Lindsay SW, et al. Habitat targeting for controlling aquatic stages of malaria vectors in Africa. Am J Trop Med Hyg. 2006;74:517–8. [PubMed] [Google Scholar]
- 16.Moller-Jacobs LL, Murdock CC, Thomas MB. Capacity of mosquitoes to transmit malaria depends on larval environment. Parasit Vectors. 2014;7:1–12. doi: 10.1186/s13071-014-0593-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mwangangi JM, Mbogo CM, Muturi EJ, Nzovu JG, Kabiru EW, Githure JI, et al. Influence of biological and physicochemical characteristics of larval habitats on the body size of Anopheles gambiae mosquitoes (Diptera: Culicidae) along the Kenyan coast. J Vector Borne Dis. 2007;44:122–7. [PMC free article] [PubMed] [Google Scholar]
- 18.Isman MB. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu Rev Entomol. 2006;51:45–66. doi: 10.1146/annurev.ento.51.110104.151146. [DOI] [PubMed] [Google Scholar]
- 19.Khater HF. Ecosmart biorational Insecticides: alternative insect control strategies. In: Perveen F, editor. Insectic Adv Integr Pest Manag. Rijeka, Croatia: InTech; 2012. [Google Scholar]
- 20.Regnault-Roger C, Vincent C, Arnason JT. Essential oils in insect control: low-risk products in a high-stakes world. Annu Rev Ento. 2011;57:405–24. doi: 10.1146/annurev-ento-120710-100554. [DOI] [PubMed] [Google Scholar]
- 21.Lee S-H, Oh H-W, Fang Y, An S-B, Park D-S, Song H-H, et al. Identification of plant compounds that disrupt the insect juvenile hormone receptor complex. Proc Natl Acad Sci U S A. 2015;112:1733–8. doi: 10.1073/pnas.1424386112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.George D, Finn R, Graham K, Sparagano O. Present and future potential of plant-derived products to control arthropods of veterinary and medical significance. Parasit Vectors. 2014;7:1. doi: 10.1186/1756-3305-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carroll SP, Loye J. PMD, a registered botanical mosquito repellent with deet-Like efficacy. J Am Mosq Control Assoc. 2006;22:507–14. doi: 10.2987/8756-971X(2006)22[507:PARBMR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Mdoe FP, Cheng S-S, Msangi S, Nkwengulila G, Chang S-T, Kweka EJ. Activity of Cinnamomum osmophloeum leaf essential oil against Anopheles gambiae ss. Parasit Vectors. 2014;7:209. doi: 10.1186/1756-3305-7-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maia MF, Moore SJ. Plant-based insect repellents: a review of their efficacy, development and testing. Malar J. 2011;10:S11. doi: 10.1186/1475-2875-10-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imbahale SS, Mukabana WR. Efficacy of neem chippings for mosquito larval control under field conditions. BMC Ecol. 2015;15:1. doi: 10.1186/s12898-015-0041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.AJ M (L), Morgan ED, Nisbet AJ. Azadirachtin, a natural product in insect control. Amsterdam: Elsevier; 2005. pp. 117–35. [Google Scholar]
- 28.Ndung’u M, Torto B, Knols BGJ, Hassanali A. Laboratory evaluation of some eastern African Meliaceae as sources of larvicidal botanicals for Anopheles gambiae. Int J Trop Insect Sci. 2004;24:311–8. [Google Scholar]
- 29.Kihampa C, Joseph CC, Nkunya MHH, Magesa SM, Hassanali A, Heydenreich M, et al. Larvicidal and IGR activity of extract of Tanzanian plants against malaria vector mosquitoes. J Vector Borne Dis. 2009;46:142–52. [PubMed] [Google Scholar]
- 30.Nyamoita MG. Toxicity of individual and blends of pure phytoecdysteroids isolated from vitex schiliebenii and vitex payos against Anopheles gambiae ss larvae. World J Org Chem. 2013;1:1–5. [Google Scholar]
- 31.Mithöfer A, Boland W. Plant defense against herbivores: chemical aspects. Annu Rev Plant Biol. 2012;63:431–50. doi: 10.1146/annurev-arplant-042110-103854. [DOI] [PubMed] [Google Scholar]
- 32.Lima MAS, Barros MCP, Pinheiro SM, do Nascimento RF, de Abreu Matos FJ, Silveira ER. Volatile compositions of two asteraceae from the north‐east of Brazil: ageratum conyzoides and acritopappus confertus (Eupatorieae). Flavour Fragr. J. 2005;20:559–61. [Google Scholar]
- 33.Melo NI, Magalhães LG, Carvalho CE, Wakabayashi KAL, Magalhães LG, Aguiar GP, et al. M. CAE 2011. Schistosomicidal activity of the essential oil of Ageratum conyzoides L. (Asteraceae) against Schistosoma mansoni adult worms. Molecules. 2011;16:762–73. doi: 10.3390/molecules16010762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamboj A, Saluja A. Ageratum conyzoides L.: a review on its phytochemical and pharmacological profile. Int J Green Pharm. 2008;2:59. doi: 10.4103/0973-8258.41171. [DOI] [Google Scholar]
- 35.Okunade AL. Ageratum conyzoides L. (Asteraceae) Fitoterapia. 2002;73:1–16. doi: 10.1016/S0367-326X(01)00364-1. [DOI] [PubMed] [Google Scholar]
- 36.Liu XC, Liu ZL. Evaluation of larvicidal activity of the essential oil of ageratum conyzoides L. aerial parts and its major constituents against aedes albopictus. J Entomol Zoolo Stud. 2014;2:345–50. [Google Scholar]
- 37.Saxena RC, Jayashree S, Padma S, Dixit OP. Evaluation of growth disrupting activity of agerantum conyzoides crude extract on Culex quinquefasciatus (Diptera: Culicidae) J Environ Biol. 1994;15:67–74. [Google Scholar]
- 38.Saxena A, Saxena RC. Effects of Agerantum conyzoides extract on the developmental stages of malaria vector, Anopheles stephensi (Diptera, Culicidae) J Environ Biol. 1992;13:207–9. [Google Scholar]
- 39.Harborne AJ. Phytochemical methods a guide to modern techniques of plant analysis. 3. London: chapman and Hall; 1998. p. 302. [Google Scholar]
- 40.WHO . Report of the WHO Informal consultation on the evaluation and testing of insecticides. Geneva: WHO; 1996. [Google Scholar]
- 41.Nyamoita MG, Ester I, Zakaria MH, Wilber L, Bwire OJ, Ahmed H. Comparison of the effects of extracts from three Vitex plant species on Anopheles gambiae ss (Diptera: Culicidae) larvae. Acta Trop. 2013;127:199–203. doi: 10.1016/j.actatropica.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 42.R Core Team . R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 43.Ghosh A, Chowdhury N, Chandra G. Plant extracts as potential mosquito larvicides. Indian J Med Res. 2012;135:581–98. [PMC free article] [PubMed] [Google Scholar]
- 44.Borthakur N, Baruah AKS, Bhagat SD. Search for precocenes in Agerantum conyzoides Linn of North East India. J Indian Chem Soc. 1987;64(9):580–1. [Google Scholar]
- 45.Nathan SS, Savitha G, George DK, Narmadha A, Suganya L, Chung PG. Efficacy of Melia azedarach L. extract on the malarial vector Anopheles stephensi Liston (Diptera: Culicidae). Bioresour. Technol. 2006;97:1316–23. doi: 10.1016/j.biortech.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 46.Nathan SS, Hisham A, Jayakumar G. Larvicidal and growth inhibition of the malaria vector Anopheles stephensi by triterpenes from Dysoxylum malabaricum and Dysoxylum beddomei. Fitoterapia. 2008;79:106–11. doi: 10.1016/j.fitote.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 47.Innocent E, Nkunya MHH, Hassanali A. Larvicidal activity of Kotschya uguenensis plant powders and methanol extracts against Anopheles gambiae ss larvae in the laboratory and in simulated ponds. J Appl Pharm Sci. 2013;3:122. [Google Scholar]
- 48.Al‐Sharook Z, Balan K, Jiang Y, Rembold H. Insect growth inhibitors from two tropical Meliaceae: effect of crude seed extracts on mosquito larvae1. J Appl Entomol. 1991;111:425–30. doi: 10.1111/j.1439-0418.1991.tb00344.x. [DOI] [Google Scholar]
- 49.Govindachari TR, Suresh G, Wesley SD. Insect antifeedant and growth regulating activities of neem seed oil - the role of major tetranortriterpenoids. J Appl Entomol. 2000;124:287–91. doi: 10.1046/j.1439-0418.2000.00480.x. [DOI] [Google Scholar]
- 50.Martinez SS, Van Emden HF. Growth disruption, abnormalities and mortality of Spodoptera littoralis (Boisduval)(Lepidoptera: Noctuidae) caused by azadirachtin. Neotrop Entomol. 2001;30:113–25. doi: 10.1590/S1519-566X2001000100017. [DOI] [Google Scholar]
- 51.Nathan SS, Kalaivani K, Murugan K. Effects of neem limonoids on the malaria vector Anopheles stephensi Liston (Diptera: Culicidae) Acta Trop. 2005;96:47–55. doi: 10.1016/j.actatropica.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 52.Sakr HH, Roshdy SH, El-Seedi HR. Hyptis brevipes (Lamiaceae) extracts strongly inhibit the growth and development of Spodoptera littoralis (Boisd.) larvae (Lepidoptera: Noctuidae) J Appl Pharm Sci. 2013;3:83. [Google Scholar]
- 53.Cespedes CL, Molina SC, Muñoz E, Lamilla C, Alarcon J, Palacios SM, et al. The insecticidal, molting disruption and insect growth inhibitory activity of extracts from Condalia microphylla Cav. (Rhamnaceae) Ind Crops Prod. 2013;42:78–86. doi: 10.1016/j.indcrop.2012.05.002. [DOI] [Google Scholar]
- 54.Varma J, Dubey NK. Prospectives of botanical and microbial products as pesticides of tomorrow. Curr Sci. 1999;76:172–8. [Google Scholar]
- 55.Trigo JR, Campos S, Pereira AM. Presença de alcalóides pirrolizidinicos em Ageratum conyzoides L. Simp. Plantas Med. do Bras. Sao Paulo. (Resumos). 1988. p. 13.
- 56.Ming LC. Ageratum conyzoides: a tropical source of medicinal and agricultural products. Perspect New Crop New Uses. 1999;469–73.
- 57.Vyas AV, Mulchandani NB. Polyoxygenated flavones from Ageratum conyzoides. Phytochemistry. 1986;25:2625–7. doi: 10.1016/S0031-9422(00)84523-9. [DOI] [Google Scholar]
- 58.Nijhout HF. Insect hormones. Princeton: Princeton Univ. Press; 1994. p. 280. [Google Scholar]
- 59.Dubrovsky EB. Hormonal cross talk in insect development. Trends Endocrinol Metab. 2005;16:6–11. doi: 10.1016/j.tem.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 60.Riddiford LM, Ashburner M. Effects of juvenile hormone mimics on larval development and metamorphosis of Drosophila melanogaster. Gen Comp Endocrinol. 1991;82:172–83. doi: 10.1016/0016-6480(91)90181-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.


